Abstract
The human antibody response to flavivirus infection is dominantly directed against a cross-reactive epitope on the fusion loop of domain II (DII-FL) of the envelope (E) protein. Although antibodies against this epitope fail to recognize fully mature West Nile virus (WNV) virions and accordingly neutralize infection poorly in vitro, their functional properties in vivo remain less well understood. Here, we show that while passive transfer of poorly neutralizing monoclonal antibodies (MAb) and polyclonal antibodies against the DII-FL epitope protect against lethal WNV infection in wild-type mice, they fail to protect mice lacking activating Fcγ receptors (FcγR) and the complement opsonin C1q. Consistent with this, an aglycosyl chimeric mouse-human DII-FL MAb (E28) variant that lacks the ability to engage FcγR and C1q also did not protect against WNV infection in wild-type mice. Using a series of immunodeficient mice and antibody depletions of individual immune cell populations, we demonstrate that the nonneutralizing DII-FL MAb E28 does not require T, B, or NK cells, inflammatory monocytes, or neutrophils for protection. Rather, E28 treatment decreased viral load in the serum early in the course of infection, which resulted in blunted dissemination to the brain, an effect that required phagocytic cells, C1q, and FcγRIII (CD16). Overall, these studies enhance our understanding of the functional significance of immunodominant, poorly neutralizing antibodies in the polyclonal human anti-flavivirus response and highlight the limitations of current in vitro surrogate markers of protection, such as cell-based neutralization assays, which cannot account for the beneficial effects conferred by these antibodies.
INTRODUCTION
West Nile virus (WNV) is a zoonotic mosquito-transmitted Flavivirus that can infect and cause disease in humans and many other vertebrate animals. The Flavivirus genus also contains other human pathogens of global relevance, including dengue virus (DENV), yellow fever virus, Japanese encephalitis virus, and tick-borne encephalitis virus. Most human WNV infections are asymptomatic, but about 20% of infected individuals experience a mild fever, and less than 1% develop severe neuroinvasive disease (67). Risk factors for symptomatic disease include an age of greater than 55 years, a compromised immune status, genetic variation in the OAS1 gene, and a CC5Δ32 genotype (17, 26, 40, 41). Although WNV first appeared in the Western Hemisphere in 1999 in New York and spread rapidly through North America, surprisingly few human clinical infections have been reported in Central and South America, despite the migration of avian hosts and appropriate vectors for transmission (35, 56).
WNV infection requires attachment to cell surface receptors, which remain poorly defined, endocytosis, and acid-catalyzed fusion of the virus within the late endosome. After translation of input-strand RNA and viral replication, progeny virion assembly occurs within the endoplasmic reticulum (ER), with the capsid protein and genomic RNA associating with premembrane (prM) and envelope (E) proteins (42). Virus particles bud into the lumen of the ER as immature virions in which the E and prM proteins interact to form 60 heterotrimeric spikes with icosahedral symmetry (84). Transit of the immature virion through the mildly acidic compartments of the trans-Golgi network (TGN) triggers an extensive rearrangement on the virion surface. This low-pH-induced transition causes the E proteins on immature virions to lie flat as antiparallel dimers on the surface of the virion (36), which in turn increases the susceptibility of prM to cleavage by a furin-like serine protease in the TGN (39, 82). Release of prM occurs in the neutral pH of the extracellular space (82). Mature flavivirus virions are relatively smooth particles that display 90 E protein dimers arranged in a herringbone pattern. While cleavage of prM is a required step in the viral life cycle, it can be an inefficient process. Moreover, partially mature flavivirus virions containing some uncleaved prM also retain infectivity (29, 33, 51).
Studies of mice and other animals have established that humoral immunity is an essential component for protection against lethal WNV infection (57). B cells and secreted antibody were found necessary for survival of mice after WNV inoculation (18, 19), and passive transfer of WNV-immune serum protected naive recipients from WNV challenge (22, 74). Moreover, preexposure prophylaxis and postexposure therapy with WNV-specific monoclonal antibody (MAb) or polyclonal antibodies conferred protection in both mice and hamsters (6, 7, 22, 48, 53, 71, 74, 75). The E protein of WNV is the principal target of neutralizing antibodies. Antibody neutralization occurs by blocking attachment to host cells, penetration of virions into cells, and the low-pH-dependent fusion of the viral and host cell membranes (58). X-ray crystallographic analysis of several flavivirus E proteins has revealed a canonical structure with three domains (domain I [DI], DII, and DIII). The generation and characterization of large panels of mouse and human MAbs against epitopes spanning the WNV E protein have enhanced our understanding of the antibody response to WNV. Although mouse MAbs that bind to all three domains of WNV E protein have been described, the most potently inhibitory MAbs recognize the lateral ridge epitope on DIII (DIII-LR) (2, 53, 64). In comparison, the human anti-E repertoire appears more focused on a poorly neutralizing epitope on the fusion loop of DII (DII-FL) (54, 75).
In this study, we examined the contribution of poorly neutralizing antibodies to protection against WNV infection. In particular, a fusion loop-specific MAb (E28), which had little detectable inhibitory activity in cell culture, protected mice against lethal WNV infection. Protection required antibody effector function, as survival benefit was lost in mice lacking activating Fc gamma receptors (FcγR) and the complement opsonin C1q, or when an aglycosyl E28 variant that cannot engage FcγR and C1q was administered to wild-type mice. In subsequent mechanistic studies, we found that E28 treatment decreased viral load in the serum early in the course of infection, an effect that required cells with phagocytic activity. Finally, we demonstrate that human and hamster polyclonal antibody responses after DENV infection that cross-react with WNV are generally poorly neutralizing and skewed to the DII-FL epitope and that passive transfer of IgG purified from DENV-immune hamsters also protects mice from lethal WNV infection. Our study establishes the functional significance of immunodominant poorly neutralizing antibodies in the polyclonal human antiflavivirus response and highlights the limitations of current neutralization assays, which cannot account for the protective effects conferred by these antibodies in vivo.
MATERIALS AND METHODS
Antibodies.
All MAb and polyclonal antibodies used in this study were purified by protein A or protein G affinity chromatography. Anti-WNV mouse MAbs E24, E28, E34, and E53 have been described previously (53, 55). Mouse isotype controls recognized dengue virus envelope (E; DENV-2 E70, IgG1) (70) and precursor membrane (prM; 2H2, IgG2a) (25) glycoproteins and do not cross-react with WNV. Chimeric versions of MAb E28 (Ch-E28 and Ch-E28 N297Q) were generated by cloning the variable (VH and VL) regions of murine MAb E28 upstream of human IgG1 constant regions and performing site-directed mutagenesis as described previously for chimeric versions of MAb E16 (53). The negative control chimeric mouse-human IgG1 MAb (Ch-4420) was specific for fluorescein isothiocyanate (FITC) (3). PK136, a hybridoma producing mouse anti-mouse-NK1.1 MAb (IgG2a), was a gift of W. Yokoyama (St. Louis, MO). RB6.8C5, a hybridoma producing rat anti-mouse-Gr-1 MAb (IgG2b), was a gift of E. Unanue (St. Louis, MO). A negative-control rat IgG was purchased from Jackson ImmunoResearch. Hamster IgG was isolated from the serum of naïve or DENV-2 infected golden Syrian hamsters (Harlan). Human serum was a gift of R. Akkina and S. Halstead (Pediatric Dengue Vaccine Initiative) and A. de Silva (Chapel Hill, NC). Serum was collected from individuals who had experienced multiple infections in the past with more than one serotype of DENV as judged by clinical history and plaque reduction neutralization test (PRNT) titer analysis. The sera had the following reciprocal 50% PRNT titer (PRNT50) values against DENV-1, DENV-2, DENV-3, and DENV-4: DENV-1 (640, 611, 213, and 319); DENV-2 (371, 320, 288, and >1,280); DENV-3 (>1,280, 300, 99, and 266); DENV-4 (>1,280, >1,280, >1,280, and 285); DENV-5 (589, >1,280, 717, and 82); DENV-6 (>1,280, >1,280, >1,280, and 187).
The following antibodies against mouse antigens were conjugated to fluorescent markers and used in flow cytometry experiments: anti-CD11b–Alexa Fluor 647 (AF647; M1/70), anti-CD45–peridinin chlorophyll protein (PerCP; 30-F11), anti-Ly6C–phycoerythrin (PE; AL-21), anti-Ly6G–PerCP-Cy5.5 (1A8). The conjugated antibodies listed above were purchased from BD Biosciences. Anti-CD3ε–AF647 (145-2C11) was purchased from BioLegend, anti-NKp46–PE (29A1.4) was purchased from eBioscience, and anti-human IgG–AF647 was purchased from Invitrogen.
Virus stocks.
The WNV strain (3000.0259) was isolated in New York in 2000 and was described previously (20). The virus was passaged twice in C6/36 Aedes albopictus cells to generate a stock virus that was used in all in vivo experiments. In vitro studies utilized a virus stock that was passaged one additional time in C6/36 or Vero cells. An in vivo-derived stock of WNV was generated from plasma 3 days after infection of AG129 mice as described below. WNV subviral particles (SVP) were generated as described previously (80) by transiently transfecting a pcDNA3.1 plasmid carrying the prM and E proteins into 293T cells and harvesting cell culture supernatant after 48 h. WNV reporter virus particles (RVP) that differed with respect to the extent of prM cleavage were generated as described previously (51).
Mouse experiments.
Mouse studies were approved and performed according to the guidelines of the Washington University School of Medicine Animal Safety Committee. All wild-type C57BL/6J mice were purchased from a commercial source (Jackson Laboratories). Congenic C1q−/− mice were originally obtained from M. Botto and G. Stahl (Imperial College, London, England, and Beth Israel Deaconess Medical Center, Boston, MA). Congenic common γ-chain-deficient (FcγR−/−) mice, which lack FcγRI, FcγRIII, and FcγRIV, and FcγRIII−/− mice were purchased commercially (Taconic). The C1q−/− × FcγR−/− and C1q−/− × FcγRIII−/− mice were generated by crossing the individual knockout mice. All mice were bred and maintained in pathogen-free barrier facilities. Purified antibodies were administered to mice diluted in endotoxin-free phosphate-buffered saline (PBS; HyClone Laboratories) by the intraperitoneal route. WNV was diluted in Hanks' balanced salt solution (Mediatech, Inc.) containing 1% heat-inactivated fetal bovine serum (FBS; Omega Scientific) and administered by subcutaneous injection in the footpad after anesthetization with xylazine and ketamine. For NK cell depletion, mice were administered the PK136 anti-NK1.1 antibody (100 μg) 2 days before and after infection with WNV. For neutrophil and inflammatory monocyte depletion, mice were given the RB6.8C5 anti-Gr-1 antibody (250 μg) 1 day before infection with WNV.
To isolate serum, blood was collected in serum Gel Z tubes (Sarstedt), allowed to clot on ice for 30 min, and centrifuged, and the liquid phase was aliquoted and stored at −80°C. Viral RNA was isolated from serum by using the QIAamp viral RNA minikit (Qiagen), stored at −80°C, and quantified utilizing TaqMan quantitative reverse transcriptase PCR (qRT-PCR) on a 7500 Fast real-time PCR system (Applied Biosystems) as described previously (18). For plasma isolation, blood was collected into chilled EDTA-coated tubes (Becton Dickinson) on ice and centrifuged at 4°C, and the liquid phase was aliquoted and stored at −80°C. For tissue harvesting, mice were anesthetized with xylazine and ketamine and perfused extensively with PBS prior to organ dissection. Tissues were placed on dry ice, weighed, and stored at −80°C. Tissues were subsequently homogenized in PBS, and virus was titrated by plaque assay on BHK21-15 cells.
