Abstract
FadD is an acyl coenzyme A (CoA) synthetase responsible for the activation of exogenous long-chain fatty acids (LCFA) into acyl-CoAs. Mutation of fadD in the symbiotic nitrogen-fixing bacterium Sinorhizobium meliloti promotes swarming motility and leads to defects in nodulation of alfalfa plants. In this study, we found that S. meliloti fadD mutants accumulated a mixture of free fatty acids during the stationary phase of growth. The composition of the free fatty acid pool and the results obtained after specific labeling of esterified fatty acids with a Δ5-desaturase (Δ5-Des) were in agreement with membrane phospholipids being the origin of the released fatty acids. Escherichia coli fadD mutants also accumulated free fatty acids released from membrane lipids in the stationary phase. This phenomenon did not occur in a mutant of E. coli with a deficient FadL fatty acid transporter, suggesting that the accumulation of fatty acids in fadD mutants occurs inside the cell. Our results indicate that, besides the activation of exogenous LCFA, in bacteria FadD plays a major role in the activation of endogenous fatty acids released from membrane lipids. Furthermore, expression analysis performed with S. meliloti revealed that a functional FadD is required for the upregulation of genes involved in fatty acid degradation and suggested that in the wild-type strain, the fatty acids released from membrane lipids are degraded by β-oxidation in the stationary phase of growth.
INTRODUCTION
The model microorganism Escherichia coli can grow using long-chain fatty acids (LCFA) as the sole carbon source. LCFA (>10 carbons) are transported into the cell by the outer membrane protein FadL and subsequently converted into their coenzyme A (CoA) thioesters by acyl-CoA synthetase, encoded by fadD (41). Degradation of acyl-CoAs proceeds via an inducible set of enzymes that catalyze the β-oxidative cleavage of the acyl-CoA into acetyl-CoAs. The first step in the β-oxidation cycle involves the conversion of acyl-CoA to enoyl-CoA via FadE. The remaining steps of hydration, oxidation, and thiolytic cleavage in fatty acid degradation are performed by a tetrameric complex consisting of two copies each of FadA and FadB (reviewed in reference 41). Strains mutated in fadD cannot produce acyl-CoA and thus cannot grow on exogenous LCFA (41). Mutant strains with a deletion of the fadL gene cannot transport LCFA across the cell envelope and, therefore, cannot grow on LCFA, yet they retain the ability to β-oxidize LCFA in vitro (9).
Mutants of Saccharomyces cerevisiae with the combined deletion of faa1 and faa4, encoding two acyl-CoA synthetases, secrete fatty acids (44). Cyanobacteria are able to incorporate exogenous fatty acids, although they seem to be devoid of an acyl-CoA synthetase activity. Instead, they contain an acyl-acyl carrier protein synthetase (Aas) activity that catalyzes the ATP-dependent esterification of fatty acids to acyl carrier protein (24). Mutants of cyanobacteria in Aas are unable to utilize exogenous fatty acids and secrete endogenous fatty acids into the culture medium (24). In both cases, S. cerevisiae mutants defective in acyl-CoA synthetases and the cyanobacterial Aas mutants, it has been established that released fatty acids originate from membrane lipids (24, 44). It was proposed that the fatty acids are released as a consequence of lipid remodeling when fatty acid residues are exchanged (24, 44).
Recently we identified a different process of lipid remodeling in Sinorhizobium meliloti that involves the polar head group of membrane lipids instead of fatty acids (50). Under growth conditions of sufficient phosphate, 95% of the membrane lipids of S. meliloti are phospholipids consisting of phosphatidylcholine, phosphatidylethanolamine (PE), monomethyl-PE, dimethyl-PE, phosphatidylglycerol (PG), and cardiolipin. However, under phosphorus-limiting conditions, S. meliloti 1021 is able to remodel existing phospholipids to convert most of them into phosphorus-free membrane lipids (50). The outer leaflet of the outer membrane in Gram-negative bacteria contains minor amounts of phospholipids but is mainly composed of lipopolysaccharides (LPS). LPS consist of three structural regions, where the lipid A region provides the hydrophobic part that anchors the LPS to the outer membrane. Remarkably, 3-hydroxy-fatty acids are part of the lipid A structure, while such fatty acids do not usually appear in phospholipids. Several studies have shown the importance of LPS for S. meliloti (7, 18, 45).
In several bacteria, a mutation in fadD provokes phenotypes apparently unrelated to the function of FadD as an enzyme required for growth on LCFA (5, 32, 48). A mutant of Streptomyces coelicolor in fadD1 shows a severe deficiency in the production of the antibiotic actinorhodin (Atc), which seems to be related to a delayed expression of the act biosynthetic genes (5). In Salmonella enterica serovar Typhimurium, a mutation in fadD represses the expression of HilA, an activator of invasion genes (32). The lack of fadD in Sinorhizobium meliloti GR4 results in multicellular swarming behavior and defects in the establishment of its symbiosis with alfalfa (48). Swarming is a type of bacterial motility characterized by a rapid and coordinated population migration across a surface and involves a complex process of differentiation in which cells usually become hyperflagellated and elongated (19). Interestingly, fadD1 mutants of Pseudomonas aeruginosa also showed an increased swarming motility (25), and fadD mutants of Vibrio cholerae showed a repression of the major virulence genes as well as an increase in motility (38).
Although the wild-type strain of S. meliloti GR4 never shows swarming motility, the S. meliloti wild-type strains 1021 and 2011 presented a surface movement resembling that of the fadD mutants in approximately 70% of experiments (34). In all three S. meliloti strains, a mutation in fadD promoted swarming motility (34). Furthermore, it was demonstrated that genes required for the biosynthesis of the siderophore rhizobactin 1021 are essential for swarming in the wild-type strains, but they are dispensable in a fadD mutant background (34). Given the function of FadD in fatty acid metabolism, we investigated the lipidic composition of S. meliloti wild-type and fadD mutant strains. We found that the fadD mutant of S. meliloti accumulated significant amounts of free fatty acids in the stationary phase of growth. Several lines of evidence support that the fatty acids accumulated in the fadD mutant are derived from complex membrane lipids. We propose that a major function of FadD is the utilization of endogenous free fatty acids that could have been released as a result of lipid remodeling.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids used in this work and their relevant characteristics are listed in Table 1. Mutations of the fadD or fadL gene in E. coli Y-Mel or in K27(pBBR1MCS-3) were transduced from strains in the Keio Collection (4) by P1vir transduction (49). Correct transfer of the fadL mutation in strains YfadL1 and KfadL1 was corroborated using K2 oligonucleotide (4) and an oligonucleotide that matched the last 21 nucleotides of the fadL gene (data not shown) by using PCR and amplification of a fragment of the expected size. E. coli strains were grown at 30°C either in Luria-Bertani (LB) broth (43) or in M9 minimal medium (33). S. meliloti strains were grown at 30°C either in complex tryptone yeast (TY) broth supplemented with 4.5 mM CaCl2 (8) or in the minimal medium Sherwood (46), with succinate (8.3 mM) replacing mannitol as the carbon source, or Robertsen (40). Antibiotics were added, when required, to the following final concentrations (in μg ml−1): carbenicillin (100) and tetracycline (20) for E. coli; streptomycin (200), kanamycin (200), and tetracycline (8) for S. meliloti.
Table 1.
