Abstract
Atrial natriuretic factor (ANF) is abundantly expressed in atrial cardiomyocytes throughout ontogeny and in ventricular cardiomyocytes in the developing heart. However, during cardiac failure and hypertrophy, ANF expression can reappear in adult ventricular cardiomyocytes. The transcription factor Nkx2-5 is one of the major transactivators of the ANF gene in the developing heart. We identified Nkx2-5 binding at three 5′ regulatory elements (kb −34, −31, and −21) and at the proximal ANF promoter by ChIP assay using neonatal mouse cardiomyocytes. 3C analysis revealed close proximity between the distal elements and the promoter region. A 5.8-kb fragment consisting of these elements transactivated a reporter gene in vivo recapitulating endogenous ANF expression, which was markedly reduced in tamoxifen-inducible Nkx2-5 gene knockout mice. However, expression of a reporter gene was increased and expanded toward the outer compact layer in the absence of the transcription repressor Hey2, similar to endogenous ANF expression. Functional Nkx2-5 and Hey2 binding sites separated by 59 bp were identified in the −34 kb element in neonatal cardiomyocytes. In adult hearts, this fragment did not respond to pressure overload, and ANF was induced in the absence of Nkx2-5. These results demonstrate that Nkx2-5 and its responsive cis-regulatory DNA elements are essential for ANF expression selectively in the developing heart.
INTRODUCTION
The 28-amino-acid peptide known as atrial natriuretic factor (ANF) is released from cardiomyocytes and regulates fluid and electrolyte balance as well as cardiovascular growth (14, 33). During cardiac development, regional myocardial gene expression is linked to the differentiation of ventricular cardiomyocytes. ANF is abundantly expressed in the atrial cardiomyocytes and in the inner (trabecular) layer of ventricular cardiomyocytes, where proliferative activity is lower than that in the outer (compact) layer between embryonic day 9.5 (E9.5) and E14.5 (7, 42).
In fully developed adult hearts, ANF is normally downregulated in the ventricles, and atrial cardiomyocytes produce the majority of ANF peptide. On the other hand, volume and/or pressure overload of the heart due to various causes leads to the reexpression of genes that are transcribed in embryonic hearts but silent in adult hearts, including ANF (so-called reactivation of a fetal cardiac gene program) (14, 33, 36). Whether common or distinct cis elements and transcription factors are responsible for gene regulation during normal cardiac development compared to diseased hearts has been studied extensively, but the process is not completely understood (11, 13, 20, 31, 39).
One known transactivator of the ANF gene in developing hearts is the homeodomain-containing transcription factor Nkx2-5 (9, 26, 48). Nkx2-5 is the most widely used marker for the cardiomyocyte lineage (2, 46). Mice with germ line deletion of Nkx2-5 are developmentally arrested around E10.5, at which time ANF expression is markedly downregulated both in atria and ventricles (30, 48). To clarify the function of Nkx2-5 beyond E10.5, we recently generated mice with a tamoxifen-inducible Nkx2-5 gene knockout (3, 47, 50). Nkx2-5 deletion beginning at the mid-embryonic stage (E12.5 and E13.5) and at E19 results in premature death, whereas after terminal differentiation of cardiomyocytes beginning at 2 weeks of age, it does not lead to lethality but to moderate cardiac contractile defects. At all these stages, levels of ANF transcripts were markedly decreased shortly after Nkx2-5 deletion.
Previous studies demonstrated Nkx2-5-dependent regulation of ANF through its proximal promoter using reporter assays and electrophoretic mobility shift assays (EMSAs) (4, 9, 18, 26), yet these findings remain to be clarified in vivo. In addition, the proximal promoter is not responsible for ventricular layer-specific expression of ANF in vivo. A recent study identified regulatory elements sufficient for ANF transcription in both physiologically normal and diseased ventricles located within a 140-kb 5′ genomic fragment from the transcription start site using a bacterial artificial chromosome (BAC) DNA fragment (13). Specific transcription factors and their binding elements in physiologically or developmentally normal versus diseased hearts in this large DNA fragment remain to be identified.
To identify Nkx2-5-binding elements in the genomic locus, including the ANF gene, we employed chromatin immunoprecipitation (ChIP) in mouse cardiomyocytes either expressing or lacking Nkx2-5 shortly after tamoxifen-induced gene targeting. We identified the ANF promoter region and four additional 5′ distal regions preferentially immunoprecipitated with Nkx2-5 antibodies from cardiomyocytes expressing Nkx2-5. Chromosome conformation capture (3C) revealed close proximity of the distal elements and the ANF promoter. Indeed, a 5.8-kb DNA fragment, including the proximal promoter and the three upstream elements (kb −34, −31, and −21 regions, 1 to 2.5 kb each), transactivated a lacZ reporter gene in hearts in vivo in an Nkx2-5-dependent manner, recapitulating expression of endogenous ANF.
MATERIALS AND METHODS
Mouse models.
Generation of mice with a tamoxifen-inducible Nkx2-5 gene knockout was described previously (3). To generate mice with a tamoxifen-inducible GATA4 gene knockout floxed--GATA4 mice (Jackson Laboratory, stock no. 008194; generated by Stephan Duncan) (52) were bred with Cre-ER mice (Jackson Laboratory, stock no. 004453; generated by Andrew McMahon) (12). Maternal injection of tamoxifen (0.5 to 1 mg/g body weight, intraperitoneally) was performed at either E10.5 or E19. Tamoxifen was injected into adult mice (6 to 9 weeks of age; body weight, 20 to 25 g) for 2 consecutive days before surgery. Generation of Hey2 germ line knockout mice was previously described (41).
To generate lacZ reporter mice, luciferase plasmid constructs (pGL2 −34−31−21-ANF promoter and −34−31−21-BNP promoter; see below) were subcloned into the pSIB vector (43). All animal care protocols fully conformed to the standards of the Association for the Assessment and Accreditation of Laboratory Animal Care, with approvals from the University of Florida Institutional Animal Care and Use Committees.
ChIP assays followed by real-time PCR.
After genotyping, neonatal hearts isolated from either flox/flox or flox/flox/Cre mice were separated and subjected to serial trypsin digestion followed by Percoll density gradients. Cardiomyocytes (3 × 106 cells per ChIP assay), fixed with 1% formaldehyde, were subjected to ChIP assays (EZ ChIP kit; Millipore) using 5 μg of affinity-purified anti-Nkx2-5 (17), anti-dimethyl-histone H3K4 (07-030; Millipore), anti-trimethyl-histone H3K27 (07-449; Millipore), or control rabbit IgG antibodies (EZ ChIP kit). TaqMan real-time PCR was performed using 10-fold serially diluted input DNA for generating a standard curve (primers and probes are listed in Table 1).
Table 1.
