Abstract
Objective
To examine toddlers’ full-day patterns of cortisol production on child care days and non–child care days, with a particular focus on whether the mid-afternoon elevations at child care persist into the evening or decrease to typical levels observed on non–child care days.
Design
A prospective observational study.
Setting
Four child care centers in a suburban, mid-Atlantic area.
Participants
Forty-two children aged 16 to 24 months attending full-day child care.
Main Exposure
Full-day child care.
Outcome Measure
Salivary cortisol samples obtained at wake-up, mid-morning, mid-afternoon, and bedtime for children on 2 child care days and 2 non–child care days.
Results
Children showed different patterns of cortisol production on child care days compared with non–child care days (, P=.001). Child care days were characterized by an afternoon increase in cortisol levels (unlike non–child care days) and decreases to bedtime values that were comparable with levels on non–child care days.
Conclusion
Results suggest that the effects of child care on children’s cortisol production are time limited across the day.
From about 6 weeks of age, infants begin to show a diurnal pattern of cortisol production with an early morning peak and an evening nadir.1 This pattern becomes increasingly stable across the first year of life,2 but remains “immature” through the preschool years.3(p126) The adultlike pattern in which a decrease is seen from mid-morning to mid-afternoon does not emerge until children are 5 to 6 years of age3; rather, infants, toddlers, and preschoolers show relatively flat mid-morning to mid-afternoon slopes in cortisol when they are at home.3,4 It is during this time of relative immaturity that the system appears particularly susceptible to environmental influences on daytime cortisol production patterns.5–7
On child care days, many children show an increase in cortisol from mid-morning to mid-afternoon,3,4 rather than the decrease that characterizes the mature pattern of cortisol production.8 This morning to afternoon increase is a robust finding that has emerged across day care centers varying in quality,9 child sex,3,9 and various napping conditions.3 However, it has not been clear whether cortisol values decrease during the evening to the bedtime nadir that is typical on days when children do not go to child care or whether values continue to be elevated at night. This is an important step in clarifying the persistence and meaning of child care effects on neuroendocrine functioning, especially given that chronic activation of the hypothalamic-pituitary-adrenal system has been associated with risk of cognitive impairments and compromised immune function.7
Gunnar and colleagues3,7,9 have studied the effects of child care on children’s production of cortisol extensively. The most robust finding is that cortisol increases are seen from mid-morning to mid-afternoon when children are in the child care environment.3,9 Although a number of variables have been identified as potentially important in mediating or moderating the effects of child care on cortisol levels, the picture is complex.
Quality of day care has been associated with the rise in cortisol, with higher-quality day care associated with smaller increases in cortisol.10 Even in good to excellent child care, however, increases in cortisol have usually been seen.9 A shy or inhibited temperament has been associated with elevated afternoon cortisol production in child care,4 but peer rejection, which represents the opposite behavioral tendencies as shyness, has also been associated with elevated afternoon levels of cortisol during the preschool day.11 Watamura et al3 ruled out the possibility that napping patterns were responsible for the effects seen. Gunnar et al11 emphasized that the effects are complex. Nonetheless, across a range of conditions, young children often show a reversed pattern of cortisol production during child care days compared with cortisol production on non–child care days.
The primary aim of the present study was to examine daily cortisol production patterns among toddlers attending full-day center-based care and to compare patterns on child care vs non–child care days. Toddlers were expected to show different daytime patterns on child care days vs non–child care days. Differences were expected in mid-afternoon values, with higher afternoon values at child care compared with afternoon values on non–child care days, replicating prior findings.9 Of particular interest was whether the mid-afternoon elevations at child care persisted into the evening or whether levels decreased, reaching a nadir as on non–child care days.
METHODS
PARTICIPANTS
Forty-four toddlers (aged 16–24 months) were recruited into the study. Children attended full-day center-based child care. Cortisol data were collected for 42 of the 44 children (1 child refused sampling and 1 child left the child care center before sampling was complete). Analyses were conducted in the 42 children who completed salivary cortisol sampling. Of this group, 27 were boys and 15 were girls. Twenty-four were European American, 10 were biracial, 7 were African American, and 1 was Asian American. Annual family income ranged widely, with some families receiving purchase-of-care subsidies and some earning up to $200 000 (range, $4056–$200 000; median, $85 000). Mean age at sampling was 21 months (SD, 2.48 months). All children had been enrolled a minimum of 2 months in their respective classrooms before measures were collected.
