Abstract
Flavobacteria and their phages were isolated from Finnish freshwaters and fish farms. Emphasis was placed on finding phages infecting the fish pathogen Flavobacterium columnare for use as phage therapy agents. The host ranges of the flavobacterial phages varied, phages infecting F. columnare being more host specific than the other phages.
TEXT
Species of the genus Flavobacterium are widely distributed in nature, and they have been found in diverse habitats (3, 19, 30, 32, 36, 37, 38, 39). In general, flavobacteria are nonpathogenic, but some species are opportunistic pathogens (2). Columnaris, a disease caused by the fish pathogen Flavobacterium columnare, can cause up to 100% mortality among salmonid fingerlings (28). Antibiotic treatment must be applied to prevent mass mortalities at fish farms. Despite effective treatment, columnaris occurs repeatedly during the summer (18). Therefore, the number of antibiotic treatments and the amount of antibiotics used can be extremely high. Increased resistance of environmental bacteria to antibiotics in fish farms and their surroundings has been reported (10, 24, 26, 31), and antibiotic-resistant fish-pathogenic flavobacteria have also emerged (7). To avoid risks related to antibiotic use, enrichment of bacteriophages could be used as an ecological method of decreasing the number of F. columnare infections. To our knowledge, this is the first study on European F. columnare phages.
In this study, a total of 53 flavobacterial isolates were received during the warm-water period (May to August) in 2008 and 2009 from water samples that included both open freshwater environments (rivers and lakes not connected to fish farming) and three inland land-based fish farms rearing mainly salmonid fingerlings in Finland (Fig. 1; Table 1). One bacterium (B67) was isolated from diseased fish in 2007. Water samples were cultured on Shieh agar (25) and 1/5× Luria Bertani agar (22), and yellow- and orange-pigmented colonies were selected for analyses. The flavobacterial isolates were subjected to PCR with universal primers (UP-PCR) (for methods, see references 5, 6,, and 13), and the 16S rRNA genes of different groups were sequenced (Table 1). All F. columnare isolates fell into the same UP-PCR group, and thus they were further analyzed with ribosomal intergenic spacer analysis (RISA) (8, 29). According to RISA, the isolates were assigned to five groups (Table 1). Based on our data, the occurrence of F. columnare seems to be connected to the fish farming environment; this organism was not isolated from natural waters. However, it is likely that the initial source of F. columnare at the farms is nature, because there is evidence that it is also present outside fish farms (20, 21; H. Kunttu, L.-R. Sundberg, and E. T. Valtonen, unpublished data).
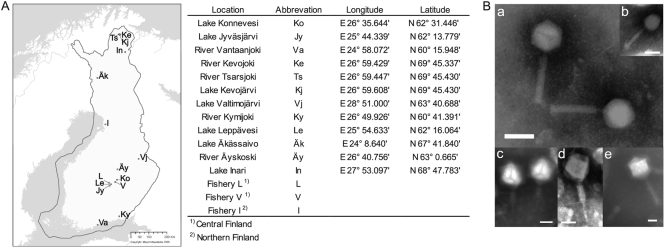
Fig. 1.
(A) Sites in Finland where flavobacteria and their phages were isolated. Sampling sites are marked on the free map obtained from the National Land Survey of Finland (Maanmittauslaitos, 2005). The sites and their abbreviations and coordinates are listed on the right. (B) Electron micrographs of purified and negatively stained tailed Flavobacterium phages. (a) FCV-1; (b) FCL-2; (c) FJy-3; (d) FKo-2; (e) FKy-1. Bar, 50 nm.
Table 1.