Primers and probes for TaqMan PCR
| Assay and numbera | Location | Sequence |
||
|---|---|---|---|---|
| Forward primer | Reverse primer | Probe | ||
| ChIP | ||||
| 1 | −43776 to −43694 | GGGATTCAGAGACTCAGCATAAGAC | GTACATACTACTACTCTCGGCCTACA | ACGCCCAGAGACCAA |
| 2 | −38496 to −38435 | TGGGAAGCACTTGTGGATCTG | TGGAGAGGATAATGGCTCTTCTGT | CTGGGCCCTGACTTC |
| 3 | −37314 to −37217 | GTTTAGGCAGCCCTGATGTCT | AGTGTTTCCTCCCCTCTTCCT | CCTGCCAAATTCCA |
| 4 | −34995 to −34929 | CTCCATGAGTGGTGTCTGCTTAA | CAGGTACGTGCACACATTGG | CATTGCCACTCTCCC |
| 5 | −31012 to −30896 | ACTGGAGGAGAAGAGCTACTGTTC | TGTGAGCACATTGCAAAATCTTTGA | CAGCCCTCAGTTCATG |
| 6 | −24911 to −24781 | CGTTAGAAGGGACAGCAAGTAAGAA | ACACCCTGACAGAAGTTGAAATTACA | CATGCTCCATTCTTCC |
| 7 | −21187 to −21108 | CTTACATTGTCCTACATGGCCCTTT | CACCCAGAGTACCAAGCAATTGATA | CCCTCCCCATTCCTCA |
| 8 | −17621 to −17557 | GCTGCAGGAAGGGCTTGAA | GGTTTATGGAGACAGCCTCTGA | CATGTGCTCAAAAGTG |
| 9 | −15519 to −15440 | GCATCCTCAAGCTGAAATCTTCACA | GTGGCTCACACAATGGTTTTGATAC | CCACCTTCCAACAACC |
| 10 | −14258 to −14201 | GATCGGATCCGTCAGTCGTTT | AGGGAAGTGGCAAGGTAGGT | CTCACCGTTACAGCCC |
| 11 | −12676 to −12608 | ACATTTGAGCCATCACCTGCTT | CTGGGAAAATGGGTGTGTACTGT | CAGCCTGCACCTCTG |
| 12 | −9543 to −9467 | CTCAGTCCACAGCTTCAGAGATAAA | TCACTCAAGACCGGACAAAGTTG | TTGCCCTCCACATCCT |
| 13 | −6843 to −6771 | CCATTTAGCTGTACTGGCTTCCTA | CTTTCCCCTGATGCCTGGAT | CCTTCCTGCACTGCCC |
| 14 | −2693 to −2600 | ACCAGAATTCTAGACCCCGGATT | GCCTCTCCATGCTCACTTCAG | CTGCCCACCATTGGCT |
| 15 | −309 to −226 | CCCAGGAAGATAACCAAGGACTCT | CACATTCTTGCTGATTTGCCTCAA | CCCCACTTCAAAGGTG |
| 16 | +514 to +586 | TGCCCTCTTGAAAAGCAAACTGA | CCCGAAGCAGCTGGATCTT | TCTGCTCGCTGGCCCT |
| 17 | +1788 to +1857 | GGTGGCCAGTTCAAACGATTT | CCGAGTGATACACGTGGATAGGT | CCACTGCTGCGTCACC |
| 18 | +2981 to +3047 | CCAGATGTCATGGGACAGACA | TGCCTTGTCACCAGTCATAGC | CCCCTGCCCTGTCACC |
| ANF 3C | ||||
| 1 | GACTGGATATGATGACGGAGCAT | TGAGACCCTGCCACCAAAATAAAA | AACCCCAGATCTCTCTCC | |
| 2 | GCTGTCTTCAGATGCACCAGAA | TGAGACCCTGCCACCAAAATAAAA | CAGATCTCTCTCCAGCCCTG | |
| 3 | CCCCTAGAACTGGAATACAGATGGT | TGAGACCCTGCCACCAAAATAAAA | CAGATCTCTCTCCAGCCCTG | |
| 4 | GAGCATTCAGGAGGTAGGAACAG | TGAGACCCTGCCACCAAAATAAAA | CTGGAGAGAGATCTGC | |
| 5 | TCTGGTGTTGCTATGTGTTCAAATTCT | TGAGACCCTGCCACCAAAATAAAA | CCTCCCCAAGATCTCT | |
Chromosome conformation capture (3C).
Formaldehyde-fixed cardiomyocytes (5 × 106 cells per 3C assay) utilized for 3C experiments were subjected to digestion with BglII following the standard protocol provided by J. Dekker (University of Massachusetts Medical School) (8). BAC DNA (20 μg) containing the entire ANF and BNP loci (RPC 123.C, clone 128E8, chromosome 4, 147272452 to 147481609; length, 209,158 bp; Invitrogen) was used as a control. TaqMan real-time PCR was performed using probes near the restriction sites (primers and probes are listed in Table 1).
Reporter assays and cloning of Hey2.
The ANF gene containing the described above BAC clone was subjected to PCR for amplification of the following genomic DNA fragments using specific forward (F) and reverse (R) primers: for the ANF promoter fragment (−451 to +56), 5′-GTCATTGCCTCCTCTCCCGC-3′ (F) and 5′-TGTCTCTGCCCACTCTGGTTTC-3′) (R); for the −34 kb fragment (−36320 to −34624 from the ANF transcription start site), 5′-TGATTACCAGCCCACCTTTGAC-3′ (F) and 5′-ACCCCCAGCCCCGTATGG-3′ (R); for the −31 kb fragment (−31783 to −29218 from the ANF transcription start site), 5′-GCACTTGCTACTAAAGGCGGG-3′ (F) and 5′-GGGGTTCAGAAGGGTCTTATCGTC-3′ (R); and for the −21 kb fragment (−21627 to −20582 from the ANF transcription start site), 5′-GGGCTGAGGGGTCACACAATC-3′ (F) and 5′-TTGGGTTTAGGGTCCACCTTATG-3′ (R). The PCR fragments were cloned into pCR-Blunt II-TOPO or pCR2.1 vector (Invitrogen), sequenced, and inserted into the basic plasmid pGL2 (Promega) using appropriate restriction enzyme sites.
Neonatal cardiomyocytes isolated as described above were plated in 12-well plates. The next day, cells were cotransfected with 2 μg of luciferase reporter constructs and 0.5 μg of Rous sarcoma virus β-galactosidase constructs using the calcium phosphate method for 2 h as described previously (18).
Mouse Hey2 cDNA was cloned from RNA that had been isolated from neonatal mouse hearts and subjected to reverse transcription using random priming followed by PCR using two sets of specific primers to amplify partial mouse Hey2 cDNA: for fragment 1, 5′-GCAGGGAGGGAGGGAGGAAG-3′ (F) and 5′-AGCACTCTCGGAATCCAATGC-3′ (R), and for fragment 2, 5′-CCGACAACTACCTCTCAGATTATGG-3′ (F) and 5′-GGCGTTTTCCTTTTCCAAGTCAG-3′ (R). Two fragments were used for amplifying full-length Hey2 cDNA by PCR. Hemagglutinin (HA)-tagged full-length Hey2 was amplified by PCR (F, 5′-GAAGCTTATGTACCCATACGATGTTCCAGATTACGCTAAGCGCCCTTGTGAGGAAACGA-3′; R, 5′-GGCGTTTTCCTTTTCCAAGTCAG-3′; underlining indicates the HA sequence). The PCR product was cloned into pcDNA3 vector (Invitrogen) and sequenced.
Next, 0.5 μg of pcDNA3 or pcDNA3HA-Hey2, 2 μg of luciferase reporter constructs, and 0.5 μg of CMV β-galactosidase constructs were cotransfected into neonatal cardiomyocytes using the calcium phosphate method for 2 h.
X-Gal staining, whole-mount in situ hybridization, and measurement of β-galactosidase activity.
Mouse embryos and hearts were stained with the β-galactosidase substrate X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) and were photographed before and/or after clearing as described previously (44). Whole-mount in situ hybridization was performed using the mouse ANF 578-bp PCR products (F, 5′-TGGGCAGAGACAGCAAACATC-3′; R, 5′-TGACACACCACAAGGGCTTAGG-3′) or BNP 429-bp PCR products (F, 5′-TGGGGAGGCGAGACAAGGG-3′; R, 5′-TCTTCCTACAACAACTTCAGTGCG-3′) as described previously (35). Heart homogenates in reporter lysis buffer (Promega) were utilized for measurement of β-galactosidase activity using o-nitrophenyl-β-d-galactopyranoside normalized by protein content (BCA protein assay kit; Pierce).
Real-time RT-PCR.
Real-time reverse transcription-PCR (RT-PCR) was performed using inventoried TaqMan gene expression assays (Applied Biosystems): ANF, Mm01255748; BNP, Mm00435304; βMHC (β myosin heavy chain), Mm00600555; lacZ, Ac03987581; GATA4, Mm00484689; and Hey2, Mm00469280. Data were normalized to β-actin expression (assay no. 4352933E). Results from triplicate experiments were averaged.
Western blotting.
The primary antibodies were GAPDH monoclonal antibody (TRK5G4-6C5; Research Diagnostics) and HA monoclonal antibody (2367; Cell Signaling).
Transverse aortic constriction (TAC) and pressure measurement.
TAC and left ventricular pressure were measured with a Millar catheter using a standard method as described previously (37, 45).
Northern blotting.
Northern blotting was performed using probes described previously (5, 47).
Statistical analyses.
Results among groups were compared using analysis of variance (ANOVA) and Fisher's post hoc test (StatView version 5.01). A P value of <0.05 is considered significant.
RESULTS
Binding of Nkx2-5 in the ANF gene (Nppa) locus.
