Abstract
Nonstructural protein σ1s is a critical determinant of hematogenous dissemination by type 1 reoviruses, which reach the central nervous system (CNS) by a strictly blood-borne route. However, it is not known whether σ1s contributes to neuropathogenesis of type 3 reoviruses, which disseminate by both vascular and neural pathways. Using isogenic type 3 viruses that vary only in σ1s expression, we observed that mice survived at a higher frequency following hind-limb inoculation with σ1s-null virus than when inoculated with wild-type virus. This finding suggests that σ1s is essential for reovirus virulence when inoculated at a site that requires systemic spread to cause disease. Wild-type and σ1s-null viruses produced comparable titers in the spinal cord, suggesting that σ1s is dispensable for invasion of the CNS. Although the two viruses ultimately achieved similar peak titers in the brain, loads of wild-type virus were substantially greater than those of the σ1s-null mutant at early times after inoculation. In contrast, wild-type virus produced substantially higher titers than the σ1s-null virus in peripheral organs to which reovirus spreads via the blood, including the heart, intestine, liver, and spleen. Concordantly, viral titers in the blood were higher following infection with wild-type virus than following infection with the σ1s-null mutant. These results suggest that differences in viral brain titers at early time points postinfection are due to limited virus delivery to the brain by hematogenous pathways. Transection of the sciatic nerve prior to hind-limb inoculation diminished viral spread to the spinal cord. However, wild-type virus retained the capacity to disseminate to the brain following sciatic nerve transection, indicating that wild-type reovirus can spread to the brain by the blood. Together, these results indicate that σ1s is not required for reovirus spread by neural mechanisms. Instead, σ1s mediates hematogenous dissemination within the infected host, which is required for full reovirus neurovirulence.
INTRODUCTION
Many viral diseases occur as a consequence of systemic dissemination within the infected host. Some viruses, such as herpes simplex virus (20, 28) and rabies virus (3, 39), spread within their hosts by neural routes. Others, including human immunodeficiency virus (42) and measles virus (47), use hematogenous pathways to spread systemically. Although the general principles of virus dissemination are understood, little is known about the viral and cellular determinants that govern virus spread. Defining the mechanisms used by viruses to disseminate within their hosts is essential to an understanding of how viruses cause systemic disease and may foster development of therapeutics that arrest viral replication prior to the seeding of target tissues.
Mammalian orthoreoviruses (reoviruses) are highly tractable models for studies of viral pathogenesis. Reoviruses are nonenveloped, icosahedral viruses that contain 10 segments of double-stranded RNA (dsRNA) (24). In newborn mice, type 1 and type 3 reoviruses invade the central nervous system (CNS) following oral or intramuscular inoculation but use different routes and produce distinct pathological consequences. Type 1 reoviruses access the CNS by hematogenous routes and infect ependymal cells, causing ependymitis and hydrocephalus (41, 45, 46). Type 3 reoviruses spread to the CNS by neural routes (41) and infect neurons, causing lethal encephalitis (22, 41). However, type 3 reoviruses also use hematogenous routes to disseminate to other organs, including the heart, liver, and spleen (1, 12). Serotype-specific differences in neurotropism and disease segregate with the viral S1 dsRNA gene segment (10, 38), which encodes attachment protein σ1 and nonstructural protein σ1s (33, 44). Receptor engagement is critical for target cell selection by many viruses, suggesting that the σ1 attachment protein is the primary determinant of viral tropism. However, σ1s is required for hematogenous dissemination of type 1 reovirus to sites of secondary replication in mice (6). Because of the serotype-specific differences in reovirus tropism, routes of spread, and outcome of infection, it is possible that the σ1s protein from different serotypes mediates serotype-specific functions.
Protein σ1s is a 14-kDa nonstructural protein encoded by the viral S1 gene segment (7, 11, 33). The σ1s open reading frame (ORF) completely overlaps the σ1 coding sequence; however, σ1s lies in a different reading frame (7–9, 11, 33). Little amino acid sequence identity exists among the σ1s proteins from the different reovirus serotypes (7, 9). The only feature of the σ1s protein that is conserved across the serotypes is a cluster of positively charged amino acids near the amino terminus (7, 9). For type 3 reovirus, this cluster functions as a nuclear localization signal (15). The σ1s protein has been implicated in reovirus-induced cell cycle arrest at the G2/M boundary (29, 30) and may function in reovirus neurovirulence by influencing reovirus-induced apoptosis in the murine CNS (16). However, interpreting these studies of σ1s function is complicated because the σ1s-null mutant virus used in previous experiments is not isogenic to the parental strain from which it was derived (32). Thus, a role for σ1s in the pathogenesis of type 3 reovirus is undefined.
