Abstract
DNA replication is tightly coordinated both with cell cycle cues and with responses to extracellular signals to maintain genome stability. We discovered that human Cdt1, an essential origin licensing protein whose activity must be restricted to G1 phase, is a substrate of the stress-activated mitogen-activated protein (MAP) kinases p38 and c-Jun N-terminal kinase (JNK). These MAP kinases phosphorylate Cdt1 both during unperturbed G2 phase and during an acute stress response. Phosphorylation renders Cdt1 resistant to ubiquitin-mediated degradation during S phase and after DNA damage by blocking Cdt1 binding to the Cul4 adaptor, Cdt2. Mutations that block normal cell cycle-regulated MAP kinase-mediated phosphorylation interfere with rapid Cdt1 reaccumulation at the end of S phase. Phosphomimetic mutations recapitulate the stabilizing effects of Cdt1 phosphorylation but also reduce the ability of Cdt1 to support origin licensing. Two other CRL4Cdt2 targets, the cyclin-dependent kinase (CDK) inhibitor p21 and the methyltransferase PR-Set7/Set8, are similarly stabilized by MAP kinase activity. These findings support a model in which MAP kinase activity in G2 promotes reaccumulation of a low-activity Cdt1 isoform after replication is complete.
INTRODUCTION
Precise and complete genome duplication presents a unique challenge during the cell division cycle. To permit efficient replication, DNA synthesis initiates at many chromosomal sites, known as origins of DNA replication. During G1 phase, origins are loaded with an inactive form of the DNA helicase core, the minichromosome maintenance (MCM) complex. Origins with loaded MCM complexes are “licensed” because they are competent for replication initiation in the subsequent S phase. MCM loading is accomplished through recruitment of MCM complexes from the nucleoplasm by the Cdt1 protein to an origin-bound assembly of the origin recognition complex (ORC) and the Cdc6 protein. ORC and Cdc6 then load MCM onto DNA (48, 57, 58).
Failure to properly control MCM loading can lead to replication errors and genome instability if insufficient origin licensing occurs in G1 or if inappropriate origin relicensing occurs after the onset of S phase. For example, high levels of Cdt1 or Cdc6 activity in S or G2 phase can promote origin relicensing, which leads to extensive rereplication and cell death; modest deregulation of either Cdc6 or Cdt1 promotes genome instability and tumorigenesis (5, 28, 44). Thus far, the best-understood mechanisms restricting origin licensing to G1 phase are cell cycle-regulated accumulation and degradation of licensing proteins and inhibition of several licensing proteins after S-phase onset through phosphorylation by cyclin-dependent kinases (CDKs) (7, 23, 34).
Given the critical need to maintain tight control and coordination of origin licensing, it is likely that additional important regulatory mechanisms have yet to be uncovered. Cell cycle progression is arrested in response to a variety of cellular stresses, including exposure to inflammatory cytokines, bacterial toxins, osmotic shock, etc. (reviewed in references 19, 38, 41, and 68). Furthermore, the signaling pathways mediating cell cycle arrest in response to such stresses are also active during G2 and M phases even in the absence of exogenous stress (14, 29, 42, 65), but little is known about how origin licensing may be influenced by these pathways. We have investigated the regulation of replication licensing factors by the stress-activated mitogen-activated protein (MAP) kinases and have discovered a direct link between these activities and control of the stability and activity of the essential licensing protein, Cdt1.
MATERIALS AND METHODS
Cell culture and manipulations.
HeLa cells were cultured in Dulbecco modified Eagle medium (DMEM) (Difco) supplemented with 10% fetal calf serum (Sigma). Xeroderma pigmentosum group A (XPA)-deficient cells (GM04312) with a documented defect in DNA repair and UV-inducible PCNA loading (3) and their XPA-positive (XPA+) derivative (GM15879) were obtained from the Coriell Institute (GM15879) and cultured in DMEM plus 10% fetal calf serum. HCT-116 cells were cultured in McCoy's medium plus 10% fetal calf serum. HeLa cells were synchronized in early S phase by double thymidine block or in prometaphase by treatment with 2 mM thymidine for 18 h followed by release into 100 nM nocodazole for 10 h. Stress treatments included supplementation to 350 to 500 mM sorbitol, 100 μg/ml tumor necrosis factor alpha (TNF-α), or 100 ng/ml lipopolysaccharide (LPS) (each from Sigma) or dimethyl sulfoxide (DMSO) as controls. Mitogen-activated protein (MAP) kinase inhibitors (Sigma) were used at the following concentrations: p38 inhibitor SB203580 at 30 μM and c-Jun N-terminal kinase (JNK) inhibitor SP600125 at 100 μM. The concentration of SB203580 was selected as the amount necessary to block the sorbitol-induced phosphorylation of MAP kinase-activated protein kinase 2 (MAPKAP-K2) in our cell lines (not shown). The MEK inhibitor was used at 50 μM (compound UO126 from Promega), and the MAPKAP-K2 inhibitor was used at 150 μM (Calbiochem). Cells were transfected with plasmids using 0.005% polyethylenimine (Sigma). DNA damage was generated by a single dose of 20 J/m2. Bromodeoxyuridine (BrdU) labeling and flow cytometry were carried out as described previously (52). Significance testing utilized the Student t test on a minimum of three biological replicates. Plasmid transfections were performed with polyethylenimine; small interfering RNA (siRNA) transfections were performed with 100 nM (each) siRNA duplex using Dharmafect 1 reagent (Dharmacon) according to the manufacturer's guidelines for 48 to 72 h for depletion of p38 and Cdt1; JNK siRNAs were used at 250 nM. Synthetic duplexed RNA oligonucleotides were synthesized by Invitrogen; their sequences are provided in Table 1.
Table 1.
Target sequences for siRNA-mediated depletion
Antibodies.
Antibodies were purchased from the following sources: p38, phospho-p38, c-Jun N-terminal kinase 1 (JNK1), and JNK2, phospho-JNK1, and JNK2, MAPKAP kinase 2, phospho-MAPKAP kinase 2, and PR-Set7/Set8 from Cell Signaling Technologies; Cdc6 (sc-9964), PCNA (PC-10), and hemagglutinin (HA) from Santa Cruz Biotechnology; MCM complex component 2 (Mcm2) (BM28) and origin recognition complex (ORC) subunit 2 (Orc2) from BD Pharmingen; p21 (Ab-10) from Neomarkers; V5 from Invitrogen; green fluorescent protein (GFP) (JL-8) from Clontech; and alpha-tubulin (DM1A) from Sigma. Antibodies to detect Cul4A and DDB1 were a gift from Y. Xiong (University of North Carolina), and Cdt2 antibody was a gift from A. Dutta (University of Virginia). Antibodies to human Cdt1 have been previously described (16). Detection of phosphorylation-dependent Cdt1 mobility shifts required precasting polyacrylamide gels at least 16 h prior to extended electrophoresis in ice-cold Tris-glycine buffer. Phosphorylated peptide antigens (PRPALPA[pT]PPA[pT]PPAA[pS]PSALK and TIM[pS]PGEMEKHL where pT is phosphorylated threonine and pS is phosphorylated serine) were designed, synthesized, and used for rabbit immunizations by 21st Century Biochemicals (Marlboro, MA).
Plasmids and recombinant protein preparation.
Plasmids for expressing glutathione S-transferase (GST)–Cdt1 fusions were generated by recombinational cloning (Gateway LR clonase; Invitrogen) between pENTR-Cdt1 derivatives and pDEST15 for N-terminal GST fusions or pDEST24 for C-terminal fusions. Plasmids for transient-transfection assays were generated by recombination with pcDNA-DEST40 which introduces a C-terminal V5 epitope tag. Stable cell lines were constructed by subcloning the Cdt1 cDNAs into pCLXSN (51) and packaging into retroviral particles by standard protocols in 293T cells followed by selection in 500 μg/ml G418 (Gibco). Silent mutations to render Cdt1 siRNA resistant (beginning with codon 519, ACA TAT GTG AAA CTC GAT [silent mutations underlined]) were introduced by standard cloning methods. GST fusions were partially purified from protease-deficient Escherichia coli (BL21; Invitrogen) as previously described (16).
Chromatin fractionations and protein interaction assays.
Chromatin fractionations were carried out as previously described (17). Whole-cell lysates for immunoprecipitation were prepared in CSK buffer (17) containing S7 micrococcal nuclease and CaCl2 to release chromatin-bound material into the soluble pool and clarified by centrifugation. Supernatants were then diluted with coimmunoprecipitation (co-IP) buffer (50 mM HEPES [pH 7.2], 33 mM potassium acetate [KAc], 0.5 mM EGTA, 0.1% NP-40, 10% glycerol, and protease and phosphatase inhibitors).
In vitro kinase assays.
Purified p38 (Cell Signaling Technologies) was incubated with recombinant protein collected from 100 to 300 ml of bacterial culture in kinase reaction buffer (50 mM Tris-HCl [pH 7.5], 10 mM MgCl2, and 1 mM dithiothreitol [DTT]). Kinase reaction mixtures included 1 μM ATP and 5 μCi [γ-32P]ATP (Perkin Elmer) at 30°C. The kinase reaction was followed by SDS-PAGE and autoradiography. For kinase reactions involving immunoprecipitated MAP kinase or complexes formed on GST-Cdt1 bound to glutathione-agarose beads, roscovitine (Sigma) was included at 25 μM to inhibit any coprecipitating cyclin-dependent kinase (CDK).
