Abstract
The RNA genome of the hepatitis C virus (HCV) diversifies rapidly during the acute phase of infection, but the selective forces that drive this process remain poorly defined. Here we examined whether Darwinian selection pressure imposed by CD8+ T cells is a dominant force driving early amino acid replacement in HCV viral populations. This question was addressed in two chimpanzees followed for 8 to 10 years after infection with a well-defined inoculum composed of a clonal genotype 1a (isolate H77C) HCV genome. Detailed characterization of CD8+ T cell responses combined with sequencing of recovered virus at frequent intervals revealed that most acute-phase nonsynonymous mutations were clustered in class I epitopes and appeared much earlier than those in the remainder of the HCV genome. Moreover, the ratio of nonsynonymous to synonymous mutations, a measure of positive selection pressure, was increased 50-fold in class I epitopes compared with the rest of the HCV genome. Finally, some mutation of the clonal H77C genome toward a genotype 1a consensus sequence considered most fit for replication was observed during the acute phase of infection, but the majority of these amino acid substitutions occurred slowly over several years of chronic infection. Together these observations indicate that during acute hepatitis C, virus evolution was driven primarily by positive selection pressure exerted by CD8+ T cells. This influence of immune pressure on viral evolution appears to subside as chronic infection is established and genetic drift becomes the dominant evolutionary force.
INTRODUCTION
Upon transmission to a new host, the hepatitis C virus (HCV) persists in approximately 65 to 80% of infected patients. HCV circulates in infected individuals as a swarm of genetically different but closely related viral variants known as a quasispecies. Heterogeneity of the HCV genomes is the result of the high virion production rate in the liver (35) and the lack of proofreading activity of the viral RNA-dependent RNA polymerase. These characteristics facilitate rapid adaptation of HCV to the host, in part through selection of preexisting minor variants from the quasispecies population (43). Viral evolution is governed by the positive or negative selection of mutations and the accumulation of neutral substitutions (genetic drift) (43). The rate of HCV evolution has been reported to be much higher during the acute phase than the chronic phase of infection (7, 26). It is assumed that this process is driven by the adaptive immune response. Neutralizing antibodies contribute to early diversification of the HCV envelope genes through positive selection of escape mutations (15), a process that is maintained during the chronic phase of infection (49). CD4+ T cells appear to exert limited selection pressure against the virus because mutations in major histocompatibility complex (MHC) class II epitopes are uncommon (19, 21). Mutational escape from CD8+ T cells is well established (8, 16, 23, 24, 45, 46). In chimpanzees, the only animal model of human HCV infection, this process has been linked to positive Darwinian selection pressure (16). At the population level, HCV adaptation to CD8+ T cell pressure can also be observed as viral genetic polymorphisms associated with specific human leukocyte antigen (HLA) types (22, 47).
A negative impact of some escape mutations on viral replication has been described in HCV infection (14, 41, 48). Thus, stability of an escape mutation seems to result from the balance between the fitness cost and the replicative advantage provided by the substituted amino acid within a class I epitope. Consequently, reversion of some escape mutations are expected upon transmission of HCV to an HLA-mismatched host as pressure from the immune system is removed. For instance, mutation toward the HCV consensus sequence after virus transmission in humans has been used to estimate loss of deleterious CD8+ T cell escape mutations (39, 41, 46). Thus, negative purification of escape mutations in class I epitopes may also contribute to the higher rate of evolution observed during acute hepatitis C.
It is well established that CD8+ T cells select for escape mutations during the acute phase of infection (12, 16, 26), but their contribution to viral evolution during the course of HCV infection has been difficult to quantify. Wide variation (from 25 to 75%) in the frequency of nonsynonymous substitutions linked to loss and/or gain of escape mutations in class I epitopes was observed between the few human studies that addressed this question (12, 26, 38). The chimpanzee model of infection may reduce sources of variability because the sequence of the transmitted virus is known and sequencing at the earliest time points after infection is feasible. One study in chimpanzees measured a large increase during acute hepatitis C in the ratio of dN (nonsynonymous changes per nonsynonymous site) to dS (synonymous changes per synonymous site), a common measure of positive selection pressure. It was proposed that this was due at least in part to CD8+ T cell selection pressure (18). Here, frequent sequencing of viral genomes and detailed characterization of the HCV-specific CD8+ T cell response were used to establish that almost all positive selection pressure occurring early in infection is concentrated on MHC class I epitopes of the virus.
MATERIALS AND METHODS
Animals.
Chimpanzees CH1530 and CH1564 were maintained under standard conditions for humane care at the New Iberia Research Center. Patr class I major histocompatibility complex (MHC) genotyping of these animals was done as previously described (10). Inoculation of both animals with full-length RNA transcripts from pHCV-H77C by direct intrahepatic injection was described previously (52, 53).
Sequence analysis of viruses recovered from infected chimpanzees.
The consensus sequence of the entire open reading frame (ORF) of HCV H77C (genotype 1a) genomes recovered from the sera of CH1530 and CH1564 was determined by reverse transcription (RT)-PCR, as previously described (6). Sequencing of clonal HCV genomes was also performed for the week 520 (CH1530) or week 439 (CH1564) time points to determine the penetrance of escape mutations into the HCV quasispecies. RNA was extracted using a QIAamp RNA extraction kit (Qiagen), and cDNA was synthesized using random hexamers and Superscript III reverse transcriptase (Invitrogen). Four overlapping fragments of the HCV genome (covering amino acids 1 to 2877 of the H77C ORF) were amplified using nested sets of PCR primers listed in Table 1. The PCR consisted of an initial denaturation step at 98°C for 30 s, followed by 30 cycles at 98°C for 10 s, 58°C to 64°C (depending on the primer set) for 20 s, and 72°C for 50 s, and a final extension step at 72°C for 300 s. The first PCR was used as a template for a second amplification using the same reaction components as described above with a 500 nM concentration of each of the inside nested primers. Nested-PCR products were subsequently cloned into Zero Blunt TOPO vector (Invitrogen). Resulting DNA plasmids were sequenced by the Laboratory for Genomics & Bioinformatics (University of Oklahoma Health Sciences Center).
Table 1.
PCR primers
| PCR fragment | NT position on H77C genomea | Sequence |
|---|---|---|
| PCR1 | FP 595–614 | TCTATGGCAATGAGGGTTGC |
| RP 3719–3738 | CCAGGTAAAGGTCCGAGGAG | |
| FPn 690–709 | CGCAATTTGGGTAAGGTCAT | |
| RPn 3279–3298 | CACGTGATGAGCTTGGTCTC | |
| PCR2 | FP 2537–2555 | CTCCTGCTTGTGGATGATG |
| RP 4722–4741 | TCAAGGCTGAAATCGACTGT | |
| FPn 2579–2598 | GGCTTTGGAGAACCTCGTAA | |
| RPn 4425–4444 | GACAGAGCAACCTCCTCGAT | |
| PCR3 | FP 4305–4323 | GATGCCACATCCATCTTGG |
| RP 6861–6879 | GGGAGGGATCAGTGAGCAT | |
| FPn 4337–4356 | CCTTGACCAAGCAGAGACTG | |
| RPn 6661–6680 | CGGGCATTTAAGATTGTCAG | |
| PCR4 | FP 6179–6199 | CATACTCAGCAGCCTCACTGT |
| RP 9171–9191 | TACTGCCCAGTTGAAGAGGTA | |
| FPn 6435–6454 | GGAGCTGAGATCACTGGACA | |
| RPn 8952–8973 | CCAGTGGTTCTATGGAGTAGCA |
FP, forward primer; RP, reverse primer; FPn, nested forward primer; RPn, nested reverse primer; Nt, nucleotide.
Generation of HCV-specific CD8+ T cell lines.
Peripheral blood mononuclear cells (PBMC) were separated on a Ficoll gradient and used directly or cryopreserved for future use. Liver tissue was obtained via transcutaneous liver biopsy and homogenized using the BD Medimachine (BD Biosciences) according to the manufacturer's instructions. CD8+ T cells were positively isolated from liver tissue homogenate or PBMC using paramagnetic beads coupled to anti-CD8 antibodies (Dynabeads; Invitrogen). Enriched intrahepatic or circulating CD8+ T cells were cocultured with irradiated (3,000 rad) autologous PBMC pulsed with peptide pools spanning the entire HCV genotype 1a polyprotein. Overlapping peptides (18-mers overlapping by 11) covering the entire HCV protein sequence (GenBank accession no. AF009606) were obtained from Genemed Synthesis. Truncated peptides used for epitope mapping were purchased from ProImmune (Oxford, United Kingdom).
