Abstract
Bacterial type IV secretion systems (T4SSs) are involved in processes such as bacterial conjugation and protein translocation to animal cells. In this work, we have switched the substrates of T4SSs involved in pathogenicity for DNA transfer. Plasmids containing part of the conjugative machinery of plasmid R388 were transferred by the T4SS of human facultative intracellular pathogen Bartonella henselae to both recipient bacteria and human vascular endothelial cells. About 2% of the human cells expressed a green fluorescent protein (GFP) gene from the plasmid. Plasmids of different sizes were transferred with similar efficiencies. B. henselae codes for two T4SSs: VirB/VirD4 and Trw. A ΔvirB mutant strain was transfer deficient, while a ΔtrwE mutant was only slightly impaired in DNA transfer. DNA transfer was in all cases dependent on protein TrwC of R388, the conjugative relaxase, implying that it occurs by a conjugation-like mechanism. A DNA helicase-deficient mutant of TrwC could not promote DNA transfer. In the absence of TrwB, the coupling protein of R388, DNA transfer efficiency dropped 1 log. The same low efficiency was obtained with a TrwB point mutation in the region involved in interaction with the T4SS. TrwB interacted with VirB10 in a bacterial two-hybrid assay, suggesting that it may act as the recruiter of the R388 substrate for the VirB/VirD4 T4SS. A TrwB ATPase mutant behaved as dominant negative, dropping DNA transfer efficiency to almost null levels. B. henselae bacteria recovered from infected human cells could transfer the mobilizable plasmid into recipient Escherichia coli under certain conditions, underscoring the versatility of T4SSs.
INTRODUCTION
Type IV secretion systems (T4SSs) are widely spread in bacteria. They show a remarkable plasticity in terms of the nature of the substrates to be secreted (DNA and/or proteins) and the destiny of the translocated substrate, which can be the external milieu or another cell, either prokaryotic or eukaryotic (1). This versatility allows them to be involved in a variety of biological processes, such as DNA transfer among bacteria (2) or effector translocation into human cells (25). This scenario is well illustrated by two T4SSs, both named Trw based on their high level of similarity, which are involved in bacterial conjugation of plasmid R388 and in human erythrocyte invasion by Bartonella spp., respectively (41). Different Bartonella species code for up to three different T4SSs which are involved in their pathogenicity and also seem to contribute to host adaptation. Specifically, B. henselae and B. tribocorum present two T4SSs: the VirB/VirD4 T4SS, involved in vascular endothelial cell infection, and the Trw T4SS, required for erythrocytic infection (9, 33). It has been recently shown that the latter mediates host-specific adhesion to erythrocytes (43). This T4SS will be named Trw-Bt to distinguish it from the R388 Trw T4SS. The similarity between Trw from R388 and that from Bartonella extends into their genetic organization and functional exchangeability of part of their components: the core components of both T4SSs are functionally exchangeable, while components of the pilus cannot be exchanged (11). This suggests that T4SS machineries may share a central structure for secretion, while peripheral components involved in specific interactions with their cognate substrates and target cells may have diverged accordingly. In spite of these similarities, no conjugal DNA transfer of R388 derivatives was detected through the Trw-Bt T4SS (11).
With the exception of the Trw T4SS of Bartonella spp., for which no substrate has been described so far, all characterized T4SSs involved in bacterial pathogenicity secrete at least one specific protein substrate. In the case of T4SSs involved in bacterial conjugation and in DNA transfer to plant cells by Agrobacterium tumefaciens, the secreted substrate is a protein with a covalently attached DNA strand. DNA secreted into the milieu by the T4SS of Neisseria gonorrhoeae also appears to be linked to a leader protein which resembles conjugative relaxases (34). Protein recruitment by the different T4SSs is based on secretion signals present in most cases in the C-terminal 50 residues of the protein (6), but in some cases other parts of the protein are also required for secretion; such is the case of the Bep proteins secreted by the VirB T4SS of Bartonella spp. (40).
It is believed that a component of the T4SS termed the coupling protein (T4CP) plays a key role in recruiting this protein substrate. T4CPs are anchored to the inner membrane and show ATPase activity. Their role in substrate recruitment was based on early genetic evidence (5) and supported by protein-protein interactions detected between T4CP and both the substrate and other T4SS components (26). They are proposed to pump out DNA during bacterial conjugation (24), but they are also required for protein substrate translocation in the absence of DNA transfer (12). However, it has to be noted that these assumptions have been made based on the study of T4CPs belonging to DNA transfer systems. T4CP homologues in T4SSs involved in protein secretion into animal cells are not involved in DNA transfer. They show multiple protein-protein interactions with other T4SS components, and they are essential for protein secretion. Only recently has a cytoplasmic protein-protein interaction been reported between the Helicobacter pylori Cagβ T4CP and its secreted substrate CagA (18).
TrwB is the T4CP of conjugative plasmid R388. This protein has been extensively characterized. It interacts with the T4SS component TrwE and also with TrwE homologues of other T4SSs involved in both DNA and protein secretion (11, 26). Recently, a mutagenesis analysis of this protein has shown that the transmembranal region is involved in interaction with TrwE. Several point mutations in this region were shown to increase this interaction with the Bartonella TrwE homologue, without affecting its functionality in conjugative DNA transfer (10).
Substrate exchange between different T4SSs has been previously reported. Conjugative systems show very specific T4CP-substrate interactions, but the T4CP can recruit its cognate substrate to different conjugative T4SSs (26). VirD4, the T4CP of the A. tumefaciens T4SS involved in DNA transfer to plant cells, can also replace conjugative T4CPs in conjugative DNA transfer (16). With respect to substrate exchange between T4SSs involved in protein translocation into animal cells, highly related T4SSs can naturally share their protein substrates, as shown for Coxiella burnetii substrates translocated by the Legionella pneumophila homologous Dot/Icm T4SS (45). It has also been shown that heterologous proteins can be secreted through T4SSs when the corresponding C-terminal secretion signal is added (30, 44). Moreover, bacterial conjugative relaxases have been shown to be translocated through T4SSs into eukaryotic cells due to some similarity in their C termini with the corresponding secretion signal, such as with MobA of plasmid RSF1010 (15, 17). In the case of TraA relaxase, translocation through the VirB/VirD4 T4SS of Bartonella henselae was accomplished by addition of the corresponding secretion signal (40).
DNA transfer through T4SSs is inherent in conjugative T4SSs and in A. tumefaciens VirB T4SS. Conjugative T4SSs have also been shown to transfer DNA into eukaryotic cells (4, 17, 46). In addition, the Dot/Icm T4SS involved in the pathogenicity of L. pneumophila was shown to transfer DNA between bacteria (27). Finally, a recent report has also shown that it is possible to send DNA to human cells through a T4SS involved in pathogenicity: the VirB/D4 T4SS of B. henselae mediates the transfer of a Bartonella cryptic plasmid into vascular endothelial cells. Although the DNA transfer frequency was very low, this could be improved significantly by adding the VirB/D4 secretion signal to the C terminus of the conjugative relaxase (38).