Bacterial strains and plasmids used in this work
| Strain or plasmid | Relevant characteristic(s)a | Reference or source |
|---|---|---|
| Escherichia colistrains | ||
| DH5α | recA1 φ80dlacZΔM15; cloning strain | 22 |
| S17-1 | thi pro recA hsd(rK− mK+); RP4 integrated in the chromosome; 2-Tc::Mu Km::Tn7 (Tpr/Smr) | 47 |
| Y-Mel | Wild-type strain | 39 |
| K-27 | fadD mutant derivative of Y-Mel | 36 |
| JW1794-1 | BW25113 fadD::kan | 4 |
| JW2341-1 | BW25113 fadL::kan | 4 |
| YfadD1 | Y-Mel fadD::kan | This work |
| YfadL1 | Y-Mel fadL::kan | This work |
| KfadL1 | K27 fadL::kan | This work |
| Sinorhizobium meliloti strains | ||
| GR4 | Wild-type strain | 12 |
| QS77 | fadD::Tn5 insertion mutant derivative of GR4, Kmr | 48 |
| 1021 | Smr of SU47 wild type | 20 |
| 1021FDC5 | fadD mutant derivative of wild-type 1021, Smr Kmr | 34 |
| Plasmids | ||
| pET17b | Expression vector, Cbr | Novagen |
| pBBR1MCS-3 | Broad-host-range vector, Tcr | 28 |
| pBBRD4 | pBBR1MCS-3 derivative harboring fadD of S. meliloti GR4, Tcr | 48 |
| pRK404 | Broad-host-range vector, Tcr | 15 |
| pDM10 | pHP13 derivative containing the Δ5-Des gene of B. subtilis | 1 |
| pNG28 | pET17b cloned in pRK404 | This work |
| pECH9 | Δ5-Des gene of B. subtilis in pET17b | This work |
| pRCanul5 | pECH9 cloned in pRK404 | This work |
Tcr, Tpr, Kmr, Smr, and Cbr indicate tetracycline, trimethoprim, kanamycin, streptomycin, and carbenicillin resistance, respectively.
DNA manipulations.
Recombinant DNA techniques were carried out using standard procedures (43). The Δ5-desaturase (Δ5-Des) gene was amplified from plasmid pDM10 (1) using the specific oligonucleotides 5′-AGGAATACATATGACTGAACAAACCATTGCAC-3′ and 5′-AAAGGATCCTCAGGCATTCTTCCGCAGC-3′. The forward primer incorporated an NdeI restriction site (underlined) overlapping the start codon of the gene, and the reverse primer incorporated a BamHI restriction site (underlined) after the stop codon. After digestion of the amplified fragment with NdeI and BamHI, it was cloned into pET17b that had been digested with the same enzymes, resulting in plasmid pECH9. Plasmid pECH9 was linearized with BamHI and cloned into pRK404 previously digested with BamHI, resulting in plasmid pRCanul5. As a control, pET17b was linearized with BamHI and cloned into pRK404 previously digested with the same enzyme, resulting in plasmid pNG28. pRCanul5 and pNG28 were mobilized into S. meliloti 1021FDC5 by biparental mating using the E. coli S17-1 donor strain as described previously (47).
In vivo labeling of S. meliloti and E. coli with [14C]acetate and analysis of lipid extracts by TLC.
The lipid compositions of the different S. meliloti and E. coli strains were determined following labeling with [1-14C]acetate. Cultures (1 ml) were inoculated at an initial optical density at 620 nm (OD620) of 0.1 from precultures grown in the same medium. After the addition of 2 μCi [1-14C]acetate (60 mCi mmol−1) to each culture, they were incubated to distinct time points in different growth phases. The cells were harvested by centrifugation, washed with 500 μl of water, and resuspended in 100 μl of water. The lipids were extracted according to the method of Bligh and Dyer (10). The chloroform phase was used for lipid analysis by one-dimensional thin-layer chromatography (TLC) using high-performance TLC silica gel 60 plates (Merck) and mobile-phase ethyl acetate-hexane-acetic acid (60:40:5 [vol/vol/vol]). Two-dimensional TLC was performed as described previously (14). Radioactivity was detected using a Storm 820 PhosphorImager (Amersham Biosciences). Image analysis and signal quantification were carried out using ImageQuant TL (Amersham Biosciences).
Fatty acid analysis.
For fatty acid analysis, 20-ml cultures were grown until early stationary phase for S. meliloti or until late stationary phase for E. coli. Cells and culture media were separated by centrifugation at 6,000 × g for 15 min. Cell pellets were resuspended in 1 ml of water and extracted according to the methods described by Bligh and Dyer (10). To distinguish between free and esterified fatty acids in cellular lipid extracts, samples were split into two equivalent parts and treated with two different reagents that resulted in either specific methylation of free fatty acids or in transmethylation of esterified fatty acids (44). Fatty acids from spent supernatants were extracted twice with equal volumes of acidified ethyl acetate (0.1-ml liter−1 glacial acetic acid), and they were subsequently converted to methyl esters as described previously (44). Ten micrograms of tridecanoic acid (C13:0) was added as an internal standard. The methyl esters were extracted twice with 1 ml of hexane each time followed by centrifugation. The upper hexane phases were pooled into a new glass vial and dried under a nitrogen stream.
For quantitative analysis, fatty acid methyl esters were dissolved in 600 μl hexane (high-performance liquid chromatography/spectrophotometric grade), and 1 μl was used for analysis by gas chromatography (GC; model 6890; Agilent Technologies, Santa Clara, CA) coupled to a mass spectrometric detector (quadrupole MSD HP 5973; Agilent Technologies, Santa Clara, CA). Samples were separated in a 5% phenyl, 95% methylpolysiloxane capillary column (HP-5MS; 25-m by 0.200-mm with 0.33-μm film thickness; Agilent Technologies, Santa Clara, CA). The oven was set at 100°C for 2 min, and the following gradient was used: 100°C to 244°C at 5°C min−1 and then holding at 244°C for 10 min. Helium was used as the carrier gas, with a flow rate of 40 cm s−1 at 100°C. The molecules were ionized by electron impact at 70 eV. Fatty acid species were identified using retention times and mass spectral information by comparison with the bacterial acid methyl esters mix standard (BAME 47080-U; Sigma-Aldrich). The relative amounts of fatty acid methyl esters were determined by comparing the areas under the peaks on the chromatogram to the area under the peak of the internal fatty acid standard (C13:0).
RNA isolation and synthesis of labeled DNA.
For RNA isolation, preinocula of 1021 and 1021FDC5 cells were grown at 30°C in 3 ml of TY broth to late exponential phase (OD620, 1.0 to 1.1). After incubation, 1 ml of each culture was pelleted, washed twice in Robertsen minimal medium, and resuspended in the same volume of the latter medium. Erlenmeyer flasks (250 ml) containing 30 ml of liquid Robertsen medium were inoculated with 0.3 ml of the rhizobial suspension and incubated at 30°C with continuous shaking (190 rpm) until reaching either an OD620 of 0.7 to 0.8 (mid-exponential phase of growth) or an OD620 of 1.1 to 1.2 (beginning of stationary phase of growth). Cells were harvested by centrifugation and washed with 0.1% Sarkosyl, and the cell pellets were immediately frozen in liquid nitrogen and conserved at −80°C until RNA isolation. For microarray hybridization and reverse transcription-quantitative real-time PCR (RT-qPCR), RNA was isolated using the Qiagen RNeasy RNA purification kit following the manufacturer's instructions. Residual DNA was removed with the RNase-free DNase I set (Roche). The quality of the RNA was checked by 1.4% agarose gel electrophoresis.
Cy3- and Cy5-labeled cDNAs were prepared according to the methods of DeRisi et al. (13) from 15 μg of total RNA. Three slide hybridizations were performed using the labeled cDNA synthesized from each of the RNA preparations from three independent bacterial cultures.
Microarray hybridization, image acquisition, and data analysis.