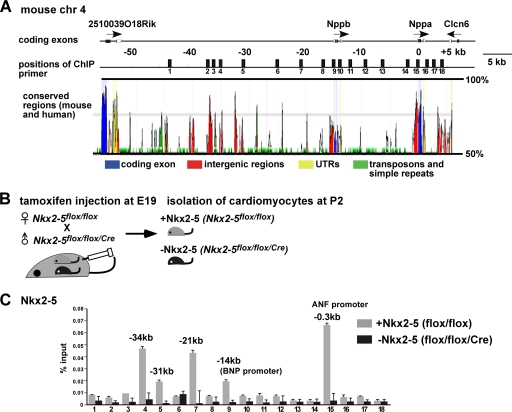
Nkx2-5 has been shown to regulate transcription of the ANF and BNP genes, which are localized in tandem and separated by approximately 14 kb on mouse chromosome 4 (Fig. 1A) (47, 48). In order to understand Nkx2-5-dependent transcriptional regulation, we examined Nkx2-5 binding to the ANF/BNP gene locus by ChIP in mouse neonatal cardiomyocytes. Two different cell populations of mouse neonatal cardiomyocytes, one expressing and one lacking Nkx2-5, were isolated from Nkx2-5flox/flox or Nkx2-5flox/flox/Cre mice after maternal injection of tamoxifen (Fig. 1B). Our previous studies demonstrated that Nkx2-5 expression was almost completely eliminated after perinatal tamoxifen injection at postnatal day 2 (P2) (3), which reduced ANF transcription by 90% at P4 (47).
Fig. 1.
Profile of Nkx2-5 occupancy at a genomic locus, including the ANF and BNP genes. (A) Organization of the genomic locus, including the ANF (Nppa) and BNP (Nppb) genes. Numbers indicate distances, in kilobases (kb), from the ANF transcription start site. Eighteen positions of ChIP-PCR primer-probe sets are indicated. (B) Diagram of experiment demonstrating tamoxifen injection at E19 into pregnant female (Nkx2-5flox/flox) mice mated with male (Nkx2-5flox/flox/Cre) mice, followed by isolation of cardiomyocytes at P2. (C) ChIP analysis of Nkx2-5 in cardiomyocytes expressing Nkx2-5 isolated from flox/flox hearts or lacking Nkx2-5 isolated from flox/flox/Cre hearts. Results are shown as percentage of input DNA recovered (means plus standard errors [SE]) from two independent experiments with PCR performed in duplicate. ANF, atrial natriuretic factor; BNP, brain natriuretic peptide.
After elimination of interspersed repeats and low-complexity DNA sequences using the RepeatMasker program (http://www.repeatmasker.org), the remaining region was utilized for designing TaqMan primer and probe sets for every 1 kb in the genomic locus from kb −44 to +3 relative to the ANF transcription start site (Fig. 1A). Validated primer and probe sets demonstrating linear amplification in serially diluted input DNA were further utilized for ChIP assays (File Builder v3.1; Applied Biosystems) (Table 1). Nkx2-5 antibodies preferentially immunoprecipitated the genomic regions at kb −34, −31, −21, −14 (BNP promoter), and −0.3 relative to the ANF transcription start site in cardiomyocytes expressing Nkx2-5 but not in cardiomyocytes lacking Nkx2-5 (Fig. 1C). Histone modification patterns examined by H3K4 or H3K27 methylation in the ANF/BNP gene locus were not significantly different in the neonatal cardiomyocytes with and without Nkx2-5 (see Fig. S1 in the supplemental material). However, the recovery from input DNA was approximately 10-fold higher with anti-H3K4me2 than anti-H3K27me3 antibodies. This could be due to lower efficiencies of immunoprecipitation using H3K27me3 antibodies or to reduced occupancy of this marker in this gene locus.
Identification of three regulatory DNA elements in the 5′ region of the ANF gene.
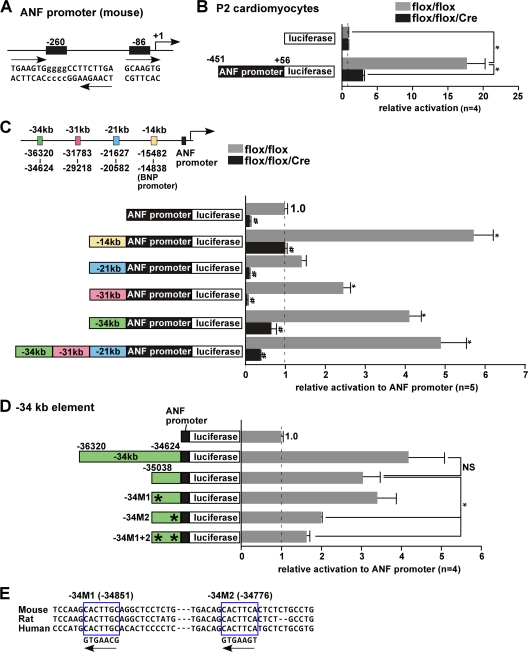
By using reporter assays and EMSAs, we and others previously established that the ANF proximal promoter region includes two potential Nkx2-5-binding sites conserved in mouse and rat (mouse bp −260 and −86 sites and rat bp −242 and −83 sites) (Fig. 2A) (9, 18, 26). In neonatal cardiomyocytes (P2) expressing endogenous levels of Nkx2-5 proteins, the proximal ANF promoter (bp −451 to +56 relative to the transcription start site) mediated 18-fold activation of a reporter gene relative to the control promoterless construct. The activation was reduced by 85% in cardiomyocytes lacking Nkx2-5 protein (Fig. 2B). Addition of the four upstream Nkx2-5-binding elements (kb −34, −31, −21, and −14 BNP promoter elements) identified in ChIP assays increased luciferase activity 1.7- to 5.5-fold in Nkx2-5 expressing cardiomyocytes (Fig. 2C). When three elements (kb −34, −31, and −21) were included in tandem (−34−31−21-ANF-luc), we observed a slight further increase in luciferase activity with strong suppression in the absence of Nkx2-5 compared to addition of each element alone. These results indicate that fragments enriched in ChIP assays include Nkx2-5-dependent promoter and enhancer activities in neonatal cardiomyocytes.
Fig. 2.
Functional characterization of the Nkx2-5-responsive sites in the proximal promoter and enhancer elements for ANF gene expression. (A) Schematics of two Nkx2-5 consensus binding sites in the mouse ANF proximal promoter located at bp −260 and −86 from the ANF transcription start site. The bp −260 site corresponds to the rat bp −242 site, including palindromic consensus binding sites, and the bp −86 site corresponds to the rat bp −87 site (18). (B) Relative luciferase reporter activities of ANF proximal promoter (−451 to +56) normalized to β-galactosidase activity, with the value of the promoterless luciferase reporter defined as 1 (values are means ± SE). *, P < 0.0001 (ANOVA). (C) Analysis of enhancer activities of the four 5′ upstream Nkx2-5-binding elements located around kb −14 (BNP promoter), −21, −31, and −34 relative to the ANF transcriptional start site in the presence of Nkx2-5 (flox/flox). The corresponding induction of luciferase reporter activities was normalized to β-galactosidase activity, with the value for the ANF(−451 to +56) luciferase reporter defined as 1 (values are means ± SE). *, P < 0.05 in comparison to the ANF promoter; #, P < 0.05 for flox/flox versus flox/flox/Cre mice (ANOVA). (D) Analysis of deletion and point mutations in the −34-ANF promoter luciferase construct demonstrating that the M2 mutation eliminates enhancer activity. (E) Sequence of two consensus Nkx2-5 binding sites located at bp −34851 and −34776 relative to the ANF transcription start site.
Functional characterization of the Nkx2-5-responsive site in the kb −34 region.
We next examined the Nkx2-5-responsive site(s) in the kb −34 element (1,696 bp), which yielded the strongest enhancer activity among the three elements with the exclusion of the BNP promoter region. Using several deletion mutants, we found that the 3′ end of the kb −34 element (414 bp) exhibited enhancer activity (Fig. 2D). This region contains two Nkx2-5 consensus binding sites, which are conserved in mice, rats, and humans (bp −34851 and −34776 sites) (Fig. 2E). Point mutations of −34851 (−34M1) and/or −34776 (−34M2) from AAG to CCG revealed that the bp −34776 site includes predominantly Nkx2-5-dependent enhancer activity.
Dynamic changes in the chromatin configuration of the ANF gene locus upon Nkx2-5 binding.