After 2 to 3 weeks of culture, cells were expanded with anti-CD3 antibodies and irradiated human PBMC as feeder cells. Three to four weeks later, cells were tested for HCV specificity by gamma interferon (IFN-γ) ELISpot or intracellular cytokine staining (ICS) assays as described below using the whole HCV-1a peptide set arranged in nine peptide pools. When positive results were obtained, peptide pools were deconvoluted to identify the targeted peptide. Amino- or carboxy-truncated peptides were used to determine the minimal epitope.
Circulating CD8+ T cells were also stimulated with single peptides that represented known or presumptive epitopes that spanned positions with nonsynonymous mutations in the first year of infection as described in Tables 2 and 3. After one or two rounds of stimulation, cells were tested for specificity by ICS assay.
Table 2.
Amino acid changes in recovered viruses following monoclonal H77C infection of chimpanzee CH1530a
| Wk p.i.b | RNA titer (log10 GE/ml)c | Difference from H77C sequence at indicated aa position: |
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E1 |
E2 |
p7 |
NS2 |
NS3 |
NS5A |
NS5B |
|||||||||||||||||||||||||||
| 265 | 395 | 400 | 403 | 404 | 408 | 410 | 444 | 480 | 765 | 790 | 828 | 901 | 937 | 978 | 1008 | 1145 | 1202 | 1211 | 1444 | 1533 | 1759 | 2116 | 2217 | 2220 | 2248 | 2279 | 2283 | 2340 | 2359 | 2668 | 2951 | ||
| L | T | V | L | T | K | N | Q | L | A | L | A | I | A | R | Q | R | G | T | F | A | V | I | A | D | N | R | R | L | G | Q | R | ||
| 2 | 3.7 | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| 8 | 4.9 | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | E | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| 12 | 5.1 | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | E | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| 20 | 4.2 | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | E | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| 31 | 4.3 | • | • | • | • | • | • | • | • | • | P | F | • | • | • | • | • | • | E | • | • | P | • | • | • | D/N | • | • | • | • | D | Q/R/K | • |
| 40 | 4.6 | • | • | • | • | • | • | • | • | • | P | F | • | • | • | • | • | • | E | • | • | P | • | • | • | N | • | • | • | • | D | Q/R/K | • |
| 52 | 4.8 | • | • | • | • | • | • | • | • | • | P | F | • | • | • | • | • | Q | E | • | • | P | • | • | • | N | • | • | • | • | D | Q/R/K | • |
| 90 | NT | • | • | A | F | A | • | S | H | • | P | F | • | V | • | • | • | Q | E | • | • | P | • | • | • | N | N/D | • | • | • | D | R | • |
| 104 | 5.3 | • | • | A | F | A | • | S | H | • | P | F | • | V | • | • | • | Q | E | • | • | P | • | • | • | N | D | • | • | • | D | R | • |
| 130 | 5.7 | • | I | A | • | • | R | • | H | • | P | F | A/V | • | • | • | R | Q | E | • | Y | P | • | • | T | N | • | Q | • | • | D | R | • |
| 183 | 5.7* | • | • | A | F | A | • | S | H | • | P | F | • | V | • | • | • | Q | E | S | Y | P | • | V | • | N | D | • | R/P | • | D | R | R/K |
| 260 | 5.9* | • | • | A | F | A | • | S | H | • | P | F | • | V | • | • | • | Q | E | S | Y | P | A | V | • | N | D | • | P | V | D | R | K |
| 364 | 5.8* | I | • | A | F | A | • | S | H | P | P | F | • | V | • | • | • | Q | E | S | Y | P | A | V | • | N | D | • | P | V | D | R | K |
| 520 | 6.3* | I | • | A | F | A | R | R | H | P | P | F | V | V | V | R/Q | • | Q | E | S | Y | P | A | V | • | N | D | • | P | V | D | R | K |
Chimpanzee CH1530 became infected following intrahepatic transfection with RNA transcripts of H77C. The sequence of H77C (GenBank accession no. AF011751) is shown at the top. Amino acids (aa) identical with H77C are indicated with dots. Dominant changes are indicated by capital letters.
p.i., postinfection with H77C.
HCV RNA titers were determined by Monitor 1.0 or Monitor 2.0. GE, genome equivalent. NT, not tested. *, samples tested by bDNA 3.0.
Table 3.
Amino acid changes in recovered viruses following monoclonal H77C infection of chimpanzee CH1564a
| Wk p.i.b | RNA titer (log10 GE/ml)c | Difference from H77C sequence at indicated aa position: |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Core |
E1 |
E2 |
p7 |
NS2 |
NS3 |
NS4B |
NS5A |
NS5B |
||||||||||||||||||||||||||||||||||||||||||||||||
| 9 | 11 | 355 | 357 | 362 | 463 | 527 | 528 | 574 | 709 | 710 | 714 | 720 | 753 | 760 | 763 | 765 | 766 | 787 | 790 | 793 | 814 | 837 | 843 | 901 | 937 | 938 | 1136 | 1150 | 1202 | 1355 | 1358 | 1612 | 1615 | 1621 | 1626 | 1745 | 1759 | 1993 | 2065 | 2105 | 2116 | 2118 | 2144 | 2153 | 2198 | 2283 | 2329 | 2340 | 2350 | 2412 | 2485 | 2486 | 2555 | 2809 | ||
| R | T | V | A | F | T | Y | S | V | I | A | I | V | I | A | H | L | V | V | L | M | V | R | M | I | A | I | H | G | G | V | S | I | K | P | Y | V | V | T | Y | M | I | S | E | E | M | R | K | L | K | A | Q | D | D | T | ||
| 11 | 4.3 | • | • | • | • | • | A | • | • | • | • | • | • | • | • | • | • | • | A | • | • | • | • | • | • | • | • | • | • | • | E | • | P | • | • | S | • | • | • | • | F | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| 21 | 3.9 | • | • | • | • | • | A | • | • | • | • | • | • | • | • | T | • | • | A | • | • | V | • | • | • | • | • | • | • | • | E | • | P | L | • | • | F | • | • | • | F | • | • | P | • | E/A | • | • | • | • | • | • | • | • | • | • |
| 38 | 4.9 | • | • | • | A/V | • | A | • | • | • | • | • | • | I | • | • | • | L/F | A | • | • | V | • | • | • | • | • | I/V | • | • | E | • | P | • | • | • | F | • | • | • | F | M/V | V | A/S/P | E/G | • | • | • | • | • | Q | • | R | • | D/N | T/A |
| 52 | 4.0 | • | P | • | • | • | A | • | • | • | • | V | L | • | • | • | N | • | A | • | • | V | • | • | • | V | • | • | • | • | E | • | P | • | • | • | F | • | • | • | F | V | V | • | • | D | • | • | • | • | R | • | H | E | • | • |
| 177 | 4.6* | K | P | I | • | • | A | H | • | L | V | V | L | I | • | • | N | • | A | A | • | V | A | H | • | V | V | • | Q | G/S | E | I | P | L | R | • | F | • | • | I | F | V | V | A | • | D | V | • | R | • | R | • | H | E | • | • |
| 282 | 5.4* | K | • | I | • | Y | A | H | N | L | • | V | L | I | V | • | N | • | A | • | • | V | A | H | I | V | V | • | Q | • | E | I | P | L | R | • | F | • | A | I | F | V | V | A | • | D | V | • | R | V | R | D | H | E | • | • |
| 439 | 5.9* | K | N | I | • | Y | A | H | N | S | • | V | L | I | V | • | N | • | A | • | F | V | A | H | I | V | V | • | Q | • | E | I | P | L | • | • | F | V/D | A | I | F | V | V | A | • | D | V | R/P | R | V | R | D | H | E | • | • |
Chimpanzee CH1564 became infected following intrahepatic transfection with RNA transcripts of H77C. The sequence of H77C (GenBank accession no. AF011751) is shown at the top. Amino acids identical with H77C are indicated with dots. Dominant changes are indicated by capital letters.
p.i., postinfection with H77C.
HCV RNA titers were determined by Monitor 1.0 or Monitor 2.0. GE, genome equivalent. *, samples tested by bDNA 3.0.
IFN-γ ELISpot assay.
CD8+ T cells were plated in AIM-HS (AIM V lymphocyte medium [Invitrogen] supplemented with 2% human AB serum [Gemini Bio-Products]) in a 96-well plate at 5 × 104 cells per well the day before the assay. Autologous PBMC or B-lymphoblastoid cell lines (B-LCL) generated as described elsewhere (10, 27) were irradiated (3,000 rad) and pulsed with HCV or control irrelevant peptides for use as antigen-presenting cells (APC). Peptide-pulsed PBMC (1 × 105) or B-LCL (5 × 103) were mixed with the CD8+ T cells and immediately transferred to ELISpot plates precoated with anti-IFN-γ antibodies (MultiScreen-IP plate; Millipore). Plates were incubated for 36 h and developed according to the manufacturer's instructions (chimpanzee IFN-γ ELISpot kit, black spot; U-CyTech).