In this work, we show that R388 derivatives can be transferred by a conjugation-like mechanism from B. henselae to human cells and also to recipient bacteria under certain conditions. Efficient DNA transfer was obtained into vascular endothelial cells, mediated by both the VirB/VirD4 T4SS of B. henselae and the relaxase of R388, suggesting that TrwC is efficiently recruited by the T4SS of the pathogen in spite of not containing the VirB/D4 secretion signal. Recruitment could be aided by the R388 T4CP TrwB, which is required for efficient DNA transfer. Thus, TrwC is a natural substrate of B. henselae VirB/D4 T4SS.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains are listed in Table 1. Escherichia coli strains were grown on Luria-Bertani broth, supplemented with agar for solid culture. E. coli strain DH5α was used in all cloning procedures. Strain DY380 was used to express the Red recombination system. Plasmids were maintained in the lacIq strain D1210. The cya-deficient strain DHM1 was used for the bacterial two-hybrid assay. Bartonella sp. strains were grown on Columbia blood agar plates at 37°C under 5% CO2 atmosphere. Selective media included the following antibiotics at the indicated concentrations: ampicillin (Ap), 100 μg/ml; chloramphenicol (Cm), 25 μg/ml; kanamycin (Km), 50 μg/ml (E. coli) or 30 μg/ml (Bartonella); nalidixic acid (Nx), 20 μg/ml; streptomycin (Sm), 300 μg/ml (E. coli) or 100 μg/ml (Bartonella); gentamicin (Gm), 10 or 100 μg/ml; and rifampin (Rf), 100 μg/ml (E. coli) or 50 μg/ml (Bartonella).
Table 1.
Bacterial strains
| Strain | Genotype | Reference |
|---|---|---|
| B. henselae | ||
| MFE133 | RSE247 ΔtrwE-Bt | This work |
| MFE137 | RSE247 ΔtrwE-Bt ΔvirB2-11 | This work |
| MFE114 | RSE247 ΔvirB2-11 | This work |
| RSE242 | RSE247 ΔvirB4 | 36 |
| RSE247 | Spontaneous Smr from “Houston-1” | 36 |
| TRB148 | RSE247 ΔvirD4 | 40 |
| CHDE105a | Spontaneous Rfr from “Houston-1” | This work |
| B. tribocorum | ||
| RSE148 | Spontaneous Smr from B. tribocorum 5065 | 41 |
| E. coli | ||
| D1210 | SmrrecA hspR hsdM rpsL lacIq | 32 |
| DH5α | Nxr F−supE44ΔlacU169 (φ80lacZΔM15)hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 15 |
| DHM1 | Nxrcya-854 recA1 gyrA96 (NaI) thi-1 hsdR17 spoT1 rfbD1 glnV44(AS) | 19 |
| DY380 | Smr λ cI857 (cro-bioA) tet (DH10B) | 22 |
Spontaneous rifampin-resistant mutant obtained by selection of B. henselae ATCC 49882T on 50 mg/liter of rifampin.
Construction of Bartonella mutant strains.
Construction of B. henselae RSE247 derivatives carrying in-frame deletion mutations in different T4SS genes was performed by the two-step double crossover strategy as described previously (36, 39). The 5′- and 3′-flanking regions of the DNA fragment to be deleted were amplified and combined by megaprime PCR. The resulting fragment was digested by BamHI and inserted into the corresponding site of pTR1000, yielding suicide plasmids pSHdvirB2-11 (deletion of virB2-virB11 [virB2-11]) and pASB17 (deletion of trwE-Bt). Deletions were verified by overspanning PCR using primers CCATTCCCTCCTATTTTTGC and ATCATTAACATTGCGCCCAGT for the virB operon and primers CTTGGTCAAGCAACCGTGC and ACAACACCACATCAATTGTG for trwE. The virB2-11 trwE-Bt double mutant was generated by deletion of trwE-Bt in the virB2-11 mutant.
Plasmid constructions.
Bacterial plasmids are listed in Table 2 (published plasmids) and Table 3 (plasmids constructed for this work). Plasmids were constructed using standard methodological techniques (35). Restriction enzymes, shrimp alkaline phosphatase, and T4 DNA ligase were purchased from Fermentas. High-fidelity Triple Master polymerase was purchased from Eppendorf. DNA sequences of all cloned PCR segments were determined. An outline of each plasmid construction is shown in Table 3. Plasmids constructed by induction of the Red recombination system in strain DY380 (22) are as follows.
Table 2.
Published plasmids used in this work
| Plasmid | Antibiotic resistance | Description | Reference or source |
|---|---|---|---|
| pCIG1026 | Apr | Codes for TrwC K502T mutant | 8 |
| pCMS1 | Apr | trwC with XhoI site before stop codon | C. Machón |
| pFJS134 | Gmr | pBBR6::oriT+trwABC | 11 |
| pHP108 | Apr | pUT18C::TrwB P18S | 10 |
| pHP127 | Cmr | pT25::TrwB P18S | 10 |
| pMTX514 | Cmr | pT25::trwB | 26 |
| pMTX601 | Apr | pUT18c::trwB | 10 |
| pMTX697 | Apr | pUT18c::trwE-Bt | 11 |
| pMTX698 | Cmr | pT25::trwE-Bt | 11 |
| pPG104 | Gmr | Source of bepD-BID sequence | 40 |
| pRS117 | Kmr | Source for PCMV-egfp cassette | 38 |
| pSU1423 | Cmr | pSU18::oriT+trwABC | 3 |
| pSU1443 | Kmr Tpr | pSU1425::Tn5tac in trwB | 23 |
| pSU1445 | Kmr Tpr | pSU1425::Tn5tac in trwC | 23 |
| pSU2007 | Kmr | R388 with Kmr cassette | 28 |
| pSU4058 | Apr | pHG327::trwL-trwD | 3 |
| pSU4134 | Kmr Tpr | pSU1425::Tn5tac1 in trwE | 26 |
| pSU4632 | Cmr | Codes for TrwB K136T mutant | 29 |
| pT25 | Cmr | Vector for Cya-T25 fusions | 20 |
| pT25zip | Cmr | Positive control for two-hybrid assay | 20 |
| pTR1000 | Kmr | Suicide vector for gene replacement | 42 |
| pUT18c | Apr | Vector for Cya-T18 fusions | 21 |
| pUT18czip | Apr | Positive control for two-hybrid assay | 21 |
Table 3.