CHILD CARE SETTINGS
Children were recruited from 4 child care centers in a suburban, mid-Atlantic area. Centers were accredited by the National Association for the Education of Young Children (NAEYC). The NAEYC Academy for Early Childhood Program Accreditation administers a voluntary accreditation system to help raise the quality of US child care centers. All centers were chosen from a NAEYC list to ensure a minimum standard of care. Included among these was a university child care center that enrolled children of faculty, staff, and community members, offering subsidized care as needed. Most children (73%) were recruited from 6 classrooms at the university child care center. The remaining children were sampled from 1 toddler classroom in each of the other 3 child care facilities. At all centers, classrooms were self-enclosed, separated by age group (infant, toddler, and preschool), and had staff to child ratios between 1:3 and 1:6. The quality of the child care environment was rated by observers using a standardized instrument, which we will describe more fully.
PROCEDURE
Saliva samples were collected from children on 2 days when children were not in child care and on 2 days when children were in child care. Trained research assistants instructed parents in how to collect children’s home samples of saliva. Parents were instructed to collect samples on non–child care days at wake-up, mid-morning (around 10 am), mid-afternoon (around 3 pm), and bedtime for 2 days. Wake-up samples were collected between 6:30 am and 9:37 am (mean, 7:50 am). Mid-morning samples were collected between 8:45 am and 12:30 pm (mean, 10:53 am). Mid-afternoon samples were collected between 1:30 pm and 7:00 pm (mean, 3:44 pm). Bedtime samples were collected between 7:10 pm and 11:13 pm (mean, 8:41 pm).
Parents were also asked to collect salivary samples at wake-up and bedtime for 2 days that the child attended child care. Wake-up samples on these days were collected between 6:00 am and 8:50 am (mean, 7:11 am). Bedtime samples were collected between 7:08 pm and 10:03 pm (mean, 8:32 pm). Trained research assistants collected mid-morning and mid-afternoon saliva samples from the children on child care days. Mid-morning samples at child care were collected between 8:35 am and 11:30 am (mean, 10:03 am). Mid-afternoon samples at child care were collected between 2:30 pm and 4:30 pm (mean, 3:27 pm). Owing to the variability of collection times within each sampling occasion, an alternative time structure (explained below) was used to more accurately represent sampling times and intervals.
Compliance caps (MEM Tracking Caps, Aardex, Corporation) were used to track the time of cortisol sampling when children were not at child care. These caps were placed on the vials that contained the dental cotton rolls used for saliva sampling. When the cap was opened, a microchip recorded the time, providing verification of sampling time. The use of such caps has been shown to enhance sampling compliance.12
Saliva samples were collected by placing one end of a dental cotton roll in a child’s mouth to wet it. The cotton was then dipped in a cup containing 0.8 g of flavored beverage crystals (cherry-flavored drink mix) and placed back in the child’s mouth. The drink crystals were used to stimulate salivation. Use of the flavored crystals has been found to affect cortisol levels only minimally when the enzyme immunoassay is used.13,14 After one end of the cotton roll was soaked with saliva, the roll was placed in a prelabeled vial and stored in the freezer until the samples were returned to the laboratory.
MEASURES
Cortisol
The saliva samples were stored in a freezer at the laboratory at −20°C until they were assayed. Samples were assayed using an enzyme immunoassay (Salimetrics, LLC). On the day of assaying, samples were thawed and centrifuged at 3000 rpm for 10 minutes and transferred into test wells with a pipette. The minimum sample test volume was 10 µL. All samples from a child were included in the same assay batch to minimize variability. Standards were included in every assay to ensure that assaying properties remained constant. A laboratory control was included in each assay as well. The intraassay and interassay coefficients of variation were 3.5% and 5.1%, respectively.