RESULTS
Cdt1 is phosphorylated by the p38 and JNK stress-activated MAP kinases.
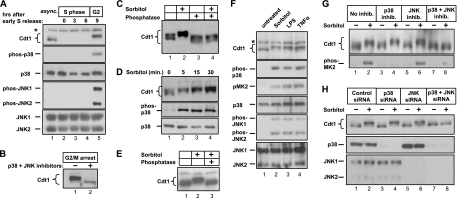
Human Cdt1 is actively degraded throughout S phase, but it reaccumulates once replication is complete (55); this pattern is evident in HeLa cells synchronized by double thymidine block and release (Fig. 1 A, lanes 2 to 5). Early work suggested that the Cdt1 inhibitor geminin is responsible for stabilizing Cdt1 during G2 and M phases (8, 49). However, subsequent investigation revealed that the reason for Cdt1 degradation in geminin-depleted cells is explained by rereplication-associated DNA damage when geminin is downregulated (30). Thus, under normal growth conditions, geminin cannot account for Cdt1 stability after the completion of S phase. Cdt1 in G2 and M phase cells is known to exhibit phosphorylation-dependent changes in gel mobility (33, 55). We note that both p38 and c-Jun N-terminal kinase (JNK) activities are naturally high in G2 and mitosis even in the absence of an exogenous stress (Fig. 1A, lane 5) (12, 14, 15, 43). To test whether Cdt1 phosphorylation late in the cell cycle is MAP kinase dependent, we held cells in prometaphase (G2/M) with nocodazole, conditions that also induce high p38 and JNK activity (64, 69), and then treated the cells with p38 and JNK inhibitors. Within 1 h of inhibitor treatment, we found that endogenous Cdt1 converts to a faster-migrating form and is also reduced in overall abundance (Fig. 1B).
Fig. 1.
Stress triggers MAPK-dependent phosphorylation of Cdt1. (A) Lysates from HeLa cells synchronized by double thymidine block and release were probed for the indicated endogenous antigens (e.g., phos-p38, phosphorylated p38). The position of a nonspecific band is indicated by the asterisk to the left of the gel. async., asynchronous; hrs, hours. (B) HeLa cells were arrested in prometaphase by thymidine-nocodazole synchronization and then treated with a combination of p38 and c-Jun N-terminal kinase (JNK) inhibitors (+) or mock treated (−) for 1 h prior to harvest and detection of endogenous Cdt1. (C) Asynchronous HeLa cells were treated with 500 mM sorbitol for 60 min. Cell lysates were resolved by SDS-PAGE, and endogenous Cdt1 was detected by immunoblotting. For lanes 3 and 4, lysates were treated with a mixture of calf intestinal phosphatase and lambda phosphatase (in the absence of phosphatase inhibitors) prior to electrophoresis. The gel was prepared and electrophoresed using conditions optimized to detect phosphorylation-dependent mobility changes as described in Materials and Methods. (D) Lysates from HeLa cells treated with sorbitol for the indicated times were immunoblotted to detect endogenous Cdt1, phosphorylated p38 (phospho-p38), and total p38. (E) Primary human diploid fibroblasts were treated with sorbitol, and lysates were incubated with protein phosphatase as indicated prior to analysis of endogenous Cdt1. (F) HeLa cells were treated with sorbitol for 30 min, 100 ng/ml lipopolysaccharide (LPS) for 60 min, or 100 μg/ml tumor necrosis factor alpha (TNFα) for 60 min. Lysates were probed for the indicated proteins by immunoblotting. (G) HeLa cells were treated with MAPK inhibitors (inhib.) for 15 min prior to sorbitol treatment, and lysates were probed with anti-Cdt1 antibody or phosphorylated MAPK-activated protein kinase 2 (phos-MK2) antibody. (The p38 inhibitor was SB203580, and the JNK inhibitor was SP600125.) (H) HeLa cells were transfected with control small interfering RNA (siRNA) targeting green fluorescent protein (GFP) (lanes 1 and 2), siRNA molecules targeting p38α and p38β (lanes 3 and 4), siRNA targeting JNK1 and JNK2 (lanes 5 and 6), or siRNA simultaneously targeting all four stress-activated MAPK isoforms (lanes 7 and 8) for 60 h and then mock treated or treated with sorbitol as indicated. Endogenous Cdt1, p38α, p38β, JNK1, and JNK2 were detected by immunoblotting. The p38α and p38β isoforms are recognized by the same antibody and migrate together by SDS-PAGE.
To determine whether exogenous activators of p38 and JNK also induce Cdt1 phosphorylation, we treated asynchronous HeLa cells with sorbitol to acutely and synchronously activate the stress kinase pathway by osmotic shock (22, 68). Strikingly, the Cdt1 protein reproducibly displayed reduced electrophoretic mobility on SDS-polyacrylamide gels in response to osmotic stress (Fig. 1C, lane 2). This mobility shift was reversed by phosphatase treatment, demonstrating that Cdt1 is inducibly phosphorylated during a stress response (Fig. 1C, lane 4). Cdt1 was robustly phosphorylated within 5 min after the addition of sorbitol and just as rapidly as the activation of p38 itself (Fig. 1D). Furthermore, this phenomenon was not an idiosyncrasy of HeLa cells, since Cdt1 was phosphorylated in a variety of cell lines, including primary diploid fibroblasts (Fig. 1E). Cdt1 also showed the same reduced gel mobility in cells treated with the inflammatory cytokine tumor necrosis factor alpha (TNF-α) or the bacterial toxin lipopolysaccharide (LPS) (Fig. 1F), two additional activators of the cellular stress response distinct from osmotic shock.
The only previously identified Cdt1 kinases are the cyclin-dependent kinases (CDKs) (45, 62), but stress signaling inhibits rather than stimulates CDK activity, suggesting that CDKs were not responsible for stress-induced Cdt1 phosphorylation (47, 68). Moreover, a Cdt1 mutant lacking the cyclin binding motif (45, 62) was still phosphorylated in sorbitol-treated cells (data not shown). To determine whether Cdt1 phosphorylation requires p38 and JNK activity, we treated cells with sorbitol in the presence of p38 and/or JNK inhibitors. Neither inhibitor alone had any detectable effect, whereas treatment with both inhibitors completely blocked Cdt1 phosphorylation as measured by the gel mobility shift (Fig. 1G, compare lanes 2 and 8). Inhibitors of the p38 target MAPKAP-K2 (MAP kinase-activated protein kinase 2) or the upstream activator of the related MAP kinases, Erk1 and Erk2, had no effect on Cdt1 (data not shown). To confirm that the effects of the pharmacological inhibitors were specific for their intended targets, we also transfected cells with small interfering RNA (siRNA) molecules to deplete both p38α and p38β (Fig. 1H, lanes 3 and 4) or both JNK1 and JNK2 (Fig. 1H, lanes 5 and 6) or to simultaneously deplete all four of these MAP kinase isoforms (Fig. 1H, lanes 7 and 8). As with the inhibitor treatments, sorbitol induced Cdt1 phosphorylation in cells expressing either p38 or JNK, but not in cells depleted of both p38 and JNK (Fig. 1H, compare lanes 2 and 8), indicating that the two kinases are redundant in this setting. We concluded that Cdt1 is phosphorylated in both acutely stressed cells and in G2/M phase and that this phosphorylation is dependent on p38 and JNK, suggesting that Cdt1 may be a MAP kinase substrate.
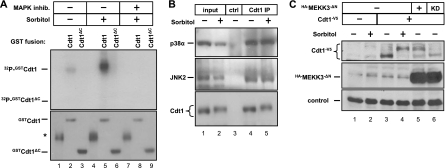
MAP kinases often form stable associations with their bona fide substrates (9, 66). To test whether Cdt1 associates with a stress-inducible kinase, we produced GST, GST-Cdt1, or a truncated version that lacks amino acids 321 to 546, GST-Cdt1ΔC. We immobilized these proteins on glutathione beads and then exposed them to cell extracts. Full-length Cdt1, but not Cdt1ΔC or GST, retrieved a sorbitol-inducible kinase from cell lysates that phosphorylated GST-Cdt1 in vitro (Fig. 2 A, compare lanes 2 and 5), and this kinase(s) was sensitive to the p38 and JNK inhibitors (Fig. 2A, compare lanes 5 and 8). Moreover, endogenous Cdt1 immunoprecipitated from cell lysates bound endogenous p38 and JNK. Cdt1 bound p38 and JNK under both stressed and unstressed conditions (Fig. 2B, lanes 4 and 5), a behavior typical of other MAP kinase substrates (9, 66).
Fig. 2.