Plasmids constructed for this work
| Plasmid name | Description | Constructiona |
||
|---|---|---|---|---|
| Vector | Insert/templ. | Enzymes/oligonucleotides 5′-3′ | ||
| pAA7 | pHP132::PCMV→eGFP | pHP132 | pHP161 | ACATCTCTTCTTAGTGTTTGACAGCTTATCATCGC |
| ACTTCGATCGCCCCGACACCCGCC | ||||
| pAA8 | pHP161::Tn5tac1×2 | pHP161 | Tn5tac1 | ClaI |
| pAA10 | pHP161::Tn5tac1 | pHP161 | Tn5tac1 | ClaI |
| pASB17 | pTR1000::B. henselae trwE flanking regions | pTR1000 | B. henselae genomic DNA | Megaprime PCRb |
| GCGGATCCCGTGCAAGCGCTCAAAAC | ||||
| TTACTCGCTCGCCTTGGTTCTCGGCGTAGTTTTGTTCTG | ||||
| AACCAAGGCGAGCGAGTAA | ||||
| GCGGATCCTCATTACCACGTAGCTCAGC | ||||
| pEF006 | pUT18C::B. henselae virB10 | pUT18C | B. henselae genomic DNA | CCAAGGATCCAATGAATGATCCAATGGATGAA |
| CCAAGAATTCATCGCTCAATGTGTGCAA | ||||
| pEF007 | pUT18C::B. henselae virD4 | pUT18C | B. henselae genomic DNA | CCAACCCGGGGTATGAAATATACAAAGACGCAA |
| CCAAGAATTCAGCTTTTCTTTGCTTGTGG | ||||
| pEF021 | pBBR6::oriT trwAB(K136T)C:: PCMV→eGFP | pHP159 | pSU4632 | ApaLI PmlI |
| pEF023 | pT25::B. henselae virB10 | pT25 | pEF006 | BamHI EcoRI |
| pEF024 | pT25::B. henselae virD4 | pT25 | pEF007 | XmaI EcoRI |
| pEF025 | pBBR6::oriT trwABC(K502T):: PCMV→eGFP | pHP159 | pCIG1026 | SphI ClaI |
| pHP128 | pSU1423::Kmr in place of first 401 bp of trwB | pSU1423 | pSU4134 | Red recombinationc |
| ATTGAGGTTTGGACACCGGAGGGGAAGGAGGATTGAGATGGATCCATCAAGAGACAGGATGAGGATCGT | ||||
| CGGTATAAGCAAGCTCACGAAGCAACACCGATTTACCGGTACCAACCCCAGAGTCCCGCTCAG | ||||
| pHP129 | pSU1423::trwB*d | pHP128 | pMTX601 | BamHI KpnI |
| pHP131 | pSU1423::trwB*(P18S) | pHP128 | pHP108 | BamHI KpnI |
| pHP132 | pBBR6::oriTtrwAB*C | pFJS134 | pHP129 | EcoRI HindIII |
| pHP134 | pBBR6::oriTtrwAB*(P18S)C | pFJS134 | pHP131 | EcoRI HindIII |
| pHP159 | pBBR6::oriTtrwAB*C:: PCMV→eGFP | pHP132 | pCEP4::eGFP | ACATATCGATTGTTTGACAGCTTATCATCGC |
| ACTTATCGATCCCCGACACCCGCC | ||||
| pHP160 | pBBR6::oriTtrwAB*(P18S)C:: eGFP←PCMV | pHP134 | pCEP4::eGFP | ACATATCGATTGTTTGACAGCTTATCATCGC |
| ACTTATCGATCCCCGACACCCGCC | ||||
| pHP161 | pBBR6::oriTtrwAB*C:: eGFP←PCMV | pHP132 | pCEP4::eGFP | ACATATCGATTGTTTGACAGCTTATCATCGC |
| ACTTATCGATCCCCGACACCCGCC | ||||
| pHP178 | pHP161 ΔtrwB::Cm | pHP161 | pMTX698 | Red recombinationc |
| GGACACCGGAGGGGAAGGAGGATTGAGATGCATCCAGACGATAGGCCTCCACATGAAGCACTTCACTGACA | ||||
| GGTCAATACCATGTGACTGAGCATTAGATAGTCCCCTCAACAGGCCTCGCCCCGCCCTGCCACTCAT | ||||
| pHP179 | pHP161 ΔtrwB | pHP178 | Self-ligation | StuI |
| pHP180 | pHP161 ΔtrwC::Km | pHP161 | pSU4134 | Red recombinationc |
| TGCAAACCGGCAACCGGCCTTTGTTGAGGGGACTATCTAAGGCCTATCAAGAGACAGGATGAGGATCGT | ||||
| CCCGTAGCACGCGCTACGGGCTTTTTCTTGTCCCTGCTTAGGCCTAACCCCAGAGTCCCGCTCAG | ||||
| pHP181 | pHP161 ΔtrwC | pHP180 | Self-ligation | StuI |
| pLA23 | TrwC-BID fusion | pCMS1 | pPG104 | CCAACTCGAGGCCCCCTCTACGAAGGAGTTGGCCCA |
| CCAACTCGAGTATCGATTACATACCAAAGGCCATTCC | ||||
| pLA24 | pHP159::TrwC-BID | pHP159 | pLA23 | SphI ClaI |
| pSHdvirB2-11 | pTR1000::B. henselae virB2-11 flanking regions | pTR1000 | B. henselae genomic DNA | Megaprime PCRb |
| CGGGATCCGCGCCACAGGAGTAACCAAT | ||||
| TTAATTCCCACCAAAGATGTTTCTCCTGGATATAGTGTCTGTCAT | ||||
| CGGGATCCAAACCTTGTAAATTGTTTTTTTCGG | ||||
| AGAAACATCTTTGGTGGGAATTAA | ||||
The column labeled “Vector” lists the vector plasmids, that labeled “Insert/templ.” lists the plasmids from which the inserts were obtained or used as templates for PCRs, and that labeled “Enzymes/oligonucleotides 5′-3′” indicates either the restriction enzymes used for cloning or the oligonucleotides used for PCR amplification of the desired fragment, with the restriction sites underlined.
Megaprime PCR: the first two primers amplified the upstream flanking region, the last two primers amplified the downstream flanking region, and both PCR products were combined to generate an in-frame deletion; the final PCR product was BamHI digested and inserted into the corresponding site of the sucide vector pTR1000.
Constructs were made by induction of Red recombination; refer to Fig. 1 and Materials and Methods for details.
trwB* carries a BamHI site right after the start codon (ATG GAT CCG ATG…) which produces the addition of 3 extra residues at the N terminus, with no phenotypic effect.
To facilitate subsequent subcloning, a BamHI site was introduced at the start of trwB in plasmid pSU1423, carrying the Dtr region of R388 (oriT + trwABC), and a Kmr cassette flanked by BamHI and KpnI restriction sites was introduced by Red recombination (pHP128); this Kmr cassette was substituted in a second step by a BamHI-KpnI fragment reconstituting the first 401 bp of the trwB open reading frame (ORF), either wild type (wt) (pHP129) or with mutation P18S, which increases interaction with TrwE-Bt (10) (pHP131). Inclusion of the BamHI site added extra codons at the beginning of trwB, thus coding for extra residues Met-Asp-Pro in front of the starting Met of wild-type TrwB. This mutant TrwB behaved as the wild type and was always used as the positive control to exclude any effect due to this N-terminal addition of 3 residues. The Dtr regions from these plasmids were transferred to the broad-host-range vector pBBR6 for their propagation in Bartonella. And finally, an enhanced green fluorescent protein gene (egfp) cassette was added by PCR, creating plasmids pHP161 and pHP160, which were introduced in B. henselae to test their transfer into human cells.
Derivatives of pHP161 carrying deletions of either trwB or trwC were created in two steps: first, an antibiotic resistance cassette flanked by StuI restriction sites was introduced by Red recombination in place of the trwB and the trwC ORFs, rendering plasmids pHP178 and pHP180, respectively. In a second step, the resistance cassette was deleted by StuI restriction, leaving in-frame deletions of trwB (pHP179) and trwC (pHP181).
To test the processivity of the transfer process, plasmids pAA7, pAA8, and pAA10 were constructed as follows. The egfp cassette from pHP161 was PCR amplified and cloned as an EarI-PvuI fragment into the corresponding sites in pHP132, producing pAA7, in which the egfp cassette is located 2,832 bp from the R388 oriT. Plasmids pAA8 and pAA10 were constructed by inserting into the ClaI site of pHP161 one and two copies, respectively, of a 4,644-bp ClaI fragment from an R6K::Tn5tac1 plasmid; the fragment contains most of Tn5tac1 (including Km resistance) from the ClaI site at its I end (GenBank accession number L11017.1), plus nucleotides (nt) 4180 to 4289 from the PilX region of R6K (accession number AJ006342.1). In these cases, the distances between the egfp cassette and the oriT are 10,786 bp (pAA8) and 15,436 bp (pAA10).
Mating assays.
Standard E. coli quantitative mating assays were performed as described previously (13): equal amounts of donor and recipient strains from overnight cultures were mixed and placed on Millipore filters on a prewarmed LB agar plate for 1 h at 37°C. Strains D1210 and DH5α were used as donors and recipients, as indicated. Results are shown as the frequency of transconjugants per donor and are the mean of 2 to 5 independent experiments.