Sm6koligo microarrays were purchased from A. Becker (University of Bielefeld, Bielefeld, Germany). Hybridizations were performed as described previously (16). For image acquisition, a GenePix 4100A scanner (Axon Instruments, Inc., Foster City, CA) was used. Quantifications of mean signal intensities for each spot were determined using the GenePix Pro 5.0 software (Axon Instruments, Inc.). Normalization and t statistic determinations were carried out using the EMMA 2.6 microarray data analysis software developed at the Bioinformatics Resource Facility Center for Biotechnology, Bielefeld University (http://www.genetik.uni-bielefeld.de/EMMA/) (17). Three independent biological replicates were performed for each experiment. Genes were regarded as differentially expressed if they showed a P value of ≤0.05, A of ≥7, and M of ≥1 or ≤(−1) (where A is the average signal-to-noise ratio and M is the log2[experiment/control] ratio).
RT-qPCR.
Total RNA (1 μg) treated with an RNase-free DNase I set (Roche) was reverse transcribed using SuperScript II reverse transcriptase (Invitrogen) and random hexamers (Roche) as primers. Quantitative real-time PCR was performed on an iCycler iQ5 (Bio-Rad, Hercules, CA). Each 25-μl reaction mixture contained either 1 μl of the cDNA or a dilution (1:10,000 for amplification of the 16S rRNA gene), 200 nM each primer, and iQ SYBR green supermix (Bio-Rad). Control PCRs of the RNA samples not treated with reverse transcriptase were also performed to confirm the absence of contaminating genomic DNA. Samples were initially denatured by heating at 95°C for 3 min, followed by a 35-cycle amplification and quantification program (95°C for 30 s, 55°C for 45 s, and 72°C for 45 s). A melting curve was conducted to ensure amplification of a single product. The oligonucleotide sequences for qPCR are listed in Table 2. The efficiency for each primer pair (E) was determined by running 10-fold serial dilutions (4 dilution series) of 1021 genomic DNA as template and generating a standard curve by plotting the log of the dilution factor against the cycle threshold (CT) value during amplification of each dilution. Amplification efficiency (E) was calculated using the formula E = [10(1/a) − 1] × 100, where a is the slope of the standard curve.
Table 2.
Sequences of oligonucleotides used for quantitative real-time PCR
| Gene | Primer sequence (5′-3′) |
|
|---|---|---|
| Forward | Reverse | |
| smc03224 (16S rRNA) | TCTACGGAATAACGCAGG | GTGTCTCAGTCCCAATGT |
| smc02227 (fadB) | TCGATGAACGTCTTCACC | CGGAGAAGGAGGATTTGC |
| smc02229 | GACATCCTCAGTCTGATG | CGTGCTCTTCGATATAGC |
The relative expression level of each gene was normalized to that of 16S rRNA, and the analysis of results was done using the comparative critical threshold (ΔΔCT) method (37).
Microarray accession number.
Detailed protocols and raw data resulting from the microarray experiments have been deposited in the ArrayExpress database with the accession number E-MEXP-2651.
RESULTS
Sinorhizobium meliloti fadD mutants accumulate free fatty acids in the stationary phase of growth.
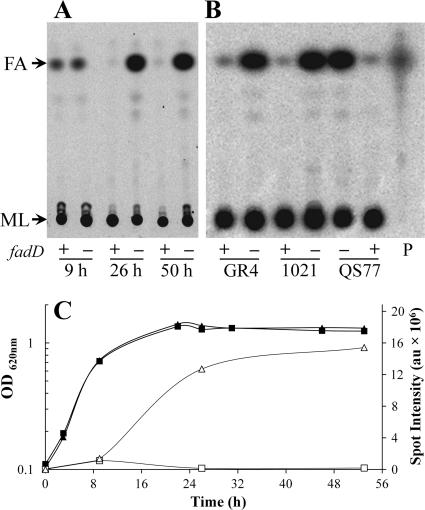
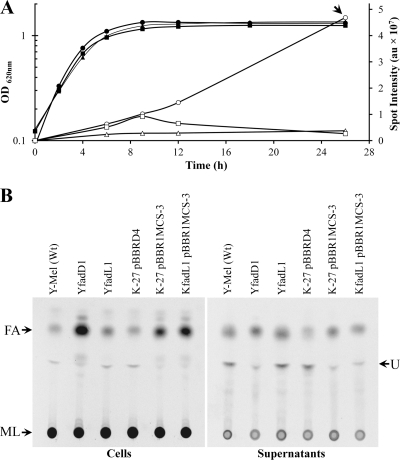
The gene fadD encodes a long-chain fatty acyl-CoA synthetase, and in order to follow fatty acid metabolism in S. meliloti wild type and a fadD mutant, we grew cultures of them in the presence of [14C]acetate. Lipid extracts obtained from cultures grown until different time points in the distinct growth phases were first analyzed by one-dimensional TLC, with a mobile phase consisting of ethyl acetate-hexane-acetic acid (60:40:5) to allow separation of the more hydrophobic compounds. Lipid extracts from cultures of S. meliloti GR4 and its fadD-deficient mutant QS77 grown to exponential phase showed similar levels of a spot that appeared to run as fatty acids (Fig. 1A and C). However, the extract of cultures grown to early or late stationary phases showed a significant increase in the spot for fatty acids in strain QS77, while this spot was barely detected in lipid extracts from the wild-type GR4 (Fig. 1A and C). Also, the accumulation of a compound migrating like fatty acids was observed in lipid extracts obtained from the S. meliloti fadD mutant strain derived from the sequenced S. meliloti 1021 strain grown to the early stationary phase of growth (Fig. 1B). Furthermore, complementation of QS77 with the fadD-bearing plasmid pBBRD4 abolished the accumulation of fatty acids, while QS77 carrying an empty plasmid (pBBR1MCS-3) still accumulated fatty acids (Fig. 1B). Therefore, the accumulation of fatty acids in the stationary phase of growth is directly related to the absence of fadD.
Fig. 1.
S. meliloti fadD mutants accumulate fatty acids in the stationary phase of growth. (A) Cellular lipid extracts obtained either from wild-type S. meliloti GR4 (fadD+) or from its fadD-deficient mutant QS77 were grown until exponential phase (OD620, 0.7; 9 h of growth), until early stationary phase (OD620, 1.2; 26 h of growth), or until late stationary phase (50 h of growth). (B) Lipid extracts of cultures grown until the early stationary phase (OD620, 1.2) of the S. meliloti strains GR4 (fadD+) and its fadD-deficient mutant derivative QS77; 1021 (fadD+) and its mutant fadD-deficient derivative 1021FDC5; QS77 carrying the empty vector pBBR1MCS-3 or carrying fadD-containing plasmid pBBRD4 (fadD+). P, [14C]palmitic acid. Membrane lipids (ML) did not migrate from the origin, and the spot for fatty acids (FA) is indicated. (C) Growth curves of strains GR4 (■) and QS77 (▴) and quantification of their fatty acids (GR4 [□] and QS77 [▵]), corresponding with the TLC plate shown in panel A. Quantification of the intensity of the spot for fatty acids is given in arbitrary units (au). Cultures were grown on Sherwood minimal medium. Similar results were obtained with cultures grown on Robertsen minimal medium.
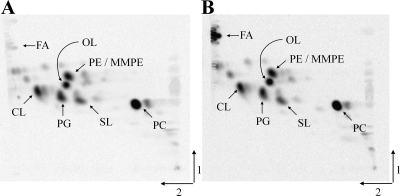
In Fig. 1C, the growth behaviors of strain GR4 and its fadD mutant are shown in relation to the content of fatty acids. Similar results were obtained with wild-type strain 1021 and its fadD-deficient mutant, 1021FDC5 (data not shown). A two-dimensional TLC analysis of lipid extracts from 1021 and its fadD-deficient mutant, 1021FDC5, grown until early stationary phase, showed no difference with respect to major membrane lipids but revealed clearly the accumulation of fatty acids in the mutant (Fig. 2).
Fig. 2.