Cortisol values 3 SDs above the mean for that time of day were considered outliers and were not included in the analyses. This is a commonly used procedure for dealing with cortisol samples that may have been sampled without adherence to sampling guidelines.15 Eleven outliers were removed from the data set (representing less than 2% of the data). Averaged values for time and context were then computed to create an average wake-up, mid-morning, mid-afternoon, and bedtime value for both child care and non–child care days. As is typically the case with cortisol data, the distribution of the data for the times measured was positively skewed. To normalize the cortisol distributions, the convention of log10 transformation was used.
Classroom Rating
Observations were conducted in each classroom to obtain a quality of care measure to be used as a control variable if indicated in preliminary analyses. The assessment instrument used was the Infant-Toddler Environmental Rating Scale–Revised (ITERS-R).16 The ITERS-R is a standardized rating scale of child care environments, consisting of 39 items organized into 7 subscales (space and furnishings, personal care routines, listening and talking, activities, interaction, program structure, and parents and staff) and a total score rated on a 7-point scale. The overall scale has a high level of internal consistency, with a Cronbach α of 0.93.15
For this study, 1 observer, blind to other data, rated the classrooms. The observer obtained reliability with an expert observer, reaching 91% agreement on items over the course of 3 classroom observations (mean weighted κ = 0.88). Total scores for the 9 classrooms rated in the present study ranged from 4.74 to 6.40 (good to excellent quality range; mean, 6.22 [SD, 0.29]).
Sleep, Health, and Eating Diary
Parents maintained a diary of child sleep patterns, food intake, as well as general health on days that the samples were collected. Diaries were monitored to ensure sampling guidelines were followed. If children were sick at the time that parents were asked to sample, the sampling was delayed until the child was well again.
STATISTICAL ANALYSIS
To address the central study question regarding patterning of cortisol production on child care vs non–child care days, data were analyzed using hierarchical linear modeling.17 Hierarchical linear modeling accounts for the nonindependence of multiple observations nested within an individual, simultaneously estimating within- and between-subject variation. The nested structure permits variability in the number and spacing of data points, allowing for inclusion of participants who are missing 1 or more points of data. In the current study, the repeated measurements of cortisol levels on child care and non–child care days were the level 1 data. The level 2 data unit was the child.
Given the between-subject variability in sampling times, it was important to create a time variable that most accurately captured the timing of and spacing between samples. Relying on mean times of assessment for each targeted occasion (wake-up, mid-morning, afternoon, and bedtime) across individuals may have resulted in a less accurate representation of diurnal changes. Thus, an alternative time structure was used for the current study, assigning individuals’ cortisol measurements to specified time-class intervals. This approach was based on that used by King et al18 to characterize time in longitudinal trauma research. Two time-class intervals were specified for each targeted occasion (wake-up, mid-morning, afternoon, and bedtime) based on the range of sampling times. This restructuring resulted in a total of 8 time-class intervals. The average time was calculated for samples falling within each specific time-class interval. The final time variable represented hours from the initial sampling interval for each sample. Using a consistent time structure across individuals allowed for recentering at meaningful points to compare cortisol values at particular times of the day.
The data were analyzed using a multivariate statistical model. Log-transformed cortisol values measured across each of the 8 times on child care and non–child care days were stacked to create the dependent variable. First, the full-day pattern of cortisol production was compared for child care and non–child care days. Second, planned comparisons were tested to further examine the nature of differences between child care and non–child care days. Specifically, hypotheses were tested regarding differences in the slopes of cortisol change between contexts, as well as differences in cortisol levels at each of the 8 times. Of particular interest was whether children’s levels of cortisol at bedtime were significantly different between contexts. The following level 1 within-individual model was used
where Log cortti is the log-transformed cortisol value for child i at time t; (home)it is a dummy indicator that is 1 for data on non–child care days and 0 for data on child care days; (CCARE)it is a dummy indicator that is 1 for data on child care days and 0 for data on non–child care days; time, time Q, and time C represent the linear, quadratic, and cubic time variables (in hours), respectively; πh1i and πc1i are the regression coefficients representing the slope of linear change in log cort at the first time point for non–child care (ie, home) and child care days, respectively; πh2i and πc2i are the regression coefficients representing the slope of quadratic change in log cort at the first time point for non–child care and child care days, respectively; πh3i and πc3i are the regression coefficients representing the slope of cubic change in log cort across time points for non–child care and child care days, respectively; and eti is the within-individual error in child i’s log cort. We examined an unconditional level 2 model with π0i, π1i, π2i, and π3i random.
