Abstract
Conjugation is an efficient way for transfer of genetic information between bacteria, even between highly diverged species, and a major cause for the spreading of resistance genes. We have investigated the subcellular localization of several conserved conjugation proteins carried on plasmid pLS20 found in Bacillus subtilis. We show that VirB1, VirB4, VirB11, VirD2, and VirD4 homologs assemble at a single cell pole, but also at other sites along the cell membrane, in cells during the lag phase of growth. Bimolecular fluorescence complementation analyses showed that VirB4 and VirD4 interact at the cell pole and, less frequently, at other sites along the membrane. VirB1 and VirB11 also colocalized at the cell pole. Total internal reflection fluorescence microscopy showed that pLS20 is largely membrane associated and is frequently found at the cell pole, indicating that transfer takes place at the pole, which is a preferred site for the assembly of the active conjugation apparatus, but not the sole site. VirD2, VirB4, and VirD4 started to localize to the pole or the membrane in stationary-phase cells, and VirB1 and VirB11 were observed as foci in cells resuspended in fresh medium but no longer in cells that had entered exponential growth, although at least VirB4 was still expressed. These data reveal an unusual assembly/disassembly timing for the pLS20 conjugation machinery and suggest that specific localization of conjugation proteins in lag-phase cells and delocalization during growth are the reasons why pLS20 conjugation occurs only during early exponential phase.
INTRODUCTION
Conjugation was one of the first tools to be used to reveal the nature of genetic material, and later it was used to gain insight into molecular genetics. Although conjugation has been studied for several decades, many key questions about the mechanism of DNA transfer between cells remain to be solved. Moreover, much less is known about the conjugation machinery and mode of conjugation in Gram-positive bacteria (12, 21, 27), a large bacterial phylum containing important human pathogens. The aim of this study was to shed light on the molecular mechanism of conjugation in the Gram-positive model bacterium Bacillus subtilis.
Several proteins have been shown to be essential or important for conjugation in Gram-negative systems, which together are thought to set up a type IV secretion system that can translocate proteins as well as DNA (7). Some of the characterized systems are the Escherichia coli F plasmid (IncF) Tra system, E. coli RP4 (IncP) Trb system, E. coli R388 (IncW) Trw system, Shigella Collb-P9 (IncI) Tra system, Agrobacterium tumefaciens VirB system, and Legionella pneumophila Dot/Icm system (8, 11). Conjugation systems are also found on integrative conjugative elements (ICEBs1 from Bacillus subtilis). In this work, we have used the Vir nomenclature, because it is widely used and refers to the best-studied system in A. tumefaciens, which can transfer a copy of DNA from the transfer region of the tumor-inducing (Ti) plasmid as single-stranded DNA (ssDNA) into plant cells; these then become transformed. There are several conserved (and thus centrally important) proteins: VirD2 is a so-called relaxase. It introduces a nick in the T-DNA border sequence and remains covalently bound at the 5′ end of the DNA transfer intermediate (15, 17, 18). After guiding the ssDNA to the coupling protein VirD4, VirD2 is cotransferred into the recipient cell (10). In all known cases (except for systems in Streptomyces), an ssDNA/protein complex is translocated into the other cell. There are three components containing ATPase motifs, VirB11, VirB4, and VirD4. VirB11 and VirD4 contain Walker A and B motifs (ATP binding site) and belong to the superfamily of P-loop ATPases (11). VirB11 in A. tumefaciens is an ATPase that forms homomultimers and possibly provides energy for DNA transfer and pilus biogenesis. Dihybrid screens have shown interactions with VirB4 and with VirB9 (8, 38). For Helicobacter pylori HP0525, a homolog of VirB11, the crystal structure was solved and it showed a hexameric assembly of the structure. The pore, which is formed by the multimeric protein in the ADP-bound form, has an external diameter of 100 Å and an internal diameter of 50 Å. It has been proposed that nucleotide binding and hydrolysis lead to conformational changes to facilitate substrate export (33).
VirD4 from A. tumefaciens is called a coupling protein (CP). The proposed functions are the recruitment of the ssDNA and protein substrate to the conjugation machinery and their translocation. In dihybrid and biochemical assays, a close contact with VirE2 (single-strand binding protein [SSB]) was detected (5). CPs of Gram-negative bacteria are known to have 2 amino-terminal transmembrane helices, a small periplasmic domain, and a large carboxy-terminal region in the cytoplasm. The X-ray crystal structure of the soluble C-terminal part of the E. coli VirD4 homolog TrwB from plasmid R388 shows a ring-like structure, similar to F1 ATPase, with a channel diameter of 20 Å (20). Purified VirD4 was detected in the soluble as well as in the membrane fractions, while exclusively protein from the soluble fraction showed ATPase activity. It was proposed that VirD4 has a translocase function, which is supported by the fact that it bears sequence homologies to DNA translocases, like SpoIIIE and FtsK. The mechanism of this process is unknown, although there are hints that interactions occur with parts of the mating pair formation (Mpf) complex (19, 30), and so it has been suggested that VirD4 recruits the transfer substrate and delivers the DNA/protein complex to the conjugation apparatus (4, 32, 34, 35).
VirB4 has homologies to the P-loop ATPase HerA. The VirB4 protein is postulated to energize the substrate export by ATP-driven conformational changes. It is essential for DNA export and seems to interact with the second Mpf-ATPase, VirB11 (4, 38). In A. tumefaciens, VirB11 interacted with VirB4, VirB8, and VirB10 and with itself (4, 11, 38). Like VirB11, VirB4 is postulated to provide energy for pilus biogenesis.
VirB1 belongs to the periplasmic factors (11) and contains a lytic transglycosylase domain (LT domain; belonging to the lysozyme superfamily). LTs catalyze the cleavage of the beta-1,4-glycosidic bond between N-acetylmuramic acid (MurNAc) and N-acetyl-d-glucosamine (GlcNAc). The proposed functions are peptidoglycan hydrolase activity and pilus/channel assembly. Dihybrid screens showed interactions with VirB1, VirB4, VirB8, VirB9, VirB10, and VirB11 (38).
We have chosen to study pLS20 as a model for conjugative plasmids in Gram-positive bacteria. pLS20 is a plasmid discovered in a natural Bacillus subtilis isolate (natto) (36) and is partitioned through a ParM-like active mechanism to daughter cells during each cell cycle, but it can also propagate via conjugation. Conjugation was shown to be independent of RecA protein and was most efficient during the early phase of logarithmic growth (22), in contrast to the acquisition of DNA through natural competence in B. subtilis, which is restricted to the entry into stationary-phase growth (9). pLS20 is 65 kb in size and can also mobilize nonconjugative plasmids, and it has been studied because it can conjugate into other Bacillus strains and can potentially be used for the transfer of large DNA fragments. Replication of pLS20 occurs via a novel mechanism: the replication region shows no similarity with other known plasmid replicons (31) and therefore has been suggested to belong to a new class of theta replicons, establishing an average of 1 to 3 copies per cell (26, 29). Its segregation employs actin-like protein Alp7a, which appears to push plasmids toward opposite cell poles via the formation of highly dynamic filaments (16). Although a miniversion of pLS20 has been used to visualize the segregation pattern, nothing is known about the localization of the full-length pLS20 plasmid or the localization of parts of its conjugation machinery. We show that full-length pLS20 behaves differently from the miniplasmid, and its localization pattern appears to be a mixture of that of bipolarly positioned low-copy-number plasmids and of an additional extremely polarly located plasmid copy (or copies). We provide evidence that the conjugation machinery assembles at a single cell pole or at a defined site along the lateral cell membrane. Most interestingly, we found that the conjugation machinery assembles in cells during extended stationary phase and during lag phase but disassembles as cells commence exponential growth, in correlation with the transfer activity of the plasmid.
