Abstract
During development pancreatic endocrine cells migrate in a coordinated fashion. This migration is necessary to form fully functional islets, but the mechanisms involved remain unknown. Therapeutic strategies to restore β-cell mass and islet functionality by reprogramming endogenous exocrine cells would be strengthened from simultaneous treatments that enhance endocrine cell clustering. We found that endocrine progenitors respond to and regulate G protein-coupled receptor (GPCR) signaling in order to cluster in islets. Rgs4, a dedicated regulator of GPCR signaling, was specifically expressed in early epithelial endocrine progenitors of both zebrafish and mouse, and its expression in the mouse endocrine progenitors was strictly dependent upon Ngn3, the key specification gene of the endocrine lineage. Rgs4 loss of function resulted in defects in islet cell aggregation. By genetically inactivating Gαi-mediated GPCR signaling in endocrine progenitors, we established its role in islet cell aggregation in both mouse and zebrafish. Finally, we identified sphingosine-1-phosphate (S1P) as a ligand mediating islet cell aggregation in both species acting through distinct but closely related receptors.
INTRODUCTION
During vertebrate development, progenitor cells of different cell lineages are often specified at great distances away from their final locations in the developing body and therefore need to migrate away from their birthplace to reach their final destinations and assemble functional units. In the vertebrate pancreas, coordinated directional migration of the endocrine cells is crucial for the formation of fully functional islets. Maturation of β cells is associated with reciprocal signaling, and hormonal secretion into the bloodstream becomes less efficient if endocrine cells are distributed in small clusters (27, 31). The elucidation of the genetic networks in pancreas morphogenesis could contribute to the efficient generation of β cells from embryonic and induced pluripotent stem (ES and iPS, respectively) cells and also to the stimulation of islet clustering after in vivo reprogramming of exocrine cells to endocrine cells (28, 53, 70, 72).
Pancreas development has been extensively studied in zebrafish and mouse. Despite differences between the two species, the role of key signaling pathways and transcription factors is conserved (26). In zebrafish, primary endocrine cells are specified and migrate exclusively as single cells. They cluster and form a single embryonic islet at the position of the developing dorsal bud (1, 8, 26). In mouse, the pancreatic epithelium proliferates and expands into the surrounding mesenchyme by extensive branching morphogenesis and tubulogenesis (20, 24, 63). Epithelial cells differentiate into Ngn3+ endocrine progenitors that undergo epithelial to mesenchymal transition and migrate into the mesenchyme to form the vascularized islets (3, 10, 49). Coordinated migration of the endocrine cells is crucial for the formation of fully functional islets (27), but very little is known about the regulation of this process.
In the mouse, the basic helix-loop-helix transcription factor Ngn3 is necessary and sufficient for the induction of the full spectrum of pancreas endocrine cell fates (11, 12, 14, 19, 52). The Ngn3-mediated endocrine program includes regulation of transcription factors such as NeuroD (17), Nkx2-2 (66), Pax4 (56), IA1 (9, 35), Atoh8 (32), MyT1 (65), and Rfx6 (57, 58). It has been suggested that Ngn3 also regulates the delamination and migratory response of mouse endocrine progenitors (10, 46), but the downstream mediators remain elusive. Using ES cell-derived pancreas progenitors, we found that expression of the regulator of G protein signaling 4 (RGS4), a Gαi/o GTPase-activating protein (GAP) that potently inhibits signaling through Gαi/o (15, 67), depended upon Ngn3 (53). RGS proteins are exclusive components of G protein-coupled receptor (GPCR) signaling and exert their effects by enhancing the intrinsic GTPase activity of activated GTP-bound Gα subunits, thereby decreasing the duration of GPCR signaling in diverse processes (47). In the mature pancreas, GPCR signaling plays an important role in the regulation of normal β-cell function (43, 48), and there is some evidence implicating it in cell fate specification during pancreas development (40, 41).
Here, we show that Rgs4 is expressed in endocrine progenitors of both zebrafish and mouse, that its expression in the mouse pancreatic epithelium is strictly dependent upon Ngn3, and that loss of function of Rgs4 results in islet fragmentation in both organisms. Furthermore, we show that disruption of Gαi-mediated GPCR signaling in endocrine progenitors results in stronger, severe islet clustering defects, and we provide evidence that implicates S1P signaling in this process in both zebrafish and mouse. These data demonstrate that S1P and GPCR signaling play a phylogenetically conserved role in endocrine pancreas morphogenesis.
MATERIALS AND METHODS
Animal strains.
Animal studies were conducted in accordance with international guidelines and after ethical approval of the competent Veterinary Service of Athens. Zebrafish transgenic lines were the Tg(ins::dsRed m1018) (8) and Tg(gut::gfp s854) (54) lines. Mouse mutant and transgenic strains were the Ngn3 strain (11), the Rgs4tm1Dgen strain from Deltagen, Gt(ROSA)26Sortm1(ptxA)Cgh from the Mutant Mouse Regional Recource Center (MMRRC), and Tg(Neurog3-cre)C1Able/J from JAX Mice. Mouse genotyping procedures were as described for Ngn3 (11), Rgs4 (http://jaxmice.jax.org/strain/005833.html), Rosa26-PTX (http://www.mmrrc.org/strains/30678/030678.html), and Ngn3-Cre (http://jaxmice.jax.org/strain/006333.html).
Zebrafish transgenesis and morpholinos.
For the rgs4::gfp transgene, a 3,765-bp fragment of the zebrafish rgs4 gene immediately upstream of the ATG was cloned upstream of enhanced green fluorescent protein (EGFP) in pEGFP-N1 (Clontech). Fifty picograms of the rgs4::gfp fragment in 4.6 nl was injected per egg. For the ins::ptx transgene, a 1,385-bp fragment upstream of the insulin ATG was cloned in pSG5 (Stratagene), replacing the simian virus 40 (SV40)/β globin promoter. The PTX cDNA (745 bp) was PCR amplified from PTX-nos1-3′UTR (where UTR is untranslated region; gift of E. Raz), introducing a stop codon at the 3′ end, and cloned downstream of the insulin (ins) promoter. A total of 150 pg of linearized plasmid in 4.6 nl was injected per egg. Knockdown of rgs4 was achieved with two morpholinos (Gene Tools): M1Rgs4 (5′-AAGCCCTTTACACATGTTGCTGATG-3′), which blocked translation, and M2Rgs4 (5′-TATTCTGGACCAGTCTTACCTGTTG-3′), which blocked splicing. Both were injected at 25 ng in 4.6 nl per egg. Three unrelated morpholinos injected at the same concentration were used as controls.
Zebrafish in situ hybridization and immunofluorescence.
Whole-mount and double fluorescent in situ hybridizations were performed according to standard protocols (21). Antisense RNA probes were used for ins (gift from A. Mayer), rgs4 (Zfin; cb436), neuroD (gift from M. Voz), sox17 and gata6 (gifts from S. Sumanas), foxA2 (gift from S. Dougan), and foxA3 (gift from L. Zhen). Primary antibodies were mouse anti-islet-1 (anti-isl1; 1:100; DSHB), mouse antiglucagon (anti-glg; 1:500; Sigma), rabbit antisomatostatin (anti-som; 1:250; Zymed), and rabbit anti-GFP (1:1,000; Molecular Probes). Secondary antibodies were Alexa 488- or Alexa 568-conjugated goat antibodies (1:500; Molecular Probes). Twenty-five to 30 zebrafish embryos were stained with each probe/stage/treatment. For treatments with chemicals, at least 40 embryos were stained.
Time-lapse imaging and cell tracking.
Live, 16-somite-stage zebrafish embryos were dechorionated, embedded in 0.8% low-melting-point agarose (Sigma), and overlaid with mineral oil and a coverslip. Images were captured every minute for 6 to 7 h under a confocal microscope (Leica SP5) with a rotating base and exported into ImageJ (http://rsb.info.nih.gov/ij/). The two-dimensional (2D) coordinates of clearly defined cells were recorded every 18 to 20 frames using the MTrackJ plug-in (http://rsb.info.nih.gov/ij/) and exported into Excel.
Zebrafish drug treatments.
Drugs were added at the 5-somite stage (11 h postfertilization [hpf]) after chorions were slightly ruptured. VPC23019 (Avanti Lipids) was added at 100 μM, JTE013 (Tocris) at up to 100 μM, AMD3100 (Sigma) at up to 100 μg/ml, retinoic acid (RA) (Sigma) at 1 μM, S1P2 agonist (S1P2Ag) (Enamine) at up to 10 μM, and FTY720-P (Cayman Chemical) at 10 μM. All treatments were repeated independently three times.
Reverse transcription-PCRs (RT-PCRs) and Western blotting.