The reporter constructs used in transfection studies contained the ANF promoter and enhancer elements adjacent to each other; thus, the constructs do not reflect the chromatin structure within the endogenous ANF gene locus. However, proximity could be established by physical contact between the enhancer and the promoter mediated by protein-protein interactions (27). In fact, protein-protein interactions of Nkx2-5 with itself and with other cardiac transcription factors have been documented (4, 6, 9, 18, 26). The chromosome conformation capture (3C) method (8) was utilized for determining proximity between the distal elements and the ANF proximal promoter. Briefly, cross-linked genomic DNA in the neonatal cardiomyocytes was digested with the restriction enzyme BglII and ligated in a large volume. Ligation frequencies were quantified by TaqMan real-time PCR using specific primers and probes (Table 1) and were compared to the ligation frequency of non-cross-linked BglII-digested BAC DNA containing the ANF locus.
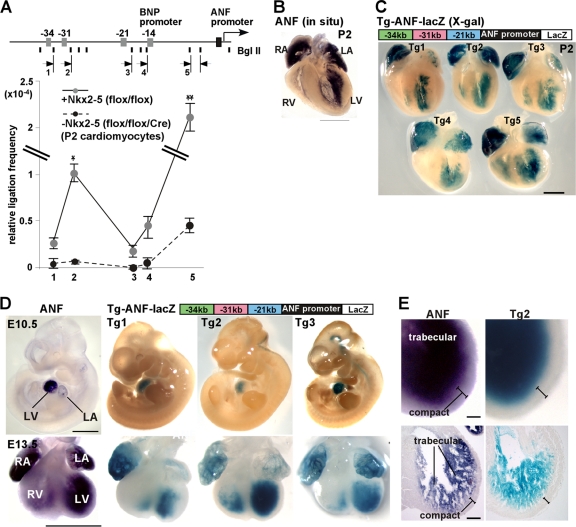
Globally, there was a higher frequency of interactions between the ANF promoter and the 5′ upstream loci (−34, −31, −21, and BNP promoter) in cardiomyocytes expressing Nkx2-5 than in those lacking Nkx2-5 (Fig. 3A). In particular, frequencies of interactions between the ANF promoter and the kb −31 element were higher than those of interactions between the ANF promoter and the kb −34 or −21 region in Nkx2-5-expressing cardiomyocytes. These results suggest that Nkx2-5 mediates close proximity between the distal regulatory elements and the proximal promoter of the ANF gene.
Fig. 3.
Proximity between upstream enhancers and the ANF proximal promoter. (A) Schematic of the ANF locus showing BglII sites and the positions of PCR primers (arrows). 3C analysis of the ANF gene locus was done with cardiomyocytes expressing (gray circles) or lacking (black circles) Nkx2-5. Data were normalized to amplification of BglII-digested and religated BAC clones containing the entire ANF locus (means ± SE) from two independent experiments with PCR performed in duplicate. * and **, P < 0.0001 and P < 0.0018 for flox/flox versus flox/flox/Cre mice (ANOVA). (B and C) Whole-mount in situ hybridization demonstrating endogenous ANF mRNA expression (B) and X-Gal staining of −34−31−21-ANF-lacZ in 5 transgenic lines (C) at P2. (D) Endogenous ANF mRNA expression in comparison to X-Gal staining in developing hearts (E10.5 and E13.5) of −34−31−21-ANF-lacZ transgenic mice (Tg1, Tg2, and Tg3). (E) Enlarged images of left ventricular expression of ANF mRNA and X-Gal staining of −34−31−21-ANF-lacZ, which is positive in the trabecular layer and negative in the compact layer. Bars = 1 mm (B and D) and 100 μm (C). LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Role of Nkx2-5-dependent proximal promoter and 5′ upstream elements in the expression of the ANF gene in embryonic and perinatal hearts.
To examine promoter/enhancer activities in vivo, we generated transgenic lacZ reporter mice (−34−31−21-ANF-lacZ), in which lacZ expression is driven by the ANF promoter and the three distal enhancer elements. The BNP promoter region was excluded from the transgenic construct in consideration of potential interference of transcription initiation from the ANF promoter in vivo. In neonatal hearts, in situ hybridization demonstrated ANF expression in atria and in the inner layer of ventricles (Fig. 3B). Expression of X-Gal-stained −34−31−21-ANF-lacZ demonstrated a similar pattern overall in a total of five different transgenic lines (Tg1 to Tg5) (Fig. 3C). Some variation in localization and intensity of staining among different transgenic lines is likely due to different genomic integration sites and copy numbers. Notably, when the ANF proximal promoter sequence was substituted for that of BNP (−34−31−21-BNP-lacZ), lacZ expression in the neonatal heart was not consistently observed in five separate transgenic lines (see Fig. S2 in the supplemental material). This suggests specificity of the −34−31−21-ANF-lacZ constructs in cardiac expression.
At E10.5 (Fig. 3D, top), expression of both ANF mRNA and X-Gal-stained −34−31−21-ANF-lacZ was mostly restricted to the heart with the exception of weak extracardiac X-Gal staining in two transgenic lines (Tg2 and Tg3). Three transgenic lines (Tg1 to Tg3) were further examined in subsequent experiments because they exhibited less extracardiac expression than the other two transgenic lines (Tg4 and Tg5) (data not shown). In E13.5 hearts, staining intensity of both mRNA and lacZ was higher in the inner trabecular layer than in the outer compact layer (Fig. 3D, bottom, and E).
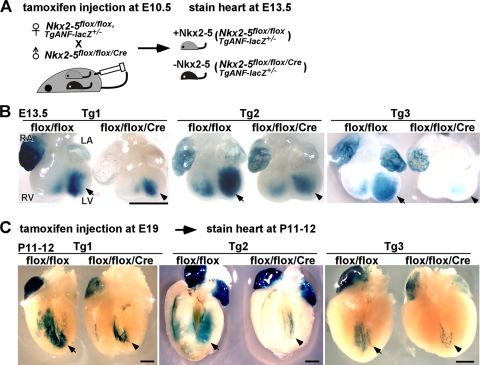
To confirm that −34−31−21-ANF-lacZ expression is Nkx2-5 dependent, as demonstrated by transient-transfection assays in cardiomyocytes, we crossed −34−31−21-ANF-lacZ mice with mice with a tamoxifen-inducible Nkx2-5 knockout. In the following experiments, X-Gal staining was performed side by side using littermates in order to eliminate stage-dependent lacZ expression. First, tamoxifen was injected at E10.5 into a pregnant female, followed by X-Gal staining at E13.5 (Fig. 4A and B). Second, tamoxifen was injected at E19 into a pregnant female, followed by X-Gal staining at P11 to P12 (Fig. 4C). At two different stages in all three transgenic lines, the intensity of X-Gal staining was reduced in Nkx2-5flox/flox/Cre mice compared to Nkx2-5flox/flox littermates.
Fig. 4.
Reduction of X-Gal staining of −34−31−21-ANF-lacZ transgenic mice in hearts lacking Nkx2-5. (A) Diagram of experimental system indicating tamoxifen injection at E10.5 and X-Gal staining at E13.5. (B) Representative images of reduced X-Gal intensity in hearts lacking Nkx2-5 in comparison to the flox/flox litters at E13.5 with tamoxifen injection at E10.5. Arrows and arrowheads indicate left ventricular X-Gal staining. Numbers of mice examined were as follows: Tg1, flox/flox = 8 and flox/flox/Cre = 5; Tg2, flox/flox = 5 and flox/flox/Cre = 3; Tg3, flox/flox = 6 and flox/flox/Cre = 3. (C) Representative images of P11-P12 flox/flox and flox/flox/Cre hearts after perinatal tamoxifen injection. Arrows and arrowheads indicate left ventricular X-Gal staining. Number of mice examined: Tg1, flox/flox = 5 and flox/flox/Cre = 2; Tg2, flox/flox = 4 and flox/flox/Cre = 3; Tg3, flox/flox = 3 and flox/flox/Cre = 4. Bars = 1 mm. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Increased expression of ANF mRNA and −34−31−21-ANF-lacZ in the left ventricle in the absence of transcription repressor Hey2.