Bacterial strains isolated and used in the study
| Bacterial straina | UP-PCR or RISA groupb | EMBL accession no.c | Sampling site | Source or reference |
|---|---|---|---|---|
| B67 | A | Fishery L | This study | |
| B28 | 1 | FR696328 | Lake Konnevesi | This study |
| B80 | 2 | FR696329 | Lake Jyväsjärvi | This study |
| B105 | 3 | FR696330 | River Vantaanjoki | This study |
| B110 | 4 | FR696331 | River Vantaanjoki | This study |
| B114 | 5 | FR696332 | River Kevojoki | This study |
| B121 | 6 | FR696333 | River Tsarsjoki | This study |
| B127 | 7 | FR696334 | Lake Kevojärvi | This study |
| B130 | 8 | FR696335 | Lake Kevojärvi | This study |
| B167 | 9 | FR696336 | Lake Jyväsjärvi | This study |
| B169 | 10 | FR696337 | Lake Jyväsjärvi | This study |
| B171 | 11 | FR696338 | Lake Valtimojärvi | This study |
| B174 | 12 | FR696339 | River Kymijoki | This study |
| B176 | 13 | FR696340 | Lake Leppävesi | This study |
| B178 | 14 | FR696341 | Lake Äkässaivo | This study |
| B180 | 15 | FR696342 | River Äyskoski | This study |
| B183 | 16 | FR696343 | Fishery L | This study |
| B185 | G | FR696344 | Fishery L | This study |
| B187 | 17 | FR696345 | Fishery L | This study |
| B207 | 18 | FR696346 | Lake Inari | This study |
| B209 | 19 | FR696347 | Lake Inari | This study |
| B214 | 20 | FR696348 | Fishery V | This study |
| B218 | 21 | Fishery V | This study | |
| B222 | 22 | FR696349 | River Kymijoki | This study |
| B223 | 23 | FR696350 | River Kymijoki | This study |
| B224 | 24 | River Kymijoki | This study | |
| B225 | 24 | FR696351 | Fishery V | This study |
| B226 | 25 | FR696352 | Fishery V | This study |
| B230 | I | Fishery V | This study | |
| B234 | C | Fishery V | This study | |
| B235 | C | Fishery V | This study | |
| B236 | ND | Fishery V | This study | |
| B237 | C | Fishery V | This study | |
| B241 | 26 | FR696353 | Fishery V | This study |
| B243 | 27 | FR696354 | Fishery V | This study |
| B244 | ND | Fishery V | This study | |
| B245 | C | Fishery V | This study | |
| B247 | C | Fishery V | This study | |
| B257 | 26 | FR696355 | Fishery V | This study |
| B259 | C | Fishery V | This study | |
| B260 | ND | FR696356 | Fishery V | This study |
| B261 | C | Fishery V | This study | |
| B262 | ND | FR696357 | Fishery V | This study |
| B263 | ND | FR696358 | Fishery V | This study |
| B267 | C | Fishery V | This study | |
| B268 | C | Fishery V | This study | |
| B269 | ND | Fishery V | This study | |
| B270 | C | Fishery V | This study | |
| B271 | C | Fishery I | This study | |
| B272 | J | Fishery I | This study | |
| B273 | C | Fishery I | This study | |
| B274 | C | Fishery I | This study | |
| B275 | C | Fishery I | This study | |
| Rz-A | A | 29 | ||
| R-B | B | 29 | ||
| Rz-C | C | 29 | ||
| S-C | C | 29 | ||
| R-D | D | 29 | ||
| S-D | D | 29 | ||
| Rz-E | E | 29 | ||
| R-E | E | 29 | ||
| S-E | E | 29 | ||
| S-F | F | F. columnare type strain NCIMB 2248 | ||
| Rz-G | G | 29 | ||
| R-G | G | 29 | ||
| S-G | G | 29 | ||
| R-H | H | 29 |
The previously studied F. columnare strains (genomic groups A to H and colony morphologies 1 to 4) are referred to in this study by placing the colony morphology after the genomic group: Rz, rhizoid (previously 1); R, rough (previously 2 and 3); and S, smooth (previously 4). For example Rz-C corresponds to the previous designation C1.
UP-PCR group (numbers) for Flavobacterium sp. or RISA group (letters) for Flavobacterium columnare. ND, not determined.
For the partial 16S rRNA sequence.