Cdt1 associates with stress-inducible MAP kinases. (A) Cells were subjected to sorbitol treatment with or without prior treatment with the p38 and JNK MAPK inhibitors as indicated. Cell lysates were incubated with recombinant GST fusion protein GST-Cdt1 (amino acids 1 to 546) or GST-Cdt1ΔC (amino acids 1 to 320) immobilized on glutathione-Sepharose beads. Bound complexes were then incubated in kinase buffer with [γ-32P]ATP, and phosphorylated substrates were detected by autoradiography. Total protein in the reaction mixture was detected by Coomassie blue staining. (The position of a nonspecific band in the control GST preparation is indicated by the asterisk to the left of the gel [lanes 1, 4, and 7]. The absence of an obvious phosphorylation-dependent mobility shift reflects the different electrophoresis conditions needed in this experiment.) (B) Endogenous Cdt1 was isolated from lysates of sorbitol-treated or mock-treated cells using anti-Cdt1 antibody or normal rabbit serum as a control (ctrl). Proteins coimmunoprecipitating with Cdt1 were analyzed by immunoblotting with antibodies to detect endogenous p38 and JNK. IP, immunoprecipitation. (C) Cells were transfected with plasmids encoding HA-MEKK3ΔN (hemagglutinin [HA]-tagged MEK kinase 3 lacking its autoinhibitory domain, constitutively active) or a kinase dead (KD) version of MEKK3ΔN and cotransfected with plasmid encoding V5-tagged Cdt1 (Cdt1-V5). Cells were treated with sorbitol as indicated and probed with V5 and HA antibodies to detect the ectopic proteins. The loading control is a nonspecific background band.
Finally, we activated p38 and JNK by a molecular genetic approach. We transfected cells with a plasmid encoding V5-tagged Cdt1 (Cdt1-V5) plus a plasmid encoding hemagglutinin (HA)-tagged MEK kinase 3 (MEKK3) lacking its autoinhibitory domain. This form of MEKK3 stimulates downstream activation of p38 and JNK even in the absence of extracellular signals (25). When coexpressed with active MEKK3, Cdt1-V5 underwent a mobility shift even in the absence of a signal (Fig. 2C, compare lanes 4 and 5). A catalytically inactive mutant of MEKK3 (kinase dead [KD]) had no effect on Cdt1-V5 (Fig. 2C, lane 6). These results combined with the results of pharmacological, genetic, and biochemical analyses above demonstrate that Cdt1 is a substrate for stress-activated MAP kinases both in vitro and in vivo.
Cdt1 phosphorylation requires five consensus MAP kinase sites.
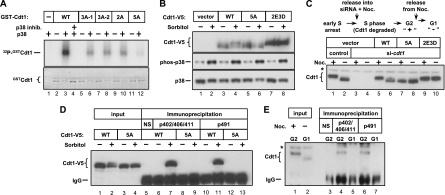
The results of the GST-pulldown experiments (Fig. 2A) suggested that stress-induced Cdt1 phosphorylation sites are C terminal to amino acid 320 and distinct from the site of CDK-mediated phosphorylation at T29 (63). Our tests of additional truncation mutants of Cdt1 narrowed the primary phosphoacceptor region to sites C terminal to amino acid 388 (data not shown). MAP kinases require a proline at the +1 position relative to the phosphoacceptor serine or threonine (4). We converted five such conserved sites in Cdt1 to alanine one at a time and expressed them as GST fusions in bacteria. Each of the single mutants was phosphorylated virtually as robustly as normal Cdt1 by p38 in vitro (data not shown). We then created several combinations of alanine substitutions generating double and triple mutants and a form of Cdt1 in which all five sites were simultaneously converted to alanine. The double and triple mutants were still phosphorylated by p38 in vitro, albeit to a lesser extent than wild-type Cdt1 was (Fig. 3 A, compare lanes 6, 8, and 10 to lane 3). In contrast, Cdt1 phosphorylation was barely detectable when all five phosphorylation sites were converted to alanines (Cdt1-5A), demonstrating that we had identified all of the major p38 target sites for in vitro phosphorylation (Fig. 3A, lane 12).
Fig. 3.
Five sites in Cdt1 are phosphorylated by stress-inducible MAP kinases. (A) Recombinant full-length N-terminal GST-Cdt1 fusion proteins were incubated with active p38 in the presence of [γ-32P]ATP followed by autoradiography. Total Cdt1 was detected by Coomassie blue staining (note the mobility shift in the phosphorylated substrates). The mutated residues in the different GST-Cdt1 constructs are as follows: S372A T402A T406A for 3A-1, T402A T406A S411A for 3A-2, S372A S491A for 2A, and S391A T402A T406A S411A S491A for 5A (in subsequent analysis, S372 was not important for Cdt1 phosphorylation in vitro and thus was not included in the 5A mutant). The substrate for the reaction in lane 1 is GST alone. WT, wild type. (B) HCT-116 cells stably expressing WT Cdt1, Cdt1-5A, or Cdt1-2E3D (S391D T402E T406E S411D S491D) were subjected to sorbitol treatment as indicated. Ectopic Cdt1 or endogenous phospho-p38 and p38 were detected by immunoblotting. (C) HeLa cells stably expressing (untagged) WT Cdt1, Cdt1-5A, or Cdt1-2E3D were synchronized in early S phase by double thymidine block and then released into transfection medium containing cdt1 siRNA or control siRNA plus nocodazole (Noc.) for 10 h. The ectopic Cdt1 expression constructs harbor synonymous mutations at the siRNA binding site. Cells were then released into G1 for 2 h prior to analysis by immunoblotting with anti-Cdt1 antibody. We confirmed that the cells had completed S phase at the G2 time points (data not shown). The position of a nonspecific band is indicated by the asterisk to the left of the gel. (D) HeLa cells stably expressing V5-tagged WT Cdt1 or Cdt1-5A were treated with 0.5 M sorbitol (+) or treated with PBS (−) as a control for 30 min. Lysates were then immunoprecipitated with crude antisera raised against phosphorylated Cdt1 peptides (phosphorylated threonines at positions 402, 206, and 411 [pT402/T406/T411] and phosphorylated serine at position 491 [p491]) and then probed for the V5 tag. The control immunoprecipitate with normal rabbit serum (NS) is indicated. The antibody heavy chain is labeled IgG. (E) HeLa cells were synchronized in G2 by sequential thymidine and nocodazole treatments and then released into G1 for 2 h. Lysates were immunoprecipitated with phospho-specific Cdt1 antisera and then probed for endogenous Cdt1. The control immunoprecipitate with normal rabbit serum (NS) is indicated. The positions of the antibody heavy chain (IgG) and a nonspecific band (asterisk) are indicated to the left of the gel.
To test whether these five sites were required for MAP kinase-mediated Cdt1 phosphorylation in vivo, we created a panel of cell lines stably expressing near-endogenous levels of normal Cdt1, Cdt1-5A, or a new mutant form in which the two threonines were converted to glutamic acid and the three serines were converted to aspartic acid (Cdt1-2E3D). Ectopically expressed Cdt1-5A showed no detectable change in gel mobility in response to sorbitol, indicating that the site(s) responsible for the shift had been altered (Fig. 3B, lane 6). Moreover, the Cdt1-2E3D mutant showed reduced gel mobility even in untreated cells (Fig. 3B, lane 7), suggesting that at least one of these mutations mimics the effects of phosphorylation on Cdt1 gel mobility. To determine whether the MAP kinase target sites are also responsible for Cdt1 phosphorylation in G2/M cells, we synchronized cells by thymidine-nocodazole arrest. For this experiment, we created stable cell lines expressing native Cdt1 (without the epitope tag) but eliminated endogenous Cdt1 by siRNA transfection during the release from thymidine into nocodazole. The Cdt1 constructs used to create these cell lines harbor synonymous mutations in the binding site for the cdt1 siRNA. Since Cdt1 is actively degraded during S phase (e.g., Fig. 1A and reference 53), the presence of cdt1 siRNA prevents the reaccumulation of endogenous Cdt1 in the subsequent G2 phase. By waiting until cells have entered early S phase before siRNA addition, this protocol allows normal origin licensing in G1 and thus normal S-phase progression, because Cdt1 is not required for replication after origin licensing is complete. All of the cells reached G2 DNA content on schedule (confirmed by flow cytometry [not shown]), but cells treated with cdt1 siRNA during S phase lacked Cdt1 protein in the subsequent G2/M phase (Fig. 3C, compare lanes 1 and 3). The cells were then released from the nocodazole block for 2 h by mitotic shake-off. As expected, both endogenous Cdt1 and normal ectopic Cdt1 (wild type [WT]) had slower gel mobility in G2 cells than in G1 cells (Fig. 3C, compare lanes 1 and 5 to lanes 2 and 6). Strikingly, mutation of the Cdt1 MAP kinase sites to unphosphorylatable alanine (Cdt1-5A) completely blocked the nocodazole-induced mobility shift (Fig. 3C, lanes 7 and 8), whereas the Cdt1-2E3D mutant constitutively remained in the slower-migrating form (Fig. 3C, lanes 9 and 10), indicating that these sites are responsible for Cdt1 gel mobility changes in G2/M. It should be noted that the gel mobility shift may be caused by phosphorylation at only a subset of the five sites and furthermore, some amino acids may be phosphorylated by MAP kinases but have no effect on Cdt1 mobility. Thus, although it is a useful indicator of Cdt1 targeting by MAP kinases, we do not presume that altered gel mobility demonstrates phosphorylation at all five sites.
