Abstract
Mycobacterium sp. strain JC1 is able to grow on methanol as a sole source of carbon and energy using methanol:N,N′-dimethyl-4-nitrosoaniline oxidoreductase (MDO) as a key enzyme for methanol oxidation. The second open reading frame (mdoR) upstream of, and running divergently from, the mdo gene was identified as a gene for a TetR family transcriptional regulator. The N-terminal region of MdoR contained a helix-turn-helix DNA-binding motif. An electrophoretic mobility shift assay (EMSA) indicated that MdoR could bind to a mdo promoter region containing an inverted repeat. The mdoR deletion mutant did not grow on methanol, but growth on methanol was restored by a plasmid containing an intact mdoR gene. In DNase I footprinting and EMSA experiments, MdoR bound to two inverted repeats in the putative mdoR promoter region. Reverse transcription-PCR indicated that the mdoR gene was transcribed only in cells growing on methanol, whereas β-galactosidase assays showed that the mdoR promoter was activated in the presence of methanol. These results indicate that MdoR serves as a transcriptional activator for the expression of mdo and its own gene. Also, MdoR is the first discovered member of the TetR family of transcriptional regulators to be involved in the regulation of the methanol oxidation, as well as to function as a positive autoregulator.
INTRODUCTION
Methylotrophic bacteria use reduced carbon compounds containing no carbon-carbon bonds as sole carbon and energy sources (4). The enzyme for the oxidation of methanol to formaldehyde in methanol-oxidizing bacteria is highly expressed in cells growing on methanol, indicating the expression of the gene for this enzyme is regulated according to the presence of methanol (19).
The regulation of methanol oxidation in Gram-negative bacteria is known to be variable. At least 26 genes are required for the oxidation of methanol in Methylobacterium extorquens AM1 (23, 37). MxaB, a putative two-component response regulator, and MxbD and MxbM, a putative sensor-regulator pair, are involved in the regulation of methanol oxidation in this bacterium (35, 36). Also, MxcQ and MxcE, another putative two component regulatory system, are required for the expression of mxaF, the gene for the large subunit of methanol dehydrogenase (MDH) (37). Further, a methanol-inducible promoter with a multi-A tract sequence upstream of mxaF is essential for the expression of mxaF (22, 39). In Paracoccus denitrificans, genes involved in methanol oxidation are located in the mxa gene cluster (38). A two-component system consisting of MxaY, a putative histidine kinase, and MxaX, a putative response regulator, is involved in the control of MDH expression (11). Another two-component system consisting of FlhR and FlhS also regulates methanol oxidation in Paracoccus denitrificans (12). However, little is known about the regulation of the genes responsible for the oxidation of methanol in Gram-positive bacteria. It is only known that the gene for NAD-dependent MDH in Bacillus methanolicus strain MGA3 is upregulated in cells growing on methanol (15).
Mycobacterium sp. strain JC1 is a Gram-positive bacterium that grows on methanol as a sole source of carbon and energy using methanol:N,N′-dimethyl-4-nitrosoaniline oxidoreductase (MDO) as a key enzyme for the oxidation of methanol (5, 25, 26, 28, 34). The gene for MDO (mdo) has been cloned and characterized, along with two complete upstream open reading frames (ORFs) and a complete downstream ORF (25). Analysis of the amino acid sequence of the second upstream ORF, which runs in the opposite direction from mdo, revealed that it coded for a TetR family transcriptional regulator, suggesting that this ORF (mdoR) may be involved in the regulation of mdo gene expression (25).
In the present study, we describe the identification and characterization of a novel transcriptional regulator, MdoR, which is involved in the regulation of methanol oxidation in Mycobacterium sp. strain JC1. Our results show that MdoR activates the expression of mdo and positively regulates its own expression.
MATERIALS AND METHODS
Bacterial strains, plasmids, primers, probes, and cultivation conditions.