Previous reports describe phages infecting the genus Flavobacterium and their interaction with the bacterial host mostly in marine environments (4, 9, 12, 14), but the phage-host relationship of the fish pathogen Flavobacterium psychrophilum has also been studied (27). A total of 49 bacteriophages were isolated from water samples (Table 2). Phages were enriched using flavobacterial isolates from freshwaters, fish farms, and previously described F. columnare strains. Phage stocks were prepared, and selected phages were grown by infecting the host bacterium (multiplicity of infection, 5 to 10) at the proper cell density, concentrated, and purified (22). Many of the isolated phages produced low-titer lysates, and they were used only for infection tests (see below for host range studies). Phage genomic DNA was extracted (for methods, see references 1 and 23) and digested with BamHI, EcoRI, HindIII, and PstI. For the genomes that were cut, the genome size was calculated from the resulting restriction profile (Table 2). For the phages that were sequenced (FKj-2, FL-1, FCL-2, and FCV-1), approximately 3,000 bp of each genome (except FCV-1, for which 1,600 bp was used) was subjected to BLAST searching (http://blast.ncbi.nlm.nih.gov/Blast.cgi; May 2011), and no significant DNA sequence similarity was found in the database. Putative protein-coding genes were analyzed using Vector NTI 11.0.0 (Invitrogen). Best matches for FCL-2 were to a hypothetical protein of the Vibrio phage VP16T (score, 57.8) and to a hypothetical protein of the Vibrio phage VP16C (score, 55.1). For FCV-1, the best match was to a hypothetical protein, B40-8030, of the Bacteroides phage B40-8 (score, 53.9).
Table 2.
Bacteriophages isolated and characterized in this study
| Phageb | Sampling site | Isolation strain | Phage family | Approximate genome size (kbp)c | RISA groupd |
|---|---|---|---|---|---|
| FJy-1 | Lake Jyväsjärvi | B80 | Myoviridae | 30 | |
| FJy-2 | Lake Jyväsjärvi | B167 | NDa | >48 | |
| FJy-3 | Lake Jyväsjärvi | B169 | Myoviridae | L | |
| FKo-1 | Lake Konnevesi | B28 | Myoviridae | 25–48 | |
| FKo-2 | Lake Konnevesi | B28 | Myoviridae | 20–30 | |
| FKe-1 | River Kevojoki | B114 | Myoviridae | ND | |
| FTs-1 | River Tsarsjoki | B121 | ND | 25–48 | |
| FKj-1 | Lake Kevojärvi | B127 | ND | >48 | |
| FKj-2 | Lake Kevojärvi | B130 | Myoviridae | 25–48 | |
| FKy-1 | River Kymijoki | B222 | Myoviridae | 20–48 | |
| FKy-2 | River Kymijoki | B223 | Podoviridae? | 25–48 | |
| FKy-3 | River Kymijoki | B224 | Myoviridae? | L | |
| FLe-1 | Lake Leppävesi | B176 | Siphoviridae? | 20–30 | |
| FL-1 | Fishery L | B183 | Myoviridae | 55 | |
| FV-1 | Fishery V | B214 | Myoviridae | 28 | |
| FV-2 | Fishery V | B218 | ND | 25–48 | |
| FV-3 | Fishery V | B225 | Myoviridae | ND | |
| FV-4 | Fishery V | B226 | Podoviridae | 25–48 | |
| FV-5 | Fishery V | B241 | ND | L | |
| FV-6 | Fishery V | B243 | ND | 30–40 | |
| FV-8 | Fishery V | B257 | ND | L | |
| FV-9 | Fishery V | B260 | Podoviridae? | L | |
| FV-10 | Fishery V | B262 | ND | L | |
| FV-11 | Fishery V | B263 | ND | >48 | |
| FV-12 | Fishery V | B278 | Podoviridae? | 60 | |
| FCL-1 | Fishery L | B67 | Myoviridae/Podoviridae | 50 | A |
| FCL-2 | Fishery L | B185 | Myoviridae | 30 | G |
| FCL-3 | Fishery L | R-G | ND | 30 | G |
| FCL-4 | Fishery L | R-G | ND | ND | G |
| FCV-1 | Fishery V | Rz-C | Myoviridae | 50 | C |
| FCV-2 | Fishery V | B235 | ND | ND | C |
| FCV-3 | Fishery V | B236 | ND | ND | C |
| FCV-4 | Fishery V | Rz-C | ND | ND | C |
| FCV-5 | Fishery V | Rz-C | ND | ND | C |
| FCV-6 | Fishery V | Rz-C | ND | ND | C |
| FCV-7 | Fishery V | Rz-C | ND | ND | C |
| FCV-8 | Fishery V | Rz-C | ND | ND | C |
| FCV-9 | Fishery V | B245 | ND | ND | C |
| FCV-10 | Fishery V | B247 | ND | ND | C |
| FCV-11 | Fishery V | Rz-C | ND | ND | C |
| FCV-12 | Fishery V | Rz-C | ND | ND | C |
| FCV-13 | Fishery V | B261 | ND | ND | C |
| FCV-14 | Fishery V | Rz-C | ND | ND | C |
| FCV-15 | Fishery V | Rz-C | ND | ND | C |
| FCV-16 | Fishery V | Rz-C | ND | ND | C |
| FCV-17 | Fishery V | Rz-C | ND | ND | C |
| FCV-18 | Fishery V | Rz-C | ND | ND | C |
| FCV-19 | Fishery V | Rz-C | ND | ND | C |
| FCV-20 | Fishery V | Rz-C | ND | ND | C |
ND, not determined.