Flow cytometry.
For preparation of splenocytes as single-cell suspensions, spleens were manually homogenized on 40-μm cell strainers into RPMI medium (Sigma). For preparation of peripheral blood leukocytes (PBL), blood was collected into EDTA-coated tubes, and erythrocytes were lysed by diluting whole blood in ACK buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM EDTA) for 5 min. Splenocytes or PBL were washed thrice in PBS with 2% FBS prior to staining with cell-type-specific conjugated MAbs for 30 min on ice. Cells were washed thrice and then analyzed on a FACSCalibur flow cytometer (BD Biosciences) using FlowJo software (Treestar).
Liposomes.
Clodronate liposomes were prepared according to published methods (60, 79). Briefly, 8 mg of cholesterol and 86 mg of phosphatidyl choline (Sigma-Aldrich) were dissolved in chloroform. Excess chloroform was removed under vacuum. Clodronic acid disodium salt (Cl2MDP; Sigma-Aldrich) was dissolved in deionized water, and the pH was adjusted to 7.3 with 5 N NaOH. The dry lipid pellet was resuspended in either 2.5 g of Cl2MDP in 10 ml of water for macrophage-depleting liposomes or PBS for control liposomes. Liposomes were washed with PBS by ultracentrifugation (Beckman L-80 ultracentrifuge with 70.1 Ti rotor; 22,000 × g for 30 min) and resuspended in a final volume of 4 ml of PBS. To confirm that clodronate-containing lipsomes effectively depleted macrophages, C57BL/6 RAG1−/− mice were injected intravenously with 250 μl of liposome suspension and splenocytes were analyzed by flow cytometry 24 h later for expression of CD11b and CD11c markers. For experiments in wild-type mice, liposomes were administered intravenously 1 day prior to and 1 day after WNV infection.
Neutralization assays. (i) PRNTs.
Classical PRNTs were performed as described previously on BHK21-15 cells after infection with 50 to 125 PFU of WNV (80), depending on the assay. Fifty percent effective concentrations (EC50) were generated by nonlinear regression analysis (GraphPad Software).
(ii) RVP.
Neutralization assays with WNV RVP expressing green fluorescent protein (GFP) were performed with Raji-DCSIGN-R cells as described previously (59).
(iii) Multistep growth curve.
WNV was mixed with individual MAbs (30 μg/ml) in Dulbecco's modified Eagle medium (DMEM) with 10% FBS for 1 h at 37°C, then added to a monolayer of BHK21-15 cells at a multiplicity of infection (MOI) of 0.001, and incubated for the specified times at 37°C. Supernatants were collected at the indicated times, aliquoted, stored at −80°C, and quantified by plaque assay on BHK21-15 cells.
(iv) Raji-DCSIGN-R cells and infectious virus.
WNV was mixed with individual MAbs (30 μg/ml) in RPMI 1640 with 5% FBS for 1 h at 37°C, added to Raji-DCSIGN-R cells (MOI, 0.001), and incubated for 28 h at 37°C. Cells were fixed with 4% paraformadehyde, permeabilized with saponin (0.1% [wt/vol]), stained for WNV antigen (primary Ab Ch-E16, secondary Ab goat anti-human IgG-AF647) and processed on a FACSArray flow cytometer (BD Biosciences) using FlowJo software (Treestar).
SVP and protein ELISA.
Nunc MaxiSorp polystyrene 96-well plates were coated overnight at 4°C with E24 MAb (10 μg/ml) in a pH 9.3 carbonate buffer. Plates were washed thrice in enzyme-linked immunosorbent assay (ELISA) wash buffer (PBS with 0.02% Tween 20) and blocked for 1 h at 37°C with ELISA block buffer (PBS, 2% bovine serum albumin, and 0.02% Tween 20). WNV SVP were captured for 1 h at room temperature. Subsequently, plates were rinsed five times in wash buffer and then incubated with anti-WNV or flavivirus monoclonal or polyclonal antibodies (human or hamster) or controls (human IgG1–anti-FITC or naïve hamster antibodies) in duplicate for 1 h at room temperature. Plates were washed five times and then incubated with biotinylated goat anti-human or hamster IgG (1:1,000 dilution; Jackson ImmunoResearch) for 1 h at room temperature in blocking buffer. After further rinsing, plates were incubated with 2 μg/ml of horseradish peroxidase-conjugated streptavidin (Vector Laboratories) for 30 min at room temperature. Plates were washed six times and developed with tetramethylbenzidine (TMB) substrate (Dako). The reaction was stopped with 2 N H2SO4, and emission (450 nm) was read on a TriStar microplate reader (Berthold Technologies). For quantification of the epitope specificity of anti-WNV E hamster IgG and human serum, plates were coated with 10 μg/ml of recombinant WNV E proteins produced in Escherichia coli (wild type, W101R mutant, or E-quadruple mutant [T76R M77E W101R L107R]) as described previously (54). The E-quadruple mutant was the gift of C. Nelson and D. Fremont (St. Louis, MO). Equivalent site density was confirmed by measuring reactivity with E24 or Ch-E16 MAb, both of which recognize a distinct epitope on DIII-LR. Endpoint titers were defined as three standard deviations above the background optical density at 450 nm as determined by regression analysis using the Prism program (GraphPad Software).
Western blotting.
Individual preparations of WNV (106 PFU) were inactivated in 0.1% NP-40 detergent at 55°C for 15 min. Subsequently, 4× lithium dodecyl sulfate sample buffer (Invitrogen) was added, and samples were heated to 95°C for 10 min, centrifuged briefly, and loaded onto a Nu-PAGE 10% bis-Tris gel (Invitrogen). After electrophoresis, the gel was rinsed in double-distilled H2O, and protein was transferred to a nitrocellulose membrane using the iBlot system (Invitrogen). Membranes were washed for 10 min in wash buffer (PBS with 0.05% Tween 20), incubated overnight at 4°C in blocking buffer (5% dry milk in wash buffer), and stained with human anti-E Ch-E16 MAb or polyclonal rabbit anti-M (Imgenex) at 1 μg/ml in blocking buffer for 2 h. Following five rinses in wash buffer, membranes were incubated with horseradish peroxidase-conjugated goat anti-human IgG (Sigma) or anti-rabbit IgG (Thermo Scientific) diluted 1:2,000 in blocking buffer for 1 h. After five additional rinses, the blots were developed with Amersham ECL reagent (GE Healthcare) and visualized after exposure to X-ray film.
Statistical analysis.
Survival studies were analyzed using the log rank test. Comparisons of viral titers utilized either the Mann-Whitney nonparametric t test or one-way analysis of variance (ANOVA), as indicated. A paired Student's t test was used for comparisons of hamster IgG and human serum titers against different recombinant E proteins. Neutralization curves were compared using the F test.
RESULTS
Poorly neutralizing MAbs protect mice from WNV infection.
While the PRNT and analogous assays are viewed as standard measures of inhibitory activity of antibodies against many viruses, an imperfect correlation has been observed for DENV between the degree of neutralization in vitro and protection in vivo (9). To explore this concept further, we assessed the protective activities in vivo of several previously characterized murine MAbs against the WNV E glycoprotein and their corresponding neutralizing capacities as judged by the PRNT assay on BHK21-15 cells. In the absence of passively transferred antibody, as seen previously (22), administration of 102 PFU of insect cell-derived WNV via a subcutaneous route to 4- to 5-week-old wild-type C57BL/6 mice resulted in a 14% survival rate (Table 1). While prophylaxis 1 day prior to infection with 40 μg of two strongly neutralizing DIII-LR MAbs (E24 [IgG2a] and E34 [IgG1], PRNT50, 4.0 and 80 ng/ml, respectively) provided strong protection (≥90% survival; P < 0.0001), passive transfer of poorly neutralizing (E53 [IgG2a]) and nonneutralizing (E28 [IgG1]) DII-FL MAbs still provided significant protection (43% [P = 0.003] and 60% [P < 0.0001], respectively), albeit it at lower levels (Table 1; Fig. 1A). Thus, the capacity for anti-WNV MAbs to protect in vivo does not correlate perfectly with results from the PRNT assay in cell culture.
Table 1.
MAb protection of 4- to 5-week-old wild-type micea
| MAb or control | Epitope on WNV E | Isotype | Neutralizing activity | MTD ± SDb | Survival (%) | P value |
|---|---|---|---|---|---|---|
| PBS | + | 10.1 ± 2.1 | 8/57 (14) | |||
| Anti-DENV-2 E70 (anti-DENV E control) | IgG1 | − | 10.2 ± 2.1 | 4/20 (20) | 0.6 | |
| 2H2 (anti-DENV prM control) | IgG2a | − | 10.2 ± 1.6 | 3/30 (10) | 0.9 | |
| E24 | DIII-LR | IgG2a | +++ | 15 | 28/29 (97) | <0.0001 |
| E28 | DII-FL | IgG1 | − | 11.1 ± 3.1 | 24/40 (60) | <0.0001 |
| E34 | DIII-LR | IgG1 | +++ | 12 | 9/10 (90) | <0.0001 |
| E53 | DII-FL | IgG2a | + | 10.4 ± 1.9 | 13/30 (43) | 0.003 |
The indicated MAbs (40 μg) were passively transferred into wild-type mice (4 to 5 weeks old) 1 day prior to infection with 102 PFU of C6/36 cell-derived WNV. Survival analysis was followed for 21 days, and P values were determined using the log rank test versus results for PBS-treated mice. The isotype control MAbs reacted specifically with DENV but not WNV proteins (data not shown). Neutralization activity was scored according to the data in Fig. 1A: +++, strong neutralization; +, partial neutralization; −, no neutralization. The epitope location on the WNV E protein is based on prior published studies (53, 55).
MTD, mean time to death (± the standard deviation) for mice that succumbed to infection.
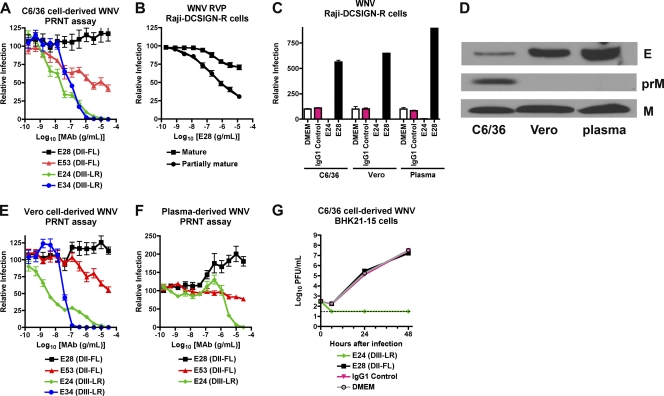
Fig. 1.