MATERIALS AND METHODS
Bacterial strains and media.
Bacillus strains (see Table S2 in the supplemental material) were grown in LB medium at 37°C for conjugation assays and at 30°C for microscopy. Selection pressure for the inserted fusions was always maintained with appropriate antibiotics. Because of the high stability of pLS20 (22), antibiotic was never added for maintenance of the plasmid. The fusion of VirD4 and cyan fluorescent protein (CFP), expressed from the chromosome (strains TCR3 and TB15), was induced with 0.01 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and the inducible fusion of VirB4 and yellow fluorescent protein (YFP; TCR04) was grown in LB medium supplemented with 0.5% xylose. For microscopy, cells were grown until stationary phase for 10 h and were resuspended into fresh LB medium (time point, 0 h).
Conjugation assays.
Mating experiments were performed as described before (22). To compare the number of trancipients in a single growth experiment, the number of trancipients at each time point was calculated. These numbers of trancipients were normalized to equal cell numbers at each time point of the experiment. Given that numbers of trancipients are relative numbers, they can be used to compare different growth stages of one strain. To determine the exact transfer rate, a dilution series (10−5, 10−6, and 10−7) of samples, taken for mating experiments at different growth stages, was plated on LB agar to determine the definite number of cells during a conjugation experiment. Different amounts of mating mixtures were spread on plates supplemented with antibiotics that selected for the according trancipient.
Constructions of plasmids and strains.
To create C-terminal green fluorescent protein (GFP), YFP, CFP, mCherry or TAP Tag fusions of VirB11 (ORF 13), VirD4 (ORF 24), VirB4 (ORF 28), VirB1 (ORF 30), and VirD2 (ORF 34) for single-crossover integration into the plasmid, the 3′ regions of the genes were amplified by PCR using primers 1 to 10 (see Table S1 in the supplemental material). PCR products of the C-terminal regions of virB11, virD4, virB4, and virD2 were cloned with the indicated enzymes into the multiple cloning site (mcs) of modified pSG1164y (25), generating pTB01, pTB02 and pTB03, pTB05, TB13, and TB14 (see Table S2 in the supplemental material). The plasmid expressing an mCherry C-terminal fusion to virB1 was constructed by exchanging dnaX with virB1 in pCT2 (14), using EcoRI and XhoI restriction sites and generating pTB04 (virB1-mCherry).
pLS20neo was a kind gift from and constructed by Shinya Kaneko (Tokyo Institute of Technology, Yokohama-shi Kanagawa, Japan) by replacing the cat gene of pLS20cat (22) with a neo gene by using in vitro gene assembly technology (unpublished results). To introduce the lacO repeat into pLS20neo, a 1-kb region of pLS20neo (bp 61101 to 62100) was amplified by PCR, using primers 11 and 12 (see Table S1 in the supplemental material), and cloned into the HindIII restriction site of pAT12 (39), resulting in pTB06.
To generate the IPTG-inducible thrC::Physpank::virD4-cerulean cfp construct pTCR3, virD4 was first subcloned in pFD2, encoding the fluorescent protein cerulean-CFP. virD4 was amplified by PCR using primer pair 14/17 and pLS20neo as template DNA, digested with KpnI and XhoI, and cloned into pFD2. In a second PCR, virD4 was amplified together with GFP, using pTCR2 as template DNA and primer pair 18/19, digested with NheI and SphI and cloned into NheI and SphI sites of pDP150, creating plasmid pTCR3. To generate the xylose-inducible amyE::Pxyl::virB4-yfp construct pTCR4, virB4 was amplified by PCR using primer pair 20/21 and pLS20neo as template DNA, digested with KpnI and XhoI, and cloned into KpnI- and XhoI-digested pSG1193.
To generate PY79 containing pLS20 (AKR04), PY79 was transformed with pLS20neo and transformants were selected with kanamycin. AKR04 was transformed with the plasmids pTB01, pTB02 pTB03, pTB04, pTB05, pTB06, pTB10, and pTB14, and transformants were selected with the appropriate antibiotics to generate strains TB01, TB02, TB03, TB04, TB05, TB06, TB10, and TB14. To create a strain expressing a LacI-CFP fusion (AKR05), PY79 was transformed with chromosomal DNA of PG26. Transformants were selected with lincomycin and erythromycin.
pLS20neo with the inserted lacO repeat from strain TB06 was conjugated as described previously (22) into strain AKR05 (lacI-cfp), as recipient, resulting in strain TB07. Strains TB08 and TB09 were created by conjugation with strain CDS4 (dnaX-mCherry) (14). Trancipients of TB07 were selected with chloramphenicol, lincomycin, and erythromycin, and trancipients of TB08 and TB09 were selected by spectinomycin and chloramphenicol. Strain TB12 (pLS-cfp dnaX-mCherry) was generated by transformation of chromosomal DNA from strain CDS4 (dnaX-mCherry) in TB07.
For the bimolecular fluorescence complementation (BiFC) experiment, gfp from construct 1164 virB4gfp was replaced with the C-terminal part of YFP (Yc) by using primer pair 15/16 and PstI and SpeI restriction sites, resulting in pTB10. In pSW02 (dnaX-yn; kind gift from S. Welling), dnaX was exchanged with virD4 by using the EcoRI and XhoI restriction sites (primers 13 and 14), resulting in pTB11. PY79/pLS20neo was transformed with pTB10, and transformants of TB10 were selected by chloramphenicol resistance. TB10 was transformed with pTB11, and transformants were selected with spectinomycin and chloramphenicol, resulting in TB11. TCR1 and TCR2 were created by conjugation of TB10 with SW02 and JS96 (13), and trancipients were selected with spectinomycin and chloramphenicol. TB15 was created by conjugation of TCR03 and TB14, and trancipients were selected by chloramphenicol, lincomycin, and erythromycin. For the simultaneous visualization of VirB1 and VirB11 in the same cell, TB01 (virB11-yfp) was transformed with pTB04 and transformants were selected with spectinomycin and chloramphenicol, resulting in strain TB16.
To generate a C-terminally truncated fusion of VirD2 and GFP, 500 bp lying 120 bp upstream of the virD2 stop codon was PCR amplified with primer pair 24/25 and pLS20neo as template, digested with ApaI and ClaI, and cloned into ApaI- and ClaI-digested pSG1164.
FISH.
To localize double-stranded pLS20 and ssDNA intermediates produced during the process of conjugation in situ, a denaturing and nondenaturing gene fluorescence in situ hybridization (FISH) assay was performed as described in reference 3, using the 5′ region of the virD2 gene as a probe. Details and variations are provided in the supplemental material.
Image acquisition.