The first letter(s) of the phage name indicates the isolation host (F, Flavobacterium sp.; FC, F. columnare); subsequent letters refer to the sampling site.
L, much larger than the typical tailed phage genome (50 kb).
The F. columnare RISA group that the phage is specific to.
Transmission electron microscopy (TEM) was used to study phage morphology. All of the flavobacterial phages that were characterized were tailed phages of the families Myoviridae, Podoviridae, and Siphoviridae (Fig. 1 and Table 2). Most of these phages had an average head size of 50 to 70 nm, but some of the myovirus isolates had capsid sizes of about 100 nm or more (FJy-3, FKo-2, FKj-2, FKy-1, and FKy-3).
Phages infecting F. columnare were isolated only from fish farms during disease outbreaks. These phages might survive inside the host cell during the cold-water period and start a lytic cycle when nutrients become available for the host cell and enough energy is available.
Studies on aquatic phage-host interplay have been conducted extensively in marine environments (15), but less is known about this interplay in freshwaters (15, 34, 35). In our study, the host ranges differed greatly between the phage isolates (Fig. 2). In the initial screening for susceptibility of the bacteria for phages, each bacterium was infected with each phage isolate by spotting the phage lysate on top agar containing the host bacterium. Each bacterium was then infected with each phage using a plaque assay. Some of the isolated phages infecting Flavobacterium species were identified as having a broad host range. These phages infected bacteria isolated from both freshwater and fish farm samples, although it has been suggested that single-host enrichments select for phages with narrow host ranges from sewage and marine environments (11, 33). Some of the phages (especially all F. columnare phages) were more host specific, as determined with our collection of Flavobacterium strains. F. columnare strains isolated from the same location as the phage were the only ones susceptible to that specific phage.
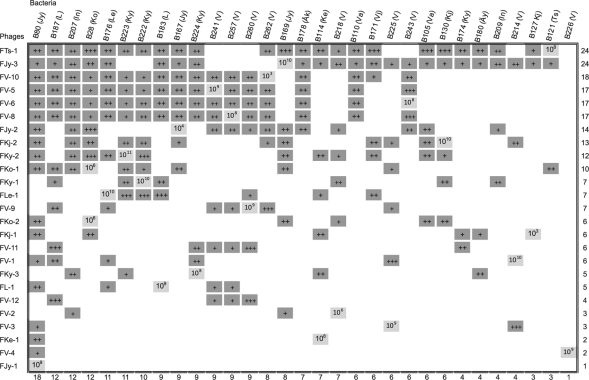
Fig. 2.
Host ranges of the phages infecting different Flavobacterium sp. strains. Dark gray squares indicate infection; white indicates no infection, and light gray squares mark the strain which was originally used for isolation of the phage. The numbers at right and bottom are the total number of different host ranges of the phage and susceptibility of bacteria to phages, respectively. The approximate titer of each phage on the bacteria is marked with plus signs (+, ≤105; ++, 106 to 108; and +++, ≥109 PFU/ml). The titer (PFU/ml) of the phage on the isolation strain is marked on the light gray squares. In parentheses after the name of the bacterial strain is the abbreviation of the place of isolation, which is also included in the phage nomenclature (Fig. 1). F. columnare phages infected only one specific F. columnare RISA group, and they are listed in Table 2.