RNA isolation, oligonucleotide design, PCRs, protein extraction, and Western blotting were performed according to standard procedures. The following primers were used (annealing temperature and program): Ngn3, TGGCGCCTCATCCCTTGGATG and CAGTCACCCACTTCTGCTTCG (58°C, 35 cycles); Rgs4, GTCGGAATACAGCGAGGAGAAC and GGAAGGATTGGTCAGGTCAAGATAG (55°C, 35 cycles); β-actin, ATGGATGACGATATCGCTGCGC and TCTGTCAGGTCCCGGCCA (60°C, 25 cycles). Primary antibodies were rabbit anti-Ngn3 (1:400; gift from H. Edlund), rabbit anti-Rgs4 (1:5,000; Abcam), and mouse anti-β-actin (1:5,000; Santa Cruz). Secondary antibodies were anti-rabbit and anti-mouse horseradish peroxidase (HRP)-conjugated goat antibodies (1:5,000; Dako).
Mouse embryo pancreas dissections and immunofluorescence.
Mouse pancreata were cryosectioned at 12 μm using standard procedures. Primary antibodies used were mouse anti-β-galactosidase (1:250; Promega), rabbit anti-Pdx1 (1:5,000; gift from C. Wright), rabbit anti-Ngn3 (1:200; gift from H. Edlund), rat anti-E-cadherin (1:400; Zymed), rabbit anti-Rfx6 (1:500; gift from G. Gradwohl), rabbit anti-C-peptide (1:200; Linco), rat anti-cytokeratin 19 (anti-CK-19; 1:250; DSHB), mouse anti-insulin (1:1,000; Sigma), mouse antiglucagon (1:500; Sigma), rabbit anti-phospho-H3 (1:500; Cell Signaling), and rat anti-platelet endothelial cell adhesion molecule 1 (anti-PECAM-1; 1:50; Pharmingen). Secondary antibodies were anti-mouse, anti-rabbit, and anti-rat Alexa-488-, Alexa-633-, and Alexa-568-conjugated goat antibodies (1:500; Molecular Probes).
Organotypic cultures of embryonic pancreata.
Dorsal pancreatic buds were dissected and cultured for 6 days on 0.4-μm-diameter filters (Millicell-CM; Millipore) on Dulbecco's modified Eagle's medium (DMEM; Gibco), 1× N2 supplement (Gibco), and 1× penicillin-streptomycin-glutamine (Gibco). Treatments were with pertussis toxin (PTX; Calbiochem) at 5 μg/ml, JTE013 (Tocris) at 20 μM, VPC23019 (Avanti Lipids) at up to 250 μM, AMD3100 (Sigma) at up to 100 μg/ml, FTY720-P (Cayman Chemical) at up to 10 μM, and S1P2Ag (Enamine) at 5 μM. Chemicals were replenished with each medium change every second day. Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assays using a fluorescent cell death detection kit (Roche) showed that these treatments did not induce cell death as staining was very low and similar to that of untreated controls.
Morphometric and statistical analysis of islet morphogenesis.
For all morphometric analyses of embryonic and newborn pancreata, 12-μm-thick cryosections at least 50 μm apart were used so that any given endocrine cell cluster or islet was scored only once. Three different pancreata and four sections from each pancreas were used for each condition and stage. Endocrine specification and clustering of pancreatic explants at 14.5 days postcoitus (dpc) were remarkably consistent among controls or similarly treated pancreata.
To determine endocrine specification and clustering, image analysis data were acquired using fixed camera settings and exported into ImageJ. For endocrine specification, cellular contours were calculated using the “analyze particles” tool with standard thresholds across samples, and the total pixels of the marked signal areas were calculated. The signal area of C-peptide-positive(C-peptide+) and glucagon-positive (glucagon+) immunofluorescence was calculated separately, added, divided by the corresponding total signal area for 4′,6′-diamidino-2-phenylindole (DAPI), and expressed as percent endocrine specification, thus normalizing for pancreatic mass. To analyze the degree of endocrine cell (C-peptide+ immunofluorescence) clustering, cluster contours were calculated using a plug-in developed for ImageJ cell sorting (S. Pagakis, personal communication) with standard thresholds across samples, and the total area of each cluster was recorded in pixels. Settings were chosen so that single cells would be included in the analysis while noise signal would be excluded. The list of the clusters with their corresponding sizes was exported into Excel. Clusters falling within a certain range of sizes were grouped, their total signal area was calculated, and the contribution of that group as a percentage of the total signal area of all clusters was plotted as a function of the size range to give rise to the cluster size distribution graph. The window of the size range was kept constant. To get a smooth distribution graph, consecutive groups were overlapping by a half-size-range window.
To determine islet association with ducts, postnatal day 1 (P1) pancreata from three wild-type (wt) and three Rgs4−/− newborns were used, and a total of 85 and 83 islets, respectively, were scored. To guard against bias, section photos were assigned numbers randomly and scored blindly. Islets were defined as C-peptide+ cellular aggregates of at least 40-μm diameter. Islets were scored as duct associated when CK-positive (CK-19+) cells were intermingled with C-peptide+ cells (13, 25, 36). There may be ducts above or below the level of the section examined, which may result in underestimation of duct association, but it is expected that this would occur to a similar extent in wt and Rgs4 homozygotes.
The SPSS Statistics, version 17.0, software package (SPSS Inc.) was used for statistical analysis. The standard Student t test was used to compare endocrine specification and islet-duct association. A Kolmogorov-Smirnov test revealed that islet size distributions of wt, RGS4−/−, PTX-treated, and S1P2Ag-treated pancreata were not normal. A nonparametric Kruskal-Wallis test determined that differences of cluster size distributions among untreated, PTX-treated, RGS4−/−, and S1P2Ag-treated explants were all statistically significant (P < 0.001), with the notable exception of differences between the RGS4−/− and S1P2Ag-treated pancreata. Differences in mean values in pairwise comparisons were also determined to be statistically significant using a Mann-Whitney U test (P < 0.001).
RESULTS
The GPCR signaling regulator Rgs4 is expressed in the mouse pancreatic epithelium in an Ngn3-dependent manner.
Rgs4 expression was strongly upregulated in mouse ES cell-derived pancreas progenitors in response to Ngn3 induction (53). To examine whether Rgs4 is actually expressed in the developing pancreas, we used the Rgs4tm1Dgen knock-in mouse strain expressing LacZ driven by the endogenous Rgs4 promoter (2) and thus fully recapitulating the Rgs4 expression pattern. Rgs4-LacZ was expressed in Ngn3+, E-cadherin-positive (E-cadherin+) and Pdx1+ epithelial cells and also in some mesenchymal cells at both 12.5 and 14.5 dpc (Fig. 1 A and B; see Fig. S1A, B, and D posted at http://www.flickr.com/photos/65375148@N07/). None of the Rgs4-LacZ+ mesenchymal cells expressed either Ngn3, Rfx6, insulin, or glucagon (data not shown), suggesting that these cells did not belong to the endocrine lineage. In a pattern consistent with that of other pan-endocrine Ngn3 effectors, where, for example, 47% of the Ngn3+ cells were Rfx6+ at 15.5 dpc (57, 58), at 14.5 dpc, 31% of Ngn3+ cells (n = 512, from three different pancreata) were Rgs4-LacZ+ (Fig. 1A). Moreover, all epithelial Rgs4-LacZ+ cells were also Rfx6+ (Fig. 1C; see Fig. S1C posted at http://www.flickr.com/photos/65375148@N07/), and none of the Rgs4-LacZ+ cells were either ins+ or glg+ (data not shown). These data suggested that Rgs4 expression in the epithelium was confined to endocrine progenitors. To examine whether epithelial Rgs4 expression was dependent upon Ngn3, we compared its expression in wt and Ngn3−/− embryonic pancreata. RT-PCR and Western blot experiments at 14.5 dpc showed that Rgs4 expression was strongly reduced at both the RNA and protein levels in Ngn3−/− pancreata (Fig. 1F). Consistent with this, Rgs4-LacZ expression was specifically and completely abolished in the pancreatic epithelium at both 12.5 and 14.5 dpc, whereas Rgs4-LacZ+ mesenchymal cells persisted (Fig. 1D and E; see Fig. S1E and F posted at the URL mentioned above). We did not detect Rgs4 expression in mature endocrine cells at any developmental time point until and including P1.
Fig. 1.
Rgs4 is expressed in the mouse pancreatic epithelium in an Ngn3-dependent manner. (A to C) LacZ expression, driven by the endogenous Rgs4 promoter in 14.5-dpc embryonic pancreata of the Rgs4tm1Dgen mouse strain was found in a subset of Ngn3+ (indicated by white arrows in panel A), E-cadherin+ (indicated by white arrows in panel B), and Rfx6+ (indicated by white arrows in panel C) cells in the developing pancreatic epithelium, as well as in some mesenchymal cells (indicated by arrowheads in panels A, B, and C). (D and E) Rgs4-LacZ expression is specifically and completely abolished from the pancreatic epithelium of Ngn3 null embryos marked by E-cadherin (D) and Pdx1 (E); however, it persists in mesenchymal cells (indicated by arrowheads in both panels). (F) Consistent with the immunofluorescence data, Rgs4 expression is significantly reduced in the RNA levels, as shown by RT-PCR (−RT represents samples not subjected to reverse transcription), and protein levels, as shown by Western blotting, in pancreata of 14.5-dpc Ngn3 null embryos (F). Scale bar, 20 μm.