RESULTS
PRELIMINARY ANALYSES
Preliminary analyses were conducted to examine potential effects of demographic and quality of care variables on cortisol production. Cortisol patterning has been shown to vary by age during early childhood,3 but no significant associations between age and cortisol concentrations emerged for any of the time points in the present study (P=.75). Furthermore, cortisol levels were not significantly associated with child sex or ethnicity, family income, or quality of child care (ie, total scores on the ITERS-R).
FULL-DAY CORTISOL PATTERNS ON CHILD CARE AND NON–CHILD CARE DAYS
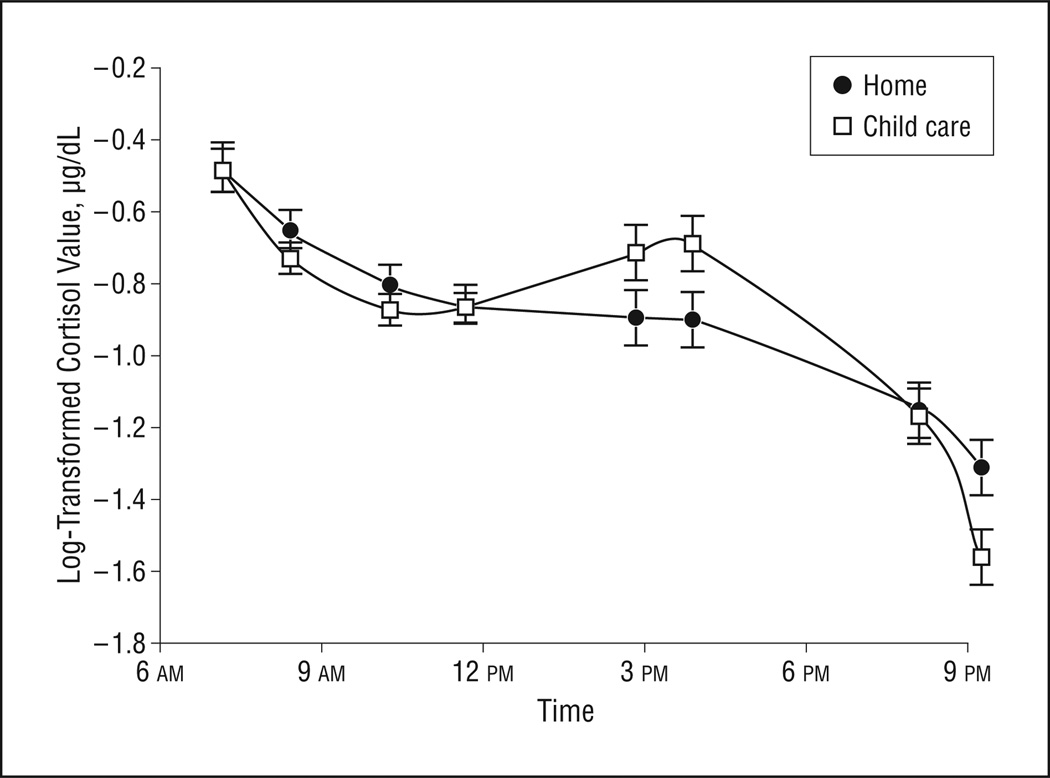
To examine context-related differences in the full-day pattern of cortisol production, we first tested whether the overall trajectories of change differed between contexts. Results of the full model are summarized in Table 1. As predicted, the overall trajectories of change in cortisol on non–child care vs child care days were significantly different (, P=.001) (Figure).
Table 1.