The bacterial strains and plasmids used in the present study are described in Table 1. Mycobacterium sp. strain JC1 DSM 3803 was grown at 37°C in standard mineral base medium (SMB) (16) supplemented with 1% (vol/vol) methanol (SMB-MeOH) or 0.2% (wt/vol) glucose (SMB-glucose). Growth was determined by turbidity measured at 436 nm. For cultivation on plates, cells were grown on solid SMB-MeOH or SMB-glucose in the presence or absence of 100 μg of hygromycin/ml. Deletion mutants of mdoR were screened on solid medium containing SMB supplemented with 10% (wt/vol) sucrose (SMB-sucrose). Escherichia coli strains were grown at 37°C in LB in the presence or absence of 50 μg of ampicillin/ml. E. coli DH5α was used as a host for all plasmid constructions. E. coli BL21(DE3) was used for the overproduction of MdoR protein. The primers and probes used in the present study are listed in Table 2.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or descriptiona | Source or reference |
|---|---|---|
| Bacterial strains | ||
| Mycobacterium sp. | ||
| Strain JC1 | Wild type (DSM 3803); Hygs | 34 |
| Strain JC1(RM3m) | mdoR deletion mutant; Hygs | This study |
| Strain JC1(RM3c) | Strain JC1(RM3m) carrying plasmid pHP11; Hygr | This study |
| E. coli | ||
| BL21(DE3) | F−ompThsdSB(rB− mB−) galdcm | Promega |
| DH5α | supE44 lac169(φ80lacZΔM15)hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Gibco-BRL |
| Plasmids | ||
| pDAS1 | 8,621-bp reporter vector containing a promoterless lacZ gene; Hygr | 32 |
| pET22b(+) | 5,493-bp T7 expression vector; Ampr | Novagen |
| pGEM-T Easy | 3,015-bp linear plasmid for direct subcloning of PCR product; Ampr | Promega |
| pHP8L | pGEM-T Easy containing a 1,531-bp PCR product containing partial mdoR gene | This study |
| pHP8R | pGEM-T Easy containing a 1,290-bp PCR product containing partial mdoR gene and partial orf2 gene | This study |
| pHP9 | pGEM-T Easy containing a 2,821-bp PCR product containing deleted mdoR gene and partial orf2 gene | This study |
| pHP10 | pKO containing a 2,684-bp BamHI-HindIII fragment containing deleted mdoR gene and partial orf2 gene | This study |
| pHP11 | pNBV1 containing 817-bp PCR product for complementation of RM3m | This study |
| pHP12 | pET22b(+) containing a 688-bp EcoRI-XhoI fragment corresponding to the mdoR gene | This study |
| pHP13 | pDAS1 harboring a 289-bp ClaI-XbaI fragment containing a putative mdoR promoter | This study |
| pKO | 8,366-bp vector containing sacB gene for sucrose counterselection; Hygr and Kanr | 33 |
| pNBV1 | 5.8-kb high-copy-number plasmid with pAL5000 replicon derived from p16R1; Hygr | 14 |
Hyrr, hygromycin resistance; Hyrs, hygromycin sensitivity; Ampr, ampicillin resistance; Kanr, kanamycin resistance.
Table 2.
Primers and probes used in this study
| Type of analysis and primer or probe | Sequence (5′-3′)a |
|---|---|
| RT-PCR | |
| MR-F | CCAAGAAGCAGCGCAACCAAT |
| MR-R | GTAGTAGTAGACGGCCGATAT |
| Construction and verification of mdoR deletion mutant | |
| L1-F | AGGTAGCGTCCATGACAGATA |
| L1-R | tctagaCACTTCTCGTCGATCGTGGAT* |
| R1-F | tctagaAGCCACACCGATTCCGATGAT* |
| R1-R | tctagaAATAGTGCAGGGATTCAAGCG* |
| MRid-F | AATGGGCCGCGAAGAACTGCG |
| MRid-R | TACAACGTACGAATGCAGGTA |
| Construction of MdoR expression plasmid | |
| MdoR-F | gaattcCATGGGTACCGCATCATCGGA† |
| MdoR-R | ctgcagCGGCTTCAGGTTGCCGATGA‡ |
| Construction of mdoR complementation plasmid | |
| CR-F | ctgcagGATCTACGGCTTCAGGTTGCC‡ |
| CR-R | ctgcagATCCAACAAGGTGGTCGCCGC‡ |
| DNase I footprinting | |
| RF-F | gaattcAGCTTGCGCATGCTCAGCCCG† |
| RF-R | ctgcagACGCGAAGCCCGCATCCAACA‡ |
| Construction of reporter plasmid | |
| RP-F | tctagaATTGGTTGCGCTGCTTCTTGG* |
| RP-R | atcgatTCGTCGATCGTCGTGTCGCAG§ |
| EMSAb | |
| MDP-F | CGGGCAGCGTGCTGCGAGT |
| MDP-R | ACTCGCAGCACGCTGCCCG |
| MRP-F | CCTCCGTACGCTGTACGTAATGCAACGTACAACGTACGAAT |
| MRP-R | ATTCGTACGTTGTACGTTGCATTACGTACAGCGTACGGAGG |
| MRIR1-F | CCTCCGTGTGCTGTACGTAATGCAACGTACAACGTACGAAT |
| MRIR1-R | ATTCGTACGTTGTACGTTGCATTACGTACAGCACACGGAGG |
| MRIR2-F | CCTCCGTACGCTGTACGTAATGCAACGTGTAACGTACGAAT |
| MRIR2-R | ATTCGTACGTTACACGTTGCATTACGTACAGCGTACGGAGG |
| MRIR12-F | CCTCCGTGTGCTGTACGTAATGCAACGTGTAACGTACGAAT |
| MRIR12-R | ATTCGTACGTTACACGTTGCATTACGTACAGCACACGGAGG |
*, the XbaI recognition sequence is indicated in lowercase, underlined text; †, the EcoRI recognition sequence is indicated in lowercase, underlined text; ‡, the PstI recognition sequence is indicated in lowercase, underlined text; §, the ClaI recognition sequence is indicated in lowercase, underlined text.