Separation of [14C]acetate-labeled lipids of wild-type S. meliloti 1021 (A) and the fadD-deficient mutant 1021FDC5 (B) by two-dimensional thin-layer chromatography. Cultures were grown until early stationary phase (final OD620, 1.2) in Sherwood minimal medium. Fatty acids (FA) and the membrane lipids phosphatidylcholine (PC), phosphatidylethanolamine (PE), monomethyl-PE (MMPE), sulfolipid (SL), ornithine lipid (OL), phosphatidylglycerol (PG), and cardiolipin (CL) are indicated.
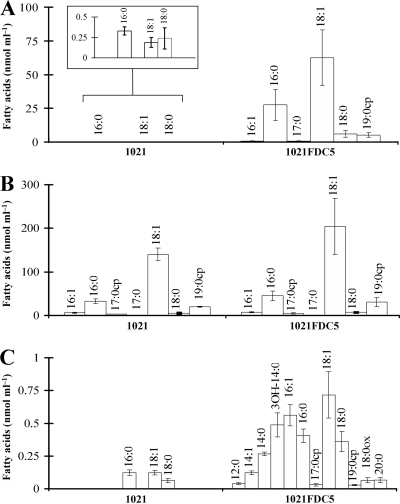
The compound accumulated in the fadD mutants comigrated in different separation systems as free fatty acids (Fig. 1 and 2). Therefore, we proceeded to analyze the content of free fatty acids on lipid extracts from wild-type 1021 and its fadD-deficient derivative, 1021FDC5, that had been grown on minimal medium to the early stationary phase. The concentrations of free fatty acids in lipid extracts obtained from cell pellets were significantly higher in the mutant than in the wild type. The lipid extract of the fadD-deficient mutant contained relative concentrations of total free fatty acids corresponding to 102.6 (standard deviation [SD], 36.7) nmol ml of culture−1, while samples of the wild-type strain contained only 0.75 (SD, 0.24) nmol ml−1 (see Table S1 of the supplemental material). Clearly, the mutation in fadD resulted in a significant accumulation of cell-associated free fatty acids. The most abundant fatty acid accumulated in the fadD mutant was cis-vaccenic acid (C18:1 Δ11), comprising more than 60% of the total fatty acids. Following in abundance were palmitic acid (C16:0; 26.4%), stearic acid (C18:0; 5.8%), and lactobacillic acid (C19:0cp; 5.0%) (Fig. 3A; see also Table S1). These are the same fatty acids found typically esterified to membrane lipids of S. meliloti (6). We quantified the esterified fatty acids in the cellular lipid extracts of 1021 and its fadD mutant and found that the patterns of esterified fatty acids present in both strains were similar (Fig. 3B; see also Table S1). Furthermore, the composition of free fatty acids that accumulated in 1021FDC5 reflected the composition of fatty acids esterified to membrane lipids (Fig. 3A and B). Also, a modest accumulation of free fatty acids was observed in supernatants of cultures of the fadD mutant. A total of 3.15 (SD, 0.55) nmol of fatty acids ml−1 was present in the culture medium of the mutant versus 0.31 (SD, 0.06) nmol ml−1 present in that of the wild type (see also Table S1). Besides the detection of fatty acids usually known to be esterified to membrane phospholipids, the supernatant of the mutant strain contained significant amounts of myristic (C14:0) and 3-hydroxymyristic (3-OH-C14:0) acids (Fig. 3C; see also Table S1). In particular, 3-OH-C14:0 amounted to 15.5% of the total free fatty acids present in the spent culture medium. This is remarkable, as 3-OH-C14:0 is the most abundant fatty acid in lipid A of S. meliloti (18, 45), and it might be found in the medium due to release from lipid A. Less abundant, each one comprising from 1 to 3.8% of the total, were the fatty acids C12:0, C14:1, C20:0, and C18:0 epoxide.
Fig. 3.
Fatty acid profiles of lipid extracts from cells and spent culture medium of wild-type S. meliloti 1021 and fadD mutant 1021FDC5. (A) Free fatty acids in cell lipid extracts. (B) Esterified fatty acids in cell lipid extracts. (C) Free fatty acids in the culture medium. Concentrations are given in nmol/ml of culture. The inset in panel A depicts magnified columns. For each strain, three independent cultures were analyzed. The error bars represent the SD.
The accumulated free fatty acids derive from complex membrane lipids.
The free fatty acids identified in cellular lipid extracts from the fadD mutant reflected the composition of the fatty acids usually esterified to membrane lipids (Fig. 3; see also Table S1 in the supplemental material). The detection of cyclopropane fatty acids (C17:0cp and C19:0cp) in the free fatty acid fraction was remarkable, as cyclopropanation in fatty acids is introduced by cyclopropane fatty acid synthases on fatty acyl residues that are esterified to membrane lipids (21). The presence of the cyclopropane fatty acids in the culture medium and in the cell lipid extracts strongly suggested that these fatty acids had been released from complex membrane lipids.
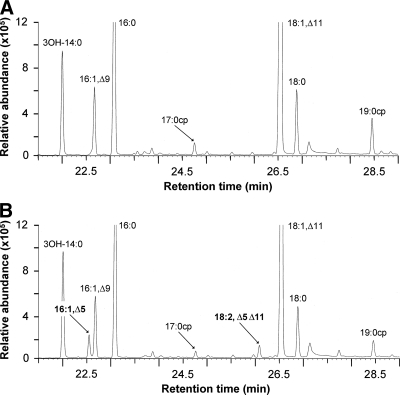
With the aim to obtain additional proof about the metabolic origin of the free fatty acids, we followed a similar strategy to that described for yeast by Scharnewski and coworkers (44). This strategy consists of introducing a fatty acid modification that occurs only when fatty acids are substituents of membrane phospholipids. We introduced the gene coding for Δ5-Des lipid desaturase from Bacillus subtilis into the fadD mutant 1021FDC5. Δ5-Des is a membrane-bound acyl desaturase that introduces a cis-double bond between the 5 and 6 positions of fatty acyl residues attached to membrane lipids (2). The analysis by gas chromatography of methyl esters obtained from free fatty acids in the spent culture medium of 1021FDC5 cells expressing the Δ5-Des gene showed the presence of two additional peaks that were not present in the same fraction of 1021FDC5 carrying an empty vector (Fig. 4). The new peaks were identified as C16:1 Δ5 and C18:2 Δ5 Δ11, based on comparison of the mass spectra we obtained with those reported for the methyl ester of C16:1 Δ5 (35) and for the methyl ester of C18:2 Δ5 Δ11 (11) (see Fig. S1 and S2 in the supplemental material, respectively). The capacity of Δ5-Des to produce C18:2 Δ5 Δ11 was demonstrated previously after overexpression of the enzyme in E. coli (11). Expression of the Δ5-Des gene in the S. meliloti fadD mutant strain resulted in an abundance of 5.5% of Δ5-modified fatty acids in the pool of esterified fatty acids, indicating successful expression of the Δ5-desaturase (data not shown). More interestingly, 1.5% of the free fatty acids of the cell lipid extract (data not shown) and 3% of free fatty acids in the culture medium (Fig. 4) contained Δ5-modified fatty acids. These findings indicate a release of the modified lipid-bound fatty acyl residues into the pool of free fatty acids and suggest that the accumulation of free fatty acids is the result of the release of fatty acyl residues from membrane lipids.
Fig. 4.
Expression of the Δ5-Des gene in S. meliloti 1021FDC5 leads to formation of Δ5-unsaturated free fatty acids. Partial gas chromatograms of methyl esters obtained from fatty acids in the spent culture medium of 1021FCD5 carrying an empty vector (A) or expressing the Δ5-Des gene (B) are shown. The different fatty acids in panel A were confirmed by comigration and by comparison of their mass spectra with authentic standards. The Δ5-modified fatty acids were identified by comparison with reported spectra (see Fig. S1 and S2 in the supplemental material).
