Abstract
The presentation of viral epitopes to cytotoxic T lymphocytes (CTLs) by swine leukocyte antigen class I (SLA I) is crucial for swine immunity. To illustrate the structural basis of swine CTL epitope presentation, the first SLA crystal structures, SLA-1*0401, complexed with peptides derived from either 2009 pandemic H1N1 (pH1N1) swine-origin influenza A virus (S-OIVNW9; NSDTVGWSW) or Ebola virus (EbolaAY9; ATAAATEAY) were determined in this study. The overall peptide–SLA-1*0401 structures resemble, as expected, the general conformations of other structure-solved peptide major histocompatibility complexes (pMHC). The major distinction of SLA-1*0401 is that Arg156 has a “one-ballot veto” function in peptide binding, due to its flexible side chain. S-OIVNW9 and EbolaAY9 bind SLA-1*0401 with similar conformations but employ different water molecules to stabilize their binding. The side chain of P7 residues in both peptides is exposed, indicating that the epitopes are “featured” peptides presented by this SLA. Further analyses showed that SLA-1*0401 and human leukocyte antigen (HLA) class I HLA-A*0101 can present the same peptides, but in different conformations, demonstrating cross-species epitope presentation. CTL epitope peptides derived from 2009 pandemic S-OIV were screened and evaluated by the in vitro refolding method. Three peptides were identified as potential cross-species influenza virus (IV) CTL epitopes. The binding motif of SLA-1*0401 was proposed, and thermostabilities of key peptide–SLA-1*0401 complexes were analyzed by circular dichroism spectra. Our results not only provide the structural basis of peptide presentation by SLA I but also identify some IV CTL epitope peptides. These results will benefit both vaccine development and swine organ-based xenotransplantation.
INTRODUCTION
Swine-origin zoonoses pose an increasing threat to human health. Their importance was recently highlighted by the emergence of a new swine-origin influenza A virus (S-OIV), also called 2009 pandemic influenza A (pH1N1) virus, which initially emerged in North America and rapidly spread all over the world in 2009, remaining epidemic through 2010 (57, 63), and by a 2009 outbreak of Ebola-Reston virus in pig populations in the Philippines (48), which was the first swine Ebola-Reston virus infection event ever reported. These zoonotic viruses remain a cause of broad concern for human public health.
Pigs are considered a “mixing bowl” for influenza viruses (IV) from different species, because pigs have receptors that bind to both avian and human IV strains (27). IV consists of eight single-stranded RNA segments encoding 12 proteins: nucleoprotein (NP), three polymerase proteins (PA, PB1, and PB2), two matrix proteins (M1 and M2), two nonstructural proteins (NS1 and NS2), two surface glycoproteins [hemagglutinin (HA) and neuraminidase (NA)], and two newly identified proteins (PB1-F2 and PB1 N40) (6, 28, 73). Reassortment of eight gene segments from different IV strains is a common cause of the emergence of new IV strains. As a mixing bowl, pigs have been the source of emergent IV for a long time, especially H1N1 (18, 52, 62, 74). In fact, swine IV, named classical H1N1 SIV, was the first IV ever isolated (56). Similar S-OIV infection events have led us to believe that pigs act as a primary host in the cross-species transmission of IV. Obviously, elimination of IV in swine would aid the control of IV in humans.
The major histocompatibility complex (MHC) class I molecules play a pivotal role in cellular immune responses against virus infection. Classical MHC class I molecules present viral peptides, termed cytotoxic T lymphocyte (CTL) epitopes, to specific T-cell receptors (TCRs) of CD8+ T cells. The subsequent formation of an immune synapse results in the proliferation of CTLs, lysis of the virus-infected cells, and eventually clearance of the virus from the host (22). Structural studies have revealed that viral peptides could interact with six pockets (A to F) in the peptide-binding groove (PBG) of MHC I and form a trimolecular complex, including MHC I heavy chain, epitope peptide, and β2-microglobulin (β2m) (43). In humans, the MHC is also termed human leukocyte antigen (HLA). Classical HLA I genes exist at three loci in the human genome, and each locus has dozens to hundreds of alleles (29). Polymorphisms of MHC I determine the distinct three-dimensional (3D) structure of the PBG. Viral epitopes bind to the PBG with different affinities in an MHC-restricted manner. Each MHC I allele is able to bind a particular profile of CTL epitopes, based on the compatibility of the binding pockets in the PBG (24). Thus far, most of the HLA I and mouse MHC I complex structures have been solved (47), and complex structures of some other species have also been elucidated in recent years, including rat, monkey, chicken, bovine, and chimpanzee (8, 19, 30, 35, 36, 41, 60). Nevertheless, little is known about the structure of swine MHC I molecules.
The genes encoding swine MHC I, termed the swine leukocyte antigen (SLA) region, were first reported by Vaiman et al. in 1970 (70, 71). The SLA region has subsequently been found in the 7p1.1 band of the short arm of the seventh chromosome and spanning a region of approximately 1.1 Mb (53). Seven classical and three nonclassical SLA I genes are linked in the SLA region; the expressed SLA I loci are SLA-1, SLA-2, and SLA-3, while SLA-4, SLA-5, SLA-9, and SLA-11 are pseudogenes (65). The SLA-1 locus has been found to have the highest expression level (40), implying that SLA-1 molecules play a dominant role in the immune process, including presentation of CTL epitopes. Currently, 116 SLA I allelic genes, including the SLA-1, SLA-2, and SLA-3 loci, have been deposited in the Immune Polymorphism Database (IPD; http://www.ebi.ac.uk/ipd/index.html). Over 43 of the reported SLA I genes are alleles of SLA-1. SLA-1*0401 molecules are commonly expressed in five swine breeds (25) and the PK-15 cell line, indicating that SLA-1*0401 is a valuable SLA I allele that has survived long-term evolutionary selection. Many studies of SLA I have been reported during the past 40 years, and the CTL immune responses involving SLA I molecules have been studied using numerous diverse methods (11, 14, 51, 54). Furthermore, since 2009, the NetMHCpan (http://www.cbs.dtu.dk/services/NetMHCpan/) method has made it possible to predict CTL epitope peptides for SLA I molecules (26).
Current vaccine regimens use inactivated influenza virus to acquire neutralizing antibodies against the external HA glycoprotein (5). However, this glycoprotein mutates rapidly through both antigenic drift and shift, and current vaccines are usually ineffective against newly emerged IV strains; therefore, new vaccines must be developed every year. New vaccine strategies are increasingly directed at conserved CTL epitopes of IV (64), as CTL responses have been proven to clear IV and reduce the severity of symptoms (44, 46), and seasonal IV-specific CTL responses can cross-react with peptides derived from S-OIV (21). Although hundreds of T-cell epitopes derived from IV were identified for humans, mice, and other animals (and deposited in the Immune Epitope Database and Analysis Resource [IEDB]), IV-derived CTL epitopes for pigs have remained elusive until now.
To examine the structural basis of SLA I antigen presentation, we solved the first crystal structures of swine MHC I SLA-1*0401 complexed with either S-OIV- or Ebola virus-derived peptides. In addition to the common characteristics of mammalian MHCs, a distinctive feature of SLA-1*0401 is that residue Arg156 has the ability to “veto” the binding of viral peptides if they do not contain a small or negatively charged residue at position P3. Although S-OIV NA449-457-NSDTVGWSW (S-OIVNW9) and Ebola virus vp35155-163-ATAAATEAY (EbolaAY9) have different sequences, EbolaAY9 binds SLA-1*0401 with a conformation very similar to that of S-OIVNW9 but with the help of 8 additional water molecules. Notably, the residues in P7 positions in both S-OIVNW9 and EbolaAY9 are exposed on the surface of the PBG, making these two peptides featured in contact with TCRs. Finally, 23 potential CTL epitopes from 2009 pandemic S-OIV were identified, three of which are cross-species epitopes also presented by HLA-A*0101 and may activate cross-species CTL responses. Based on the structures of SLA-1*0401 solved here and the binding affinities of the peptides determined by the in vitro refolding method, the peptide binding motif of SLA-1*0401 was proposed and examined by testing the thermostabilities of SLA-1*0401 with key peptides.
MATERIALS AND METHODS
Synthesis of viral peptides.
A total of 39 viral peptides were used in these experiments (Tables 1 and 2). Thirty-eight nonapeptides matching the 2009 pH1N1 S-OIV were predicted by NetMHCpan-2.0 (http://www.cbs.dtu.dk/services/NetMHCpan) and synthesized by SciLight Biotechnology. In addition, the sequence information for EbolaAY9 (ATAAATEAY) was provided by Ilka Hoof of Technical University of Denmark, and this peptide was synthesized as described above. The purities of the peptides were >90%, as assessed by high-performance liquid chromatography.
Table 1.
Predicted peptides and their binding to SLA-1*0401, evaluated by in vitro refolding
| Virus, protein, and position | Amino acid sequence | %Randoma | Stabilityb |
|
|---|---|---|---|---|
| R156 | A156 | |||
| Ebola virus | ||||
| vp35 | ||||
| 155–163 | ATAAATEAY | 0.01 | ++ | ++ |
| Influenza A virus | ||||
| NA | ||||
| 449–457 | NSDTVGWSW | 32 | ++ | ++ |
| 265–274 | KSVEMNAPNY | 0.25 | − | / |
| 304–312 | VSFNQNLEY | 0.03 | − | / |
| HA | ||||
| 87–95 | LSTASSWSY | 0.25 | + | / |
| 126–134 | SSFERFEIF | 1.50 | − | / |
| 215–223 | YVFVGSSRY | 0.10 | − | ++ |
| M1 | ||||
| 1–10 | MSLLTEVETY | 0.5 | − | / |
| NS1 | ||||
| 82–89 | ASVPTSRY | 0.17 | − | / |
| PA | ||||
| 455–464 | ATEYIMKGVY | 0.30 | ++ | ++ |
| 557–565 | QVSRPMFLY | 0.25 | + | / |
| 679–687 | GTFDLGGLY | 0.03 | − | ++ |
| PB1 | ||||
| 315–323 | RMFLAMITY | 0.3 | − | / |
| 488–497 | GTFEFTSFFY | 0.15 | − | / |
| PB2 | ||||
| 564–572 | WSQDPTMLY | 0.25 | − | ++ |
| NP | ||||
| 145–153 | ATYQRTRAL | 0.5 | − | / |
| 480–488 | MSNEGSYFF | 0.8 | − | / |
% Random is a base value for estimating the binding affinities of peptides with the NetMHCpan server: Rank threshold for strongly binding peptides, 0.100; rank threshold for weakly binding peptides, 1.000.