Taken together, these data showed that Rgs4 is expressed in endocrine progenitors in the pancreatic epithelium as a downstream Ngn3 effector.
Rgs4 participates in islet formation during mouse embryo development.
Rgs4 null mutants are viable and fertile (2, 18, 48), but Rgs4 exerts key functions in adult mouse physiology by regulating fatty acid and glucose homeostasis, parasympathetic signaling in heart rate control (2, 18), and insulin release from β cells (48). Since Rgs4 expression was not detected in endocrine cells at any developmental time point until and including P1, the expression and role of Rgs4 in the adult pancreas must be attributed to reactivation in mature β cells during postnatal pancreas maturation.
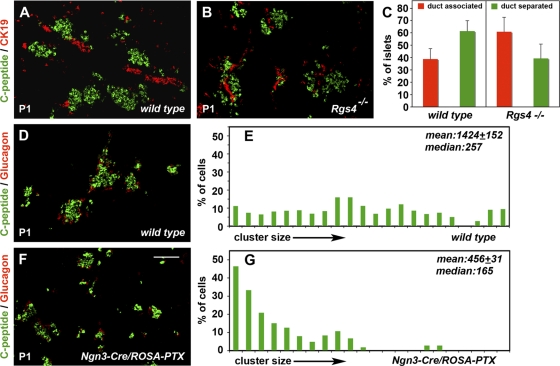
To assess the role of Rgs4 in islet morphogenesis, we examined whether endocrine specification and islet formation were affected in Rgs4 null P1 pups. Separate immunofluorescence experiments for both C-peptide and glucagon combined with image analysis revealed that endocrine specification (see Fig. S3H posted at http://www.flickr.com/photos/65375148@N07/) and gross islet morphology (Fig. 2 A and B) were not altered in Rgs4 null pancreata at P1. Furthermore, endothelial development was not affected, as revealed by PECAM-1 immunofluorescence (data not shown). Endocrine progenitor cells detach and migrate away from the ducts to form mature islets, and therefore the degree to which they remain duct associated has been widely used as a criterion of their migratory behavior (13, 25, 36). Thus, we compared the degree of islet association with ducts in wt and Rgs4−/− pancreata at P1. Similar to the findings of other studies (13, 25, 36), the majority of islets in newborn wt mice were not associated with CK-19+ ductal cells (Fig. 2C), but islets of Rgs4−/− littermates had a stronger tendency (P < 0.005) to remain duct associated (Fig. 2C), suggesting a migratory defect.
Fig. 2.
Disruption of Rgs4 and Gαi signaling in endocrine progenitors resulted in islet formation defects. (A to C) Quantification of islet association with ducts in wild-type and Rgs4 null pancreata at postnatal day 1 (P1). Duct-separated islets (C-peptide+) are round with no ductal cells (CK-19+) interspersed with them (A). Duct-associated islets tend to be more elongated, with ductal cells closely associated or interspersed within them (B). The ratio of duct-associated to duct-separated islets shifts in favor of the former in the pancreata of Rgs4 null newborn (P1) mice (C) in a statistically significant manner (P < 0.005). (D to G) Analysis of islet formation was carried out in wild-type and Ngn3-Cre/ROSA-PTX pancreata at P1 by C-peptide and glucagon immunofluorescence (D and F) followed by image analysis. The percentages of endocrine (C-peptide+) cells belonging to a specific size range were plotted against the size ranges to generate distribution graphs of the cluster sizes (E and G). Statistical analysis showed that the mean (± standard error of the mean) and median cluster sizes became significantly smaller in Ngn3-Cre/ROSA-PTX pancreata (G) than in wild-type pancreata (E). The difference was statistically significant (Mann-Whitney U test, P < 0.001). Scale bar, 100 μm.
Endocrine cell migration to form islets is a long process, barely completed by birth. Since endocrine cells remain to a significant extent dispersed before birth, it is difficult to quantitate clustering defects during gestation. To corroborate the observed phenotype, bypass possible functional compensation from systemic factors, and set the stage for the identification of signaling molecules involved in the process, we turned to explant cultures of mouse embryonic pancreata. Dorsal pancreata dissected at 12.5 or 14.5 dpc and cultured for 6 days developed normally by proliferating, undergoing secondary transition, and forming islets at the end of that period (see Fig. S2A to C, D, and E posted at http://www.flickr.com/photos/65375148@N07/). Islet clustering was more advanced after 6 days of culture of 14.5-dpc embryonic pancreata, and thus we cultured 14.5-dpc wt and Rgs4−/− dorsal pancreata for 6 days and compared the degree of endocrine specification and islet clustering at the end of that period using immunofluorescence and image analysis. Endocrine specification, as revealed separately by both C-peptide and glucagon immunofluorescence and quantitation of the total signal area using image analysis, was not affected in the Rgs4−/− cultured pancreata (see Fig. S3G posted at the URL mentioned above). Proliferation levels as shown by phospho-histone H3 immunofluorescence were the same in wild-type and Rgs4−/− pancreata, and levels of cell death were equally low in wt and Rgs4−/− pancreata (data not shown; see Fig. S3I, J, and L posted at the URL mentioned above). Additionally, epithelial development revealed by E-cadherin immunofluorescence was similar in wt and Rgs4−/− pancreata (see Fig. S3A and B posted at the URL mentioned above). However, both the mean and median sizes of islet clusters in Rgs4−/− pancreata were reduced compared to those of wt controls in a statistically significant manner (P < 0.001) (compare Fig. 3 A and D to B and E).
Fig. 3.
Disruption of Rgs4 and Gαi signaling in embryonic pancreas explants resulted in islet formation defects. Immunofluorescence on 14.5-dpc pancreatic explants after 6 days (ds) in culture showed that, compared to untreated controls (A), disruption of Rgs4 function (B) or disruption of Gαi-dependent GPCR signaling in the presence of 5 μg/ml PTX (C) disrupted islet clustering. Bar, 100 μm. The percentages of endocrine (C-peptide+) cells belonging to a specific size range were plotted against the size ranges to generate distribution graphs of the cluster sizes (D to F). Image processing and statistical analysis showed that the mean (± standard error of the mean) and median cluster sizes became progressively smaller when control pancreata (D) were compared to Rgs4−/− (E) and PTX-treated pancreata (F). These differences were statistically significant either in three-way (Kruskal-Wallis test) or pairwise (Mann-Whitney U test) comparisons (P < 0.001).
Thus, Rgs4 depletion resulted in a measurable but relatively mild phenotype in islet clustering in both P1 pancreata and cultured embryonic explants. However, islets did form, suggesting that endocrine cells delaminated but that their migratory capacity was compromised. Since RGS proteins are exclusive GPCR signaling components, these experiments implicated Rgs4 as well as GPCR signaling in the control of islet formation.
Disruption of Gαi-mediated GPCR signaling in mouse embryonic pancreata leads to islet clustering defects.
The mild Rgs4 knockout phenotype could be due to functional redundancy with other proteins belonging to this large family of more than 20 members. Notably, Rgs8 and Rgs16 are expressed in pancreas progenitors and in maturing endocrine cells in a temporal pattern complementary to that of Rgs4 (64). To bypass functional compensation from other RGS proteins and assess the possible involvement of Gαi subunits, known mediators of chemotactic regulation in various cell types (5), we used pertussis toxin (PTX) which specifically inactivates Gαi subunits by ADP ribosylation (22). Pancreatic 14.5-dpc explants were cultured for 6 days in the presence of PTX (5 μg/ml), and the degree of endocrine specification and islet clustering was determined at the end of that period using immunofluorescence and image analysis. At this concentration endocrine specification revealed separately by both C-peptide and glucagon immunofluorescence and quantitation of the total signal area using image analysis was not affected in the PTX-treated pancreata (see Fig. S3G posted at http://www.flickr.com/photos/65375148@N07/). Proliferation levels as shown by phospho-histone H3 immunofluorescence were the same in wild-type and PTX-treated pancreata, and levels of cell death were equally low in wt and Rgs4−/− pancreata (data not shown; see Fig. S3I, K, and L posted at the URL mentioned above). Additionally, epithelial development as revealed by E-cadherin immunofluorescence was not affected (see Fig. S3A and C posted at the URL mentioned above). However, islet clustering was dramatically affected in PTX-treated pancreata compared to either untreated wt controls or Rgs4−/− explants as endocrine cells remained dispersed, and islets did not form. The size distribution of the clusters shifted dramatically toward smaller clusters, concomitant with a drastic reduction of both the mean and median cluster sizes (P < 0.001) (compare Fig. 3C and F to A and D), thus implicating Gαi subunits and GPCR signaling in mouse islet morphogenesis. Consistent with the notion that the mild Rgs4 null phenotype could be due to functional compensation from other Rgs genes, the PTX phenotype was substantially stronger (compare Fig. 3B and E with C and F). To investigate whether these effects reflected an endocrine progenitor cell-autonomous requirement for Gαi signaling, we intercrossed heterozygous ROSA26PTX (43) transgenic mice with heterozygous Ngn3-Cre transgenic mice (51) to permanently excise the stop cassette in double heterozygotes and express PTX exclusively in Ngn3+ progenitors and their descendants. We then analyzed islet formation in wt and doubly heterozygous transgenic littermates at P1 separately by both C-peptide and glucagon immunofluorescence combined with image analysis. We found that endocrine specification was not affected (see Fig. S3H posted at http://www.flickr.com/photos/65375148@N07/), but islet size distribution shifted dramatically to smaller sizes in the Ngn3-Cre/ROSA-PTX double-heterozygote pancreata (Fig. 2F and G), concomitant with a drastic reduction of both the mean and median cluster sizes. Again, this phenotype was substantially stronger than the corresponding Rgs4 null phenotype (compare Fig. 2B with F). This indicated an endocrine precursor cell-autonomous requirement for Gαi-mediated GPCR signaling in islet clustering. The results of the analysis at P1 were strikingly similar to those observed in wt and PTX-treated explant pancreata (compare Fig. 2D to G with 3A, C, D, and F), suggesting that this requirement was confined to endocrine cells and underlining the suitability of embryonic pancreas explant cultures to address aspects of pancreas development.