Fatty acid release also occurs in Escherichia coli.
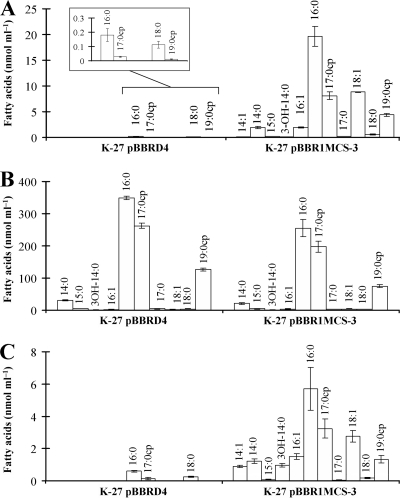
E. coli is the model organism in which bacterial fatty acid metabolism has been studied most extensively. Since mutants in fadD of E. coli were available, we wanted to explore if the phenomenon of release of fatty acids from membrane lipids also occurred in this organism. We first analyzed lipid extracts after labeling of E. coli cultures in minimal medium. As observed for S. meliloti, there was no difference with respect to the accumulation of free fatty acids during the exponential phase of growth (Fig. 5A). Also, no significant accumulation of fatty acids could be observed in an E. coli fadD mutant in the early stationary phase of growth (OD, 1.2; 9 h of growth) (Fig. 5A). However, at 26 h of growth (15 h after entering the stationary phase), significant amounts of free fatty acids were detected in lipid extracts of the fadD mutants (Fig. 5). In contrast to the fadD mutant, lipid extracts of the late stationary phase of the E. coli wild type or the fadD mutant complemented with fadD from S. meliloti did not show fatty acid accumulation (Fig. 5). We next quantified free fatty acids and esterified fatty acids in the E. coli fadD mutant carrying a vector or carrying the same vector with fadD of S. meliloti from cultures grown in minimal medium until late stationary phase. The cellular lipid extract of the fadD mutant contained a total of 46.2 (SD, 3.4) nmol of free fatty acids ml of culture−1, while the lipid extract of the complemented strain contained only a total of 0.33 (SD, 0.08) nmol ml of culture−1 (see Table S2 in the supplemental material). Remarkably, cis-vaccenic acid (C18:1 Δ11) amounted to 19% of the total in the free fatty acid pool, which is in stark contrast to its presence at less than 1% in the pool of esterified fatty acids (Fig. 6A and B). The other free fatty acids of the lipid extract reflected the composition of the esterified fatty acids (Fig. 6A and B; see also Table S2). The free fatty acids present in the culture medium of the E. coli fadD mutant were similar to the free fatty acids present in the cellular lipid extract, with the major difference being that 3-hydroxymyristic acid (3-OH-C14:0) was 5% of the free fatty acids in the culture medium and was only 0.1% in the cell-associated lipid extract (Fig. 6; see also Table S2). The sum of cyclopropane fatty acids (C17:0cp and C19:0cp) in both fractions of free fatty acids consisted of about 25% of the total fatty acids (Fig. 6A and C; see also Table S2). The presence of these cyclopropane fatty acids in the free form strongly suggests that they were released from membrane phospholipids, since the cyclopropane fatty acid synthase of E. coli only modifies monounsaturated fatty acids that are lipid bound (21). The spectrum of free fatty acids found in the E. coli fadD mutant was similar to the spectrum of esterified fatty acids, but it was not identical. This can be explained by the fact that esterified fatty acids continue to be modified, but not the free fatty acids that have been released from the membrane lipids during the distinct growth phases. Since cultures of E. coli were collected in late stationary phase, most of the monounsaturated fatty acids in the lipid-bound fraction had been converted to their cyclopropane forms.
Fig. 5.
fadD mutants of E. coli accumulate free fatty acids in the late stationary phase of growth. (A) Growth curves of strains Y-Mel (■), K-27(pBBRD4) (▴), and K-27(pBBR1MCS-3) (•) and quantification of fatty acids for Y-Mel (□), K-27(pBBRD4) (▵), and K-27(pBBR1MCS-3) (○). Quantifications of the intensities of the spots for fatty acids are given in arbitrary units (au). (B) Lipid extracts from cells or supernatants of E. coli strains Y-Mel (Wt), YfadD1 (fadD deficient), YfadL1 (fadL deficient), K-27(pBBRD4) (fadD deficient complemented with fadD from S. meliloti), K-27(pBBR1MCS-3) (fadD deficient), and KfadL1(pBBR1MCS-3) (fadD and fadL deficient). Lipid extracts were obtained from cultures grown until late stationary phase (26 h). FA, fatty acids; ML, membrane lipids (did not migrate from the origin); U, unidentified lipid. Cultures were grown on M9 minimal medium. The arrow in panel A indicates when samples shown in panel B were taken.
Fig. 6.
Fatty acid profiles of lipid extracts from cells and spent culture media of E. coli K-27 (fadD deficient) carrying S. meliloti fadD-containing plasmid pBBRD4 and K-27 carrying empty vector pBBR1MCS-3. (A) Free fatty acids in cell lipid extracts. (B) Esterified fatty acids in cell lipid extracts. (C) Free fatty acids in the culture medium. The inset in panel A depicts magnifications of the columns. For each strain two independent cultures were analyzed.
The accumulation of free fatty acids observed in both E. coli and S. meliloti fadD mutants could be explained as a result of lysis of some cells during the stationary phase of growth. However, comparison of OD and CFU values of wild-type and mutant strains at the point where fatty acids were measured does not support this hypothesis (data not shown). Moreover, if fatty acids accumulating in the fadD mutant were from an extracellular origin, i.e., from cellular lysis, the accumulation of fatty acids should also be observed in an E. coli fadL mutant, which is unable to incorporate exogenously supplied LCFA. To test this hypothesis, E. coli fadD, fadL, and fadD fadL mutants were constructed by transduction. These mutants, in contrast to wild-type E. coli, were unable to grow in oleate as a sole carbon source. Furthermore, the incorporation of [14C]palmitic acid in the mutant strains was as expected for strains with deficiencies in fadD or fadL (42). Also in our hands, the fadD mutant incorporated fatty acids only into phosphatidylethanolamine (data not shown). In contrast, due to the impaired uptake of palmitic acid, we hardly observed any incorporation of fatty acids into the phospholipids of the fadL mutant (data not shown). Whereas free fatty acids accumulated in a fadD mutant, a fadL mutant did not show such an accumulation of fatty acids in cells or in spent medium (Fig. 5B). The fact that fatty acids do not accumulate and must therefore be consumed in a fadL mutant strongly suggests that fatty acids that accumulate in the fadD mutant are of intracellular origin. The consumption of fatty acids in a fadL mutant is in agreement with its reported capability of performing β-oxidation in vitro (9). Furthermore, a fadD fadL double mutant also accumulated fatty acids in the cell-associated fraction (Fig. 5B), indicating again the intracellular nature of the accumulated fatty acids, because in this strain transport of fatty acids from the outside is impaired.
FadD is required for the upregulation of S. meliloti fatty acid degradation (fad) genes in the stationary phase of growth.