Fluorescence microscopy was performed with an Olympus AX70 microscope and an AXIO Observer A1 (Zeiss) or an AXIO Imager (Zeiss) with a Cool Snap ES2 camera (Photometrics), using total internal reflection fluorescence (TIRF) objectives with a numerical aperture of 1.45. Cells were grown in LB medium and mounted on thin agarose pads (1% prepared in S750 minimal medium supplemented with 0.1% Casamino Acids) on object slides. Membranes were stained with FM4-64 (2.5 μg/ml). Images were processed by using Metamorph 6.3 and ImageJ 1.43 software (Image Processing and Analysis). FRAP experiments were performed on a Zeiss Axio Observer Z with a Cascade II 518 camera (Photometrics) and a 405-nm laser beam.
Western blot analysis.
For Western blot analysis of TAP-tagged VirB4, cultures were grown in liquid media with the appropriate antibiotics. Equal numbers of cells were harvested at each time point during exponential growth. Blotting with specific antisera was performed using a semidry procedure. Antibodies against the CBP-epitope of the TAP tag were used to detect the fusion protein, and anti-MreB antiserum was used as a control.
RESULTS
Genes present on pLS20.
pLS20 is a 65-kb plasmid that was isolated by Tanaka et al. (36) from B. subtilis (natto) IFO3335 and was characterized as a natural fertility plasmid. Growth stage-dependent kinetics of conjugational transfer frequencies in liquid compared to solid media were determined. pLS20 transfers poorly on solid media, or in minimal media, but shows maximal transfer kinetics of (3.3 ± 0.78) × 10−3 trancipients/donor/15 min during the transition from fresh inoculation of the culture to exponential growth. We have systematically analyzed all open reading frames (ORFs) present on the plasmid, with special interest for potential conjugation proteins. A cartoon of pLS20 is shown in Fig. S1 of the supplemental material. We found that pLS20 carries genes for all conserved ATPase motif proteins: orf13, virB11; orf24, virD4; orf28, virB4. A BLAST search showed strongest homology of VirD4 to the TraG/TraD system from Clostridium perfringens. VirD4 is predicted to have 2 N-terminal transmembrane domains (based on SMART and EMBL databases) or even 4 transmembrane domains (HMMTOP database). For VirB4, BLAST searches showed highest homologies to a predicted TraC-like protein from Enterococcus faecalis T1 and a VirB4 component from Lactobacillus casei. orf30 encodes a VirB1 ortholog with highest homologies to putative lipoproteins from Listeria spp. or Bacillus thuringiensis, as well as significant homologies to the secreted cell wall dl-endopeptidase Yddh/CwIT from B. subtilis. VirB1 contains one predicted transmembrane helix at the N terminus (HMMTOP, residues 24 to 48; SMART, 25 to 47). Catalytic residues of the N-acetyl-d-glucosamine binding site of LTs are conserved. The C-terminal region of the protein contains conserved domains with homologies to cell wall-associated hydrolyases and invasion-associated secreted endopeptidases and bacteriophage-like amidases. VirB1 thus appears to be able to confer glycosidase as well as peptidase activity. orf34 has similarity to relaxases and is thus likely to encode a VirD2 ortholog. It contains no apparent transmembrane domains and is likely a soluble protein. More proteins found on pLS20 are described in the supplemental material.
Localization pattern of pLS20 in live bacterial cells.
We wanted to visualize a conjugative plasmid from a Gram-positive organism and proteins involved in conjugation to gain further insights into this process. The group of J. Pogliano has characterized an actin-like partitioning system on pLS20 and has used a minimal variant of the plasmid to visualize its localization (16). We wanted to visualize the full-length plasmid, which may behave differently from its minimal version, and to colocalize it with the DNA replication machinery to determine its place of replication. To do this, we used the lacO array (integrated close to the replication region in pLS20)/LacI-CFP system, which yielded fully conjugation-proficient pLS20. Figure 1A shows that exponentially growing cells contain 3 to 4 copies (up to 5) of pLS20, which agrees well with the measured copy number of 3 (26). In exponential growth phase, all cells contained several pLS20 foci. Of these, 30% of the cells showed a focus at one pole and 3% at both poles (Table 1). These data suggest that pLS20 plasmids do not undergo cohesion but are rapidly distributed within the cell. In stationary-phase cells, only one or two copies are detectable (Fig. 1C), showing that the copy number is growth phase-dependent and drops as cells exit exponential growth. Of stationary-phase cells, 42% showed a focus at one pole, 46% showed foci at both poles, and 12% contained one or two foci at nonpolar positions at the membrane; only 27% of all cells contained additional foci between the poles. Thus, pLS20 is predominantly found at the cell pole, but also at additional membrane-proximal sites away from a pole, in stationary-phase cells and in cells during lag phase. These data suggest that the cell pole plays a dominant role for pLS20 during early growth or growth arrest. Additionally, most pLS20 copies appear to localize at the cell membrane. To verify this, we used TIRF microscopy, which visualizes fluorescence that is very close to the cell surface. Figure 1E shows that a large number of plasmids were still seen in the TIRF mode, in which some signals were lost that were not close to the membrane plane imaged.
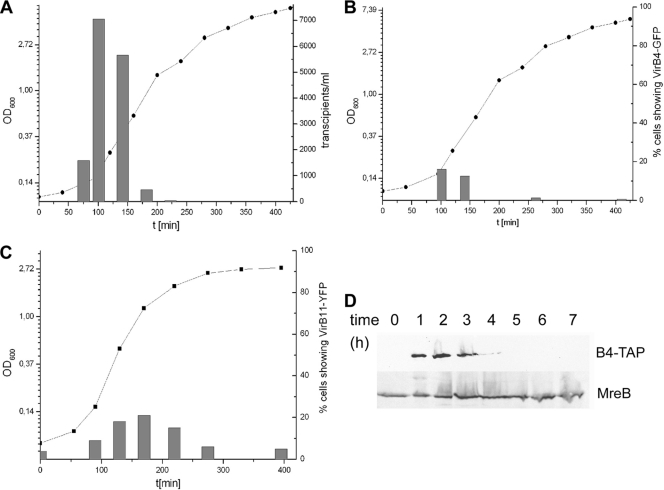
Fig. 1.
Localization of pLS20 (tagged with LacI-CFP bound to a pLS20-integrated lacO array) in live Bacillus subtilis cells. (A to C) Mid-exponential-phase B. subtilis cells expressing LacI-CFP (A) or lacking pLS20 (lacO) (B), and pLS20 in cells in late stationary phase (24 h) (C). (D) PLS20 in spores. (E) Epifluorescence and TIRF images of the same cells. (F) Simultaneous visualization of pLS20 (CFP) with DnaX-mCherry. (Overlay) DnaX-mCherry, red; pLS20 (CFP) green. (G) Time-lapse microscopy of pLS20. Images were captured at the time points (in seconds) indicated above the panels. White arrowheads indicate stationary polar plasmids, while red triangles indicate areas of highly motile plasmids. (H and I) FISH experiments using an ssDNA probe to visualize pLS20 in cells carrying pLS20 under denaturing conditions (H) or under nondenaturing conditions (I). Left panels, green channel; right panels, outline of cell in red channel. Bars, 2 μm.
Table 1.