Multilevel Modeling Coefficients of Change in Salivary Cortisol Across Child Care and Non–Child Care Daysa
| Effect | Change in Salivary Cortisol |
P Value |
|
|---|---|---|---|
| Coefficient (SE) | t41 | ||
| Home intercept, βh00 | −0.472 (0.071) | −6.69 | <.001 |
| Child care intercept, βc00 | −0.483 (0.058) | −8.27 | <.001 |
| Home linear slope, βh10 | −0.166 (0.039) | −4.24 | <.001 |
| Child care linear slope, βc10 | −0.250 (0.037) | −6.81 | <.001 |
| Home quadratic slope, βh20 | 0.023 (0.007) | 3.32 | .002 |
| Child care quadratic slope, βc20 | −0.048 (0.006) | 7.41 | <.001 |
| Home cubic slope, βh30 | −0.001 (0.000) | −3.34 | .001 |
| Child care cubic slope, βc30 | −0.003 (0.000) | −8.25 | <.001 |
Abbreviation: SE, standard error.
Overall home vs child care trajectories differed significantly (, P=.001).
Figure.
Cortisol level patterns on child care days vs non–child care days for the full sample. Error bars represent standard error.
To examine the nature of the differences in cortisol change between contexts, the slopes of the trajectories were first compared. As predicted, there was a significant difference in the cubic function between contexts (, P=.001). As can be seen in the Figure, child care days were characterized by a morning decrease, then an afternoon rise, followed by a bedtime drop in cortisol levels, whereas non–child care days showed a morning decrease, then a flattening across the afternoon, followed by a similar decline at bedtime. Thus, the differences appear to be specific to the afternoon times.
Finally, comparisons between child care vs non–child care coefficients were tested for each of the 8 time points (Table 2). As predicted, there were significant differences in the afternoon, such that cortisol levels were higher on child care days than on non–child care days. This effect held for samples collected both earlier (, P=.005) and later (, P=.002) in the afternoon. As expected, there were no significant differences between contexts for samples collected at wake-up and mid-morning (P>.05). Of particular interest was whether cortisol levels remained higher on child care days than non–child care days or returned to typical levels by bedtime. As can be seen in Table 2, there were no significant differences in cortisol levels at bedtime samples collected earlier in the evening (, P=.77). However, there were significant differences between contexts for samples collected later in the evening (, P=.02), such that cortisol levels on child care days were lower than cortisol levels on non–child care days. As the restructuring of the time intervals resulted in a lower number of samples per time point, this particular finding should be interpreted with caution.
Table 2.
Between-Context Comparisons of Fixed Effects Across Time Points
| Sample | Mean Time of Cortisol Measurement |
Coefficient |
χ2 | |
|---|---|---|---|---|
| Home | Child Care | |||
| Wake-up | ||||
| Early | 7:09 am | −0.47 | −0.48 | 0.02 |
| Late | 8:26 am | −0.65 | −0.73 | 1.62 |
| Mid-morning | ||||
| Early | 10:16 am | −0.80 | −0.87 | 1.12 |
| Late | 11:40 am | −0.85 | −0.86 | 0.02 |
| Afternoon | ||||
| Early | 2:50 pm | −0.89 | −0.72 | 7.94a |
| Late | 3:52 pm | −0.90 | −0.69 | 10.38a |
| Bedtime | ||||
| Early | 8:05 pm | −1.14 | −1.17 | 0.09 |
| Late | 9:14 pm | −1.31 | −1.56 | 5.76b |
P<.01.
P<.05.
COMMENT
Consistent with previous studies of cortisol patternsonnon–child care days,17 toddlers showed highest levels at wake-up and lowest levels at bedtime. On days when children attended child care, however, they showed increases in cortisol production from mid-morning to mid-afternoon and decreases from afternoon to bedtime. Most importantly, children’s cortisol values at bedtime were as low on child care days as they were on non–child care days despite the afternoon rises. Although afternoon elevations have been well documented among preschool samples,10,16,19 this study provides the first data, to our knowledge, regarding post–child care cortisol levels on days that children attend full-day day care. These findings suggest that bedtime cortisol levels are at least as low on child care days as on non–child care days.