R156, wild-type SLA-1*0401 heavy chain with arginine at position 156; A156, mutated SLA-1*0401 heavy chain with alanine in place of arginine at position 156; ++, peptide binds strongly and can tolerate anion-exchange chromatography; −, peptide does not bind SLA-1*0401; +, peptide binds SLA-1*0401 but cannot tolerate anion-exchange chromatography; /, peptide was not tested.
Table 2.
Selected potential CTL epitope peptides from the 2009 pH1N1 influenza A virus, based on the SLA-1*0401 binding motif
| Protein and position | Amino acid sequence | Published MHC allelea | Published CTL response (reference[s])a | Stability with SLA-1*0401b |
|---|---|---|---|---|
| NA | ||||
| 25–33 | QIGNIISIW | / | / | ++ |
| 266–274 | SVEMNAPNY | / | / | ++ |
| 414–423 | GLDCIRPCFW | / | / | ++ |
| HA | ||||
| 16–24 | NADTLCIGY | / | / | ++ |
| 343–351 | IAGFIEGGW | B58 | / | −c |
| 358–366 | WTGMVDGWY | A1 | / | − |
| 445–454 | LLENERTLDY | / | / | ++ |
| M1 | ||||
| 6–15 | EVETPTRSEW | / | / | ++ |
| 36–45 | NTDLEALMEW | / | / | ++ |
| M2 | ||||
| 83–91 | AVDVDDGHF | / | / | ++ |
| NP | ||||
| 9–17 | MIGGIGRFY | / | / | +d |
| 44–52 | CTELKLSDY | A1 | Positive (3, 10, 12) | ++ |
| 378–386 | TLELRSRYW | / | / | ++ |
| NS1 | ||||
| 194–202 | VSENIQRFAW | / | / | ++ |
| PA | ||||
| 437–445 | HIASMRRNY | / | / | ++ |
| 531–539 | RLEPHKWEKY | / | / | ++ |
| PB1 | ||||
| 347–355 | KMARLGKGY | A1(23), A3, A26, B8, B27, B58, B62 | Positive (72) | + |
| 372–380 | MLASIDLKY | A1, Mamu-A*02(23) | − | |
| 542–551 | ATAQMALQLF | Mamu-A*01, Mamu-A*02 | / | + |
| 591–599 | VSDGGPNLY | A1, A26, A80, B15, B18, B58, Mamu-A*02 | Positive (2, 12) | ++ |
| PB2 | ||||
| 197–205 | KIAPLMVAY | / | / | ++ |
| 213–222 | VAGGTGSVY | / | / | + |
/, no information available.
++, peptide binds strongly and can tolerate anion-exchange chromatography; −, peptide does not bind SLA-1*0401; +, peptide binds SLA-1*0401 but cannot tolerate anion-exchange chromatography.
Preparation of proteins.
The SLA-1*0401 gene (EU170457) was cloned from PK-15 cells. The PCR primers and conditions were described previously (15). The PCR product was sequenced, ligated into the pET21a vector (Novagen), and transformed into Escherichia coli strain BL21(DE3). Recombinant SLA-1*0401 was expressed in inclusion bodies and purified as described previously (7).
The gene fragment encoding the mature peptide of swine β2-microglobulin (sβ2m) was amplified from plasmid p2X-β2m, which we constructed previously (15). The PCR product was sequenced, ligated into the pET21a vector, and transformed into E. coli strain BL21(DE3). Recombinant sβ2m was also expressed in inclusion bodies and purified as previously described (7).
Refolding of SLA-1*0401 with S-OIV or Ebola viral peptides.
To form a complex with each peptide (Tables 1 and 2), SLA-1*0401 and sβ2m were refolded using the gradual dilution method, as previously described (9). As a negative control, SLA-1*0401 and sβ2m were also refolded without peptide. After 48 h of incubation at 277 K, the remaining soluble portion of the complex was concentrated and then purified by chromatography on a Superdex200 16/60 column followed by Resource-Q anion-exchange chromatography (GE Healthcare), as previously described (9).
Crystallization and data collection.
Two viral peptides, S-OIVNW9 [NW9; NSDTVGWSW, derived from A/Beijing/01/2009(H1N1) NA protein in the region from 449 to 457] and EbolaAY9 (AY9; ATAAATEAY, derived from Ebola virus VP35 protein in the region from 155 to 163), were selected for crystallization with SLA-1*0401 heavy chain and sβ2m. SLA-1*0401–S-OIVNW9 and SLA-1*0401–EbolaAY9 complexes were concentrated to 8 mg/ml in a buffer containing 20 mM Tris (pH 8.0) and 50 mM NaCl for crystallization. After being mixed with reservoir buffer at a 1:1 ratio, the purified SLA-1*0401–sβ2m-peptide complex (pSLA-1*0401) was crystallized by the hanging-drop vapor diffusion method at 291 K. Index kits (Hampton Research, Riverside, CA) were used to screen the crystals. After several days, crystals of SLA-1*0401–S-OIVNW9 and SLA-1*0401–EbolaAY9 were obtained with solutions 43 (25% [wt/vol] polyethylene glycol 3350, 0.1 M bis-Tris [pH 6.5]) and 38 (30% [vol/vol] JeffamineM-600 [pH 7.0], 0.1 M bis-Tris [pH 7.0]). Diffraction data were collected using an in-house X-ray source (Rigaku MicroMax007 desktop rotating anode X-ray generator with a Cu target operated at 40 kV and 30 mA) and an R-Axis IV++ imaging-plate detector at a wavelength of 1.5418 Å. In each case, the crystal was first soaked in reservoir solution containing 15% glycerol as a cryoprotectant for several seconds and then flash-cooled in a stream of gaseous nitrogen at 100 K (50). The collected intensities were indexed, integrated, corrected for absorption, scaled, and merged using HKL2000 (49).
Structure determination and refinement.
The structures of SLA-1*0401–S-OIVNW9 and SLA-1*0401–EbolaAY9 (pSLA-1*0401) were solved by molecular replacement using the MOLREP program with HLA-A*1101 (PDB code, 1Q94) as the search model. Extensive model building was performed by hand using COOT (13), and restrained refinement was performed using REFMAC5. Further rounds of refinement were performed using the phenix.refine program implemented in the PHENIX package (1) with isotropic ADP refinement and bulk solvent modeling, which improved the R and Rfree factors from 0.194 and 0.209 to 0.151 and 0.177, respectively. The stereochemical quality of the final model was assessed with the PROCHECK program (33). Data collection and refinement statistics are listed in Table 3.
Table 3.
X-ray diffraction data processing and refinement statistics
| Parameter or statistic | EbolaAY9a | S-OIVNW9a |
|---|---|---|
| Data processing | ||
| Space group | C121 | P1211 |
| Cell parameters (Å) | a = 88.68, b = 40.24, c = 103.63 | a = 96.73, b = 37.65, c = 111.43 |
| Resolution range (Å) | 23.8–2.10 | 28.3–2.59 |
| Total reflections | 125,529 | 103,966 |
| Unique reflections | 21,633 | 23,574 |
| Completeness (%) | 99.2 (98.4) | 99.9 (100.0) |
| Rmerge (%)b | 6.0 (27.6) | 12.6 (49.9) |
| I/σ | 28.321 (6.706) | 11.833 (2.889) |
| Refinement | ||
| R factor (%)c | 19.2 | 20 |
| Rfree (%) | 23 | 26 |
| RMSD | ||
| Bonds (Å) | 0.003 | 0.003 |
| Angles (°) | 0.763 | 0.735 |
| Average B factor | 33.560 | 26.865 |
| Most favored (%) | 98 | 96 |
| Disallowed (%) | 0.0 | 0.0 |
Numbers in parentheses indicate the highest-resolution shell.
Rmerge = ∑h∑Iih − <Ih>/∑h∑I<Ih>, where <Ih> is the mean intensity of the observations Iih of reflection h.
R factor = ∑(Fobs−Fcalc)/∑Fobs; Rfree is the R factor for a subset (5%) of reflections that was selected prior to refinement calculations and not included in the refinement.
Preparation of the Arg156-to-Ala mutant of pSLA-1*0401.
To investigate the function of Arg156 in SLA-1*0401, Arg156 was mutated to Ala156 by overlap PCR (primers used for mutation were 5′-GGCGGAGCGTGCGAGGAGCTAC-3′ and 5′-GTAGCTCCCGCTACGCTCCGCC-3′, the underlined sequences mutated the codon encoding Ala). The resulting protein was termed SLA-1*0401-Ala156. SLA-1*0401-Ala156 was inserted into the pET21a vector and expressed in BL21(DE3) cells. Recombinant SLA-1*0401-Ala156 was expressed in inclusion bodies and further purified, as described previously (9). SLA-1*0401-Ala156 was refolded with sβ2m and each viral peptide. In addition, the complexes formed by refolding were further purified by gel filtration and anion-exchange chromatography as described above (9).