Neutralizing activity of MAbs in cell culture against different preparations of WNV. (A, E, and F) PRNT assay results. E28 (DII-FL, IgG1), E53 (DII-FL, IgG2a), E24 (DIII-LR, IgG2a), and E34 (DIII-LR, IgG1) were tested for neutralization of C6/36 cell-derived (A), Vero cell-derived (E), or plasma-derived (F) WNV (50 to 125 PFU) by classical PRNT assay on BHK21-15 cells. Data shown are combined results of two independent experiments performed in triplicate. The data were normalized to data from six control wells in each experiment with no MAb. (B) RVP neutralization assay on Raji-DCSIGN-R cells. E28 was incubated with RVP prior to infection of Raji-DCSIGN-R cells. RVP were prepared normally (mixture of mature, immature, and partially mature) or in cells overexpressing the furin protease (mature) to create a more homogeneous population of mature virions. The data shown are the combined results of three independent experiments performed in duplicate, and the results were normalized to those from two control wells in each experiment with no MAb. (C) WNV neutralization assay on Raji-DCSIGN-R cells. WNV (MOI, 0.001) derived from C6/36 cells, Vero cells, or plasma of infected AG129 mice was mixed with medium (DMEM) or 30 μg/ml of MAb E24 or E28 prior to infection of Raji-DCSIGN-R cells. One day later, cells were stained with Ch-E16 and processed by flow cytometry. The data shown are representative of three independent experiments performed in triplicate and were normalized to those from three control wells for each virus with no MAb. (D) Western blot with anti-E (top) or anti-prM/M (bottom) antibodies using WNV derived from C6/36 cells, Vero cells, or plasma from infected AG129 mice. For anti-E blotting, an equivalent number of PFU were loaded as judged by plaque assay on BHK21-15 cells. (G) Inhibition of WNV replication by MAbs in the multistep growth analysis. C6/36 cell-derived WNV was mixed with medium (DMEM) or 30 μg/ml of MAb E24 or E28 prior to infection of BHK21-15 cells. At the indicated time points, supernatant was harvested for titration by plaque assay. Data shown are combined data from three independent experiments performed in triplicate.
In vitro characterization of poorly neutralizing MAbs.
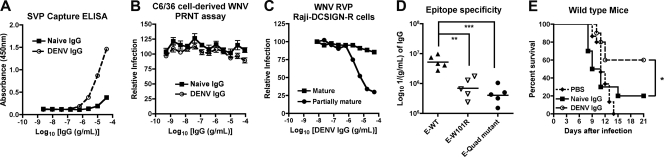
The inability of the PRNT assay to predict MAb protection in vivo warranted further investigation. Previous studies established that the relative maturity of WNV virions as reflected by the degree of cleavage of prM protein affects the neutralizing activity of MAbs that recognize some (e.g., DII-FL) but not other (DIII-LR) epitopes (51). As seen previously (51), E53 neutralized infection in Raji-DCSIGN-R cells of mature WNV RVP produced in a cell line overexpressing furin to a lesser extent than those generated in the parent cells, which produce a mixture of mature and partially immature virions. In comparison, E24 neutralized both types of RVP equivalently (data not shown). In contrast to the classical PRNT assay with BHK21-15 cells and similar to results with E53, E28 consistently showed a low but measurable neutralization (∼30%) of mature RVP in Raji-DCSIGN-R cells (Fig. 1B). The modest ability of E28 to neutralize WNV RVP was not a cell-type-specific effect, as E28 (30 μg/ml) failed to neutralize infection of Raji-DC-SIGNR cells with C6/36-derived fully infectious WNV, and in fact enhanced infection compared to controls (Fig. 1C).
To assess whether the maturation state of WNV also affected neutralization in the PRNT assay, we propagated WNV in Vero cells, which produce virions that are more mature than those derived from C6/36 insect cells (Fig. 1D) (24). Similar to data with insect cell-propagated WNV, DIII-LR-specific MAbs E24 and E34 completely neutralized Vero cell-derived WNV with similar PRNT50 values, whereas E28 showed no appreciable inhibitory activity across a wide dose range (Fig. 1E). Again, E53 showed partial neutralizing activity of Vero cell-derived WNV, achieving ∼50% overall inhibition, although its potency was shifted 500-fold (P < 0.0001) to a requirement for higher concentrations of MAb. Thus, E28 lacked neutralizing activity of insect or mammalian cell-generated WNV on BHK21-15 cells, whereas a decreased state of maturity of WNV RVP was associated with some inhibitory activity in Raji-DCSIGN-R cells.
Since the source of WNV affected the level of neutralization observed in different cell types, we assessed the capacity of MAbs to inhibit WNV infection with virus produced in vivo. Because the recovery of infectious extracellular WNV from plasma of wild-type mice is challenging due to limited viremia, we infected highly susceptible AG129 mice, which lack receptors required for type I and type II interferon signaling. Plasma-derived WNV was not neutralized by E28 at any concentration tested (Fig. 1F); these results may be explained by the completely mature phenotype of WNV in plasma, as uncleaved prM was not observed by Western blotting (Fig. 1D). Of note, E24 was 600-fold less potent (P < 0.0001) at neutralizing infection of plasma-derived WNV (PRNT50, 2.4 μg/ml) than insect-derived WNV (PRNT50, 4.0 ng/ml). This could be due to the lower specific infectivity of plasma-derived compared to cell culture-derived WNV, as judged by the enhanced amount of E protein per equivalent PFU (Fig. 1D) or, alternatively, to the existence of a variant virus that escapes neutralization. Finally, higher concentrations of E28 paradoxically augmented infection of plasma WNV preparations. Although the mechanism for this remains uncertain, we speculate that plasma-derived WNV may preferentially support MAb-induced virus aggregation, as BHK21-15 cells lack expression of FcγR, precluding the possibility of “classical” antibody-dependent enhancement of infection (30). Collectively, these results suggest that the PRNT assay on BHK21-15 cells does not always predict the protective activity of WNV in mice, even after accounting for different sources of virus, including those derived in vivo.
To further characterize the intrinsic neutralization capacity of anti-WNV MAbs on BHK21-15 cells, we performed multistep growth curve analysis. While the addition of E24 (30 μg/ml) prior to infection resulted in complete neutralization, equivalent concentrations of E28 provided no reduction in viral titer compared to the isotype antibody or medium controls (Fig. 1G). Despite conferring protection in mice in passive transfer studies, and in contrast to E24, E28 had limited inhibitory activity in cell culture that was maturation state and cell type dependent.
E28 MAb prophylaxis reduces viral load in wild-type mice.
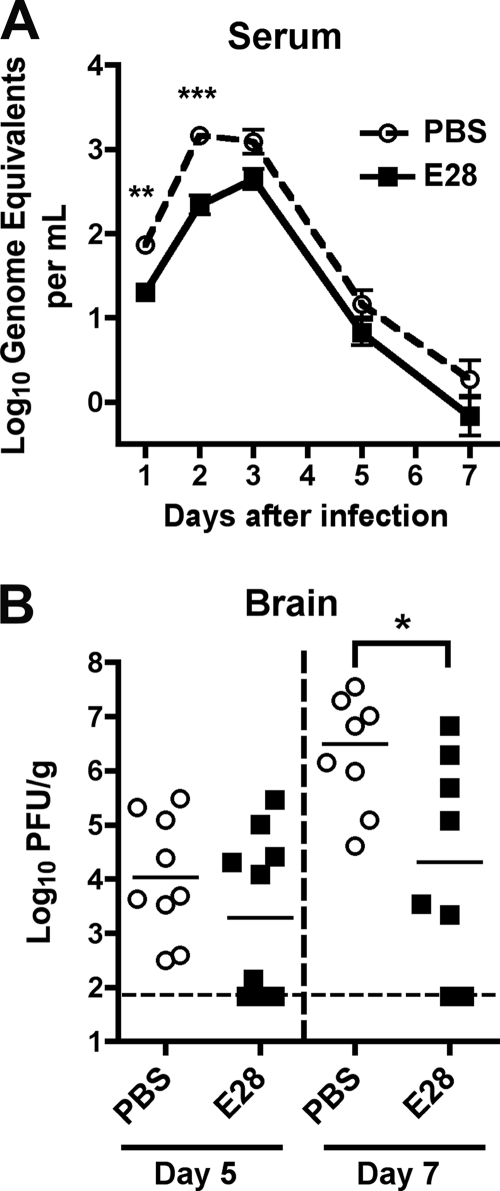
To gain further insight into how prophylaxis with the poorly neutralizing E28 MAb protects against WNV infection in vivo, passive transfer experiments were repeated, but mice were sacrificed and their organs collected for viral burden analysis at different time points. Mice receiving E28 MAb had lower levels of WNV in serum at days 1 and 2 postinfection (∼3.4-fold [P < 0.002] and ∼6.5-fold [P < 0.0001] respectively) than the PBS-treated animals as measured by quantitative RT-PCR of viral RNA (Fig. 2A). Correspondingly, mice treated with E28 MAb had decreased (∼100-fold [P < 0.04]) levels of infectious WNV in the brain at day 7 after infection (Fig. 2B). Although differences of WNV in the brain did not attain statistical significance at day 5 (P > 0.2), nearly half of the E28 MAb-treated mice had levels at or below the limit of detection of the assay, whereas all PBS-treated mice had measurable WNV titers. Thus, treatment with E28 MAb altered the course of WNV infection at an early stage, which impacted dissemination and replication at later phases of pathogenesis.
Fig. 2.
Protective effect of E28 MAb in mice. (A) WNV burden in the serum of 5-week-old wild-type C57BL/6 mice that were administered PBS or E28 (40 μg) via the intraperitoneal route 1 day prior to WNV infection (C6/36 cell derived; 102 PFU) via the subcutaneous route. On the indicated days, serum was harvested and viral burden was determined by qRT-PCR assay. The data are expressed as genome equivalents per ml of serum and reflect 8 to 20 animals per condition per time point. Asterisks indicate values that are statistically significant (**, P < 0.01; ***, P < 0.001). (B) WNV burden in the brains of 5-week-old wild-type mice. Mice were treated as described above. At days 5 or 7 after WNV infection, brains were harvested and viral burdens were determined by plaque assay on BHK21-15 cells. The data are expressed as PFU per gram and reflect 8 to 10 animals per condition per time point. The following percentages of mice had viral burdens below detection (<65 PFU/g): day 5 PBS, 0%; day 5 E28, 40%; day 7 PBS, 0%; day 7 E28, 25%. Asterisks indicate values that are statistically significant (P < 0.05). All analyses utilized the Mann-Whitney nonparametric t test.
C1q and activating Fcγ receptors are required for in vivo protection by E28 MAb.