We then raised phosphospecific antibodies using two different Cdt1 peptides. One peptide was triply phosphorylated at the cluster of MAP kinase sites at amino acids 402, 406, and 411; the second peptide included Ser491. The antisera were used for immunoprecipitation with lysates of naïve or sorbitol-treated cell lines expressing V5-tagged Cdt1, and those immunoprecipitates were then probed for the V5 epitope. Both the phospho-402/406/411 (phosphorylated amino acids at positions 402, 406, and 411) and the phospho-491 antibodies efficiently precipitated normal Cdt1 from lysates of sorbitol-treated cells (Fig. 3D, lanes 7 and 11). Moreover, Cdt1-5A was not recognized by either antibody even in lysates of sorbitol-treated cells, indicating that Cdt1 is phosphorylated both on Ser491 and in the 402/406/411 region in vivo. Both phospho-specific antibodies also immunoprecipitated endogenous Cdt1 from lysates of nocodazole-arrested cells, but not from lysates of G1 cells (Fig. 3E, compare lanes 4 and 6 with lanes 5 and 7). The use of these antibodies confirms that Cdt1 is phosphorylated on at least 2 of the 5 (and most likely at least 4 of the 5) MAP kinase sites in the C-terminal region of Cdt1 in vivo both during an acute cellular stress response and during G2 phase.
MAP kinase-dependent phosphorylation stabilizes Cdt1.
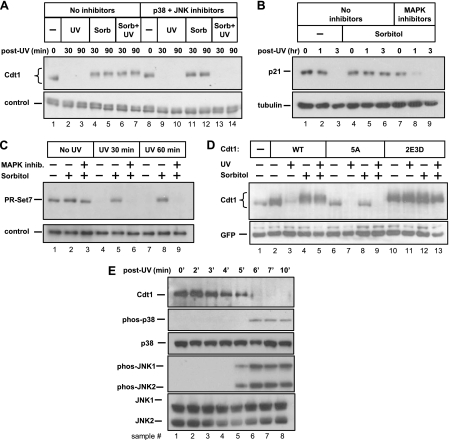
Since treatment of nocodazole-arrested cells with MAP kinase inhibitors led to a decrease in Cdt1 abundance (Fig. 1B), we considered the possibility that MAP kinase phosphorylation directly affects Cdt1 stability. We treated cells with sorbitol to acutely induce Cdt1 phosphorylation and then UV irradiated these cells to rapidly and synchronously trigger Cdt1 polyubiquitination by CRL4Cdt2 (32). In the absence of sorbitol, DNA damage induced the expected degradation of Cdt1 (Fig. 4 A, lanes 2 and 3). Strikingly, however, brief sorbitol pretreatment completely blocked the degradation of Cdt1 induced by UV irradiation (Fig. 4A, lanes 6 and 7). Cdt1 phosphorylation induced by presynchronization with nocodazole also blocked UV-induced degradation (data not shown). Stress signaling did not block UV-induced degradation of Cdc6 and thus did not prevent DNA damage (data not shown). Incubation of cells with p38 and JNK inhibitors prior to UV irradiation not only blocked Cdt1 phosphorylation as before but also restored Cdt1 susceptibility to UV-induced degradation (Fig. 4A, compare lanes 13 and 14 to lanes 6 and 7).
Fig. 4.
MAPK-mediated phosphorylation prevents UV-mediated degradation of three substrates of the CRL4Cdt2 ubiquitin ligase. (A) Asynchronous HeLa cells were subjected to UV irradiation, sorbitol treatment, and combined treatments in the presence and absence of MAPK inhibitors as indicated. Sorbitol (Sorb) and/or inhibitors were added 15 min prior to UV irradiation. The cells were harvested at the indicated times (in minutes), and the lysates were analyzed by immunoblotting with anti-Cdt1 antibody. A nonspecific band serves as a loading control. (B) Lysates of cells irradiated and/or treated with p38 and JNK inhibitors as described above for panel A for the indicated times (in hours) were analyzed for endogenous p21 and tubulin proteins by immunoblotting. (C) Lysates of cells irradiated and/or treated with p38 and JNK inhibitors as in panel A for the indicated times were analyzed for endogenous PR-Set7/Set8 by immunoblotting. A nonspecific band serves as a loading control. (D) HeLa cells transfected with plasmids encoding WT Cdt1, Cdt1-5A (unphosphorylatable) or Cdt1-2E3D (phosphomimetic) mutants were treated with UV and sorbitol as indicated and analyzed by immunoblotting with anti-Cdt1 antibody. (E) HeLa cells were UV irradiated, and whole-cell lysates from time points from 0 to 10 min postirradiation were probed for the indicated endogenous antigens.
Cdt1 degradation during S phase and after UV damage requires interaction with the Cul4 E3 ubiquitin ligase complex through an adaptor protein, Cdt2 (31, 37). Two other substrates of the human CRL4Cdt2 complex have been identified, the p21 CDK inhibitor and the PR-Set7 methyltransferase (also known as Set8) (1, 2, 13, 67). Interestingly, p21 is also a substrate for stress-activated MAP kinases (40). We observed—as had others before (2, 10)—that p21 protein is downregulated after UV irradiation (Fig. 4B, lanes 2 and 3). We further found that like Cdt1, p21 was not targeted for degradation by CRL4Cdt2 in cells pretreated with sorbitol to activate the stress MAP kinases (Fig. 4B, lanes 5 and 6). Also like Cdt1, inhibitors of stress-activated MAP kinases reversed this effect, rendering p21 once again susceptible to degradation (Fig. 4B, lanes 8 and 9). In addition, we observed the same MAP kinase-dependent stabilization of PR-Set7 (Fig. 4C). Cellular stress did not affect the abundance of the CRL4Cdt2 E3 ligase components themselves, arguing against global stabilization of CRL4Cdt2 substrates by downregulating the E3 ligase subunits (data not shown).
To further investigate the mechanism of Cdt1 stabilization, we tested whether the phosphorylation sites in Cdt1 are required for UV resistance. We transfected cells with plasmids encoding normal Cdt1, the unphosphorylatable Cdt1-5A mutant, or the phosphomimetic 2E3D mutant. Although normal Cdt1 was resistant to UV-induced degradation after MAP kinase activation, Cdt1-5A was still susceptible to degradation even in the presence of activated MAP kinase (Fig. 4D, compare lanes 5 and 9). Furthermore, Cdt1-2E3D was not sensitive to UV-induced degradation under any conditions (Fig. 4D, lanes 11 and 13). We note that both p38 and JNK are also activated by UV irradiation (19, 68), but Cdt1 is degraded after DNA damage. In the absence of Cdt1 phosphorylation before UV treatment, however, Cdt1 was mostly degraded before the kinases were activated (Fig. 4E, lanes 5 and 6), indicating that the rapid kinetics of Cdt1 degradation after UV treatment preclude stabilization by UV-activated p38 or JNK. Thus, Cdt1 phosphorylation at one or more of the five MAP kinase sites blocks subsequent DNA damage-induced degradation.
Phosphorylation protects Cdt1 from CRL4Cdt2 after DNA damage and in G2 phase by interfering with binding to Cdt2.
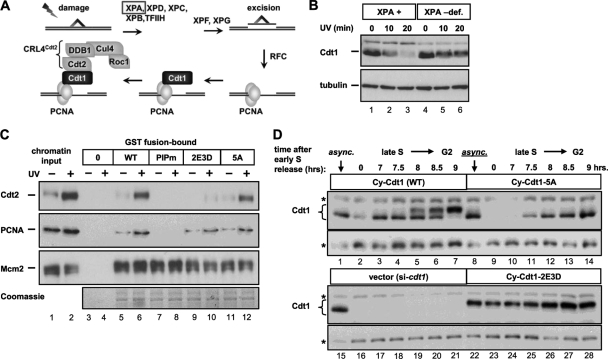
Interaction of Cdt1 with CRL4Cdt2 is dependent on Cdt1 binding to the sliding clamp PCNA, and PCNA must also be loaded onto DNA for a productive interaction between Cdt1 and the Cul4 adaptor Cdt2. This requirement for loaded PCNA applies to both S-phase Cdt1 degradation and to UV-induced degradation of Cdt1 (6, 54, 59). For example, human cells that fail to efficiently load PCNA during UV-induced nucleotide excision repair also fail to efficiently degrade Cdt1 (Fig. 5 A and B). To investigate the mechanism of Cdt1 stabilization by MAP kinases, we prepared recombinant Cdt1-GST fusions expressing wild-type Cdt1, the unphosphorylatable Cdt1-5A, and the phosphomimetic Cdt1-2E3D. For a control, we included a mutant form of Cdt1 that cannot bind PCNA, Cdt1PIPm (30). We tested these proteins for binding to PCNA and Cdt2 in chromatin fractions of UV-treated cells that were sonicated to produce soluble chromatin fragments. As expected, UV induced the chromatin loading of both PCNA and Cdt2 (Fig. 5C, lanes 1 and 2) but had no effect on chromatin loading of MCM subunit 2 (Mcm2) (18). Disruption of the PCNA binding site eliminated PCNA binding to Cdt1, which correspondingly eliminated recruitment of Cdt2 to the Cdt1-PCNA complex (Fig. 5C, lane 8), demonstrating that this in vitro binding assay recapitulates the interactions that occur in vivo. Both wild-type Cdt1 and Cdt1-5A bound PCNA and Cdt2 equally well (Fig. 5C, lanes 6 and 12). Importantly, the phosphomimetic Cdt1-2E3D showed normal binding to PCNA but severely impaired recruitment of Cdt2 (Fig. 5C, lane 10). None of the mutations affected Cdt1 binding to Mcm2 in this assay. Overall, we conclude that MAP kinase phosphorylation sites induce Cdt1 stabilization by blocking the Cdt1-Cdt2 interaction and not the Cdt1-PCNA interaction.
Fig. 5.