In this study, we used plasmid-based reverse genetics to generate a σ1s-null reovirus to determine how σ1s influences systemic dissemination of type 3 reovirus. Following intramuscular inoculation, mice infected with the σ1s-null mutant survive at a higher frequency than those infected with wild-type virus. This result suggests that σ1s is a determinant of reovirus virulence when reovirus is inoculated at a site that requires systemic dissemination. Wild-type and mutant viruses produced equivalent titers in the spinal cord, indicating that σ1s is not required for reovirus invasion of the CNS. Although the two viruses produced similar peak titers in the brain, titers of the σ1s-null mutant in the brain were markedly lower than those of wild-type virus at early times postinfection. Viral blood titers were substantially higher following infection with wild-type virus than those produced by the σ1s-null mutant. In addition, titers of σ1s-null virus in organs that reovirus accesses via the bloodstream, including the heart, intestine, liver, and spleen, were substantially lower than those produced by wild-type virus. These data suggest that σ1s is essential for reovirus hematogenous spread within an infected host but dispensable for dissemination by neural routes. Sectioning the sciatic nerve prior to hind-limb inoculation diminished but did not eliminate spread of wild-type virus to the brain. However, dissemination of σ1s-null virus to the brain was almost completely abolished following sciatic nerve transection. These data indicate that reovirus accesses the brain by a combination of hematogenous and neural routes. Collectively, the results described in this study suggest that reovirus trafficking to the brain via hematogenous dissemination precedes spread by neural routes and that blood-borne viral transport is essential for reovirus neurovirulence.
MATERIALS AND METHODS
Cell lines.
HeLa cells were maintained in Dulbecco modified Eagle medium supplemented to contain 10% fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/ml of penicillin, 100 μg/ml streptomycin, and 25 ng/ml of amphotericin B (Invitrogen). L929 cells were maintained in Joklik's minimum essential medium supplemented to contain 10% FBS, 2 mM l-glutamine, 100 U/ml of penicillin, 100 μg/ml streptomycin, and 25 ng/ml of amphotericin B.
Viruses.
Recombinant reoviruses were generated using plasmid-based reverse genetics (19). Monolayers of L929 cells at approximately 90% confluence (3 × 106 cells) in 60-mm-diameter dishes (Corning) were infected with rDIs-T7 pol at a multiplicity of infection (MOI) of ∼0.5 50% tissue culture infective doses (TCID50) per cell. At 1 h postinfection, cells were cotransfected with nine plasmid constructs representing cloned gene segments from the strain type 3 Dearing (T3D) genome—pT7-L1T3D (2 μg), pT7-L2T3D (2 μg), pT7-L3T3D (2 μg), pT7-M1T3D (1.75 μg), pT7-M2T3D (1.75 μg), pT7-M3T3D (1.75 μg), pT7-S2T3D (1.5 μg), pT7-S3T3D (1.5 μg), and pT7-S4T3D (1.5 μg)—in combination with 2 μg of pBacT7-S1T3D or pBacT7-S1T3D σ1s-null. For each, 3 μl of TransIT-LT1 transfection reagent (Mirus) was used per μg of plasmid DNA. Following 5 days of incubation, recombinant virus was isolated from transfected cells by plaque purification using monolayers of L929 cells (43). For generation of σ1s-deficient virus, pBacT7-S1T3D (T3D S1; GenBank accession number HM159619) was altered by QuikChange (Stratagene) site-directed mutagenesis. To confirm sequences of the mutant virus, viral RNA was extracted from purified virions and subjected to OneStep reverse transcription-PCR (RT-PCR) (Qiagen) using S1-specific primers. Primer sequences are available from the corresponding author upon request. PCR products were analyzed following electrophoresis in Tris-borate-EDTA agarose gels or purified and subjected directly to sequence analysis. The presence of a noncoding signature mutation in the L1 gene of viruses generated by plasmid-based rescue was confirmed using RT-PCR and L1-specific primers (19).
Purified reovirus virions were generated using second- or third-passage L929 cell lysate stocks of twice-plaque-purified reovirus as described previously (13). Viral particles were Freon extracted from infected cell lysates, layered onto 1.2- to 1.4-g/cm3 CsCl gradients, and centrifuged at 62,000 × g for 18 h. Bands corresponding to virions (1.36 g/cm3) (36) were collected and dialyzed in virion storage buffer (150 mM NaCl, 15 mM MgCl2, 10 mM Tris-HCl [pH 7.4]). The concentration of reovirus virions in purified preparations was determined from the following equivalence: 1 optical density at 260 nm (OD260) unit = 2.1 × 1012 virions (36). Viral titer was determined by plaque assay using L929 cells (43).
Virus replication assays.