Taken together, the results above established that Gαi-mediated GPCR signaling in mouse endocrine progenitors is necessary for cells to coalesce and form islets.
Zebrafish embryonic endocrine progenitors express rgs4.
In the developing zebrafish pancreas, endocrine progenitor cells continuously appear in a relatively wide area of the foregut epithelium and move posteriorly to coalesce into the single embryonic islet (8, 26). Concomitant with this movement there is an anterior-to-posterior maturation sequence, illustrated by overlapping expression domains of endocrine markers corresponding to progressively more mature cells (Fig. 4 F) (58). To investigate whether the role of Rgs4 is evolutionarily conserved in endocrine pancreas morphogenesis, we first analyzed rgs4 expression during embryo pancreas development in zebrafish. Expression of rgs4 was, indeed, detected in the pancreas area by in situ hybridization next to isl1+ cells. (Fig. 4A and B) (60).
Fig. 4.
Rgs4 is expressed in early endocrine progenitor cells in zebrafish. (A, B, and F) Rgs4 was expressed in the developing zebrafish gut (black arrows in panels A and B). As shown by simultaneous rgs4 in situ hybridization (black arrow in panel B) and isl1 immunofluorescence (white arrows in panel B), rgs4 is expressed in the developing pancreas in a domain anterior to but distinct from the isl1 domain that encompasses late and mature endocrine cells (B). Cells in the pancreatic anlage at 24 hpf are arranged according to maturation stage, with more mature cells occupying more posterior positions (F). As development proceeds the expression of late and mature endocrine markers expands anteriorly. Anterior is to the left. (C to E) Double fluorescent whole-mount in situ hybridization of zebrafish embryos at 21, 24, and 28 hpf for rgs4 (red) and neurod (green). At 21 hpf, rgs4 is expressed adjacent and more anterior to neurod-expressing endocrine progenitor cells (C). At 24 hpf there is partial overlap between rgs4 and neurod expression domains (D), whereas by 28 hpf rgs4 expression lies entirely within the neurod expression domain (E). Anterior is to the left. (G to I) In ins::dsRed embryos injected with a transgene, containing 3.8 kb of the rgs4 promoter upstream of the GFP cDNA (rgs4::gfp), GFP expression at 24 hpf was confined outside but more anterior to the islet (green arrow in panel G), but at 36 hpf GFP expression was seen within the islet (green arrow in panel H), occasionally colocalizing with ins+ cells (yellow arrows in panel I). Anterior is to the left. Scale bars, 25 μm (B), 20 μm (E and I).
We then performed a time course expression analysis using rgs4 in situ hybridization and immunofluorescence for isl1, a marker of late endocrine progenitors and mature endocrine cells (58). Cells expressing rgs4 remained at all times distinct from isl1+ cells and were located at the anterior tip of the isl1+ cell population (Fig. 4B; see also Fig. S4A to C posted at http://www.flickr.com/photos/65375148@N07/). Similarly, rgs4-expressing cells remained distinct from ins+ cells (60). Expression of rgs4 peaked at 24 hpf and completely disappeared by 36 hpf (see Fig. S4A to C posted at the URL mentioned above), a time point when expression of late endocrine progenitor markers persists (58).
We then sought to define the relationship of rgs4 expression with neurod, an early endocrine progenitor marker (34). To bypass the lack of specific zebrafish rgs4 antibodies, a time course of rgs4/neurod expression with double in situ fluorescent hybridization was performed. This revealed that rgs4-expressing cells appeared initially in a domain anterior to the neurod-expressing cells. Progressively, rgs4-expressing cells were incorporated in the neurod expression domain (Fig. 4C to E), suggesting that they were early endocrine progenitor cells. Collectively, these data suggested that rgs4 is transiently expressed in early endocrine pancreas progenitor cells.
To directly address whether descendants of the rgs4-expressing cells were indeed incorporated in the mature islet, we generated an rgs4::gfp reporter construct that was injected into ins::dsRed (8) fertilized zebrafish eggs to generate transient rgs4::gfp transgenics. We first assessed whether this reporter construct recapitulated the true rgs4 expression pattern. During zebrafish development rgs4 is also expressed in distinct nuclei of the central nervous system (CNS) and in the pronephros (61). At low DNA concentrations (50 pg per egg), gfp expression was exclusively confined to true rgs4 expression sites (n = 107) (data not shown; see Fig. S4D posted at the URL mentioned above), including the developing gut where roughly 10% of the transgenic embryos showed expression. Having established the fidelity of the reporter construct, we sought to determine whether it could be used as a short-term lineage marker. Cells carrying the rgs4::gfp transgene were expected to retain gpf expression longer than expression of the endogenous gene due to the long half-life of the gfp used (4, 37). This was indeed confirmed in our experimental setup since rgs4::gfp expression in the islet region was retained at least up to 36 hpf, at a time when rgs4 endogenous expression was completely abolished. We then analyzed the localization of the gut gfp+ cells vis-à-vis dsRed+ cells using confocal microscopy and double rgs4::gfp/ins::dsRed transgenic embryos. At 24 hpf, gfp+ gut cells were found exclusively next to ins+ cells (5/5 embryos) (Fig. 4G), and at 36 hpf gfp+ cells were found incorporated in the islet as either ins− (3/5 embryos) or ins+ cells (2/5 embryos) (Fig. 4H and I).
Taken together these data suggested that rgs4-expressing cells were early endocrine progenitors, and this raised the possibility that rgs4 and consequently GPCR signaling are involved in islet formation in zebrafish as well.
Disruption of rgs4- and Gαi-mediated GPCR signaling results in defects of directional endocrine cell migration in the zebrafish embryo.
To assess rgs4 and GPCR involvement in embryonic zebrafish islet formation, we first knocked down rgs4 in the ins::dsRed genetic background. To ensure specificity, low concentrations of two different morpholinos, an AUG and a splice morpholino, were used. Analysis at 24, 48, and 72 hpf gave very similar results for both morpholinos. Embryos looked morphologically normal, with the exception of a dilated heart phenotype at 72 hpf attributed to interference with the cardiac progenitor migration guided by GPCR signaling (29). Wild-type embryos (n > 200) or control morpholino-injected embryos (three different sequence-unrelated morpholinos; n > 200) formed a single islet containing insulin-positive (insulin+) cells at 24 hpf and also glucagon+ (glg+) and somatostatin+ (som+) cells at 72 hpf (Fig. 5 A to C). In contrast, at 24 and 48 hpf, 36% (n = 89) of the morphants had split islets forming two and, occasionally, three smaller islets (Fig. 5E). Among the morphants surviving to 72 hpf (n = 40), 30% had two or three islets containing ins+, som+, and glg+ cells (Fig. 5F and G). Islets were forming at the proper and/or more anterior positions and were of variable sizes, irrespective of their anterior-posterior position.
Fig. 5.