For the EMSA sequences, underlining indicates the inverted repeat sequences. Boldface italics within the (underlined) inverted repeat sequences indicates the base transversion of the inverted repeat sequences CGTAC and GTACG.
RNA isolation and RT-PCR.
Total RNA was isolated from cells harvested at the mid-exponential-growth phase using TRIzol reagent (Invitrogen), as previously described (32). To identify mdoR transcripts in cells grown in SMB-MeOH and SMB-glucose, reverse transcription-PCR (RT-PCR) using two primers, MR-F and MR-R, was performed according to the manufacturer's instructions (Invitrogen). The resulting cDNA that covers a part of the mdoR gene was used directly for PCR. The PCR mixture contained 20 pmol of each primer, 100 ng of cDNA template, and 0.5 U of ExTaq polymerase (Takara) in a final volume of 20 μl. Amplification was carried out as follows: primary denaturation for 3 min at 95°C; followed by 30 cycles of denaturation for 40 s at 95°C, annealing for 40 s at 52°C, and elongation for 40 s at 72°C; with a final postelongation step for 10 min at 72°C.
Overproduction and purification of MdoR in E. coli.
To overproduce MdoR in E. coli, two primers, MdoR-F and MdoR-R containing a 6-mer extension of EcoRI and XhoI sites (underlined, Table 2), respectively, were synthesized. The amplified 688-bp PCR products were digested with EcoRI and XhoI and cloned into pET22b(+) (Novagen) to produce the plasmid, pHP12. pHP12 harboring a complete mdoR gene was subsequently introduced into E. coli BL21(DE3) and induced for MdoR expression with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 4 h at 30°C. The overexpressed His-tagged MdoR protein was purified on a Ni-NTA column (TaKaRa), according to the manufacturer's instructions.
EMSA.
Electrophoretic mobility shift assay (EMSA) was performed using a previously described method with some modifications (8). To detect the binding of MdoR to a putative promoter region of mdo, 21-mer oligonucleotide primers (MDP-F and MDP-R) covering an inverted repeat (5′-GCAGCGTGCTGC-3′) (underlined) in the putative mdo promoter were synthesized. To identify whether MdoR binds to the putative mdoR promoter region, 41-mer oligonucleotide primers were synthesized covering two inverted repeats in the putative promoter region of mdoR (5′-CGTACGCTGTACG-3′ and 5′-CGTACAACGTACG-3′) (underlined), which are present at nucleotides 22 to 34 and nucleotides 43 to 55 upstream of the mdoR translation start site, respectively, with (MRIR1-F, MRIR1-R, MIR2-F, MIR2-R, MIR12-F, and MIR12-R) or without (MRP-F and MRP-R) transversing bases (indicated by boldface italic letters in Table 2) in the inverted repeat. MDP-F, MRP-F, MRIR1-F, MIR2-F, and MIR12-F were then hybridized to MDP-R, MRP-R, MRIR1-R, MIR2-R, and MIR12-R, respectively, and the resulting 21- and 41-bp fragments were used for EMSA after labeling with [γ-32P]ATP.
DNase I footprinting assay.
DNA fragments containing the putative promoter of mdoR were prepared by PCR using primers RF-F and RF-R with 6-mer EcoRI and XhoI sites (underlined, Table 2). The resulting 304-bp fragments were end labeled with [γ-32P]ATP and digested with EcoRI or XhoI to prepare strand-specific end-labeled DNA fragments. The end-labeled fragments were mixed with MdoR in the binding buffer used for EMSA. The mixture was then subjected to DNase I footprinting analysis following previously described methods (3, 30).
Construction of reporter plasmid and transformation.
Amplification of the putative promoter of mdoR covering two inverted repeats present 18 to 58 bp upstream of the mdoR translation start codon was done using primers RP-F and RP-R with 6-mer XbaI and ClaI sites (underlined, Table 2), respectively. The 289-bp PCR products were eluted from an agarose gel after electrophoresis, purified with the QIAquick gel extraction kit (Qiagen), and cloned into the XbaI and ClaI sites of the promoterless pDAS1 vector (32) to create a transcriptional fusion to the lacZ gene, resulting in reporter plasmid, pHP13. Vectors were introduced into Mycobacterium sp. strain JC1 wild type or an mdoR deletion mutant by electrotransformation according to the method of Seo et al. (32).
Construction and complementation of mdoR deletion mutant.