No infection of F. columnare strains by Flavobacterium sp. phages was observed, and vice versa. However, 11 Flavobacterium sp. phage lysates (FTs-1, FKo-2, FL-1, FV-1, FKy-1, FKy-2, FKy-3, FV-3, FV-4, FV-5, FV-6, and FV-8) inhibited the growth or lysed the underlying bacterial culture on all F. columnare strains tested but produced no individual plaques (data not shown). It could be that the phages were able to bind to the bacteria and cause death but were not able to produce progeny, or the clear spots could be an indication of bacteriocin activity. The causative agent of this strong inhibition or lysis should be studied further for the possibility of developing antimicrobial agents. One of the phages (FCL-1) was isolated from a fish suffering from columnaris. The presence of the F. columnare phages in the fish indicates the possibility of controlling a fish disease by enrichment of these phages. A number of successful reports on phage therapy against fish diseases have been published; in these studies, the phages were applied directly to the water (16, 17). Another possible benefit related to phages is that they could be developed for use as diagnostic tools. In the present study, we show that bacteriophages and flavobacteria are widespread in northern freshwaters. We found phages of F. columnare to be host specific, making them good candidates for phage therapy.
Nucleotide sequence accession numbers.
All sequences have been submitted to the EMBL Nucleotide Sequence Database (http://www.ebi.ac.uk/embl/) under the accession numbers given in Table 1 and FR714876 (FCL-2), FR714877 (FL-1), FR714878 (FKj-2), and FR865436 (FCV-1).
Acknowledgments
Petri Papponen, Katja Ryymin, and Irene Helkala are thanked for their excellent technical assistance. Some bacterial strains used in this study were kindly donated by Päivi Rintamäki and Finnish food safety authority EVIRA. Station manager Kari Saikkonen and field master Esa Karpoff from The Kevo Research Station (University of Turku) are acknowledged for providing water samples.
This work was supported by Finnish Centre of Excellence Program of the Academy of Finland (2006–2011) grant 1129648 (J.K.H.B.), Academy of Finland grant 127500 (L.-R.S.), and a grant from the Maj and Tor Nessling Foundation (J.K.H.B.).
Footnotes
Published ahead of print on 2 September 2011.
REFERENCES
- 1. Bamford J. K., Bamford D. H. 1991. Large-scale purification of membrane-containing bacteriophage PRD1 and its subviral particles. Virology 181:348–352 [DOI] [PubMed] [Google Scholar]
- 2. Bernardet J. F., Bowman J. P. 2006. The genus Flavobacterium, p. 481–531 In Dworkin M., Falkow S., Rosenberg E., Schleifer K.-H., Stackebrandt E. (ed.), The prokaryotes: a handbook on the biology of bacteria, 3rd ed., vol. 7 Springer-Verlag, New York, NY [Google Scholar]
- 3. Bernardet J. F., et al. 1996. Cutting a Gordian knot: emended classification and description of the genus Flavobacterium, emended description of the family Flavobacteriaceae, and proposal of Flavobacterium hydatis nom. nov. (basonym, Cytophaga aquatilis Strohl and Tait 1978). Int. J. Syst. Evol. Microbiol. 46:128 [Google Scholar]
- 4. Borriss M., Helmke E., Hanschke R., Schweder T. 2003. Isolation and characterization of marine psychrophilic phage-host systems from Arctic sea ice. Extremophiles 7:377–384 [DOI] [PubMed] [Google Scholar]
- 5. Brandt K. K., Petersen A., Holm P. E., Nybroe O. 2006. Decreased abundance and diversity of culturable Pseudomonas spp. populations with increasing copper exposure in the sugar beet rhizosphere. FEMS Microbiol. Ecol. 56:281–291 [DOI] [PubMed] [Google Scholar]