Disruption of Rgs4 and Gαi signaling results in endocrine cell migration defects in zebrafish embryos. (A to C) dsRed expression in the wild-type ins::dsRed zebrafish embryos marks a single islet containing only ins+ cells at 24 hpf (white arrow in panel A). By 72 hpf the single islet contains glg+ and som+ cells as well (white arrows in panels B and C). (D) Cell tracking of ins+ cells of the ins::dsRed embryos reveals that specified endocrine cells migrate directionally to generate a single islet. Successive time points (frames) are approximately 20 min apart and noted in the trajectories as round points. Arrows denote rearward direction of movement and the endpoint of the recording. (E to G) dsRed expression in the rgs4 morphants of the ins::dsRed line shows the formation of ectopic islets at 24 hpf (arrows in panel E) that by 72 hpf contain both glg+ and som+ cells (arrows in panels F and G). (H) Cell tracking of ins+ cells in rgs4 morphants of the ins::dsRed line reveals that specified endocrine cells lose directional migration. Successive time points (frames) are 20 min apart and noted as round points. Arrows denote rearward direction of movement and the endpoint of the recording. (I to L) Injection of wild-type or ins::dsRed zebrafish embryos with a transgene containing 1.4 kb of the insulin promoter upstream of a PTX cDNA (ins::ptx) resulted in the ectopic appearance of isl1+ cells (compare panel I to panel K; arrows in panel K) in wt embryos and ins+ cells in ins::dsRed transgenic embryos (compare panel J to panel L; arrows in panel L). Scale bar, 50 μm. Anterior is to the left.
To analyze these effects, we first determined whether endoderm and gut tube formation were affected. Expression of sox17 (21) and foxa2 (44) in wt (n > 50) and rgs4 morpholino-injected embryos (n > 50) were indistinguishable at 10 hpf, indicating that endoderm migration was not affected (see Fig. S5A to D posted at http://www.flickr.com/photos/65375148@N07/). Similarly, expression of the anterior and posterior endoderm markers foxa3 (25) and gata6 (44) at 48 hpf and 24 hpf, respectively, was not affected in rgs4 morpholino-injected embryos (n > 40 for each probe and condition), confirming that gut patterning was not affected either (see Fig. S5E to H posted at the URL mentioned above). Finally, gut tube formation as revealed by the expression pattern of the gut::gfp transgene (54) was indistinguishable in wt (n > 40) and rgs4 morpholino-injected (n > 40) embryos (see Fig. S5I and J posted at the URL mentioned above). By comparison, treatment with 100 nM RA from 11 hpf onwards (n > 40) resulted in duplication of gut tube structures at 24 hpf (see Fig. S5K posted at the URL mentioned above). Therefore, endoderm migration, endoderm patterning, and gut tube integrity were not affected in rgs4 morpholino-injected embryos, suggesting that the morpholino effects on islet formation were specific. We then determined whether endocrine specification was affected. The number of isl1+ endocrine cells in wt embryos (n = 25) and rgs4 morphants (n = 21) was essentially the same (25 ± 3.5 in the wt versus 24 ± 3 in the morphants), ruling out specification defects.
To examine whether the rgs4 knockdown phenotype resulted from migratory defects of the endocrine cells, we performed time-lapse fluorescence microscopy and cell tracking analysis between 16 and 22 hpf in the ins::dsRed genetic background. In wt embryos ins+ cells were continuously appearing, moving in an anterior to posterior direction to form a single islet (1, 8, 26) (Fig. 5D; see also the movie posted at http://www.flickr.com/photos/65375148@N07/). In contrast, in the affected rgs4 morphants, all tracked ins+ cells moved randomly, failing to form a single islet (Fig. 5H; see the movie posted at the URL mentioned above). Diverging trajectories were detected very early in the time-lapse sequence (Fig. 5H, cells 1 and 2), supporting an early role of rgs4 in directional cell migration.
We then assessed the possible involvement of Gαi subunits in the directional migration of early zebrafish endocrine cells by permanently uncoupling them from GPCRs using pertussis toxin (PTX). To that end we generated an ins::ptx transgene (16) and injected it in fertilized wt or ins::dsRed zebrafish eggs. At low DNA concentrations (150 ng per egg), 25% (n = 24) of the injected wt embryos and 25% (n = 40) of the injected ins::dsRed embryos had isl1+ (compare Fig. 5I with K) or ins+ (compare Fig. 5J with 5L) ectopic anterior cells, respectively, that failed to move posteriorly and incorporate in the main islet. This was never observed at that stage in wt embryos (n > 200) or embryos injected with just the insulin promoter (n = 40) (Fig. 5I and J). Therefore, Gαi-mediated GPCR signaling in the endocrine cells is necessary for their directional migration in zebrafish.
Sphingosine-1-phosphate (S1P) signaling is implicated in endocrine pancreas morphogenesis in mouse and zebrafish.
During mouse pancreas development, individual endocrine cells migrate over significant distances to form vascularized islets (3, 10, 20, 49). Similarly, in zebrafish endocrine precursors directionally migrate in an anterior-to-posterior direction to coalesce into a single islet (8, 26). The experiments outlined above suggested a role of GPCR signaling in the endocrine precursors for islet formation but did not suggest possible ligand(s) involved in this process.
The G protein-coupled CXCR4 receptor and its ligand CXCL12 have been widely implicated in directed cell migration in both zebrafish and mouse (42, 62), including the migration of germ cells in zebrafish (68) and migration of angioblasts near the presumptive pancreatic domain of the definitive endoderm (23). Accordingly, we tested whether CXCR4/CXCL12 signaling was involved in pancreas endocrine cell migration by treating ins::dsRed transgenic zebrafish embryos at 11 hpf with up to 100 μg/ml of the specific CXCR4 antagonist AMD3100 (71) and scoring islet formation at 24 hpf. Blocking CXCR4 activity had no effect on either pancreas endocrine or endoderm formation in zebrafish (data not shown). We also cultured 14.5-dpc pancreatic explants in the presence of up to 100 μg/ml of AMD3100 (71) for 6 days and assayed islet formation using C-peptide and glucagon immunofluorescence combined with image analysis. We found that endocrine specification and islet clustering in AMD3000-treated explants were indistinguishable from those of untreated controls (data not shown), suggesting that CXCR4/CXCL12 signaling may not be involved in endocrine cell migration.
Phospholipids also signal through GPCR receptors and are involved in cell migration in both mouse and zebrafish in diverse cellular contexts (29, 59). S1P receptors engage diverse Gα subunits, including Gαi subunits (59). Thus, we first tested in zebrafish whether blocking the main S1P receptors using the specific antagonists VPC23019 for S1P receptors 1 and 3 (S1P1/3) (6) and JTE013 for S1P2 (38) would disrupt islet formation. To avoid gastrulation-related defects, we treated ins::dsRed transgenic zebrafish embryos from 11 hpf onwards with either VPC23019 or JTE013 and scored islet morphogenesis at 24 hpf. Treatment with 100 μM VPC23019 resulted in nearly complete islet cell dispersal (compare Fig. 6 A and B) in 62% of the treated embryos (n = 29) that persisted at least until 72 hpf without affecting the number of endocrine cells or general endoderm patterning (compare Fig. 6D and E). JTE013 had no effect on islet morphogenesis even at concentrations up to 100 μM. These results suggested that S1P signaling mediated by S1P1/3 is necessary in directional migration of early zebrafish insulin+ cells to form an islet. If S1P is acting as a chemoattractant for endocrine progenitor cells, an agonist should have an effect similar to that of the antagonist through disruption of the chemotactic gradient. Thus, we treated ins::dsRed transgenic zebrafish embryos from 11 hpf onwards with a 10 μM concentration of the activated S1P1/3-specific agonist FTY720 (FTY720-P) (33). Similar to treatment with the antagonist, this treatment also resulted in islet cell dispersal (compare Fig. 6A and C) in 75% of the treated embryos (n = 65) that persisted at least until 72 hpf without affecting the number of endocrine cells or general endoderm patterning (compare Fig. 6D and F). In contrast, the presence of a 10 μM concentration of the S1P2-specific agonist (S1P2Ag) (45) had no effect on islet morphogenesis (data not shown).
Fig. 6.
Sphingosine-1-phosphate (S1P) signaling participates in islet cell clustering in both zebrafish and mouse. (A to C) The single islet marked by dsRed expression in untreated ins::dsRed zebrafish embryos at 24 hpf (A) was dispersed at 24 hpf in embryos treated from 11 hpf onwards with the S1P1/3 antagonist VPC23019 (B) or the S1P1/3 agonist FTY720-P (C). (D to F) Zebrafish embryos carrying both the ins::dsRed and gut::gfp transgenes were treated with 100 μM VPC23019 (E) or 10 μM FTY720-P (F) from 11 hpf onwards and analyzed at 72 hpf for defects in islet clustering and general endoderm formation. GFP expression in the untreated embryos marked the entire gut tube, including endoderm organs like liver (L), stomach (S), pancreas (P), and gut (G), while dsRed marks the single islet within the pancreas (D). In VPC23019 (E)- and FTY720-P (F)-treated embryos, gut formation and organ morphogenesis were unaffected, while endocrine cells remained dispersed. (G and H) Immunofluorescence on 14.5-dpc pancreatic explants after 6 days in culture in the presence of 5 μM S1P2Ag disrupted islet clustering (G). The percentages of endocrine (C-peptide+) cells belonging to a specific size range were plotted against the size ranges to generate a distribution graph of the cluster sizes. Image processing and statistical analysis showed that the mean and median cluster sizes decreased dramatically (H) compared to untreated controls (Fig. 4A and D). The difference with the untreated control and PTX-treated pancreata was statistically significant (Mann-Whitney U test) (P < 0.001). Scale bars, 50 μm (C) 100 μm (F and G).