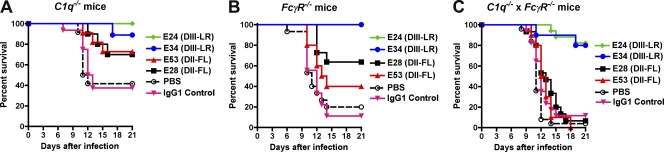
Since E28 MAb had little neutralizing activity in cell culture assays yet protected against WNV infection and pathogenesis, we hypothesized that Fc-mediated effector functions of the MAb contributed to the observed in vivo effects. To test this, congenic mice deficient in the complement component C1q were administered MAbs 1 day prior to WNV infection. Older 8- to 12-week-old mice were used for these studies, as these immunodeficient strains are inherently more susceptible to WNV pathogenesis than wild-type mice (14, 43, 53) and older C57BL/6 mice (up through 24 weeks) are more resistant to infection (12, 22). In mice lacking C1q, which initiates the antibody-dependent classical pathway of complement activation, 400 μg of E53 MAb prevented (P = 0.02) mortality caused by WNV infection (Table 2). Whereas a lower dose (40 μg) of E28 MAb increased the survival time of C1q−/− mice by 3 days (P < 0.02), this dose of E53 or E28 failed to provide statistically significant protection against WNV mortality (Table 2 and Fig. 3A). The partial, albeit limited protection by E28 in C1q−/− mice was somewhat surprising, as this MAb is of the IgG1 isotype, which binds mouse C1q with low affinity (8). However, some mouse IgG1 MAbs bind complement better than others (23). Consistent with a functionally significant interaction, in vitro experiments showed that the addition of mouse C1q limited E28-mediated enhancement of infection in cells expressing FcγR (data not shown).
Table 2.
MAb protection of 8- to 12-week-old mice lacking antibody effector functionsa
| Mouse genotype and MAb or control | Dose (μg) | Epitope on WNV E | Isotype | MTD ± SDb | Survival (%) | P value |
|---|---|---|---|---|---|---|
| C1q−/− | ||||||
| PBS | 11.0 ± 0.6 | 5/12 (42) | ||||
| Anti-DENV-2 E70 (anti-DENV E control) | 40 | IgG1 | 11.4 ± 1.7 | 6/16 (38) | 0.9 | |
| 2H2 (anti-DENV prM control) | 400 | IgG2a | 12.0 ± 2.3 | 3/8 (38) | 0.9 | |
| E24 | 40 | DIII-LR | IgG2a | NA | 12/12 (100) | 0.002 |
| E24 | 400 | DIII-LR | IgG2a | NA | 11/11 (100) | 0.003 |
| E28 | 40 | DII-FL | IgG1 | 14.0 ± 2.0 | 7/10 (70) | 0.11 |
| E34 | 40 | DIII-LR | IgG1 | 17 | 8/9 (89) | 0.02 |
| E53 | 40 | DII-FL | IgG2a | 13.0 ± 2.0 | 8/11 (73) | 0.09 |
| E53 | 400 | DII-FL | IgG2a | 16 | 9/10 (90) | 0.02 |
| FcγR−/− | ||||||
| PBS | 10.6 ± 2.0 | 3/15 (20) | ||||
| Anti-DENV-2 E70 (anti-DENV E control) | 40 | IgG1 | 11.4 ± 1.6 | 1/9 (11) | 0.9 | |
| 2H2 (anti-DENV prM control) | 400 | IgG2a | 12.3 ± 3.6 | 5/12 (42) | 0.3 | |
| E24 | 40 | DIII-LR | IgG2a | NA | 10/10 (100) | 0.0001 |
| E24 | 400 | DIII-LR | IgG2a | NA | 11/11 (100) | <0.0001 |
| E28 | 40 | DII-FL | IgG1 | 12.5 ± 1.0 | 7/11 (64) | 0.008 |
| E34 | 40 | DIII-LR | IgG1 | NA | 5/5 (100) | 0.006 |
| E53 | 40 | DII-FL | IgG2a | 11.8 ± 1.6 | 4/10 (40) | 0.16 |
| E53 | 400 | DII-FL | IgG2a | 9.8 ± 1.0 | 8/12 (67) | 0.04 |
| C1q−/− × FcγR−/− | ||||||
| PBS | 11.2 ± 1.1 | 1/25 (4) | ||||
| Anti-DENV2-E70 (anti-DENV E control) | 40 | IgG1 | 11.8 ± 1.6 | 2/17 (12) | 0.1 | |
| 2H2 (anti-DENV prM control) | 400 | IgG2a | 11.0 ± 2.0 | 1/10 (10) | 0.5 | |
| E24 | 40 | DIII-LR | IgG2a | 16.0 ± 2.6 | 14/17 (82) | <0.0001 |
| E24 | 400 | DIII-LR | IgG2a | NA | 11/11 (100) | <0.0001 |
| E28 | 40 | DII-FL | IgG1 | 13.1 ± 2.2 | 1/15 (7) | 0.01 |
| E28 | 400 | DII-FL | IgG1 | 12.8 ± 1.8 | 0/8 (0) | 0.09 |
| E34 | 40 | DIII-LR | IgG1 | 15.0 ± 5.7 | 8/10 (80) | <0.0001 |
| E53 | 40 | DII-FL | IgG2a | 12.9 ± 2.2 | 0/10 (0) | 0.08 |
| E53 | 400 | DII-FL | IgG2a | 12.9 ± 3.1 | 0/8 (0) | 0.4 |
| C1q−/− × FcγRIII−/− | ||||||
| PBS | 11.3 ± 2.6 | 14/29 (48) | ||||
| Anti-DENV-2 E70 (anti-DENV E control) | 40 | IgG1 | 12.0 ± 4.6 | 4/8 (50) | 0.9 | |
| E24 | 40 | DIII-LR | IgG2a | NA | 10/10 (100) | 0.007 |
| E28 | 40 | DII-FL | IgG1 | 11.3 ± 3.1 | 6/16 (38) | 0.5 |
| E53 | 40 | DII-FL | IgG2a | 13.0 ± 3.3 | 16/24 (67) | 0.13 |
The indicated MAb (or PBS) was passively transferred to 8- to 12-week-old mice 1 day prior to infection with 102 PFU of C6/36 cell-derived WNV. Survival was followed, and P values were determined using the log rank test for comparison to PBS-treated mice.
MTD, mean time to death (± the standard deviation) for mice that succumbed to infection. NA, not applicable, as none of the animals in the treatment group succumbed to infection.
Fig. 3.
Efficacies of different anti-WNV MAbs in C1q−/− and FcγR−/− mice. Eight- to 12-week-old C1q−/− (A), FcγR−/− (B), or C1q−/− × FcγR−/− C57BL/6 mice (C) were passively transferred 40 μg of E24 (DIII-LR, IgG2a), E34 (DIII-LR, IgG1), E53 (DII-FL, IgG2a), E28 (DII-FL, IgG1), an isotype control (IgG1), or PBS 1 day prior to infection with 102 PFU of C6/36 cell-derived WNV. Animals were followed for survival over a period of 3 weeks. The number of animals for each antibody condition ranged as follows: C1q−/−, 9 to 16; FcγR−/−, 5 to 15; C1q−/− × FcγR−/−, 10 to 25. Statistically significant differences are described in the text and were based on comparisons to the PBS-treated mice.
Mice lacking the common γ-chain of activating FcγR lack surface expression and signaling from FcγRI (CD64), FcγRIII (CD16), and FcγRIV (52). To assess the impact of FcγR-mediated effector functions on the protective capacity of poorly neutralizing MAbs, prophylaxis studies were repeated in mice lacking the common γ-chain (FcγR−/−). Passive transfer of 40 μg of E28 and 400 μg of E53 protected FcγR−/− mice against lethal WNV infection (Table 2 and Fig. 3B). Because we hypothesized that complement and FcγR-mediated effector functions might jointly contribute to the protective capacity of poorly neutralizing MAbs, we generated C1q−/− × FcγR−/− mice. Notably, 400 μg doses of E28 and E53 MAbs did not prevent lethality in C1q−/− × FcγR−/− mice, although robust protection was observed with strongly neutralizing DIII-LR MAbs (Table 2 and Fig. 3C). A requirement for FcγRIII for E28- and E53-mediated protection was inferred from passive transfer experiments with C1q−/− × FcγRIII−/− mice, as statistically significant protection also was lost (Table 2). Collectively, these results suggest that Fc-mediated effector functions (C1q and FcγR) are required for the protective activity of poorly neutralizing anti-WNV antibodies.
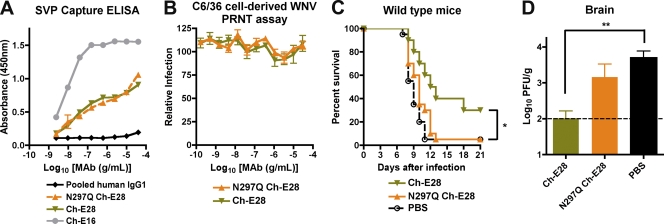
To confirm independently that Fc effector functions of poorly neutralizing MAbs are required for protection in vivo, we engineered chimeric versions of E28 that retained or lost the ability to interact with C1q and FcγR. The variable (VH and VL) regions of mouse E28 were cloned upstream of the human IgG1 constant regions, and a chimeric mouse-human MAb (Ch-E28) was expressed as the wild type and aglycosyl variant; the latter contains a mutation (N297Q) in the heavy chain that eliminates binding to C1q and all FcγR (72). Wild-type Ch-E28 and N297Q Ch-E28 bound to WNV virions equivalently in a capture ELISA (Fig. 4A), yet still lacked neutralizing activity as judged by PRNT assay on BHK21-15 cells with C6/36-derived WNV (Fig. 4B). While wild-type Ch-E28 provided significant protection against mortality (P = 0.03) in 4- to 5-week-old wild-type mice infected with WNV, N297Q Ch-E28 failed to do so (Fig. 4C). Accordingly, treatment with Ch-E28 but not Ch-E28 N297Q was associated with reduced WNV levels in the brain at day 7 after infection (Fig. 4D). These experiments confirm that Fc effector functions are required for in vivo protection by the poorly neutralizing MAb E28.
Fig. 4.
Functional activity of wild-type or aglycosyl chimeric E28 MAbs in vitro and in vivo. (A) A capture ELISA was used to detect binding of wild-type or N297Q Ch-E28 MAbs to WNV SVP. Microtiter plates were coated with murine E24 and incubated with SVP, and absorbance was measured with increasing concentrations of the indicated chimeric (Ch-E28, N297Q Ch-E28, or Ch-E16) or control human IgG1 antibodies. One representative of three independent experiments is shown. (B) PRNT assay. Ch-E28 and N297Q Ch-E28 were tested for neutralization of C6/36 cell-derived WNV by standard PRNT assay on BHK21-15 cells. Data shown are combined results of two independent experiments performed in triplicate. The data were normalized to data from six control wells in each experiment with no MAb. (C) PBS or 40 μg of Ch-E28 or N297Q Ch-E28E24 was passively transferred to 4-week-old wild-type C57BL/6 mice 1 day prior to infection with 102 PFU of C6/36 cell-derived WNV. Animals were followed for survival over a period of 3 weeks. The number of animals for each antibody condition ranged from 13 to 19. Asterisks indicate values that were statistically significant by the log rank test (P < 0.05). (D) WNV burden in the brains of 4-week-old wild-type mice. Mice were treated as described above. At day 7 after WNV infection, brains were harvested and viral burdens were determined by plaque assay on BHK21-15 cells. The data are expressed as PFU per gram and reflect results for 22 animals per condition. The following percentages of mice had viral burdens below detection (<100 PFU/g): PBS, 14%; Ch-E28, 59%; N297Q Ch-E28, 36%. **, statistically significant by a one-way ANOVA (P < 0.01). The viral burden between Ch-E28 and N297Q Ch-E28 approached statistical significance (P = 0.05).
B, T, and NK cells are not required for E28 MAb-mediated protection.