Phosphorylation blocks Cdt1 association with Cdt2 and controls Cdt1 stability during S phase. (A) In the case of UV-induced damage, a collection of nucleotide excision repair factors that includes the Xeroderma pigmentosum group A (XPA) protein remove the damaged region, creating a substrate for PCNA loading, Cdt1 interaction, and recruitment of the CRL4Cdt2 ubiquitin E3 ligase via the Cdt2 adaptor. TFIIH, transcription factor IIH; RFC, replication factor complex. (B) UV-induced Cdt1 degradation was monitored by immunoblotting whole-cell lysates of cells derived from patients with a profound XPA deficiency (XPA –def.) that are hence also PCNA loading deficient (3). Cdt1 degradation was compared to degradation in derivatives of those cells reconstituted with the normal XPA cDNA (XPA +). (C) C-terminal GST fusions of wild-type and mutant Cdt1 were partially purified from E. coli and incubated with sonicated chromatin fragments from untreated and UV-irradiated cells. Cdt1PIPm is Cdt1 with a three-amino acid substitution in the PCNA-interacting motif (30). Association of endogenous PCNA, Cdt2, and Mcm2 with GST alone (lanes 3 and 4) or GST-Cdt1 fusion proteins was monitored by immunoblotting, and the isolated GST-Cdt1 proteins were detected by Coomassie blue staining. (D) Stable derivatives of HeLa cells were created with a cyclin-dependent kinase (CDK)-resistant form of Cdt1 that lacks the cyclin/CDK binding site at amino acids 68 to 70 (Cy motif). These Cdt1 constructs also bear mutations in MAP kinase phosphorylation sites as indicated. These cell lines were then synchronized in early S phase by double thymidine block (time zero). Cells were released from the early S block into transfection medium to deplete endogenous Cdt1, and samples were collected from 7 h until 9 h after release to monitor Cdt1 phosphorylation and accumulation during late S and G2. Whole-cell lysates were probed to detect Cdt1 by immunoblotting. The positions of nonspecific bands that serve as loading controls are indicated by asterisks to the left of the gel.
Since Cdt1 degradation during S phase can also be triggered by CRL4Cdt2 (in conjunction with CRL1Skp2 [54]), the mechanism of Cdt1 protection from UV-induced degradation may also apply to S-phase degradation. Likewise, since the stress MAP kinases are active in G2 phase, phosphorylation of Cdt1 in G2 may have effects similar to the effects of phosphorylation of Cdt1 in acutely stressed cells. We therefore tested whether mutation of the MAP kinase phosphorylation sites affects Cdt1 stability during the transition between S phase and G2 phase. To separate Cdt1 ubiquitination by CRL4Cdt2 from ubiquitination by CRL1Skp2, we combined the phosphorylation site mutations with a mutation in the CDK binding site at positions 68 to 70 of Cdt1 (Cy motif). This mutation of the Cy motif effectively blocks Cdt1 phosphorylation by CDKs, and thus, Cdt1 does not bind the Cul1 adaptor Skp2 during S phase (45). We generated stable cell lines expressing these new forms (Cy-Cdt1, Cy-Cdt1-5A, and Cy-Cdt1-2E3D) and eliminated endogenous Cdt1 by siRNA transfection during release from an early S-phase block as in Fig. 3C. By 7 h after release from the thymidine arrest, S phase was nearly complete, and Cy-Cdt1 began to reaccumulate (Fig. 5D, lane 3). Between 8 and 9 h postrelease, the amount of Cy-Cdt1 protein was substantially increased relative to early S phase (Fig. 5D, compare lanes 5 to 7 to lane 2). In contrast, the Cy-Cdt1-5A mutant protein did not show the same phosphorylation shift in S phase or G2, and the kinetics of Cdt1 reaccumulation were consistently delayed relative to normal Cdt1 (Fig. 5D, compare lanes 10 to 14 to lanes 3 to 7). In marked contrast, the phosphomimetic Cy-Cdt1-2E3D protein was not efficiently downregulated in S phase (Fig. 5D, lanes 23 to 28). These patterns of S-phase degradation and reaccumulation indicate that MAP kinase-dependent Cdt1 phosphorylation influences Cdt1 stability not only in response to DNA damage but also during S phase and G2.
p38 and JNK activities contribute to stress-induced inhibition of MCM loading.
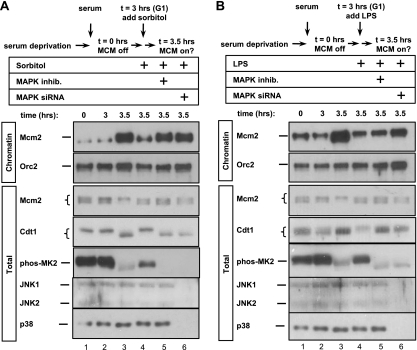
The finding that an essential origin licensing protein is regulated by p38 and JNK MAP kinases prompted us to investigate a potential role for these activities in regulating origin licensing. To test this idea, we utilized HCT-116 cells which can be readily synchronized in G1 by serum deprivation and restimulation. We prepared both whole-cell lysates (total) and chromatin-enriched fractions from G1 cultures. Chromatin association for these experiments is defined as resistance to extraction with nonionic detergent but subsequent susceptibility to solubilization with nuclease (see Materials and Methods for details.) In low-serum medium, Mcm2 was abundant but not significantly associated with chromatin (Fig. 6 A, lane 1), and serum stimulation for 3.5 h induced robust MCM chromatin association (Fig. 6A, lane 3). We also probed whole-cell lysates of these cells with antibody to phosphorylated MAPKAP-K2 and observed, as had been reported previously (26), that at least one isoform of stress MAP kinase, p38, is active in quiescent cells (Fig. 6A, lane 1). Interestingly, phosphorylation of both MAPKAP-K2 and Cdt1 sharply declined just as Mcm2 became chromatin loaded (Fig. 6A, compare lanes 2 and 3). Addition of sorbitol 3 h after serum addition but just prior to MCM loading acutely activated stress MAP kinases and efficiently blocked MCM loading but had no effect on ORC subunit 2 (Orc2) chromatin association (Fig. 6A, compare lanes 3 and 4). These results are consistent with prior observations that osmotic stress by NaCl addition to G1 cells prevents MCM loading (35). To determine whether p38 and JNK have a role in this inhibition, we either treated the cells with MAP kinase inhibitors 15 min prior to sorbitol addition (Fig. 6A, lane 5) or transfected cells with siRNAs to deplete p38α and p38β plus JNK1 and JNK2 during the serum deprivation period (Fig. 6A, lane 6). Depletion of both p38 and JNK or inhibiting their activities had no effect on MCM loading in unstressed cells (data not shown), but strikingly, inhibition of MAP kinase activity prior to sorbitol treatment rescued MCM loading in G1 (Fig. 6A, compare lanes 5 and 6 with lane 4). We obtained very similar results using lipopolysaccharide (LPS) to activate MAP kinases instead of sorbitol (Fig. 6B). Thus, activation of p38 and JNK during G1 can block MCM loading.
Fig. 6.
Stress MAP kinases inhibit MCM loading. (A) HCT-116 cells were synchronized in G0/G1 by serum deprivation and then restimulated with serum for the indicated number of hours. Where indicated, sorbitol was added to a concentration of 0.35 M 3 h after stimulation with serum. A mixture of p38 and JNK inhibitors was added 15 min before sorbitol addition to the cells in lane 5. The cells in lane 6 were transfected with a mixture of four siRNAs targeting p38α and p38β plus JNK1 and JNK2 24 h prior to serum stimulation. Whole-cell lysates (total) and chromatin fractions were analyzed for the indicated endogenous proteins by immunoblotting. (B) As in panel A except that LPS was added to 100 ng/ml 3 h after serum stimulation instead of sorbitol.
The phosphomimetic Cdt1 mutant has reduced function.
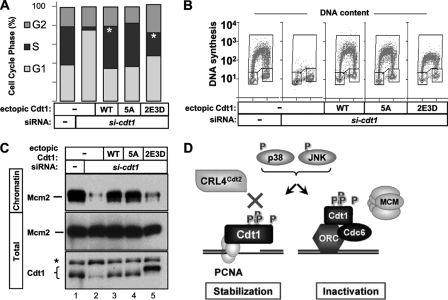
Since Cdt1 is a direct target of p38 and JNK, we sought to determine whether stress-induced phosphorylation affects Cdt1 function in loading MCM complexes. We suppressed endogenous Cdt1 expression by siRNA transfection in asynchronous cells stably expressing normal or mutant Cdt1. We then evaluated DNA content and DNA synthesis by bromodeoxyuridine (BrdU) incorporation 80 h after transfection. The distribution of G1, S, and G2 populations in the five cell lines is plotted in Fig. 7 A; plots from a representative experiment are shown in Fig. 7B. As expected, profound depletion of Cdt1 protein resulted in a severe reduction in the S-phase population due to failed origin licensing in G1, and expression of normal Cdt1 complemented this defect (Fig. 7A and B). The unphosphorylatable Cdt1-5A mutant supported apparently normal DNA synthesis, meaning that neither phosphorylation of Cdt1 at these sites nor the presence of these specific serines and threonines is strictly required for S-phase entry and progression (Fig. 7A, 5A bar, and Fig. 7B, 5A panel). On the other hand, cells expressing only the phosphomimetic Cdt1-2E3D had half as many S-phase cells as cells expressing normal Cdt1 and an increase in both G1 and G2 populations (Fig. 7A). Moreover, cells expressing Cdt1-2E3D did not produce the same high levels of BrdU incorporation as cells expressing normal Cdt1. (BrdU intensity is plotted on a logarithmic scale.) Thus, even when these mutant cells did enter S phase (we presume that HCT-116 cells, like all other tumor-derived cell lines tested [27, 46, 52, 60], fail the origin licensing checkpoint), DNA synthesis was much less efficient.
