Abstract
A lytic phage (øZCW1) was isolated from an algicidal bacterium Pseudoalteromonas sp. strain SP48 that specifically kills the toxic dinoflagellate Alexandrium tamarense. We demonstrated that øZCW1 could trigger the growth of A. tamarense by inhibiting the growth of algicidal bacterium SP48. In contrast, the growth of A. tamarense was suppressed when cocultured with either SP48 or the øZCW1-resistant mutant of SP48. This study provides the first evidence of the indirect impact of bacteriophage on bloom-forming microalgae via phage lysis of alga-killing bacteria.
TEXT
Massive growth of phytoplankton often results in algal blooms in aquatic systems, and blooms of harmful algae can exert negative effects on aquatic ecosystems (12). Although harmful algal blooms (HABs) occur worldwide, the initiation and termination of HABs are still not well understood (2). Bacteria are known to actively interact with HAB species (6, 7). Many algicidal bacteria have been isolated in association with HAB-causing algae, and these bacteria can inhibit the growth of harmful algae (3, 13, 21, 22, 33). The algicidal activity of these bacteria can be influenced by other coexisting organisms in nature, such as competition of nonalgicidal bacteria and prey by heterotrophic protists (14, 15). An unexplored area is the impact of viral lysis on algicidal bacteria and the subsequent (or indirect) effect of such a viral activity on bloom-forming algae.
Viruses are the most abundant biological entities in the aquatic ecosystem, and they often outnumber bacteria by 15-fold (25). Viruses are regarded as one of the major elements in the ocean for regulating biogeochemical cycling, mediating gene transfer, influencing climate change, and modulating community structure (5, 8, 26). Viral infection can account for a significant portion of microbial mortality and is believed to be as important as grazing by protists in keeping microbial biomass in balance (27). Algicidal bacteria become abundant during harmful algal blooms (4, 20). Rapid growth and high cell density of algicidal bacteria make them more vulnerable to phage infection and lysis (29, 32). The lytic activity of phage on algicidal bacteria could reduce the algal lysis activity of bacteria. We hypothesize that phage can influence the formation, growth, and termination of bloom-forming algae by interacting with algicidal bacteria.
A marine bacterium that kills the toxic dinoflagellate Alexandrium tamarense was isolated and designated Pseudoalteromonas sp. strain SP48 in our previous study (24). Pseudoalteromonas sp. strain SP48 produces heat-tolerant algicidal compounds and has a strong algicidal activity against A. tamarense. The aim of this study is to investigate the impact of bacteriophage on bloom-forming algae by modulation of the population dynamics of algicidal bacteria.
Isolation and characterization of bacteriophage and phage-resistant bacterium mutant.
Lytic bacteriophage infecting Pseudoalteromonas sp. SP48 was isolated from surface seawater collected from Xiamen Sea, China (24.4°N, 118.1°E) by using the double-layer plaque assay of Adams (1). A bacteriophage that formed clear, round plaques (ca. 2.5 mm in diameter) on strain SP48 was isolated and designated φZCW1. The morphology of φZCW1 was examined using a JEM2100 transmission electron microscope (TEM). φZCW1 is a myovirus with an isometric head (ca. 123 nm in diameter) and a long, contractile tail (ca. 235 nm long) (Fig. 1). φZCW1 is a double-stranded DNA virus, and it has a latent period of ca. 1.5 h and a burst size of ca. 91 (see Fig. S1 in the supplemental material).
Fig. 1.
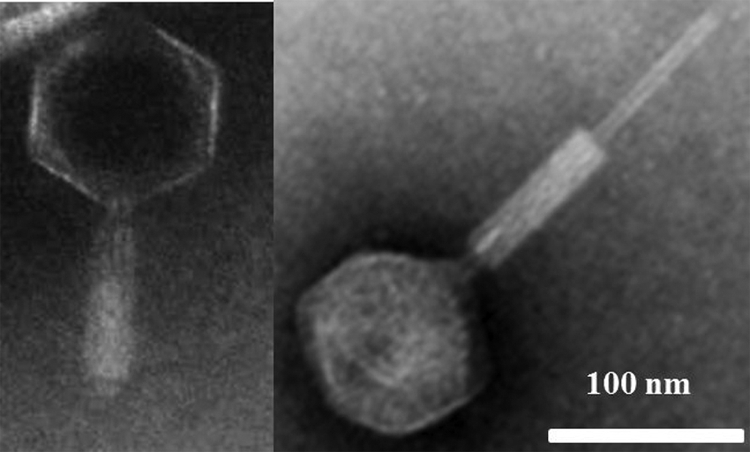
Micrographs of phage φZCW1 with a contractile tail (left panel) and contracted tail (right panel) as viewed in a JEM2100 transmission electron microscope.