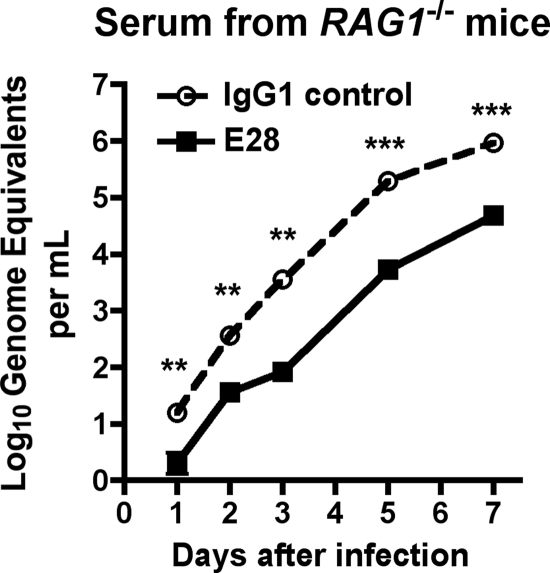
We hypothesized that E28 MAb could promote viral clearance directly through enhanced complement and FcγR-dependent uptake, as described previously for MAbs against WNV NS1 (14, 15), or that E28-dependent immune complex formation and uptake could promote antigen presentation and adaptive immune responses. To address the latter possibility, passive transfer studies were repeated in RAG1−/− mice, which lack B and T cells; as these mice are highly vulnerable to lethal WNV infection (22, 83), a lower infecting dose of virus (1 PFU) was used, and protection was assessed by a virologic rather than survival endpoint. Notably, RAG1−/− mice receiving E28 MAb showed lower levels (≥5-fold [P < 0.008]) of WNV in serum over time than the isotype control (Fig. 5). While this experiment does not completely eliminate a possible role of B and T lymphocytes in contributing to E28-mediated protection in vivo, it establishes that these cells are not required for protection in vivo.
Fig. 5.
Protective effect of E28 MAb in RAG1−/− mice. The graph shows the WNV burden in the serum of 8- to 12-week-old congenic RAG1−/− C57BL/6 mice that were administered E28 (500 μg) or an isotype control MAb via the intraperitoneal route 1 day prior to WNV infection (C6/36 cell derived; 1 PFU) via the subcutaneous route. On the indicated days, serum was harvested and viral burden was determined by qRT-PCR assay. The data are expressed as genome equivalents per ml of serum and reflect results for 5 to 10 animals per condition per time point. Asterisks indicate values that are statistically significant by the Mann-Whitney nonparametric t test (**, P < 0.01; ***, P < 0.001).
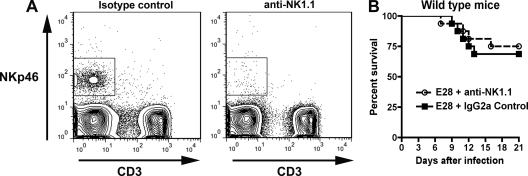
Our prophylaxis experiments with C1q−/− and C1q−/− × FcγRIII−/− mice suggested that some effector functions utilized by E28 for protection occur through FcγRIII-dependent pathways. Given that NK cells express high levels of FcγRIII and use this receptor for antibody-dependent cellular cytotoxicity (ADCC) in vivo (62), we hypothesized that NK cells might be essential for E28-mediated protection. To assess this, 4- to 5-week-old wild-type mice were administered 100 μg of a murine IgG2a control MAb or PK136, an anti-NK1.1 MAb that depletes NK cells (73). At days 2 and 4 after treatment, mice given PK136 showed significant depletion of NK cells, as expected (Fig. 6A). However, depletion of NK cells had no impact on E28 MAb-mediated protection as judged by survival (Fig. 6B) in mice treated with PK136 (100 μg) 1 day prior to WNV infection and again 3 days after infection. Thus, NK cells are not necessary for E28-mediated protection from WNV.
Fig. 6.
Effect of depletion of NK cells on protective activity of E28 MAb. Wild-type mice were depleted of NK cells after treatment with anti-NK1.1 antibody (100 μg) 2 days before and after infection with WNV. (A) Depletion of NK cells was confirmed by flow cytometry after staining with anti-NKp46 and anti-CD3. (B) WNV infection of NK cell-depleted mice. Sixteen wild-type mice (5 weeks old) were treated with either anti-NK1.1 or an isotype control antibody (2H2; anti-DENV prM), administered E28 (40 μg), infected with WNV (C6/36 cell derived; 102 PFU), and monitored for survival. No statistically significant difference in mortality was observed based on the log rank test (P > 0.6).
Phagocytic cells contribute to E28 MAb-mediated protection.
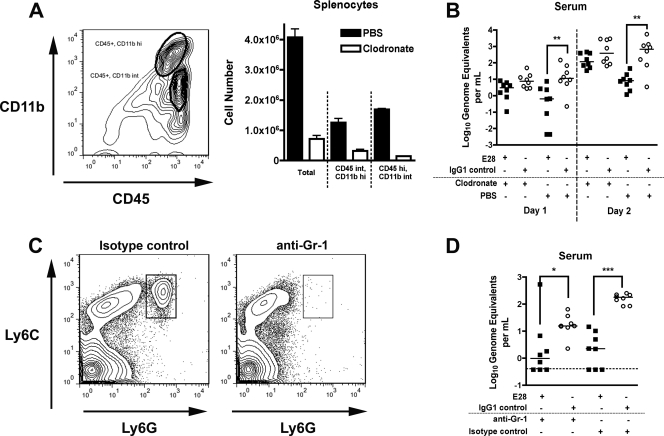
As B, T, and NK cells were not required for protection, we hypothesized that E28 might opsonize virus for phagocytosis by cells expressing FcγR and complement receptors. To test their contribution to E28-mediated protection after WNV infection, we nonselectively depleted phagocytes using clodronate liposomes (77). To confirm depletion, splenocytes were analyzed for expression of the myeloid cell markers CD11b and CD45 1 day after liposome injection. As expected, treatment with clodronate-containing liposomes depleted CD45int CD11bhi and CD45hi CD11bint cells (Fig. 7A), which correspond to myeloid cells with phagocytic capacity. To determine whether phagocytes were necessary for E28-mediated protection, 8- to 12-week-old wild-type mice were administered liposomes containing clodronate or PBS and 500 μg of E28 or isotype control MAb 1 day prior to infection with 1 PFU of WNV and then given a second dose of liposomes on day 1 postinfection. The experimental design was altered from the initial survival studies, because wild-type mice treated with clodronate liposomes are more vulnerable to WNV infection (5, 60). Clodronate liposome treatment resulted in a loss of protection by E28 MAb as judged by elevated levels of WNV in serum at days 1 and 2 postinfection, compared to mice given liposomes containing PBS (Fig. 7B). Thus, phagocytes are required for the early reduction in viremia mediated by E28.
Fig. 7.
Effect of depletion of phagocytes and neutrophils on the protective activity of E28 MAb. Eight- to 12-week-old wild-type mice were administered E28 (500 μg) or an isotype control MAb via the intraperitoneal route 1 day prior to WNV infection (C6/36 cell derived; 1 PFU) via the subcutaneous route. (A and B) Depletion of phagocytes with clodronate-containing liposomes. (A, left) Gating strategy for CD11b+ and CD45+ cell analysis by flow cytometry. (Right) Quantitative depletion of CD11b+ and CD45+ phagocyte cell populations in the spleens of RAG1−/− mice by clodronate-containing liposomes. Depletion with clodronate-containing liposomes reduced the number of CD45int CD11bhi and CD45hi CD11bint splenocytes by 74% and 91%, respectively. (B) Effects of clodronate-containing or PBS-containing liposomes on the E28-mediated reduction in viremia. Wild-type mice were treated with clodronate- or PBS-containing liposomes 1 day prior to and after infection with WNV. On the indicated days, serum was harvested and viral burden was determined by qRT-PCR assay. The data are expressed as genome equivalents per ml of serum and reflect 8 animals per condition per time point. Asterisks indicate values that are statistically significant (**, P < 0.01). (C and D) Effect of depletion of neutrophils and inflammatory monocytes on the protective activity of E28 MAb. Neutrophils and inflammatory monocytes were depleted from wild-type mice after treatment with anti-Gr-1 antibody (250 μg) 1 day before infection with WNV. (C) Depletion of neutrophils and inflammatory monocytes was confirmed by flow cytometry after staining with anti-Ly6C and Ly6G. (D) Effect of neutrophil and inflammatory monocyte depletion on E28-mediated reduction in viremia. At day 2 after infection, serum was harvested and the viral burden was determined by qRT-PCR assay. The data are expressed as genome equivalents per ml of serum and reflect results for 8 animals per condition per time point. Asterisks indicate values that are statistically significant (*, P < 0.05; ***, P < 0.001). All analyses utilized the Mann-Whitney test.
Neutrophils and inflammatory monocytes are innate immune cells that express FcγR and complement receptors and could contribute to E28 MAb-mediated clearance. To determine if these cells were necessary, mice were administered RB6.8C5 (250 μg), a rat anti-mouse Gr-1 MAb that depletes cells expressing Ly6C and Ly6G (Fig. 7C), including neutrophils, inflammatory monocytes, suppressor monocytes, and plasmacytoid dendritic cells (21). RB6.8C5 or control antibody and E28 (500 μg) or control antibody were given 1 day prior to infection. Notably, E28 MAb treatment decreased serum viremia on day 2 postinfection regardless of whether mice had received RB6.8C5 or control antibody (Fig. 7D), indicating that Gr-1-expressing cells were not required for E28-mediated protective effects in vivo. Combined with the clodronate liposome data, these results suggest that phagocytic CD11b+ macrophages may be the primary cell type responsible for much of the protective activity of E28 in vivo.
Poorly neutralizing, E28-like polyclonal antibodies protect mice from WNV infection.
While the WNV epidemic spread rapidly across North America, only a few human clinical infections have been reported in Central and South America despite the presence of the virus in avian and mosquito hosts (28, 56). Although this could reflect reporting bias, we hypothesized that prior widespread exposure to DENV and other endemic flaviviruses could result in production of polyclonal DII-FL-specific antibodies that failed to neutralize WNV infection by conventional PRNT analysis but still protected humans in vivo. Indeed, DII-FL antibodies are immunodominant in humans after infection with several flaviviruses (16, 54, 69, 75). To test whether polyclonal antibodies had E28-like epitope specificity and functional activity and would behave similarly against WNV in vivo, golden Syrian hamsters were inoculated with DENV-2, and polyclonal IgG was isolated by affinity chromatography from serum that was collected 4 weeks after infection. Hamsters were used rather than mice, because in the latter their antibody response is skewed toward the DIII-LR epitope (54). Notably, DENV-2-immune hamster IgG bound more avidly to WNV virions than naïve IgG in a capture ELISA (Fig. 8A). DENV-2 IgG failed to neutralize WNV infection by PRNT assay on BHK21-15 cells (Fig. 8B; PRNT50, <1/15 serum dilution), although some inhibition of Raji-DCSIGN-R cell infection was detected with partially mature but not mature WNV RVP (Fig. 8C). Thus, polyclonal DENV immune hamster IgG has similar in vitro neutralization characteristics as MAb E28.
Fig. 8.