Determination of complex thermostability using CD spectroscopy.
The thermostabilities of SLA-1*0401 with six key peptides were tested by circular dichroism (CD) spectroscopy. CD spectra were measured at 20°C on a Jasco J-810 spectropolarimeter equipped with a water-circulating cell holder. The protein concentration was 8 μM in pH 8.0 Tris buffer (20 mM Tris and 50 mM NaCl). Thermal denaturation curves were determined by monitoring the CD value at 218 nm by using a 1-mm-optical-path-length cell as the temperature was raised from 25 to 80°C at a rate of 1°C/min. The temperature of the sample solution was directly measured with a thermistor. The fraction of unfolded protein was calculated from the mean residue ellipticity (θ) by the standard method. The unfolded fraction (%) is expressed as (θ − θN)/(θU − θN), where θN and θU are the mean residue ellipticity values in the fully folded and fully unfolded states. The midpoint transition temperature (Tm) was determined by fitting data to the denaturation curves using the Origin 8.0 program (OriginLab) as described previously (67).
Protein structure accession numbers.
The crystal structures have been deposited in the Protein Data Bank (http://www.pdb.org/pdb/home/home.do) with accession numbers 3QQ3 and 3QQ4.
RESULTS
Overall structure of the pSLA-1*0401 complex.
Analysis of the crystal structure of the pSLA-1*0401 complexes showed two SLA-1*0401 molecules complexed with S-OIVNW9 in each asymmetric unit, termed SLA-1*0401–S-OIVNW9, and one SLA-1*0401 molecule complexed with EbolaAY9 in each asymmetric unit, termed SLA-1*0401–EbolaAY9. The 3D structures of SLA-1*0401–S-OIVNW9 and SLA-1*0401–EbolaAY9 contained the SLA-1*0401 heavy (H) chain (residues 1 to 276), sβ2m (residues 1 to 98), and both S-OIVNW9 and EbolaAY9 peptides, respectively (Fig. 1). The root mean square deviation (RMSD) for all of the Cα atoms in SLA-1*0401–S-OIVNW9 and SLA-1*0401–EbolaAY9 was 0.685 Å. The polar and nonpolar interactions of peptides with the PBG were analyzed and are listed in Table 4. In the two structures, the SLA-1*0401 H chain was composed of α1 (residues 1 to 90), α2 (residues 91 to 180), and α3 (residues 181 to 275) domains; the α1 and α2 domains form the PBG (Fig. 2). The H-chain α1 and α2 domains can be divided into two portions. One portion (α1, residues 50 to 54 and 57 to 85; α2, residues 138 to 150 and 152 to 174) forms helices located at the top of the PBG, and the remaining portion (residues 4 to 13, 20 to 28, 31 to 37, 46 to 47, 93 to 103, 110 to 118, 121 to 126, and 133 to 135) forms an eight-stranded β-sheet at the bottom. Both the α3 domain (residues 186 to 193, 199 to 208, 214 to 219, 222 to 223, 229 to 230, 234 to 235, 241 to 248, 257 to 262, and 270 to 274) and sβ2m (residues 6 to 11, 21 to 30, 36 to 41, 45 to 46, 48 to 49, 53 to 54, 60 to 68, 76 to 81, and 89 to 93) consist of two 7-stranded β sheets (Fig. 1 and 2). The areas of sβ2m buried in the SLA-1*0401 H chain are 1,342.3 Å2 for SLA-1*0401–S-OIVNW9 and 1,418.1 Å2 for SLA-1*0401–EbolaAY9. Strands A, B, D, and E of sβ2m and the loops between them broadly interact with the SLA-1*0401 H chain. In particular, residues Gln8, Tyr10, Arg12, Tyr26, His31, Asp52, Phe55, Trp60, and His99 form strong contacts with the H chain.
Fig. 1.
Overview of SLA-1*0401 structures. The A, B, and C chains in PDB 3QQ3 and C chain in PDB 3QQ4 are used to show the overall structure of SLA-1*0401. The H chain, composed of the α1, α2, and α3 domains, is shown as a cartoon. The α1 domain is in gray, α2 is in bright orange, and α3 is in light pink. sβ2m is shown as a cartoon in pale yellow; the peptides S-OIVNW9 (NSDTVGWSW) and EbolaAY9 (ATAAATEAY) are superimposed Cα traces, shown as stick models and colored by atom type (C, cyan [S-OIV-NW9] and green [EbolaAY9]; N, blue; O, red).
Table 4.
Hydrogen bonds and van der Waals interactions between peptides and SLA-1*0401 heavy chain
| Complex | Peptide |
Hydrogen bond partner |
van der Waals contact residuesa | ||
|---|---|---|---|---|---|
| Residue | Atom | Residue | Atom | ||
| EbolaAY9 | P1-Ala | N | Tyr7 | OH | Leu5, Tyr7, Tyr59, Glu63, Tyr159, Leu163, Ser167, Tyr171 (43) |
| Ser167 | OG | ||||
| Tyr171 | OH | ||||
| O | Tyr159 | OH | |||
| P2-Thr | N | Glu63 | OE1 | Tyr7, Tyr9, Met45, Glu63, Asn66, Val67, Tyr99, Tyr159, Leu163 (47) | |
| OG1 | Glu63 | OE1 | |||
| O | Asn66 | ND2 | |||
| P3-Ala | O | Asn66 | ND2 | Asn66, Thr70, Tyr99, Arg156, Tyr159 (36) | |
| P4-Ala | O | Arg156 | NH2 | Asn66, Arg156 (10) | |
| P5-Ala | N | Arg156 | NH2 | Asn66, Glu69, Thr70, Thr73 (10) | |
| P6-Thr | OG1 | Arg114 | NH2 | Thr73, Tyr74, Arg114, Trp147, Glu152, Arg156 (38) | |
| Glu152 | OE2 | ||||
| P7-Glu | N | Glu152 | OE1 | Thr73, Trp147, Ala150, Glu152, Arg155 (30) | |
| O | Trp147 | NE1 | |||
| P8-Ala | O | Trp147 | NE1 | Lys146, Trp147 (6) | |
| P9-Tyr | O | Lys146 | NZ | Tyr74, Gly77, Thr80, Leu81, Tyr84, Leu95, Ser97, Arg114, Asp116, | |
| OH | Tyr74 | OH | Thr143, Lys146, Trp147 (87) | ||
| Ser97 | OG | ||||
| Asp116 | OD1 | ||||
| OXT | Tyr84 | OG1 | |||
| Thr143 | OG1 | ||||
| S-OIVNW9 | P1-Asn | N | Tyr7 | OH | Leu5, Tyr7, Tyr59, E63, Leu163, Arg170, Tyr171 (60) |
| Ser167 | OG | ||||
| Tyr171 | OH | ||||
| O | Tyr159 | OG | |||
| ND2 | Ser167 | ND2 | |||
| P2-Ser | N | Glu63 | OE1 | Tyr7, Tyr9, Glu63, Asn66, Tyr99 (41) | |
| OG | Glu63 | OE1 | |||
| O | Asn66 | ND2 | |||
| P3-Asp | O | Asn66 | ND2 | Tyr9, Asn66, Tyr99, Arg156, Tyr159 (60) | |
| N | Tyr99 | OH | |||
| OD2 | Arg156 | NH1 (salt bridge) | |||
| P4-Thr | Asn66, Arg156 (14) | ||||
| P5-Val | N | Asn66 | Asn66, Glu69, Thr70, Arg156 (30) | ||
| P6-Gly | OD1 | Thr73, Glu152, Arg156 (8) | |||
| P7-Trp | N | Glu152 | Thr73, Lys146, Trp147, Ala150, Arg155, Arg156 (45) | ||
| P8-Ser | O | Trp147 | OE2 | Lys146, Trp147 (7) | |
| P9-Trp | OXT | Tyr84 | NE1 | Thr73, Tyr74, Gly77, Thr80, Leu81, Tyr84, Leu95Arg114, Asp116, | |
| Thr143 | OH | Tyr123, Thr143, Lys146, Trp147 (92) | |||
| O | Lys145 | OG1 | |||
| NE1 | Asp116 | NZ | |||
| OD1 | |||||
Numbers in parentheses are the amounts of van der Waals force.
Fig. 2.
Structure-based sequence alignment of SLA-1*0401 and representatives of other crystallized MHC I molecules. Black arrows above the alignment indicate β-strands; cylinders denote α-helices. Residues highlighted in red are absolutely conserved. Residues highlighted in green are species-specific amino acids that differ between swine and other animals. Residues highlighted in blue are conserved in SLA-3 but seldom appear in SLA-1 or SLA-2. Residues at position 156 are highlighted in yellow and marked by a star. Green numbers denote residues that form disulfide bonds. The total amino acid (AA) identities between SLA-1*0401 and the listed MHC I molecules are given beside the names, and the amino acid identities of each region are labeled on the right-hand side. The alignment was generated using the program ClustalX (66) and drawn with ESPript (20).
Species-specific characteristics of SLA I determined by alignment with MHC I from other vertebrates.
