Abstract
Numerous studies have shown both the detrimental and beneficial effects of reactive oxygen species (ROS) in animals, plants, and fungi. These organisms utilize controlled generation of ROS for signaling, pathogenicity, and development. Here, we show that ROS are essential for the pathogenic development of Sclerotinia sclerotiorum, an economically important fungal pathogen with a broad host range. Based on the organism's completed genome sequence, we identified two S. sclerotiorum NADPH oxidases (SsNox1 and SsNox2), which presumably are involved in ROS generation. RNA interference (RNAi) was used to examine the function of SsNox1 and SsNox2. Silencing of SsNox1 expression indicated a central role for this enzyme in both virulence and pathogenic (sclerotial) development, while inactivation of the SsNox2 gene resulted in limited sclerotial development, but the organism remained fully pathogenic. ΔSsnox1 strains had reduced ROS levels, were unable to develop sclerotia, and unexpectedly correlated with significantly reduced oxalate production. These results are in accordance with previous observations indicating that fungal NADPH oxidases are required for pathogenic development and are consistent with the importance of ROS regulation in the successful pathogenesis of S. sclerotiorum.
INTRODUCTION
Sclerotinia sclerotiorum is an economically important necrotrophic fungal pathogen with a worldwide distribution of more than 400 dicotyledonous host species (42). Included among these hosts are several agriculturally important plants, including soybean, canola, and sunflower (15). S. sclerotiorum is a particularly devastating pathogen, in part due to the ineffectiveness of traditional control measures. Spray regimes have been largely unsuccessful, and breeding programs have had limited success, presumably since resistance is genetically complex. Crop rotation strategies are hampered by the long-term viability (years) of sclerotia and the inherent broad host range of the fungus. Thus, while considerable effort has been made to prevent Sclerotinia diseases, there are currently no effective control regimes. As the development and pathogenesis of S. sclerotiorum are complex and not well understood, intervention with the disease cycle of this pathogen has proven difficult.
We are interested in identifying the factors that are responsible for or contribute to the pathogenic success of this fungus. Work from our lab as well as those of others has established that a primary determinant contributing to the pathogenic success of this fungus is its ability to synthesize and secrete high concentrations (∼10 mM) of oxalic acid (OA). Mutants of this fungus that are defective in OA production are nonpathogenic (13). We have extensively investigated the function of OA in the pathogenicity of this fungus (4, 5, 6, 17, 29, 30, 41).
Oxalate was proposed to enhance S. sclerotiorum virulence in at least three ways (7). First, oxalate reduces pH. Several cell wall-degrading enzymes secreted during invasion of plant tissues (e.g., polygalacturonase) have maximal activities at low pHs (2). Second, oxalate may be directly toxic to host plants (27). Finally, chelation of cell wall Ca2+ by the oxalate anion has been proposed both to compromise the function of Ca2+-dependent defense responses and to weaken the plant cell wall (2).
In recent years, we have shown that OA has several unique functions that contribute to the success of this phytopathogen. For example, OA is an elicitor of reactive oxygen species (ROS)-induced plant programmed cell death (17) but, paradoxically, can also dampen the host oxidative burst (4). We have recently shown that during the initial stages of colonization, Sclerotinia (via OA) generates reducing conditions that suppress host defense responses, including the oxidative burst and callose deposition, akin to the actions of hemibiotrophic pathogens (41). Once infection is established, however, Sclerotinia induces the generation of plant reactive oxygen (ROS) and programmed cell death, which is of direct benefit to this necrotrophic pathogen. In contrast, a nonpathogenic OA-deficient mutant failed to alter host redox status or these defense responses. We have concluded that ROS regulation and signaling play a key role in the pathogenicity of S. sclerotiorum and, thus, that interference with this process is a means to impair pathogenicity. ROS regulation is also known to play an important role in sclerotial differentiation (11), a key function in the development and pathogenicity of this fungus. We therefore functionally investigated the S. sclerotiorum NADPH oxidase (Nox) family, whose members are established generators of ROS.
NADPH oxidases are a primary source for generating superoxide, an important precursor of several ROS, including hydrogen peroxide. For the activation of Nox enzymes, cytosolic regulatory components (Rac, p67phox, p47phox, and p40phox) are recruited into the integral membrane protein flavocytochrome b558, consisting of the catalytic subunits gp91phox and p22phox (26, 32). The activated Nox enzyme complex generates superoxide from oxygen by utilizing NADPH as an electron donor, thereby leading to the formation of other species of reactive oxygen. Among NADPH oxidases in animals, the most well known is Nox2 (gp91phox), which is involved in several diseases (33, 32). As in animals, the plant NADPH oxidase Rboh (respiratory burst oxidase homolog), the gp91phox homolog, is considered to be a primary, but not the only, source of ROS (39). ROS from the Arabidopsis NADPH oxidases (AtrbohD and AtrbohF) are associated with host defense via the hypersensitive response (39), possibly by antagonizing salicylic acid-dependent prodeath signals (40). ROS-dependent abscisic acid (ABA) signaling by these enzymes also regulates cytosolic Ca2+ levels, thereby controlling stomatal closure, seed germination, and root elongation (19). In addition, an Arabidopsis NADPH oxidase (AtrbohC) regulates plant cell expansion through the activation of Ca2+ channels during root hair development (10).
NADPH oxidases have been found in most fungal species, excluding the hemiascomycete yeasts and other unicellular fungi (36). Interest in fungal NADPH oxidases has recently increased. Several studies have shown the importance of fungal NADPH oxidases in sexual and asexual development (3, 22, 23). In several plant-pathogenic fungi, deletion of nox genes resulted in developmental defects in structures that are key for infection, such as appressoria and sclerotia (8, 34). It has also been shown in the symbiotic interaction between the endophytic fungus Epichloë festucae and perennial ryegrass that ROS generated from fungal NADPH oxidases function to modulate plant-fungus symbiosis (37).
This study examined the contribution of S. sclerotiorum NADPH oxidases to pathogenesis, growth, and development. We identified two predicted NADPH oxidase genes in the S. sclerotiorum genome sequence (Ssnox1 and Ssnox2) (http://www.broadinstitute.org). Following the cloning and characterization of Ssnox1 and Ssnox2, these genes were functionally evaluated by RNA interference (RNAi)-mediated gene silencing. Both of these genes were found to be required for sclerotial development. In addition, Ssnox1 silenced mutants were severely compromised in their ability to cause disease. We also noticed a significant reduction in the production of oxalate by Ssnox1 mutants. Tomato plants challenged with the Ssnox1 strain showed an increased plant oxidative burst, suggesting that the decreased virulence of the Ssnox1 strain may result in an increased or active defense response in host plants that does not occur in wild-type strains. Ssnox2 mutant-infected plants showed negligible decreases in the generation of superoxide and were still pathogenic. Hence, SsNox1 of S. sclerotiorum is important for both virulence and fungal development and is correlated with oxalate production.
MATERIALS AND METHODS
Fungal strains, growth conditions, and media.