Monolayers of cells in 24-well plates (Corning) were adsorbed in triplicate with each reovirus strain at an MOI of 0.01, 0.1, or 1 PFU/cell at room temperature for 1 h in serum-free medium, washed once with phosphate-buffered saline (PBS), and incubated in serum-containing medium for various intervals. Cells were frozen and thawed twice prior to determination of viral titer by plaque assay using L929 cells (43). Viral yields were calculated according to the following formula: log10yieldtx = log10(PFU/ml)tx − log10(PFU/ml)t0, where tx is the time postinfection.
Assessment of σ1s expression by indirect immunofluorescence.
Monolayers of HeLa cells (2 × 105 cells/well) grown on coverslips in 24-well plates were adsorbed with reovirus at an MOI of 50 PFU/cell at room temperature for 1 h. Following removal of the inoculum, cells were washed with PBS and incubated in complete medium at 37°C for 18 h to permit completion of a single cycle of viral replication. Monolayers were fixed with 1 ml of methanol at −20°C for at least 30 min, washed twice with PBS, and blocked with 0.1% gelatin, 0.1% Tween 20 (Sigma), and 20% normal goat serum (Vector Laboratories) in PBS. For detection of σ1s, cells were washed with PBS and incubated with mouse monoclonal anti-σ1s antibody 2F4 (32) at a dilution of 1:500 in PBS with 0.1% gelatin, 2% normal goat serum, and 0.1% Tween 20. For detection of reovirus proteins, cells were washed once with PBS and stained with polyclonal rabbit anti-reovirus serum at a 1:1,000 dilution in PBS–0.5% Triton X-100 at room temperature for 1 h. Monolayers were washed twice with PBS–0.5% Triton X-100 and incubated with a 1:1,000 dilution of Alexa 488- or Alexa 546-labeled anti-rabbit or anti-mouse IgG (Invitrogen), respectively. Monolayers were washed with PBS, and infected cells were visualized by indirect immunofluorescence using an Axiovert 200 fluorescence microscope (Carl Zeiss).
Infection of mice.
C57BL/6J mice were obtained from Jackson Laboratory. Swiss Webster mice were obtained from Harlan Biosciences. Animal husbandry and experimental procedures were performed in accordance with Public Health Service policy and approved by the Vanderbilt University School of Medicine Institutional Animal Care and Use Committee.
Two-day-old mice were inoculated intramuscularly or intracranially with purified reovirus diluted in PBS. Intramuscular inoculations (10 μl) were delivered into the left hind limb (hamstring muscle) using a Hamilton syringe and 30-gauge needle. Intracranial inoculations (5 μl) were delivered into the left cerebral hemisphere using a Hamilton syringe and 30-gauge needle (40). For analysis of viral virulence, mice were monitored for weight loss and symptoms of disease for 25 days postinoculation. For survival experiments, mice were euthanized when found to be moribund (defined by rapid or shallow breathing, lethargy, or paralysis). Death was not used as an endpoint. Data from these experiments are reported as “percent survival.” For analysis of virus replication, mice were euthanized at various intervals following inoculation, and organs were collected into 1 ml of PBS and homogenized by freezing, thawing, and sonication. For analysis of viremia, mice were euthanized and decapitated at various intervals following inoculation, and whole blood was collected from the neck into a 1-ml syringe containing 100 μl Alsever's solution (Sigma). Viral titers in organ homogenates were determined by plaque assay using L929 cells.
Sciatic nerve sectioning.
Two-day-old C57/BL6 mice were anesthetized by hypothermia. The proper depth of anesthesia was assessed visually by absence of a withdrawal reflex and lack of response to external stimuli. The area around the incision site was cleaned with Betadine using a cotton swab. A 0.5-cm incision was made along the back of the thigh using surgical scissors (Fine Science Tools). The sciatic nerve was isolated using forceps (Fine Science Tools), and a segment of the nerve was excised using surgical scissors. The incision was closed by bringing the skin flaps into apposition and applying liquid skin (Webster Veterinary). The pups were warmed gradually by placement in a room temperature cage. Following recovery of activity, pups were moved to a cage that had been placed atop a warming pad (Braintree Scientific). Pups were then inoculated intramuscularly with 1 × 106 PFU of rsT3D or rsT3D σ1s-null reovirus prior to returning them to the dam. Mice were euthanized at 2 or 4 days following inoculation, and organs were collected into 1 ml of PBS and homogenized by freezing, thawing, and sonication.
Quantification of viral RNA using RT-qPCR.