To construct a vector for mutagenesis, a 1,531-bp DNA fragment containing part of the mdoR gene was prepared by PCR using the primers L1-F and L1-R with a 6-mer XbaI site (underlined, Table 2) and cloned into the pGEM-T Easy vector to create pHP8L. A 1,290-bp DNA fragment containing a partial mdoR gene and a partial orf2 gene was also amplified by PCR using the primers R1-F and R1-R with a 6-mer XbaI site (underlined, Table 2), followed by cloning into the pGEM-T Easy vector to yield pHP8R. The pHP8R plasmid was digested with XbaI, and the resulting 1,290-bp XbaI fragment was inserted into pHP8L digested with the same enzyme to produce pHP9. The pHP9 plasmid was then digested with BamHI and HindIII to yield a 2,684-bp BamHI-HindIII fragment containing a partially deleted mdoR and part of orf2. The fragment was subsequently inserted into pKO digested with BamHI and HindIII to construct pHP10. The pHP10 plasmid was then introduced into Mycobacterium sp. strain JC1 by electrotransformation. Transformed cells were cultivated overnight in SMB-glucose, and a portion of the cultures was plated onto solid SMB-glucose containing hygromycin. Hygromycin-resistant cells were then isolated and inoculated into 2 ml of SMB-glucose. After 4 days of cultivation, the resulting culture was plated on solid SMB-sucrose. Mutants that were able to grow on SMB-sucrose were selected and designated as mdoR-deletion mutant RM3m. The fidelity of the deletion event in RM3m was confirmed by PCR using the primers MRid-F and MRid-R.
For the complementation of MdoR activity in RM3m, the entire length of mdoR was amplified by using primers CR-R and CR-F with a PstI site (underlined, Table 2), and PCR products digested with PstI were cloned into pNBV1, producing the plasmid pHP11. pHP11 was then introduced into RM3m by electrotransformation, and the complemented mutant, RM3c, was selected by resistance to hygromycin.
β-Galactosidase assay.
β-Galactosidase assays were performed as described previously (32), using cell extracts prepared from cells of Mycobacterium sp. strain JC1 carrying pHP13 and grown to the late exponential growth phase in SMB-MeOH and SMB-glucose. Activity is expressed as Miller units per mg of total cell protein.
RESULTS AND DISCUSSION
MdoR belongs to the TetR family of transcriptional regulators.
Since the Mycobacterium sp. strain JC1 mdo gene was significantly induced in the presence of methanol (25), it seemed likely that the mdo gene was transcriptionally regulated. Further, a previous study had found that the amino acid sequence deduced from the nucleotide sequence of the orf1 gene (GenBank accession no. GQ161963), which has the opposite orientation of mdo, was homologous to the TetR family of transcriptional regulators (25). Since bacterial genes encoding transcriptional regulators are often located in the vicinity of their target genes, orf1 was designated as mdoR in the present study.
The amino acid sequence deduced from the nucleotide sequence of the mdoR gene was 40, 38, 38, and 35% identical to the amino acid sequences of a tetracycline repressor domain protein of Kribbella flavida DSM 17836 (GenBank accession no. EEJ20442), VarR of Streptomyces virginiae (GenBank accession no. AB046994), Pip of Streptomyces coelicolor (GenBank accession no. AF193856), and RifQ of Amycolatopsis mediterranei S699 (GenBank accession no. AF040570), respectively (2, 10, 24). Consistent with most TetR family regulators having molecular masses ranging from 21 to 25 kDa (31), MdoR has a calculated molecular mass of 24,860 Da and consists of 225 amino acids. A helix-turn-helix DNA-binding motif, which is the characteristic feature of the TetR family regulators (27), is present at the N-terminal region of MdoR, corresponding to a region between amino acids 44 and 66 (Fig. 1).
Fig. 1.
Multiple alignment of MdoR of Mycobacterium sp. strain JC1 with several TetR family regulators. The reference sequences used included a tetracycline repressor domain protein (TetR) of Kribbella flavida DSM 17836, VarR of Streptomyces virginiae, Pip of Streptomyces coelicolor, and RifQ of Amycolatopsis mediterranei S699. The asterisk indicates conservation of identical amino acids. Conserved and semiconserved substitutions are indicated as a colon and a period, respectively.
MdoR positively regulates the expression of the mdo gene in the presence of methanol.
In a previous study, an inverted repeat sequence (underlined), 5′-GCAGCGTGCTGC-3′, was identified 39 to 50 bp upstream of the transcriptional start site of the mdo gene (25). It is well known that inverted repeat sequences in the promoter region of genes in various bacteria act as binding sites for the TetR family transcriptional regulators (9, 18, 20). Therefore, MdoR was purified and used to determine binding to the putative mdo promoter region covering the inverted repeat.
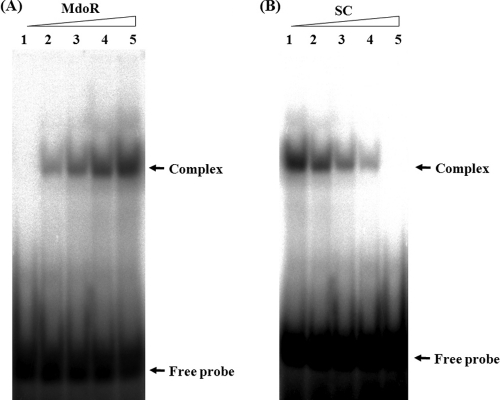
EMSA results indicated that MdoR bound to the DNA fragment containing the inverted repeat of the mdo promoter in Mycobacterium sp. strain JC1 (Fig. 2A). The specificity of binding between MdoR and the promoter region of mdo was identified by EMSA using excessive amounts of specific competitors (Fig. 2B). Nonspecific competitors did not affect the binding between MdoR and DNA fragment containing the inverted repeat. Analogous to previous reports that the inverted repeats in the promoter regions of genes that are under the control of TetR transcriptional regulator act as TetR binding sites, these results suggest that MdoR may recognize and bind the inverted repeat sequence in the mdo promoter region to regulate mdo gene expression in Mycobacterium sp. strain JC1.