Twenty bacterial strains were used to test the host specificity of φZCW1 (Table 1). Susceptibility of bacteria to φZCW1 was determined by the appearance of plaques on double-layer agar plates. φZCW1 caused lysis of only Pseudoalteromonas sp. SP48 and not the other bacterial strains tested (Table 1), demonstrating the high host specificity of this phage.
Table 1.
φZCW1 host range assay and bacterial strains used in this study
| Bacterial strain tested | Algicidal activitya | Geographic origin of strain | Susceptibilityb |
|---|---|---|---|
| Pseudoalteromonas sp. SP48 | + | East China Sea | + |
| Alteromonas sp. SP31 | + | East China Sea | − |
| Alteromonas sp. SP44 | + | East China Sea | − |
| Pseudoalteromonas sp. DHQ17 | + | East China Sea | − |
| Pseudoalteromonas sp. DHQ4 | + | East China Sea | − |
| Pseudoalteromonas sp. DHQ5 | + | East China Sea | − |
| Pseudoalteromonas sp. DHY3 | + | East China Sea | − |
| Pseudoalteromonas sp. HH2 | + | East China Sea | − |
| Pseudoalteromonas sp. HH5 | + | East China Sea | − |
| Idiomarina sp. SP96 | + | East China Sea | − |
| Alteromonas sp. DH46 | + | East China Sea | − |
| Vibrio sp. DH51 | + | East China Sea | − |
| Halomonas sp. DH77 | + | East China Sea | − |
| Marinobacter sp. CN46T | + | South China Sea | − |
| Marinobacter sp. CN74T | + | South China Sea | − |
| Marinobacter sp. B17 | − | East China Sea | − |
| Marinobacterium sp. AN9 | − | South Korea | − |
| Marinobacterium sp. KCTC12756 | − | Deokjeok Island, South Korea | − |
| Marinobacterium sp. F19 | − | East China Sea | − |
| Novosphingobium sp. F2 | − | East China Sea | − |
Symbols: +, with algicidal activity; −, without algicidal activity.
Symbols: +, sensitive to viral infection; −, resistant to viral infection.
A φZCW1-resistant SP48 mutant, SP48-M, was obtained from a bacterial colony recovered from a clear plate by the plaque assay by the method of Huang et al. (11). The phage-resistant activity of SP48-M was sustained for several hundred generations.
Phage-bacterium-microalga assemblies.
In order to understand the interactions between the HAB species A. tamarense, algicidal bacterium SP48 (that specifically kills A. tamarense), and the lytic phage φZCW1 (that specifically kills SP48), different assemblages of these microbial consortia were established (Table 2). Experiment 1 contained 100 ml of exponentially growing A. tamarense culture (ca. 13,000 cells ml−1). For experiments 2, 3, and 4, exponentially growing bacterial cultures were washed in f/2 medium (9) by centrifugation (3,000 × g, 15 min) three times to remove extracellular algicidal compounds. Experiment 2 included the mixture of 100 ml of exponentially growing A. tamarense (ca. 13,000 cells ml−1) and SP48 (ca. 4.7 × 107 cells ml−1). For experiments 3 and 4, washed bacterial cultures, strains SP48 (ca. 4.9 × 107 cells ml−1) and SP-M (ca. 1 × 108 cells ml−1), were mixed with bacteriophage φZCW1 separately with a phage/bacterium ratio of 1:10. The mixture was added to 100 ml exponentially growing A. tamarense. Aliquots (10 ml) of Zobell 2216E medium (BD Bioscience) were added to all four assemblages to support the growth of bacteria (24). Each experiment was carried out in triplicate. Axenic A. tamarense ATGD98-006 cultures used in this study were obtained by removing free-living and attached bacterial symbionts using detergents and antibiotics (23). The axenic cultures were grown in f/2 medium (without silicate) at 20°C on a 12-h light-dark cycle (12-h light–12-h dark) with a photo flux density of 50 μmol m−2 s−1. The algicidal activity of Pseudoalteromonas sp. SP48 and lytic activity of bacteriophage φZCW1 were evaluated by microscopic counting of bacterial cells and virus-like particles, respectively, following the protocol of Patel et al. (19). To count A. tamarense, samples were fixed with Lugol's iodine followed by direct microscopic counting (24).