Fig. 7.
The phosphomimetic Cdt1 mutant is hypomorphic for MCM loading and DNA replication. (A) HCT-116 cells stably expressing WT Cdt1, Cdt1-5A (unphosphorylatable), or Cdt1-2E3D (phosphomimetic mutant) were treated with siRNA targeting endogenous Cdt1 or GST as a control for 80 h and labeled with bromodeoxyuridine (BrdU) for the final hour. DNA content and DNA synthesis were evaluated by flow cytometry using anti-BrdU and propidium iodide. The average proportion of cells in each cell cycle phase was quantified from gated populations of three independent experiments. The standard errors of the means for the cell populations in the G1, S, and G2 cell cycle phases, respectively, are the following: 3.5, 5.1, and 3.0 for control siRNA; 6.5, 2.9, and 6.1 for empty vector plus siRNA targeting cdt1 (si-cdt1); 3.8, 0.6, and 3.9 for WT Cdt1 plus si-cdt1; 0.8, 1.8, and 1.2 for Cdt1-5A plus si-cdt1; and 5.9, 8.4, and 3.0 for Cdt1-2E3D plus si-cdt1. Asterisks indicate statistical significance (P < 0.05) determined by paired Student's t test. (B) Two-dimensional histograms of cell lines transfected with control siRNA or cdt1 siRNA expressing siRNA-resistant WT Cdt1, Cdt1-5A, Cdt1-2E3D, or empty vector. The y axis shows DNA synthesis as measured by fluorescein isothiocyanate (FITC)-conjugated anti-BrdU signal on a logarithmic scale, and the linear x axis shows DNA content measured by propidium iodide staining. (C) The cell lines in panel A were depleted of endogenous Cdt1 with siRNA for 80 h. Chromatin fractions and whole-cell lysates (total) were analyzed for Mcm2 and Cdt1. (D) Model of MAPK-dependent phosphorylation of Cdt1. MAPK-dependent phosphorylation of Cdt1 has two biological consequences: protection from CRL4Cdt2-mediated degradation and functional inhibition. P, phosphate; ORC, origin recognition complex.
We next measured the ability of the phosphomimetic Cdt1 mutant to support MCM chromatin loading. Whole-cell lysates and chromatin fractions were prepared from the asynchronous cultures shown in Fig. 7A and probed for endogenous Mcm2 and for Cdt1 (Fig. 7C). Cdt1 knockdown resulted in a severe defect in MCM chromatin association, and wild-type Cdt1 and Cdt1-5A fully complemented this phenotype (Fig. 7C, lanes 2 to 4). In marked contrast to both wild-type Cdt1 and Cdt1-5A, the Cdt1-2E3D mutant was severely compromised for MCM loading (Fig. 7C, lane 5). Reduced MCM loading is predicted to result in fewer licensed origins, which could readily explain the markedly lower rates of BrdU incorporation in Fig. 7B. If the negative charge substitutions at the five phosphorylation sites indeed mimic the effects of Cdt1 phosphorylation by MAP kinases—as they clearly do for Cdt1 stability—then MAP kinase-mediated phosphorylation inhibits Cdt1 function in MCM loading.
DISCUSSION
We have demonstrated here that the Cdt1 DNA replication licensing factor is a substrate for stress-activated mitogen-activated protein (MAP) kinases. The consequences of this phosphorylation are 2-fold: resistance to CRL4Cdt2-mediated degradation and reduced activity for minichromosome maintenance (MCM) loading.
Though p38 and JNK MAP kinases have been extensively studied in response to acute cellular stresses, additional studies have shown that these kinases are active in G2 during normal cell cycles even in the absence of exogenous stress (12, 14, 29, 42, 65). Cdt1 is phosphorylated by MAP kinases during G2 phase, and our findings indicate that phosphorylation contributes to both inactivation of Cdt1 and Cdt1 reaccumulation once DNA replication is complete. Since Cdt1 reaccumulates in G2/M, but MCM complexes are not normally reloaded until the next G1 phase, we suggest that MAP kinase kinase-mediated Cdt1 phosphorylation serves to inactivate licensing in cooperation with the inhibitory effects of Cdk-mediated phosphorylation of multiple origin licensing proteins and accumulation of geminin.
Neither phosphorylated Cdt1 nor the Cdt1-2E3D phosphomimetic are degraded in UV-irradiated cells. Why would cellular stress inactivate Cdt1 by phosphorylation rather than trigger Cdt1 destruction as occurs after DNA damage? Cdt1 destruction requires either phosphorylation by cyclin A/Cdk2 or interaction with loaded PCNA (54), and neither is necessarily available during a cellular stress response. Thus, inhibition by phosphorylation may have evolved as an alternative mechanism to inactivate origin licensing. Inhibiting Cdt1 by phosphorylation rather than through degradation could also provide opportunities for rapid recovery from stress or rapid reactivation of the G2/M-stabilized Cdt1 once cells complete mitosis. Phosphorylated Cdt1 is also resistant to DNA damage-induced degradation because the mechanism by which Cdt1 is degraded after DNA damage is the same mechanism that targets Cdt1 during S phase. We note that the stabilization of PR-Set7 and p21, combined with the finding that p21 is also a confirmed MAP kinase substrate (40), suggests a general mechanism to control CRL4Cdt2 targets. Moreover, the purpose of active stabilization of Cdt1 (and possibly other CRL4Cdt2 targets) during G2 could be to facilitate efficient licensing in the subsequent G1 phase or possibly to permit an alternate late cell cycle function. For example, the PR-Set7/Set8 methyltransferase is one CRL4Cdt2 target that we demonstrate here is protected by MAP kinase activity, and PR-Set7/Set8 has documented roles in both origin licensing and chromatin compaction during G2 and M phases (11). Rapid stabilization of PR-Set7/Set8 and other CRL4Cdt2 targets after S phase completes may promote efficient execution of late cell cycle events. The fact that null alleles of the Drosophila melanogaster Cdt1 ortholog, “double-parked,” enter but cannot execute mitosis (71) hints at a possible late cell cycle role for Cdt1, but rigorous testing for such a role requires further investigation. The Cdk inhibitor p21 clearly has roles in more than one cell cycle phase also.
The fact that the Cdt1 phosphomimetic mutant very closely recapitulates the effects of bona fide phosphorylation on Cdt1 stability strongly argues that these mutations also recapitulate functional effects of Cdt1 phosphorylation and by inference that phosphorylated Cdt1 has less activity than unphosphorylated Cdt1 does. Despite the hyperstability of the phosphomimetic protein, cells expressing Cdt1-2E3D do not show a significantly higher propensity to rereplicate than cells expressing normal Cdt1 either when expressed alone or in combination with geminin depletion, and the same holds true for Cdt1-5A (data not shown). We interpret these results in light of the opposing effects of phosphorylation on Cdt1 function and stability and as further support for reduced function of the phosphomimetic mutant. The coincidence of Cdt1 phosphorylation in G2 phase when MCM loading is inhibited provides additional circumstantial evidence for a model in which MAP kinase-dependent phosphorylation facilitates the accumulation of Cdt1 at the end of S phase but holds Cdt1 in a low-activity form. In that regard, Cdt1 phosphorylation during G2 may serve as an additional means to avoid relicensing and rereplication when S-phase destruction is no longer an option due to the lack of loaded PCNA.
While this article was being prepared, Miotto and Struhl reported Cdt1 phosphorylation at T29 by JNK1 in response to acute cellular stress (50). Those authors found that phosphorylation of Cdt1 interfered with its recruitment of the HBO1 acetylase to origins. Interestingly, the T29 phosphorylation site which has previously been thought to be a CDK site (63) may not be efficiently recognized by p38 (Fig. 3A), nor is it responsible for the G2 or stress-induced gel mobility shift (Fig. 3B and C). The T29 site is distinct from the five phosphorylation sites that we investigated in this study, though one of the five (S391) was also identified in the Miotto and Struhl study by mass spectrometry (peptides containing the other 4 sites were not measured). We confirmed phosphorylation of the majority of the five C-terminal sites using site-specific phosphorylation-sensitive antibodies; thus, it may be that Cdt1 is phosphorylated on as many as six amino acids by one or both stress MAP kinases. JNK could be primarily responsible for T29 phosphorylation to block HBO1 binding, while both p38 and JNK phosphorylate the five sites near the MCM binding domain to block MCM loading by an additional mechanism.