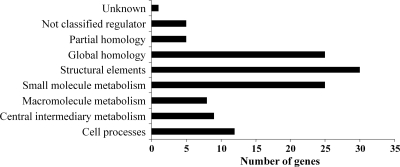
The differences observed in accumulation of fatty acids between the fadD mutant and wild-type strains prompted us to compare the transcriptomes of these two strains in cultures grown in liquid minimal medium at the beginning of the stationary phase. A total of 128 genes appeared to be differentially expressed under these conditions, with the majority of them (120 genes) showing downregulation in the fadD mutant compared to the wild-type strain (see Table S3 in the supplemental material). Numbers of downregulated genes in each functional category are presented as a histogram in Fig. 7. Many of the downregulated genes (26%) have unknown functions or display partial or global homology to genes deposited in databases (Fig. 7). Moreover, we found downregulation of a significant number of genes involved in different metabolic activities (Fig. 7; see also Table S3), suggesting a lower metabolic rate in the fadD mutant compared to the wild-type strain. Thus, we found downregulation of genes involved in protein metabolism (up to 29 genes coding for ribosomal proteins, 3 elongation factors, and 3 chaperone genes), elements of different respiratory chains and associated functions (11 genes), the active methyl cycle (4 genes), and fatty acid degradation (3 genes). The lower expression of the genes belonging to the operon smc02229-fadA-fadB and of genes encoding elements of different respiratory chains in the fadD mutant than in the wild-type strain during the stationary phase could indicate a lower level of fatty acid degradation in the mutant and explain the higher accumulation of fatty acids observed in this strain under these conditions. SMc02229 has been annotated as a putative acyl-CoA dehydrogenase, which could catalyze the conversion of acyl-CoA to enoyl-CoA, the first step in the β-oxidation cycle, whereas FadB and FadA participate in the remaining steps of hydration, oxidation, and thiolytic cleavage in fatty acid degradation. Therefore, we decided to analyze the expression of smc02229 and fadB by performing RT-qPCR in 1021 and 1021FDC5 cultures in the exponential and stationary phases of growth (Table 3). The results obtained indicated that at the beginning of the stationary phase of growth (OD620, 1.2), both genes showed higher expression in the wild-type strain than the fadD mutant, thereby confirming the microarray data. On the other hand, when the analyses were performed in cultures of the wild-type and mutant strains in the exponential phase of growth (OD620, 0.8), similar expression levels were observed for the two genes (Table 3). These results are in agreement with the observed pattern of accumulation of fatty acids (Fig. 1), which showed a similar level in wild-type and mutant strains during the exponential phase but which were consumed in the wild type during the stationary phase. The RT-qPCR data were also used to obtain the relative expression levels of the fad genes in the stationary phase versus exponential phase of growth in each of the two genetic backgrounds. These analyses revealed that in the wild-type strain the expression levels of smc02229 and fadB were higher in the stationary than in the exponential phase of growth (33.8 ± 10.7 and 3.4 ± 1, respectively). In contrast, in the fadD mutant decreased expression of these genes was observed in stationary phase (−3.88 ± 0.43 and −2.31 ± 0.29, respectively), which may have been due to the lower metabolic rate shown by this strain after entering the stationary phase of growth. These results suggest that, in S. meliloti, FadD is required for the upregulation of fad genes at the beginning of the stationary phase of growth, which in turn allows for the utilization of endogenous free fatty acids released from membrane lipids.
Fig. 7.
Distribution in functional categories of downregulated genes in the S. meliloti fadD mutant compared to the wild-type strain. Functional categories were assigned according to the S. meliloti Genome Project (http://iant.toulouse.inra.fr/bacteria/annotation/cgi/rhime.cgi).
Table 3.
Relative expression of fatty acid degradation genes in S. meliloti cultures of two strains at exponential and stationary phases of growth
| Gene | Relative expression ratio, 1021 vs. 1021FDC5a |
||
|---|---|---|---|
| Microarrays (OD, 1.2)b | RT-qPCR (OD, 1.2)c | RT-qPCR (OD, 0.8)c | |
| smc02229 | 11.96 | 80.64 ± 28.93 | −1.65 ± 0.22 |
| fadA | 3.5 | ND | ND |
| fadB | 2.8 | 11.68 ± 4.9 | 1.18 ± 0.1 |
Data correspond to results obtained from cultures of the two strains grown in Robertsen medium. Similar results were obtained from cultures grown in Sherwood medium (data not shown). Cultures in stationary phase (OD, 1.2) were used for the microarray experiments; cultures in either the stationary or exponential (OD, 0.8) growth phase were used in the RT-qPCRs. ND, not determined.
Fold changes in gene expression obtained in the microarray experiments were calculated as 2M (see text).
Fold changes in gene expression obtained from RT-qPCR were calculated from CT values obtained from real-time PCR experiments using the comparative ΔΔCT method (37). The smc03224 gene coding for 16S rRNA was used as an internal control. Data are averages from two independent biological experiments with three technical replicates. The minus sign indicates a decreased fold change in 1021. The standard errors of the means from the RT-qPCRs are shown.
Among the nonclassified regulators that appeared downregulated in the microarray study was smc01260 (see Table S3 in the supplemental material). Interestingly, Kazakov et al. (26), using a comparative genomic approach, identified SMc01260 as the candidate transcriptional factor that might control the fatty acid utilization pathway in S. meliloti 1021. The smc01260 regulon proposed by Kazakov et al. (26) comprises 9 operons: smc02162 (fadD), smc02229-smc02228 (fadA)-smc02227 (fadB), smc00977-smc00976, smc00041, smc02377 (etf), smc00729 (etfB1)-smc00728 (etfA1)-smc00727 (hbdA), smc02479 (mdh)-smc02480 (sucC)-smc02481 (sucD)-smc02482 (sucA)-smc02483 (sucB)-smc02484-smc02485-smc02486-smc02487 (lpdA2), smc02391, and smc02150. Genes in four of these operons, underlined in the previous list, appeared downregulated in the S. meliloti fadD mutant (see Table S3). The operons belonging to this regulon are preceded by a common palindromic 18-bp DNA motif named the ILV box, which is supposed to be the binding site for the transcriptional regulator SMc01260 (26). Upstream of smc01260, we also identified an 18-bp sequence that differed by only 1 nucleotide from the consensus of the ILV box (see Fig. S3 in the supplemental material), suggesting autoregulation. Our experimental data are in agreement with the computationally identified regulatory network for fatty acid degradation in S. meliloti.
DISCUSSION
We have determined that mutation of fadD in S. meliloti or in E. coli provokes the accumulation of a mixture of free fatty acids in the stationary phase of growth. The composition of the free fatty acid pool found in the experiments concerning expression of a lipid-specific Δ5-desaturase is in agreement with membrane phospholipids being the origin of the released fatty acids. Radiolabeling experiments indicated that fatty acids are released from both the wild type and mutant (Fig. 1, 9 h of growth). However, the accumulation observed in the stationary phase of growth occurred in fadD mutants, due to their inability to activate and consume the released fatty acids. Furthermore, we found that a fadL mutant of E. coli did not accumulate fatty acids, while a fadD fadL double mutant still accumulated them. These results demonstrate that released fatty acids are of intracellular origin and that their subsequent consumption does not require uptake. So far, the reason for this fatty acid release is not known.
The higher expression of the fad genes observed in wild-type S. meliloti than in the fadD mutant suggests that released fatty acids are directed to fatty acid degradation. The utilization of these fatty acids by the wild type could provide an extra carbon source when other sources are depleted. Alternatively, acyl-CoAs could be converted into storage compounds in the stationary phase of growth. For example, Streptomyces coelicolor forms triacylglycerols as storage compounds, and long-chain acyl-CoAs are used as acyl donors in triacylglycerol biosynthesis (3); however, formation of triacylglycerols has not been reported in S. meliloti. In either case, the wild type should have a metabolic advantage over a fadD mutant in the stationary phase. Although survival rates of the wild-type strain and fadD mutants were very similar at entry (for S. meliloti) or 15 h after entering into the stationary phase (for E. coli), in the case of E. coli we found a significant reduction (66%) in the survival rate of the fadD mutant compared to that of the wild type after extended times in stationary phase (41 h after entering the stationary phase).