Table 2.
Different assemblage setups for experiments
| Expt | Harmful alga | Bacteria | Bacteriophage |
|---|---|---|---|
| Expt 1 | A. tamarense | ||
| Expt 2 | A. tamarense | Pseudoalteromonas sp. SP48 | |
| Expt 3 | A. tamarense | Pseudoalteromonas sp. SP48 | φZCW1 |
| Expt 4 | A. tamarense | Pseudoalteromonas sp. SP48-M | φZCW1 |
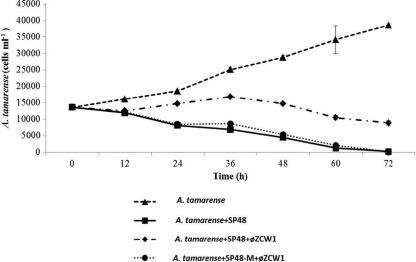
In experiment 1, dinoflagellate A. tamarense grew from 13,600 to 38,566 cells ml−1 in 72 h (Fig. 2). The growth of A. tamarense was inhibited and decreased from 13,600 cells ml−1 to an undetectable level in 72 h in experiment 2 (with algicidal bacterium SP48). The growth inhibition of A. tamarense by strain SP48 was reduced in experiment 3 (with SP48-specific phage). The cell density of A. tamarense in experiment 3 increased slightly in 36 h and decreased to 8,866 cells ml−1 in 72 h. In experiment 4, the growth of A. tamarense was inhibited and decreased to an undetectable level in 72 h with the addition of phage-resistant bacterium SP48-M (Fig. 2).
Fig. 2.
Population dynamics of harmful alga A. tamarense in different assemblages. A. tamarense represents growing A. tamarense culture without any additions. A. tamarense+SP48 represents coculture of A. tamarense and bacterium Pseudoalteromonas sp. strain SP48. A. tamarense+SP48+φZCW1 represents coculture of A. tamarense and bacterium Pseudoalteromonas sp. SP48 and bacteriophage φZCW1. A. tamarense+SP48-M+φZCW1 represents coculture of A. tamarense and bacterium Pseudoalteromonas sp. SP48-M and bacteriophage φZCW1. Each experiment was carried out in triplicate. The error bars represent the standard deviations for the values at different time points.
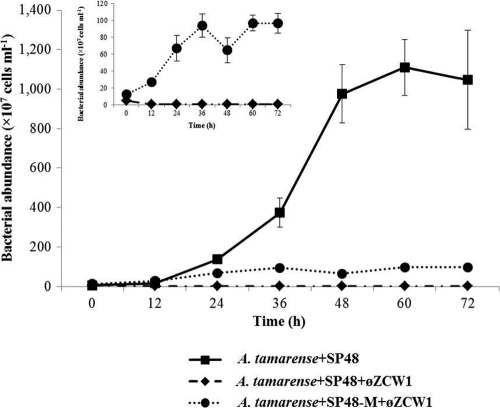
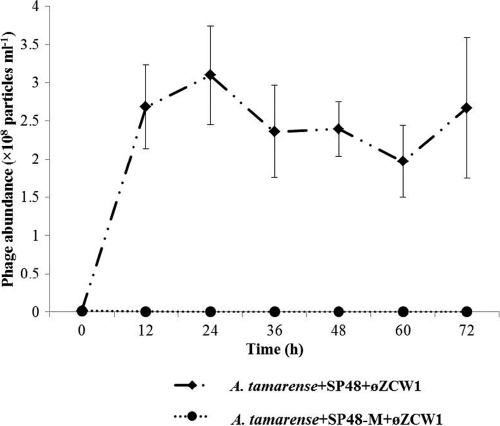
Bacterial abundance in experiment 2 increased from 4.7 × 107 to 1.1 × 1010 cells ml−1 at 60 h (Fig. 3). In contrast, addition of phage φZCW1 in experiment 3 inhibited the growth of algicidal bacterium SP48 (Fig. 3). The bacterial abundance stabilized at 107 cells ml−1, whereas the number of bacteria reached 1 × 1010 cells ml−1 when bacteriophage was not added. The φZCW1-resistant bacterium SP48-M was able to grow (inset in Fig. 3) in experiment 4 but to a lower degree than in experiment 2, which tested A. tamarense and SP48 but no phage. Abundance of phage φZCW1 increased from 1.4 × 106 to 2.7 × 108 particles ml−1 in experiment 3 during the first 12 h and remained relatively stable within 72 h (Fig. 4). Viruses could not be enumerated in experiment 4, where the mutant host strain SP48-M was mixed with phage φZCW1.