Copy numbers and localization patterns of pLS20 in different growth phases
| Growth phase | No. of plasmids/cell | % of cells with plasmid(s): |
||
|---|---|---|---|---|
| At one pole | At both poles | Between poles | ||
| Exponential | 3.5 | 30 | 3 | 100 |
| Stationary | 1.3 | 42 | 46 | 27 |
| Competence | 2.5 | 6.5 | 92.5 | 11 |
We also followed the localization of pLS20 in sporulating cells. B. subtilis spores all contained several pLS20 copies (Fig. 1D), showing that pLS20 is inherited through sporulation with high efficiency.
To obtain information on the mode of replication of the conjugative plasmid, we colocalized pLS20 and DnaX, a central component of the replication machinery. We found overlapping pLS20-CFP and DnaX-mCherry signals in only 2% of the cells, while 98% of the signals were nonoverlapping (Fig. 1F). Note that most experiments were performed with rich (LB) medium at 30°C (because cells carrying pLS20 do not grow and conjugate well in minimal medium [data not shown]), where cells have overlapping rounds of chromosomal replication and therefore multiple replication machineries. These data indicate that pLS20 may not employ the chromosomal replication machinery for its own propagation, in agreement with its unique replication mechanism.
Time-lapse microscopy revealed two different populations of pLS20. Polar pLS20-CFP foci were generally static and did not move during the entire experiment, while nonpolar plasmids were generally mobile and moved between the 7-s time intervals (Fig. 1G; also see Movie S1 in the supplemental material). These data suggest that polar pLS20 is stably anchored to the membrane, while internal plasmids are free to diffuse within a certain area of the cell.
Nondenaturing FISH has revealed which of the two DNA strands is translocated by the A. tumefaciens conjugation machinery (3). We also performed FISH experiments on pLS20, which verified that under denaturing conditions, the plasmid is found at the cell poles and at the lateral membrane (Fig. 1H). However, we were not able to detect a specific signal under nondenaturing conditions (Fig. 1I), showing that ssDNA from pLS20 transferred during conjugation is less accessible for FISH than in the Gram-negative system.
VirB4 and VirD4 localize as discrete foci at the cell membrane and to the cell pole during late stationary phase and during lag phase.
We generated fluorescent protein fusions to several conserved conjugation proteins encoded on pLS20. Fusions were integrated via single-crossover recombination into pLS20. To test if the fusions were functional, transformants were used as donors to transfer pLS20 into a new recipient. Because only a single copy was transferred, the resultant conjugants contained solely recombinant pLS20 copies. Conjugation efficiencies of resultant strains were very similar to wild-type levels (Table 2), showing that all protein fusions generated are fully functional, and they were expressed as sole sources of the protein within cells.
Table 2.
Transfer frequencies of pLS20neo carrying different FP fusionsa
| FP fusion | OD600 | Frequency of transfer (trancipients/donor/15 min) | Avg frequency b |
|---|---|---|---|
| VirD4-GFP | 0.23 | 1.8 × 10−3 | 1.6 × 10−3 |
| 0.37 | 1.3 × 10−3 | ||
| VirB4-GFP | 0.25 | 8.3 × 10−4 | 6.1 × 10−4 |
| 0.41 | 3.9 × 10−4 | ||
| VirD2-YFP | 0.25 | 5.9 × 10−4 | 4.3 × 10−4 |
| 0.42 | 2.7 × 10−4 | ||
| VirD2ΔC-YFP | 0.22 | 1.46 × 10−4 | 3.93 × 10−3 |
| 0.23 | 7.72 × 10−3 | ||
| VirB11-YFP | 0.24 | 2.8 × 10−4 | 2.1 × 10−4 |
| 0.34 | 1.3 × 10−4 |
Values are averages of two experiments at the same OD. Note that transfer frequencies of VirB11 varied greatly (due to a reason we cannot explain) such that a statistical evaluation was impossible.
Average frequency at the two different ODs.
For VirB4, we found homogeneous fluorescence in >95% of all cells during mid-exponential growth (Fig. 2A), showing that the protein is expressed. This was verified by Western blot analysis (Fig. 3D). Note that during exponential growth, B. subtilis PY79 cells form almost exclusively chains (Fig. 2A, gray triangle), and few single cells (swimmers, 5 to 10% of all cells [white triangles]). During mid-exponential growth, few single cells (less than 1% of all cells) were observed that contained single VirB4-GFP foci, and these were generally at the cell membrane (Fig. 2B; note that in this image small cells are highly overrepresented). During late exponential phase, no more cells containing VirB4-GFP foci were detectable, although single cells were still present (data not shown) and, likewise, only homogeneous fluorescence was observed in cells during early stationary phase (10 h after inoculation in fresh medium) (Fig. 2C). To address the question, whether single cells containing VirB4-GFP foci during mid-log-phase growth may not yet have commenced an active cell cycle, we monitored the localization of VirB4-GFP in cells expressing DnaX-mCherry, a central component of the replication machinery. Small cells that displayed VirB4-GFP very rarely contained DnaX-mCherry foci (however, due to a reason unclear to us, many [about 60%] of the cells had high mCherry background fluorescence), while all cell chains contained DnaX-mCherry foci but no VirB4-GFP foci (Fig. 2D). Colocalization of discrete DnaX-mCherry foci and VirB4-GFP was only observed in less than 1% of all cells analyzed (Fig. 2D). Thus, cells that are actively replicating their chromosomes and are therefore commencing exponential growth do not contain VirB4-GFP assemblies, while cells that probably have not yet initiated replication frequently do contain assemblies. These findings raised the possibility that B. subtilis cells assemble VirB4 molecules at the cell membrane as they transition from the stationary phase to growth (lag phase) and disassemble them as they induce an active cell cycle. We therefore monitored VirB4-GFP fluorescence in cells freshly resuspended in rich medium (note that in minimal medium, we observed extremely poor conjugation activity and few cells containing VirB4-YFP foci). Strikingly, 20 to 30% of the cells contained a single focus or, rarely, up to 2 foci. In 75% of these cases, a focus was located at a cell pole, while in 25% a focus was positioned at a random position, but in 90% of all cases, the focus was clearly located at the membrane (Fig. 2E). Therefore, VirB4 assembles at the membrane, and frequently at the pole, during lag phase. Interestingly, this is the time when conjugation activity is highest, and in fact, the number of cells showing VirB4-GFP foci correlated with the conjugation activity of the culture (Fig. 3A and B). We wished to find out if VirB4 assembles at some point during stationary phase, or if foci are freshly induced as the cell obtains new medium. Intriguingly, 24 h after inoculation of the culture (i.e., about 14 h after reaching stationary phase), 10% of the cells contained a single polar VirB4-GFP focus and 3% had a focus at the lateral membrane; 1% contained two polar foci (Fig. 2F). Thus, VirB4 also assembles during stationary phase in a subset of the cells, where cells are not active for conjugation (Fig. 3A), and in additional cells when fresh medium is supplied, where conjugation is induced.
Fig. 2.
(A to D) Localization of VirB4 and VirD4. Images show VirB4-GFP in the mid-exponential growth phase (A, B, and D), early stationary phase (10 h) (C), or with simultaneous visualization of VirB4-GFP and DnaX-mCherry during mid-exponential growth (D). Gray triangles indicate cell chains expressing DnaX-mCherry, and white triangles indicate small cells with VirB4-GFP foci. (E and F) VirB4-GFP at lag phase (1 to 2 h after inoculation) (E) or at mid-stationary phase (24 h) (F). Polar VirB4-GFP assemblies are indicated by white triangles. (G to J) VirD4-GFP during lag phase (G), early stationary phase (10 h) (H), or mid-stationary phase (24 h) (I), and simultaneous visualization of VirD4-GFP and DnaX-mCherry during mid-exponential growth (J). Gray triangles indicate cell chains expressing DnaX-mCherry (faint foci), and white triangles indicate small cells with VirD4-GFP foci. Bars, 2 μm.