- 6. Bulat S., Mironenko N., Lapteva M., Strelchenko P. 1994. Polymerase chain reaction with universal primers (UP-PCR) and its application to plant genome analysis, p. 113–129 In Adams R. P., Mille J. S., Golenberg E. M., Adams J. E. (ed.), Conservation of plant genes II: utilization of ancient and modern DNA. Missouri Botanical Garden Press, St. Louis, MO [Google Scholar]
- 7. Ekman E. 2003. Natural and experimental infections with Flavobacterium psychrophilum in salmonid fish. Ph.D. dissertation Swedish University, Uppsala, Sweden [Google Scholar]
- 8. Hartmann M., Frey B., Kolliker R., Widmer F. 2005. Semi-automated genetic analyses of soil microbial communities: comparison of T-RFLP and RISA based on descriptive and discriminative statistical approaches. J. Microbiol. Methods 61:349–360 [DOI] [PubMed] [Google Scholar]
- 9. Holmfeldt K., Middelboe M., Nybroe O., Riemann L. 2007. Large variabilities in host strain susceptibility and phage host range govern interactions between lytic marine phages and their Flavobacterium hosts. Appl. Environ. Microbiol. 73:6730–6739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huys G., et al. 2000. Characterization of oxytetracycline-resistant heterotrophic bacteria originating from hospital and freshwater fishfarm environments in England and Ireland. Syst. Appl. Microbiol. 23:599–606 [DOI] [PubMed] [Google Scholar]
- 11. Jensen E. C., et al. 1998. Prevalence of broad-host-range lytic bacteriophages of Sphaerotilus natans, Escherichia coli, and Pseudomonas aeruginosa. Appl. Environ. Microbiol. 64:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiang S. C., Kellogg C. A., Paul J. H. 1998. Characterization of marine temperate phage-host systems isolated from Mamala Bay, Oahu, Hawaii. Appl. Environ. Microbiol. 64:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lübeck M., Alekhina I. A., Lübeck P. S., Jensen D. F., Bulat S. A. 1999. Delineation of Trichoderma harzianum into two different genotypic groups by a highly robust fingerprinting method, UP-PCR, and UP-PCR product cross-hybridization. Mycol. Res. 103:289–298 [Google Scholar]
- 14. Middelboe M., Holmfeldt K., Riemann L., Nybroe O., Haaber J. 2009. Bacteriophages drive strain diversification in a marine Flavobacterium: implications for phage resistance and physiological properties. Environ. Microbiol. 11:1971–1982 [DOI] [PubMed] [Google Scholar]
- 15. Middelboe M., Jacquet S., Weinbauer M. 2008. Viruses in freshwater ecosystems: an introduction to the exploration of viruses in new aquatic habitats. Freshw. Biol. 53:1069–1075 [Google Scholar]
- 16. Nakai T., Park S. C. 2002. Bacteriophage therapy of infectious diseases in aquaculture. Res. Microbiol. 153:13–18 [DOI] [PubMed] [Google Scholar]
- 17. Park S. C., Nakai T. 2003. Bacteriophage control of Pseudomonas plecoglossicida infection in ayu Plecoglossus altivelis. Dis. Aquat. Organ. 53:33–39 [DOI] [PubMed] [Google Scholar]
- 18. Pulkkinen K., et al. 2010. Intensive fish farming and the evolution of pathogen virulence: the case of columnaris disease in Finland. Proc. Biol. Sci. 277:593–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qu J. H., Yuan H. L., Li H. F., Deng C. P. 2009. Flavobacterium cauense sp. nov., isolated from sediment of a eutrophic lake. Int. J. Syst. Evol. Microbiol. 59:2666–2669 [DOI] [PubMed] [Google Scholar]
- 20. Revetta R. P., Rodgers M. R., Kinkle B. K. 2005. Isolation and identification of freshwater bacteria antagonistic to Giardia intestinalis cysts. J. Water Health 3:83–85 [PubMed] [Google Scholar]
- 21. Rickard A., McBain A., Ledder R., Handley P., Gilbert P. 2003. Coaggregation between freshwater bacteria within biofilm and planktonic communities. FEMS Microbiol. Lett. 220:133–140 [DOI] [PubMed] [Google Scholar]