The S. sclerotiorum wild type (strain 1980) and Nox silenced mutants were incubated on potato dextrose agar (PDA) or broth (PDB) at 25°C. Infections were conducted on detached tomato (Rutger) leaves. For pharmacological studies, wild-type S. sclerotiorum was incubated on PDA for 2 to 3 days. Agar plugs were then transferred to PDA containing 40 mM N-acetyl-cysteine (NAC) or 1 mM diphenyleneiodonium (DPI), and growth and sclerotial development were monitored over time. Oxalic acid levels were determined after growth of the designated S. sclerotiorum strains on PDA or PDB. After incubation, 10 mM EDTA (pH 7.6) was added to the agar plug, and the mixture was melted at 95°C. Samples in the PDB were directly analyzed. Oxalic acid was quantified using an oxalate detection kit according to the manufacturer's recommendations (Trinity Biotech, Wicklow, Ireland).
RNAi constructs and fungal transformation.
The fungal RNAi vector pSilent-1 was obtained from Oded Yarden. Sequences of S. sclerotiorum Nox1 and Nox2 were obtained by aligning the human gp91phox amino acid sequence with the sequence of the S. sclerotiorum genome (http://www.broadinstitute.org/annotation/genome/sclerotinia_sclerotiorum/MultiHome.html). nox1 and nox2 were amplified from S. sclerotiorum genomic DNA and cloned into pGEM-T. To silence nox1, sense and antisense fragments were amplified using two sets of primers (XhoINOX1F [AACTCGAGCGAAAGCCATCGATGAAG] and HindIIINOX1R [AAAAGCTTCGACATCGGCTCCTACAC] and KpnINOX1F [AAGGTACCCGAAAGCCATCGATGAG] and BglIINOX1R [AAAGATCTCGACATCGGCTCCTACAC]) and introduced into pSilent-1 with a spacer fragment to make a hairpin RNA structure. To silence nox2, sense and antisense fragments were amplified using two sets of primers (XhoINOX2F [AACTCGAGTGCTAGATCAGCTGCCTTGA] and HinIIINOX2R [AAAAGCTTCCTCGGGACAACAAAAGAAA] and KpnINOX2F [AAGGTACCTGCTAGATCAGCAGCCTTGA] and BglIINOX2R [AAAGATCTCCTCGGGACAACAAAAGAAA]), and PCR sets were used to construct the silencing vector. These silencing vectors for nox1 and nox2 and the pSilent-1 vector control were used to transform S. sclerotiorum. Generation of protoplast and fungal transformation was performed as described by Rollins (31). Total RNA was extracted with TRIzol according to the manufacturer's instructions (Invitrogen, Carlsbad, CA).
Semiquantitative RT-PCR analyses.
For semiquantitative reverse transcription-PCR (RT-PCR), total RNA was DNase treated and subjected to first-strand DNA synthesis using Moloney murine leukemia virus (MMLV) reverse transcriptase. Nox1 and Nox2 were amplified using SsNOX1F (CGAAAGCCATCGATGAAG) and SsNOX1R (CGACATCGGCTCCTACAC) and SsNOX2F (TGCTAGATCAGCTGCCTTGA) and SsNOX2R (CCTCGGGACAACAAAAGAAA), respectively. As an internal control, SsEF-1F (TCCTATCTCCGGTTTCAACG) and SsEF-1R (GCAAGCAATGTGAGCAGTGT) were used.
ROS detection assay.
In order to detect superoxide in tomato leaves (1 to 2 days after fungal inoculation), leaves were placed in 0.5 mg/ml nitroblue tetrazolium (NBT) (10 mM potassium phosphate buffer, pH 7.5) aqueous solution for 2 h. After incubation, leaves were rinsed in 70% ethanol and mounted in 50% glycerol.
For detection of superoxide in fungal structures, tissues were incubated in 0.5 mg/ml NBT (10 mM potassium phosphate buffer, pH 7.5) aqueous solution for 2 h. After incubation, fungal tissues were observed with light microscopy. A colorimetric NBT assay was also performed. After incubation with NBT, fungi were ground in liquid nitrogen. The reduced NBT was solubilized with the same volume of 2 M KOH and a 1.3× volume of dimethyl sulfoxide (DMSO) for 30 min.
ROS staining was conducted on agar plugs of the wild-type isolate 1980 shortly after sclerotial initials were observed. Aliquots of protoplasts generated from sclerotial initials were then stained with 2′,7′-dichlorodihydrofluorescein diacetate (DCHF-DA) to visualize H2O2.
Microscopy.
Microscopic observations were made using an Olympus IX-81 microscope with differential interference contrast optics (10× UPlanFL N objective, 0.30 numerical aperture [NA]). Digital images were captured with an Olympus DP70 camera. All images were collected using Olympus DP controller and manager software under the same conditions (image size, 1,360 by 1,024; exposure time, 60.0 s; ISO sensitivity, ISO200; exposure mode, auto) and were saved as TIF files.
RESULTS
Generation of ROS by S. sclerotiorum is necessary for sclerotial development.
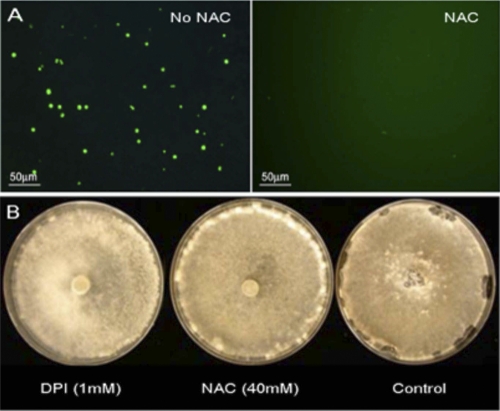
The sclerotium is a multicellular, highly melanized structure for overwintering and long-term survival and plays an important role in disease propagation. Since it has been postulated and later shown that ROS levels impact fungal development (11), we monitored intracellular ROS production from sclerotial initials using the oxidant-sensitive fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate (DCHF-DA). Fluorescence emission was higher in sclerotial initials, suggesting that sclerotial initiation is associated with generation of intracellular ROS (Fig. 1A). Inhibition of ROS is thus predicted to negatively impact sclerotial development. Indeed, when wild-type S. sclerotiorum was treated with 40 mM N-acetyl-cysteine (NAC), an effective antioxidant and ROS scavenger, or 1 mM diphenyleneiodonium (DPI), an inhibitor of flavoenzymes such as NADPH oxidase, sclerotial initial formation was considerably reduced and sclerotial maturation was prevented (Fig. 1B). Similar results were also reported with Botrytis cinerea grown on DPI (34). There were no significant differences in the extents of the radial growth of S. sclerotiorum under these conditions (data not shown). Together, these experiments suggest that ROS generation and signaling is required for proper development, including sclerotial formation, and that an NADPH oxidase may play an important role in this signaling pathway.
Fig. 1.