Intracellular cytokine staining assay.
Peptide-pulsed autologous B-LCL were mixed with CD8+ T cells at a 1:1 ratio with 1 μg/ml of anti-CD28 and anti-CD49d antibodies to provide costimulation (BD Pharmingen). After 1 h of incubation, GolgiPlug (BD Biosciences) was added to each well, and cells were further incubated for 16 h at 37°C under 7% CO2. At the end of the incubation, cells were washed once in phosphate-buffered saline (PBS) supplemented with 2.5% fetal calf serum (FCS) and 0.01% sodium azide (fluorescence-activated cell sorter [FACS] buffer) and blocked for 20 min in blocking buffer (FACS buffer supplemented with 20% human AB serum). Cells were then stained with mouse monoclonal antibodies to CD8 (allophycocyanin conjugated; BD Pharmingen) and CD4 (Pacific Blue conjugated; BioLegend). After two washes with FACS buffer, cells were stained with a Live/Dead fixable blue stain kit (Invitrogen), washed twice, and permeabilized using BD Cytofix/Cytoperm solution (BD Biosciences). Cells were stained with mouse monoclonal antibodies to CD3 (peridinin chlorophyll protein conjugated; BD Pharmingen) and IFN-γ (phycoerythrin conjugated; BD Pharmingen) and visualized on a Becton Dickinson LSR flow cytometer. Data were analyzed using FlowJo software (Tree Star, Inc.).
HCV mRNA production and transfection.
The fragments corresponding to nucleotides 915 to 2769 (encoding HCV proteins E1, E2, and p7; amino acids 192 to 809) or nucleotides 3420 to 6359 (encoding HCV proteins NS3, NS4A, and NS4B; amino acids 1027 to 2006) of the HCV genome were amplified by PCR with the Phusion Hot Start polymerase using 10 ng of a plasmid encoding the full-length HCV sequence (GenBank accession no. AF009606; kind gift of Charles Rice, The Rockefeller University, New York, NY) (25) as a template, forward primer 5′-ATATCTAGAACCATGTACCAAGTGCGCAATTCCTCGGGGCTTACCA-3′ and reverse primer 5′-TATAAGCTTTTATCATGCGTATGCCCGCTGAGGCAACG-3′, or forward primer 5′-ATATCTAGAACCATGGCGCCCATCACGGCGTACGCCCAGCAGA-3′ and reverse primer 5′-ATAAAGCTTTCATTAAATCCCAGGCAGTTGTGGCATGAGCTTG-3′, respectively. The resulting DNA fragments were inserted at the corresponding sites of the pGem-3zf vector (Promega), yielding pGem-3z-H77-E1-p7 and pGem-3z-H77-NS3-NS4 plasmids, which were verified by sequencing as described above. Site-directed mutagenesis was used to introduce the G1394A nucleotide substitution (underlined in the following primers) into the pGem-3z-H77-E1-p7 plasmid using the forward primer 5′-GCTCACTGGGGAATCCTGGCGGGCA-3′ and the reverse primer 5′-TGCCCGCCAGGATTCCCCAGTGAGC-3′ and the QuikChange II targeted mutagenesis kit (Stratagene), yielding pGem-3z-H77-E1-p7-1394A plasmid. The C4937T and CG4937TC nucleotide substitutions (underlined in the following primers) were introduced into the pGem-3z-H77-NS3-NS4 plasmid using the forward primer 5′-GGTATGAGCTCACGCCTGCCGAGACTACAGTTAG-3′ and the reverse primer 5′-CTAACTGTAGTCTCGGCAGGCGTGAGCTCATACC-3′ or the forward primer 5′-GGTATGAGCTCACGCCTCCCGAGACTACAGTTAG-3′ and the reverse primer 5′-CTAACTGTAGTCTCGGGAGGCGTGAGCTCATACC-3′, yielding pGem-3z-NS3-NS4-4937T and pGem-3z-NS3-NS4-4937TC plasmids, respectively. All mutations were verified by the sequencing of positive clones as described above. As a control, the enhanced green fluorescent protein (EGFP) ORF was subcloned into the pGem3z vector using the BamHI and NotI restriction enzymes. Plasmids were then linearized and used for the generation of capped and poly(A) tailed mRNA in vitro using the mMESSAGE mMACHINE T7 ultra kit (Ambion) according to the manufacturer's instructions. After cleaning with the RNeasy MinElute cleanup kit (Qiagen), mRNA integrity was assessed on a formaldehyde-denaturing agarose gel and mRNA was stored at −80°C until ready for use. Autologous B-LCL were electroporated with 5 μg of viral mRNA using the BTX ECM 600 electroporator (Genetronics, Inc.) at 400 V and 50 F. Immediately after electroporation, cells were transferred into 2 ml of RPMI 1640 medium (Invitrogen) supplemented with 10% FCS and cultured for 24 h in a 24-well plate at 37°C under 7% CO2. At the end of the incubation, B-LCL were washed once, resuspended in AIM-HS, and mixed with the CD8+ T cells at a 1:1 ratio in an ICS assay as described above.
Sequence analysis.
The numbers of dS and dN were estimated using the Nei-Gojobori method (33). In preliminary analyses, estimation of these quantities by more complex methods (28, 54) yielded essentially identical results, as is expected because the number of substitutions per site was generally low; therefore, we used the Nei-Gojobori method because it has a lower variance (34).
An HCV genotype 1a consensus was obtained from the alignment of 208 near-full-length polyprotein sequences assembled from the European HCV database (euHCVdb; data not shown) (9). This sequence was used to assess mutations away from and toward the subtype 1a consensus after infection of animals with clonal H77C genotype 1a genomes.
RESULTS
Replication and evolution of clonal HCV genomes.
Chimpanzees CH1530 and CH1564 were experimentally infected with HCV by direct intrahepatic inoculation of clonal viral genomes transcribed from an infectious molecular clone. The sequence of this molecular clone (designated H77C to indicate clonality of the genomes; GenBank accession no. AF011751) was determined from the sequence of the dominant quasispecies amplified from the plasma of patient H with acute hepatitis C (53). CH1530 remained viremic through 2.7 years (140 weeks) of observation (32). CH1564 was initially infected by direct intrahepatic injection of clonal genotype 2a genomes (52). It was superinfected 6 weeks later with the clonal H77C. Within 2 weeks of superinfection, only H77C genomes were detected in serum. The pattern of viremia during the first 28 weeks of infection for this chimpanzee was described previously (52).
Infection of these animals with clonal HCV H77C genomes provided a unique opportunity to compare the influences of cellular immunity on virus evolution during the acute and chronic phases of infection. We therefore extended the period of observation to 8.5 years for CH1564 and 10 years for CH1530. HCV titers were consistently in a narrow range, from 105 to 106 genome equivalents (GE)/ml of serum, during the extended period of observation for CH1530 (Table 2). Viremia in CH1564 ranged from 104 to 106 GE/ml of serum (Table 3). A trend toward increasing viremia over 8 to 10 years of infection was observed in both animals.
HCV genomes recovered from serial serum samples were sequenced to compare the rates of evolution during the acute and chronic phases of infection. Consensus nucleotide sequences of nearly full-length HCV genomes (complete open reading frame) are shown in Tables S1 (CH1530) and S2 (CH1564) in the supplemental material. The numbers of nucleotide and amino acid substitutions were remarkably similar for viruses from both animals. Compared with the clonal HCV genomes that initiated infection, a total of 90 nucleotide substitutions were observed over 10 years of persistent virus replication in CH1530 (see Table S1). Thirty-four of them resulted in unique amino acid substitutions at 32 different positions (Table 2). For CH1564, 126 nucleotide substitutions were observed over 8.5 years (see Table S2), with 58 of them causing amino acid changes at 55 different positions (Table 3). Comparison of HCV genomes from the two animals revealed eight shared amino acid substitutions. Only one of these common substitutions occurred within the first year of infection. Replacement of glycine with glutamic acid at position 1202 (G1202E) was observed at week 8 in CH1530 and week 11 in CH1564. Glutamic acid is encoded at this position by the majority of genotype 1a viruses (205 out of 208 full-length polyprotein sequences [data not shown]). Additionally, the G1202E change also occurred in other chimpanzees inoculated with the same H77C sequence (6) (data not shown), and so it is likely that this mutation toward the consensus sequence was required for optimal in vivo HCV replication (5, 6).