Functional activity in vitro and in vivo of polyclonal IgG derived from naïve or DENV-2-infected hamsters. (A) A capture ELISA was used to detect binding of naive or DENV-2-immune polyclonal IgG to WNV SVP. Microtiter plates were coated with murine E24, incubated with SVP, and detected with increasing concentrations of the indicated affinity-purified polyclonal hamster IgG. One representative of three independent experiments is shown. (B) PRNT assay. Naive and DENV-2-immune polyclonal IgG were tested for neutralization of C6/36 cell-derived WNV by PRNT assay on BHK21-15 cells. Data shown are the combined results of two independent experiments performed in triplicate. The data were normalized to data from six control wells in each experiment with no MAb. (C) RVP neutralization assay on Raji-DCSIGN-R cells. Increasing concentrations of naive or DENV-2-immune polyclonal IgG were incubated with RVP for 1 h prior to infection of Raji-DCSIGN-R cells. RVP were prepared normally (mixture of mature, immature, and partially mature) or in cells overexpressing the furin protease (mature) to create virions of different maturation states. Data shown are representative results of two independent experiments performed in duplicate. (D) Epitope specificity of DENV-2-immune hamster IgG against WNV E protein. Shown is a comparison of the antibody titer from DENV-2-immune hamsters for wild-type (E-WT) and DII-FL loss-of-function variants (E-W101R and E-quadruple [Quad] mutant). Titers of antibodies were compared from individual DENV-2-infected hamsters. Note that the plating densities of E-WT, E-W101R, and the E-quadruple mutant were equivalent, as no differences in binding were observed with E24 (DIII-LR) MAb when tested in parallel (data not shown). Asterisks indicate values that are statistically significant by Student's paired t test (**, P < 0.01; ***, P < 0.001). (E) PBS or 400 μg of pooled naïve or DENV-2-immune affinity-purified IgG was passively transferred to 4- to 5-week-old wild-type C57BL/6 mice 1 day prior to infection with 102 PFU of C6/36 cell-derived WNV. Animals were followed for survival over a period of 3 weeks. The number of animals for each condition group ranged from 10 to 15. Asterisks indicate values that are statistically significant by the log rank test (*, P < 0.05).
The vast majority of DII-FL-specific MAbs, including E28, lose binding to recombinant E protein, which encodes a single W101R mutation (54). However, E53 retains binding to E-W101R but does not bind a recombinant E protein with three additional mutations within and proximal to the fusion loop (T76R, M77E, W101R, and L107R [E-quadruple mutant]). Polyclonal DENV-2-immune hamster IgG that cross-reacted with WNV E protein was skewed toward the DII-FL epitope (W101R, 83% ± 13% binding [P < 0.005]; quadruple mutant, 90% ± 7.7% binding [P < 0.002]) as determined in direct binding assays to wild-type and mutant recombinant E protein (Fig. 8D). As most of the DENV-2-immune hamster cross-reactive IgG recognized the DII-FL epitope and did not neutralize WNV infection in BHK21-15 cells, we tested it for protective activity in vivo. Similar to results with E28 MAb, passive transfer of DENV-2-immune hamster IgG 1 day prior to WNV infection significantly (P = 0.03) protected 4- to 5-week-old mice compared to naïve IgG (Fig. 8E). Thus, poorly neutralizing cross-reactive polyclonal antibodies derived from heterologous flavivirus infection can mitigate WNV infection in vivo.
The cross-reactive antibody repertoire of serum from DENV immune humans is skewed toward the DII-FL epitope.
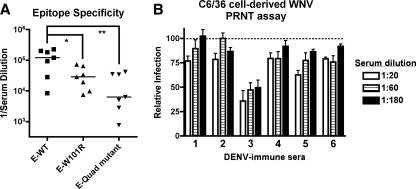
Having demonstrated that polyclonal anti-DENV-2 hamster IgG was nonneutralizing yet protective against WNV infection in mice, we questioned whether similar trends would be observed with human serum from DENV-immune individuals. Serum with cross-reactivity to WNV was obtained from patients with a history of infection of at least two serotypes of DENV (S. Halstead and A. de Silva, personal communication). Analogous to that seen with hamster IgG, polyvalent DENV-immune human serum that cross-reacts with WNV E protein was skewed toward the DII-FL epitope (Fig. 9A). Although variability was observed in the loss of binding to E-W101R (51% ± 42% binding; P < 0.05), loss of binding to the E-quadruple mutant was more consistent (82% ± 14% binding; P < 0.005). Only one of the six sera tested neutralized WNV infection by >50% (PRNT50, 1/180 for sample 3). None of the remaining five human immune sera neutralized WNV by 50% at the lowest serum dilution tested (Fig. 9B; PRNT50, <1/20 serum dilution), establishing that humans infected with DENV produce DII-FL antibodies that cross-react to WNV yet poorly neutralize infection. However, given the limited available quantities of human sera, we could not directly test the protective capacity in passive transfer experiments in mice.
Fig. 9.
Sera from humans with a history of multiple DENV infections contain cross-reactive, poorly neutralizing antibodies directed against the DII-FL epitope. The cross-reactivities of these human immune sera for DENV are described in Materials and Methods. (A) Epitope specificity of DENV-immune human serum against WNV E protein. Antibody titers from DENV-immune human serum for wild-type (E-WT) and DII-FL loss-of-function variants (E-W101R and E-quadruple [Quad] mutant) are shown. Note that the plating densites of E-WT, E-W101R, and the E-quadruple mutant were equivalent, as no differences in binding were observed with Ch-E16 (DIII-LR) MAb when tested in parallel (data not shown). Asterisks indicate values that are statistically significant based on Student's paired t test (*, P < 0.05; **, P < 0.01). (B) WNV PRNT assay. DENV-immune human sera from six individuals with a history of heterotypic infections was tested for neutralization of C6/36 cell-derived WNV by PRNT assay on BHK21-15 cells. Data shown are combined results of two independent experiments performed in triplicate. The data were normalized to data from 12 control wells in each experiment with naïve human serum.
DISCUSSION
A prior study suggested that DII-FL MAbs neutralize WNV poorly in vitro yet still can protect mice from WNV infection (55). Here, we examined in greater detail the ability of two DII-FL MAbs, E53 and E28, to inhibit infection in vitro and in vivo. These DII-FL MAbs neutralized WNV differently according to the cell type used and the maturity of the virus stock, with fully mature WNV more resistant to neutralization. The DII-FL MAbs protected wild-type mice from lethal WNV infection, and prophylaxis with E28 MAb decreased WNV viremia at days 1 and 2 after infection and reduced infection in the brain at 7 days after infection. The protective activities of DII-FL MAbs were essentially abolished in mice lacking both C1q and FcγR or in wild-type mice treated with an aglycosyl MAb variant, establishing that Fc effector functions mediate in vivo protection of poorly neutralizing MAbs. While studies with deficient mice and depleting antibodies indicated that B, T, and NK cells were not necessary for this protection, phagocytes were required. Cross-reactive polyclonal antibodies from DENV-immune hamsters and humans also were poorly neutralizing against WNV in vitro and directed against the DII-FL epitope. Nonetheless, and analogous to data with E28 MAb, these cross-reactive polyclonal antibodies conferred protection in passive transfer studies in mice.
Using multiple in vitro assays with WNV derived from different cell sources, we confirmed the poorly neutralizing capacity of DII-FL MAbs against WNV. While the PRNT assay on BHK21-15 or Vero cells remains the “gold standard” measure of antibody neutralization of flaviviruses (61), imperfect correlations between titers in vitro and protection in vivo have been observed (9). One hypothesis as to why poorly neutralizing antibodies protect in vivo is that the PRNT assay, which is usually performed with C6/36-cell derived viral stocks, does not account for the all of the neutralizing activity of different antibodies against flaviviruses. To determine whether the source of the WNV stock affected MAb inhibitory activity by PRNT assay, we tested WNV prepared in mammalian Vero cells or in vivo from the plasma of mice. Plasma- rather than serum-derived WNV was used to avoid maturation artifacts associated with ex vivo activation of clotting cascade serine proteases, which might adventitiously cleave prM. Vero cell- and plasma-derived WNV was essentially mature and resistant to DII-FL MAb neutralization by PRNT assay, consistent with studies showing that DII-FL MAbs poorly bind or neutralize mature forms of WNV (13, 51).
Because a prior study suggested differential inhibitory activities of anti-WNV MAbs mapping to DI and DII based on use of RVP and Raji-DCSIGN-R cells (55), we expanded our analysis to determine whether the poor neutralizing activities of MAbs showed cell-type-specific effects. E28 did not neutralize insect or mammalian cell-derived WNV in Raji-DCSIGN-R cells; indeed, it paradoxically enhanced infection through an unknown mechanism, as these cells lacked expression of activating FcγR. Therefore, Raji-DCSIGN-R cells were not inherently better than BHK21-15 cells at revealing the neutralization potential of E28. However, when less mature forms of RVP were prepared, E28 showed a modest inhibitory activity on Raji-DCSIGN-R cells, analogous to that described with E53 (51). Thus, the neutralization capacity of DII-FL MAbs was cell type and maturation state dependent, and the PRNT assay provided an incomplete evaluation of the inherent inhibitory activities of these antibodies. This idea is important to consider, as the humoral response to vaccines against WNV (1) and DENV (49) is currently evaluated primarily using the PRNT assay.
The results of the cell culture studies suggested that DII-FL MAbs, such as E28, were at most weakly neutralizing and possibly nonneutralizing, depending on the source of virus and assay used for evaluation. Nonetheless, E28 and other poorly neutralizing DII-FL MAbs were protective in passive transfer experiments in mice (55). The ability of poorly neutralizing MAbs to protect animals against virus infection is not inherently novel, as it has been observed with flaviviruses (27, 34, 55), alphaviruses (10, 38, 46, 66), coronaviruses (50), reoviruses (76), and rhabdoviruses (37). These other studies were observational, however, and the mechanism of protection in animals remained uncharacterized. Based on its in vitro properties, we selected E28 for an in-depth analysis of the mechanism of protection in mice. Studies with both strongly and poorly neutralizing antibodies against herpesviruses (81), flaviviruses (65), retroviruses (32, 47), and poxviruses (4) established that Fc effector functions were required for reducing viral burden and clinical morbidity. Analogously, our passive transfer experiments with C1q−/− × FcγR−/− mice or an aglycosyl E28 variant in wild-type mice demonstrated the necessity of Fc effector functions for protection by weakly or nonneutralizing antibodies.
As the Fc portion of an antibody can engage complement and FcγRs, we repeated infections in C1q−/−, FcγR−/−, and C1q−/− × FcγR−/− mice to gain further mechanistic insights into protective mechanisms. While some protective capacity was lost in C1q−/− mice, E28 and E53 failed to protect C1q−/− × FcγRIII−/− mice, implicating this FcγR as a key component of the survival phenotype conferred by poorly neutralizing MAbs. How do Fc effector functions enhance the protective potential of weakly neutralizing antibodies? As a previous study had demonstrated that C5 was not required for protective antibody effects on WNV (44), virion lysis appeared an unlikely mechanism. However, DII-FL MAbs may become neutralizing in the presence of C1q, as it reduces the stoichiometric threshold required for antibody neutralization (45). Further mechanistic studies were aimed at identifying the cell type(s) in vivo that conferred the protective activity. As E28 still decreased viremia in WNV-infected RAG1−/− mice, it seems unlikely that immune complex uptake, enhanced antigen presentation, and priming of the adaptive B and T cell responses can explain the protective effects in vivo. The experiments in RAG1−/− mice were supported by prophylaxis studies with E53, which showed no change in the kinetics of CD4+ or CD8+ T cell activation or quality of the neutralizing antibody response after WNV infection in wild-type mice (M. Vogt and M. Diamond, unpublished studies).