Antioxidants impair sclerotial development in S. sclerotiorum. (A) Sclerotial initials generate large amounts of ROS. An agar-mycelium plug of the wild-type isolate 1980 was inoculated onto potato dextrose agar (PDA) and grown until the appearance of sclerotial initials. Aliquots of protoplasts generated from sclerotial initials were then stained with 2′,7′-dichlorodihydrofluorescein diacetate (DCHF-DA) to visualize ROS within the cells in response to N-acetyl-cysteine (NAC), as described in Materials and Methods. Images shown are representative of three independent experiments. (B) An agar-mycelium plug of the wild-type isolate 1980 was inoculated on PDA with or without 40 mM NAC or 1 mM diphenyleneiodonium (DPI). Sclerotial development was monitored and photographed.
Transcript levels of S. sclerotiorum Nox1 increase in planta and during sclerotial development.
From the available, completed genomic sequence of S. sclerotiorum, we indentified two predicted nox genes that harbored significant homology to human gp91phox and Nox homologs in several filamentous fungi. These two nox genes were amplified from genomic DNA of S. sclerotiorum. The predicted amino acid (aa) sequences of S. sclerotiorum Nox1 (541 aa) and Nox2 (581 aa) were aligned with Epichloë festucae NoxA (GenBank accession no. AB236860.1), E. festucae NoxB (AB236861.1), Aspergillus nidulans NoxA (AY174088.1), Podospora anserina Nox1 (AF364817.1), P. anserina Nox2 (AY372210.1), Neurospora crassa NoxA (AABX02000003.1), and N. crassa NoxB (AABX02000010.1). The Ssnox1 sequence is 74%, 69%, 73%, and 72% homologous to E. festucae NoxA, A. nidulans NoxA, N. crassa NoxA, and P. anserine Nox1, respectively. SsNox2 is 77%, 78%, and 79% homologous to E. festucae Nox2, N. crassa NoxB, and P. anserina Nox2, respectively. A 36% similarity level was noted between Ssnox1 and Ssnox2. Both S. sclerotiorum Nox coding regions contain hallmark features of NADPH oxidases, including a six-transmembrane (TM) region, a flavin adenine dinucleotide (FAD) binding motif, and four NADPH binding motifs (see Fig. S1 in the supplemental material). Since these are highly conserved regions among multicellular organisms, it is expected that the two NADPH oxidases of S. sclerotiorum function similarly to other fungal homologs.
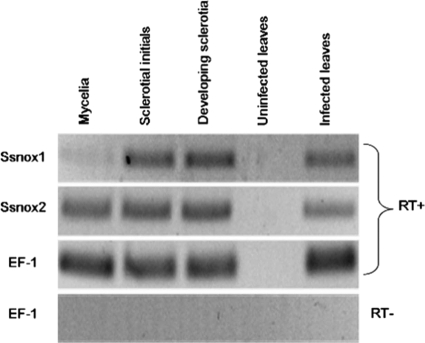
Semiquantitative RT-PCR was used to examine the expression levels of S. sclerotiorum nox genes during sclerotial development in vitro and in disease development in tomato leaves. Ssnox1 and Ssnox2 showed distinct patterns of expression during both processes. Ssnox2 was constitutively expressed throughout sclerotium formation, as well as during interaction with the plant, showing no detectable expression changes (Fig. 2). However, transcript levels of Ssnox1 were low during hyphal growth and dramatically increased during the induction of sclerotial initials and developing sclerotia. Furthermore, transcript levels of Ssnox1 also increased during fungal interaction with plant tissue (Fig. 2). These data show that Ssnox1 and Ssnox2 expression patterns are not redundant and that their functions are likely distinct but partially overlap. Importantly, Ssnox1 appears to play a more important role than Ssnox2 in disease development and progression.
Fig. 2.
Expression analysis of Nox genes of S. sclerotiorum during development and plant infection. RNA was extracted from fungal and leaf tissues in the indicated stages, 4 days after inoculation with an agar plug (5 mm in diameter) colonized with S. sclerotiorum or uncolonized PDA. RT-PCR was performed with gene-specific primers for SsNox1, SsNox2, or elongation factor 1α (EF-1) as described in Materials and Methods. RT−, no-template control.
Sclerotinia nox RNAi mutants.
RNA interference (RNAi)-mediated gene silencing has proven to be an efficient method to inhibit the expression of genes in many eukaryotes, including fungi (25). To genetically investigate the role of Ssnox1 and Ssnox2 in development and pathogenesis, the RNAi silencing vector pSilent-1 (25) was used to express hairpin structures of Ssnox1 and Ssnox2. We constructed two silencing vectors (pSNOX1 and pSNOX2) carrying the hygromycin B resistance gene as the selectable marker. As a control, an empty vector (pSilent-1) was used. These constructs were introduced into wild-type S. sclerotiorum via protoplast transformation (31). RNA blots and semiquantitative RT-PCR analyses (Fig. 3) were used to confirm silencing in the transformants. Ssnox1 (A2) and Ssnox2 (B4) silenced mutants were selected for further study (Fig. 3).
Fig. 3.
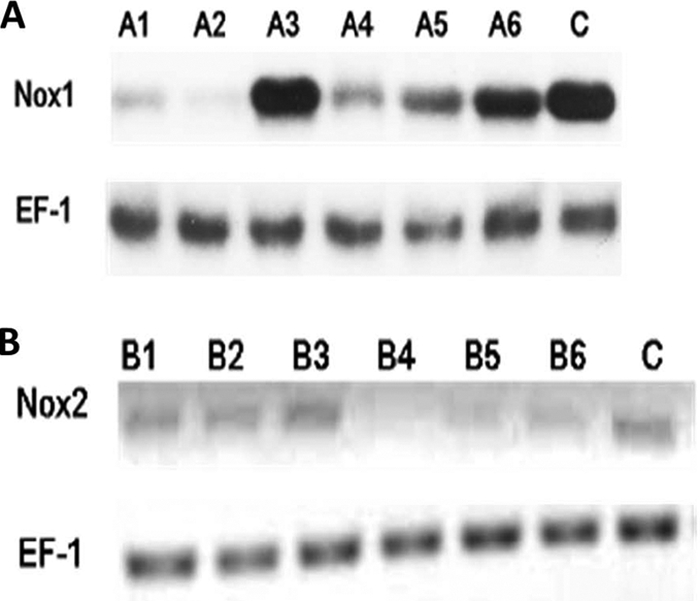
Silencing of S. sclerotiorum nox1 and nox2. (A) A Northern blot to determine Nox1 mRNA expression was analyzed with the Nox1 silenced transformants. Elongation factor-1 (EF-1) served as an internal control. A1, A2, A4, and A5 (silenced transformants) show reduced RNA expression compared to the wild-type control and nonsilenced transformants (A3 and A6). (B) RT-PCR analysis of Nox1 mRNA expression in the Nox2 silenced transformants. EF-1 served as an internal control. B1 to B3 are nonsilenced transformants, and B4 to B6 are silenced transformants. C, wild-type control.