Fig. 2.
EMSA for putative mdo promoter region. (A) EMSA with the putative mdo promoter region and purified MdoR. A 7.4-fmol aliquot of 32P-labeled 21-bp DNA fragment covering the inverted repeat (GCAGCGTGCTGC) was incubated with 0, 1.0, 2.1, 4.2, and 8.4 pmol of MdoR (lanes 1 to 5, respectively). (B) EMSA for mdo promoter in the presence of specific competitors. Purified MdoR (16.8 pmol) was incubated with 7.4-fmol of 32P-labeled 21-bp DNA fragment covering inverted repeat (GCAGCGTGCTGC) in the absence (lane 1) and presence of 1-, 5-, 10-, and 50-fold molar excesses of cold 21-bp DNA fragments covering the inverted repeat (specific competitor [SC]; lanes 2 to 5, respectively).
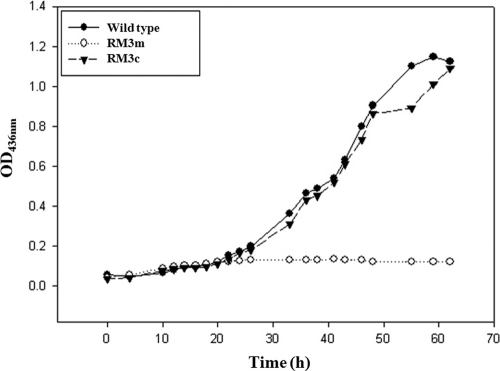
To investigate whether MdoR is important for regulation in vivo, an mdoR deletion mutant was constructed. The mdoR deletion mutant was confirmed by diagnostic PCR in Materials and Methods. The mdoR deletion mutant RM3m could not grow on methanol as a sole carbon and energy source, but growth of this strain on methanol was restored by pHP11 encoding an intact mdoR gene (Fig. 3). We have previously reported that MDO is the key enzyme for methanol oxidation in Mycobacterium sp. strain JC1 (25). Thus, it may be that RM3m was unable to grow on methanol because MDO was not expressed in these cells due to the absence of functional MdoR.
Fig. 3.
Growth of Mycobacterium sp. strain JC1 in the presence of methanol. Wild type, mdoR deletion mutant (RM3m), and strain JC1 (RM3m) carrying plasmid pHP11 (RM3c) were cultivated aerobically at 37°C in SMB-MeOH.
The present results strongly indicate that MdoR binds the mdo promoter region and positively regulates the expression of the mdo gene in Mycobacterium sp. strain JC1 growing with methanol. Most TetR family regulators play a negative role in the expression of genes in many bacteria, except for TetR in Clostridium tetani (21), AtrA-g in Streptomyces griseus (13), and PhaD in Pseudomonas putida (7), which positively regulate genes for the production of tetanus toxin and streptomycin and for the metabolism of polyhydroxyalkanoate, respectively.
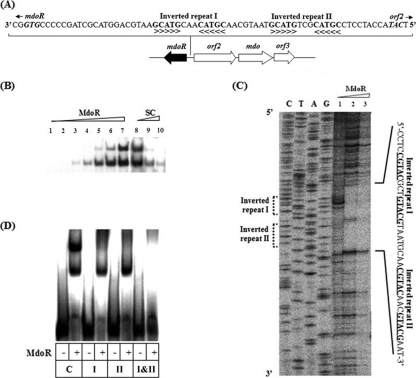
MdoR specifically binds to two inverted repeats in the putative mdoR promoter region.
To investigate whether MdoR specifically binds to its own promoter sequence, the DNA-binding activity of purified MdoR was assessed by DNase I footprinting and EMSA against a DNA fragment covering the two inverted repeats in the putative mdoR promoter. Two inverted repeat sequences (underlined), 5′-CGTACAACGTACG-3′ and 5′-CGTACGCTGTACG-3′, were located 22 to 34 bp and 43 to 55 bp upstream of the mdoR translational start site (Fig. 4 A). The EMSA results first showed that MdoR bound to two sites in the putative mdoR promoter in Mycobacterium sp. strain JC1 (Fig. 4B). DNase I footprinting experiments then revealed that the nucleotide positions 19 to 63 bp upstream of the mdoR start codon, which correspond to the position of the two inverted repeat sequences, were protected by MdoR from degradation by DNase I (Fig. 4C), indicating that MdoR binds to the two inverted repeats in the putative promoter of mdoR. This was confirmed by EMSA using MdoR and DNA fragments containing putative mdoR promoters with various base transversions in the two inverted repeat sequences (Fig. 4D). EMSA with DNA fragments covering the two inverted repeats with base transversion also showed that the A and C bases present at positions 4 and 5 in each inverted repeat were critical for MdoR binding (Fig. 4D). The specificity of binding between MdoR and the putative mdoR promoter was measured in competitive EMSA in the presence of excessive amount of specific competitors (Fig. 4B). The results indicate that MdoR specifically recognizes and binds to both inverted repeats within the putative mdoR promoter.