To analyze their diversity, typical human, mouse, rat, monkey, bovine, and chicken class I molecules were aligned with SLA-1, SLA-2, and SLA-3 alleles (Fig. 2). Although the genome sequences indicate that the SLA-1 and SLA-3 loci are more similar to each other than to SLA-2 (40), the amino acid sequences of SLA-1 and SLA-2 are more homologous by phylogenetic analysis. The H chain of SLA-1*0401 is >88% identical to other SLA-1 molecules and 89% identical to SLA-2 molecules. However, SLA-1*0401 is only 85 to 88% identical to SLA-3 molecules. Comparison of SLA I and other class I sequences revealed that, with the exception of SLA-2*jh01, Lys19and Ala163 are highly conserved in SLA-3 but seldom appear in SLA-1 and SLA-2 molecules (Fig. 2). In SLA I alleles, only the α3 domains are highly conserved, and α1 domains are more variable than α2 domains. NCBI BLAST database searches demonstrated that there are 13 common amino acid differences among the SLA I alleles and other crystallized mammalian class I molecules. Importantly, the variation arises mainly in the α3 domain, though a few (six amino acids) appeared in the α1 and α2 domains. The RMSD between pSLA-1*0401 and other class I molecules annotated in the Protein Data Bank (PDB) (http://www.pdb.org/pdb/home/home.do) is <1.6 Å, with the exception of chicken B21, for which the RMSD is >2 Å. HLA-A*1101 has the highest identity with SLA-1*0401 (78%), with an RMSD of 0.682 Å. Although there are ∼20 amino acid residue differences between the sβ2m and human β2m (hβ2m) sequences, only three of those residues interact with the H chain. Most differences that affect the interaction between sβ2m and the SLA-1*0401 H chain are at the N terminus of sβ2m. Val1 and Lys6 in sβ2m form only 3 contacts with the SLA-1*0401 H chain, whereas Ile1 and Lys6 in hβ2m can form 47 contacts with the HLA I H chain.
Comparison of the pockets and viral peptide binding interface of SLA-1*0401 and HLA I.
The PBG in HLA I was previously classified into six pockets, A to F (55). Structural analysis indicated that the PBG of pSLA-1*0401 also contains these six pockets; therefore, the same nomenclature was provisionally adopted here for the analysis of pSLA-1*0401.
Pocket A in pSLA-1*0401 consists of residues Leu5, Tyr7, Tyr59, Glu63, Tyr159, Leu163, Ser167, and Tyr171 (Fig. 3A). The positions of Tyr7 and Tyr171 were conserved in mammalian class I molecules (30) and formed hydrogen bonds with the amino group of the P1 residues of the bound peptides. Leu5 and Ser167 seldom appear in classical HLA-A. However, Leu5 is found in HLA-B*44, and Ser167 is found in nonclassical HLA (42) and H-2kb (45). In most class I molecules, the residue at position 167 is Trp, which has a large side chain; however, in pSLA-1*0401, Ser167 forms two hydrogen bonds with the amino group of P1-Asn of S-OIVNW9 and one hydrogen bond with P1-Ala of EbolaAY9 (Table 4). Due to the change from Ser167 to Trp167, the N terminus of the PBG in pSLA-1*0401 appears to be more open than in other crystallized class I molecules except cattle MHC class I N*01801 (35).
Fig. 3.
Composition of the pockets of SLA-1*0401. Pockets are shown as surface representations in light pink. The residues comprising these pockets are shown as stick models and labeled. Residues of bound peptides accommodated by these pockets are shown as stick models and colored as in Fig. 1. The hydrogen bonds between peptides and pockets are shown as a yellow dashed line. (A) Pocket A with the P1 residue (Asn of S-OIVNW9). (B) Pocket B with the P2 anchor residue (Thr of S-OIVNW9). (C) Pocket E with the P6 residue (P6-Gly in S-OIVNW9 does not have side chain contacts with pocket E, so this displays P6-Thr of EbolaAY9). (D) Pocket F with the PC anchor residue (Trp of S-OIVNW9).
In pSLA-1*0401, the P2-Ser of S-OIVNW9 and P2-Thr of EbolaAY9 are inserted into pocket B in the same way. The main amino group (N) of both P2 residues is tethered by hydrogen bonds from Glu63 in the PBG. Both hydroxyls of the P2 side chains form hydrogen bonds to Glu63 and Asn66 (Table 4). Pocket B in pSLA-1*0401 consists of the residues Tyr7, Tyr9, Ala24, Met45, Glu63, Asn66, and Val67, as it also does in HLA-A*1101 (Fig. 2 and 3B) (34). This result demonstrates that, as in HLA-A*1101, pocket B of pSLA-1*0401 is able to accommodate residues with neutral side chains (Ser, Thr, Ala, Ile, Leu, Met, or Val) at P2 (34).
Pocket E in pSLA-1*0401 accommodates the side chain of the P6 residue (Fig. 3C). In pSLA-1*0401–EbolaAY9, the side chain of the P6 residue (Thr) is inserted into pocket E and forms hydrogen bonds with Arg114 and Glu152 (Table 4). In pSLA-1*0401–S-OIVNW9, the P6 Gly residue interacts with pocket E even without any side chain. The side chains of the P7 and P8 residues extend outward into the solvent, where they may be recognized by TCRs.
Pocket F of pSLA-1*0401 is composed of the highly conserved residues Thr73, Tyr84, Tyr123, Thr143, Lys146, and Trp147, as well as the less conserved residues Tyr74, Gly77, Thr80, Leu81, Tyr84, Leu95, Ser97, Arg114, Asp116, and Ile124 (Fig. 3D) (61). The PC anchor residues for SLA-1*0401 are similar to those for HLA-A*01, HLA-B*35, and HLA-B*57, which have large residues with an aromatic ring (32, 59, 61). The aromatic rings in S-OIVNW9 and EbolaAY9 are held in close contact with residues in pocket F by strong hydrogen bonds and van der Waals contacts (Table 4). P9-Tyr in EbolaAY9 forms more hydrogen bonds with residues Tyr74 and Ser97 than P9-Trp in S-OIVNW9 by using hydroxyls on its aromatic ring (Table 4). However, P9-Trp is larger than P9-Tyr and has a more complementary shape for pocket F. Therefore, it forms more van der Waals contacts than P9-Tyr (Table 4).
The detailed analysis of distinct C and D pockets of pSLA-1*0401 is described below. The unconserved residues composing pockets A, B, E, and F of pSLA-1*0401 partially contribute to the distinct peptide presentation and TCR contact of SLA I.
A flexible Arg156 residue in pocket D functions as a “one-ballot veto” for epitope binding.
Pockets C and D in SLA-1*0401–S-OIVNW9 are very different from those in SLA-1*0401–EbolaAY9. In SLA-1*0401–S-OIVNW9, pockets C and D are integrated as one cavity, whereas pockets C and D in SLA-1*0401–EbolaAY9 are separate. This conformational change is due to the flexible side chain of Arg156, located in pocket D (Fig. 4 A and B). In SLA-1*0401–S-OIVNW9, Arg156 forms a strong salt bridge with the P3 Asp of S-OIVNW9. The side chain of P3 Asp interacts with the side chain of Arg156 and pushes it close to the α2 helix of SLA-1*0401 (Fig. 4A and C). However, in SLA-1*0401–EbolaAY9, the side chain of P3 Ala is too short to interact with Arg156 in pocket D. Instead, the flexible side chain of Arg156 extends into the PBG and binds the oxygen atom of the main chain of P4 Ala (Fig. 4B and C).
Fig. 4.
The flexible Arg156 in the D pocket contacts two peptides in different ways. (A) In SLA-1*0401–S-OIVNW9, P3 Asp interacts with the side chain of Arg156 and pushes it close to the α2 helix of SLA-1*0401. (B) In SLA-1*0401–EbolaAY9, the flexible side chain of Arg156 extends into the PBG and forms a hydrogen bond with an oxygen atom of the main chain of P4 Ala. (C) Superimposition of the SLA-1*0401–S-OIVNW9 andSLA-1*0401–EbolaAY9 structures showing the conformational variation of Arg156. The flexible side chain of Arg156 extends into the PBG. The peptide and Arg156 backbone in the NA449-457 structure are shown as a stick model in cyan; the peptide and Arg156 in vp35155-163 are labeled in green. Hydrogen bonds are illustrated as yellow dotted lines. Red circles are used to highlight the variation.
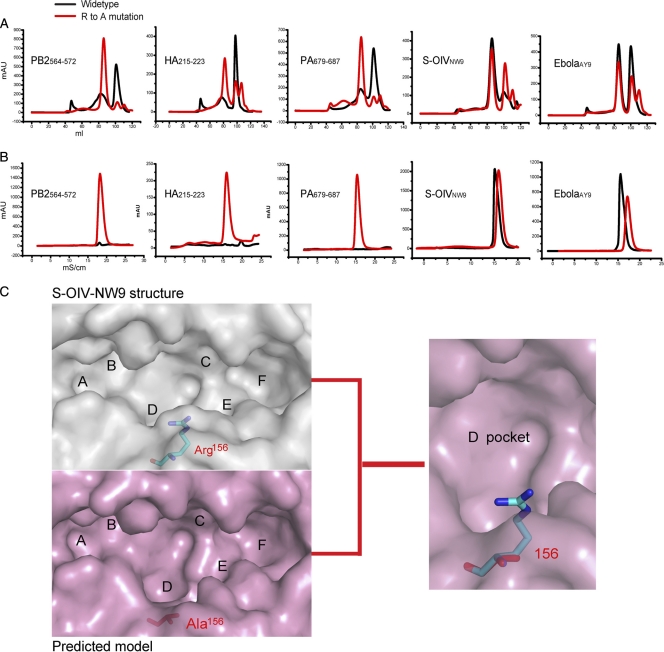
To investigate the role of Arg156 in the 3D structure of pSLA-1*0401, Arg156 was mutated to Ala156, and the mutant protein was termed SLA-1*0401–H-Ala156. SLA-1*0401–H-Ala156 and sβ2m were refolded with the viral epitopes S-OIVNW9, EbolaAY9, HA215–223, PA455–464, PA679–687, and PB2564-572 (Table 2 and Fig. 5 A and B). The refolding results demonstrate that SLA-1*0401–H-Ala156 bound to S-OIVNW9 or EbolaAY9 in the same way as the wild-type SLA-1*0401. However, binding to HA215–223, PA455–464, PA679–687, and PB2564-572 led to dramatic changes. All tested peptides tolerated anion-exchange chromatography and bound to SLA-1*0401–H-Ala156 to form stable complexes (Fig. 5A and B). We further modeled the 3D structure of pSLA-1*0401–H-Ala156 using SWISS-MODEL (http://swissmodel.expasy.org) (Fig. 5C). When Ala156 replaced Arg156, pocket D became larger, and its polarity was reduced. Therefore, pocket D in the mutated protein has fewer steric limitations and could accommodate more residues (Fig. 5C).