To examine the role of nox genes during hyphal growth, the nox silenced mutants, the empty vector control, and the wild-type isolates were grown on potato dextrose agar (PDA). Extents of hyphal growth were not significantly different in all strains (Fig. 4). However, closer examination revealed that hyphae of the Ssnox2 mutant were highly branched compared to those of the wild-type, vector control, and Ssnox1 strains (Fig. 5). The Ssnox2 branching is reminiscent of the previously reported E. festucae noxA deletion mutant phenotype (37). Taken together, this suggests that NADPH oxidases may be involved in the regulation of hyphal branching.
Fig. 4.
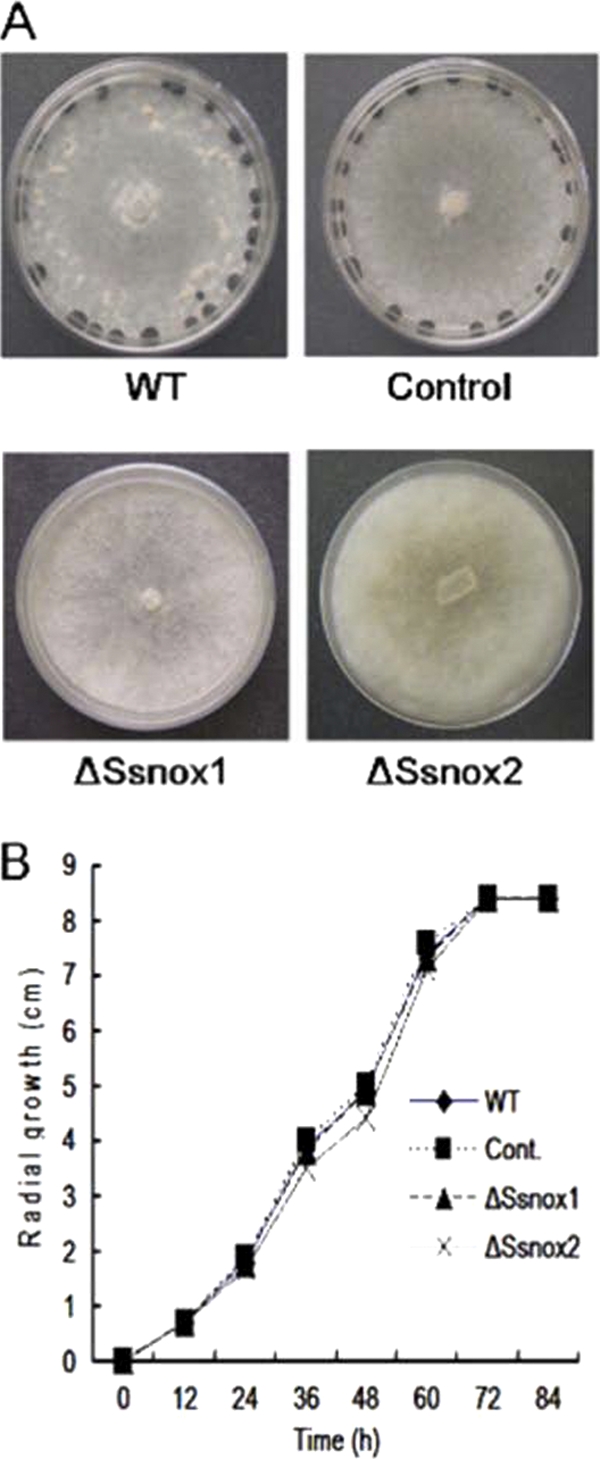
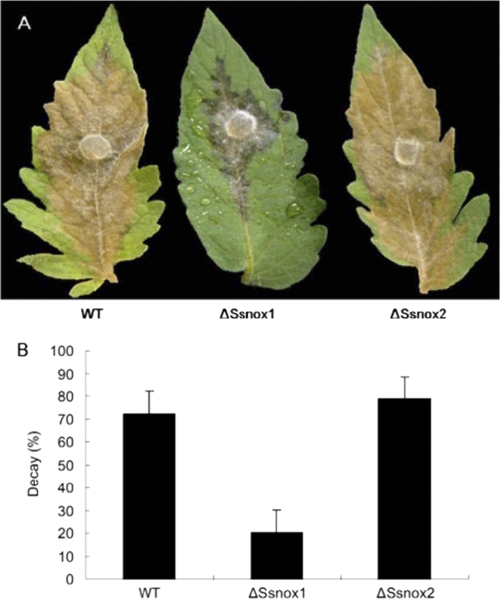
Phenotypes of nox knockdown mutants of S. sclerotiorum. (A) Sclerotial development of Nox silenced mutants (ΔSsnox1 and ΔSsnox2) on PDA. Defects in sclerotial development were observed in the ΔSsnox1 and ΔSsnox2 mutants, while the wild type (WT) and a transformant (control) containing an empty vector developed only sclerotia. Photographs were taken 3 weeks postinoculation. (B) Comparison of hyphal growth rates on PDA. Agar plugs (5 mm in diameter) obtained from growing hyphal tips of each tested strain were inoculated at the center of the petri dish. Colony diameters were measured at 12-h intervals. Cont., control. Data were obtained from three independent assays; error bars are omitted for clarity.
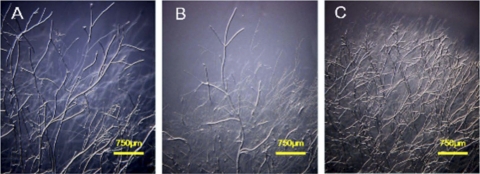
Fig. 5.
In vitro hyphal development of ΔSsnox1 and ΔSsnox2 mutants. The two Nox silenced mutants and wild-type strains were cultured on PDA for 2 days. Vegetative hyphae were observed under a dissecting microscope. (A) Wild type; (B) ΔSsnox1 mutant; (C) ΔSsnox2 mutant. Bars = 750 μm.
To determine whether sclerotial development was affected in these mutant strains, we monitored sclerotium formation on PDA. Consistent with the pharmacological study, ΔSsnox1 mutants were unable to produce sclerotia, and only occasionally were Ssnox2 mutants able to produce sclerotia (Fig. 4). In contrast, wild-type S. sclerotiorum and strains carrying the empty vector showed normal sclerotial development. The defect in sclerotial development of Nox silenced strains was not due to aberrant physiological growth defects since the extents of radial growth of all strains were nearly identical (Fig. 5). Together with the results of DPI/NAC treatments, these data support the requirement of NADPH oxidases for proper sclerotial development.
Production of superoxide in the ΔSsnox1 mutant is impaired.
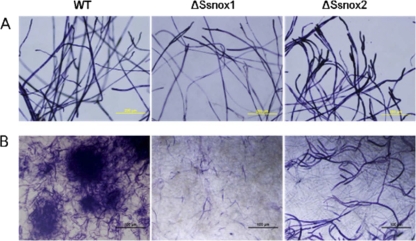
NADPH oxidases generate superoxide. Therefore, we reasoned that in a nox mutant background, the accumulation of superoxide is likely to be reduced. To determine whether inactivation of SsNox1 and SsNox2 leads to altered ROS generation, we examined ROS production during hyphal growth and sclerotium formation in both mutants. Nitroblue tetrazolium (NBT) staining was used to detect superoxide (4). The wild-type and Nox silenced mutant strains were cultured on PDA and stained with NBT 4 days later, when sclerotium formation initiates. Dark-blue formazan precipitates were observed in the wild-type fungus during hyphal growth/sclerotial initiation (Fig. 6). During hyphal growth, no significant differences in superoxide production were observed between Ssnox2 silenced mutants and the wild type, whereas the Ssnox1 silenced mutant had a significant reduction in superoxide accumulation (Fig. 6). This significant reduction in staining indicates that superoxide production was impaired in this mutant. Generation of superoxide in Ssnox2 mutants was also reduced, but not to the extent observed in Ssnox1 mutants (Fig. 6).