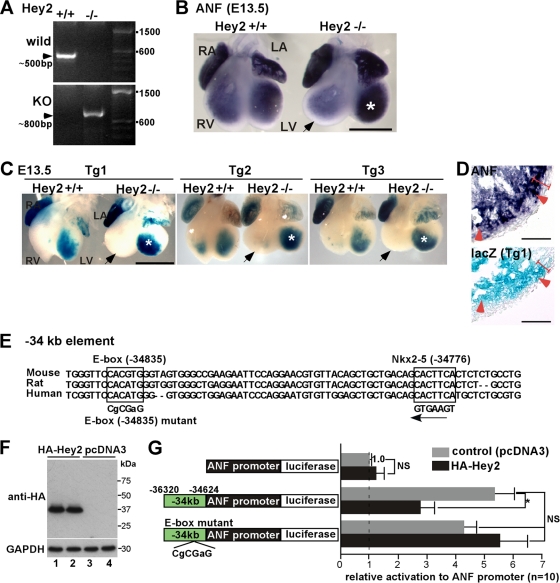
Multiple previous studies have demonstrated that the ANF promoter alone is sufficient for driving expression of reporter genes in the ventricle but is lacking a trabecular-layer-specific reporter gene expression pattern (11, 13), suggesting that the ANF promoter combined with upstream elements is necessary to transactivate the lacZ gene in the trabecular layer of the ventricle. Because Nkx2-5 is expressed throughout the ventricular layer (17, 22, 28), we hypothesized that a repressor interferes with Nkx2-5 specifically in the compact layer. Hey2 (also referred to as CHF1, HRT2, Hesr-2, and HERP1) is a member of the basic helix-loop-helix transcription factor family that preferentially binds to the E-box [CAC(G/A)TG] and is predominantly expressed in the ventricular compact layer (10, 21, 54).
In Hey2 germ line knockout mice (determined by genotyping using PCR) (Fig. 5A), expression of ANF mRNA was increased in the left ventricle, consistent with previous studies (Fig. 5B, asterisks) (21, 54). Intensity of X-Gal staining of −34−31−21-ANF-lacZ in the left ventricle was also increased in Hey2−/− mice in all three transgenic lines (Fig. 5C). Tissue sections revealed ectopic expression of ANF mRNA and −34−31−21-ANF-lacZ in the compact layer (Fig. 5D). These results indicate that the −34−31−21-ANF construct includes Hey2-reponsive elements, which contributes to the elimination of lacZ expression in the compact layer of the left ventricle.
Fig. 5.
Enhanced X-Gal staining of −34−31−21-ANF-lacZ transgenic mice in the left ventricle of transcription repressor hearts lacking Hey2. (A) PCR genotyping of Hey2+/+ (lane 1) and germ line Hey2−/− mice (lane 2). (B) Representative images of in situ hybridization of ANF in Hey2+/+ versus Hey2−/− litters. ANF expression in the left ventricle of Hey2−/− is marked with an asterisk. The right ventricle was rounder and larger in Hey2 knockout mice, in which ANF mRNA was slightly reduced (arrow). (C) Increased X-Gal intensity in the left ventricle of hearts lacking Hey2 (asterisk) in comparison to the control litters at E13.5 in Tg1 to Tg3. The right ventricles of Hey2−/− mice are marked with arrows. Representative images from a total of 9 Hey2+/+ and 8 Hey2−/− heterozygous −34−31−21-ANF-lacZ-positive embryos are shown. (D) Enlarged images of tissue sections demonstrate ectopic expression of ANF and lacZ in the compact layer (arrowheads). The compact layer is defined morphologically as the appearance of a compact band of uniform tissue, while the endocardial noncompacted trabecular layer consists of trabecular meshwork with deep endomyocardial spaces (16). (E) An E-box sequence is located at −34835 bp, close to the Nkx2-5-binding sequence at −34776 bp. The mutated E-box sequence used in the −34-ANF luciferase reporter construct is indicated. (F) Western blotting demonstrates HA-tagged Hey2 protein expression in the transfected cells (lanes 1 and 2) in comparison to control cells transfected with pcDNA3. (G) Effects of Hey2 on ANF(−451 to +56), −34-ANF promoter, and the E-box mutant of −34-ANF luciferase constructs. Induction of luciferase reporter activities was normalized to β-galactosidase activity, with the value in ANF(−451 to +56) luciferase reporter cotransfected with empty pcDNA3 plasmid defined as 1 (values are means ± SE). *, P < 0.05 (ANOVA). Bars = 1 mm (B and C) and 50 μm (D). LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
A consensus Hey2-binding E-box sequence conserved in mice, rats, and humans was found at the bp −34835 site, close to the Nkx2-5-responsive site in the kb −34 element (59 bp 5′ of the Nkx2-5-responsive bp −34776 site) (Fig. 2D and E and Fig. 5E). Cotransfection of a Hey2 expression plasmid with −34-ANF promoter-luciferase reporter plasmid into neonatal cardiomyocytes resulted in substantial reduction of luciferase activities compared to cotransfection of empty plasmid (Fig. 5F and G). At the same dose, we did not observe a reduction of ANF promoter-luciferase expression, consistent with a previous report (53). Point mutations in the E box (CACGTG to CGCGAG) at the bp −34835 site attenuated the reduction of luciferase activity following ectopic expression of Hey2, indicating that the bp −34835 site includes predominant Hey2-dependent repressor activity in the kb −34 element (Fig. 5G).
A number of in vitro studies, including ours, demonstrated that a GATA transcription factor, GATA4, transactivates the ANF gene by binding to the ANF promoter region (9, 26, 32). Hey2-dependent ANF repression has also been reported to be mediated by a reduction of GATA4 binding to the ANF promoter region (10, 19, 53). Contrary to in vitro studies, ANF expression was not reduced (34) but instead was slightly increased in GATA4 germ line knockout mice (52), which die around E7.0 to E9.5. To clarify these discrepancies at a later stage of heart development when two separate layers in the ventricles are evident, we employed the same method but replaced floxed-Nkx2-5 with floxed-GATA4 mice (52). Tamoxifen was injected at E10.5 and E11.5, and E13.5 hearts were examined for expression of GATA4, ANF, and BNP by quantitative RT-PCR. Despite the marked reduction of GATA4 after tamoxifen injection, ANF and BNP expression was not reduced but was instead slightly upregulated, consistent with a previous study (52) (see Fig. S3 in the supplemental material). Thus, in embryonic hearts, GATA4 is not required for transactivation of ANF.
Dispensable function of Nkx2-5 in reinduction of ANF in perinatal failing hearts.
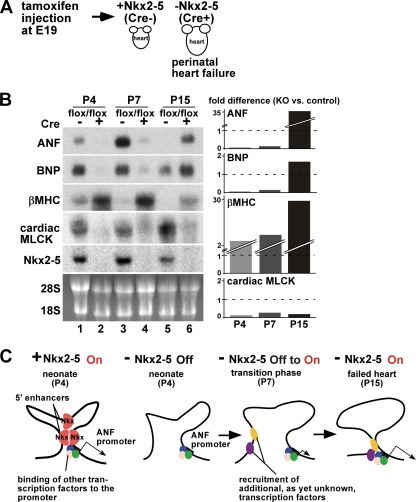
Perinatal deletion of Nkx2-5 causes progressive heart failure with an increased heart weight/body weight ratio within 4 days, leading to premature death at 2 to 4 weeks of age (Fig. 6A) (3). Thus, it was intriguing to examine whether ANF is reexpressed in the failing heart in the absence of Nkx2-5, which is critical for ANF transcription during cardiac development. Expression of ANF mRNA was examined at P4, P7, and P15 by Northern blotting; it was initially below the level of detection shortly after Nkx2-5 deletion at P4, slightly detectable at P7, and markedly upregulated at P15 (Fig. 6B). Reactivation of another fetal cardiac gene, encoding β myosin heavy chain (βMHC), was also increased from P4 to P15 in hearts lacking Nkx2-5, likely representing cardiac contractile dysfunction in these mice.
Fig. 6.
Distinct role of Nkx2-5 in the regulation of the ANF genes under physiologically normal and pathological conditions. (A) Diagram of the experimental system indicating tamoxifen injection at E19. Nkx2-5 deletion causes progressive heart failure within a few weeks (3). (B) Northern blotting shows time-dependent changes in ANF, BNP, and βMHC mRNA in comparison to decreased cardiac MLCK and Nkx2-5 mRNA levels at P4 (lanes 1 and 2), P7 (lanes 3 and 4), and P15 (lanes 5 and 6) in control (flox/flox) versus Nkx2-5 knockout (flox/flox/Cre) hearts. RNA was purified from two or three pooled hearts. (Lanes 1 and 2 are reproduced from reference 47 with permission of the publisher.) Differences in the expression in Nkx2-5 knockout versus control hearts normalized to 28S RNA are shown. (C) Model for transcription of the ANF gene in the heart with (+) or without (−) Nkx2-5 under normal physiological conditions at P4 and at early (P7) and later stages (P15) of failing hearts. Other transcription factors potentially binding at the promoter region (blue, green, and pink) may be GATA4/5/6, serum-responsive factor, TBX5, MEF2C, Baf60C, glucocorticoid receptor, adrenergic signaling through AP1 and SP1, endothelin 1, and Zac1 (14, 23, 25, 36, 49, 55). cis elements and responsive transcription factors (yellow and purple) that mediate reactivation of the ANF gene at P7 and P15 remain to be identified. P, postnatal day; MHC, myosin heavy chain; MLCK, myosin light chain kinase.