Mating assays using Bartonella strains were carried out on a Millipore filter on Columbia blood agar plates for 6 h. Bartonella cells were previously grown on Columbia blood agar plates for 3 to 4 days with appropriate antibiotic selection; bacteria from one plate were collected and washed with 1 ml of phosphate-buffered saline (PBS), pelleted, and resuspended in 50 μl PBS. In matings involving both Bartonella and E. coli, 25 μl of the Bartonella suspension was mixed with 20 μl of E. coli from overnight liquid culture, washed with 1 ml of PBS, and resuspended in 20 μl of PBS. For matings involving donor Bartonella rescued from infection experiments, infections of EA.hy926 cells with B. henselae carrying the appropriate plasmids were carried out as explained below; after 3 days of infection, the cultures were washed and either bacteria from the supernatants were recovered and used directly for the matings or, for matings using donor Bartonella attached to or inside human cells, the washed infected culture cells were resuspended in 1 ml of ice-cold distilled water to promote an osmotic shock. Lysates were centrifuged at 1,000 rpm for 3 min, and bacteria were recovered by centrifugation of supernatants at 4,000 rpm for 6 min, resuspended in 200 μl PBS, and mixed with the same volume of the E. coli culture used as a recipient.
Plasmids were routinely introduced in Bartonella by conjugation, following the same procedure as for quantitative matings but streaking the mating mix directly on selective medium.
Western blots.
The amount of TrwB and TrwC in the cells was estimated by Western blotting of total protein extracts, as described previously (10). E. coli D1210 cells containing the indicated plasmids were grown overnight. Cells were collected, centrifuged, resuspended in a 1/10 volume of 2× SDS gel loading buffer (35), and frozen at −20°C. Samples (20 μl) were boiled for 5 min and applied to SDS-PAGE gels. After the run, gels were transferred to nitrocellulose filters. Filters were stained with 0.1% Coomassie brilliant blue R250 in 50% methanol to estimate protein transfer. After incubation with the primary antibody, secondary antibody (peroxidase-conjugated anti-rabbit IgG; ICN) was used at a 1:10,000 dilution. Detection was performed with a Supersignal kit (Pierce) and bands were analyzed on a Bio-Rad ChemiDoc apparatus. Anti-TrwB (10) and anti-TrwC (14) primary antibodies were used at 1:5,000 and 1:10,000 dilutions, respectively.
Bacterial two-hybrid assay.
In vivo protein-protein interactions were tested by the bacterial two-hybrid assay as described previously (11). In this assay, the strength of the interactions is reflected in the expression of lacZ. Strain DHM1 was grown at 30°C and cotransformed with plasmids coding for T25 and T18 fusion proteins. Transformants were grown overnight and spread on sectors of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside)-containing plates to observe and compare the blue color.
Cell culture and transfection.
Human cell lines used in this work were human embryonic kidney 293T cells (ATCC CRL-11268) and the immortalized hybridoma EA.hy926 (ATCC CRL-2922), a fusion cell line of human umbilical vein endothelial cells (HUVEC) and adenocarcinomic human alveolar basal epithelial cells (A549). Cells were grown on Dulbecco's modified Eagle's medium (DMEM) plus Glutamax (Gibco) supplemented with 10% fetal bovine serum (Cambrex) at 37°C in 5% CO2. 293T cells were transfected with 7 μg of DNA plus 14 μl of JetPei (Genycell). GFP expression was checked with a Nikon Eclipse E400 fluorescence microscope.
Cell infection and flow cytometry.
Human EA.hy926 cells were grown in 6-well plates (80,000 cells per well) in 3 ml of medium for 16 h. Then, DMEM was replaced by M199 medium (Gibco) with 10% fetal bovine serum. B. henselae strains were grown for 3 to 4 days on Columbia blood agar plates, collected, and washed with PBS. Bacteria were added to EA.hy926 cells in M199 at a multiplicity of infection (MOI) of 400. To quantify intracellular survival and growth, the infection mixture was incubated for 24 h, treated with Gm at 100 μg/ml for 2 h, and lysed by osmotic shock with water after 3 washes with PBS. The last wash was plated to check that no bacteria remained alive. For later time points, fresh medium with Gm at 10 μg/ml was added after a PBS wash, and at 48 h and 72 h postinfection the cells were again washed 3 times with PBS and lysed by osmotic shock. The lysates were serially diluted and spread in Columbia blood agar plates to determine the number of intracellular bacteria.
When the infected cultures were used to detect DNA transfer, they were incubated for 3 days in the absence of antibiotics. Cells were then washed with PBS, treated with trypsin, centrifuged, resuspended in 200 μl PBS, and analyzed by using a Cytomics FC50 flow cytometer (Beckman Coulter) to quantify GFP-positive cells. In the experiments made in the presence of DNase, 5 μg/ml of DNase I (Roche) per well was added during the incubation period.
RESULTS
Mobilization of DNA into human cells through the T4SS of Bartonella henselae.
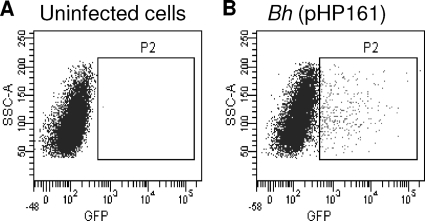
We have assayed DNA mobilization from bacteria into human cells by using a combination of part of the R388 conjugative machinery and Bartonella T4SS. To detect DNA transfer, we added a eukaryotic egfp expression cassette to the plasmid containing the R388 Dtr region (oriT+trwABC), obtaining plasmid pHP161 (Fig. 1A and Table 3), which we introduced into B. henselae. We confirmed the expression of egfp in transfected human 293T cells and the lack of egfp expression in bacteria (not shown). After infection of EA.hy926 cells with B. henselae containing pHP161, we detected GFP-positive cells by flow cytometry (Fig. 2). The efficiency of DNA transfer varied from 0.5 to 3.5% of GFP-positive cells and was not affected by the presence of DNase I in the culture medium (data not shown). The processivity of the DNA transfer process (a feature of bacterial conjugation) was assessed by comparing the transfer frequencies of equivalent plasmids with different distances between the oriT (start of DNA transfer) and the egfp cassette (transfer required to observe eGFP-positive cell). As shown in Table 4, no significant differences in GFP-positive cells could be observed when the transfer distance varied from 2.8 to 15.4 kb.
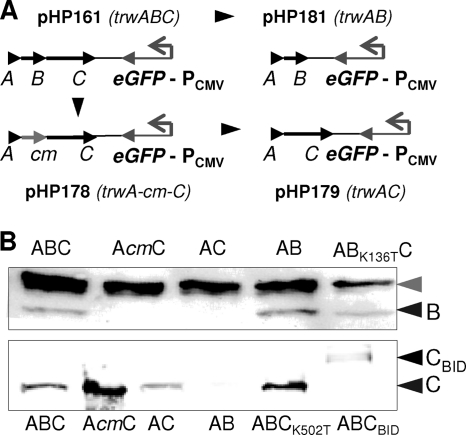
Fig. 1.
(A) Plasmids used to test DNA transfer into human cells. These plasmids contain the pBBR6 replication origin, a gentamicin resistance gene, the indicated R388 Dtr region (oriT, trwA, trwB, trwC), and a eukaryotic egfp expression cassette (PCMV-egfp-SV40 polyadenylation signal). (B) Western blots with anti-TrwB (top) and anti-TrwC (bottom) primary antibodies to detect TrwB and TrwC steady-state levels in E. coli carrying plasmids coding for the indicated R388 proteins. Black arrows point to TrwB, TrwC, and TrwC-BID; the gray arrow indicates a unspecific band detected by the anti-TrwB antibody (10).
Fig. 2.
Fluorescence-activated cell sorting (FACS) graph plotting cell granularity (side scatter A [SSC-A]) versus eGFP fluorescence intensity (in abscissas). (A) Uninfected cells, which determined eGFP background. The square marks the population considered positive. (B) Cells infected by B. henselae containing plasmid pHP161 (oriT+trwABC).