Remarkably, the phenotype of fatty acid accumulation that we observed in fadD mutants of S. meliloti and of E. coli was also reported for mutants of S. cerevisiae and of cyanobacteria that are also deficient in their respective systems of fatty acid activation (24, 44). In both cases, release of fatty acids from membrane lipids was postulated to be due to a process of lipid remodeling consisting of an exchange of fatty acyl residues (24, 44). So far, little is known about this type of lipid remodeling in the phospholipids of bacteria. Direct evidence for phospholipid remodeling by fatty acyl exchange in E. coli was obtained by Kol and coworkers (27). They demonstrated that exogenously added short-chain PE to a PE-deficient mutant resulted in the typical PE-fatty acyl profile of the wild-type strain. Similar experiments were carried out in Synechocystis PCC6803. Exogenously added dioleoylphosphatidylglycerol to a PG-deficient mutant led to the formation of new retailored PG species according to the functional needs of the cyanobacterium (29). Release of fatty acids from phospholipids involves the activity of phospholipases type A. A future challenge will be to assign the corresponding open reading frames (ORFs) that are responsible for such activities. While there is no ORF assigned for phospholipase A in the genome of S. meliloti, in E. coli two different phospholipase A activities have been described, but their biological roles remain unknown (41).
The present study and previous ones showed that a major role of fatty acid activation is the utilization of endogenous fatty acids in organisms as diverse as S. cerevisiae, cyanobacteria, S. meliloti, and E. coli. Probably, this mechanism is conserved in most organisms, as suggested by Kaczmarzyk and Fulda (24). FadD has been studied mainly as an activity required for degradation of exogenous LCFA, and addition of exogenous fatty acids to the wild-type S. meliloti increases the expression of the fadD gene (data not shown). However, the important role of FadD in utilization of endogenous free fatty acids was not recognized previously in bacteria.
Fatty acids may act as environmental cues that control swarming motility in different bacteria. In Proteus mirabilis, oleic acid (C18:1 Δ9) enhances swarming, whereas some saturated fatty acids inhibit this type of surface motility (31). Also, in Serratia marcescens, saturated fatty acids negatively regulate swarming (30). Interestingly, in this bacterium a relationship between cellular fatty acid composition and swarming phenotypes has been observed, leading to the hypothesis that modulation of cellular fatty acid composition and hence homeostasis of membrane fluidity may play a crucial role in controlling swarming motility. Seemingly contradictory to the previous results, swarming of Pseudomonas aeruginosa was not altered by the saturated fatty acids palmitic (C16:0) or stearic acid (C18:0), while oleic and vaccenic (C18:1 Δ11) acids inhibited swarming (23). In the case of S. meliloti, none of the fatty acids tested (palmitic acid, stearic acid, or oleic acid at a concentration of 5 μg ml−1) was able to induce swarming of the GR4 strain (data not shown). While the addition of palmitic or stearic acid did not interfere with the swarming phenotype of strain QS77, oleic acid inhibited its swarming phenotype (data not shown). However, we still cannot rule out a function of fatty acids in swarming of S. meliloti, since the wild type is able to metabolize fatty acids while the fadD mutant cannot use them. Further analysis of the lipidic composition presented under swarming-inducing conditions by different S. meliloti strains will reveal whether membrane fluidity is involved in swarming, as postulated by Lai et al. (30).
In E. coli, acyl-CoAs resulting from FadD activity on long-chain fatty acids are able to bind to FadR, a repressor of the GntR family, which controls the expression of the fatty acid degradation genes. Binding of the acyl-CoAs to FadR causes release of the regulatory protein from the operator and thus derepression of the fad genes (41). Our data indicate that formation of acyl-CoAs is also required for activation of the fad regulon in S. meliloti, although the regulator probably involved, SMc01260, belongs to the MerR family. Our experimental data are in agreement with the computationally identified regulatory network for fatty acid degradation in S. meliloti as well as with Kazakov's proposal (26), in which an acyl-CoA intermediate of the fatty acid degradation pathway might be the physiological effector controlling the expression of the smc01260 regulon.
Supplementary Material
ACKNOWLEDGMENTS
Plasmid pDM10 was kindly provided by Diego de Mendoza (Universidad de Rosario, Rosario, Argentina). Escherichia coli mutant strains JW1794-1 and JW2341-1 were generously provided by José Adelfo Escalante (Instituto de Biotecnología, UNAM, Cuernavaca, Mexico). P1 bacteriophage and oligonuclotide K2 were kindly provided by Francisco Bolivar and Héctor Castañeda (Instituto de Biotecnología, UNAM, Cuernavaca, Mexico). We thank Maria Gregoria Medina (Centro de Investigaciones Químicas) for excellent technical assistance and Pieter van Dillewijn (Estación Experimental del Zaidín, CSIC) for sharing data concerning fadD gene expression.
This work was supported by grants from CONACyT/Mexico (49738-Q and 82614), from the Spanish MICINN(BIO2007-62988), and from Consejería de Innovación, Ciencia y Empresa (Junta de Andalucía; CVI 03541) to M.J.S. and from Laboratorio Nacional de Estructura de Macromoléculas (2006-C01-56431) to L.A. J.N. was supported by a postdoctoral contract from Consejería de Innovación, Ciencia y Empresa (Junta de Andalucía). A.P.-C. was supported during a Ph.D. program (Programa de Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de México) by a scholarship from Consejo Nacional de Ciencia y Tecnología (Mexico).
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 16 September 2011.
REFERENCES
- 1. Aguilar P. S., Cronan J. E., Jr., de Mendoza D. 1998. A Bacillus subtilis gene induced by cold shock encodes a membrane phospholipid desaturase. J. Bacteriol. 180:2194–2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Altabe S. G., Aguilar P., Caballero G. M., de Mendoza D. 2003. The Bacillus subtilis acyl lipid desaturase is a Δ5 desaturase. J. Bacteriol. 185:3228–3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arabolaza A., Rodriguez E., Altabe S., Alvarez H., Gramajo H. 2008. Multiple pathways for triacylglycerol biosynthesis in Streptomyces coelicolor. Appl. Environ. Microbiol. 74:2573–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baba T., et al. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio Collection. Mol. Syst. Biol. 2:2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Banchio C., Gramajo H. 2002. A stationary-phase acyl-coenzyme A synthetase of Streptomyces coelicolor A3(2) is necessary for the normal onset of antibiotic production. Appl. Environ. Microbiol. 68:4240–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Basconcillo L. S., McCarry B. E. 2008. Comparison of three GC/MS methodologies for the analysis of fatty acids in Sinorhizobium meliloti: development of a micro-scale, one-vial method. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 871:22–31 [DOI] [PubMed] [Google Scholar]
- 7. Becker A., Fraysse N., Sharypova L. 2005. Recent advances in studies on structure and symbiosis-related function of rhizobial K-antigens and lipopolysaccharides. Mol. Plant Microbe Interact. 18:899–905 [DOI] [PubMed] [Google Scholar]
- 8. Beringer J. E. 1974. R factor transfer in Rhizobium leguminosarum. J. Gen. Microbiol. 84:188–198 [DOI] [PubMed] [Google Scholar]
- 9. Black P. N., DiRusso C. C. 2003. Transmembrane movement of exogenous long-chain fatty acids: proteins, enzymes, and vectorial esterification. Microbiol. Mol. Biol. Rev. 67:454–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911–917 [DOI] [PubMed] [Google Scholar]