These data suggest that Nkx2-5 is necessary for ANF expression in physiological development at P4 but may not be necessary for reactivation of the ANF gene in the heart failure stage (P15) (Fig. 6C). Reduction of X-Gal staining of −34−31−21-ANF-lacZ in P11-P12 hearts in the absence of Nkx2-5 (Fig. 4C) also suggests that the Nkx2-5-binding elements do not play a role in reactivation of the ANF gene.
Nkx2-5-independent ANF transcription in pressure-overloaded adult hearts.
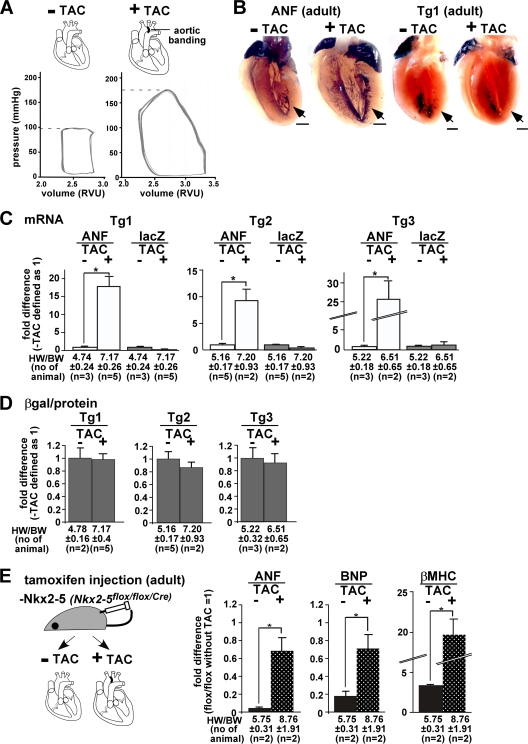
To further examine a potential role for Nkx2-5 in gene regulation in diseased hearts, we utilized the pressure-overloaded hypertrophy model induced by transverse aortic constriction (TAC) in adult mice (Fig. 7A). In normal adult hearts, endogenous ANF mRNA expression was restricted with respect to the surface of the trabecular layers. In contrast, in pressure-overloaded hearts following TAC, ANF expression expanded toward the outer layer after 2 weeks and was accompanied by an increase in heart size and wall thickness (Fig. 7B, ANF). In contrast, X-Gal-stained −34−31−21-ANF-lacZ expression was not changed with 70 mmHg increases in the maximum left ventricular systolic pressure following TAC (Fig. 7B, Tg1).
Fig. 7.
Nkx2-5-independent ANF transcription in pressure-overloaded adult hearts. (A) Diagram of experiments, demonstrating pressure overload (transverse aortic constriction [TAC]), and a representative LV pressure-volume curve with and without TAC, demonstrating increased LV systolic pressure 2 weeks after TAC. Volume is shown in relative volume units (RVU) without additional calibration. (B) Endogenous ANF mRNA expression compared to X-Gal staining of −34−31−21-ANF-lacZ transgenic mice in adult hearts with or without pressure overload (TAC). Bars = 1 mm. (C) Difference, as determined by TaqMan real-time RT-PCR, between ANF and lacZ mRNA expression, with expression without TAC defined as 1. Increase of ANF but not lacZ mRNA expression in pressure-overloaded hearts is demonstrated in three transgenic lines. (D) Difference of lacZ enzymatic activities normalized to the amount of proteins with or without TAC in three transgenic lines. lacZ expression without TAC was defined as 1. The heart weight-to-body weight ratios (HW/BW) (means ± SE) and the number of mice examined are indicated. (E) Diagram of experiments utilizing mice lacking Nkx2-5 with or without TAC in adult mice. The difference in ANF, BNP, and βMHC mRNA expression in Nkx2-5 knockout hearts with and without TAC relative to the control flox/flox heart without TAC (defined as 1) is shown. Increase in ANF, BNP, and βMHC mRNA expression in the pressure-overloaded hearts is demonstrated in Nkx2-5 knockout hearts. The HW/BW ratios (means ± SE) and the number of mice examined are indicated.
Quantitative measurements of ANF mRNA confirmed 10- to 25-fold upregulation relative to control sham-operated mice (without TAC) with increased heart weight/body weight ratios after pressure overloading in multiple mice in all three transgenic lines (Fig. 7C). In contrast, there was no increase in lacZ mRNA (Fig. 7C) or lacZ enzymatic activity in the heart lysates (Fig. 7D).
In contrast to what has been observed in embryonic and perinatal mice (3, 50), Nkx2-5 deletion at later stages (after 2 weeks of age) does not cause lethality despite the presence of mild contraction defects (47). Tamoxifen-injected adult Nkx2-5flox/flox/Cre mice were subjected to pressure overloading for 1 week and compared to sham-operated mice (Fig. 7E). ANF transcription in Nkx2-5flox/flox/Cre mice was reduced 0.04-fold compared to control Nkx2-5flox/flox littermates after tamoxifen injection without TAC but was upregulated after pressure overloading in the absence of Nkx2-5 (increased 0.04- to 0.69-fold in comparison to flox/flox control mice). In addition, levels of the cardiac hypertrophic markers BNP and βMHC were increased in hearts lacking Nkx2-5 after TAC (Fig. 7E).
Taken together, these studies demonstrate that −34−31−21-ANF-lacZ construct lacks DNA regulatory elements that respond to pressure overload as well as to perinatal heart failure. These data also suggest that Nkx2-5 binding may not be necessary for ANF reactivation following left ventricular pressure overloading, despite several studies suggesting that Nkx2-5 is involved in the reactivation of ANF transcription during pressure overloading (1, 40, 51).
DISCUSSION
Transcription factor Nkx2-5 has been widely used as a marker gene for the cardiomyocyte lineage (2, 46) and has been shown to regulate a critical set of genes to maintain proper cardiac formation and function, including ANF (47, 48, 50). To our knowledge, this study is the first to systemically examine Nkx2-5-responsive regulatory DNA elements in the ANF gene locus using biologically relevant mouse neonatal cardiomyocytes. These elements, including four 5′ regulatory elements (kb −34, −31, −21, and −14) and the ANF proximal promoter, were identified using ChIP assays. A 5.8-kb genomic fragment including these four elements transactivated a reporter gene (−34−31−21-ANF-lacZ) and yielded expression patterns that were comparable to those of the endogenous ANF gene in embryonic, neonatal, and adult hearts under normal physiological conditions in vivo and markedly downregulated in mice with Nkx2-5 deleted.
The specific chromosomal configurations mediated by Nkx2-5 could be explained by homodimerization of Nkx2-5 proteins that bind to different sites (18) and/or heterodimerization with other transcription factors, including TBX5, SRF, and Zac1 (4, 6, 55). One possibility is that these factors bind to nearby genomic elements, interact with each other, and thereby dynamically modify chromosomal configurations to form an active chromatin hub (38). Nkx2-5 binding at the proximal ANF promoter has previously been demonstrated in in vitro transfection assays and in EMSAs (18, 26). The binding of Nkx2-5 to the promoter in in vivo, which contains two consensus Nkx2-5 binding sites (TNAAGTG), was confirmed in this study. The newly identified functional Nkx2-5 binding site in the kb −34 enhancer activity has the sequence TGAAGTG (bp −34776 site). Interestingly, the Nkx2-5 binding sites at bp −34776 and bp −260 are identical. Despite an extensive analysis of mutations in the consensus Nkx2-5 binding sites as well as in the core binding sequence (AAG), we could not map the Nkx2-5 binding site(s) in the kb −31 enhancer element. This could be interpreted as an indication that Nkx2-5 binding in the kb −31 element occurs via a nonconsensus sequence or that Nkx2-5 is recruited to this site by other DNA-binding proteins.