Although NK cells appear unnecessary for protection against primary WNV infection in mice (68), those studies were performed in naïve animals, which lack preexisting anti-WNV antibodies. As our passive transfer experiments suggested that FcγRIII contributes to E28-mediated protection and that NK cell ADCC requires FcγRIII (62), it seemed plausible that NK cells might be required for the survival benefit conferred by E28. However, mice depleted of NK cells showed no significant change in E28-mediated protection compared to those receiving an isotype control, nondepleting MAb.
As complement and FcγRs also can promote phagocytosis of antibody-opsonized antigens, we speculated that a specific cell type capable of phagocytosis of viral particles or infected cells conferred E28-mediated protection. After nonselectively depleting phagocytes from wild-type mice with clodronate-containing liposomes, E28 no longer reduced WNV viremia during the first 2 days of infection. Since clodronate-containing liposomes deplete several cell types with phagocytic potential (78), we tested the role of specific populations of phagocytes by antibody depletion. Gr-1 is expressed on neutrophils and inflammatory monocytes (21), both of which have phagocytic potential. As E28 MAb prophylaxis retained its ability to decrease viremia in mice treated with depleting concentrations of anti-Gr-1 antibody, neutrophils and inflammatory monocytes were likely not the key phagocytic cell types that conferred protection. While additional experiments are needed to absolutely identify the target cell type, our data collectively suggest that a complement- and FcγR-expressing innate immune phagocyte (e.g., tissue macrophage or activated dendritic cell) mediates protection of E28 in mice.
While the detailed studies with E28 were informative from a mechanistic standpoint, it was important to confirm whether poorly neutralizing polyclonal antibodies shared the same phenotype. Purified polyclonal IgG from the serum of DENV-immune hamsters was E28-like, as it recognized SVP in a capture ELISA, was specific largely for the DII-FL epitope on WNV E protein, and was poorly neutralizing in both PRNT and RVP assays. Analogously, these cross-reactive polyclonal antibodies nonetheless protected wild-type mice from WNV infection. The anti-WNV antibody repertoire in many human patients is skewed similarly toward the DII-FL epitope (54, 75), which is somewhat surprising, given that MAbs recognizing this epitope neutralize WNV poorly and do not bind fully mature virus (51). In screening sera from humans with a remote history of DENV infection, a single prior exposure elicited a minor cross-reactive antibody response, whereas individuals with evidence of multiple heterotypic infections perhaps unsurprisingly elicited higher titers of WNV-reactive antibodies (Vogt and Diamond, unpublished), which neutralized WNV poorly and bound the DII-FL epitope. The similarity of the DENV-immune human sera to the protective hamster IgG suggests that immunodominant DII-FL antibodies elicited in response to natural DENV infection (16), while nonneutralizing against WNV and undetected by the PRNT assay, could still confer protection via Fc effector function mechanisms. While further epidemiologic studies are warranted, widespread exposure to DENV and other endemic flaviviruses in tropical Central and South America could result in production of polyclonal DII-FL-specific antibodies that fail to neutralize WNV infection by conventional PRNT analysis but still protect humans in vivo, and thus contribute to the lack of severe cases of human WNV infection in this region. Indeed, one recent serological surveillance study in Mexico supports this hypothesis (63).
Overall, this study describes a mechanism for the protective effects in vivo of anti-WNV antibodies that are poorly neutralizing in in vitro assays. For WNV, both MAbs and polyclonal antibodies directed against the DII-FL epitope protected wild-type mice from lethal WNV infection in vivo. This activity was dependent upon the Fc effector functions of the antibodies and required phagocytic cells, C1q, and FcγRIII. The ability of cross-reactive antibodies elicited by heterologous flavivirus infection to protect against WNV in vivo could explain the fewer-than-anticipated number of reported cases of WNV infection in Central and South America and analogously, the historical observation of reduced numbers of St. Louis encaphalitis virus or Japanese encephalitis virus infections in regions of the Americas and Southeast Asia where many people are immune to DENV (11, 31). Finally, the inability of the PRNT assay to predict the functional capacities of specific classes of protective antiflavivirus antibodies should give pause to its use as the definitive assay for measuring the immune response to the multitude of flavivirus vaccines that are in clinical testing or under development.
ACKNOWLEDGMENTS
We thank R. Akkina, S. Halstead, A. de Silva, C. Nelson, D. Fremont, W. Yokoyama, J. Elliott, B. Calderon, and E. Unanue for providing key reagents, technical expertise, and experimental suggestions and direction and A. Fuchs and J. Brien for insightful discussions.
This work was supported by grants and contracts from the NIH: grant U01 AI061373 and R01-AI077955 (M.S.D.), the Midwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research (U54 AI057160), HHSN272201D000401/HHSN27200004/D04 (R.B.T.), and the intramural program of NIAID.
Footnotes
Published ahead of print on 14 September 2011.
REFERENCES
- 1. Beasley D. W. 2011. Vaccines and immunotherapeutics for the prevention and treatment of infections with West Nile virus. Immunotherapy 3: 269–285 [DOI] [PubMed] [Google Scholar]
- 2. Beasley D. W., Barrett A. D. 2002. Identification of neutralizing epitopes within structural domain III of the West Nile virus envelope protein. J. Virol. 76: 13097–13100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bedzyk W. D., Johnson L. S., Riordan G. S., Voss E. W., Jr 1989. Comparison of variable region primary structures within an anti-fluorescein idiotype family. J. Biol. Chem. 264: 1565–1569 [PubMed] [Google Scholar]
- 4. Benhnia M. R., et al. 2009. Vaccinia virus extracellular enveloped virion neutralization in vitro and protection in vivo depend on complement. J. Virol. 83: 1201–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ben-Nathan D., Huitinga I., Lustig S., van Rooijen N., Kobiler D. 1996. West Nile virus neuroinvasion and encephalitis induced by macrophage depletion in mice. Arch. Virol. 141: 459–469 [DOI] [PubMed] [Google Scholar]
- 6. Ben-Nathan D., et al. 2003. Prophylactic and therapeutic efficacy of human intravenous immunoglobulin in treating West Nile virus infection in mice. J. Infect. Dis. 188: 5–12 [DOI] [PubMed] [Google Scholar]
- 7. Ben-Nathan D., et al. 2009. Using high titer West Nile intravenous immunoglobulin from selected Israeli donors for treatment of West Nile virus infection. BMC Infect. Dis. 9: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bindon C. I., Hale G., Bruggemann M., Waldmann H. 1988. Human monoclonal IgG isotypes differ in complement activating function at the level of C4 as well as C1q. J. Exp. Med. 168: 127–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blaney J. E., Jr., Matro J. M., Murphy B. R., Whitehead S. S. 2005. Recombinant, live-attenuated tetravalent dengue virus vaccine formulations induce a balanced, broad, and protective neutralizing antibody response against each of the four serotypes in rhesus monkeys. J. Virol. 79: 5516–5528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boere W. A., et al. 1985. Mechanisms of monoclonal antibody-mediated protection against virulent Semliki Forest virus. J. Virol. 54: 546–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bond J. O., Hammon W. M. 1970. Epidemiologic studies of possible cross protection between dengue and St. Louis encephalitis arboviruses in Florida. Am. J. Epidemiol. 92: 321–329 [DOI] [PubMed] [Google Scholar]
- 12. Brien J. D., Uhrlaub J. L., Hirsch A., Wiley C. A., Nikolich-Zugich J. 2009. Key role of T cell defects in age-related vulnerability to West Nile virus. J. Exp. Med. 206: 2735–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cherrier M. V., et al. 2009. Structural basis for the preferential binding of immature flaviviruses by a fusion-loop specific antibody. EMBO 28: 3269–3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chung K. M., et al. 2006. Antibodies against West Nile virus non-structural (NS)-1 protein prevent lethal infection through Fc gamma receptor-dependent and independent mechanisms. J. Virol. 80: 1340–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chung K. M., Thompson B. S., Fremont D. H., Diamond M. S. 2007. Antibody recognition of cell surface-associated NS1 triggers Fc-γ receptor-mediated phagocytosis and clearance of West Nile virus-infected cells. J. Virol. 81: 9551–9555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crill W. D., Hughes H. R., Delorey M. J., Chang G. J. 2009. Humoral immune responses of dengue fever patients using epitope-specific serotype-2 virus-like particle antigens. PLoS One 4: e4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Diamond M. S., Klein R. S. 2006. A genetic basis for human susceptibility to West Nile virus. Trends Microbiol. 14: 287–289 [DOI] [PubMed] [Google Scholar]
- 18. Diamond M. S., Shrestha B., Marri A., Mahan D., Engle M. 2003. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J. Virol. 77: 2578–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Diamond M. S., et al. 2003. A critical role for induced IgM in the protection against West Nile Virus infection. J. Exp. Med. 198: 1853–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ebel G. D., Carricaburu J., Young D., Bernard K. A., Kramer L. D. 2004. Genetic and phenotypic variation of West Nile virus in New York, 2002–2003. Am. J. Trop. Med. Hyg. 71: 493–500 [PubMed] [Google Scholar]
- 21. Egan C. E., Sukhumavasi W., Bierly A. L., Denkers E. Y. 2008. Understanding the multiple functions of Gr-1(+) cell subpopulations during microbial infection. Immunol. Res. 40: 35–48 [DOI] [PubMed] [Google Scholar]
- 22. Engle M., Diamond M. S. 2003. Antibody prophylaxis and therapy against West Nile Virus infection in wild type and immunodeficient mice. J. Virol. 77: 12941–12949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ey P. L., Prowse S. J., Jenkin C. R. 1979. Complement-fixing IgG1 constitutes a new subclass of mouse IgG. Nature 281: 492–493 [DOI] [PubMed] [Google Scholar]
- 24. Fuchs A., et al. 2010. Direct complement restriction of flavivirus infection requires glycan recognition by mannose binding lectin. Cell Host Microbe 8: 186–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gentry M. K., Henchal E. A., McCown J. M., Brandt W. E., Dalrymple J. M. 1982. Identification of distinct antigenic determinants on dengue-2 virus using monoclonal antibodies. Am. J. Trop. Med. Hyg. 31: 548–555 [DOI] [PubMed] [Google Scholar]
- 26. Glass W. G., et al. 2006. CCR5 deficiency increases risk of symptomatic West Nile virus infection. J. Exp. Med. 203: 35–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gould E. A., Buckley A., Barrett A. D., Cammack N. 1986. Neutralizing (54K) and non-neutralizing (54K and 48K) monoclonal antibodies against structural and non-structural yellow fever virus proteins confer immunity in mice. J. Gen. Virol. 67: 591–595 [DOI] [PubMed] [Google Scholar]