We then tested the effects of S1P antagonists and agonists on mouse pancreas explants by culturing 14.5-dpc dorsal pancreas explants in the presence of the compounds for 6 days and analyzing their effects on islet morphogenesis at the end of that period. Neither the S1P1/3 antagonist VPC23019 at up to 250 μM nor the S1P1/3 agonist FTY720-P at up to 10 μM had any effect on either endocrine specification or islet formation (see Fig. S3G posted at the URL mentioned above). We then investigated possible involvement of the S1P2 receptor in islet cell clustering. Treatment of the pancreas explants with 5 μM S1P2Ag (45) did not affect either endocrine specification or epithelial or endothelial development (see Fig. S3D and G posted at the URL mentioned above) or levels of cell death (data not shown). However, the size distribution of the endocrine clusters shifted dramatically toward smaller clusters, concomitant with a drastic reduction of both the mean and median cluster sizes (P < 0.001) (compare Fig. 4A and D with 6G and H). This reduction was stronger than that observed in the Rgs4−/− pancreas explants but comparable to the reduction observed in PTX-treated explants (compare Fig. 4B and E with 6G and 4C and F with 6H). Blocking the S1P2 receptor using the JTE013 antagonist at 20 μM resulted in endocrine progenitor cell loss, most likely due to reduced survival of the cells, and these effects will be analyzed elsewhere (J. Serafimidis et al., unpublished data). Notably, the mouse phenotype of S1P signaling disruption strongly resembled that of Gαi-mediated GPCR signaling disruption.
Thus, these findings established the implication of S1P signaling in the chemotactic response of both zebrafish and mouse endocrine progenitors through distinct (S1P1/3 for zebrafish and S1P2 for the mouse) but closely related receptors of S1P.
DISCUSSION
Pancreas endocrine progenitors need to acquire directional sensing, polarity, and motility in order to coalesce and form islets in both zebrafish and mouse. Directional cell movement depends on receptors sensing a chemoattractant gradient and transmitting that signal to intracellular effectors. Here, we provide evidence identifying S1P and Gαi-mediated GPCR signaling as players in this process.
We first found that a dedicated GPCR signaling regulator, Rgs4, was expressed in both mouse and zebrafish endocrine progenitors. Expression of mouse Rgs4 in the pancreatic epithelium strictly depends upon Ngn3, and it is restricted in epithelial endocrine progenitors. Rfx6 is a pan-endocrine molecular marker downstream of Ngn3, and it is expressed continuously in both endocrine progenitors and mature endocrine cells. It has been shown that 47% of Ngn3+ cells also expressed Rfx6 at 15.5 dpc, whereas we found that 31% of Ngn3+ cells were expressing Rgs4 at 14.5 dpc. Thus, whereas we cannot exclude the possibility that only a subset of Ngn3+ cells express Rgs4, this comparison favors, in our view, the interpretation that all endocrine progenitors express Rgs4 but only transiently while still in the epithelium. The expression domain of rgs4 in the zebrafish islet region is more anterior, partially overlaps with that of neurod, and appears smaller than that of early- and late-progenitor markers such as neurod and isl1. Zebrafish Rgs4 expression is switched off earlier than either neurod or isl1. Transient lineage tracing revealed that all rgs4::gfp cells ended up within the forming islet, and, collectively, these findings suggested that rgs4 marks very early endocrine progenitors. It is not known whether all endocrine progenitors express rgs4; a direct comparison with the neurod and isl1 expression domains would suggest that a subset of endocrine progenitors express rgs4. On the other hand, it is not known whether all neurod or isl1 cells become endocrine cells. Thus, the small rgs4 expression domain could be due to early, transient expression in all progenitors.
In short, whereas it remains unknown, particularly in zebrafish, whether all endocrine progenitors express Rgs4, its knockdown resulted exclusively in migratory defects in zebrafish and disruption of islet clustering in the mouse, showing that its expression is necessary in both species for islet formation and dispensable for endocrine specification. The mild mouse phenotype could be due to functional redundancy with other proteins belonging to the same large family (55). Rgs4 is an exclusive GPCR signaling component (55), and we reasoned that a disruption of GPCR signaling in the endocrine progenitors by genetic means could implicate this signaling pathway in zebrafish endocrine progenitor migration and mouse islet formation. Indeed, transient transgenic expression of PTX in zebrafish embryos under the insulin promoter resulted in cells that did not incorporate into the islet, suggesting that Gαi-mediated GPCR signaling is necessary for endocrine cell migration in zebrafish. Induced specific expression of PTX in Ngn3+ cells and their descendants did not affect endocrine specification but resulted in strong islet cell dispersal, implicating Gαi-mediated GPCR signaling in mouse islet formation. Consistent with the possibility of functional redundancy among RGS proteins, this phenotype was much stronger than the Rgs4 null phenotype. Small islets did form, however, suggesting that this was not a delamination phenotype. Unfortunately, it was not possible to assess the functional consequences in the adult of small islet formation since Gαi-mediated GPCR signaling and Rgs4 are also involved in glucose-stimulated insulin secretion in mature β cells (48). Given that we did not detect Rgs4 expression in endocrine cells during development and through P1, Rgs4 must be reactivated sometime during postnatal life as the presence of Rgs4 mRNA has been convincingly demonstrated in the adult islets (48). Rgs4 null mutants are viable and fertile and generally normal (2) but show a higher degree of glucose intolerance and associated decreased insulin secretion in the pancreas (18). Additionally, Rgs4 knockout specifically in the beta cells resulted in deregulated insulin secretion (48). Thus, the late function of Rgs4 is fully separable from its developmental functions described here.
The results obtained using cultures of embryonic wt, Rgs4 null, and PTX-treated pancreata (compare Fig. 2 and 3) were very similar to those obtained from P1 pancreata, suggesting that PTX effects were restricted to endocrine precursors and, most importantly, that pancreas embryo cultures could be used to investigate possible GPCR ligands involved in islet formation. Embryonic pancreas explant cultures have the advantage of isolating the developing tissue from systemic influences. This approach can uncover signals that do play a role but are not indispensable or are acting in functional redundancy with systemically delivered signals. Nevertheless, their identification can be particularly useful in directing differentiation of pluripotent stem cells to specific pancreatic lineages or enhancing islet clustering after, e.g., exocrine cell reprogramming. Thus, we used the developing zebrafish embryo and mouse embryo pancreatic explants to identify signals involved. Disruption of S1P signaling in zebrafish embryos by S1P1/3 agonist and antagonist treatments resulted in severe islet cell dispersal without affecting endocrine specification. This was consistent with a chemotactic function for S1P. The same compounds had no effect in mouse islet clustering, but an agonist of the closely related receptor S1P2 resulted in strong defects in islet clustering without affecting endocrine specification. These experiments implicated S1P in islet clustering in both organisms, acting through distinct but closely related receptors.
Endothelial cells have been postulated to attract migrating endocrine cells in the mouse (30). Accordingly, GPCR ligand(s) such as S1P emanating from the developing blood vessels could establish the signaling gradients required to attract endocrine progenitors. In the simplest model S1P, and possibly other chemoattractants, would act directly on Rgs4-expressing endocrine progenitors. Alternatively, S1P may be acting on local mesenchyme cells to regulate endocrine migration indirectly or through a relay mechanism. Agonist treatments resulted in endocrine cell dispersal, consistent with S1P being the chemoattractant itself and thus favoring the former interpretation. S1P has been shown to indirectly mediate budding and proliferation of the early mouse pancreatic endoderm through the stimulation of pancreatic mesenchymal cell proliferation (7, 50), suggesting that the same signal(s) may be used repeatedly during organ development but playing different roles.
A mechanism establishing an intracellular activity gradient in response to an extracellular chemoattractant gradient is necessary for directional migration of the endocrine progenitors. Rgs4, a modulator of GPCR signaling, is well positioned to establish that condition. Rgs4 is expressed in early zebrafish and mouse endocrine progenitors. Increased expression of Rgs4 in endocrine progenitors would be expected to inhibit S1P signaling at the forming trailing edge (low agonist concentration) while permitting signaling at the forming leading edge (high agonist concentration). Therefore, the activated Gβγ subunits in the future leading edge could preferentially stimulate phosphatidylinositol 3-kinase (PI3K), resulting in the asymmetric accumulation of its lipid by-products at that location to promote directed migration (69). Accordingly, antagonizing the receptor, disrupting the chemotactic gradient by providing a diffuse signal (agonist), inactivating Rgs4, or disrupting Gαi-mediated signaling in either zebrafish or mouse would disrupt or even out the activity gradient, leading to the observed defects in directional migration. As Rgs4 is expressed only early on in zebrafish and in epithelial early endocrine progenitors in the mouse, its function may not be required beyond the asymmetry-establishing stage, or other functionally equivalent RGS proteins may take over. Endocrine cell clustering was not completely abolished in either organism, implying the operation of additional, functionally overlapping mechanisms.