Table 4.
Transfer efficiencies of DNA fragments of different lengths to human cellsa
| Plasmid name | Distance (bp) between oriT and egfp cassette | % GFP-positive cells |
|---|---|---|
| pAA7 | 2,832 | 0.42 |
| pAA10 | 10,786 | 0.77 |
| pAA8 | 15,436 | 0.49 |
Data are the mean of three independent assays.
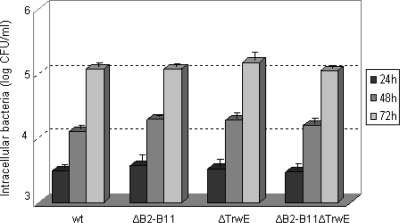
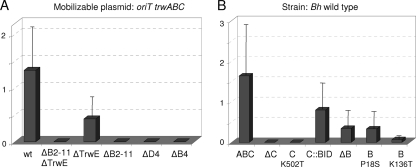
The observed GFP expression could be due to intracellular bacterial lysis or causes other than DNA transfer from bacteria. To determine if the mobilizable plasmid was being secreted through the B. henselae T4SS, we assayed strains with in-frame deletion mutants in several T4SS genes: a virB4 deletion mutant, a virD4 deletion mutant (ΔvirD4 strain), a whole virB operon deletion mutant (ΔvirB2-11 strain), a trwE-Bt deletion mutant (ΔtrwE-Bt strain), and a double trwE-Bt virB2-11 deletion mutant (ΔvirB2-11 ΔtrwE-Bt strain). The ΔvirD4 and ΔvirB4 mutant strains have been previously described (Table 1). Although strains lacking a functional VirB/D4 T4SS are deficient in invasome formation (40), they are able to get internalized by endocytosis into cultured cells with the same efficiency as the wild-type strain (36). The ΔtrwE-Bt, ΔvirB2-11, and ΔvirB2-11 ΔtrwE-Bt mutant strains were constructed by the two-step double-crossover strategy as explained in Materials and Methods. We determined their efficiencies of infection compared with that of the wild-type strain. As shown in Fig. 3, there were no significant differences in the intracellular infection and subsequent growth between wild-type and mutant strains. We compared the DNA transfer efficiencies in all the strains. The results of the transfer experiments (Fig. 4A) show that the ΔvirB2-11 ΔtrwE-Bt double mutant is not able to transfer DNA to human cells, confirming that DNA transfer occurs necessarily through one or both T4SSs. In the case of the ΔtrwE-Bt mutant, there is roughly one-third of DNA transfer seen for the wild type. However, the virB mutants, including the mutant lacking only virD4, are all DNA transport deficient. These data indicate that DNA transfer occurs through the VirB T4SS of B. henselae.
Fig. 3.
Number of intracellular bacteria recovered from infected EA.hy926 cells after 24, 48, and 72 h postinfection.
Fig. 4.
Percentage of GFP-positive cells infected by the indicated B. henselae mutant strains carrying plasmid pHP161 (oriT trwABC) (panel A) or wild-type B. henselae carrying plasmids which expressed the indicated TrwB or TrwC variants (panel B). The bars represent means with standard deviation from at least three independent experiments done in triplicate. Strains in panel A are identified by the deleted trw-Bt or vir genes, e.g., the ΔB2-11 is the strain with a virB2-to-virB11 deletion, and so on. Plasmids in panel B are named by their difference from pHP161, containing wild-type trwABC genes, as follows: ABC, wild-type plasmid; ΔB and ΔC, deletion of trwB and trwC, respectively; C::BID, TrwC-BID fusion protein; TrwB and TrwC point mutants are indicated.
Role of the R388 components in DNA transfer.
To determine if DNA transfer through the B. henselae T4SS was driven by the R388 conjugation machinery, we constructed mobilizable plasmids lacking either trwB or trwC, coding for the T4CP and relaxase of R388, respectively, both essential for bacterial conjugation. The plasmids constructed are shown in Fig. 1A. Table 5 shows complementation assays of R388 trwB and trwC mutants which indicate that, as expected, the plasmids without trwB do not complement a mutation in trwB but complement mutations in trwC and, conversely, that the plasmid without trwC complements mutations in trwB but not in trwC. We also checked the TrwB and TrwC levels expressed by these plasmids by Western blotting (Fig. 1B). As expected, TrwB was produced to wild-type levels by the ΔtrwC plasmid and was not detectable in ΔtrwB mutants. However, when we checked the TrwC levels in ΔtrwB plasmids, we found that plasmid pHP179, which was constructed leaving the start codon of trwC under the same translation signals as in R388, produced low levels of TrwC, while pHP178, probably driving trwC expression from the Cm resistance cassette, produced more TrwC than the wild type (Fig. 1). Accordingly, we selected pHP178 as our ΔtrwB plasmid to test the effect of TrwB absence without diminishing TrwC levels.
Table 5.
Complementation of R388 trwB and trwC mutants
| Plasmids in donora | R388 Trw region | Transfer frequencyb |
|---|---|---|
| pSU1443 | R388 TrwB− | <1 × 10−7 |
| pSU1443 + pHP161 | R388 TrwB− + TrwABC | 1 × 10−2 |
| pSU1443 + pHP178 | R388 TrwB− + TrwAC, Cmr | <1 × 10−7 |
| pSU1443 + pHP179 | R388 TrwB− + TrwAC | 2 × 10−7 |
| pSU1443 + pHP181 | R388 TrwB− + TrwAB | 8 × 10−3 |
| pSU1445 | R388 TrwC− | <1 × 10−7 |
| pSU1445 + pHP161 | R388 TrwC− + TrwABC | 4 × 10−2 |
| pSU1445 + pHP178 | R388 TrwC− + TrwAC, Cmr | 3 × 10−2 |
| pSU1445 + pHP179 | R388 TrwC− + TrwAC | 3 × 10−2 |
| pSU1445 + pHP181 | R388 TrwC− + TrwAB | <1 × 10−7 |
| pSU4058 + pHP159 | R388 T4SS + TrwABC | 7 × 10−1 |
| pSU4058 + pLA24 | R388 T4SS + TrwABC-BID | 8 × 10−1 |
Donor strain: E. coli DH5α. Recipient: E. coli D1210.
Transfer frequency is expressed as the number of transconjugants per donor.
We tested mobilization of these plasmids from B. henselae into human cells (Fig. 4B). It can be observed in the first place that TrwC is essential for DNA transfer. Moreover, a TrwC K502T point mutation, which abolishes DNA helicase activity essential for conjugation (8), was also unable to transfer DNA; this mutation did not affect protein stability according to Western blot analysis (Fig. 1B). This finding proves that DNA mobilization from B. henselae to human cells is a conjugative process. In an attempt to increase DNA transfer efficiencies, we added the VirB/VirD4 secretion signal to the C terminus of TrwC by fusing the C-terminal part of the VirB/VirD4 substrate BepD, containing the BID domain required for secretion (40) (Table 3). This TrwC-BID fusion was checked for its activity in conjugation and shown to act as the wild-type protein in complementation assays (Table 4). However, when we assayed DNA transfer into human cells, we found DNA transfer rates lower than those found with wild-type TrwC (Fig. 4B). TrwC-BID protein levels shown by Western blotting were very low (Fig. 1B), suggesting that the protein was unstable; this could be the reason for the decrease in DNA transfer.