Fig. 3.
Transfer kinetics of pLS20 and localization relative to growth phase. (A) Conjugation frequency of pLS20 at different time points during growth. OD600, optical density at 600 nm. (B) Number of cells showing VirB4-GFP foci at different time points during growth (localization frequency). (C) Number of cells showing VirB11-YFP foci at different time points during growth (localization frequency). (D) Western blot at different time points during growth (hours after inoculation are indicated) of VirB4-TAP by using TAP tag-specific antibodies (or against MreB, loading control).
We wanted to investigate if the localization of VirB4 was associated with swimmers (motile single cells) or chain formers. Because during exponential phase most PY79 cells are chain formers, we overproduced SwrA from the amylase locus, which results in the formation of a majority of swimmers (>90%) (24). However, even under these conditions, most cells during lag phase showed VirB4-GFP foci, and few cells contained foci during exponential phase (data not shown). Because the pattern of localization was not considerably different between chain formers and swimmers, it is clearly associated with the growth phase and not with motility.
To test if several proteins from the presumed conjugation machinery follow a similar localization pattern, we monitored the subcellular localization of VirD4. Indeed, VirD4-GFP was visible as a membrane-proximal focus in cells during lag phase and again frequently at a single cell pole (Fig. 2G). Few cells containing foci were seen during mid-exponential growth (Fig. 2J), and no foci were observed during early stationary phase (Fig. 2H). However, discrete foci were easily detectable after 24 h (mid-stationary phase) (Fig. 2I). As for VirB4, DnaX-mCherry and VirD4-GFP showed exclusive localization and were present in the same cell only in extremely few cases (Fig. 2J), revealing that VirD4 assembles at the membrane during late stationary phase and even more so during lag phase (when early stationary phase cells are resuspended in fresh medium, VirD4-GFP also assembles within 60 to 120 min, like VirB4-GFP), but it disassembles as cells enter exponential growth. These data suggest that the entire conjugation machinery or at least the central part of it may assemble and disassemble at a defined subcellular site within stationary/lag-phase cells.
To test for the specificity of localization, we expressed VirB4-YFP or VirD4-CFP from ectopic loci on the chromosome in B. subtilis cells lacking pLS20. In both cases, homogeneous cytosolic fluorescence was observed throughout all growth phases (Fig. 4A and B, respectively), and no membrane-attached foci were detectable even in lag-phase cells (under low or high inducer concentrations). As a control, both ectopically induced fusions were induced in cells carrying pLS20. In both cases, conjugation proteins localized in lag-phase cells and no longer in exponentially growing cells (see Fig. S4 in the supplemental material). Thus, VirB4 and VirD4 require at least a part of the pLS20 conjugation machinery to obtain their specific localization in lag-phase cells. These data reinforce the idea that two central components of the conjugation machinery specifically assemble at the membrane to mediate conjugation during the early growth phase.
Fig. 4.
Colocalization of VirB4 and VirD4 and their interaction. (A) VirB4-YFP expressed from the amylase locus during lag phase in the absence of pLS20. (B) VirD4-CFP expressed from the thr locus during lag phase in the absence of pLS20. (C) Cells expressing VirB4-mCherry from the original gene locus on pLS20 and VirD4-CFP from the thr locus during lag phase. White arrowheads indicate colocalization of VirD4 and VirB4. (D to H) BiFC experiments, with VirD4-Yn expressed during lag phase (D), VirB4-Yc and VirD4-Yn expressed during lag phase (white lines indicate septa between cells) (E), VirB4-Yc and VirD4-Yn expressed during mid-exponential phase (F), VirB4-Yc and MreC-Yn expressed during lag phase (G), and VirB4-Yc and DnaX-Yn expressed during lag phase (H). White arrowheads indicate small cells with a BiFC focus. (I) VirD2-CFP expressed during lag phase. (J and K) VirD2-CFP during early stationary phase (10 h after inoculation) (J) or VirD2-CFP during early exponential phase (K). White arrowheads indicate single cells containing a VirD2-CFP focus; gray arrowheads indicate large cells lacking VirD2-CFP signals. (L) Cells expressing truncated VirD2-GFP. Bars, 2 μm.
VirB4 and VirD4 colocalize and interact at defined sites at the membrane.
In order to investigate if VirB4 and VirD4 localize to the same site(s), we expressed an mCherry fusion of VirB4 from its original locus on pLS20 and VirD4-CFP from an ectopic locus on the chromosome. VirD4-CFP was expressed at a low inducer concentration. With full induction, polar foci were not detectable, due to high background fluorescence. During lag phase and until early exponential phase (3 h after inoculation), VirB4-mCherry and VirD4-GFP foci were observed, 80% of which colocalized at a single cell pole or at other sites along the cell membrane or within the cell (Fig. 4C), indicating that VirB4 and VirD4 assemble at the same site(s) within cells during lag phase. To test if both proteins form a functional complex, we employed BiFC. The N-terminal part of YFP was fused to VirD4, and the C-terminal part was fused to VirB4, both expressed from their original locus on pLS20. Each construct introduced into pLS20 by itself did not show any fluorescence (Fig. 4D and data not shown), but when expressed together, clear foci were detectable, mostly close to the cell membrane and at a cell pole, in lag-phase cells (Fig. 4E). Mid-exponential-phase growing cells did not show any foci; only a few small cells showed a fluorescent focus (Fig. 4F). During early stationary phase, no foci were detectable (data not shown). As controls, we performed BiFC with VirB4-Yc and DnaX-Yn, but no foci were found even during lag phase (Fig. 4H). We also used the membrane protein MreC as a control, because MreC localizes at many positions along the membrane and also to the cell poles (13). However, no BiFC foci were detected (Fig. 4G), showing that VirB4 and VirD4 specifically interact at single defined sites at the cell membrane in lag-phase cells, suggesting that they functionally interact in the conjugation apparatus.
VirD2, VirB1, and VirB11 also localize to the membrane in a growth phase-dependent manner, but with different patterns.
The finding that most lag-phase cells contain a polarly localized or membrane-localized plasmid copy suggested that the entire conjugation machinery may be present at these sites, such that ssDNA can be directly threaded into the export channel. If this were true, we would expect the VirD2 relaxase protein to also be present at this site, although it was possible that the two theoretical proteins required might not be resolved against background fluorescence. However, we clearly observed VirD2-CFP foci at a single cell pole or at other positions in early-lag-phase cells (Fig. 4I). During mid-exponential phase, foci were only observed in the few remaining single cells, while in cell chains, only faint diffuse fluorescence was detectable (Fig. 4K). Thus, like VirB4 and VirD4, the relaxase is present at the presumed sites of transfer at the membrane only during commencement of growth. In contrast to VirB4 and VirD4, VirD2-CFP foci were again detectable in early-stationary-phase cells (10 h) (Fig. 4J), indicating that early steps in conjugation may already commence at this time point, while the entire machinery assembles at a later time point (24 h). The C-terminal region of A. tumefaciens VirD2 has been shown to be important for efficient DNA transfer and for interaction with the type IV secretion system (37). We tested whether this also holds true for B. subtilis by integrating a truncated GFP fusion into the original virD2 locus. Interestingly, truncated VirD2-GFP still localized to the pole or to the lateral membrane (Fig. 4L), indistinguishably from wild-type VirD2, and the conjugation frequency of pLS20 carrying truncated VirD2 was not considerably affected (Table 2), showing that the mode of action of pLS20 VirD2 differs markedly from that of the Gram-negative system.