Most amino acid substitutions that appeared in genomes of these two viruses over 8 to 10 years of persistent replication were not shared. A substantial percentage of the amino acid changes occurred before week 52, including 9 of 34 (26%) for CH1530 and 33 of 58 (57%) for CH1564 (Tables 2 and 3). Nonsynonymous substitutions appeared earlier in HCV genomes from CH1564 (11 weeks postinfection [p.i.]) than CH1530 (31 weeks p.i.), even though both chimpanzees were infected with the same clonal H77C virus. Differences in pace and identity of amino acid substitution during the first year of infection in animals CH1530 and CH1564 suggest that a variable force, perhaps adaptive immunity, was dominant in shaping early virus evolution. To assess this possibility, immune responses were characterized in both animals.
Immune selection pressure in HCV-infected chimpanzees.
Antibodies can exert selection pressure against HCV envelope glycoprotein E2 in human infections. Antibody-driven variation in E2 is less common in chimpanzees (3, 40). Consistent with this observation, animal CH1564 did not develop envelope-specific antibodies (data not shown) and the E2 hypervariable region 1 (HVR1) remained intact in viruses from this animal (Table 3). Animal CH1530 did develop a neutralizing antibody response (32). Of the 34 amino acid substitutions observed in the HCV H77C polyprotein (Table 2), 6 were in the E2 HVR1 and presumably driven by antibody selection pressure as previously reported (32, 55).
Mutations within class I epitopes were also characterized during the acute and chronic phases of infection. Circulating HCV-specific T cells were detected in the blood of these animals during the first year of infection by lymphoproliferation assay. Recombinant HCV genotype 1 antigens stimulated a measurable proliferative response at weeks 11 and 15 after H77C challenge for chimpanzee CH1564 and at weeks 24 and 25 for chimpanzee CH1530 (F. V. Chisari and R. Thimme, personal communication). Analysis of CD8+ T cell immunity during the acute phase of infection in animal CH1564 using a pool of 65 synthetic peptides representing human and chimpanzee class I epitopes revealed a response to a single epitope from the HCV core protein (designated C137) at 11 and 22 weeks after H77C challenge (Chisari and Thimme, personal communication). This epitope, which spanned amino acids 137 to 144 (IPLVGAPL) of H77C, did not acquire escape mutations. Importantly, the 65 epitopes included in the analysis were those known at the time and were not selected based on the class I genotype of CH1564. Because the CD8+ T cell response to HCV is somewhat idiosyncratic and epitopes are not easily predicted even when the class I genotype is known (51), it is likely that the response to epitope C137 was a surrogate of a broader response not represented by the 65 peptides. Typing of Patr class I haplotype of animals CH1530 and CH1564 (Table 4) revealed that they were genetically capable of responding to additional epitopes that were identified in more recent studies of HCV-infected chimpanzees. These included 4 potential epitopes in CH1530 (3 restricted by Patr-A*0101 and one by B*2401) and 5 in CH1564 (3 restricted by Patr-B*1301 and one by B*0901) (Table 4). Importantly, CD8+ T cells targeting C137 and the additional 8 predicted epitopes described in Table 4 could not be expanded from the peripheral blood or chronically infected liver of the relevant chimpanzee, and so recognition was not confirmed. Through 8 to 10 years of follow-up, only one of these unconfirmed epitopes (NS4B2663 targeted by animal CH1530) acquired a fixed amino acid change (Q2668R) that might have facilitated escape from CD8+ T cell recognition (Tables 2 and 4).
Table 4.
Summary of class I-restricted epitopes
| Animal | Epitopea | Patr restriction haplotype | Name of epitope | Sequence | Mutatedb | Escapedc |
|---|---|---|---|---|---|---|
| CH1530 | Predicted | A*0101 | E2617 | HYPCTINYTIFK | − | − |
| A*0101 | NS21011 | LLGPADGMVSK | − | − | ||
| A*0101 | NS4B1724 | MLAEQFKQK | − | − | ||
| B*2401 | NS5B2663 | CDLDPQARVAI | + | NT | ||
| Targeted | A*0701 | E1347 | MIAGAHWGVLd | − | − | |
| A*0701 | p7758 | SLAGTHGLVSFLf | + | + | ||
| A*0701 | p7789 | ALYGMWPLLLf | + | + | ||
| B*0802 | NS31524 | GCAWYELTPd | − | + | ||
| CH1564 | Predicted | B*1301 | Core41 | GPRLGVRAT | − | − |
| B*1301 | Core137 | IPLVGAPL | − | − | ||
| B*1301 | E2542 | TRPPLGNWF | − | − | ||
| B*0901 | NS31123 | CTCGSSDLY | − | − | ||
| B*1301 | NS31373 | IPFYGKAI | − | − | ||
| Targeted | A*0701 | E1347 | MIAGAHWGVLd | + | − | |
| B*0901 | E2462 | LTDFAQGWe | + | + | ||
| A*0701 | p7758 | SLAGTHGLVSFLf | + | + | ||
| A*0701 | p7789 | ALYGMWPLLLf | + | + | ||
| B*0901 | NS31565 | LTHIDAHFLd | − | − | ||
| A*0602 | NS31617 | TLHGPTPLLYe | + | + | ||
| A*0602 | NS4B2055 | MWSGTFPINAYf | + | + |
Predicted epitopes are epitopes described in other studies and restricted by a Patr allele expressed by the indicated chimpanzee; targeted epitopes are epitopes for which cognate CD8+ T cells were isolated from the indicated chimpanzee.
Amino acid change(s) occurred over time within minimal epitopes.
Amino acid change(s) within or outside epitopes impaired recognition by cognate CD8+ T cells. NT, not tested.
Epitopes for which specific CD8+ T cells were expanded only from the intrahepatic compartment.
Epitopes for which specific CD8+ T cells were expanded only from the peripheral blood.
Epitopes for which specific CD8+ T cells could be expanded from the peripheral blood and the intrahepatic compartment.
To better define the scope of CD8+ T cell immunity in these animals, HCV-specific CD8+ T cell lines were established from the chronically infected liver or peripheral blood at week 452 (CH1530) or 439 (CH1564) (summarized in Table 4). HCV-specific cytotoxic T lymphocytes (CTL) from CH1530 targeted four epitopes, including three restricted by Patr-A*0701 (designated E1347, p7758, and p7789) and one restricted by Patr-B*0802 (NS31524). The response was slightly broader in CH1564. CD8+ T cell lines from this animal recognized the three Patr-A*0701-restricted epitopes also targeted by CH1530. Four additional epitopes restricted by Patr-A*0602 (NS31617 and NS5A2055) and Patr-B*0901 (NS31565 and E2462) alleles unique to CH1564 were also identified. Only one of the newly defined epitopes (NS31617) was among the 65 peptides used to probe the acute-phase CD8+ T cell response, as it overlapped with a known human HLA-A0101-restricted epitope. The majority of these confirmed epitopes (8 of 11) acquired mutations (Table 2 and 3) at the time when cellular immunity was evident, as measured by lymphoproliferation to recombinant HCV antigens. Although lymphoproliferation is usually considered a measure of T helper cell immunity, it likely reflects the timing of the associated CD8+ T cell response.
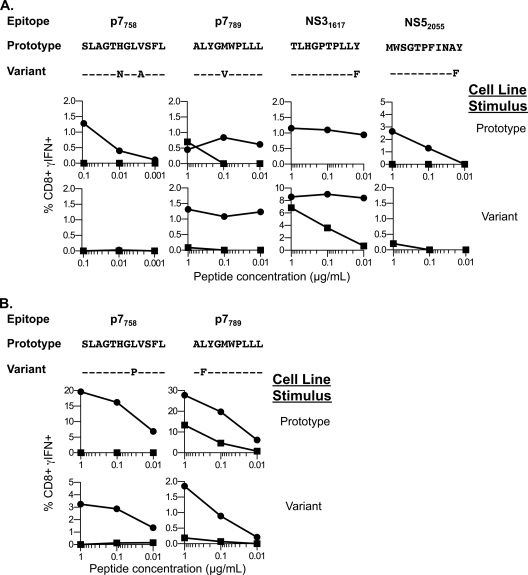
Functional recognition of the wild type and variant epitopes by the CD8+ T cells was examined over a range of serial peptide dilutions. In CH1564, mutations with the potential to impair CD8+ T cell recognition (Fig. 1) were found within epitopes by week 11 (E2462, p7758, NS31617, NS5A2055) or week 21 (p7789) of infection. Diminished recognition of the variant peptides in these assays is the consequence of a decrease in binding affinity to MHC and/or of the affinity of the peptide-MHC complexes for the T cell receptor, as previously described for escape mutations during HCV or human immunodeficiency virus (HIV) infections (8, 16, 31). Of note, three of the five mutations in this animal were previously proven to impair epitope binding to the class I molecule (T463A, Y1626F, and Y2065F) (30). Three of the escape mutations (in epitopes E2462 [T463A], p7789 [M793V], and NS5A2055 [Y2065F]) were permanently fixed at their first appearance in the quasispecies (Table 3). The other two epitopes (p7758 and NS31617) underwent a series of iterative changes over several months before fixation of stable escape mutations (Table 3). For instance, a proline-to-serine (P1621S) substitution at amino acid 5 of epitope NS31617 was first observed at week 11 (Table 3). This variant, nonetheless, disappeared from the quasispecies by week 21, when it was replaced by an Y1626F escape variant that remained fixed through 439 weeks (8.5 years) of follow-up. Accumulations of iterative amino acid substitutions in epitopes as late as 1 year after infection were probably the result of an equilibrium between efficiency of escape from ongoing CD8+ T cell pressure and fitness costs imposed by the escape mutations as demonstrated before (14, 41, 48). For example, in CH1564, the presence of CD8+ T cells specific for the P1621S variant with minimal cross-reactivity with the inoculum or the fixed Y1626F variant (Fig. 1F) suggests that two consecutive CD8+ T cell responses, first against the inoculum and then against the P1621S variant, were required to eventually select for the Y1626F variant.