- 22. Sambrook J., Russell D. W. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 23. Santos M. A. 1991. An improved method for the small scale preparation of bacteriophage DNA based on phage precipitation by zinc chloride. Nucleic Acids Res. 19:5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schmidt A. S., Bruun M. S., Dalsgaard I., Larsen J. L. 2001. Incidence, distribution, and spread of tetracycline resistance determinants and integron-associated antibiotic resistance genes among motile aeromonads from a fish farming environment. Appl. Environ. Microbiol. 67:5675–5682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shieh H. Studies on the nutrition of a fish pathogen, Flexibacter columnaris. Microbios Lett. 13:129–133 [Google Scholar]
- 26. Sorum H. 2006. Antimicrobial drug resistance in fish pathogens, p. 213–238 In Aarestrup F. M. (ed.), Antimicrobial resistance in bacteria of animal origin. ASM Press, Washington, DC [Google Scholar]
- 27. Stenholm A. R., Dalsgaard I., Middelboe M. 2008. Isolation and characterization of bacteriophages infecting the fish pathogen Flavobacterium psychrophilum. Appl. Environ. Microbiol. 74:4070–4078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Suomalainen L., Tiirola M., Valtonen E. 2005. Effect of Pseudomonas sp. MT 5 baths on Flavobacterium columnare infection of rainbow trout and on microbial diversity on fish skin and gills. Dis. Aquat. Organ. 63:61–68 [DOI] [PubMed] [Google Scholar]
- 29. Suomalainen L. R., Kunttu H., Valtonen E. T., Hirvela-Koski V., Tiirola M. 2006. Molecular diversity and growth features of Flavobacterium columnare strains isolated in Finland. Dis. Aquat. Organ. 70:55–61 [DOI] [PubMed] [Google Scholar]
- 30. Tamaki H., et al. 2003. Flavobacterium limicola sp. nov., a psychrophilic, organic-polymer-degrading bacterium isolated from freshwater sediments. Int. J. Syst. Evol. Microbiol. 53:519–526 [DOI] [PubMed] [Google Scholar]
- 31. Tamminen M., et al. 2011. Tetracycline resistance genes persist at aquaculture farms in the absence of selection pressure. Environ. Sci. Technol. 45:386–391 [DOI] [PubMed] [Google Scholar]
- 32. Van Trappen S., Mergaert J., Swings J. 2003. Flavobacterium gelidilacus sp. nov., isolated from microbial mats in Antarctic lakes. Int. J. Syst. Evol. Microbiol. 53:1241–1245 [DOI] [PubMed] [Google Scholar]
- 33. Wichels A., Gerdts G., Schütt C. 2002. Pseudoalteromonas spp. phages, a significant group of marine bacteriophages in the North Sea. Aquat. Microb. Ecol. 27:233–239 [Google Scholar]
- 34. Wilhelm S. W., Matteson A. R. 2008. Freshwater and marine virioplankton: a brief overview of commonalities and differences. Freshw. Biol. 53:1076–1089 [Google Scholar]
- 35. Wommack K. E., Colwell R. R. 2000. Virioplankton: viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev. 64:69–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yi H., Oh H. M., Lee J. H., Kim S. J., Chun J. 2005. Flavobacterium antarcticum sp. nov., a novel psychrotolerant bacterium isolated from the Antarctic. Int. J. Syst. Evol. Microbiol. 55:637–641 [DOI] [PubMed] [Google Scholar]
- 37. Yoon J. H., Kang S. J., Lee J. S., Oh T. K. 2007. Flavobacterium terrigena sp. nov., isolated from soil. Int. J. Syst. Evol. Microbiol. 57:947–950 [DOI] [PubMed] [Google Scholar]
- 38. Zhang D. C., Wang H. X., Liu H. C., Dong X. Z., Zhou P. J. 2006. Flavobacterium glaciei sp. nov., a novel psychrophilic bacterium isolated from the China no. 1 glacier. Int. J. Syst. Evol. Microbiol. 56:2921–2925 [DOI] [PubMed] [Google Scholar]
- 39. Zhu F., Wang S., Zhou P. 2003. Flavobacterium xinjiangense sp. nov. and Flavobacterium omnivorum sp. nov., novel psychrophiles from the China no. 1 glacier. Int. J. Syst. Evol. Microbiol. 53:853–857 [DOI] [PubMed] [Google Scholar]