Fig. 5.
Arg156 vetoes peptides by its side chain. The results of peptides co-refolding with the wild type are shown as black lines, and results for SLA-1*0401–H-Ala156 are shown as red lines (A and B). The peptides used are indicated in each graph. (A) The refolded complexes were analyzed by chromatography on a Superdex200 16/60 column. (B) Anion-exchange chromatography results. (C) 3D model of the mutant H chain (Arg156 to Ala156) built by the SWISS-MODEL program (light purple). Ala156 is shown as a stick model, in red. Arg156 in SLA-1*0401–S-OIVNW9 is shown as in Fig. 4. Structural alignments reveal that the D pocket of SLA-1*0401–than that of SLA-1*0401–S-OIVNW9.
These results reveal that Arg156 “vetoes” the binding of peptides HA215–223, etc., to pSLA-1*0401. All the tested peptides have similar or identical P2, P6, and PC residues which are favored by pockets B, E, and F of SLA-1*0401. The short side chain of Ala provides no additional binding affinity to the peptides. The only reason that peptides such as HA215–223 cannot bind to SLA-1*0401 is that Arg156 rejects them by repulsion. S-OIVNW9 and EbolaAY9 are able to bind with SLA-1*0401 stably because they can bind Arg156. Therefore, only peptides with P3 residue suitable for Arg156 are able to form stable complexes with pSLA-1*0401.
S-OIVNW9 and EbolaAY9 bind pSLA-1*0401 with markedly similar conformations with the help of different water molecules.
The two peptides both adopt M-shaped conformations (Fig. 6 A). The solvent-accessible surface areas of S-OIVNW9 and EbolaAY9 are 351.6Å2 and 239.5Å2, respectively. Remarkably, the P4, P7, and P8 positions in S-OIVNW9 and EbolaAY9 are exposed on the surface. These three residues may play a crucial role in contacting TCRs. In particular, the P7 residues in both S-OIVNW9 and EbolaAY9 are prominently exposed, suggesting that this residue is pivotal to the specificity of swine TCR recognition. Compared to other peptides of influenza virus presented by human or mouse MHC I molecules, S-OIVNW9 peptide presentation is similar to the class of featured molecules with dominant immunogenicity (Fig. 6B and C).
Fig. 6.
Conformational comparison of S-OIVNW9, EbolaAY9, and peptides of IV with “featured” and “featureless” conformations. The PBGs of the compared structures are superimposed in Cα traces and shown as cartoons. The α2 helixes were hidden to display the peptides. Peptides are shown as cartoons with the side chain. (A) EbolaAY9 (green) was inserted deeper into the PBG than S-OIVNW9 (cyan). The hydrogen bonds were shown as dashed line (yellow for EbolaAY9, red for S-OIVNW9). (B) S-OIVNW9 compared with the “featured” peptide of PA224-232 (light blue; PDB code, 1YN6). (C) S-OIVNW9 compared with “featureless” peptide M158-66 (yellow, 1HHI).
Although the sequences of S-OIVNW9 and EbolaAY9 are quite different, no significant conformational change of the peptides in the PBG of pSLA-1*0401 could be found by superimposing the two peptides, except that EbolaAY9 was inserted deeper into the PBG than S-OIVNW9 (Fig. 6A). This is because EbolaAY9, especially P6 residue, can form more downward-facing hydrogen bonds with residues in PBG than S-OIVNW9 (Fig. 6A). In S-OIVNW9, P6-Gly has no side chain and cannot form any bonds with residues in the E pocket.
The formation of a hydrogen bond net with water molecules has been observed in HLA I and can stabilize an epitope in the PBG (58). In the two structures of pSLA-1*0401, different numbers of water molecules are bound to each viral epitope. The well-defined electron density map of S-OIVNW9 and EbolaAY9 highlights these differences (Fig. 7A and B). In SLA-1*0401–EbolaAY9, 12 water molecules form hydrogen bonds in the PBG (Fig. 7D), whereas only three water molecules are bound in pSLA-1*0401–S-OIVNW9 (Fig. 7C). EbolaAY9 contains five Ala residues, which makes it more difficult to directly form enough hydrogen bonds for binding. Therefore, EbolaAY9 uses nine extra water molecules to create hydrogen bonds to SLA-1*0401. Within the groove of SLA-1*0401, the bound water molecules interact with the H-chain polymorphic residues and help to stabilize multiple peptides.
Fig. 7.
Electron densities of bound peptides and water molecules in the two structures of SLA-1*0401. The final 2Fo-Fc-stimulated annealing omit maps of S-OIVNW9 (A) and EbolaAY9 (B), contoured at 1.0 σ, are shown. Residues of the SLA-1*0401 H chain which contact peptides and water molecules to form a hydrogen bond net are shown as stick models in white. The blue balls represent water molecules. Hydrogen bonds are illustrated as dotted lines. (C and D) Water molecules assisting S-OIVNW9 (C) and EbolaAY9 (D) to bind to PBG.
Cross-species presentation of peptide by SLA-1*0401 and HLA-A*0101 with different conformations.
Although SLA-1*0401 is more homologous to HLA-A*1101, the cross-species presentation of IV peptides (Table 2) indicates that the peptide binding motif of SLA-1*0401 is similar to that of HLA-A*0101. The key residues in the PBG of HLA-A*1101 that anchor peptide residues are different from those in SLA-1*0401 and HLA-A*0101. The structure of HLA-A*0101 containing a peptide (pHLA-A*0101; PDB code 3BO8) from melanoma-associated antigen 1 (MAGE-A1) illustrates that its anchor residues at P2, P3, and PC (peptide sequence, EADPTGHSY) have the same properties as pSLA-1*0401 (Fig. 8). In pSLA-1*0401 and pHLA-A*0101, pockets B, D, and F accommodate anchor residues and have similar surface electrostatic potential (Fig. 8A and B). Pockets B and F are hydrophobic cavities with weak negative potential, and pocket D generally has a strong positive potential.
Fig. 8.
Different peptide conformations presented by pSLA-1*0401 and pHLA-A*0101 (PDB code, 3BO8). (A) The surface of the PBG in pHLA-A*0101 is colored according to electrostatic potential (calculated in the absence of the peptide or bound water molecules); blue denotes positive potential, and red indicates negative potential. The MAGE peptide is in pale yellow. (B) The electrostatic potential surface of the PBG in pSLA-1*0401 and peptide S-OIVNW9 (cyan). (C) Comparison of the B pockets between pSLA-1*0401 (white) and pHLA-A*0101 (pale yellow), hydrogen bonds in pSLA-1*0401 (yellow dotted line), and hydrogen bonds in pHLA-A*0101 (red dotted lines). The substituted residues are shown as stick models and labeled in red, and the names of residues of SLA-1*0401 are in front. The same residues are shown as line models. (D) Comparison of D pockets. (E) Comparison of F pockets and the conformations of P9 anchor residues. (F) Comparison of conformations of S-OIVNW9 and MAGE peptides. P7 positions are highlighted by the pink oval.
The B pockets of pSLA-1*0401 and pHLA-A*0101 differ by substitutions at positions 9 and 67, where Tyr9 and Val67 in pSLA-1*0401 are changed to Phe9 and Met66 in pHLA-A*0101, respectively (Fig. 8C). Although the two alterations have different sizes and polarities, they have little effect on the small P2 anchor residues because they induce only weak van der Waals contacts.
The compositions of the D pockets of pSLA-1*0401 and pHLA-A*0101 are essentially the same (Fig. 8D). Arg114 and Arg156 cause pocket D to have a strong positive potential and show preference for anchor residues with negative charge. In both pSLA-1*0401 and pHLA-A*0101, Arg156 anchors P3 negatively charged residues with a strong salt bridge and is critical for stable peptide binding (Fig. 8D). This is quite different from HLA-A*1101, residue 156 of which is Gln (Fig. 2).
There are four residue alterations between F pockets of pSLA-1*0401 and pHLA-A*0101: Tyr74 →Asp, Gly77→Asn, Leu95→Ile, and Ser97→Ile. Although these substitutions are insufficient to affect the preference of F pockets, they change the shape of the F pocket and alter the conformations of PC residues (Fig. 8A, B, and E). Position 77 seems to be of particular importance, as the side chain of Asn77 in pHLA-A*0101 restricts the orientation of the aromatic ring of P9-Tyr and makes an included angle of about 120° with the aromatic ring of P9-Trp in pSLA-1*0401 (Fig. 8E). In contrast, Asp74, Asp77, and Asp116 in HLA-A*1101 endow the F pocket with negative potential and a preference for positively charged anchor residues.
Although peptides can be cross-species presented by pSLA-1*0401 and pHLA-A*0101, the conformations of the presented peptides have obvious variations, especially in the central region (Fig. 8F). In pHLA-A*0101, the main chain of the MAGE peptide is dragged toward the α2 helix by Arg156, where Arg156 forms a strong salt bridge with P3-Glu and hydrogen bonds with P7-His and P5-Thr. In SLA-1*0401–S-OIVNW9, Arg156 forms a salt bridge only with P3-Asp. P5-Val interacts with Asn66 in the α1 helix, and P7-Trp contacts Glu152 in the α2 helix with a hydrogen bond (Fig. 8F). Due to the presence of an Ala residue at position 152 in HLA-A*0101, Arg156 is more flexible and can pull the side chain of P7 residue of the MAGE peptide down, which may affect the TCR recognition of pHLA-A*0101.