Fig. 6.
Superoxide accumulation in Nox silenced mutants. Strains were inoculated on PDA. Superoxide accumulation was observed at 4 days postinoculation (sclerotial initial). For detection, NBT staining was used and the intensity was monitored under a light microscope. Blue staining represents the accumulation of superoxide. The wild type shows extensive precipitation of blue formazan, and the ΔSsnox1 and ΔSsnox2 mutants show lesser accumulations of blue formazan. Bars = 100 μm.
The SsNox1 mutant is compromised in virulence, and oxalate production is significantly reduced.
We were interested in determining the phenotypes of our mutant strains with respect to pathogenicity. NADPH oxidase mutants of Magnaporthe oryzae and Botrytis cinerea have been shown to be less pathogenic than the corresponding wild-type fungal strains (8, 34). In accordance, the virulence of the Ssnox1 mutant in tomato plants was also found to be significantly reduced. However, the Ssnox2 mutant was as virulent as the wild type (Fig. 7). This correlated with the results from the RT-PCR analysis of SsNox genes showing that SsNox1 expression was induced during infection and that SsNox2 levels were not altered and were constitutively expressed (Fig. 2). Therefore, SsNox1 appears to play a more prominent role during infection and pathogenic development and, as will be discussed below, is also linked to oxalate production.
Fig. 7.
Pathogenicity of the ΔSsnox1 and ΔSsnox2 RNAi mutants. (A) Lesion development on tomato leaves. Leaves were inoculated with an agar plug (5 mm in diameter) of each strain. Inoculation with wild-type fungus served as the control. Photographs were taken at 3 days after inoculation. (B) Relative lesion areas on tomato leaves. Lesion areas in panel A were quantitatively analyzed with ImageJ 1.38x software.
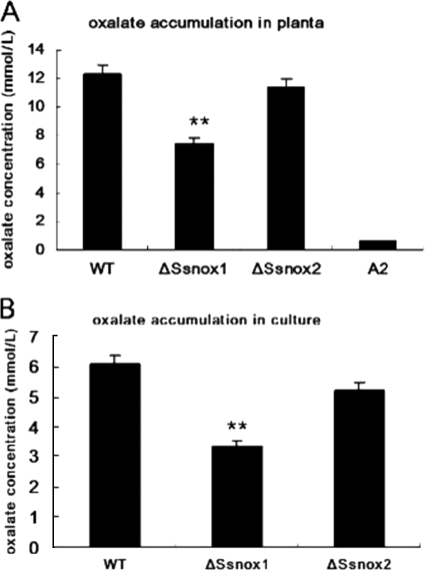
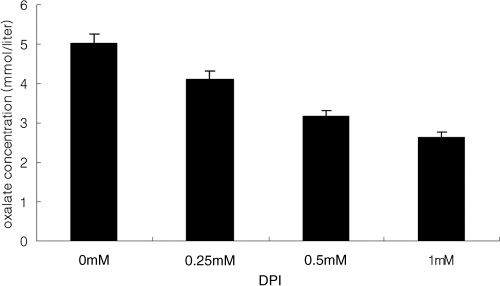
Recent studies in Aspergillus species showed that the accumulation of ROS was linked to the production of the secondary metabolite aflatoxin (16, 28). Based on this result and the well-established importance of disease development with the multifunctional secondary metabolite oxalic acid, we asked whether reduced levels of ROS in the Ssnox1 mutant affects oxalate production. Oxalate levels were measured in both the mutant and wild-type strains at 3 days postculturing in potato dextrose broth (PDB). The ΔSsnox1 mutant produced about half as much oxalate as wild-type S. sclerotiorum (Fig. 8), although not to the extent of the OA− A2 mutant (4). However, the Ssnox2 mutant's OA levels were approximately the same as those of the wild type (Fig. 8). Oxalate production was then determined during fungal interaction with the plant. At 2 days postinfection, plugs were removed from the leaf tissue and oxalate levels determined. Consistent with in vitro data, the production of oxalate was reduced in the Ssnox1 (nonpathogenic) mutant similarly to in the wild type during host challenge. The Ssnox2 mutant showed no difference in its production of oxalate from that of the wild type and was equally pathogenic (Fig. 7 and 8). Importantly, when the Ssnox1 mutant was supplemented with potassium oxalate (KOX; pH 7.0), though not complete, an increase in virulence was observed. This treatment had no effect on wild-type virulence (Fig. 9). KOH alone had no detectable effects on the leaf for the duration of this experiment (data not shown). These observations suggest a direct correlation between ROS generation, oxalate, and disease development. Since DPI blocks sclerotium formation (Fig. 1B), we examined the production of oxalate by growing fungi in PDB treated with DPI (0 to 1 mM) (Fig. 10). Results showed that DPI treatment inversely correlated with the production of oxalate in a dose-dependent manner. When ROS decreased, levels of oxalate also decreased. This further suggests that NADPH oxidases are linked to oxalate production, consistent with the limited ability of the fungus to cause disease.
Fig. 8.
Oxalate accumulation in the ΔSsnox1 and ΔSsnox2 mutants. (A) Oxalic acid accumulation in planta. Leaves were inoculated with a PDA plug (5 mm in diameter) colonized with the indicated strains. Wild-type fungus served as a positive control, and an oxalate-deficient mutant (A2) was used as the negative control. (B) Oxalic acid production in potato dextrose broth (PDB). Each strain was cultured for 3 days, and the resulting liquid culture was analyzed for oxalate accumulation.
Fig. 9.
Oxalate partially restores the virulence of the Nox1 silenced mutant. Nicotiana benthamiana leaves were infiltrated with either water or potassium oxalate (KOX) and then immediately inoculated with agar plugs colonized with wild-type S. sclerotiorum or the ΔSsnox1 mutant. 1, wild type plus water; 2, wild type plus KOX (pH 7.0); 3, ΔSsnox1 mutant plus water; and 4, ΔSsnox1 mutant plus KOX (pH 7.0) at room temperature. Lesion development was monitored over 2 days. Photographs were taken 1.5 days postinoculation.
Fig. 10.
Oxalate accumulation and ROS inhibition following DPI treatment. S. sclerotiorum wild-type isolate 1980 was cultured in PDB treated with 0 mM to 1 mM DPI and analyzed for oxalate accumulation.