Fig. 4.
DNase I footprinting and EMSA for putative mdoR promoter. (A) Putative promoter region of mdoR. The putative Shine-Dalgarno sequence is indicated in boldface and underlined. The inverted repeats are represented in boldface with symbols (> or <). The predicted start codons of mdoR and orf2 are shown in boldface and italic letters. (B) EMSA with MdoR and putative mdoR promoter region. A 8.2-fmol aliquot of 32P-labeled 41-bp DNA fragment covering two inverted repeats was incubated with 0, 0.5, 1.0, 2.1, 4.2, 8.4, and 16.8 pmol of purified MdoR (lanes 1 to 7, respectively). Purified MdoR (16.8 pmol) was incubated with 8.2 fmol of 32P-labeled 41-bp DNA fragment covering two inverted repeats in the absence (lane 8) and the presence of 10- and 50-fold molar excesses of cold 41-bp DNA fragments (specific competitor [SC]; lanes 9 and 10, respectively). (C) DNase I footprinting of putative mdoR promoter. The 304-bp DNA fragments were end labeled with [γ-32P]ATP and incubated with 0, 8.4, and 16.8 pmol of purified MdoR (lanes 1 to 3, respectively). (D) Effect of base transversion in the inverted repeat on the binding of MdoR to the putative mdoR promoter. EMSA was carried out with 41-bp DNA fragments covering two inverted repeats with no base transversion (C lanes) and with the DNA fragment with base transversion in the inverted repeat I (I lanes), inverted repeat II (II lanes), and inverted repeats I and II (I&II lanes) in the presence (+) or absence (−) of MdoR (16.8 pmol).
MdoR positively regulates its own gene expression.
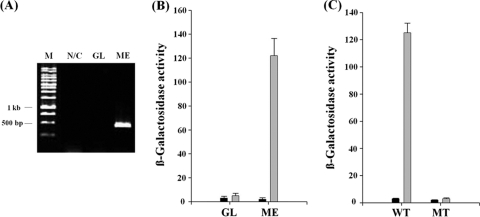
Many TetR family transcriptional regulators regulate the expression of their own gene (10, 17). Therefore, we tested whether the MdoR in Mycobacterium sp. strain JC1 also regulates the expression of its own gene during growth on methanol. RT-PCR of total RNA prepared from cells grown in methanol as a template and MR-F and MR-R as primers produced a 0.4-kp product (Fig. 5A, lane 3). No products were generated when total RNA prepared from cells grown with glucose was used as a template (Fig. 5A, lane 2), indicating that mdoR is expressed only in cells growing on methanol. β-Galactosidase assays with cell extracts prepared from Mycobacterium sp. strain JC1 harboring a reporter plasmid pHP13, which contains a fusion of the lacZ gene to the 289-bp putative mdoR promoter region, revealed that reporter activity was 24-fold higher in cells grown on methanol than in the cells grown on glucose (Fig. 5B). Further, strong β-galactosidase activity was detected in cell extracts prepared from Mycobacterium sp. strain JC1 containing pHP13 when the culture medium was switched from SMB-glucose to SMB-methanol, whereas the activity in cell extracts from a mdoR-deletion mutant containing pHP13 was negligible (Fig. 5C).
Fig. 5.
Inducible transcription of mdoR in cells grown on methanol. (A) RT-PCR of mdoR in Mycobacterium sp. strain JC1. RT-PCR was carried out with the primers MR-F and MR-R and total RNAs from cells grown with glucose (GL) and methanol (ME). RT-PCR product using primers MR-F and MR-R without reverse transcriptase (N/C) was used as a negative control. (B) Effect of growth substrate on the induction of mdoR promoter in Mycobacterium sp. strain JC1. β-Galactosidase activity was performed with cells harboring pDAS1 (■) and pHP13 (▩) that contain a promoterless lacZ gene and a putative mdoR promoter-lacZ fusion fragment, respectively, after grown on glucose (GL) or methanol (ME). (C) Effect of MdoR on the induction of mdoR promoter in Mycobacterium sp. strain JC1. β-Galactosidase activity was measured with wild-type (WT) and mdoR-defective mutant (MT) cells harboring pDAS1 (■) or pHP13 (▩) after growth on glucose to the mid-exponential phase, followed by incubation under 1% (vol/vol) methanol for 3 h. The units of β-galactosidase activity were expressed as nmol of o-nitrophenyl-β-d-galactopyranoside hydrolyzed min−1 mg of protein−1.