Structure-based screening of conserved and cross-species IV epitope peptides.
CTL epitopes with high affinity for pMHC can form stable complexes by in vitro co-refolding (37, 38). Therefore, using SLA-1*0401–EbolaAY9 as a positive control, a total of 16 predicted peptides covering S-OIV and all influenza viruses were refolded with SLA-1*0401 and sβ2m (Table 1). However, only two peptide (S-OIVNW9 and PA455–464) formed stable complexes with SLA-1*0401 and sβ2m. S-OIVNW9 and PA455–464 could be purified by gel filtration and anion-exchange chromatography, similarly to the positive controls (Table 1). Two nonapeptides, HA87–95 and PA557–565, formed less stable pSLA complexes and could be collected by gel filtration but did not tolerate the strongly ionic environment during anion-exchange chromatography (Table 1). The other 13 peptides bound to SLA-1*0401 with lower affinities and did not form a stable pSLA peak after gel filtration. Based on the SLA-1*0401–S-OIVNW9 and SLA-1*0401–EbolaAY9 structures and binding results, a predicted motif for binding SLA-1*0401 was defined: residues with a large aromatic ring form the C termini, and a neutral residue (Ser, Thr, Ala, Ile, Leu, Met, or Val) occupies position 2, whereas in position 3 either a negative charged residue (Asp or Glu) or a residue without a side chain (Ala or Gly) should occur.
Based on the identified motifs for SLA-1*0401, a total of 22 peptides matching IV strains were refolded using the method mentioned above. Sixteen of the 22 peptides stably bound SLA-1*0401 (Table 2). All of these peptides could be purified by gel filtration and anion-exchange chromatography, like vp35155-163, the positive-control peptide. The other three peptides bound to SLA-1*0401 with lower affinity and did not form a stable pMHC peak after gel filtration. These peptides all have a small amino acid at P3 and an acidic amino acid at P6. Moreover, their low affinities might be explained by the fact that Glu152 in pocket E repels P6 residues with the same charge. Four peptides formed less stable pSLA complexes, which could be collected by gel filtration but did not tolerate the strongly ionic environment during anion-exchange chromatography (Table 2). These peptides have a small P3 anchor residue combined with small P6 secondary anchor (Ala or Gly) residues, which may have weaker binding affinities with the PBG of pSLA-1*0401.
Based on the structures of SLA-1*0401 and results of binding stabilities of total 39 peptides, four residues in peptides (P2, P3, P6, and PC) were defined as anchor or secondary anchor residues, and the peptide binding motif of SLA-1*0401 was proposed (Fig. 9). P3 and PC anchor residues are selected more strictly by SLA-1*0401 than P2 and P6 anchor residues, which indicates that P3 and PC residues are the primary anchor residues of the peptides binding with SLA-1*0401.
Fig. 9.
Peptide-binding motif of SLA-1*0401 and thermal stability analysis of pSLA-1*0401 molecules. The surface of PBG is shown as a 40% transparency. Anchor residues (P2, P3, P6, and PC) are in red and indicated by anchor symbols, which also indicate the directions in which the anchor residues point: down toward the peptide binding platform, toward the α1 helix, or toward the α2 helix. Values in parentheses are the frequencies at which the amino acids are found at the indicated positions among the 23 SLA-1*0401-binding peptides. The predicted motif of SLA-1*0401 was proposed based on the structures and peptide-binding results.
Interestingly, three peptides (NP44-52, CTELKLSDY, S-OIVCY9; PB1347–355, KMARLGKGY, S-OIVKY9; and PB1591–599, VSDGGPNLY, S-OIVVY9) were identical to the human CTL epitopes presented by HLA-A*0101 (2, 3, 10, 12, 23, 72), which is in agreement with our structural analysis of the cross-species presentation of peptide by SLA-1*0401 and HLA-A*0101.
Thermostabilities of the complexes of SLA-1*0401 with key peptides.
The binding stabilities of key peptides, including S-OIVNW9, EbolaAY9, and cross-species peptides, were further analyzed by using CD spectra (Fig. 10). Tms were determined from melting curves as described previously (67). SLA-1*0401–S-OIVNW9 and SLA-1*0401–EbolaAY9 form the most stable complexes, with Tms of 47.1°C and 47.5°C, respectively. pSLA-1*0401 complexed with two other cross-species peptides (S-OIVCY9 and S-OIVVY9) has similar thermostabilities. The Tms of SLA-1*0401–S-OIVCY9 and SLA-1*0401–S-OIVVY9 are 43.3°C and 43.1°C, respectively. The thermostabilities of pSLA-1*0401 complexes that could not tolerate anion-exchange chromatography are clearly lower than those of S-OIVNW9, EbolaAY9, S-OIVCY9, and S-OIVVY9. For example, the Tm of SLA-1*0401–S-OIVKY9 is 37.3°C, which was the lowest among the tested peptides. The SLA-1*0401 affinity of peptide NP9–17 (MIGGIGRFY, S-OIVMY9) is similar to that of S-OIVKY9, and this peptide also has a low Tm (38.3°C). The different thermostabilities of EbolaAY9, S-OIVKY9 and S-OIVMY9 indicate that polar P6 residues which can form hydrogen bonds with pocket E can greatly improve the stability of pSLA-1*0401 complexes. Furthermore, the varied thermostabilities of S-OIVNW9, S-OIVCY9, and S-OIVVY9 could be caused by PC-Trp having a greater binding affinity than PC-Tyr.
Fig. 10.
Thermostabilities of pSLA-1*0401 complexes. The thermostabilities of SLA-1*0401 with six peptides (S-OIVNW9, EbolaAY9, S-OIVCY9, S-OIVVY9, S-OIVKY9, and S-OIVMY9) were tested by CD spectroscopy. The temperature was increased by 1°C/min. The curves for the unfolded fractions were determined by monitoring the CD value at 218 nm (67).
DISCUSSION
SLA I plays a crucial role in cellular immune antigen presentation in pigs and in xenotransplantation of pig organs into humans in place of donor human tissues. The structural and biophysical analyses of pSLA-1*0401 in this study provide a basis for future related research. Conserved CTL epitopes are valuable targets for overcoming the antigenic drift and shift of IV; however, before this study, there was no information about the peptide binding properties of SLAs because of the absence of structure-based evidence of the peptide binding motifs of SLAs. The mechanism of presentation of viral epitopes by MHC/HLA in both humans and mice has been thoroughly studied (16, 17, 43, 68). The SLA I and HLA I sequences are <80% homologous at the amino acid level, indicating evolutionary divergence. The first crystal structure of SLA-1*0401 defined here provides insights into viral epitope presentation of SLA I. Surprisingly, based on our structures, we have identified potential epitopes matching S-OIV and other IV for this common allele, and some of the epitopes can even be presented cross-species by human HLA, e.g., HLA-A*0101.
A comparison of SLA-1*0401 with HLA-A*1101 revealed 78% homology, and the RMSD between SLA-1*0401 and HLA-A*1101 was <0.7. This indicates that the arrangement and orientation of the carbon skeletons are similar, as seen in the pMHC of other species. After examining the sequences and structures of the SLA I and HLA I molecules, we found some amino acid differences between the two species (Ala121/Lys and Ser236/Ala in the H chain and Val1/Ile, Pro33/Ser, and His98/Met in β2m). Our results revealed fewer van der Waals interactions and hydrogen bonds between the H chain and β2m in SLA I than in HLA I. Leu5, Ser167, and Gly77 frequently appear in SLA I molecules but are seldom found in HLA I, which might indicate distinct selective evolution in pigs and humans. In SLA-1*0401, Leu5 is found at the bottom of pocket A, and Ser167 is at the N terminus of pocket A and makes P1 residues more exposed in the PBG of SLA-1*0401; the Gly77 position in pocket F of SLA-1*0401 leads to a conformational change of the heterocycle of the PC residue compared to HLA-A*0101 (Fig. 8D).
A pronounced feature of SLA-1*0401 structure is determined by Arg156. Arg156 appears at a frequency of over 15% in SLA I alleles. In the two SLA-1*0401 structures reported here, the Arg156 is located in the D pocket, showing a distinctive alteration. Its flexible side chain is able to contact the two viral peptides in different manners. When Arg156 is mutated to Ala156, SLA-1*0401 can broaden its peptide-binding spectrum, showing binding to some peptides that do not bind to the wild type. This result indicates that Arg156 in SLA-1*0401 has the veto power for binding viral peptides. The great flexibility of Arg156 allows viral peptides to bind in various ways which are difficult to predict without 3D structures. This reinforces the importance of 3D structure determination of pMHC for epitope identification (39). This study also reveals that the peptide binding motif of HLA-A*0101 is similar to that of SLA-1*0401, due to their common Arg156 and their similar B and F pockets (32). In the absence of restriction from Glu152 in SLA-1*0401, Arg156 in HLA-A*0101 can contact more peptide positions with greater freedom and provide distinct and specific submotifs (31). The nonmammalian MHC structure of chicken BF2*2101 also shows a flexible Arg9, located in pocket B next to a huge central pocket, allowing BF2*2101 to bind to different peptides with different conformations. The flexibility of Arg9 allows the peptide to bind the BF2*2101 molecule with particular combinations of amino acids at positions P2 and PC-2 (30). Importantly, these results together suggest the presence of an Arg residue (irrespective of its position at 9 or 156) located in a pocket of the PBG; one must use caution in making computer-based epitope peptide predictions due to Arg's flexible and salient side chain.