DISCUSSION
A growing body of evidence has established that reactive oxygen species flux and redox regulation have key regulatory functions with respect to fungal disease development and host plant-resistant responses (3, 8, 12, 22, 23, 34, 36, 37). A principal means by which ROS are generated is via NADPH oxidases. From the available genome sequence of S. sclerotiorum, we identified two homologs of human gp91phox in S. sclerotiorum. Based on data reported thus far, filamentous fungi generally have two Nox subfamilies (NoxA/Nox1, NoxB/Nox2), while others, including Fusarium species, M. oryzae, P. anserina, Aspergillus terreus, and Phaeosphaeria nodorum, have a third Nox subfamily member (NoxC/Nox3). NoxA/Nox1 and NoxB/Nox2 are homologous to human gp91phox, whereas NoxC/Nox3 has a predicted calcium binding elongation factor (EF)-hand motif in the NH2 termini also found in mammalian Nox5 and plant Rboh proteins (32).
In this study, we identified and characterized the two predicted nox genes (Ssnox1 and Ssnox2) in S. sclerotiorum. Both S. sclerotiorum Nox coding regions contain a six-transmembrane (TM) region, a putative FAD binding motif, and four NADPH binding motifs similar to those of plant and animal NADPH oxidases. However, neither SsNox protein has a putative calcium binding EF-hand motif at its NH2-terminal end, consistent with the membership of its gene in the two-member subgroup of fungal Nox genes. Increased ROS has been observed in several fungal developmental structures, including cleistothecia, appressoria, and hyphal tips (8, 22, 23, 36). Similarly, generation of ROS was observed in sclerotial initials and infection cushions in S. sclerotiorum. To more directly assess the formation of reactive oxygen metabolites, we determined intracellular peroxide levels with DCHF-DA, which fluoresces when oxidized. Protoplasts generated from sclerotial initials treated with DCHF-DA were specifically fluorescent (vegetative hyphae were not), correlating ROS production with development. Consistent with this observation, the ROS scavenger NAC or the NADPH oxidase inhibitor DPI prevented DCF fluorescence and blocked sclerotial formation. Georgiou and colleagues (11) have also shown that hydroxyl radical scavengers and antioxidants inhibit the sclerotial development of several fungi, including S. sclerotiorum. Antioxidants, including N,N′-diphenyl-1,4-phenylene diamide, 1,3-dimethyl-2-thiourea, ammonium pyrroline dimethyl-dithiocarbamate, and NAC, blocked chemiluminescence enhanced by lucigenin (indicating the formation of ROS) during the differentiation process and conidiation of Neurospora crassa (14), similar to our observations. Exposure to the antioxidant ascorbic acid and the catalase mimic Mn(III)tetrakis(1-methyl-4-pyridyl)porphyrin also inhibited germination and appressorium formation of M. oryzae (8). Taken together, our data coupled with previous reports indicate the importance of ROS signaling for various fungal developmental structures.
To genetically examine the two S. sclerotiorum nox genes, we utilized an RNAi approach. We produced several knockdown mutations in both genes. Silencing Ssnox1 resulted in strains unable to form sclerotia. In other fungi, NoxA/Nox1 was shown to be involved in the development of sexual structures. For example, in A. nidulans, NoxA knockout mutants are defective in the differentiation of cleistothecia, although hyphal growth and asexual development were normal (22). Studies with N. crassa and Podospora anserina also showed a block in sexual development when Nox1 homologs were deleted. In contrast to NoxA of A. nidulans, Nox1 of N. crassa is also involved in asexual development and hyphal growth (3, 23). In S. sclerotiorum, asexual sclerotium formation is required for generation of the apothecium, a fungal sexual fruiting body. Thus, Ssnox1 RNAi mutants are unable to develop apothecia via their inability to form the necessary sclerotial precursor. In contrast to NoxA/Nox1, NoxB/Nox2 is directly required for ascospore germination in N. crassa and P. anserina (3, 23). Importantly, this result is analogous to what was reported for the closely related necrotroph Botrytis cinerea, where both nox genes were involved in sclerotial formation. B. cinerea noxB mutants produced few, small, and abnormal sclerotia and failed to develop apothecia (34). Transcript levels of Sclerotinia NADPH oxidases also correlated with ROS generation in the nox silenced mutants. Generation of ROS in the Ssnox1 was significantly reduced during sclerotial development but not in the Ssnox2 mutants. These results suggest that Ssnox1 contributes to the induction of ROS in sclerotial initials and that Ssnox2 plays a minimal role in the accumulation of ROS during sclerotial development. The data presented show that Ssnox genes and ROS signaling are key determinants in S. sclerotiorum development and pathogenicity.
NADPH oxidase activity is associated with virulence.
Egan and colleagues demonstrated that the Magnaporthe oryzae nox1 and nox2 genes play a pivotal role in appressorium differentiation, a specialized infection structure required for fungal penetration into host tissue (8). Nox-generated ROS within the M. oryzae appressorium facilitates oxidative cross-linking of proteins, thereby strengthening the appressorium cell wall to withstand the enormous turgor pressure generated during breaching of the host cuticular barrier (38). Deletion of nox genes resulted in weakened appressorial cell walls and the inability to penetrate and cause infection (8). Consistent with these findings, BcNox2 is similarly required for appressorium penetration of the plant cuticle in B. cinerea (34). In our study, silencing of nox1 significantly reduced virulence in S. sclerotiorum, while Ssnox2 silenced mutants were similar to the wild type with respect to virulence. Recent studies with Epichloë festucae, a fungal mutualist of ryegrass, provided interesting and, at first glance, perhaps unexpected evidence for involvement of ROS in a plant-fungal mutualistic relationship (36, 37). A restriction enzyme-mediated integration (REMI/knockout) screen revealed that one of the two E. festucae nox genes (NoxA), when interrupted, resulted in the conversion from a mutualistic to a pathogenic lifestyle (37). Thus, while wild-type E. festucae exhibits a symbiotic association with the perennial ryegrass, inactivation of E. festucae NoxA resulted in pathogenicity, increased fungal biomass, and disease (37). Importantly, ROS accumulation was detected at the interface between the extracellular matrix and host cell walls of meristematic tissue in the interaction of ryegrass and E. festucae (wild type) (37). Further, this group showed a decrease in ROS accumulation at the interface between ryegrass and the noxA mutant (37). These observations suggest that wild-type E. festucae may require ROS (generated from NADPH oxidase) for signaling in the host plant (37). This ROS signal from wild-type E. festucae may moderate the plant defense response to maintain mutualism. It will be of particular interest to unravel the plant components that are modulated by fungal ROS to promote this phenotype. Though the interactions between S. sclerotiorum and E. festucae with their respective hosts differ in outcome, both organisms utilize similar strategies involving nox regulation to subdue defenses and gain unimpeded entry into the host plant.
NADPH oxidases and oxalate production.