Rac1, a member of the Rho family of GTPases, is an intracellular transducer of multiple signaling pathways, including growth factor, integrin, and GPCR signaling. Expression of a dominant negative form of the small Rho GTPase Rac1 in insulin-producing cells inhibited migration of the islet cells away from the duct (13). GPCR signaling may activate small Rho GTPases that regulate actin cytoskeleton dynamics and lamellipodium formation (39) in a Gα-subunit-dependent manner in several cell types (5). Accordingly, permanent blocking of Gαi/o-mediated activation using PTX in mouse embryonic pancreas explants resulted in a dramatically stronger phenotype.
Furthermore, GPCR signaling may elicit effects on cell motility through transactivation of receptor tyrosine kinases (RTKs) (5) and the Wnt pathway. Notably, the mouse null mutant of the epidermal growth factor receptor (EGFR) RTK exhibited migration defects of the pancreatic endocrine cells (36), and the fzd-2 receptor and its ligand wnt-5 have been implicated in endocrine cell migration in both zebrafish and mouse embryos (25), and thus coordination of GPCR, Wnt, and RTK signaling may drive directional endocrine migration.
It will be important to elucidate the full spectrum and roles of GPCRs and the signals involved in pancreatic and, particularly, endocrine development. This will contribute to efficient conversion of ES cells and iPS cells into mature β cells either for cell therapy of diabetes or for the enhancement of islet formation after in vivo reprogramming of exocrine to endocrine cells. The conservation of the role of GPCR signaling in islet formation in mouse and zebrafish suggests that the latter can be used to test novel pharmacological agents that enhance islet formation in vivo.
ACKNOWLEDGMENTS
We thank A. Efstratiadis (Biomedical Research Foundation of the Academy of Athens [BRFAA]), V. Episkopou (Clinical Science Centre, Medical Research Council [MRC], United Kingdom), T. Wilkie (University of Texas Southwestern Medical Center), and D. Stainier (University of California, San Francisco [UCSF]) for discussions and comments; S. Pagakis (BRFAA) for help with the time-lapse and image analysis; F. Guillemot (National Institute for Medical Research, United Kingdom) for the Ngn3 mice; D. Stainier (UCSF) and P. Ingham (MRC, Sheffield, United Kingdom) for zebrafish lines; M. Ioannou (BRFAA) for mouse embryos; and A. Kastania (BRFAA) for the statistical analysis. We also thank A. Mayer (Medical College of Wisconsin), S. Sumanas (Children's Hospital Medical Center, Cincinnati, OH), S. Dougan (University of Georgia), L. Zhen (NUS, Singapore), and M. Voz (University of Liege) for gifts of in situ probes; E. Raz (ZMBE) for the PTX cDNA; and C. Wright (Vanderbilt University), H. Edlund (Umea University), and G. Gradwohl (IGBMC) for antibody gifts.
This work was funded by Juvenile Diabetes Research Foundation (regular research grant 1-2007-680 to A.G.), European Foundation for the Study of Diabetes (EFSD Novonordisk), and partly by Human Frontier Science Program (to D.B.).
We declare that we have no competing interests.
Footnotes
Published ahead of print on 12 September 2011.
REFERENCES
- 1. Biemar F., et al. 2001. Pancreas development in zebrafish: early dispersed appearance of endocrine hormone expressing cells and their convergence to form the definitive islet. Dev. Biol. 230:189–203 [DOI] [PubMed] [Google Scholar]
- 2. Cifelli C., et al. 2008. RGS4 regulates parasympathetic signaling and heart rate control in the sinoatrial node. Circ. Res. 103:527–535 [DOI] [PubMed] [Google Scholar]
- 3. Cole L., Anderson M., Antin P. B., Limesand S. W. 2009. One process for pancreatic beta-cell coalescence into islets involves an epithelial-mesenchymal transition. J. Endocrinol. 203:19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corish P., Tyler-Smith C. 1999. Attenuation of green fluorescent protein half-life in mammalian cells. Protein Eng. 12:1035–1040 [DOI] [PubMed] [Google Scholar]
- 5. Cotton M., Claing A. 2009. G protein-coupled receptors stimulation and the control of cell migration. Cell Signal. 21:1045–1053 [DOI] [PubMed] [Google Scholar]
- 6. Davis M. D., Clemens J. J., Macdonald T. L., Lynch K. R. 2005. Sphingosine 1-phosphate analogs as receptor antagonists. J. Biol. Chem. 280:9833–9841 [DOI] [PubMed] [Google Scholar]
- 7. Edsbagge J., et al. 2005. Vascular function and sphingosine-1-phosphate regulate development of the dorsal pancreatic mesenchyme. Development 132:1085–1092 [DOI] [PubMed] [Google Scholar]
- 8. Field H. A., Dong P. D., Beis D., Stainier D. Y. 2003. Formation of the digestive system in zebrafish. II. Pancreas morphogenesis. Dev. Biol. 261:197–208 [DOI] [PubMed] [Google Scholar]
- 9. Gierl M. S., Karoulias N., Wende H., Strehle M., Birchmeier C. 2006. The zinc-finger factor Insm1 (IA-1) is essential for the development of pancreatic beta cells and intestinal endocrine cells. Genes Dev. 20:2465–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gouzi M., Kim Y. H., Katsumoto K., Johansson K., Grapin-Botton A. 2011. Neurogenin3 initiates stepwise delamination of differentiating endocrine cells during pancreas development. Dev. Dyn. 240:589–604 [DOI] [PubMed] [Google Scholar]
- 11. Gradwohl G., Dierich A., LeMeur M., Guillemot F. 2000. Neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc. Natl. Acad. Sci. U. S. A. 97:1607–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grapin-Botton A., Majithia A. R., Melton D. A. 2001. Key events of pancreas formation are triggered in gut endoderm by ectopic expression of pancreatic regulatory genes. Genes Dev. 15:444–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greiner T. U., Kesavan G., Stahlberg A., Semb H. 2009. Rac1 regulates pancreatic islet morphogenesis. BMC Dev. Biol. 9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gu G., Dubauskaite J., Melton D. A. 2002. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129:2447–2457 [DOI] [PubMed] [Google Scholar]
- 15. Heximer S. P., Watson N., Linder M. E., Blumer K. J., Hepler J. R. 1997. RGS2/G0S8 is a selective inhibitor of Gqα function. Proc. Natl. Acad. Sci. U. S. A. 94:14389–14393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang H., Vogel S. S., Liu N., Melton D. A., Lin S. 2001. Analysis of pancreatic development in living transgenic zebrafish embryos. Mol. Cell Endocrinol. 177:117–124 [DOI] [PubMed] [Google Scholar]
- 17. Huang H. P., et al. 2000. Regulation of the pancreatic islet-specific gene BETA2 (neuroD) by neurogenin 3. Mol. Cell. Biol. 20:3292–3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iankova I., et al. 2008. Regulator of G protein signaling-4 controls fatty acid and glucose homeostasis. Endocrinology 149:5706–5712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johansson K. A., et al. 2007. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev. Cell 12:457–465 [DOI] [PubMed] [Google Scholar]
- 20. Jorgensen M. C., et al. 2007. An illustrated review of early pancreas development in the mouse. Endocr. Rev. 28:685–705 [DOI] [PubMed] [Google Scholar]
- 21. Kanai-Azuma M., et al. 2002. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development 129:2367–2379 [DOI] [PubMed] [Google Scholar]
- 22. Kaslow H. R., Burns D. L. 1992. Pertussis toxin and target eukaryotic cells: binding, entry, and activation. FASEB J. 6:2684–2690 [DOI] [PubMed] [Google Scholar]
- 23. Katsumoto K., Kume S. 2011. Endoderm and mesoderm reciprocal signaling mediated by CXCL12 and CXCR4 regulates the migration of angioblasts and establishes the pancreatic fate. Development 138:1947–1955 [DOI] [PubMed] [Google Scholar]
- 24. Kesavan G., et al. 2009. Cdc42-mediated tubulogenesis controls cell specification. Cell 139:791–801 [DOI] [PubMed] [Google Scholar]
- 25. Kim H. J., et al. 2005. Wnt5 signaling in vertebrate pancreas development. BMC Biol. 3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kinkel M. D., Prince V. E. 2009. On the diabetic menu: zebrafish as a model for pancreas development and function. Bioessays 31:139–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Konstantinova I., et al. 2007. EphA-Ephrin-A-mediated beta cell communication regulates insulin secretion from pancreatic islets. Cell 129:359–370 [DOI] [PubMed] [Google Scholar]
- 28. Kroon E., et al. 2008. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 26:443–452 [DOI] [PubMed] [Google Scholar]