Expression of endogenous ANF mRNA and −34−31−21-ANF-lacZ is restricted to the inner trabecular layer of ventricles, while Nkx2-5 is expressed throughout the layers (17, 22, 28). Therefore, in the outer compact layer, Nkx2-5 may not be able to access the binding sites because of a closed chromatin structure of the binding element(s), or Nkx2-5 might be functionally inactivated by a repressor(s). Ectopic lacZ reporter gene expression in the outer compact layer in Hey2 knockout mice indicates that the −34−31−21-ANF-lacZ reporter construct contains Hey2-responsive elements. One Hey2-binding E-box sequence was identified in close proximity to the Nkx2-5 binding site in the kb −34 element.
The underlying mechanisms of Hey2 binding at the −34 kb region leading to reduction of ANF transcription remain to be understood. Hey2 binding at the kb −34 element might modify the chromatin structure of the ANF locus and reduce interactions between kb −34 and ANF promoter regions in the compact layer. We performed 3C assays using neonatal cardiomyocytes isolated from both trabecular and compact layers, which may result in relatively low frequencies of interaction between kb −34 and the promoter regions examined in this study. Hey2 has been shown to recruit the histone deacetylase HDAC1 and the corepressor N-Cor to chromatin (15). A previous study failed to show histone deacetylase-dependent inhibition of the ANF-proximal promoter (10); however, this inhibition remains to be determined in the presence of the distal elements. Another puzzling finding is that the −34−31−21-ANF-lacZ reporter construct as well as ANF mRNA was upregulated only in the left ventricle, despite the presence of Hey2 in the compact layers of both ventricles, consistent with previous studies (21, 54). Mechanisms governing regional gene regulation between inner trabecular versus outer compact layers in right versus left ventricles remain to be elucidated. Of note, expression of lacZ was excluded from the atrioventricular canal (data not shown), where another inhibitor, Tbx2, has been shown to interact with Nkx2-5 at the ANF promoter (11).
Hey2-dependent ANF gene repression has been shown to associate with reduction of GATA4 binding to the ANF promoter region in in vitro studies (10, 19, 53). However, in vivo, we did not observe a reduction of ANF transcripts shortly after GATA4 deletion at E13.5 in developing hearts, which is consistent with previous studies in germ line GATA4 knockout mice (34, 52).
The ANF enhancer regions identified in this study are located within a 140-kb 5′ genomic fragment from the transcription start site, as determined by using a BAC DNA fragment, a region previously shown to be sufficient for ANF transcription both during development and in pressure-overloaded hearts (13). Binding of Nkx2-5 to the distal elements (kb −34, −31, and −21, and the proximal promoter) is required only for physiological expression of ANF. Combining the results from previous studies (11, 13, 20, 31, 39) and this study, the kb −34, −31, and −21 elements with the proximal promoter region are not involved in or sufficient for the reactivation of ANF, and the other regulatory region(s) within the 140-kb BAC are responsible for the ANF activation in diseased hearts.
cis elements and responsive transcription factors that mediate reactivation of the ANF gene in diseased hearts remain to be discovered. A number of signaling pathways and transcription factors, including GATA4/5/6, serum-responsive factor, TBX5, MEF2C, Baf60C, glucocorticoid receptor, adrenergic signaling through AP1 and SP1, endothelin 1, and Zac1, have been reported to regulate ANF gene expression under physiologically normal or pathological conditions (14, 23, 25, 36, 49, 55). To our knowledge, however, cis elements of all these transcription factors have been examined within the ANF promoter region, which is insufficient for the reactivation of ANF under pathological conditions. Whether these candidate transcription factors bind to the region outside the promoter or new factors are recruited for reactivation of the ANF gene remains to be elucidated.
A previous study demonstrated reduction of Hey2 mRNA 1 week after pressure overloading by TAC (29). We consistently found that Hey2 mRNA was reduced by 50% 2 weeks after TAC (see Fig. S4 in the supplemental material). Therefore, reduction of repressor Hey2 and subsequent reduction of its occupancy in the ANF gene locus outside the regions examined in this study may play a role in reactivation of the ANF gene in pressure-overloaded hearts. Another candidate transcription repressor, neuron-restrictive silencer factor (NRSF), has been shown to regulate ANF expression by binding to the 3′ noncoding exon under normal physiological conditions (24).
Nkx2-5 binding at the BNP proximal promoter was demonstrated by ChIP. We detected a nearly 10-fold higher transcription level mediated by the proximal BNP promoter compared to the ANF promoter in neonatal cardiomyocytes, which was reduced by 27% in the absence of Nkx2-5 (see Fig. S5 in the supplemental material). However, the lacZ reporter construct, including three 5′ elements and the BNP-proximal promoter, had only marginal activity in the heart (see Fig. S2). Based on the genomic structure of the ANF and BNP genes in tandem, the presence of common regulatory elements is plausible, but they are unlikely to be located within the kb −34, −31, and −21 elements. Transient-transfection assays in neonatal cardiomyocytes demonstrated that these two promoters include enhancer activities. It would be interesting to examine these effects in hearts in vivo.
In summary, we demonstrated Nkx2-5 binding at specific genomic sites in the ANF gene locus. These binding elements were found to be sufficient for transactivating a reporter gene in hearts mirroring endogenous ANF gene expression. We further showed that the repressor Hey2 contributes to spatial expression pattern of the reporter gene in the developing heart. These results suggest that Nkx2-5 mediates formation of a chromatin hub in the ANF genomic locus in the developing heart. This active chromatin hub involving Nkx2-5-driven regulation of ANF transcription is not sufficient for the reactivation of ANF expression in the pressure-overloaded heart or during heart failure.
Supplementary Material
ACKNOWLEDGMENTS
We greatly appreciate K.R. Chien, J. T. Lu, K. Sato, N. Horikoshi, H. Matsunami, R. Fiske, K. Fortin, S. P. Oh, C. Ketcham, and P. Sayeski for valuable suggestions.
This work was supported by the National Institutes of Health (HL081577 to H.K.).
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
Published ahead of print on 19 September 2011.
REFERENCES
- 1. Bar H., Kreuzer J., Cojoc A., Jahn L. 2003. Upregulation of embryonic transcription factors in right ventricular hypertrophy. Basic Res. Cardiol. 98:285–294 [DOI] [PubMed] [Google Scholar]
- 2. Beltrami A. P., et al. 2003. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114:763–776 [DOI] [PubMed] [Google Scholar]
- 3. Briggs L. E., et al. 2008. Perinatal loss of Nkx2-5 results in rapid conduction and contraction defects. Circ. Res. 103:580–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bruneau B. G., et al. 2001. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell 106:709–721 [DOI] [PubMed] [Google Scholar]
- 5. Chan J. Y., et al. 2008. Identification of cardiac-specific myosin light chain kinase. Circ. Res. 102:571–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen C. Y., et al. 1996. Activation of the cardiac alpha-actin promoter depends upon serum response factor, Tinman homologue, Nkx-2.5, and intact serum response elements. Dev. Genet. 19:119–130 [DOI] [PubMed] [Google Scholar]
- 7. Christoffels V. M., et al. 2000. Chamber formation and morphogenesis in the developing mammalian heart. Dev. Biol. 223:266–278 [DOI] [PubMed] [Google Scholar]
- 8. Dekker J., Rippe K., Dekker M., Kleckner N. 2002. Capturing chromosome conformation. Science 295:1306–1311 [DOI] [PubMed] [Google Scholar]
- 9. Durocher D., Charron F., Warren R., Schwartz R. J., Nemer M. 1997. The cardiac transcription factors Nkx2-5 and GATA-4 are mutual cofactors. EMBO J. 16:5687–5696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fischer A., et al. 2005. Hey basic helix-loop-helix transcription factors are repressors of GATA4 and GATA6 and restrict expression of the GATA target gene ANF in fetal hearts. Mol. Cell Biol. 25:8960–8970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Habets P. E., et al. 2002. Cooperative action of Tbx2 and Nkx2.5 inhibits ANF expression in the atrioventricular canal: implications for cardiac chamber formation. Genes Dev. 16:1234–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hayashi S., McMahon A. P. 2002. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev. Biol. 244:305–318 [DOI] [PubMed] [Google Scholar]