Regarding the mechanism for Cdt1 inhibition, analysis of chromatin-enriched cellular fractions from synchronized G1 cells revealed no stress-induced differences in Cdt1 chromatin association nor substantial induction of the Cdt1 inhibitor geminin (data not shown). We also found no effect of phosphorylation on the ability of Cdt1 to bind its partner licensing proteins or geminin in endogenous coimmunoprecipitation experiments (data not shown), and GST fusions to the phosphomimetic mutant bind Cdc6, ORC, and MCM subunits (Fig. 5C and data not shown). Four of the five phosphorylation sites (S391, T402, T406, and S411) lie in a flexible region of Cdt1 (36, 39) that is sufficient for Mcm6 binding in vitro (70). Cdt1 is a highly dynamic protein both in vivo (72) and in a reconstituted MCM loading assay using purified Saccharomyces cerevisiae proteins (56) consistent with a model in which Cdt1 rapidly shuttles between the nucleoplasm and an origin-bound ORC-Cdc6 loading machine. Cdt1 phosphorylation may disrupt conformational changes required during these cycles of MCM recruitment, loading, and release. The newly revealed importance of these particular amino acids in Cdt1 function may also be useful in future mechanistic studies of the MCM loading event.
It had previously been shown that activation of the stress kinase pathway during G1 is sufficient to block S-phase entry, but MCM loading was not examined in that study (24). Moreover, Iizuka et al. found that osmotic stress can block MCM loading, but that study did not explore a role for stress-activated MAP kinases (35). On the basis of the low activity of the phosphomimetic mutant, we suggest that Cdt1 phosphorylation by stress-activated kinases is one event that links MAP kinase activity to inhibition of origin licensing. If so, then our discovery (plus that of Miotto and Struhl [50]) mark the first posttranslational modifications of Cdt1 shown to control mammalian Cdt1 function in MCM loading. Interestingly, the unphosphorylatable Cdt1-5A mutant does not render cells resistant to acute stress (data not shown), and we hypothesize that Cdt1 is not the only licensing protein regulated during a stress response. For instance, each of the MCM subunits themselves has conserved candidate MAP kinase phosphorylation sites that could be additional targets (unpublished observations).
We have primarily utilized osmotic stress to activate p38 and JNK in this study, but a great many extracellular signals and cell cycle checkpoints are channeled through p38 and/or JNK. The knowledge that once activated, these kinases regulate Cdt1 and origin licensing will shed important light on the cellular effects of such signaling pathways. The stress MAP kinases are important not only for the response to acute cellular insults and in G2 phase which we have investigated here but they also play key roles in pathways controlling differentiation, migration, proliferation, apoptosis, cell morphology, and the immune response, and the coordination of these responses is extraordinarily complex (19, 38, 41, 68). This new interface of stress signaling activities with origin licensing suggests that the replication licensing system is intimately integrated with multiple pathways of information flow within the cell to preserve genome integrity under a wide variety of situations. Continued investigation may reveal other specific examples of such coordination that will contribute to a comprehensive understanding of dynamic replication licensing control in vivo.
ACKNOWLEDGMENTS
We are grateful to G. Johnson and colleagues for the MEKK3 plasmids and helpful technical advice, to Y. Xiong and A. Dutta for the generous gifts of antibodies, to K. Raiford, J. Bernardo, R. DeAlwise, K. Brantley, and J. Jones for technical assistance, and to members of the Cook lab, R. Duronio, and C. Vaziri, for comments on the manuscript.
This research was supported by NIH R01GM083024 to J.G.C.
Footnotes
Published ahead of print on 19 September 2011.
REFERENCES
- 1. Abbas T., et al. 2010. CRL4(Cdt2) regulates cell proliferation and histone gene expression by targeting PR-Set7/Set8 for degradation. Mol. Cell 40:9–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abbas T., et al. 2008. PCNA-dependent regulation of p21 ubiquitylation and degradation via the CRL4Cdt2 ubiquitin ligase complex. Genes Dev. 22:2496–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aboussekhra A., et al. 1995. Mammalian DNA nucleotide excision repair reconstituted with purified protein components. Cell 80:859–868 [DOI] [PubMed] [Google Scholar]
- 4. Alvarez E., et al. 1991. Pro-Leu-Ser/Thr-Pro is a consensus primary sequence for substrate protein phosphorylation. Characterization of the phosphorylation of c-myc and c-jun proteins by an epidermal growth factor receptor threonine 669 protein kinase. J. Biol. Chem. 266:15277–15285 [PubMed] [Google Scholar]
- 5. Arentson E., et al. 2002. Oncogenic potential of the DNA replication licensing protein CDT1. Oncogene 21:1150–1158 [DOI] [PubMed] [Google Scholar]
- 6. Arias E. E., Walter J. C. 2006. PCNA functions as a molecular platform to trigger Cdt1 destruction and prevent re-replication. Nat. Cell Biol. 8:84–90 [DOI] [PubMed] [Google Scholar]
- 7. Arias E. E., Walter J. C. 2007. Strength in numbers: preventing rereplication via multiple mechanisms in eukaryotic cells. Genes Dev. 21:497–518 [DOI] [PubMed] [Google Scholar]
- 8. Ballabeni A., et al. 2004. Human geminin promotes pre-RC formation and DNA replication by stabilizing CDT1 in mitosis. EMBO J. 23:3122–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bardwell L. 2006. Mechanisms of MAPK signalling specificity. Biochem. Soc. Trans. 34:837–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bendjennat M., et al. 2003. UV irradiation triggers ubiquitin-dependent degradation of p21WAF1 to promote DNA repair. Cell 114:599–610 [DOI] [PubMed] [Google Scholar]
- 11. Brustel J., Tardat M., Kirsh O., Grimaud C., Julien E. 2011. Coupling mitosis to DNA replication: the emerging role of the histone H4-lysine 20 methyltransferase PR-Set7. Trends Cell Biol. 21:452–460 [DOI] [PubMed] [Google Scholar]
- 12. Campos C. B. L., Bédard P. A., Linden R. 2002. Activation of p38 mitogen-activated protein kinase during normal mitosis in the developing retina. Neuroscience 112:583–591 [DOI] [PubMed] [Google Scholar]
- 13. Centore R. C., et al. 2010. CRL4(Cdt2)-mediated destruction of the histone methyltransferase Set8 prevents premature chromatin compaction in S phase. Mol. Cell 40:22–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cha H., Wang X., Li H., Fornace A. J. 2007. A functional role for p38 MAPK in modulating mitotic transit in the absence of stress. J. Biol. Chem. 282:22984–22992 [DOI] [PubMed] [Google Scholar]
- 15. Chuang J. Y., et al. 2008. Phosphorylation by c-Jun NH2-terminal kinase 1 regulates the stability of transcription factor Sp1 during mitosis. Mol. Biol. Cell 19:1139–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cook J. G., Chasse D. A., Nevins J. R. 2004. The regulated association of Cdt1 with minichromosome maintenance proteins and Cdc6 in mammalian cells. J. Biol. Chem. 279:9625–9633 [DOI] [PubMed] [Google Scholar]
- 17. Cook J. G., et al. 2002. Analysis of Cdc6 function in the assembly of mammalian prereplication complexes. Proc. Natl. Acad. Sci. U. S. A. 99:1347–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cortez D., Glick G., Elledge S. J. 2004. Minichromosome maintenance proteins are direct targets of the ATM and ATR checkpoint kinases. Proc. Natl. Acad. Sci. U. S. A. 101:10078–10083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cuadrado A., Nebreda A. R. 2010. Mechanisms and functions of p38 MAPK signalling. Biochem. J. 429:403–417 [DOI] [PubMed] [Google Scholar]
- 20. Curtin J. F., Cotter T. G. 2004. JNK regulates HIPK3 expression and promotes resistance to Fas-mediated apoptosis in DU 145 prostate carcinoma cells. J. Biol. Chem. 279:17090–17100 [DOI] [PubMed] [Google Scholar]
- 21. Dai Y., Rahmani M., Grant S. 2003. Proteasome inhibitors potentiate leukemic cell apoptosis induced by the cyclin-dependent kinase inhibitor flavopiridol through a SAPK/JNK- and NF-kappaB-dependent process. Oncogene 22:7108–7122 [DOI] [PubMed] [Google Scholar]
- 22. de Nadal E., Alepuz P. M., Posas F. 2002. Dealing with osmostress through MAP kinase activation. EMBO Rep. 3:735–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Diffley J. F. 2004. Regulation of early events in chromosome replication. Curr. Biol. 14:R778–R786 [DOI] [PubMed] [Google Scholar]
- 24. Ellinger-Ziegelbauer H., Kelly K., Siebenlist U. 1999. Cell cycle arrest and reversion of Ras-induced transformation by a conditionally activated form of mitogen-activated protein kinase kinase kinase 3. Mol. Cell. Biol. 19:3857–3868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fanger G. R., Johnson N. L., Johnson G. L. 1997. MEK kinases are regulated by EGF and selectively interact with Rac/Cdc42. EMBO J. 16:4961–4972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Faust D., et al. 2005. p38alpha MAPK is required for contact inhibition. Oncogene 24:7941–7945 [DOI] [PubMed] [Google Scholar]
- 27. Feng D., Tu Z., Wu W., Liang C. 2003. Inhibiting the expression of DNA replication-initiation proteins induces apoptosis in human cancer cells. Cancer Res. 63:7356–7364 [PubMed] [Google Scholar]