In the absence of TrwB, there is a decreased but detectable level of DNA transfer (about 1/10 of GFP-positive cells compared to results for the plasmid coding for TrwB) (Fig. 4B). The same result was obtained when we assayed the TrwB P18S mutant, lying in the transmembranal domain of TrwB, which shows stronger interactions with B. tribocorum TrwE while maintaining its conjugative functions (10). Thus, TrwB, although not absolutely essential, is involved in DNA transfer to human cells, requiring the TrwB region involved in T4SS interaction. When TrwB ATPase mutation K136T (8) was used, DNA transfer rates dropped to almost null levels, suggesting a dominant negative effect of this mutation. We confirmed by Western blotting that the steady-state level of the TrwB mutant was similar to that of the wild-type protein (Fig. 1B).
Protein-protein interactions between T4CP and T4SS.
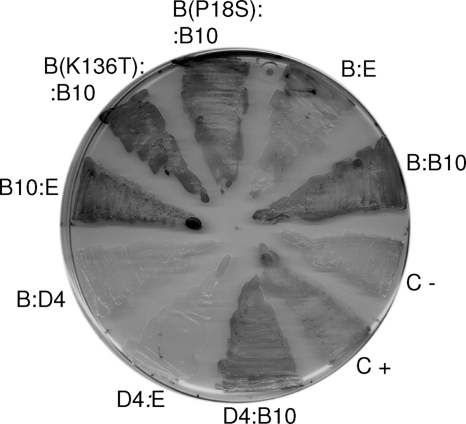
The bacterial two-hybrid assay has been used in previous works to determine that T4CP TrwB of R388 interacts strongly with the R388 T4SS component TrwE (the VirB10 homologue) and also interacts weakly with its TrwE-Bt homologue in B. tribocorum (11). In view of the above results, we tested possible interactions between TrwB and the VirB10 component of B. henselae. Figure 5 shows the results obtained. TrwB interacts with B. henselae VirB10, and in fact this interaction is stronger than that with TrwE-Bt. The assayed TrwB mutations P18S and K136T maintain this strong interaction. In addition, we tested VirD4 interactions and found that VirD4 interacts with its cognate VirB10 counterpart but not with the homologue TrwE-Bt or with R388 TrwB (Fig. 5).
Fig. 5.
Bacterial two-hybrid assay used to detect protein-protein interactions. Pairs of plasmids encoding fusions of the indicated proteins to the T18 and T25 domains of adenylate cyclase were transformed into test strain DHM1. Transformants were streaked on X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside)-containing plates. Blue color reflects interaction between the fused proteins. C+ and C−, plasmids pT25zip + pUT18Czip and pUT18C, respectively. Abbreviations for fused proteins: B, R388 TrwB; point TrwB mutations are indicated in parentheses; E, TrwE-Bt; B10, B. henselae VirB10; D4, B. henselae VirD4.
Conjugative DNA transfer from Bartonella T4SS.
In a previous work, we assayed conjugative transfer of R388 derivatives from E. coli harboring the Trw-Bt T4SS of B. tribocorum, because of its high level of similarity with the Trw T4SS of R388. However, no conjugative DNA transfer of R388 derivatives was observed (11). We now tested conjugal DNA transfer of R388 and derivatives from B. tribocorum itself in order to assure that both sets of T4SS genes (the trw-Bt and virB genes) were being correctly expressed. Mating assays were performed as described in Materials and Methods. To test the system, R388 was transferred from B. tribocorum into either B. henselae or E. coli. The results (Table 6) show that R388 is efficiently transferred into E. coli, while the frequency of transfer drops more than a thousand times when Bartonella is used as the recipient. Next, we assayed the mobilization of plasmid pHP132, carrying the R388 Dtr region cloned into a Bartonella-replicating vector, by the chromosomally encoded T4SS of B. tribocorum, into E. coli. No transconjugants were obtained (Table 6) either by expressing the R388 T4SS from the recipient cell or by assaying the mutation in TrwB shown to increase its interaction with the B. tribocorum TrwE homologue (P18S) (10).
Table 6.
Mating assays using Bartonella tribocoruma
| Plasmid in donor | Recipient | Transfer frequencyb |
|---|---|---|
| pSU2007 (R388) | B. henselae | 8 × 10−5 |
| pSU2007 (R388) | E. coli DH5α | 1 × 10−1 |
| pHP132 (oriTtrwABC) | E. coli DH5α | <1 × 10−8 |
| pHP134 [oriTtrwAB(P18S)C] | E. coli DH5α | <1 × 10−8 |
| pHP132 (oriTtrwABC) | E. coli DH5α (R388 TrwB−) | <1 × 10−8 |
Matings were performed as explained in Materials and Methods.
Transfer frequency is expressed as the number of transconjugants per donor.
The trw-Bt genes are induced once the bacteria enter the human cell (41) and the virB genes are induced in the appropriate infection medium (31). We assayed the conjugal mobilization of pHP132 from donor B. henselae cells rescued from infection assays, as explained in Materials and Methods (Table 7). B. henselae was used in place of B. tribocorum due to its better infection efficiency. When bacteria were recovered from the supernatants of infection assays and used as donors, we obtained about 10−8 transfer efficiency in only 2 out of 8 mating assays; the few transconjugants obtained were checked for the presence of the transferred plasmid and confirmed to be true transconjugants. When human cells were washed and broken, so that recovered bacteria were either intracellular or attached to the infected cells, we obtained a surprisingly high level of conjugal mobilization, about 1% of transconjugants; however, this result was obtained only with bacteria obtained from infection assays rendering high levels of DNA transfer to human cells (>3%). We do not know the reason for this striking difference. Transconjugants were again checked for the presence of the mobilizable plasmid and confirmed to be true transconjugants. The transfer was dependent on the R388 machinery, since no transconjugants were obtained without TrwC (plasmid pHP181).
Table 7.
Mating assays using Bartonella henselae recovered from cell infections and E. coli DH5αa
| Donor bacterium recoveryb | Plasmid in donor | Transfer frequencyc |
|---|---|---|
| From infection medium | pHP161 (oriTtrwABC) | 1 × 10−8d |
| pHP181 (oriTtrwAB) | <1 × 10−8 | |
| From broken cells | pHP161 (oriTtrwABC) | 1 × 10−2e |
| pHP181 (oriTtrwAB) | <1 × 10−6 |
Matings were performed as explained in Materials and Methods.
Bacteria used as donors were recovered from cell infection experiments, either directly from the infection medium or by washing and breaking the cells.
Transfer frequency is expressed as the number of transconjugants per donor.
Transconjugants obtained in only 2 out of 8 assays.
Transconjugants obtained only when infection assays rendered high levels (>3%) of DNA transfer to human cells.
DISCUSSION
Among all known families of bacterial secretion systems, T4SSs stand out due to their versatility, enabling them to form part of such different bacterial processes as horizontal DNA transfer, symbiosis, and pathogenicity (2). Molecular studies of different T4SSs point so far to a conserved core complex through which substrates as different as a protein or a DNA molecule can be secreted either to the outer milieu or to another cell, prokaryotic or eukaryotic. In addition, several reports have demonstrated the possibility of switching substrates between different T4SSs (see the introduction). Recently, Schröder et al. (38) demonstrated that Bartonella can transfer a cryptic plasmid occurring in the bartonellae into EA.hy926 cells via its VirB/VirD4 T4SS, although at a low efficiency of 0.02%. Fusion of the BID domain required for secretion with the plasmid-encoded DNA transport protein Mob resulted in a 100-fold increase in DNA transfer. All these facts prompted us to test the translocation of derivatives of conjugative plasmid R388 through the T4SS of Bartonella spp., with the aim of testing heterologous DNA transfer and characterizing the molecular requirements of the transfer process. We have obtained conjugative DNA transfer of R388 derivatives from B. henselae into specific human cell types through the T4SS of this human pathogen with the same high efficiency as that driven by the BID-containing Mob protein. This is the first report of T4SS-mediated heterologous DNA transfer into human cells. The efficiency of DNA transfer was measured by expression of a eukaryotic egfp cassette. On average, around 1 to 2% of the cells were GFP positive. This is probably an underestimation of the rate of substrate transfer, since GFP-positive cells require DNA mobilization plus egfp expression, for which the substrate must enter the nucleus and the complementary strand must be synthesized. Another interesting finding is that B. henselae cells recovered from human cell infections are able to transfer this DNA to E. coli in conjugation assays (Table 7). This result underscores the flexibility of T4SSs with respect to the recipient cell for substrate secretion.