Fig. 1.
CD8+ T cell recognition of intact versus mutated class I epitopes in chimpanzee CH1564 infected with clonal H77C. CD8+ T cell lines from chimpanzee CH1564 were tested for recognition of epitopes encoded by the genotype 1a inoculum (H77C) or variant sequences encoded by the dominant quasispecies circulating at week 439 postinfection. A box delineates the sequence of each minimal epitope. Results are presented as the number of IFN-γ secreting cells per million input CD8+ T cells or as the frequency of IFN-γ-producing CD8+ T cells, as determined by an ELISpot or ICS assay, respectively. (A) Patr-B*0901-restricted E2462 epitope; (B) Patr-A*0701-restricted p7789 epitope; (C) Patr-A*0602-restricted NS5A2055 epitope; (D) Patr-A*0701-restricted p7758 epitope; (E) Patr-A*0602-restricted NS31617 epitope. Data are representative of duplicate experiments. (F) Sequences of the dominant quasispecies at the indicated time points are shown at the top. The week 11 variant peptide sequence TLHGSTPLLY was used to generate a CD8+ T cell line as described in Materials and Methods. Specificity was assessed by ICS for recognition of the H77C inoculum (crosses), the week 11 variant peptide (circles), or the week 21 variant peptide (triangles) over a range of peptide dilutions.
Mutations in class I epitopes were acquired several months later in CH1530. Serial sequencing of viral genomes revealed that mutations appeared sometime after week 20, but before week 31, of infection in the two class I epitopes that escaped T cell recognition (p7758 [A765P] and p7789 [L790F]) (Fig. 2 A). In an additional class I epitope (NS31524) that escaped T cell recognition, the only amino acid substitution (A1533P) occurred outside the epitope immediately adjacent to the carboxy-terminal residue. This mutation altered processing of the epitope as CD8+ T cells recognized targets expressing the wild-type (1533A) but not the mutant (1533P) NS3 gene (Fig. 2B).
Fig. 2.
CD8+ T cell recognition of intact versus mutated class I epitopes in chimpanzee CH1530 infected with clonal H77C. CD8+ T cell lines from chimpanzee CH1530 were tested for recognition of epitopes encoded by the genotype 1a inoculum (H77C) or variant sequences encoded by the dominant quasispecies circulating at week 520 postinfection. A box delineates the sequence of each minimal epitope. Results are presented as the frequency of IFN-γ-producing cells among the CD8+ T cells as determined by an ICS assay. (A) CD8+ T cell recognition of the Patr-A*0701-restricted p7758 and p7789 epitopes (left and right, respectively). (B) Processing and presentation of the Patr-B*0802-restricted NS31524 epitope. Autologous B-LCL were transfected with an mRNA encoding the NS3 to NS4 sequence of H77C (1533A mRNA), the same HCV fragment encoding the NS3 protein with the A1533P mutation (1533P mRNA), or the EGFP protein as a negative control. Transfected B-LCL were then tested for recognition by NS31524- or NS41723-specific cell lines by IFN-γ ICS assay. Results were normalized to the results with stimulation obtained with B-LCL transfected by 1533A mRNA. Data are representative of duplicate experiments.
Finally, two epitopes did not escape CD8+ T cell recognition despite 8 to 10 years of persistent HCV replication. Sequencing of the virus from animal CH1564 throughout the follow-up revealed that a Patr-B*0901-restricted NS31565 epitope did not mutate, even though cognate CD8+ T cells were present in the persistently infected liver (Table 4 and data not shown). Similarly, CD8+ T cells from both animals recognized the Patr-A*0701-restricted E1347 epitope. In one animal (CH1530), this epitope did not mutate (Table 4 and data not shown). In CH1564, a position 9 valine-to-isoleucine substitution (V355I) appeared in the epitope by week 177, approximately 2 years later than the bona fide escape mutations that appeared in other epitopes between 11 and 21 weeks of infection (Table 3). It did not represent an escape mutation because CD8+ T cell recognition of valine- and isoleucine-substituted peptides was the same, and the E1347 epitope was efficiently processed and presented in target cells that expressed both versions of the E1 gene (Fig. 3).
Fig. 3.
CD8+ T cell recognition of a variant epitope in chimpanzee CH1564 infected with clonal H77C. (Left panel) A CTL line derived from the liver of CH1564 was tested for recognition of the V355I variant peptide on autologous target cells by IFN-γ ICS assay. (Right panel) Autologous B-LCL were transfected with mRNA encoding the E1 to p7 wild-type sequence of H77 (355V mRNA), the same HCV fragment encoding the E1 protein with the V355I mutation (355I), or the EGFP protein as a negative control. Transfected B-LCL were then tested for recognition by E1347- or p7758-specific cell lines by IFN-γ ICS assay. Results were normalized to the results with stimulation obtained with B-LCL transfected by 355V mRNA. Data are representative of duplicate experiments.
To address whether the escape variants that remained fixed through the duration of follow-up were also immunogenic, we generated CD8+ T cell lines from the peripheral blood against the inoculum or escape variant sequences using minimal epitopes. As shown in Fig. 4, even when cell lines were expanded using the variant peptides, recognition of the inoculum sequence was favored. Thus, no CD8+ T cell response specific to the viral variants that eventually became fixed in the quasispecies could be detected in either animal.
Fig. 4.
Absence of CD8+ T cell response against the fixed variants. The H77C inoculum amino acid sequence (prototype) and sequences of the corresponding fixed viral escape variants are shown at the top of the figure. Dashes indicate identity with the H77C sequence. Peptides used to generate CD8+ T cell lines are indicated on the right (stimulus). Cell lines were tested by intracellular cytokine staining for recognition of the H77C inoculum (circles) or the variant peptides (squares) over a range of peptide dilutions. Results are presented as the frequency of IFN-γ-producing cells among the CD8+ T cells. (A) Data for CH1564. The cell line generated with the NS31617 prototype sequence could be tested only against the H77C sequence due to the low number of cells. No cell lines could be obtained against p7758 and NS5A2055 variant peptides. (B) Data for CH1530.
Interestingly, T cell escape mutations were among the first to appear in HCV genomes from both animals, with the exception of the very early G1202E substitution observed in viruses from them. The median week of first occurrence for amino acid replacements in class I epitopes was 21, while the median week of first occurrence in the remainder of the polyprotein was 130. The difference between the two medians was highly significant (P = 0.001; Mann-Whitney test) (Fig. 5). Six amino acids were replaced in HVR1, all in the virus isolated from CH1530 (Table 2) (55). The median time of first occurrence for the amino acid replacements in HVR1 was 104 weeks; this was not significantly different from the median time of occurrence in the remainder of the polyprotein excluding both CD8+ T cell epitopes and HVR1 (177 weeks) (P > 0.05; Mann-Whitney test). In accordance with this result, Liu et al. demonstrated recently that antibody-driven mutations arose 1 to 2 years after infection (29).
Fig. 5.

Week of first occurrence of amino acid replacements in HCV genomes. Distribution of frequencies (counts) of amino acid replacements in presented CD8+ T cell epitopes (red) and the remainder of the polyprotein (green) by week of first occurrence in the consensus sequences.
Impact of the HCV-specific CD8+ T cell response on viral evolution.
CD8+ T cell selection pressure on evolution of HCV genomes was estimated by comparing the percentage of nonsynonymous substitutions falling within class I epitopes with that of the remainder of the genome. CD8+ T cell selection pressure was first detected at week 11 of infection in CH1564. At this time point, a total of 6 nonsynonymous mutations were detected in HCV genomes and 4 of them (67%) were within class I epitopes (Table 5). Similar results were obtained in animal CH1530. At week 31, when CD8+ T cell pressure was first detected, 5 of 8 nonsynonymous mutations (62%) were in class I epitopes (Table 5). In both animals, these percentages began to drop by 1 year of infection as other nonsynonymous mutations accumulated outside class I epitopes (Table 5), although in CH1530, we observed only a single amino acid change between weeks 31 and 52. This analysis of virus genomes obtained by early and frequent sampling suggested that CD8+ T cells are a dominant selective force during acute HCV infection.