Fig. 3.
Population dynamics of algicidal bacterium Pseudoalteromonas sp. SP48 and phage-resistant bacterium mutant SP48-M in different assemblages. A. tamarense+SP48 represents coculture of A. tamarense and bacterium Pseudoalteromonas sp. SP48. A. tamarense+SP48+φZCW1 represents coculture of A. tamarense and bacterium Pseudoalteromonas sp. SP48 and bacteriophage φZCW1. A. tamarense+SP48-M+φZCW1 represents coculture of A. tamarense and bacterium Pseudoalteromonas sp. SP48-M and bacteriophage φZCW1. Each experiment was carried out in triplicate. The error bars represent the standard deviations for the values at different time points.
Fig. 4.
Population dynamics of bacteriophage φZCW1 in different assemblages. A. tamarense+SP48+φZCW1 represents coculture of A. tamarense and bacterium Pseudoalteromonas sp. SP48 and bacteriophage φZCW1. A. tamarense+SP48-M+φZCW1 represents coculture of A. tamarense and bacterium Pseudoalteromonas sp. SP48-M and bacteriophage φZCW1. Each experiment was carried out in triplicate. The error bars represent the standard deviations for the values at different time points.
Lytic viruses are believed to play an important role in regulating the microbial abundance and distribution via the “kill-the-winner” model (28, 31). Viruses actively interact with heterotrophic bacteria, cyanobacteria, and eukaryotic algae and are capable of regulating population structure and genetic diversity of bacteria and microalgae in the marine environment (17, 18, 30). However, little is known about the potential impact of phage-host interaction on the population dynamics of eukaryotic microalgae. We demonstrated that bacteriophage can influence the growth of eukaryotic microalgae by killing the bacteria that are lethal to algae. In the mixture of A. tamarense, Pseudoalteromonas sp. SP48, and the lytic phage φZCW1 (experiment 3), φZCW1 was able to reduce the algicidal activity of strain SP48 by killing the host bacteria. The inhibition of algal growth was relaxed at the first 36 h due to the active viral lysis of SP48. A dramatic increase of viral particles in experiment 3 further supports the lytic activity of φZCW1. Unexpectedly, algal abundance showed a slight decrease after 36 h. This might be caused by development of a Pseudoalteromonas sp. SP48 mutant strain resistant to φZCW1. Bacterial strains resistant to viruses can be an important factor in affecting the lytic efficiency of viruses. On the other hand, when φZCW1-sensitive bacterium SP48 was replaced with φZCW1-resistant bacterium SP48-M (experiment 4), the cell numbers of A. tamarense decreased to an undetectable level after 72 h of coincubation of these three microbial consortia. In this case, strain SP48-M was able to kill A. tamarense because SP48-M is resistant to phage φZCW1. The biomass of SP48-M was lower than the biomass of SP48 in experiment 2. The growth rate of phage-resistant bacterial mutant is in general significantly lower than that of the wild type (10). Obvious reduced growth capability of phage-resistant strains has been detected in some other studies (16). However, SP48-M still showed great algicidal activity against A. tamarense and activity as great as that of Pseudoalteromonas sp. SP48. This is ascribed to the algicidal mechanism of Pseudoalteromonas sp. SP48, which secretes algicidal compounds. When the bacterial concentration is high enough to produce sufficient accumulation of algicidal compounds, the algicidal activity shows a less direct relationship to bacterial abundance.