In the peptide-MHC refolding tests, a total of 23 viral peptides derived from S-OIV peptides were identified as SLA-1*0401-binding peptides that may act as candidate CTL epitopes, 17 of which strongly bound to SLA-1*0401. Three viral peptides (NP44–52, CTELKLSDY; PB1347-355, KMARLGKGY; and PB1591-599, VSDGGPNLY) were identical to the previously defined human CTL epitopes that can be presented by HLA-A*0101 (12, 23), further confirming our structural observation of the similar PBGs of these two MHCs. PB1347-355 and PB1591-599, identified in SLA-1*0401, could also bind to other HLA I molecules (4, 72). We used the IEDB to determine whether these viral peptides that match S-OIV could be presented by other class I molecules. PB1347-355 and PB1591-599 both bind to SLA-1*0401 and are also potential CTL epitopes for HLA-A1, -A3, -A26, -B8, -B27, -B58, and -B62 and, in the case of PB1347-355, for HLA-A80, -B15, and -B18 (http://www.immuneepitope.org). PB1347-355 (KMARLGKGY) not only is highly conserved in IV strains but also may cross-induce immunity in humans (23). PB1591-599 was shown to bind the monkey MHC class I allele Mamu-A*02 and stimulate CTL responses (Table 2). These conserved peptides have the potential to stimulate cross-species immune responses and could be used to protect swine and humans from variable forms of the virus. Potential cross-species epitope presentation by different MHC molecules may also explain the existence of hot-spot CTL epitope regions in a given viral protein, which could direct choice of the immune-dominant antigens as vaccines.
In pSLA-1*0401, the side chains from the P4, P7, and P8 residues of viral epitopes protrude from the PBG, and their Cα atoms are almost at the same height as those in the MCH α-helix. Based on studies of pHLA-TCR structures (68, 69), the protruding side chains at the P4, P7, and P8 positions may be recognized by TCRs and play a role in TCR docking. In particular, the P7 residues in S-OIVNW9 and EbolaAY9 are prominently exposed, suggesting that this residue in pSLA-1*0401 is pivotal to the specificity of swine TCR recognition. However, the side chain of the P7 residue of MAGE-A1 is pulled down by Arg156 and does not show any outstanding features for TCR recognition. These results indicate that the cross-species-presented epitopes may stimulate CTL responses in both pigs and humans but with distinct characteristics based on the different conformations of the peptide presented.
The first structures of pSLA-1*0401 determined here provide us a clear view of the CTL epitope peptide presentation of swine MHC I and help widen our understanding of antigen presentation in different animals. Future comparative studies of antigen presentation may also help depict the evolution and origin of T-cell-based adaptive immunity. More importantly, the rational screening of the potential pSLA-1*0401-restricted CTL epitope peptides based on our structures may be beneficial for the development of T-cell-based vaccines against viruses that have crossed between pigs and humans.
ACKNOWLEDGMENTS
This work was supported by grants from the Ministry of Science and Technology of China (Transgenic Special Grant 2009ZX08009-150B; grant 2008ZX10101; project 973, grant 2007CB815805), by a Special Grant for Protein Science of the National 973 Project (grant 2010CB911902), and by the Chinese Universities Scientific Fund (project 2011JS015). G.F.G. is a leading principal investigator of the National Natural Science Foundation of China (NSFC) Innovative Research Group (grant 81021003). The funders had no role in study design, data collection and analysis, the decision to publish, or the preparation of the manuscript. We declare no financial or commercial conflict of interest.
We thank Yi Shi and Yan Wu for thoughtful discussions. We thank Joel Haywood for his comments on the manuscript. We sincerely thank Ilka Hoof of Technical University of Denmark for providing the peptide information.
Footnotes
Published ahead of print on 7 September 2011.
REFERENCES
- 1. Adams P. D., et al. 2002. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 58: 1948–1954 [DOI] [PubMed] [Google Scholar]
- 2. Assarsson E., et al. 2008. Immunomic analysis of the repertoire of T-cell specificities for influenza A virus in humans. J. Virol. 82: 12241–12251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boon A. C., et al. 2002. The magnitude and specificity of influenza A virus-specific cytotoxic T-lymphocyte responses in humans is related to HLA-A and -B phenotype. J. Virol. 76: 582–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bourgault Villada I., et al. 2000. Identification in humans of HPV-16 E6 and E7 protein epitopes recognized by cytolytic T lymphocytes in association with HLA-B18 and determination of the HLA-B18-specific binding motif. Eur. J. Immunol. 30: 2281–2289 [DOI] [PubMed] [Google Scholar]
- 5. Bui H. H., Peters B., Assarsson E., Mbawuike I., Sette A. 2007. Ab and T cell epitopes of influenza A virus, knowledge and opportunities. Proc. Natl. Acad. Sci. U. S. A. 104: 246–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen W., et al. 2001. A novel influenza A virus mitochondrial protein that induces cell death. Nat. Med. 7: 1306–1312 [DOI] [PubMed] [Google Scholar]
- 7. Chen W., et al. 2010. Crystal structure of a bony fish beta2-microglobulin: insights into the evolutionary origin of immunoglobulin superfamily constant molecules. J. Biol. Chem. 285: 22505–22512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chu F., et al. 2007. First glimpse of the peptide presentation by rhesus macaque MHC class I: crystal structures of Mamu-A*01 complexed with two immunogenic SIV epitopes and insights into CTL escape. J. Immunol. 178: 944–952 [DOI] [PubMed] [Google Scholar]
- 9. Chu F., et al. 2005. Complex assembly, crystallization and preliminary X-ray crystallographic studies of rhesus macaque MHC Mamu-A*01 complexed with an immunodominant SIV-Gag nonapeptide. Acta Crystallogr. F Struct. Biol. Cryst. Commun. 61: 614–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Bree G. J., et al. 2005. Selective accumulation of differentiated CD8+ T cells specific for respiratory viruses in the human lung. J. Exp. Med. 202: 1433–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diaz I., et al. 2009. In silico prediction and ex vivo evaluation of potential T-cell epitopes in glycoproteins 4 and 5 and nucleocapsid protein of genotype-I (European) of porcine reproductive and respiratory syndrome virus. Vaccine 27: 5603–5611 [DOI] [PubMed] [Google Scholar]
- 12. DiBrino M., et al. 1993. HLA-A1 and HLA-A3 T cell epitopes derived from influenza virus proteins predicted from peptide binding motifs. J. Immunol. 151: 5930–5935 [PubMed] [Google Scholar]
- 13. Emsley P., Cowtan K. 2004. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60: 2126–2132 [DOI] [PubMed] [Google Scholar]
- 14. Gao F. S., et al. 2006. Reconstruction of a swine SLA-I protein complex and determination of binding nonameric peptides derived from the foot-and-mouth disease virus. Vet. Immunol. Immunopathol. 113: 328–338 [DOI] [PubMed] [Google Scholar]
- 15. Gao F. S., Xu C. B., Long Y. H., Xia C. 2009. Secondary structure and 3D homology modeling of swine leukocyte antigen class 2 (SLA-2) molecules. Immunobiology. 214: 475–482 [DOI] [PubMed] [Google Scholar]
- 16. Gao G. F., et al. 1997. Crystal structure of the complex between human CD8alpha(alpha) and HLA-A2. Nature 387: 630–634 [DOI] [PubMed] [Google Scholar]
- 17. Garcia K. C., et al. 1998. Structural basis of plasticity in T cell receptor recognition of a self peptide-MHC antigen. Science 279: 1166–1172 [DOI] [PubMed] [Google Scholar]
- 18. Garten R. J., et al. 2009. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 325: 197–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gleimer M., et al. 2011. Although divergent in residues of the peptide binding site, conserved chimpanzee Patr-AL and polymorphic human HLA-A*02 have overlapping peptide-binding repertoires. J. Immunol. 186: 1575–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gouet P., Robert X., Courcelle E. 2003. ESPript/ENDscript: extracting and rendering sequence and 3D information from atomic structures of proteins. Nucleic Acids Res. 31: 3320–3323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Greenbaum J. A., et al. 2009. Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population. Proc. Natl. Acad. Sci. U. S. A. 106: 20365–20370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haskins K., Kappler J., Marrack P. 1984. The major histocompatibility complex-restricted antigen receptor on T cells. Annu. Rev. Immunol. 2: 51–66 [DOI] [PubMed] [Google Scholar]
- 23. Heiny A. T., et al. 2007. Evolutionarily conserved protein sequences of influenza a viruses, avian and human, as vaccine targets. PLoS One 2: e1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hirohashi Y., et al. 2002. An HLA-A24-restricted cytotoxic T lymphocyte epitope of a tumor-associated protein, survivin. Clin. Cancer Res. 8: 1731–1739 [PubMed] [Google Scholar]
- 25. Ho C. S., et al. 2009. Nomenclature for factors of the SLA system, update 2008. Tissue Antigens 73: 307–315 [DOI] [PubMed] [Google Scholar]
- 26. Hoof I., et al. 2009. NetMHCpan, a method for MHC class I binding prediction beyond humans. Immunogenetics 61: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ito T., et al. 1998. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J. Virol. 72: 7367–7373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kingsford C., Nagarajan N., Salzberg S. L. 2009. 2009 Swine-origin influenza A (H1N1) resembles previous influenza isolates. PLoS One 4: e6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Klein J., Figueroa F., Nagy Z. A. 1983. Genetics of the major histocompatibility complex: the final act. Annu. Rev. Immunol. 1: 119–142 [DOI] [PubMed] [Google Scholar]