By using mutant B. henselae strains and mobilizable plasmids carrying different R388 elements, we showed that DNA transfer is dependent on both the VirB/VirD4 T4SS of B. henselae and the conjugal machinery of R388. An important result is the total absence of DNA transfer without TrwC, the R388 relaxase, and pilot protein; moreover, a TrwC point mutation in the DNA helicase activity, known to be required in conjugation, was also unable to transfer DNA (Fig. 4B). These results prove that DNA is being mobilized by a conjugation-like mechanism, implying that the transferred substrate is a TrwC-DNA complex. In fact, our results show the same transfer efficiency for molecules of different lengths (Table 4), as expected for conjugative DNA transfer; it can be anticipated that the transfer process will allow the introduction of long DNA molecules.
The results with B. henselae in-frame deletion mutants indicate that the VirB/VirD4 T4SS of B. henselae is essential for DNA transfer, while a ΔtrwE-Bt mutant is only slightly impaired in DNA transfer (Fig. 4A). We cannot exclude that some DNA transfer may occur through the Trw-Bt T4SS, but this decrease could be due to the interference of the unassembled Trw-Bt T4SS with the normal functioning of the VirB/VirD4 T4SS. Thus, TrwC seems to work as an efficient natural substrate for the VirB/VirD4 T4SS.
T4SS substrates are recruited through a T4CP. In our hybrid DNA transfer system, the T4CPs of both partners are present: R388 TrwB and VirD4 from B. henselae. Both T4CPs were shown to interact with VirB10 (Fig. 5), so they could be competing for the same secretion channel. Our results (Fig. 4) show that in the absence of TrwB, DNA transfer is significantly decreased. Thus, TrwC could be preferentially recruited by TrwB, but in its absence, it could be recruited by VirD4. Although TrwC does not have a BID domain, its C terminus is positively charged, which could account for low-efficiency recruitment by VirD4. The addition of the BID domain to its C terminus did not improve DNA transfer, but this is probably due to the instability of the fusion protein (Fig. 1B). VirD4 probably plays a structural role in the VirB/D4 T4SS which cannot be replaced by TrwB, explaining the transfer-deficient phenotype of the virD4 mutant. The TrwB ATPase mutant shows a dominant negative phenotype which could be due to TrwC sequestering. It is noteworthy that the TrwB P18S mutant, altered in the transmembranal region involved in interactions with the VirB10 homologues, provokes the same drop in DNA transfer as the absence of the T4CP. This result highlights the importance of the T4CP-VirB10 interaction for DNA transfer. VirB10 homologues are thought to be T4SS sensors which allow the opening of the T4SS channel for substrate translocation (7). So, any change in the interaction with the T4CP could affect secretion.
Given that R388 is a broad-host-range plasmid which replicates efficiently in Bartonella spp., R388 transfer to human cells could happen in nature. It is easy to imagine that during evolution, the opportunity has occurred for T4SS-containing pathogens to recruit conjugative machineries for transferring DNA into the cells they infect. Injection of genes into animal cells could help the pathogens in their infection process in the long term, as in a similar way A. tumefaciens subverts plant cells to make them produce metabolites for the long-term benefit to the bacteria. However, no known pathogenesis-associated T4SS recruits DNA as its dedicated substrate. It is possible that such transfer does occur but we have not detected it yet. Intriguingly, the T4CP homologue of H. pylori Cag T4SS is a DNA binding protein (37).
In addition to the biological significance of this finding, biotechnological applications can be developed in the near future utilizing T4SS-based customized DNA delivery systems. The discovery of pathogens with T4SSs increases along with genome database growth. If T4SSs from different human pathogens can be used to send DNA into the cells they infect specifically, many different cellular types could be genetically modified in this way. In addition, since this is a conjugation-like DNA transfer process, it is expected to proceed continuously, thus allowing DNA transfer of molecules containing full-length human genes with their own regulatory sequences; this is an important improvement over existing DNA delivery methods based on viruses, which impose strict size limits by the viral capsids in the DNA to be introduced. Finally, the R388 conjugative system has an additional advantage: TrwC, which is transported covalently linked to the DNA into the host cell, has site-specific integrase activity in the recipient cell (12). TrwC can be targeted to the nucleus, and there are sequences in the human genome with high homology to the TrwC target DNA sequence which function as recombination targets (1), where TrwC could integrate the incoming DNA. In the future, it could be possible to develop a tool combining in vivo DNA delivery and site-specific integration of any DNA molecule into the human genome of specific human cells. This would be an invaluable tool for gene therapy.
ACKNOWLEDGMENTS
This work is dedicated to the memory of Marco Faustmann.
This work was supported by grants BIO2008-00133 and BIO2007-63656 from the Spanish Ministry of Science and Innovation to M.L. and F.J.S., respectively, and by grant 31003A-109925 from the Swiss National Science Foundation to C.D.
We acknowledge Sonja Huser for constructing the ΔvirB2-11 mutant.
Footnotes
Published ahead of print on 9 September 2011.