Table 5.
Summary of amino acid changes within first year of infection
| Wk of detection | No. (%) of aa changes ina: |
|||||
|---|---|---|---|---|---|---|
| CH1530 |
CH1564 |
|||||
| Total | Toward | Escaped | Total | Toward | Escaped | |
| First occurrenceb | 8 (100) | 1 (13) | 5 (62) | 6 (100) | 2 (33) | 4 (67) |
| 52 | 9 (100) | 1 (11) | 5 (56) | 33 (100) | 3 (9) | 9 (27) |
Total, total number of amino acid changes from inoculum sequence; toward, number of changes toward genotype 1a consensus observed; escaped, number of changes occurring within or resulting in loss of recognition of a CD8+ T cell epitopes. Frequencies of total amino acid changes are indicated in parentheses.
First time point at which escape mutations were detected (week 31 for CH1530 and week 11 for CH1564).
To quantify more precisely the contribution of CD8+ T cells to the rapid pace of amino acid substitution in the acute phase of infection, we computed dN and dS rates over time. The dN/dS ratio was initially high for both chimpanzees (>3) and then rapidly declined to stabilize at approximately 0.5 for 3 to 4 years (Fig. 6). The dN/dS ratio continued to decline at a much lower rate over the remaining 5 to 7 years of follow-up.
Fig. 6.

Plot of dN/dS ratio for HCV genomes from chimpanzees CH1530 and CH1564. The mean dN/dS ratio for viruses from the two animals was calculated as described in Materials and Methods and plotted versus time postinfection (in weeks). Statistical analysis was performed as described in Materials and Methods.
It was previously proposed that a high dN/dS ratio in clonal HCV genomes during the early phase of infection in chimpanzees was driven by T cell selection pressure (18). To address this issue, we reanalyzed dS and dN in class I-restricted epitopes versus those of the remainder of the genomic consensus sequences generated at each time point after infection. The 8 epitopes mapped by intrahepatic CD8+ T cell lines were included in this statistical analysis. The nine predicted but unconfirmed epitopes were also included to avoid bias in a scenario where intrahepatic CD8+ T cells were present but missed due to variability in liver sampling or failure to expand in cell culture.
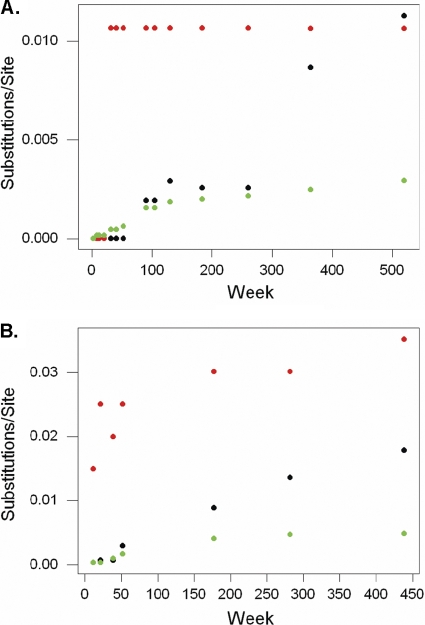
Data are presented in Fig. 7 A and B for CH1530 and CH1564, respectively. After an initial lag, dN within class I HCV epitopes increased dramatically early in infection. Thereafter, it remained steady, indicating fixation of the nonsynonymous substitutions with little or no reversion to the sequence of the clonal genomes used as inoculum (Fig. 7). In contrast, when dN was computed for the remainder of the polyprotein (i.e., excluding class I epitopes), a steady increase was observed over time, but it remained below dS, as expected for sequences that are not under positive selection pressure. Importantly, after 8 to 10 years of infection, dN in the remainder of the genome never approached the rate observed in the class I epitopes (Fig. 7).
Fig. 7.
Synonymous (dS) and nonsynonymous (dN) substitutions in HCV genomes from chimpanzees infected with clonal H77C. For chimpanzees CH1530 (A) and CH1564 (B), plots of nucleotide substitutions per site versus week postinfection are shown. dS is shown for the complete polyprotein gene (black) and dN is shown for presented CD8+ T cell epitopes (red) and the remainder of the polyprotein gene (green).
We plotted the dN/dS ratio versus time postinfection for both animals in the class I-restricted epitopes and in the remainder of the polyprotein gene. Values were averaged when data were available from both animals for the same time point. Importantly, in the epitopes, dN/dS was initially very high (nearly 50:1), providing an independent measure of the dominant impact of CD8+ T cells on HCV genome diversification early in HCV infection. After its peak, this ratio declined steeply over the first year and then continued to decline at a much more gradual rate (Fig. 8).
Fig. 8.

Comparison of dN/dS ratios for class I epitopes and the remainder of the HCV genome in chimpanzees infected with clonal H77C. Plot of dN/dS ratio (mean of data from chimpanzees CH1530 and CH1564) versus time postinfection (in weeks). The ratio for presented class I epitopes (red) and the remainder of the polyprotein gene (green) is shown.
For the remainder of the genome, dN/dS showed a steady decline from an initial low ratio of about 1:1 (Fig. 8). This decline was documented by a significant negative correlation between the dN/dS ratio in the remainder of the gene and weeks postinfection (r = −0.689; P < 0.01). Some of this gradual nonsynonymous change outside class I epitopes was probably due to positive pressure from other selective forces like antibodies that developed in animal CH1530. However, it may also reflect in part gradual purifying selection against amino acids encoded by the transmitted H77C virus. Purifying selection pressure was therefore estimated by scoring changes toward the genotype 1a HCV consensus sequence over 8 or 10 years of infection. Because CD8+ T cell selection pressure is lost after virus transmission to a histoincompatible host, the replacement of some nonconsensus amino acids in the H77C virus should reflect the loss of class I escape mutations that affect the fitness of the virus. Over 8 to 10 years of infection, a total of 20 amino acid changes outside HVR1 were toward the genotype 1a consensus sequence (9 in CH1530 and 11 in CH1564) (Table 6). A total of 10 of these 20 substitutions, including 5 of the 8 shared amino acid changes (G1202E, V1759A, I2116V, R2283P, and L2340V), were found in viruses from both animals (Table 6). Longitudinal analysis of viral sequences within CH1530 and CH1564 demonstrated that only 4 of the 20 mutations toward consensus occurred within the first year of infection, and 2 of them were due to the common G1202E substitution that was observed early in both animals. Thus, the majority of the mutations toward the overall subtype 1a consensus, including 4 of 5 common to viruses from both animals that might be considered most deleterious, occurred over a period of several years, well after the acute phase of infection.
Table 6.
Amino acid changes toward genotype 1a consensusa
| Animal | Sequence | aa at position: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 444 | 480 | 978 | 1008 | 1202 | 1759 | 2116 | 2283 | 2340 | ||||
| CH1530 | H77C | Q | L | R | Q | G | V | I | R | L | ||
| Consensus | H | P | Q | R | E | A | V | P | V | |||
| 528 | 753 | 787 | 1202 | 1358 | 1759 | 2116 | 2198 | 2283 | 2340 | 2412 | ||
| CH1564 | H77C | S | I | V | G | S | V | I | M | R | L | A |
| Consensus | N | V | A | E | P | A | V | V | P | V | D | |
Total amino acid changes toward consensus observed in both animals during the entire period of observation. Consensus was obtained by a multialignment of 208 genotype 1a sequences retrieved from the European HCV database. Amino acid changes toward consensus occurring within the first year of infection are in bold.
DISCUSSION
In this study, we examined the impact of CD8+ T cell selection pressure on the evolution of clonal HCV genomes during acute and chronic infection in chimpanzees. We observed an epitope-specific spike in the dN/dS ratio that peaked at about 50:1 during the acute phase of infection. Importantly, when the class I epitopes were removed from the analysis, no evidence of positive selection pressure could be observed on the remainder of the viral genome. The study also showed that purifying selection is a relatively uncommon event and occurs over several years of chronic infection in the chimpanzees. Altogether, our results demonstrate that Darwinian selection pressure exerted by CD8+ T cells is the dominant force driving early evolution of clonal HCV genomes through selection of escape mutations, whereas late evolution is mostly driven by genetic drift.