- 30. Koch M., et al. 2007. Structures of an MHC class I molecule from B21 chickens illustrate promiscuous peptide binding. Immunity 27: 885–899 [DOI] [PubMed] [Google Scholar]
- 31. Kondo A., et al. 1997. Two distinct HLA-A*0101-specific submotifs illustrate alternative peptide binding modes. Immunogenetics 45: 249–258 [DOI] [PubMed] [Google Scholar]
- 32. Kumar P., Vahedi-Faridi A., Saenger W., Ziegler A., Uchanska-Ziegler B. 2009. Conformational changes within the HLA-A1:MAGE-A1 complex induced by binding of a recombinant antibody fragment with TCR-like specificity. Protein Sci. 18: 37–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Laskowski R. A., Moss D. S., Thornton J. M. 1993. Main-chain bond lengths and bond angles in protein structures. J. Mol. Biol. 231: 1049–1067 [DOI] [PubMed] [Google Scholar]
- 34. Li L., Bouvier M. 2004. Structures of HLA-A*1101 complexed with immunodominant nonamer and decamer HIV-1 epitopes clearly reveal the presence of a middle, secondary anchor residue. J. Immunol. 172: 6175–6184 [DOI] [PubMed] [Google Scholar]
- 35. Li X., et al. 2011. Two distinct conformations of a rinderpest virus epitope presented by cattle MHC class I N*01801 (BoLA-A11): a host strategy to present featured peptides. J. Virol. 85: 6038–6048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu J., et al. 2011. Diverse peptide presentation of rhesus macaque MHC class I Mamu-A*02 revealed by two peptide-complex structures and insights into SIV immune escape. J. Virol. 85: 7372–7383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu J., et al. 2010. The membrane protein of severe acute respiratory syndrome coronavirus acts as a dominant immunogen revealed by a clustering region of novel functionally and structurally defined cytotoxic T-lymphocyte epitopes. J. Infect. Dis. 202: 1171–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu J., et al. 2010. Novel immunodominant peptide presentation strategy: a featured HLA-A*2402-restricted cytotoxic T-lymphocyte epitope stabilized by intrachain hydrogen bonds from severe acute respiratory syndrome coronavirus nucleocapsid protein. J. Virol. 84: 11849–11857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu J., Zhang S., Tan S., Zheng B., Gao G. F. 2011. Revival of the identification of cytotoxic T-lymphocyte epitopes for immunological diagnosis, therapy and vaccine development. Exp. Biol. Med. (Maywood) 236: 253–267 [DOI] [PubMed] [Google Scholar]
- 40. Lunney J. K., Ho C. S., Wysocki M., Smith D. M. 2009. Molecular genetics of the swine major histocompatibility complex, the SLA complex. Dev. Comp. Immunol. 33: 362–374 [DOI] [PubMed] [Google Scholar]
- 41. Macdonald I. K., et al. 2010. MHC class I bound to an immunodominant Theileria parva epitope demonstrates unconventional presentation to T cell receptors. PLoS Pathog. 6: e1001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Macdonald W. A., et al. 2003. A naturally selected dimorphism within the HLA-B44 supertype alters class I structure, peptide repertoire, and T cell recognition. J. Exp. Med. 198: 679–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Madden D. R. 1995. The three-dimensional structure of peptide-MHC complexes. Annu. Rev. Immunol. 13: 587–622 [DOI] [PubMed] [Google Scholar]
- 44. McMichael A. J., Gotch F. M., Noble G. R., Beare P. A. 1983. Cytotoxic T-cell immunity to influenza. N. Engl. J. Med. 309: 13–17 [DOI] [PubMed] [Google Scholar]
- 45. Meijers R., et al. 2005. Crystal structures of murine MHC Class I H-2 D(b) and K(b) molecules in complex with CTL epitopes from influenza A virus: implications for TCR repertoire selection and immunodominance. J. Mol. Biol. 345: 1099–1110 [DOI] [PubMed] [Google Scholar]
- 46. Mullbacher A., Lobigs M., Alsharifi M., Regner M. 2006. Cytotoxic T-cell immunity as a target for influenza vaccines. Lancet Infect. Dis. 6: 255–256 [DOI] [PubMed] [Google Scholar]
- 47. Natarajan K., Li H., Mariuzza R. A., Margulies D. H. 1999. MHC class I molecules, structure and function. Rev. Immunogenet. 1: 32–46 [PubMed] [Google Scholar]
- 48. Normile D. 2009. Emerging infectious diseases: scientists puzzle over Ebola-Reston virus in pigs. Science 323: 451. [DOI] [PubMed] [Google Scholar]
- 49. Otwinowski Z., Minor W. 1997. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276: 307–326 [DOI] [PubMed] [Google Scholar]
- 50. Parkin S., Hope H. 1998. Macromolecular cryocrystallography: cooling, mounting, storage and transportation of crystals. J. Appl. Crystallogr. 31: 945–953 [Google Scholar]
- 51. Pauly T., et al. 1995. Classical swine fever virus-specific cytotoxic T lymphocytes and identification of a T cell epitope. J. Gen. Virol. 76(Pt. 12): 3039–3049 [DOI] [PubMed] [Google Scholar]
- 52. Pensaert M., Ottis K., Vandeputte J., Kaplan M. M., Bachmann P. A. 1981. Evidence for the natural transmission of influenza A virus from wild ducts to swine and its potential importance for man. Bull. World Health Organ. 59: 75–78 [PMC free article] [PubMed] [Google Scholar]
- 53. Renard C., et al. 2006. The genomic sequence and analysis of the swine major histocompatibility complex. Genomics 88: 96–110 [DOI] [PubMed] [Google Scholar]
- 54. Rodriguez A., et al. 1996. A porcine CD8+ T cell clone with heterotypic specificity for foot-and-mouth disease virus. J. Gen. Virol. 77(Pt. 9): 2089–2096 [DOI] [PubMed] [Google Scholar]
- 55. Saper M. A., Bjorkman P. J., Wiley D. C. 1991. Refined structure of the human histocompatibility antigen HLA-A2 at 2.6 A resolution. J. Mol. Biol. 219: 277–319 [DOI] [PubMed] [Google Scholar]
- 56. Shope R. E. 1931. Swine influenza: III. Filtration experiments and etiology. J. Exp. Med. 54: 373–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Smith G. J., et al. 2009. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 459: 1122–1125 [DOI] [PubMed] [Google Scholar]
- 58. Smith K. J., et al. 1996. Bound water structure and polymorphic amino acids act together to allow the binding of different peptides to MHC class I HLA-B53. Immunity 4: 215–228 [DOI] [PubMed] [Google Scholar]
- 59. Smith K. J., et al. 1996. An altered position of the alpha 2 helix of MHC class I is revealed by the crystal structure of HLA-B*3501. Immunity 4: 203–213 [DOI] [PubMed] [Google Scholar]
- 60. Speir J. A., Stevens J., Joly E., Butcher G. W., Wilson I. A. 2001. Two different, highly exposed, bulged structures for an unusually long peptide bound to rat MHC class I RT1-Aa. Immunity 14: 81–92 [DOI] [PubMed] [Google Scholar]
- 61. Stewart-Jones G. B., et al. 2005. Structures of three HIV-1 HLA-B*5703-peptide complexes and identification of related HLAs potentially associated with long-term nonprogression. J. Immunol. 175: 2459–2468 [DOI] [PubMed] [Google Scholar]
- 62. Sugimura T., Yonemochi H., Ogawa T., Tanaka Y., Kumagai T. 1980. Isolation of a recombinant influenza virus (Hsw 1 N2) from swine in Japan. Arch. Virol. 66: 271–274 [DOI] [PubMed] [Google Scholar]
- 63. Sun Y., et al. 2010. In silico characterization of the functional and structural modules of the hemagglutinin protein from the swine-origin influenza virus A (H1N1)-2009. Sci. China Life Sci. 53: 633–642 [DOI] [PubMed] [Google Scholar]
- 64. Tan P. T., et al. 2010. Conservation and diversity of influenza A H1N1 HLA-restricted T cell epitope candidates for epitope-based vaccines. PLoS One 5: e8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tennant L. M., Renard C., Chardon P., Powell P. P. 2007. Regulation of porcine classical and nonclassical MHC class I expression. Immunogenetics 59: 377–389 [DOI] [PubMed] [Google Scholar]
- 66. Thompson J. D., Gibson T. J., Plewniak F., Jeanmougin F., Higgins D. G. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25: 4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tobita T., et al. 2003. A role for the P1 anchor residue in the thermal stability of MHC class II molecule I-Ab. Immunol. Lett. 85: 47–52 [DOI] [PubMed] [Google Scholar]
- 68. Turner S. J., et al. 2005. Lack of prominent peptide-major histocompatibility complex features limits repertoire diversity in virus-specific CD8+ T cell populations. Nat. Immunol. 6: 382–389 [DOI] [PubMed] [Google Scholar]
- 69. Tynan F. E., et al. 2007. A T cell receptor flattens a bulged antigenic peptide presented by a major histocompatibility complex class I molecule. Nat. Immunol. 8: 268–276 [DOI] [PubMed] [Google Scholar]
- 70. Vaiman M., Renard C., LaFage P., Ameteau J., Nizza P. 1970. Evidence for a histocompatibility system in swine (SL-A). Transplantation 10: 155–164 [DOI] [PubMed] [Google Scholar]
- 71. Viza D., Sugar J. R., Binns R. M. 1970. Lymphocyte stimulation in pigs: evidence for the existence of a single major histocompatibility locus, PL-A. Nature 227: 949–950 [DOI] [PubMed] [Google Scholar]
- 72. Wang M., et al. 2007. CTL epitopes for influenza A including the H5N1 bird flu; genome-, pathogen-, and HLA-wide screening. Vaccine 25: 2823–2831 [DOI] [PubMed] [Google Scholar]
- 73. Wise H. M., et al. 2009. A complicated message: Identification of a novel PB1-related protein translated from influenza A virus segment 2 mRNA. J. Virol. 83: 8021–8031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhou N. N., et al. 1999. Genetic reassortment of avian, swine, and human influenza A viruses in American pigs. J. Virol. 73: 8851–8856 [DOI] [PMC free article] [PubMed] [Google Scholar]