- 29. Kupperman E., An S., Osborne N., Waldron S., Stainier D. Y. 2000. A sphingosine-1-phosphate receptor regulates cell migration during vertebrate heart development. Nature 406:192–195 [DOI] [PubMed] [Google Scholar]
- 30. Lammert E., Cleaver O., Melton D. 2001. Induction of pancreatic differentiation by signals from blood vessels. Science 294:564–567 [DOI] [PubMed] [Google Scholar]
- 31. Lammert E., et al. 2003. Role of VEGF-A in vascularization of pancreatic islets. Curr. Biol. 13:1070–1074 [DOI] [PubMed] [Google Scholar]
- 32. Lynn F. C., Sanchez L., Gomis R., German M. S., Gasa R. 2008. Identification of the bHLH factor Math6 as a novel component of the embryonic pancreas transcriptional network. PLoS One 3:e2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mandala S., et al. 2002. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296:346–349 [DOI] [PubMed] [Google Scholar]
- 34. Mavropoulos A., et al. 2005. sox4b is a key player of pancreatic alpha cell differentiation in zebrafish. Dev. Biol. 285:211–223 [DOI] [PubMed] [Google Scholar]
- 35. Mellitzer G., et al. 2006. IA1 is NGN3-dependent and essential for differentiation of the endocrine pancreas. EMBO J. 25:1344–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miettinen P. J., et al. 2000. Impaired migration and delayed differentiation of pancreatic islet cells in mice lacking EGF-receptors. Development 127:2617–2627 [DOI] [PubMed] [Google Scholar]
- 37. Miyatsuka T., Kosaka Y., Kim H., German M. S. 2011. Neurogenin3 inhibits proliferation in endocrine progenitors by inducing Cdkn1a. Proc. Natl. Acad. Sci. U. S. A. 108:185–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Parrill A. L., Sardar V. M., Yuan H. 2004. Sphingosine 1-phosphate and lysophosphatidic acid receptors: agonist and antagonist binding and progress toward development of receptor-specific ligands. Semin. Cell Dev. Biol. 15:467–476 [DOI] [PubMed] [Google Scholar]
- 39. Petrie R. J., Doyle A. D., Yamada K. M. 2009. Random versus directionally persistent cell migration. Nat. Rev. Mol. Cell Biol. 10:538–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Prasadan K., et al. 2011. The expression and function of glucose-dependent insulinotropic polypeptide in the embryonic mouse pancreas. Diabetes 60:548–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rachdi L., Marie J. C., Scharfmann R. 2003. Role for VPAC2 receptor-mediated signals in pancreas development. Diabetes 52:85–92 [DOI] [PubMed] [Google Scholar]
- 42. Raz E., Mahabaleshwar H. 2009. Chemokine signaling in embryonic cell migration: a fisheye view. Development 136:1223–1229 [DOI] [PubMed] [Google Scholar]
- 43. Regard J. B., et al. 2007. Probing cell type-specific functions of Gi in vivo identifies GPCR regulators of insulin secretion. J. Clin. Invest. 117:4034–4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reiter J. F., Kikuchi Y., Stainier D. Y. 2001. Multiple roles for Gata5 in zebrafish endoderm formation. Development 128:125–135 [DOI] [PubMed] [Google Scholar]
- 45. Rosen H. High throughput screening for S1P receptor agonists and antagonists, S1P2 agonist. The Scripps Research Institute Molecular Screening Center, La Jolla, CA. http://mlpcn.florida.scripps.edu/index.php/probes/probe-reports.html [Google Scholar]
- 46. Rosenberg L. C., et al. 2010. The transcriptional activity of Neurog3 affects migration and differentiation of ectopic endocrine cells in chicken endoderm. Dev. Dyn. 239:1950–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ross E. M., Wilkie T. M. 2000. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu. Rev. Biochem. 69:795–827 [DOI] [PubMed] [Google Scholar]
- 48. Ruiz de Azua I., et al. 2010. RGS4 is a negative regulator of insulin release from pancreatic beta-cells in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 107:7999–8004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rukstalis J. M., Habener J. F. 2007. Snail2, a mediator of epithelial-mesenchymal transitions, expressed in progenitor cells of the developing endocrine pancreas. Gene Expr. Patterns 7:471–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sand F. W., et al. 2011. Growth-limiting role of endothelial cells in endoderm development. Dev. Biol. 352:267–277 [DOI] [PubMed] [Google Scholar]
- 51. Schonhoff S. E., Giel-Moloney M., Leiter A. B. 2004. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev. Biol. 270:443–454 [DOI] [PubMed] [Google Scholar]
- 52. Schwitzgebel V. M., et al. 2000. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development 127:3533–3542 [DOI] [PubMed] [Google Scholar]
- 53. Serafimidis I., Rakatzi I., Episkopou V., Gouti M., Gavalas A. 2008. Novel effectors of directed and Ngn3-mediated differentiation of mouse embryonic stem cells into endocrine pancreas progenitors. Stem Cells 26:3–16 [DOI] [PubMed] [Google Scholar]
- 54. Shin C. H., et al. 2008. Multiple roles for Med12 in vertebrate endoderm development. Dev. Biol. 317:467–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sjogren B., Neubig R. R. 2010. Thinking outside of the “RGS box”: new approaches to therapeutic targeting of regulators of G protein signaling. Mol. Pharmacol. 78:550–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Smith S. B., et al. 2003. Neurogenin3 and hepatic nuclear factor 1 cooperate in activating pancreatic expression of Pax4. J. Biol. Chem. 278:38254–38259 [DOI] [PubMed] [Google Scholar]
- 57. Smith S. B., et al. 2010. Rfx6 directs islet formation and insulin production in mice and humans. Nature 463:775–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Soyer J., et al. 2010. Rfx6 is an Ngn3-dependent winged helix transcription factor required for pancreatic islet cell development. Development 137:203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Spiegel S., English D., Milstien S. 2002. Sphingosine 1-phosphate signaling: providing cells with a sense of direction. Trends Cell Biol. 12:236–242 [DOI] [PubMed] [Google Scholar]
- 60. Stetsyuk V., et al. 2007. Calsenilin is required for endocrine pancreas development in zebrafish. Dev. Dyn. 236:1517–1525 [DOI] [PubMed] [Google Scholar]
- 61. Thisse B., et al. 2001. Expression of the zebrafish genome during embryogenesis. Zebrafish Model Organism Database (ZFIN) direct data submission. University of Oregon, Eugene, OR: (http://zfin.org). [Google Scholar]
- 62. Tiveron M. C., Cremer H. 2008. CXCL12/CXCR4 signalling in neuronal cell migration. Curr. Opin. Neurobiol. 18:237–244 [DOI] [PubMed] [Google Scholar]
- 63. Villasenor A., Chong D. C., Henkemeyer M., Cleaver O. 2010. Epithelial dynamics of pancreatic branching morphogenesis. Development 137:4295–4305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Villasenor A., et al. 2010. Rgs16 and Rgs8 in embryonic endocrine pancreas and mouse models of diabetes. Dis. Models Mech. 3:567–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang S., et al. 2008. Myt1 and Ngn3 form a feed-forward expression loop to promote endocrine islet cell differentiation. Dev. Biol. 317:531–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Watada H., Scheel D. W., Leung J., German M. S. 2003. Distinct gene expression programs function in progenitor and mature islet cells. J. Biol. Chem. 278:17130–17140 [DOI] [PubMed] [Google Scholar]
- 67. Watson N., Linder M. E., Druey K. M., Kehrl J. H., Blumer K. J. 1996. RGS family members: GTPase-activating proteins for heterotrimeric G-protein alpha-subunits. Nature 383:172–175 [DOI] [PubMed] [Google Scholar]
- 68. Weidinger G., Wolke U., Koprunner M., Klinger M., Raz E. 1999. Identification of tissues and patterning events required for distinct steps in early migration of zebrafish primordial germ cells. Development 126:5295–5307 [DOI] [PubMed] [Google Scholar]
- 69. Weiner O. D. 2002. Regulation of cell polarity during eukaryotic chemotaxis: the chemotactic compass. Curr. Opin. Cell Biol. 14:196–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Xu X., et al. 2008. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132:197–207 [DOI] [PubMed] [Google Scholar]
- 71. Yano T., Liu Z., Donovan J., Thomas M. K., Habener J. F. 2007. Stromal cell derived factor-1 (SDF-1)/CXCL12 attenuates diabetes in mice and promotes pancreatic beta-cell survival by activation of the prosurvival kinase Akt. Diabetes 56:2946–2957 [DOI] [PubMed] [Google Scholar]
- 72. Zhou Q., Brown J., Kanarek A., Rajagopal J., Melton D. A. 2008. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 455:627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]