- 11. Bonamore A., Macone A., Colotti G., Matarese R. M., Boffi A. 2006. The desaturase from Bacillus subtilis, a promising tool for the selective olefination of phospholipids. J. Biotechnol. 121:49–53 [DOI] [PubMed] [Google Scholar]
- 12. Casadesús J., Olivares J. 1979. Rough and fine linkage mapping of the Rhizobium meliloti chromosome. Mol. Gen. Genet. 174:203–209 [DOI] [PubMed] [Google Scholar]
- 13. DeRisi J. L., Iyer V. R., Brown P. O. 1997. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science 278:680–686 [DOI] [PubMed] [Google Scholar]
- 14. de Rudder K. E. E., Thomas-Oates J. E., Geiger O. 1997. Rhizobium meliloti mutants deficient in phospholipid N-methyltransferase still contain phosphatidylcholine. J. Bacteriol. 179:6921–6928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ditta G., et al. 1985. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid 13:149–153 [DOI] [PubMed] [Google Scholar]
- 16. Domínguez-Ferreras A., et al. 2006. Transcriptome profiling reveals the importance of plasmid pSymB for osmoadaptation of Sinorhizobium meliloti. J. Bacteriol. 188:7617–7625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dondrup M., et al. 2003. EMMA: a platform for consistent storage and efficient analysis of microarray data. J. Biotechnol. 106:135–146 [DOI] [PubMed] [Google Scholar]
- 18. Ferguson G. P., Datta A., Carlson R. W., Walker G. C. 2005. Importance of unusually modified lipid A in Sinorhizobium stress resistance and legume symbiosis. Mol. Microbiol. 56:68–80 [DOI] [PubMed] [Google Scholar]
- 19. Fraser G. M., Hughes C. 1999. Swarming motility. Curr. Opin. Microbiol. 2:630–635 [DOI] [PubMed] [Google Scholar]
- 20. Galibert F., et al. 2001. The composite genome of the legume symbiont Sinorhizobium meliloti. Science 293:668–672 [DOI] [PubMed] [Google Scholar]
- 21. Grogan D. W., Cronan J. E., Jr 1997. Cyclopropane ring formation in membrane lipids of bacteria. Microbiol. Mol. Biol. Rev. 61:429–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557–580 [DOI] [PubMed] [Google Scholar]
- 23. Inoue T., Shingaki R., Fukui K. 2008. Inhibition of swarming motility of Pseudomonas aeruginosa by branched-chain fatty acids. FEMS Microbiol. Lett. 281:81–86 [DOI] [PubMed] [Google Scholar]
- 24. Kaczmarzyk D., Fulda M. 2010. Fatty acid activation in cyanobacteria mediated by acyl-acyl carrier protein synthetase enables fatty acid recycling. Plant Physiol. 152:1598–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kang Y., Zarzycki-Siek J., Walton C. B., Norris M. H., Hoang T. T. 2010. Multiple FadD acyl-CoA synthetases contribute to differential fatty acid degradation and virulence in Pseudomonas aeruginosa. PLoS One 5:e13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kazakov A. E., et al. 2009. Comparative genomics of regulation of fatty acid and branched-chain amino acid utilization in proteobacteria. J. Bacteriol. 191:52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kol M. A., et al. 2004. Uptake and remodeling of exogenous phosphatidylethanolamine in E. coli. Biochim. Biophys. Acta 1636:205–212 [DOI] [PubMed] [Google Scholar]
- 28. Kovach M. E., et al. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176 [DOI] [PubMed] [Google Scholar]
- 29. Laczko-Dobos H., et al. 2010. Remodeling of phosphatidylglycerol in Synechocystis PCC6803. Biochim. Biophys. Acta 1801:163–170 [DOI] [PubMed] [Google Scholar]
- 30. Lai H. C., et al. 2005. The RssAB two-component signal transduction system in Serratia marcescens regulates swarming motility and cell envelope architecture in response to exogenous saturated fatty acids. J. Bacteriol. 187:3407–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liaw S. J., Lai H. C., Wang W. B. 2004. Modulation of swarming and virulence by fatty acids through the RsbA protein in Proteus mirabilis. Infect. Immun. 72:6836–6845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lucas R. L., et al. 2000. Multiple factors independently regulate hilA and invasion gene expression in Salmonella enterica serovar Typhimurium. J. Bacteriol. 182:1872–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miller J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 34. Nogales J., et al. 2010. Transcriptome profiling of a Sinorhizobium meliloti fadD mutant reveals the role of rhizobactin 1021 biosynthesis and regulation genes in the control of swarming. BMC Genomics 11:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Olagbemiro T. O., Birkett M. A., Mordue A. J., Pickett J. A. 1999. Production of (5R,6S)-6-acetoxy-5-hexadecanolide, the mosquito oviposition pheromone, from the seed oil of the summer cypress plant, Kochia scoparia (Chenopodiaceae). J. Agric. Food Chem. 47:3411–3415 [DOI] [PubMed] [Google Scholar]
- 36. Overath P., Pauli G., Schairer H. U. 1969. Fatty acid degradation in Escherichia coli. An inducible acyl-CoA synthetase, the mapping of old-mutations, and the isolation of regulatory mutants. Eur. J. Biochem. 7:559–574 [PubMed] [Google Scholar]
- 37. Pfaffl M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ray S., Chatterjee E., Chatterjee A., Paul K., Chowdhury R. 2011. A fadD mutant of Vibrio cholerae is impaired in the production of virulence factors and membrane localization of the virulence regulatory protein TcpP. Infect. Immun. 79:258–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rickenberg H. V., Lester G. 1955. The preferential synthesis of beta-galactosidase in Escherichia coli. J. Gen. Microbiol. 13:279–284 [DOI] [PubMed] [Google Scholar]
- 40. Robertsen B. K., Aman P., Darvill A. G., McNeil M., Albersheim P. 1981. Host-symbiont interactions. V. The structure of acidic extracellular polysaccharides secreted by Rhizobium leguminosarum and Rhizobium trifolii. Plant Physiol. 67:389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rock C. O. 2008. Fatty acids and phospholipids metabolism in prokaryotes, p. 59–96In Vance D. E., Vance J. E.(ed.), Biochemistry of lipids, lipoproteins and membranes, 5th ed. Elsevier, Amsterdam, Netherlands [Google Scholar]
- 42. Rock C. O., Jackowski S. 1985. Pathways for the incorporation of exogenous fatty acids into phosphatidylethanolamine in Escherichia coli. J. Biol. Chem. 260:12720–12724 [PubMed] [Google Scholar]
- 43. Sambrook J., Russell D. W. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 44. Scharnewski M., Pongdontri P., Mora G., Hoppert M., Fulda M. 2008. Mutants of Saccharomyces cerevisiae deficient in acyl-CoA synthetases secrete fatty acids due to interrupted fatty acid recycling. FEBS J. 275:2765–2778 [DOI] [PubMed] [Google Scholar]
- 45. Sharypova L. A., Niehaus K., Scheidle H., Holst O., Becker A. 2003. Sinorhizobium meliloti acpXL mutant lacks the C28 hydroxylated fatty acid moiety of lipid A and does not express a slow migrating form of lipopolysaccharide. J. Biol. Chem. 278:12946–12954 [DOI] [PubMed] [Google Scholar]
- 46. Sherwood M. T. 1970. Improved synthetic medium for the growth of Rhizobium. J. Appl. Bacteriol. 33:708–713 [DOI] [PubMed] [Google Scholar]
- 47. Simon R., Priefer U., Pühler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. BioTechnology 1:784–791 [Google Scholar]
- 48. Soto M. J., Fernández-Pascual M., Sanjuán J., Olivares J. 2002. A fadD mutant of Sinorhizobium meliloti shows multicellular swarming migration and is impaired in nodulation efficiency on alfalfa roots. Mol. Microbiol. 43:371–382 [DOI] [PubMed] [Google Scholar]
- 49. Thomason L. C., Costantino N., Court D. L. 2007. E. coli genome manipulation by P1 transduction. Curr. Prot. Mol. Biol. 79:1.17.1–1.17.8 [DOI] [PubMed] [Google Scholar]
- 50. Zavaleta-Pastor M., et al. 2010. Sinorhizobium meliloti phospholipase C required for lipid remodeling during phosphorus limitation. Proc. Natl. Acad. Sci. U. S. A. 107:302–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.