In short, our results suggest that viruses may influence nonhost microbes through a “chain reaction” mechanism. By lysing specific hosts that are lethal to other microorganisms, viruses can influence microbial population dynamics indirectly. Such a chain reaction caused by phage or viruses has not been tested in the natural environment. The phage-bacterium-alga model may be a good system to study the ecological interactions between phage, bacteria, and algae to provide a better understanding of the formation and termination of algal blooms in aquatic systems.
Supplementary Material
Acknowledgments
This work was supported by the National Nature Science Foundation (40930847, 40876061, and 31070442) and the Program for Changjiang Scholars and Innovative Research Team in University (40821063).
We thank Russell T. Hill (University of Maryland Center for Environmental Science) for kindly helping us with the English in the article.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 2 September 2011.
REFERENCES
- 1. Adams M. 1959. Assay of phage by agar layer method, p. 450–454 In Bacteriophages. Interscience Publishers, Inc., New York, NY [Google Scholar]
- 2. Adams N., Lesoing M., Trainer V. 2000. Environmental conditions associated with domoic acid in razor clams on the Washington coast. J. Shellfish Res. 19:1007–1015 [Google Scholar]
- 3. Amaro A. M., Fuentes M. S., Ogalde S. R., Venegas J. A., Suarez-Isla B. A. 2005. Identification and characterization of potentially algal-lytic marine bacteria strongly associated with the toxic dinoflagellate Alexandrium catenella. J. Eukaryot. Microbiol. 52:191–200 [DOI] [PubMed] [Google Scholar]
- 4. Barlaan E. A., Furukawa S., Takeuchi K. 2007. Detection of bacteria associated with harmful algal blooms from coastal and microcosm environments using electronic microarrays. Environ. Microbiol. 9:690–702 [DOI] [PubMed] [Google Scholar]
- 5. Brussaard C. P. D. 2004. Viral control of phytoplankton populations—a review. J. Eukaryot. Microbiol. 51:125–138 [DOI] [PubMed] [Google Scholar]
- 6. Doucette G. J. 1995. Interaction between bacteria and harmful algae: a review. Nat. Toxins. 3:65–74 [DOI] [PubMed] [Google Scholar]
- 7. Doucette G. J., McGovern E. R., Babinchak J. A. 1999. Algicidal bacteria active against Gymnodinium breve (Dinophyceae). I. Bacterial isolation and characterization of killing activity. J. Phycol. 35:1447–1454 [Google Scholar]
- 8. Fuhrman J. A., Suttle C. A. 1993. Viruses in marine planktonic systems. Oceanography 6:51–63 [Google Scholar]
- 9. Guillard R. R. L. 1975. Culture of phytoplankton for feeding marine invertebrates, p. 29–60 In Smith W. L., Canley M. H. (ed.), Culture of marine invertebrate animals. Plenum Press, New York, NY [Google Scholar]
- 10. Haaber J., Middelboe M. 2009. Viral lysis of Phaeocystis pouchetii: implications for algal population dynamics and heterotrophic C, N and P cycling. ISME J. 3:430–441 [DOI] [PubMed] [Google Scholar]
- 11. Huang C., Zhang Y., Jiao N. 2010. Phage resistance of a marine bacterium, Roseobacter denitrificans OCh114, as revealed by comparative proteomics. Curr. Microbiol. 61:141–147 [DOI] [PubMed] [Google Scholar]
- 12. Landsberg J. H. 2002. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 10:113–390 [Google Scholar]
- 13. Lovejoy C., Bowman J. P., Hallegraeff G. M. 1998. Algicidal effects of a novel marine Pseudoalteromonas isolate (class Proteobacteria, gamma subdivision) on harmful algal bloom species of the genera Chattonella, Gymnodinium, and Heterosigma. Appl. Environ. Microbiol. 64:2806–2813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mayali X., Azam F. 2004. Algicidal bacteria in the sea and their impact on algal blooms. J. Eukaryot. Microbiol. 51:139–144 [DOI] [PubMed] [Google Scholar]