Comparison of samples obtained in the later evening hours suggested that children’s cortisol levels may actually be lower on child care days than non–child care days just before sleep. Given that this difference was only significant for bedtime samples collected later in the evening, it may be an effect of separating the bedtime samples into 2 time intervals, resulting in fewer data per time point. On the other hand, children’s systems may be downregulating cortisol production in the evening to compensate for elevated afternoon levels. Replication of this finding would be important before further speculation regarding a possible homeostatic mechanism.
Although elevations of cortisol on child care days do not appear to be associated with higher bedtime values of cortisol production, it is unclear whether these heightened levels of cortisol production carry more lasting effects on children’s development. In animal studies, chronic activation of the hypothalamic-pituitary-adrenal axis has been linked to deleterious effects on brain development, poorer immune functioning, and compromised stress-response systems.20 Roisman and colleagues21 examined the effects of early care experiences (ie, maternal insensitivity and time in child care) on later hypothalamic-pituitary-adrenal axis functioning. More time in child care before age 3 years was associated with lower awakening levels of cortisol at age 15 years. More longitudinal studies of this nature are needed to further examine whether the cortisol increases at child care reflect normative, context-specific responses or confer risk of problems later in life.
There are limitations to the conclusions that can be drawn from the results of the present study. Although afternoon elevations have been documented previously,4 the assessment of full-day patterning among toddlers should be replicated. Additionally, the age range included here was narrow. As age effects related to mid-afternoon cortisol production at child care have been noted in other studies,15 investigation of full-day patterning across early childhood (infancy, preschool, and school age) is warranted. Furthermore, findings may not be generalizable to different types of child care settings (eg, home-based child care and relative care) or to child care centers that are rated poor to adequate by standard quality of care measures. The present study assessed cortisol levels among children who attended center-based care facilities of good quality as rated by the ITERS-R.
Previous research has indicated that young children show mid-morning to mid-afternoon increases in cortisol on child care days, unlike the pattern they show on non–child care days.3,9 The present study extended these findings, demonstrating that young children’s cortisol level at bedtime on child care days returns to levels at least as low as on non–child care days. Thus, the effects of child care on daytime patterning of cortisol production are not seen in elevated evening levels, suggesting time-limited elevations across the day. Nevertheless, it will be important to examine whether there are long-term consequences of the altered diurnal patterns, despite the apparent return to low levels at bedtime.
Acknowledgments
Funding/Support: This project was supported by grants R01MH052135, R01MH074374, and R01MH084135 from the National Institute of Mental Health. Dr Dozier participated in a National Institute of Mental Health–funded network (Megan Gunnar, PhD, principal investigator), which provided intellectual support for the ideas developed. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Footnotes
Author Contributions: Study concept and design: Sumner and Dozier. Acquisition of data: Sumner. Analysis and interpretation of data: Bernard and Dozier. Drafting of the manuscript: Sumner, Bernard, and Dozier. Critical revision of the manuscript for important intellectual content: Bernard and Dozier. Statistical analysis: Bernard. Obtained funding: Dozier. Study supervision: Dozier.
Financial Disclosure: None reported.