The third essential ATPase motif protein, VirB11, showed a different localization pattern. The fusion protein started to accumulate as discrete foci at the cell pole and at the lateral membrane in lag-phase cells, 1 h after inoculation (Fig. 5A). These foci became brighter, and 2 h after inoculation, VirB11-YFP formed additional irregular foci along the entire cell membrane (Fig. 5B). As cells entered exponential growth (3 h after inoculation), VirB11-YFP foci localized in a punctate helical pattern throughout the membrane and also at the newly formed septum (Fig. 5C) (showing that cells had commenced and completed a cell cycle), where other Vir proteins are rarely observed (Fig. 2 and 4). During this early exponential phase, most cells showed a focus or a gradient accumulation of VirB11-YFP at a single cell pole (Fig. 5C). In many cells, a helical pattern of VirB11-YFP foci was apparent. In mid-exponential growth, 20% of all cells exclusively showed the helical pattern of localization (but no more polar foci), which disappeared during late exponential phase (Fig. 3C). Thus, VirB11 also accumulates at the presumed conjugation site during lag phase and also throughout the membrane during exponential growth, when the above-mentioned conjugation proteins have already disassembled. To find out if VirB11 diffuses through the cell membrane or is somewhat anchored at its specific sites, we performed FRAP (fluorescence recovery after photobleaching) analysis. In contrast to the membrane proteins ATPase and succinate dehydrogenase from B. subtilis, which show half-time recoveries of 3 to 4 min (23), VirB11-YFP fluorescence did not markedly recover over a 35-min period (Fig. 5D). These data show that VirB11 is somewhat statically positioned within the membrane and does not show considerable diffusion rates.
Fig. 5.
Localization of VirB11 and VirB1. (A) VirB11-YFP 1 h after inoculation. (B) VirB11-YFP 2 h after inoculation. (C) VirB11-YFP 3 h after inoculation. (D) FRAP analysis of VirB11-YFP during early exponential phase. The dashed circle marks the area of bleaching, and numbers in top right corners of panels indicate minutes after bleaching. (E) Simultaneous visualization of VirB11-YFP and DnaX-mCherry in cells 4 h after inoculation (mid-log phase). White triangles indicate cells containing DnaX-mCherry foci and VirB11-YFP foci. (F) VirB1-mCherry 2 h after inoculation. (G) VirB1-mCherry 4 h after inoculation (mid-exponential phase). White lines indicate septa between cells. (H) Colocalization of VirB11-YFP and VirB1-mCherry (several sites are indicated by white triangles). Please note that there was no bleeding of YFP into the mCherry channel, and vice versa (data not shown). Bars, 2 μm.
VirB1 is a protein that is assumed to be associated with the cell wall. It has a predicted transmembrane helix at its N terminus, so the protein including its C terminus are supposedly outside the cell. Due to this fact, we fused VirB1 to mCherry, which in contrast to GFP variants can be transported across the membrane. Strikingly, VirB1 accumulated at the cell poles and at septa but also showed faint and homogeneous localization along the lateral sides of the cells during lag phase (Fig. 5F). However, accumulation at the poles was only observed for a subset of the cells (about 10%), indicating that conjugation employing VirB1 occurs only in a minority of the cells. Different from most other investigated conjugation proteins, and similar to VirB11, VirB1-mCherry still localized in cells during mid-exponential phase (Fig. 5G).
We also succeeded in generating a strain expressing VirB11-YFP and VirB1-mCherry, which is functional (several other dual labels were not functional and/or were mislocalized). Figure 5H shows that both signals frequently colocalized (in about 75% of the cells having either signal), and in the remaining cells, either one of the constructs was visible at a position where the other was not present. Therefore, VirB11 and VirB1 are present largely at the same position, like VirB4 and VirD4, suggesting that the conjugation machinery coassembles at the same subcellular sites.
Synthesis of VirB4 is temporally regulated.
As mentioned above, VirB4 and VirD4 coassembled at the membrane only in cells resuming growth, but not during exponential growth or early stationary phase. This was also true for VirB1 and VirB11 (Fig. 5). VirD2 accumulates in early stationary phase and during lag phase, but not during exponential growth. The assembly of the entire machinery completely coincides with conjugation activity (Fig. 3A to C), suggesting that conjugation is driven through assembly of the machinery during transition from nongrowth to growth. In this respect, it is interesting to note that PY79 cells carrying pLS20 have a reproducibly longer lag phase than cells lacking the plasmid (see Fig. S3 in the supplemental material), which was most noticeable for cells growing at 30°C. These data suggest that pLS20 alters the physiology of the host cells during the transition from the stationary phase to growth, extending the period in which it can be transferred into recipient cells.
We wanted to analyze if VirB4 assembles and disassembles due to induction and arrest of protein synthesis, and so we performed Western blot analysis with a VirB4-TAP tag fusion (GFP antiserum resulted in high background in cells carrying pLS20, precluding analysis of all generated FP fusions) that was expressed from the original virB4 locus. Figure 3D shows that VirB4-TAP was undetectable in stationary-phase cells but was rapidly induced upon resuspension in fresh medium. VirB4 disappeared during mid-exponential phase. Thus, VirB4 is synthesized at the time of assembly but disappears later than the time of disassembly of VirB4-YFP foci. VirD4-GFP was detectable throughout the cells during mid-exponential growth based on homogeneous cytosolic staining, and VirB11-YFP still localized to the membrane in a punctate helical pattern during mid-exponential phase, suggesting that also in these cases, disassembly of the conjugation machinery is not driven by the degradation of these components. It will be interesting to identify the factor(s) driving the unusual behavior of pLS20.
DISCUSSION
Our work provides evidence that the conjugation machinery in the Gram-positive model organism B. subtilis assembles at a single site at the membrane, frequently at the cell pole. We found that the pLS20 plasmid as well as VirD2, VirB4, VirD4, and VirB1 localize at the pole, in cells during extended stationary phase, and in an even more pronounced fashion in cells entering growth. VirB11 protein is present at many sites along the cell membrane and is also frequently concentrated at a single cell pole. Using BiFC, we showed that VirD4 and VirB4 functionally interact, suggesting that the type IV secretion system-like apparatus operates from a single defined subcellular site. However, VirD2, VirB4, and VirD4 no longer localized in cells that had commenced exponential growth, although they appeared to be continued to be synthesized. The assembly and disassembly of the machinery occur in parallel with the induction and decline of conjugation activity of pLS20 (22). When cells from early stationary phase, in which all monitored conjugation proteins are delocalized except for VirD2, are resuspended into fresh medium, the conjugation machinery assembles within as little as 60 min and is functional, revealing rapid assembly kinetics. Thus, our work provides striking evidence for a machinery that commences to assemble in non-exponentially growing cells, to become active during the transition to growth, and is then rendered inactive as an active cell cycle becomes operative.