During the course of these studies, we noticed that Ssnox1 expression is linked to oxalate production. As discussed, OA is an established pathogenicity factor in S. sclerotiorum (4, 6, 13). Oxalate levels in Ssnox1 mutants were significantly lower than in wild-type strains and Ssnox2 mutants during in vitro culture and, importantly, during interaction with plant tissue. Further, DPI, an inhibitor of NADPH oxidase, directly reduced the production of oxalate. Thus, genetically and biochemically, ROS are linked to oxalate and both are linked to virulence. In Aspergillus, the generation of ROS correlates with the production of the secondary metabolite aflatoxin (28, 32). Moreover, exogenous application of the antioxidant caffeic acid significantly reduced the production of aflatoxin (16). In studies from our lab, a superoxide dismutase (SOD) mutant of S. sclerotiorum also exhibited reduced levels of oxalate production and reduced virulence (S. Veluchamy, K. S. Kim, B. Williams, and M. B. Dickman, unpublished data). These observations are consistent with the established link between NADPH oxidase, superoxide generation, and activation of SOD (32). Superoxide generated from NADPH oxidases is converted to hydrogen peroxide via SOD. Therefore, the similar decreases in net oxalate production when NADPH oxidase and SOD are impaired suggest that these two enzymes function in the same pathway. Importantly, addition of OA to Ssnox1 mutants increased virulence (Fig. 9).
An obvious key question is how would this occur? Recently, we noted two bicupin proteins in the genome sequence of S. sclerotiorum (SS1G_10796.1, 47% identical to Ceriporiopsis subvermispora Oxo1 [CsOxo1], and SS1G_08814.1, 46% identical to CsOxo2). It has been suggested that cupins function as oxalate oxidases in Ceriporiopsis subvermispora (9). Oxalate oxidase (also known as germin-like in plants) catalyzes the generation of hydrogen peroxide (H2O2) from oxalate. Although it has yet to be shown whether S. sclerotiorum bicupin proteins have oxalate oxidase activity, they could be associated with oxalate-mediated ROS generation. Previous work in the Dickman lab has shown that oxalate-induced H2O2 in plant tissue is involved in programmed cell death (17). This observation suggests that fungally produced OA is associated with ROS generation in plants. While mechanistic details are as yet unknown, our observations and previous studies show a consistent link between ROS generation and the production of OA.
There are several potential routes for oxalate biosynthesis. In Sclerotinium rolfsii, the oxidation of glyoxylate by glyoxylate dehydrogenase was suggested as a major pathway for oxalate biosynthesis (1). In addition, exogenous application of 14CO2 in Aspergillus niger showed that the biosynthesis of oxalate occurs by hydrolysis of oxaloacetate (18). Hammel and colleagues (13a) also provided evidence for oxalate production via oxidation of glycolaldehyde by the Phanerochaete chrysosporium glyoxal oxidase. Several cofactors, such as NAD+, are necessary to maintain these pathways. For example, the tricarboxylic acid (TCA) cycle, considered one of the main pools for the production of secondary metabolites, including oxalate and glyoxylate, has three rate-limiting enzymes dependent on NAD+ (24), and glyoxylate dehydrogenase also uses one NAD+ molecule to produce one oxalate molecule. Therefore, recycling of cofactors for a steady-state supply of NAD+ and consumption of NADH could be required to produce oxalate. Transhydrogenase, which converts NADP+ plus NADH to NADPH plus NAD+, can contribute to this recycling. For example, NADP+ from NADPH oxidase and NADH from the TCA cycle and oxidation of glyoxylate can be transformed into NADPH and NAD+, respectively, by transhydrogenase, thereby providing cofactors for ROS generation and oxalate production. Hence, conversion of NADPH to NADP by NADPH oxidase may impact production of secondary metabolites, as noted by Lalucque and Silar (20), and transhydrogenase may contribute to this process. In line with this, S. sclerotiorum has a putative transhydrogenase (SS1G_09058.1) which has the characteristic NAD(P) transhydrogenase beta subunit. Thus, this transhydrogenase may play a role in the generation of NADPH and NAD+ from NADP+ and NADH, respectively. Interestingly, transhydrogenase homologs have not been found in available genomes of the hemiascomycete yeasts and other unicellular fungi, from which nox genes are also absent (36). Moreover, the filamentous fungi for which NADPH oxidases have been reported and genome sequences are known also contain transhydrogenase homologs. This possibility, however, at this point is purely speculative and will require further experimentation.
In summary, prevention of NADPH oxidase activity by inactivation of Ssnox1 or chemical inhibition of SsNox1 resulted in reduced oxalate levels that correlated with limited virulence. Notably, oxalate supplementation restored the virulence of Ssnox1 silenced mutants to nearly wild-type levels. These results strongly suggest a novel and functional relationship between NADPH oxidase/ROS and the production of oxalate, both of which impact fungal pathogenicity. We have shown that OA can suppress the host oxidative burst (4). The decrease in oxalate production leads to strongly localized ROS production in the host plant (41). Mechanistic studies regarding how SsNox1 regulates oxalate production will give valuable insight into how fungal NADPH oxidases contribute to the pathogenic success of S. sclerotiorum and quite possibly other fungi.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the National Science Foundation (0923918 to M.B.D.) and the Binational Agricultural Research & Development Fund (US-4041-07C to M.B.D.).
We thank Brett Williams for stimulating discussions.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 2 September 2011.