A previous study in chimpanzees infected with clonal HCV genomes reported an increase in the dN/dS ratio during the early phase of infection (18). The results of our study provide direct evidence that this was caused by CD8+ T cell Darwinian selection pressure. Selection of escape variants by CD8+ T cells has also been described in HCV-infected humans (4). Thus, a similar measurable impact of CD8+ T cells on early HCV evolution might be expected. However, two recent human studies did not reveal an increase in the dN/dS ratio (26, 38). An increased dN/dS ratio in chimpanzees but not humans could indicate that acute-phase CD8+ T cells exert more effective selection pressure in the animals. It is more likely, however, that the difference is related to the timing or frequency of virus sampling and sequencing. In most humans with acute hepatitis C, the sequence of the transmitted virus and variants that emerge during the early phase of infection are not always available for analysis, as the first sampling time point is usually several weeks after infection. At these early times, some or most of the substitutions might already have accumulated. Lastly, it should be acknowledged that the clonal HCV genome used to establish infection in these chimpanzees is not necessarily equivalent to the complex quasispecies swarm of viruses transmitted between humans and might lead to an underestimation of the dS rate in this model. Nevertheless, this approach may have revealed measurable changes caused by CD8+ T cell selection pressure that would not be obvious in humans because of the difficulty to precisely identify the founder virus, transmission-associated genetic bottlenecks, or shifts in quasispecies dominance unrelated to immunity. Of note, the increase in the dN/dS ratio was most obvious when the analysis focused on the class I epitopes. Further studies are needed to determine if early selection pressure is also directed against class I epitopes of the virus in humans with acute hepatitis C.
Our data also provide statistical evidence that amino acid changes were fixed earlier within class I epitopes than in the remainder of the viral genome (Fig. 5). It is noteworthy that approximately 65% of the earliest nonsynonymous mutations in chimpanzees were located in class I epitopes compared with 20 to 50% in humans (12, 26, 38). Several hypotheses could explain this variation between the species. First, it is conceivable that some CD8+ T cell responses selecting for escape mutations could be missed in human studies due to limited access to the liver compartment. HCV-specific CD8+ T cells are enriched in the infected liver (50) and might sometimes be difficult to expand from the peripheral blood. Alternatively, it is possible that the early measurable impact of CD8+ T cells on virus evolution is diluted with time. Later sampling points in human subjects could underestimate the percentage of mutations located in class I epitopes. For instance, in animal CH1564, the high percentage of mutations in class I epitopes at week 11 postinfection (67%) was substantially reduced by week 52 (27%) as other nonsynonymous mutations accumulated in the viral genome (Table 5). This dilution is probably the result of the burst of fixation of escape mutations early in infection (as shown in Fig. 7) and the lack of additional CD8+ T cell pressure. Failure of CD8+ T cells to apply selection pressure elsewhere on the HCV genome is consistent with the sharp decline in the dN/dS ratio (Fig. 3 and 8) and the absence of new escape mutations after the first year of infection, as documented in human studies (8, 11, 38). This contrasts with observations during chronic HIV or simian immunodeficiency virus (SIV) infection where escape mutations continued to accumulate over time. This difference might explain why the importance of CD8+ T cell-driven selection pressure on HIV genome evolution was apparent despite infrequent sampling (1, 36, 38).
Purifying selection pressure as defined by amino acid changes toward an HCV consensus sequence has been described in class I epitopes of viruses from humans infected through a single-source transmission event (39). Some of these changes may represent loss of escape mutations due to absence of CD8+ T cell pressure in the new host. According to this hypothesis, particularly deleterious mutations in the H77 genome, including those selected by CD8+ T cells in the virus donor, should mutate toward the consensus sequence in both animals soon after transmission. However, over 10 years of follow-up, only one of the eight common changes toward consensus, the G1202E substitution in HCV protein NS3, occurred in both animals during the acute phase of infection. Overall, purifying selection accounted only for about 10% (4 of 42) of amino acid substitutions observed during the first year of infection in our study. The majority of amino acid changes toward consensus occurred after more than 3 years of active replication. These results are roughly similar to those reported by others in humans (12, 26), where 15% (26) to 24% (12) of the mutations outside the envelope proteins during the acute infection were toward the consensus sequence. Together these results suggest that escape mutations most likely reverted very slowly over several years of replication. It also raises the possibility that when class I escape mutations are present in the inoculum, most are not obviously deleterious to replication or establishment of persistent infection. Consistent with this hypothesis, all eight of the new escape mutations characterized in this study did not revert back to the original clonal H77 sequence even after 8 to 10 years of chronic virus replication. Even though we could not rule out that CD8+ T cell pressure on early escaped epitopes is maintained throughout chronic infection, our results also suggest that many escape mutations are either neutral or stabilized by second-site mutations that attenuate the impact on virus replication as previously described for SIV or HIV infection (13, 20, 37, 42). For HCV, fitness cost of selected escape mutations have been investigated using HCV genomes selected for robust growth in cell culture models (14, 44, 48), and many of the mutations did impair replication. However, the possibility that this effect was compensated by changes at other positions of the genome has not been examined. Accordingly, it is possible that some of the escape mutations described in animals CH1530 and CH1564 have a negative impact on viral replication. However, maintenance of the escape mutations despite high viral titers suggests good in vivo fitness of the selected genomes harboring multiple amino acid changes. A study of an HCV transmission pair did document loss of an escape mutation in one epitope within a few weeks of infection (46), but whether this is a common or rare event remains an open question. Passage of viruses with a larger set of well-characterized escape mutations like those described in this study to naïve histoincompatible animals may provide a direct measure of how commonly reversion occurs and the impact of this process on replication and persistence.
Stability of the escape mutations as observed in CH1530 and CH1564 is also probably influenced by the apparent lack of immunogenicity of the fixed viral variants (Fig. 4). CD8+ T cell responses specific to the escaped epitopes have been described before in chimpanzees during the early phase of infection and are consistent with the iterative changes observed in the p7758 and NS31617 epitopes in CH1564 (Fig. 1F). This phenomenon has also been reported in humans during the chronic phase of HCV or HIV infection (2, 8, 17) but seems to be an infrequent event. Lack of immunogenicity can be easily explained for the peptides harboring mutations that abrogated either class I binding or processing, as the epitopes will no longer be presented for recognition by the T cell receptor. Whether the apparent poor immunogenicity of other viral variants during chronic HCV infection is due to the waning of the HCV-specific CD4+ T cell response or an antagonist effect of the escaped sequence as previously suggested (8) requires further studies.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants R37 AI47367 to C. M. Walker and R01 GM43940 to A. L. Hughes. B.C. was the recipient of a Postdoctoral Research Fellowship from the American Liver Foundation. Jens Bukh was the recipient of a professorship at the University of Copenhagen, with external funding from the Lundbeck Foundation. This research was supported by the Intramural Research Program of the NIAID, NIH. Work at New Iberia Research Center was supported by grants 5RR015087, RR016483, and RR014491 from the NCRR.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 7 September 2011.