In agreement with these findings, we found that pLS20 is mostly membrane associated and frequently found at the cell pole. Cells contain on average 3 to 4 plasmids during exponential growth, but only 1 to 2 during stationary phase. It is likely that one plasmid is present at the conjugation machinery, such that conjugation can occur very rapidly. Indeed, the 65-kb plasmid can conjugate in as little as 2 min (see Fig. S2 in the supplemental material). Corroborating the identification of an entirely novel replication mechanism, we found that pLS20 rarely colocalized with the chromosomal replication machinery. PLS20 codes for several replication proteins (a primase, a ligase, and an SSB paralog) and may use only a few components of the host replication setup. Interestingly, we found that polar pLS20 was generally statically located at its position while nonpolar plasmids were mobile, suggesting that the conjugation machinery stably anchors the plasmid, even in cells that are not actively conjugating (i.e., in the absence of recipient cells).
Our work also provides evidence for different patterns of localization of Vir proteins, as summarized in Table 3. VirB11, the third ATPase motif protein besides VirB4 and VirD4, accumulated at the cell pole soon after resuspension of stationary-phase cells into fresh medium and later during exponential growth localized to many discrete foci along the entire cell membrane, although a higher concentration was seen toward a single cell pole. The putative cell wall hydrolase VirB1 was present at the entire surface of the cell pole, while VirB4, VirD2, and VirD4 formed discrete foci. Our findings may reconcile data from A. tumefaciens and other bacteria in which it has also been reported that some proteins localized all along the lateral cell membrane (2) while others were only present at the cell pole (3, 28). It is interesting that the VirB4 component of a conjugative transposon from B. subtilis also localizes to the cell poles (6), indicating that transposon conjugation may also occur at the cell pole. Our data suggest that the pLS20 conjugation machinery is composed of a core machinery and different modules that are also present at other places in the cell, where they are nonactive. Core proteins VirB1, VirB4, and VirD4 have also been identified on another conjugative plasmid in a Gram-positive bacterium, and they also interact with each other (1), suggesting that many components of and connections within the conjugation machineries are conserved. PLS20 VirB11 protein did not diffuse through the cell membrane but showed a rather static arrangement. These data suggest that at least VirB11 is anchored by an unknown factor and/or is attached to the cell membrane.
Table 3.
Patterns of localization of Vir proteins: localization at the pole and membrane as foci
| Protein | Localizationa during growth phase |
|||
|---|---|---|---|---|
| Lag | Mid-exponential | Early stationary | Late stationary | |
| VirB4 | × | − | − | × |
| VirD4 | × | − | − | × |
| VirB11 | × | (×) (helices) | − | − |
| VirD2 | × | − | × | + |
| VirB1b | × | × | − | − |
×, present; −, absent.
Did not form discrete foci.
We asked ourselves why a conjugative plasmid may regulate its transfer to another cell at a time when there are fewer siblings around (dilution into fresh medium) and not when cell density is high and many recipient cells are available (i.e., the time point when competence is induced). We speculate that a large plasmid that requires at least 2 min for transfer (see Fig. S2 in the supplemental material) and has an active partitioning system may distribute to recipients early during growth, when nutrients are readily available. During growth, it is efficiently propagated through its active mitotic-like segregation machinery and has no need for conjugation. It is also possible that the cell wall becomes amenable for the assembly of the complex when it commences to remodel and extend, while stationary-phase cells are known for their increased robustness and changes in peptidoglycan architecture, which may hinder conjugation. Also, B. subtilis cells grow as predominantly nonmotile chains of cells, facilitating cell/cell contact, but become highly motile when they enter stationary phase.
Intriguingly, we found that cells carrying pLS20 have an extended lag phase. PLS20-carrying cells commenced exponential growth considerably later than plasmid-free cells, while the doubling times of both strains were indistinguishable. These findings indicate that the presence of the plasmid may alter the response of cells to the addition of fresh medium. This way, the plasmid may increase the time to find a suitable recipient cell. It will be interesting to determine if the plasmid interferes with the physiology of the host cell simply by being a metabolic burden or by changing the activity of host proteins or the transcription of host genes.
It will be also illuminating to identify the trigger that induces the assembly of the conjugation machinery and the signal(s) that leads to disassembly. Interestingly, we found that VirD2 accumulates at the cell pole in early-stationary-phase cells, while VirD4 and VirB4 assemble about 12 h later, during extended stationary phase. These findings suggest that early steps in conjugation are induced as cells exit growth and that the core conjugation machinery is set up during continued stationary phase. The entire machinery then appears to be set up as cells gain a new nutritional supply and the incentive to commence growth. Thus, the Gram-positive conjugation machinery appears to be built in a somewhat hierarchical order. It will also be important to identify the factors that induce membrane targeting of VirD4 and VirB4. On their own, they localize throughout the cytosol and, therefore, are apparently soluble proteins, although VirD4 contains 2 predicted transmembrane helices at its N terminus. Most of the proteins we have analyzed in this study appear to be synthesized from early growth into stationary phase, so there appears to be an assembly factor for the machinery that needs to be identified.
Supplementary Material
ACKNOWLEDGMENTS
We thank Shinya Kaneko, Tokyo Institute of Technology, Nagatsuta, Yokohama-shi Kanagawa, Japan, for providing pLS20neo before publication and Stefanie Welling for providing the DnaX-Yc construct.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 746) and the SGBM Freiburg.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 23 September 2011.