REFERENCES
- 1. Agúndez L., et al. 2011. Nuclear targeting of a bacterial integrase which mediates site-specific recombination between bacterial and human target sequences. Appl. Environ. Microbiol. 77:201–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alvarez-Martínez C. E., Christie P. J. 2009. Biological diversity of prokaryotic type IV secretion systems. Microbiol. Mol. Biol. Rev. 73:775–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bolland S., Llosa M., Avila P., de la Cruz F. 1990. General organization of the conjugal transfer genes of the IncW plasmid R388 and interactions between R388 and IncN and IncP plasmids. J. Bacteriol. 172:5795–5802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buchanan-Wollaston V., Passiatore J. E., Cannon F. 1987. The mob and oriT mobilization functions of a bacterial plasmid promote its transfer to plants. Nature 328:172–175 [Google Scholar]
- 5. Cabezón E., Sastre J. I., de la Cruz F. 1997. Genetic evidence of a coupling role for the TraG protein family in bacterial conjugation. Mol. Gen. Genet. 254:400–406 [DOI] [PubMed] [Google Scholar]
- 6. Cambronne E. D., Roy C. R. 2006. Recognition and delivery of effector proteins into eukaryotic cells by bacterial secretion systems. Traffic 7:929–939 [DOI] [PubMed] [Google Scholar]
- 7. Cascales E., Christie P. J. 2004. Agrobacterium VirB10, an ATP energy sensor required for type IV secretion. Proc. Natl. Acad. Sci. U. S. A. 101:17228–17233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. César C. E., Machón C., de la Cruz F., Llosa M. 2006. A new domain of conjugative relaxase TrwC responsible for efficient oriT-specific recombination on minimal target sequences. Mol. Microbiol. 62:984–996 [DOI] [PubMed] [Google Scholar]
- 9. Dehio C. 2008. Infection-associated type IV secretion systems of Bartonella and their diverse roles in host cell interaction. Cell. Microbiol. 10:1591–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Paz H. D., et al. 2010. Functional dissection of the conjugative coupling protein TrwB. J. Bacteriol. 192:2655–2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Paz H. D., et al. 2005. Functional interactions between type IV secretion systems involved in DNA transfer and virulence. Microbiology 151:3505–3516 [DOI] [PubMed] [Google Scholar]
- 12. Draper O., César C. E., Machón C., de la Cruz F., Llosa M. 2005. Site-specific recombinase and integrase activities of a conjugative relaxase in recipient cells. Proc. Natl. Acad. Sci. U. S. A. 102:16385–16390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grandoso G., et al. 2000. Two active-site tyrosyl residues of protein TrwC act sequentially at the origin of transfer during plasmid R388 conjugation. J. Mol. Biol. 295:1163–1172 [DOI] [PubMed] [Google Scholar]
- 14. Grandoso G., Llosa M., Zabala J. C., de la Cruz F. 1994. Purification and biochemical characterization of TrwC, the helicase involved in plasmid R388 conjugal DNA transfer. Eur. J. Biochem. 226:403–412 [DOI] [PubMed] [Google Scholar]
- 15. Grant S. G., Jessee J., Bloom F. R., Hanahan D. 1990. Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc. Natl. Acad. Sci. U. S. A. 87:4645–4649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hamilton C. M., et al. 2000. TraG from RP4 and TraG and VirD4 from Ti plasmids confer relaxosome specificity to the conjugal transfer system of pTiC58. J. Bacteriol. 182:1541–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heinemann J. A., Sprague G. F., Jr 1989. Bacterial conjugative plasmids mobilize DNA transfer between bacteria and yeast. Nature 340:205–209 [DOI] [PubMed] [Google Scholar]
- 18. Jurik A., et al. 2010. The coupling protein Cagbeta and its interaction partner CagZ are required for type IV secretion of the Helicobacter pylori CagA protein. Infect. Immun. 78:5244–5251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karimova G., Dautin N., Ladant D. 2005. Interaction network among Escherichia coli membrane proteins involved in cell division as revealed by bacterial two-hybrid analysis. J. Bacteriol. 187:2233–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karimova G., Pidoux J., Ullmann A., Ladant D. 1998. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc. Natl. Acad. Sci. U. S. A. 95:5752–5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karimova G., Ullmann A., Ladant D. 2001. Protein-protein interaction between Bacillus stearothermophilus tyrosyl-tRNA synthetase subdomains revealed by a bacterial two-hybrid system. J. Mol. Microbiol. Biotechnol. 3:73–82 [PubMed] [Google Scholar]
- 22. Lee E. C., et al. 2001. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics 73:56–65 [DOI] [PubMed] [Google Scholar]
- 23. Llosa M., Bolland S., Grandoso G., de la Cruz F. 1994. Conjugation-independent, site-specific recombination at the oriT of the IncW plasmid R388 mediated by TrwC. J. Bacteriol. 176:3210–3217(Erratum, 176:6414.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Llosa M., Gomis-Rüth F.-X., Coll M., de la Cruz F. 2002. Bacterial conjugation: a two-step mechanism for DNA transport. Mol. Microbiol. 45:1–8 [DOI] [PubMed] [Google Scholar]
- 25. Llosa M., Roy C., Dehio C. 2009. Bacterial type IV secretion systems in human disease. Mol. Microbiol. 73:141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Llosa M., Zunzunegui S., de la Cruz F. 2003. Conjugative coupling proteins interact with cognate and heterologous VirB10-like proteins while exhibiting specificity for cognate relaxosomes. Proc. Natl. Acad. Sci. U. S. A. 100:10465–10470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luo Z. Q., Isberg R. R. 2004. Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc. Natl. Acad. Sci. U. S. A. 101:841–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martínez E., de la Cruz F. 1988. Transposon Tn21 encodes a RecA-independent site-specific integration system. Mol. Gen. Genet. 211:320–325 [DOI] [PubMed] [Google Scholar]
- 29. Moncalián G., et al. 1999. Characterization of ATP and DNA binding activities of TrwB, the coupling protein essential in plasmid R388 conjugation. J. Biol. Chem. 274:36117–36124 [DOI] [PubMed] [Google Scholar]
- 30. Nagai H., et al. 2005. A C-terminal translocation signal required for Dot/Icm-dependent delivery of the Legionella RalF protein to host cells. Proc. Natl. Acad. Sci. U. S. A. 102:826–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Quebatte M., et al. 2010. The BatR/BatS two-component regulatory system controls the adaptive response of Bartonella henselae during human endothelial cell infection. J. Bacteriol. 192:3352–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sadler J. R., Tecklenburg M., Betz J. L. 1980. Plasmids containing many tandem copies of a synthetic lactose operator. Gene 8:279–300 [DOI] [PubMed] [Google Scholar]
- 33. Saenz H. L., et al. 2007. Genomic analysis of Bartonella identifies type IV secretion systems as host adaptability factors. Nat. Genet. 39:1469–1476 [DOI] [PubMed] [Google Scholar]
- 34. Salgado-Pabon W., Jain S., Turner N., van der Does C., Dillard J. P. 2007. A novel relaxase homologue is involved in chromosomal DNA processing for type IV secretion in Neisseria gonorrhoeae. Mol. Microbiol. 66:930–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sambrook J., Russell D. W. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 36. Schmid M. C., et al. 2004. The VirB type IV secretion system of Bartonella henselae mediates invasion, proinflammatory activation and antiapoptotic protection of endothelial cells. Mol. Microbiol. 52:81–92 [DOI] [PubMed] [Google Scholar]
- 37. Schröder G., et al. 2002. TraG-like proteins of DNA transfer systems and of the Helicobacter pylori type IV secretion system: inner membrane gate for exported substrates? J. Bacteriol. 184:2767–2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schröder G., Schülein R., Quebatte M., Dehio C. 2011. Conjugative DNA transfer into human cells by the VirB/VirD4 type IV secretion system of the bacterial pathogen Bartonella henselae. Proc. Natl. Acad. Sci. U. S. A. 108:14643–14648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schulein R., Dehio C. 2002. The VirB/VirD4 type IV secretion system of Bartonella is essential for establishing intraerythrocytic infection. Mol. Microbiol. 46:1053–1067 [DOI] [PubMed] [Google Scholar]
- 40. Schulein R., et al. 2005. A bipartite signal mediates the transfer of type IV secretion substrates of Bartonella henselae into human cells. Proc. Natl. Acad. Sci. U. S. A. 102:856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seubert A., Hiestand R., de la Cruz F., Dehio C. 2003. A bacterial conjugation machinery recruited for pathogenesis. Mol. Microbiol. 49:1253–1266 [DOI] [PubMed] [Google Scholar]
- 42. Truttmann M. C., Rhomberg T. A., Dehio C. 2011. Combined action of the type IV secretion effector proteins BepC and BepF promotes invasome formation of Bartonella henselae on endothelial and epithelial cells. Cell. Microbiol. 13:284–299 [DOI] [PubMed] [Google Scholar]
- 43. Vayssier-Taussat M., et al. 2010. The Trw type IV secretion system of Bartonella mediates host-specific adhesion to erythrocytes. PLoS Pathog. 6:e1000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vergunst A. C., et al. 2005. Positive charge is an important feature of the C-terminal transport signal of the VirB/D4-translocated proteins of Agrobacterium. Proc. Natl. Acad. Sci. U. S. A. 102:832–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Voth D. E., et al. 2009. The Coxiella burnetii ankyrin repeat domain-containing protein family is heterogeneous, with C-terminal truncations that influence Dot/Icm-mediated secretion. J. Bacteriol. 191:4232–4242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Waters V. L. 2001. Conjugation between bacterial and mammalian cells. Nat. Genet. 29:375–376 [DOI] [PubMed] [Google Scholar]