- 28. Gubler D. J. 2007. The continuing spread of West Nile virus in the western hemisphere. Clin. Infect. Dis. 45: 1039–1046 [DOI] [PubMed] [Google Scholar]
- 29. Guirakhoo F., Heinz F. X., Mandl C. W., Holzmann H., Kunz C. 1991. Fusion activity of flaviviruses: comparison of mature and immature (prM-containing) tick-borne encephalitis virions. J. Gen. Virol. 72: 1323–1329 [DOI] [PubMed] [Google Scholar]
- 30. Halstead S. B. 2003. Neutralization and antibody-dependent enhancement of dengue viruses. Adv. Virus Res. 60: 421–467 [DOI] [PubMed] [Google Scholar]
- 31. Hammon W. M., Tigertt W. D., Sather G. E., Berge T. O., Meiklejohn G. 1958. Epidemiologic studies of concurrent virgin epidemics of Japanese B encephalitis and of mumps on Guam, 1947–1948, with subsequent observations including dengue, through 1957. Am. J. Trop. Med. Hyg. 7: 441–467 [DOI] [PubMed] [Google Scholar]
- 32. Hessell A. J., et al. 2009. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat. Med. 15: 951–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Junjhon J., et al. 2010. Influence of pr-M cleavage on the heterogeneity of extracellular dengue virus particles. J. Virol. 84: 8353–8358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kaufman B. M., et al. 1989. Monoclonal antibodies for dengue virus prM glycoprotein protect mice against lethal dengue infection. Am. J. Trop. Med. Hyg. 41: 576–580 [DOI] [PubMed] [Google Scholar]
- 35. Komar N., Clark G. G. 2006. West Nile virus activity in Latin America and the Caribbean. Rev. Panam. Salud Publica 19: 112–117 [DOI] [PubMed] [Google Scholar]
- 36. Kuhn R. J., et al. 2002. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 108: 717–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lefrancois L. 1984. Protection against lethal viral infection by neutralizing and nonneutralizing monoclonal antibodies: distinct mechanisms of action in vivo. J. Virol. 51: 208–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Levine B., et al. 1991. Antibody-mediated clearance of alphavirus infection from neurons. Science 254: 856–860 [DOI] [PubMed] [Google Scholar]
- 39. Li L., et al. 2008. The flavivirus precursor membrane-envelope protein complex: structure and maturation. Science 319: 1830–1834 [DOI] [PubMed] [Google Scholar]
- 40. Lim J. K., et al. 2009. Genetic variation in OAS1 is a risk factor for initial infection with West Nile virus in man. PLoS Pathog. 5: e1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lim J. K., et al. 2008. Genetic deficiency of chemokine receptor CCR5 is a strong risk factor for symptomatic West Nile virus infection: a meta-analysis of 4 cohorts in the US epidemic. J. Infect. Dis. 197: 262–265 [DOI] [PubMed] [Google Scholar]
- 42. Lindenbach B. D., Rice C. M. 2001. Flaviviridae: the viruses and their replication, p. 991–1041. In Knipe D. M., Howley P. M. (ed.), Fields virology, vol. 1 Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 43. Mehlhop E., Diamond M. S. 2006. Protective immune responses against West Nile virus are primed by distinct complement activation pathways. J. Exp. Med. 203: 1371–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mehlhop E., Fuchs A., Engle M., Diamond M. S. 2009. Complement modulates pathogenesis and antibody-dependent neutralization of West Nile virus infection through a C5-independent mechanism. Virology 393: 11–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mehlhop E., et al. 2009. Complement protein C1q reduces the stoichiometric threshold for antibody-mediated neutralization of West Nile virus. Cell Host Microbe 6: 381–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mendoza Q. P., Stanley J., Griffin D. E. 1988. Monoclonal antibodies to the E1 and E2 glycoproteins of Sindbis virus: definition of epitopes and efficiency of protection from fatal encephalitis. J. Gen. Virol. 69: 3015–3022 [DOI] [PubMed] [Google Scholar]
- 47. Michaud H. A., et al. 2010. A crucial role for infected-cell/antibody immune complexes in the enhancement of endogenous antiviral immunity by short passive immunotherapy. PLoS Pathog. 6: e1000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morrey J. D., et al. 2006. Humanized monoclonal antibody against West Nile virus E protein administered after neuronal infection protects against lethal encephalitis in hamsters. J. Infect. Dis. 194: 1300–1308 [DOI] [PubMed] [Google Scholar]
- 49. Murphy B. R., Whitehead S. S. 2011. Immune response to dengue virus and prospects for a vaccine. Annu. Rev. Immunol. 29: 587–619 [DOI] [PubMed] [Google Scholar]
- 50. Nakanaga K., Yamanouchi K., Fujiwara K. 1986. Protective effect of monoclonal antibodies on lethal mouse hepatitis virus infection in mice. J. Virol. 59: 168–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nelson S., et al. 2008. Maturation of West Nile virus modulates sensitivity to antibody-mediated neutralization. PLoS Pathog. 4: e1000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nimmerjahn F., Ravetch J. V. 2006. Fcγ receptors: old friends and new family members. Immunity 24: 19–28 [DOI] [PubMed] [Google Scholar]
- 53. Oliphant T., et al. 2005. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nature Med. 11: 522–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Oliphant T., et al. 2007. The induction of epitope-specific neutralizing antibodies against West Nile virus. J. Virol. 81: 11828–11839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Oliphant T., et al. 2006. Antibody recognition and neutralization determinants on domains I and II of West Nile Virus envelope protein. J. Virol. 80: 12149–12159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Petersen L. R., Hayes E. B. 2008. West Nile virus in the Americas. Med. Clin. North Am. 92: 1307–1322 [DOI] [PubMed] [Google Scholar]
- 57. Pierson T. C., Diamond M. S. 2008. Molecular mechanisms of antibody-mediated neutralization of flavivirus infection. Exp. Rev. Mol. Med. 10: e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pierson T. C., Fremont D. H., Kuhn R. J., Diamond M. S. 2008. Structural insights into the mechanisms of antibody-mediated neutralization of flavivirus infection: implications for vaccine development. Cell Host Microbe 4: 229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pierson T. C., et al. 2007. The stoichiometry of antibody-mediated neutralization and enhancement of West Nile virus infection. Cell Host Microbe 1: 135–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Purtha W. E., Chachu K. A., Virgin H. W., Diamond M. S. 2008. Early B-cell activation after West Nile virus infection requires alpha/beta interferon but not antigen receptor signaling. J. Virol. 82: 10964–10974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Putnak J. R., et al. 2008. Comparative evaluation of three assays for measurement of dengue virus neutralizing antibodies. Am. J. Trop. Med. Hyg. 79: 115–122 [PubMed] [Google Scholar]
- 62. Ravetch J. V., Bolland S. 2001. IgG Fc receptors. Annu. Rev. Immunol. 19: 275–290 [DOI] [PubMed] [Google Scholar]
- 63. Rodriguez Mde L., et al. 2010. Serologic surveillance for West Nile virus and other flaviviruses in febrile patients, encephalitic patients, and asymptomatic blood donors in northern Mexico. Vector Borne Zoonotic Dis. 10: 151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sanchez M. D., et al. 2005. Characterization of neutralizing antibodies to West Nile virus. Virology 336: 70–82 [DOI] [PubMed] [Google Scholar]
- 65. Schlesinger J. J., Chapman S. 1995. Neutralizing F(ab′)2 fragments of protective monoclonal antibodies to yellow fever virus (YF) envelope protein fail to protect mice against lethal YF encephalitis. J. Gen. Virol. 76: 217–220 [DOI] [PubMed] [Google Scholar]
- 66. Schmaljohn A. L., Johnson E. D., Dalrymple J. M., Cole G. A. 1982. Non-neutralizing monoclonal antibodies can prevent lethal alphavirus encephalitis. Nature 297: 70–72 [DOI] [PubMed] [Google Scholar]
- 67. Sejvar J. J., et al. 2003. Neurologic manifestations and outcome of West Nile virus infection. JAMA 290: 511–515 [DOI] [PubMed] [Google Scholar]
- 68. Shrestha B., Samuel M. A., Diamond M. S. 2006. CD8+ T cells require perforin to clear West Nile virus from infected neurons. J. Virol. 80: 119–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stiasny K., Kiermayr S., Holzmann H., Heinz F. X. 2006. Cryptic properties of a cluster of dominant flavivirus cross-reactive antigenic sites. J. Virol. 80: 9557–9568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sukupolvi-Petty S., et al. 2010. Structure and function analysis of therapeutic monoclonal antibodies against dengue virus type 2. J. Virol. 84: 9227–9239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sultana H., et al. 2009. Fusion loop peptide of the West Nile virus envelope protein is essential for pathogenesis and is recognized by a therapeutic cross-reactive human monoclonal antibody. J. Immunol. 183: 650–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tao M. H., Morrison S. L. 1989. Studies of aglycosylated chimeric mouse-human IgG. Role of carbohydrate in the structure and effector functions mediated by the human IgG constant region. J. Immunol. 143: 2595–2601 [PubMed] [Google Scholar]
- 73. Teixeira H. C., Kaufmann S. H. 1994. Role of NK1.1+ cells in experimental listeriosis. NK1+ cells are early IFN-gamma producers but impair resistance to Listeria monocytogenes infection. J. Immunol. 152: 1873–1882 [PubMed] [Google Scholar]
- 74. Tesh R. B., et al. 2002. Efficacy of killed virus vaccine, live attenuated chimeric virus vaccine, and passive immunization for prevention of West Nile virus encephalitis in hamster model. Emerg. Infect. Dis. 8: 1392–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Throsby M., et al. 2006. Isolation and characterization of human monoclonal antibodies from individuals infected with West Nile Virus. J. Virol. 80: 6982–6992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tyler K. L., Mann M. A., Fields B. N., Virgin H. W., IV 1993. Protective antireovirus monoclonal antibodies and their effects on viral pathogenesis. J. Virol. 67: 3446–3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. van Rooijen N. 1994. Liposome mediated modulation of macrophage functions. Adv. Exp. Med. Biol. 355: 69–74 [DOI] [PubMed] [Google Scholar]
- 78. van Rooijen N., Hendrikx E. 2010. Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol. Biol. 605: 189–203 [DOI] [PubMed] [Google Scholar]
- 79. Van Rooijen N., Sanders A. 1994. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 174: 83–93 [DOI] [PubMed] [Google Scholar]
- 80. Vogt M. R., et al. 2009. Human monoclonal antibodies induced by natural infection against West Nile virus neutralize at a postattachment step. J. Virol. 83: 6494–6507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wright D. E., Colaco S., Colaco C., Stevenson P. G. 2009. Antibody limits in vivo murid herpesvirus-4 replication by IgG Fc receptor-dependent functions. J. Gen. Virol. 90: 2592–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yu I. M., et al. 2008. Structure of the immature dengue virus at low pH primes proteolytic maturation. Science 319: 1834–1837 [DOI] [PubMed] [Google Scholar]
- 83. Zhang S., et al. 2009. The development of resistance to passive therapy by a potently neutralizing humanized West Nile virus monoclonal antibody. J. Infect. Dis. 200: 202–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhang Y., et al. 2003. Structures of immature flavivirus particles. EMBO J. 22: 2604–2613 [DOI] [PMC free article] [PubMed] [Google Scholar]