These results indicate that the expression of the mdoR gene in Mycobacterium sp. strain JC1 growing on methanol is under positive autoregulation. In this respect, MdoR differs from other TetR family transcriptional regulators studied to date, which are all negative autoregulators (1, 6, 29). Therefore, MdoR is the first TetR family member with a positive autoregulatory function.
ACKNOWLEDGMENT
This study was supported by research grant 313-2008-2-C00776 from the Korea Research Foundation.
Footnotes
Published ahead of print on 9 September 2011.
REFERENCES
- 1. Aramaki H., Sagara Y., Hosoi M., Horiuchi T. 1993. Evidence for autoregulation of camR, which encodes a repressor for the cytochrome P-450cam hydroxylase operon on the Pseudomonas putida CAM plasmid. J. Bacteriol. 175:7828–7833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. August P. R., et al. 1998. Biosynthesis of the ansamycin antibiotic rifamycin: deductions from the molecular analysis of the rif biosynthetic gene cluster of Amycolatopsis mediterranei S699. Chem. Biol. 5:69–79 [DOI] [PubMed] [Google Scholar]
- 3. Benoit L., Tom M. 1994. DNase I footprinting. Methods Mol. Biol. 30:1–10 [DOI] [PubMed] [Google Scholar]
- 4. Chistoserdova L., Kalyuzhnaya M. G., Lidstrom M. E. 2009. The expanding world of methylotrophic. Annu. Rev. Microbiol. 63:477–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cho J. W., Yim H. S., Kim Y. M. 1985. Acinetobacter isolates growing with carbon monoxide. Kor. J. Microbiol. 23:1–8 [Google Scholar]
- 6. Chuanchuen R., Gaynor J. B., Karkhoff-Schweizer R., Schweizer H. P. 2005. Molecular characterization of MexL, the transcriptional repressor of the mexJK multidrug efflux operon in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 49:1844–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Eugenio L. I., et al. 2010. The PhaD regulator controls the simultaneous expression of the pha genes involved in polyhydroxyalkanoate metabolism and turnover in Pseudomonas putida KT2442. Environ. Microbiol. 12:1591–1603 [DOI] [PubMed] [Google Scholar]
- 8. Dhandayuthapani S., Mudd M., Deretic V. 1997. Interactions of OxyR with the promoter region of the oxyR and ahpC genes from Mycobacterium leprae and Mycobacterium tuberculosis. J. Bacteriol. 179:2401–2409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fidopiastis P. M., Miyamoto C. M., Jobling M. G., Meighen E. A., Ruby E. G. 2002. LitR, a new transcriptional activator in Vibrio fischeri, regulates luminescence and symbiotic light organ colonization. Mol. Microbiol. 45:131–143 [DOI] [PubMed] [Google Scholar]
- 10. Folcher M., et al. 2001. A transcriptional regulator of a pristinamycin resistance gene in Streptomyces coelicolor. J. Biol. Chem. 276:1479–1485 [DOI] [PubMed] [Google Scholar]
- 11. Harms N., Reijnders W. N., Koning S., van Spanning R. J. M. 2001. Two-component system that regulates methanol and formaldehyde oxidation in Paracoccus denitrificans. J. Bacteriol. 183:664–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harms N., et al. 1993. Identification of a two component regulatory system controlling methanol dehydrogenase synthesis in Paracoccus denitrificans. Mol. Microbiol. 8:457–470 [DOI] [PubMed] [Google Scholar]
- 13. Hirano S., Tanaka K., Ohnishi Y., Horinouchi S. 2008. Conditionally positive effect of the TetR-family transcriptional regulator AtrA on streptomycin production by Streptomyces griseus. Microbiology 154:905–914 [DOI] [PubMed] [Google Scholar]
- 14. Howard N. S., Gomez J. E., Ko C., Bishai W. R. 1995. Color selection with a hygromycin-resistance-based Escherichia coli-mycobacterial shuttle vector. Gene 166:181–182 [DOI] [PubMed] [Google Scholar]
- 15. Jakobsen Ø M., et al. 2006. Upregulated transcription of plasmid and chromosomal ribulose monophosphate pathway genes is critical for methanol assimilation rate and methanol tolerance in the methylotrophic bacterium Bacillus methanolicus. J. Bacteriol. 188:3063–3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim Y. M., Hegeman G. D. 1981. Purification and some properties of carbon monoxide dehydrogenase from Pseudomonas carboxydohydrogena. J. Bacteriol. 148:904–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kojic M., Venturi V. 2001. Regulation of rpoS gene expression in Pseudomonas: involvement of a TetR family regulator. J. Bacteriol. 183:3712–3720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krug A., Wendisch V. F., Bott M. 2005. Identification of AcnR, a TetR-type repressor of the acotinase gene acn in Corynebacterium glutamicum. J. Biol. Chem. 280:585–595 [DOI] [PubMed] [Google Scholar]