REFERENCES
- 1. Balmforth A. J., Thomson A. 1984. Isolation and characterization of glyoxylate dehydrogenase from the fungus Sclerotium rolfsii. Biochem. J. 218:113–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bateman D. F., Beer S. V. 1965. Simultaneous production and synergistic action of oxalic acid and polygalacturonase during pathogenesis by Sclerotium rolfsii. Phytopathology 55:204–211 [PubMed] [Google Scholar]
- 3. Cano-Domínguez N., Alvarez-Delfin K., Hansberg W., Aguirre J. 2008. NADPH oxidases NOX-1 and NOX-2 require the regulatory subunit NOR-1 to control cell differentiation and growth in Neurospora crassa. Eukaryot. Cell 7:1352–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cessna S. G., Sears V. E., Dickman M. B., Low P. S. 2000. Oxalic acid, a pathogenicity factor for Sclerotinia sclerotiorum, suppresses the oxidative burst of the host plant. Plant Cell 12:2191–2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dickman M. B., Mitra A. 1992. Arabidopsis thaliana as a model for studying Sclerotinia sclerotiorum pathogenesis. Physiol. Mol. Plant Pathol. 41:255–263 [Google Scholar]
- 6. Dickman M. B. 2007. Approaches for crop improvement to soilborne fungal diseases through biotechnology: Sclerotinia sclerotiorum as a case study. Australas. Plant Pathol. 36:116–123 [Google Scholar]
- 7. Dutton M. V., Evans C. S. 1996. Oxalate production by fungi: its role in pathogenicity and ecology in the soil environment. Can. J. Microbiol. 42:881–895 [Google Scholar]
- 8. Egan M. J., Wang J. Y., Jones M. A., Smirnoff N., Talbot N. J. 2007. Generation of reactive oxygen species by fungal NADPH oxidases is required for rice blast disease. Proc. Natl. Acad. Sci. U. S. A. 104:11772–11777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Escutia M. R., et al. 2005. Cloning and sequencing of two Ceriporiopsis subvermispora bicupin oxalate oxidase allelic isoforms: implications for the reaction specificity of oxalate oxidases and decarboxylases. Appl. Environ. Microbiol. 71:3608–3616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Foreman J., et al. 2003. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422:442–446 [DOI] [PubMed] [Google Scholar]
- 11. Georgiou C. D., Patsoukis N., Papapostolou I., Zervoudakis G. 2006. Sclerotial metamorphosis in filamentous fungi is induced by oxidative stress. Integr. Comp. Biol. 46:691–712 [DOI] [PubMed] [Google Scholar]
- 12. Giesbert S., Schürg T., Scheele S., Tudzynski P. 2008. The NADPH oxidase Cpnox1 is required for full pathogenicity of the ergot fungus Claviceps pupurea. Mol. Plant Pathol. 9:317–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Godoy G., Steadman J. R., Dickman M. B., Dam R. 1990. Use of mutants to demonstrate the role of oxalic acid in pathogenicity of Sclerotinia sclerotiorum on Phaseolus vulgaris. Physiol. Mol. Plant Pathol. 37:179–191 [Google Scholar]
- 13a. Hammel K. E., Mozuch M. D., Jensen K. A., Kersten P. J. 1994. H2O2 recycling during oxidation of the arylglycerol-β-aryl ether lignin structure by lignin peroxidase and glyoxal oxidase. Biochemistry 33:13349–13354 [DOI] [PubMed] [Google Scholar]
- 14. Hansberg W., de Groot H., Sies H. 1993. Reactive oxygen species associated with cell differentiation in Neurospora crassa. Free Radic. Biol. Med. 14:287–293 [DOI] [PubMed] [Google Scholar]
- 15. Hegedus D. D., Rimmer S. R. 2005. Sclerotinia sclerotiorum: when “to be or not to be” a pathogen? FEMS Microbiol. Lett. 251:177–184 [DOI] [PubMed] [Google Scholar]
- 16. Kim J. H., et al. 2008. Elucidation of the functional genomics of antioxidant-based inhibition of aflatoxin biosynthesis. Int. J. Food Microbiol. 122:49–60 [DOI] [PubMed] [Google Scholar]
- 17. Kim K. S., Min J. Y., Dickman M. B. 2008. Oxalic acid is an elicitor of plant programmed cell death during Sclerotinia sclerotiorum disease development. Mol. Plant Microbe Interact. 21:605–612 [DOI] [PubMed] [Google Scholar]
- 18. Kubicek C. P., Schreferl-Kunar G., Wöhrer W., Röhr M. 1988. Evidence for a cytoplasmic pathway of oxalate biosynthesis in Aspergillus niger. Appl. Environ. Microbiol. 54:633–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kwak J. M., et al. 2003. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 22:2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lalucque H., Silar P. 2003. NADPH oxidase: an enzyme for multicellularity? Trends Microbiol. 11:9–12 [DOI] [PubMed] [Google Scholar]
- 21. Reference deleted.
- 22. Lara-Ortíz T., Riveros-Rosas H., Aguirre J. 2003. Reactive oxygen species generated by microbial NADPH oxidase NoxA regulate sexual development in Aspergillus nidulans. Mol. Microbiol. 50:1241–1255 [DOI] [PubMed] [Google Scholar]
- 23. Malagnac F., Lalucque H., Lepère G., Silar P. 2004. Two NADPH oxidase isoforms are required for sexual reproduction and ascospore germination in the filamentous fungus Podospora anserina. Fungal Genet. Biol. 41:982–997 [DOI] [PubMed] [Google Scholar]
- 24. McCormack J. G., Denton R. M. 1987. The role of Ca2+ in the regulation of intramitochondrial energy production in heart. Biomed. Biochim. Acta 46:S487–S492 [PubMed] [Google Scholar]
- 25. Nakayashiki H., et al. 2005. RNA silencing as a tool for exploring gene function in ascomycete fungi. Fungal Genet. Biol. 42:275–283 [DOI] [PubMed] [Google Scholar]
- 26. Nauseef W. M. 2008. Biological roles for the NOX family NADPH oxidases. J. Biol. Chem. 283:16961–16965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Noyes R. D., Hancock J. G. 1981. Role of oxalic acid in the Sclerotinia wilt of sunflower. Physiol. Plant Pathol. 18:123–132 [Google Scholar]
- 28. Reverberi M., et al. 2008. Modulation of antioxidant defense in Aspergillus parasiticus is involved in aflatoxin biosynthesis: a role for the ApyapA gene. Eukaryot. Cell 7:988–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rollins J. A., Dickman M. B. 1998. Increase in endogenous and exogenous cyclic AMP levels inhibits sclerotial development in Sclerotinia sclerotiorum. Appl. Environ. Microbiol. 64:2539–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rollins J. A., Dickman M. B. 2001. pH signaling in Sclerotinia sclerotiorum: identification of a pacC/RIM1 homolog. Appl. Environ. Microbiol. 67:75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rollins J. A. 2003. The Sclerotinia sclerotiorum pac1 gene is required for sclerotial development and virulence. Mol. Plant Microbe Interact. 16:785–795 [DOI] [PubMed] [Google Scholar]
- 32. Scott B., Eaton C. J. 2008. Role of reactive oxygen species in fungal cellular differentiations. Curr. Opin. Microbiol. 11:488–493 [DOI] [PubMed] [Google Scholar]
- 33. Segal A. W. 2005. How neutrophils kill microbes. Annu. Rev. Immunol. 23:197–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Segmüller N., et al. 2008. NADPH oxidases are involved in differentiation and pathogenicity in Botrytis cinerea. Mol. Plant Microbe Interact. 21:808–819 [DOI] [PubMed] [Google Scholar]
- 35. Reference deleted.
- 36. Takemoto D., Tanaka A., Scott B. 2006. A p67Phox-like regulator is recruited to control hyphal branching in a fungal-grass mutualistic symbiosis. Plant Cell 18:2807–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tanaka A., Christensen M. J., Takemoto D., Park P., Scott B. 2006. Reactive oxygen species play a role in regulating a fungus-perennial ryegrass mutualistic association. Plant Cell 18:1052–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thines E., Weber R. W., Talbot N. J. 2000. MAP kinase and protein kinase A-dependent mobilization of triacylglycerol and glycogen during appressorium turgor generation by Magnaporthe grisea. Plant Cell 12:1703–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Torres M. A., Dangl J. L., Jones J. D. G. 2002. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen species intermediates in the plant defense response. Proc. Natl. Acad. Sci. U. S. A. 99:517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Torres M. A., Jones J. D. G., Dangl J. L. 2005. Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat. Genet. 37:1130–1134 [DOI] [PubMed] [Google Scholar]
- 41. Williams B., Kabbage M., Kim H. J., Britt R., Dickman M. B. 2011. Tipping the balance: Sclerotinia sclerotiorum secreted oxalic acid suppresses host defenses by manipulating the host redox environment. PLoS Pathog. 7:e1002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yajima W., Kav N. N. V. 2006. The proteome of the phytopathogenic fungus Sclerotinia sclerotiorum. Proteomics 6:5995–6007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.