- 15. Mayali X., Doucette G. J. 2002. Microbial community interactions and population dynamics of an algicidal bacterium active against Karenia brevis (Dinophyceae). Harmful Algae 1:277–293 [Google Scholar]
- 16. Middelboe M. 2000. Bacterial growth rate and marine virus-host dynamics. Microb. Ecol. 40:114–124 [DOI] [PubMed] [Google Scholar]
- 17. Middelboe M., et al. 2001. Effects of bacteriophages on the population dynamics of four strains of pelagic marine bacteria. Microb. Ecol. 42:395-406 [DOI] [PubMed] [Google Scholar]
- 18. Nagasaki K., et al. 2004. Isolation and characterization of a novel single-stranded RNA virus infecting the bloom-forming diatom Rhizosolenia setigera. Appl. Environ. Microbiol. 70:704–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patel A., et al. 2007. Virus and prokaryote enumeration from planktonic aquatic environments by epifluorescence microscopy with SYBR Green I. Nat. Protoc. 2:269–276 [DOI] [PubMed] [Google Scholar]
- 20. Riemann L., Steward G. F., Azam F. 2000. Dynamics of bacterial community composition and activity during a mesocosm diatom bloom. Appl. Environ. Microbiol. 66:578–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Simon N., Biegala I. C., Smith E. A., Vaulot D. 2002. Kinetics of attachment of potentially toxic bacteria to Alexandrium tamarense. Aquat. Microb. Ecol. 28:249–256 [Google Scholar]
- 22. Skerratt J. H., Bowman J. P., Hallegraeff G., James S., Nichols P. D. 2002. Algicidal bacteria associated with blooms of a toxic dinoflagellate in a temperate Australian estuary. Mar. Ecol. Prog. Ser. 244:1–15 [Google Scholar]
- 23. Su J., Yang X., Zheng T., Hong H. 2007. An efficient method to obtain axenic cultures of Alexandrium tamarense–a PSP-producing dinoflagellate. J. Microbiol. Methods 69:425–430 [DOI] [PubMed] [Google Scholar]
- 24. Su J. Q., et al. 2007. Isolation and characterization of a marine algicidal bacterium against the toxic dinoflagellate Alexandrium tamarense. Harmful Algae 6:799–810 [Google Scholar]
- 25. Suttle C. A. 2007. Marine viruses—major players in the global ecosystem. Nat. Rev. Microbiol. 5:801–812 [DOI] [PubMed] [Google Scholar]
- 26. Suttle C. A. 1994. The significance of viruses to mortality in aquatic microbial communities. Microb. Ecol. 28:237–243 [DOI] [PubMed] [Google Scholar]
- 27. Suttle C. A. 2005. Viruses in the sea. Nature 437:356–361 [DOI] [PubMed] [Google Scholar]
- 28. Thingstad T. F. 2000. Elements of a theory for the mechanisms controlling abundance, diversity, and biogeochemical role of lytic bacterial viruses in aquatic systems. Limnol. Oceanogr. 45:1320–1328 [Google Scholar]
- 29. Thingstad T. F., Lignell R. 1997. Theoretical models for the control of bacterial growth rate, abundance, diversity and carbon demand. Aquat. Microb. Ecol. 13:19–27 [Google Scholar]
- 30. Wang K., Chen F. 2004. Genetic diversity and population dynamics of cyanophage communities in the Chesapeake Bay. Aquat. Microb. Ecol. 34:105–116 [Google Scholar]
- 31. Weinbauer M. G. 2004. Ecology of prokaryotic viruses. FEMS Microbiol. Rev. 28:127–181 [DOI] [PubMed] [Google Scholar]
- 32. Wommack K. E., Colwell R. R. 2000. Virioplankton: viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev. 64:69–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yoshinaga I., Kawai T., Ishida Y. 1997. Analysis of algicidal ranges of the bacteria killing the marine dinoflagellate Gymnodinium mikimotoi isolated from Tanabe Bay, Wakayama Pref., Japan. Fisheries Sci. 63:94–98 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.