- 19. Lidstorm M. E., Stirling D. I. 1990. Methylotrophs: genetics and commercial applications. Annu. Rev. Microbiol. 44:27–57 [DOI] [PubMed] [Google Scholar]
- 20. Lucas C. E., Balthazar J. T., Hagman K. E., Shafer W. M. 1997. The MtrR repressor binds the DNA sequence between the mtrR and mtrC genes of Neisseria gonorrhoeae. J. Bacteriol. 179:4123–4128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marvaud J. C., Eisel U., Binz T., Niemann H., Popoff M. R. 1998. TetR is a positive regulator of the tetanus toxin gene in Clostridium tetani and is homologous to botR. Infect. Immun. 66:5698–5702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morris C. J., Lidstrom M. E. 1992. Cloning of a methanol-inducible moxF promoter and its analysis in moxB mutants of Methylobacterium extorquens AM1. J. Bacteriol. 174:4444–4449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morris C. J., Kim Y. M., Perkins K. E., Lidstrom M. E. 1995. Identification and nucleotide sequence of mxaA, mxaC, mxaK, mxaL, and mxaD genes from Methylobacterium extorquens AM1. J. Bacteriol. 177:6825–6831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Namwat W., Lee C. K., Kinoshita H., Yamada Y., Nihira T. 2001. Identification of the varR gene as a transcriptional regulator of virginiamycin S resistance in Streptomyces virginiae. J. Bacteriol. 183:2025–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park H., Lee H., Ro Y. T., Kim Y. M. 2010. Identification and functional characterization of a gene for the methanol:N,N′-dimethyl-4-nitrosoaniline oxidoreductase from Mycobacterium sp. strain JC1 (DSM 3803). Microbiology 156:463–471 [DOI] [PubMed] [Google Scholar]
- 26. Park S. W., et al. 2003. Growth of mycobacteria on carbon monoxide and methanol. J. Bacteriol. 185:142–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ramos J. L., et al. 2005. The TetR family of transcriptional repressors. Microbiol. Mol. Biol. Rev. 69:326–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ro Y. T., et al. 1997. Growth on methanol of a carboxydobacterium Acinetobacter sp. JC1 DSM 3803. J. Microbiol. 35:30–39 [Google Scholar]
- 29. Sala C., et al. 2003. Mycobacterium tuberculosis FurA autoregulates its own expression. J. Bacteriol. 185:5357–5362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sanger F., Nicklen S., Coulson A. R. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. U. S. A. 74:5463–5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Santangelo M. P., et al. 2002. Negative transcriptional regulation of the mce3 operon in Mycobacterium tuberculosis. Microbiology 148:2997–3006 [DOI] [PubMed] [Google Scholar]
- 32. Seo J. G., et al. 2007. Cloning, characterization and expression of a gene encoding dihydroxyacetone synthase in Mycobacterium sp. strain JC1 DSM 3803. Microbiology 153:4174–4182 [DOI] [PubMed] [Google Scholar]
- 33. Sherman D. R., et al. 2001. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding α-crystallin. Proc. Natl. Acad. Sci. U. S. A. 98:7534–7539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Song T., et al. 2002. Reclassification of a carboxydobacterium, Acinetobacter sp. strain JC1 DSM 3803, as Mycobacterium sp. strain JC1 DSM 3803. J. Microbiol. 40:237–240 [Google Scholar]
- 35. Springer A. L., Auman A. J., Lidstrom M. E. 1998. Sequence and characterization of mxaB, a response regulator involved in regulation of methanol oxidation, and of mxaW, a methanol-regulated gene in Methylobacterium extorquens AM1. FEMS Microbiol. Lett. 160:119–124 [DOI] [PubMed] [Google Scholar]
- 36. Springer A. L., Morris C. J., Lidstrom M. E. 1997. Molecular analysis of mxbD and mxbM, a putative sensor-regulator pair required for oxidation of methanol in Methylobacterium extorquens AM1. Microbiology 143:1737–1744 [DOI] [PubMed] [Google Scholar]
- 37. Springer A. L., Chou H. H., Fan W. H., Lee E., Lidstrom M. E. 1995. Methanol oxidation mutants in Methylobacterium extorquens AM1: identification of new genetic complementation groups. Microbiology 141:2985–2993 [DOI] [PubMed] [Google Scholar]
- 38. Van Spanning R. J. M., et al. 1991. Isolation and characterization of moxJ, moxG, and moxR genes of Paracoccus denitrificans: inactivation of moxJ, moxG, and moxR and the resultant effect on methylotrophic growth. J. Bacteriol. 173:6948–6961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang M., FitzGerald K. A., Lidstrom M. E. 2005. Identification of an upstream regulatory sequence that mediates the transcription of mox genes in Methylobacterium extorquens AM1. Microbiology 151:3723–3728 [DOI] [PubMed] [Google Scholar]