REFERENCES
- 1. Allen T. M., et al. 2005. Selective escape from CD8+ T-cell responses represents a major driving force of human immunodeficiency virus type 1 (HIV-1) sequence diversity and reveals constraints on HIV-1 evolution. J. Virol. 79: 13239–13249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bailey J. R., Williams T. M., Siliciano R. F., Blankson J. N. 2006. Maintenance of viral suppression in HIV-1-infected HLA-B*57+ elite suppressors despite CTL escape mutations. J. Exp. Med. 203: 1357–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bassett S. E., Thomas D. L., Brasky K. M., Lanford R. E. 1999. Viral persistence, antibody to E1 and E2, and hypervariable region 1 sequence stability in hepatitis C virus-inoculated chimpanzees. J. Virol. 73: 1118–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bowen D. G., Walker C. M. 2005. Mutational escape from CD8+ T cell immunity: HCV evolution, from chimpanzees to man. J. Exp. Med. 201: 1709–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bukh J., et al. 2002. Mutations that permit efficient replication of hepatitis C virus RNA in Huh-7 cells prevent productive replication in chimpanzees. Proc. Natl. Acad. Sci. U. S. A. 99: 14416–14421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bukh J., et al. 2008. Previously infected chimpanzees are not consistently protected against reinfection or persistent infection after reexposure to the identical hepatitis C virus strain. J. Virol. 82: 8183–8195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cantaloube J. F., et al. 2003. Evolution of hepatitis C virus in blood donors and their respective recipients. J. Gen. Virol. 84: 441–446 [DOI] [PubMed] [Google Scholar]
- 8. Chang K. M., et al. 1997. Immunological significance of cytotoxic T lymphocyte epitope variants in patients chronically infected by the hepatitis C virus. J. Clin. Invest. 100: 2376–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Combet C., et al. 2007. euHCVdb: the European hepatitis C virus database. Nucleic Acids Res. 35: D363–D366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cooper S., et al. 1999. Analysis of a successful immune response against hepatitis C virus. Immunity 10: 439–449 [DOI] [PubMed] [Google Scholar]
- 11. Cox A. L., et al. 2005. Comprehensive analyses of CD8+ T cell responses during longitudinal study of acute human hepatitis C. Hepatology 42: 104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cox A. L., et al. 2005. Cellular immune selection with hepatitis C virus persistence in humans. J. Exp. Med. 201: 1741–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crawford H., et al. 2007. Compensatory mutation partially restores fitness and delays reversion of escape mutation within the immunodominant HLA-B*5703-restricted Gag epitope in chronic human immunodeficiency virus type 1 infection. J. Virol. 81: 8346–8351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dazert E., et al. 2009. Loss of viral fitness and cross-recognition by CD8+ T cells limit HCV escape from a protective HLA-B27-restricted human immune response. J. Clin. Invest. 119: 376–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dowd K. A., Netski D. M., Wang X. H., Cox A. L., Ray S. C. 2009. Selection pressure from neutralizing antibodies drives sequence evolution during acute infection with hepatitis C virus. Gastroenterology 136: 2377–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Erickson A. L., et al. 2001. The outcome of hepatitis C virus infection is predicted by escape mutations in epitopes targeted by cytotoxic T lymphocytes. Immunity 15: 883–895 [DOI] [PubMed] [Google Scholar]
- 17. Feeney M. E., et al. 2005. HIV-1 viral escape in infancy followed by emergence of a variant-specific CTL response. J. Immunol. 174: 7524–7530 [DOI] [PubMed] [Google Scholar]
- 18. Fernandez J., et al. 2004. Long-term persistence of infection in chimpanzees inoculated with an infectious hepatitis C virus clone is associated with a decrease in the viral amino acid substitution rate and low levels of heterogeneity. J. Virol. 78: 9782–9789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fleming V. M., Harcourt G., Barnes E., Klenerman P. 2010. Virological footprint of CD4+ T-cell responses during chronic hepatitis C virus infection. J. Gen. Virol. 91: 1396–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Friedrich T. C., et al. 2004. Extraepitopic compensatory substitutions partially restore fitness to simian immunodeficiency virus variants that escape from an immunodominant cytotoxic-T-lymphocyte response. J. Virol. 78: 2581–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fuller M. J., et al. 2010. Selection-driven immune escape is not a significant factor in the failure of CD4 T cell responses in persistent hepatitis C virus infection. Hepatology 51: 378–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gaudieri S., et al. 2006. Evidence of viral adaptation to HLA class I-restricted immune pressure in chronic hepatitis C virus infection. J. Virol. 80: 11094–11104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guglietta S., et al. 2005. Positive selection of cytotoxic T lymphocyte escape variants during acute hepatitis C virus infection. Eur. J. Immunol. 35: 2627–2637 [DOI] [PubMed] [Google Scholar]
- 24. Guglietta S., et al. 2009. Impact of viral selected mutations on T cell mediated immunity in chronically evolving and self limiting acute HCV infection. Virology 386: 398–406 [DOI] [PubMed] [Google Scholar]
- 25. Kolykhalov A. A., et al. 1997. Transmission of hepatitis C by intrahepatic inoculation with transcribed RNA. Science 277: 570–574 [DOI] [PubMed] [Google Scholar]
- 26. Kuntzen T., et al. 2007. Viral sequence evolution in acute hepatitis C virus infection. J. Virol. 81: 11658–11668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lawlor D. A., Warren E., Ward F. E., Parham P. 1990. Comparison of class I MHC alleles in humans and apes. Immunol. Rev. 113: 147–185 [DOI] [PubMed] [Google Scholar]
- 28. Li W. H. 1993. Unbiased estimation of the rates of synonymous and nonsynonymous substitution. J. Mol. Evol. 36: 96–99 [DOI] [PubMed] [Google Scholar]
- 29. Liu L., et al. 2010. Acceleration of hepatitis C virus envelope evolution in humans is consistent with progressive humoral immune selection during the transition from acute to chronic infection. J. Virol. 84: 5067–5077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McKinney D. M., et al. 2000. Identification of five different Patr class I molecules that bind HLA supertype peptides and definition of their peptide binding motifs. J. Immunol. 165: 4414–4422 [DOI] [PubMed] [Google Scholar]
- 31. McMichael A. J., Phillips R. E. 1997. Escape of human immunodeficiency virus from immune control. Annu. Rev. Immunol. 15: 271–296 [DOI] [PubMed] [Google Scholar]
- 32. Meunier J. C., et al. 2005. Evidence for cross-genotype neutralization of hepatitis C virus pseudo-particles and enhancement of infectivity by apolipoprotein C1. Proc. Natl. Acad. Sci. U. S. A. 102: 4560–4565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nei M., Gojobori T. 1986. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 3: 418–426 [DOI] [PubMed] [Google Scholar]
- 34. Nei M., Kumar S. 2000. Molecular evolution and phylogenetics. Oxford University Press, New York, NY [Google Scholar]
- 35. Neumann A. U., et al. 1998. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-α therapy. Science 282: 103–107 [DOI] [PubMed] [Google Scholar]
- 36. O'Connor D. H., et al. 2004. A dominant role for CD8+-T-lymphocyte selection in simian immunodeficiency virus sequence variation. J. Virol. 78: 14012–14022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peyerl F. W., et al. 2003. Simian-human immunodeficiency virus escape from cytotoxic T-lymphocyte recognition at a structurally constrained epitope. J. Virol. 77: 12572–12578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pfafferott K., et al. 2011. Constrained pattern of viral evolution in acute and early HCV infection limits viral plasticity. PLoS One 6: e16797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ray S. C., et al. 2005. Divergent and convergent evolution after a common-source outbreak of hepatitis C virus. J. Exp. Med. 201: 1753–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ray S. C., et al. 2000. Hypervariable region 1 sequence stability during hepatitis C virus replication in chimpanzees. J. Virol. 74: 3058–3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Salloum S., et al. 2008. Escape from HLA-B*08-restricted CD8 T cells by hepatitis C virus is associated with fitness costs. J. Virol. 82: 11803–11812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schneidewind A., et al. 2009. Transmission and long-term stability of compensated CD8 escape mutations. J. Virol. 83: 3993–3997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Simmonds P. 2004. Genetic diversity and evolution of hepatitis C virus—15 years on. J. Gen. Virol. 85: 3173–3188 [DOI] [PubMed] [Google Scholar]
- 44. Söderholm J., et al. 2006. Relation between viral fitness and immune escape within the hepatitis C virus protease. Gut 55: 266–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tester I., et al. 2005. Immune evasion versus recovery after acute hepatitis C virus infection from a shared source. J. Exp. Med. 201: 1725–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Timm J., et al. 2004. CD8 epitope escape and reversion in acute HCV infection. J. Exp. Med. 200: 1593–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Timm J., et al. 2007. Human leukocyte antigen-associated sequence polymorphisms in hepatitis C virus reveal reproducible immune responses and constraints on viral evolution. Hepatology 46: 339–349 [DOI] [PubMed] [Google Scholar]
- 48. Uebelhoer L., et al. 2008. Stable cytotoxic T cell escape mutation in hepatitis C virus is linked to maintenance of viral fitness. PLoS Pathog. 4: e1000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. von Hahn T., et al. 2007. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology 132: 667–678 [DOI] [PubMed] [Google Scholar]
- 50. Walker C. M. 2010. Adaptive immunity to the hepatitis C virus. Adv. Virus Res. 78: 43–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ward S., Lauer G., Isba R., Walker B., Klenerman P. 2002. Cellular immune responses against hepatitis C virus: the evidence base 2002. Clin. Exp. Immunol. 128: 195–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yanagi M., Purcell R. H., Emerson S. U., Bukh J. 1999. Hepatitis C virus: an infectious molecular clone of a second major genotype (2a) and lack of viability of intertypic 1a and 2a chimeras. Virology 262: 250–263 [DOI] [PubMed] [Google Scholar]
- 53. Yanagi M., Purcell R. H., Emerson S. U., Bukh J. 1997. Transcripts from a single full-length cDNA clone of hepatitis C virus are infectious when directly transfected into the liver of a chimpanzee. Proc. Natl. Acad. Sci. U. S. A. 94: 8738–8743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yang Z., Nielsen R. 2000. Estimating synonymous and nonsynonymous substitution rates under realistic evolutionary models. Mol. Biol. Evol. 17: 32–43 [DOI] [PubMed] [Google Scholar]
- 55. Yea C., et al. 2007. Monitoring of hepatitis C virus quasispecies in chronic infection by matrix-assisted laser desorption ionization-time of flight mass spectrometry mutation detection. J. Clin. Microbiol. 45: 1053–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.