REFERENCES
- 1.Larson MC, White BP, Cochran A, Donzella B, Gunnar M. Dampening of the cortisol response to handling at 3 months in human infants and its relation to sleep, circadian cortisol activity, and behavioral distress. Dev Psychobiol. 1998;33(4):327–337. [PubMed] [Google Scholar]
- 2.Gunnar MR, Brodersen L, Krueger K, Rigatuso J. Dampening of adrenocortical responses during infancy: normative changes and individual differences. Child Dev. 1996;67(3):877–889. [PubMed] [Google Scholar]
- 3.Watamura SE, Donzella B, Kertes DA, Gunnar MR. Developmental changes in baseline cortisol activity in early childhood: relations with napping and effortful control. Dev Psychobiol. 2004;45(3):125–133. doi: 10.1002/dev.20026. [DOI] [PubMed] [Google Scholar]
- 4.Watamura SE, Donzella B, Alwin J, Gunnar MR. Morning-to-afternoon increases in cortisol concentrations for infants and toddlers at childcare: age differences and behavioral correlates. Child Dev. 2003;74(4):1006–1020. doi: 10.1111/1467-8624.00583. [DOI] [PubMed] [Google Scholar]
- 5.Dozier M, Peloso E, Lindhiem O, et al. Developing evidence-based interventions for foster children: an example of a randomized clinical trial with infants and toddlers. J Soc Issues. 2006;62(4):767–785. [Google Scholar]
- 6.Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. 2007;32(8–10):892–905. doi: 10.1016/j.psyneuen.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27(1–2):199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- 8.Davis EP, Bruce J, Gunnar MR. The anterior attention network: associations with temperament and neuroendocrine activity in 6-year-old children. Dev Psychobiol. 2002;40(1):43–56. doi: 10.1002/dev.10012. [DOI] [PubMed] [Google Scholar]
- 9.Watamura SE, Sebanc AM, Gunnar MR. Rising cortisol at childcare: relations with nap, rest, and temperament. Dev Psychobiol. 2002;40(1):33–42. doi: 10.1002/dev.10011. [DOI] [PubMed] [Google Scholar]
- 10.Dettling AC, Parker SW, Lane S, Sebanc A, Gunnar MR. Quality of care and temperament determine whether cortisol levels rise over the day for children in full-day childcare. Psychoneuroendocrinology. 2000;25:819–836. doi: 10.1016/s0306-4530(00)00028-7. [DOI] [PubMed] [Google Scholar]
- 11.Gunnar MR, Sebanc AM, Tout K, Donzella B, van Dulmen M. Peer rejection, temperament, and cortisol reactivity in preschoolers. Dev Psychobiol. 2003;43(4):346–358. doi: 10.1002/dev.10144. [DOI] [PubMed] [Google Scholar]
- 12.Kudielka BM, Broderick JE, Kirschbaum C. Compliance with saliva sampling protocols: electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosom Med. 2003;65(2):313–319. doi: 10.1097/01.psy.0000058374.50240.bf. [DOI] [PubMed] [Google Scholar]
- 13.Gordon MK, Peloso E, Auker A, Dozier M. Effect of flavored beverage crystals on salivary cortisol enzyme immunoreactive assay measurements. Dev Psychobiol. 2005;47(2):189–195. doi: 10.1002/dev.20081. [DOI] [PubMed] [Google Scholar]
- 14.Talge NM, Donzella B, Kryzer EM, Gierens A, Gunnar MR. It’s not that bad: error introduced by oral stimulants in salivary cortisol research. Dev Psychobiol. 2005;47(4):369–376. doi: 10.1002/dev.20097. [DOI] [PubMed] [Google Scholar]
- 15.Dettling AC, Gunnar MR, Donzella B. Cortisol levels of young children in full-day childcare centers: relations with age and temperament. Psychoneuroendocrinology. 1999;24(5):519–536. doi: 10.1016/s0306-4530(99)00009-8. [DOI] [PubMed] [Google Scholar]
- 16.Harms T, Clifford R, Cryer D. Infant-Toddler Environment Rating Scale. New York, NY: Teachers College Press; 1990. [Google Scholar]
- 17.Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- 18.King DW, King LA, McArdle JJ, Grimm K, Jones RT, Ollendick TH. Characterizing time in longitudinal trauma research. J Trauma Stress. 2006;19(2):205–215. doi: 10.1002/jts.20112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tout K, de Haan M, Campbell EK, Gunnar MR. Social behavior correlates of cortisol activity in childcare: gender differences and time-of-day effects. Child Dev. 1998;69(5):1247–1262. [PubMed] [Google Scholar]
- 20.Vermeer HJ, van Ijzendoorn MH. Children’s elevated cortisol levels at daycare: a review and meta-analysis. Early Child Res Q. 2006;21(3):390–401. [Google Scholar]
- 21.Roisman GI, Susman E, Barnett-Walker K, et al. NICHD Early Child Care Research Network. Early family and child-care antecedents of awakening cortisol levels in adolescence. Child Dev. 2009;80(3):907–920. doi: 10.1111/j.1467-8624.2009.01305.x. [DOI] [PubMed] [Google Scholar]