REFERENCES
- 1. Abajy M. Y., et al. 2007. A type IV secretion-like system is required for conjugative DNA transport of broad-host-range plasmid pIP501 in Gram-positive bacteria. J. Bacteriol. 189:2487–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aguilar J., Zupan J., Cameron T. A., Zambryski P. C. 2010. Agrobacterium type IV secretion system and its substrates form helical arrays around the circumference of virulence-induced cells. Proc. Natl. Acad. Sci. U. S. A. 107:3758–3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atmakuri K., Cascales E., Burton O. T., Banta L. M., Christie P. J. 2007. Agrobacterium ParA/MinD-like VirC1 spatially coordinates early conjugative DNA transfer reactions. EMBO J. 26:2540–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Atmakuri K., Cascales E., Christie P. J. 2004. Energetic components VirD4, VirB11 and VirB4 mediate early DNA transfer reactions required for bacterial type IV secretion. Mol. Microbiol. 54:1199–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Atmakuri K., Ding Z., Christie P. J. 2003. VirE2, a type IV secretion substrate, interacts with the VirD4 transfer protein at cell poles of Agrobacterium tumefaciens. Mol. Microbiol. 49:1699–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berkmen M. B., Lee C. A., Loveday E. K., Grossman A. D. 2010. Polar positioning of a conjugation protein from the integrative and conjugative element ICEBs1 of Bacillus subtilis. J. Bacteriol. 192:38–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cascales E., Christie P. J. 2004. Definition of a bacterial type IV secretion pathway for a DNA substrate. Science 304:1170–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cascales E., Christie P. J. 2003. The versatile bacterial type IV secretion systems. Nat. Rev. Microbiol. 1:137–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen I., Dubnau D. 2004. DNA uptake during bacterial transformation. Nat. Rev. Microbiol. 2:241–249 [DOI] [PubMed] [Google Scholar]
- 10. Christie P. J. 1997. Agrobacterium tumefaciens T-complex transport apparatus: a paradigm for a new family of multifunctional transporters in eubacteria. J. Bacteriol. 179:3085–3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Christie P. J., Atmakuri K., Krishnamoorthy V., Jakubowski S., Cascales E. 2005. Biogenesis, architecture, and function of bacterial type IV secretion systems. Annu. Rev. Microbiol. 59:451–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Antonio C., Farias M. E., de Lacoba M. G., Espinosa M. 2004. Features of the plasmid pMV158-encoded MobM, a protein involved in its mobilization. J. Mol. Biol. 335:733–743 [DOI] [PubMed] [Google Scholar]
- 13. Defeu Soufo H. J., Graumann P. L. 2006. Dynamic localization and interaction with other Bacillus subtilis actin-like proteins are important for the function of MreB. Mol. Microbiol. 62:1340–1356 [DOI] [PubMed] [Google Scholar]
- 14. Defeu Soufo C., et al. 2008. Cell-cycle-dependent spatial sequestration of the DnaA replication initiator protein in Bacillus subtilis. Dev. Cell 15:935–941 [DOI] [PubMed] [Google Scholar]
- 15. de la Cruz F., Frost L. S., Meyer R. J., Zechner E. L. 2010. Conjugative DNA metabolism in Gram-negative bacteria. FEMS Microbiol. Rev. 34:18–40 [DOI] [PubMed] [Google Scholar]
- 16. Derman A. I., et al. 2009. Phylogenetic analysis identifies many uncharacterized actin-like proteins (Alps) in bacteria: regulated polymerization, dynamic instability and treadmilling in Alp7A. Mol. Microbiol. 73:534–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Francia M. V., et al. 2004. A classification scheme for mobilization regions of bacterial plasmids. FEMS Microbiol. Rev. 28:79–100 [DOI] [PubMed] [Google Scholar]
- 18. Garcillan-Barcia M. P., Francia M. V., de la Cruz F. 2009. The diversity of conjugative relaxases and its application in plasmid classification. FEMS Microbiol. Rev. 33:657–687 [DOI] [PubMed] [Google Scholar]
- 19. Gilmour M. W., Gunton J. E., Lawley T. D., Taylor D. E. 2003. Interaction between the IncHI1 plasmid R27 coupling protein and type IV secretion system: TraG associates with the coiled-coil mating pair formation protein TrhB. Mol. Microbiol. 49:105–116 [DOI] [PubMed] [Google Scholar]
- 20. Gomis-Ruth F. X., et al. 2001. The bacterial conjugation protein TrwB resembles ring helicases and F1-ATPase. Nature 409:637–641 [DOI] [PubMed] [Google Scholar]
- 21. Grohmann E., Muth G., Espinosa M. 2003. Conjugative plasmid transfer in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 67:277–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Itaya M., Sakaya N., Matsunaga S., Fujita K., Kaneko S. 2006. Conjugational transfer kinetics of pLS20 between Bacillus subtilis in liquid medium. Biosci. Biotechnol. Biochem. 70:740–742 [DOI] [PubMed] [Google Scholar]
- 23. Johnson A. S., van Horck S., Lewis P. J. 2004. Dynamic localization of membrane proteins in Bacillus subtilis. Microbiology 150:2815–2824 [DOI] [PubMed] [Google Scholar]
- 24. Kearns D. B., Losick R. 2005. Cell population heterogeneity during growth of Bacillus subtilis. Genes Dev. 19:3083–3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kidane D., Sanchez H., Alonso J. C., Graumann P. L. 2004. Visualization of DNA double-strand break repair in live bacteria reveals dynamic recruitment of Bacillus subtilis RecF, RecO and RecN proteins to distinct sites on the nucleoids. Mol. Microbiol. 52:1627–1639 [DOI] [PubMed] [Google Scholar]
- 26. Koehler T. M., Thorne C. B. 1987. Bacillus subtilis (natto) plasmid pLS20 mediates interspecies plasmid transfer. J. Bacteriol. 169:5271–5278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kopec J., Bergmann A., Fritz G., Grohmann E., Keller W. 2005. TraA and its N-terminal relaxase domain of the Gram-positive plasmid pIP501 show specific oriT binding and behave as dimers in solution. Biochem. J. 387:401–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kumar R. B., Das A. 2002. Polar location and functional domains of the Agrobacterium tumefaciens DNA transfer protein VirD4. Mol. Microbiol. 43:1523–1532 [DOI] [PubMed] [Google Scholar]
- 29. Kuroki A., Ohtani N., Tsuge K., Tomita M., Itaya M. 2007. Conjugational transfer system to shuttle giant DNA cloned by Bacillus subtilis genome (BGM) vector. Gene 399:72–80 [DOI] [PubMed] [Google Scholar]
- 30. Llosa M., Zunzunegui S., de la Cruz F. 2003. Conjugative coupling proteins interact with cognate and heterologous VirB10-like proteins while exhibiting specificity for cognate relaxosomes. Proc. Natl. Acad. Sci. U. S. A. 100:10465–10470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meijer W. J., de Boer A. J., van Tongeren S., Venema G., Bron S. 1995. Characterization of the replication region of the Bacillus subtilis plasmid pLS20: a novel type of replicon. Nucleic Acids Res. 23:3214–3223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pansegrau W., Lanka E. 1996. Enzymology of DNA transfer by conjugative mechanisms. Prog. Nucleic Acid Res. Mol. Biol. 54:197–251 [DOI] [PubMed] [Google Scholar]
- 33. Savvides S. N., et al. 2003. VirB11 ATPases are dynamic hexameric assemblies: new insights into bacterial type IV secretion. EMBO J. 22:1969–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schroder G., et al. 2002. TraG-like proteins of DNA transfer systems and of the Helicobacter pylori type IV secretion system: inner membrane gate for exported substrates? J. Bacteriol. 184:2767–2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Szpirer C. Y., Faelen M., Couturier M. 2000. Interaction between the RP4 coupling protein TraG and the pBHR1 mobilization protein Mob. Mol. Microbiol. 37:1283–1292 [DOI] [PubMed] [Google Scholar]
- 36. Tanaka T., Koshikawa T. 1977. Isolation and characterization of four types of plasmids from Bacillus subtilis (natto). J. Bacteriol. 131:699–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Kregten M., Lindhout B. I., Hooykaas P. J., J. van der Zaal B. 2009. Agrobacterium-mediated T-DNA transfer and integration by minimal VirD2 consisting of the relaxase domain and a type IV secretion system. translocation signal. Mol. Plant Microbe Interact. 22:1356–1365 [DOI] [PubMed] [Google Scholar]
- 38. Ward D. V., Draper O., Zupan J. R., Zambryski P. C. 2002. Peptide linkage mapping of the Agrobacterium tumefaciens vir-encoded type IV secretion system reveals protein subassemblies. Proc. Natl. Acad. Sci. U. S. A. 99:11493–11500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Webb C. D., et al. 1998. Use of time-lapse microscopy to visualize rapid movement of the replication origin region of the chromosome during the cell cycle in Bacillus subtilis. Mol. Microbiol. 28:883–892 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.