- 28. Green B. M., Finn K. J., Li J. J. 2010. Loss of DNA replication control is a potent inducer of gene amplification. Science 329:943–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gutierrez G. J., Tsuji T., Chen M., Jiang W., Ronai Z. A. 2010. Interplay between Cdh1 and JNK activity during the cell cycle. Nat. Cell Biol. 12:686–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hall J. R., et al. 2008. Cdt1 and Cdc6 are destabilized by rereplication-induced DNA damage. J. Biol. Chem. 283:25356–25363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Havens C. G., Walter J. C. 2011. Mechanism of CRL4Cdt2, a PCNA-dependent E3 ubiquitin ligase. Genes Dev. 25:1568–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Higa L. A., Mihaylov I. S., Banks D. P., Zheng J., Zhang H. 2003. Radiation-mediated proteolysis of CDT1 by CUL4-ROC1 and CSN complexes constitutes a new checkpoint. Nat. Cell Biol. 5:1008–1015 [DOI] [PubMed] [Google Scholar]
- 33. Hodgson B., Li A., Tada S., Blow J. J. 2002. Geminin becomes activated as an inhibitor of Cdt1/RLF-B following nuclear import. Curr. Biol. 12:678–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hook S. S., Lin J. J., Dutta A. 2007. Mechanisms to control rereplication and implications for cancer. Curr. Opin. Cell Biol. 19:663–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Iizuka M., et al. 2008. Hbo1 links p53-dependent stress signaling to DNA replication licensing. Mol. Cell. Biol. 28:140–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jee J., et al. 2010. Structure and mutagenesis studies of the C-terminal region of licensing factor Cdt1 enable the identification of key residues for binding to replicative helicase Mcm proteins. J. Biol. Chem. 285:15931–15940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jin J., Arias E. E., Chen J., Harper J. W., Walter J. C. 2006. A family of diverse Cul4-Ddb1-interacting proteins includes Cdt2, which is required for S phase destruction of the replication factor Cdt1. Mol. Cell 23:709–721 [DOI] [PubMed] [Google Scholar]
- 38. Johnson G. L., Nakamura K. 2007. The c-jun kinase/stress-activated pathway: regulation, function and role in human disease. Biochim. Biophys. Acta 1773:1341–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Khayrutdinov B. I., et al. 2009. Structure of the Cdt1 C-terminal domain: conservation of the winged helix fold in replication licensing factors. Protein Sci. 18:2252–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim G.-Y., et al. 2002. The stress-activated protein kinases p38 and JNK1 stabilize p21Cip1 by phosphorylation. J. Biol. Chem. 277:29792–29802 [DOI] [PubMed] [Google Scholar]
- 41. Kyriakis J. M., Avruch J. 2001. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81:807–869 [DOI] [PubMed] [Google Scholar]
- 42. Lee K., Kenny A. E., Rieder C. L. 2010. p38 mitogen-activated protein kinase activity is required during mitosis for timely satisfaction of the mitotic checkpoint but not for the fidelity of chromosome segregation. Mol. Biol. Cell 21:2150–2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee K., Song K. 2008. Basal c-Jun N-terminal kinases promote mitotic progression through histone H3 phosphorylation. Cell Cycle 7:216–221 [DOI] [PubMed] [Google Scholar]
- 44. Liontos M., et al. 2007. Deregulated overexpression of hCdt1 and hCdc6 promotes malignant behavior. Cancer Res. 67:10899–10909 [DOI] [PubMed] [Google Scholar]
- 45. Liu E., Li X., Yan F., Zhao Q., Wu X. 2004. Cyclin-dependent kinases phosphorylate human Cdt1 and induce its degradation. J. Biol. Chem. 279:17283–17288 [DOI] [PubMed] [Google Scholar]
- 46. Liu P., et al. 2009. Replication licensing promotes cyclin D1 expression and G1 progression in untransformed human cells. Cell Cycle 8:125–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Manke I. A., et al. 2005. MAPKAP kinase-2 is a cell cycle checkpoint kinase that regulates the G2/M transition and S phase progression in response to UV irradiation. Mol. Cell 17:37–48 [DOI] [PubMed] [Google Scholar]
- 48. Masai H., Matsumoto S., You Z., Yoshizawa-Sugata N., Oda M. 2010. Eukaryotic chromosome DNA replication: where, when, and how? Annu. Rev. Biochem. 79:89–130 [DOI] [PubMed] [Google Scholar]
- 49. Mihaylov I. S., et al. 2002. Control of DNA replication and chromosome ploidy by geminin and cyclin A. Mol. Cell. Biol. 22:1868–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Miotto B., Struhl K. 18 August 2011. JNK1 phosphorylation of Cdt1 inhibits recruitment of HBO1 histone acetylase and blocks replication licensing in response to stress. Mol. Cell doi:10.1016/j.molcel.2011.06.021. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Naviaux R., Costanzi E., Haas M., Verma I. 1996. The pCL vector system: rapid production of helper-free, high-titer, recombinant retroviruses. J. Virol. 70:5701–5705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nevis K. R., Cordeiro-Stone M., Cook J. G. 2009. Origin licensing and p53 status regulate Cdk2 activity during G1. Cell Cycle 8:1952–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nishitani H., Lygerou Z., Nishimoto T., Nurse P. 2000. The Cdt1 protein is required to license DNA for replication in fission yeast. Nature 404:625–628 [DOI] [PubMed] [Google Scholar]
- 54. Nishitani H., et al. 2006. Two E3 ubiquitin ligases, SCF-Skp2 and DDB1-Cul4, target human Cdt1 for proteolysis. EMBO J. 25:1126–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nishitani H., Taraviras S., Lygerou Z., Nishimoto T. 2001. The human licensing factor for DNA replication Cdt1 accumulates in G1 and is destabilized after initiation of S-phase. J. Biol. Chem. 276:44905–44911 [DOI] [PubMed] [Google Scholar]
- 56. Randell J. C., Bowers J. L., Rodriguez H. K., Bell S. P. 2006. Sequential ATP hydrolysis by Cdc6 and ORC directs loading of the Mcm2-7 helicase. Mol. Cell 21:29–39 [DOI] [PubMed] [Google Scholar]
- 57. Remus D., Diffley J. F. 2009. Eukaryotic DNA replication control: lock and load, then fire. Curr. Opin. Cell Biol. 21:771–777 [DOI] [PubMed] [Google Scholar]
- 58. Sclafani R. A., Holzen T. M. 2007. Cell cycle regulation of DNA replication. Annu. Rev. Genet. 41:237–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Senga T., et al. 2006. PCNA is a cofactor for Cdt1 degradation by CUL4/DDB1-mediated N-terminal ubiquitination. J. Biol. Chem. 281:6246–6252 [DOI] [PubMed] [Google Scholar]
- 60. Shreeram S., Sparks A., Lane D. P., Blow J. J. 2002. Cell type-specific responses of human cells to inhibition of replication licensing. Oncogene 21:6624–6632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Singhirunnusorn P., Suzuki S., Kawasaki N., Saiki I., Sakurai H. 2005. Critical roles of threonine 187 phosphorylation in cellular stress-induced rapid and transient activation of transforming growth factor-beta-activated kinase 1 (TAK1) in a signaling complex containing TAK1-binding protein TAB1 and TAB2. J. Biol. Chem. 280:7359–7368 [DOI] [PubMed] [Google Scholar]
- 62. Sugimoto N., et al. 2004. Cdt1 phosphorylation by cyclin A-dependent kinases negatively regulates its function without affecting geminin binding. J. Biol. Chem. 279:19691–19697 [DOI] [PubMed] [Google Scholar]
- 63. Takeda D. Y., Parvin J. D., Dutta A. 2005. Degradation of Cdt1 during S phase is Skp2-independent and is required for efficient progression of mammalian cells through S phase. J. Biol. Chem. 280:23416–23423 [DOI] [PubMed] [Google Scholar]
- 64. Takenaka K., Moriguchi T., Nishida E. 1998. Activation of the protein kinase p38 in the spindle assembly checkpoint and mitotic arrest. Science 280:599–602 [DOI] [PubMed] [Google Scholar]
- 65. Tang J., Yang X., Liu X. 2008. Phosphorylation of Plk1 at Ser326 regulates its functions during mitotic progression. Oncogene 27:6635–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tanoue T., Nishida E. 2003. Molecular recognitions in the MAP kinase cascades. Cell Signal. 15:455–462 [DOI] [PubMed] [Google Scholar]
- 67. Tardat M., et al. 2010. The histone H4 Lys 20 methyltransferase PR-Set7 regulates replication origins in mammalian cells. Nat. Cell Biol. 12:1086–1093 [DOI] [PubMed] [Google Scholar]
- 68. Thornton T. M., Rincon M. 2009. Non-classical p38 map kinase functions: cell cycle checkpoints and survival. Int. J. Biol. Sci. 5:44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang T.-H., et al. 1998. Microtubule-interfering agents activate c-Jun N-terminal kinase/stress-activated protein kinase through both Ras and apoptosis signal-regulating kinase pathways. J. Biol. Chem. 273:4928–4936 [DOI] [PubMed] [Google Scholar]
- 70. Wei Z., et al. 2010. Characterization and structure determination of the Cdt1 binding domain of human minichromosome maintenance (Mcm) 6. J. Biol. Chem. 285:12469–12473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Whittaker A. J., Royzman I., Orr-Weaver T. L. 2000. Drosophila double parked: a conserved, essential replication protein that colocalizes with the origin recognition complex and links DNA replication with mitosis and the down-regulation of S phase transcripts. Genes Dev. 14:1765–1776 [PMC free article] [PubMed] [Google Scholar]
- 72. Xouri G., et al. 2007. Cdt1 associates dynamically with chromatin throughout G1 and recruits Geminin onto chromatin. EMBO J. 26:1303–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]