- 13. Horsthuis T., et al. 2008. Distinct regulation of developmental and heart disease-induced atrial natriuretic factor expression by two separate distal sequences. Circ. Res. 102:849–859 [DOI] [PubMed] [Google Scholar]
- 14. Houweling A. C., van Borren M. M., Moorman A. F., Christoffels V. M. 2005. Expression and regulation of the atrial natriuretic factor encoding gene Nppa during development and disease. Cardiovasc. Res. 67:583–593 [DOI] [PubMed] [Google Scholar]
- 15. Iso T., et al. 2001. HERP, a novel heterodimer partner of HES/E(spl) in Notch signaling. Mol. Cell Biol. 21:6080–6089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jenni R., Oechslin E., Schneider J., Attenhofer Jost C., Kaufmann P. A. 2001. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart 86:666–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kasahara H., Bartunkova S., Schinke M., Tanaka M., Izumo S. 1998. Cardiac and extracardiac expression of Csx/Nkx2.5 homeodomain protein. Circ. Res. 82:936–946 [DOI] [PubMed] [Google Scholar]
- 18. Kasahara H., et al. 2001. Characterization of homo- and heterodimerization of cardiac Csx/Nkx2.5 homeoprotein. J. Biol. Chem. 276:4570–4580 [DOI] [PubMed] [Google Scholar]
- 19. Kathiriya I. S., et al. 2004. Hairy-related transcription factors inhibit GATA-dependent cardiac gene expression through a signal-responsive mechanism. J. Biol. Chem. 279:54937–54943 [DOI] [PubMed] [Google Scholar]
- 20. Knowlton K. U., et al. 1995. Divergent pathways mediate the induction of ANF transgenes in neonatal and hypertrophic ventricular myocardium. J. Clin. Invest. 96:1311–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koibuchi N., Chin M. T. 2007. CHF1/Hey2 plays a pivotal role in left ventricular maturation through suppression of ectopic atrial gene expression. Circ. Res. 100:850–855 [DOI] [PubMed] [Google Scholar]
- 22. Komuro I., Izumo S. 1993. Csx: a murine homeobox-containing gene specifically expressed in the developing heart. Proc. Natl. Acad. Sci. U. S. A. 90:8145–8149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kuwahara K., Nakao K. 2010. Regulation and significance of atrial and brain natriuretic peptides as cardiac hormones. Endocr. J. 57:555–565 [DOI] [PubMed] [Google Scholar]
- 24. Kuwahara K., et al. 2001. The neuron-restrictive silencer element-neuron-restrictive silencer factor system regulates basal and endothelin 1-inducible atrial natriuretic peptide gene expression in ventricular myocytes. Mol. Cell Biol. 21:2085–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. LaPointe M. C. 2005. Molecular regulation of the brain natriuretic peptide gene. Peptides 26:944–956 [DOI] [PubMed] [Google Scholar]
- 26. Lee Y., et al. 1998. The cardiac tissue-restricted homeobox protein Csx/Nkx2.5 physically associates with the zinc finger protein GATA4 and cooperatively activates atrial natriuretic factor gene expression. Mol. Cell Biol. 18:3120–3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Q., Barkess G., Qian H. 2006. Chromatin looping and the probability of transcription. Trends Genet. 22:197–202 [DOI] [PubMed] [Google Scholar]
- 28. Lints T. J., Parsons L. M., Hartley L., Lyons I., Harvey R. P. 1993. Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development 119:419–431 [DOI] [PubMed] [Google Scholar]
- 29. Liu Y., Yu M., Wu L., Chin M. T. 2010. The bHLH transcription factor CHF1/Hey2 regulates susceptibility to apoptosis and heart failure after pressure overload. Am. J. Physiol. Heart Circ. Physiol. 298:H2082–H2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lyons I., et al. 1995. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes Dev. 9:1654–1666 [DOI] [PubMed] [Google Scholar]
- 31. Mayer B., et al. 2002. Molecular cloning and functional characterization of the upstream rat atrial natriuretic peptide promoter. J. Hypertens. 20:219–228 [DOI] [PubMed] [Google Scholar]
- 32. McBride K., Nemer M. 2001. Regulation of the ANF and BNP promoters by GATA factors: lessons learned for cardiac transcription. Can. J. Physiol. Pharmacol. 79:673–681 [PubMed] [Google Scholar]
- 33. McGrath M. F., de Bold M. L., de Bold A. J. 2005. The endocrine function of the heart. Trends Endocrinol. Metab. 16:469–477 [DOI] [PubMed] [Google Scholar]
- 34. Molkentin J. D., Lin Q., Duncan S. A., Olson E. N. 1997. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 11:1061–1072 [DOI] [PubMed] [Google Scholar]
- 35. Moorman A. F., Houweling A. C., de Boer P. A., Christoffels V. M. 2001. Sensitive nonradioactive detection of mRNA in tissue sections: novel application of the whole-mount in situ hybridization protocol. J. Histochem Cytochem. 49:1–8 [DOI] [PubMed] [Google Scholar]
- 36. Oka T., Xu J., Molkentin J. D. 2007. Re-employment of developmental transcription factors in adult heart disease. Semin. Cell Dev. Biol. 18:117–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pacher P., Nagayama T., Mukhopadhyay P., Batkai S., Kass D. A. 2008. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat. Protoc. 3:1422–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Palstra R. J., et al. 2003. The beta-globin nuclear compartment in development and erythroid differentiation. Nat. Genet. 35:190–194 [DOI] [PubMed] [Google Scholar]
- 39. Rockman H. A., et al. 1991. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc. Natl. Acad. Sci. U. S. A. 88:8277–8281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saadane N., Alpert L., Chalifour L. E. 1999. Expression of immediate early genes, GATA-4, and Nkx-2.5 in adrenergic-induced cardiac hypertrophy and during regression in adult mice. Br. J. Pharmacol. 127:1165–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sakata Y., et al. 2002. Ventricular septal defect and cardiomyopathy in mice lacking the transcription factor CHF1/Hey2. Proc. Natl. Acad. Sci. U. S. A. 99:16197–16202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sedmera D., et al. 2003. Spatiotemporal pattern of commitment to slowed proliferation in the embryonic mouse heart indicates progressive differentiation of the cardiac conduction system. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 274:773–777 [DOI] [PubMed] [Google Scholar]
- 43. Seki T., Hong K. H., Oh S. P. 2006. Nonoverlapping expression patterns of ALK1 and ALK5 reveal distinct roles of each receptor in vascular development. Lab. Invest. 86:116–129 [DOI] [PubMed] [Google Scholar]
- 44. Seki T., Yun J., Oh S. P. 2003. Arterial endothelium-specific activin receptor-like kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ. Res. 93:682–689 [DOI] [PubMed] [Google Scholar]
- 45. Shibata R., et al. 2004. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat. Med. 10:1384–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Singh A. M., et al. 2007. Chibby, an antagonist of the Wnt/beta-catenin pathway, facilitates cardiomyocyte differentiation of murine embryonic stem cells. Circulation 115:617–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Takeda M., et al. 2009. Slow progressive conduction and contraction defects in loss of Nkx2-5 mice after cardiomyocyte terminal differentiation. Lab. Invest. 89:983–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tanaka M., Chen Z., Bartunkova S., Yamasaki N., Izumo S. 1999. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development 126:1269–1280 [DOI] [PubMed] [Google Scholar]
- 49. Temsah R., Nemer M. 2005. GATA factors and transcriptional regulation of cardiac natriuretic peptide genes. Regul. Pept. 128:177–185 [DOI] [PubMed] [Google Scholar]
- 50. Terada R., et al. 2011. Ablation of Nkx2-5 at mid-embryonic stage results in premature lethality and cardiac malformation. Cardiovasc. Res. 91:289–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thompson J. T., Rackley M. S., O'Brien T. X. 1998. Upregulation of the cardiac homeobox gene Nkx2-5 (CSX) in feline right ventricular pressure overload. Am. J. Physiol. 274:H1569–H1573 [DOI] [PubMed] [Google Scholar]
- 52. Watt A. J., Battle M. A., Li J., Duncan S. A. 2004. GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proc. Natl. Acad. Sci. U. S. A. 101:12573–12578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xiang F., et al. 2006. Transcription factor CHF1/Hey2 suppresses cardiac hypertrophy through an inhibitory interaction with GATA4. Am. J. Physiol. Heart Circ. Physiol. 290:H1997–H2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xin M., et al. 2007. Essential roles of the bHLH transcription factor Hrt2 in repression of atrial gene expression and maintenance of postnatal cardiac function. Proc. Natl. Acad. Sci. U. S. A. 104:7975–7980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yuasa S., et al. 2010. Zac1 is an essential transcription factor for cardiac morphogenesis. Circ. Res. 106:1083–1091 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.