Total RNA was extracted from 200 μl of whole blood/Alsever's mixture using the High Pure viral RNA kit (Roche). RNA was eluted into a final volume of 40 μl. Reverse transcription-quantitative PCR (RT-qPCR) was performed using the ABI 7000 sequence detection system (Applied Biosystems) and EZ RT-PCR System (Roche) according to the manufacturer's instructions with minor modifications. Reovirus RNA was quantified using 10 μl of RNA extract. Forward (S4 83F, 5′-CGCTTTTGAAGGTCGTGTATCA-3′) and reverse (S4 153R, 5′-CTGGCTGTGCTGAGATTGTTTT-3′) primers corresponding to the viral S4 gene were used for reverse transcription and quantitative PCR amplification. The S4-specific fluorogenic probe used was 5′-dFAM-AGCGCGCAAGAGGGATGGGA-BHQ-1-3′ (Biosearch Technologies). Reverse transcription was performed at 50°C for 2 min, followed by incubation at 60°C for 30 min. The reaction was terminated by incubation at 95°C for 5 min. Subsequently, 40 cycles of quantitative PCR were performed at 95°C for 15 s followed by incubation at 60°C for 30 s. Standard curves relating threshold cycle values to copies of plasmid DNA template were generated using 10-fold dilutions of a T3D S4-encoding plasmid (pT7-T3D S4) (19). The concentration of viral RNA in each sample was extrapolated from standard curves. The final S4 RNA copy number was calculated by multiplying by 4 the copy number obtained by extrapolation from the standard curve to account for using one-quarter of the extracted RNA as a template.
Preparation of murine cortical neuron cultures.
Primary cultures of mouse cortical neurons were established using cerebral cortices of C57/BL6 embryos at developmental day E15 (1). Fetuses were decapitated, brains were removed, and cortical lobes were dissected and submerged in Hanks' balanced salt solution (Gibco) on ice. Cortices were incubated in 0.6 mg/ml trypsin solution at room temperature for 30 min, washed twice, and manually dissociated twice with a Pasteur pipette. Viable cells were plated at a density of 2.75 × 105 cells/ml in 24-well plates or on glass coverslips (BD Biosciences) placed in 24-well plates. Wells were treated prior to plating with a 10-μg/ml poly-d-lysine solution (BD Biosciences) and a 1.64-μg/ml laminin solution (BD Biosciences). Cultures were incubated for the first 24 h in neurobasal medium (Gibco) supplemented to contain 10% FBS (Gibco), 0.6 mM l-glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin. Cultures were thereafter maintained in neurobasal medium supplemented to contain 1× B27 (Gibco), 50 U/ml penicillin, and 50 μg/ml streptomycin. One-half of the medium was replaced with fresh medium every 3 to 4 days. Neurons were allowed to mature for 7 days prior to use.
Statistical analysis.
A log-rank test was used for comparison of survival curves. For experiments in which viral titers were determined in an organ or blood, the Mann-Whitney test was used to calculate two-tailed P values. This test is appropriate for experimental data that display a non-Gaussian distribution (31). When all values are less than the limit of detection, a Mann-Whitney test P value cannot be calculated. Statistical analyses were performed using Prism software (GraphPad Software, Inc.).
RESULTS
Construction and characterization of a σ1s-deficient type 3 reovirus.
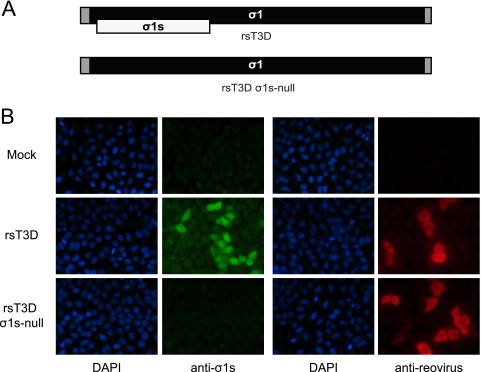
To determine the function of σ1s in the pathogenesis of type 3 reovirus, we used plasmid-based reverse genetics to engineer a recombinant reovirus deficient in σ1s expression (19). The start codon for the σ1s open reading frame was disrupted by introducing a single nucleotide change (71AUG to 71ACG) into the plasmid encoding the cDNA for the S1 gene segment derived from prototype type 3 reovirus strain T3D (Fig. 1A). This mutation alters the σ1s translational start site; however, the coding sequence of the overlapping σ1 open reading frame is not affected. Viable viruses were recovered that contain nine gene segments from T3D in combination with either the wild-type or σ1s-null T3D S1 gene segment. Analysis of genomic dsRNA from each virus verified that the rescued viruses contain the expected combination of gene segments (data not shown). The sequence of the S1 gene segment and the integrity of the σ1s translational start site from each virus were confirmed by direct sequencing of viral RNA using serotype-specific S1 primers (data not shown). The S1 genes of both strains contained no additional mutations.
Fig. 1.
Construction and characterization of a σ1s-deficient type 3 reovirus. (A) Schematic of the reovirus S1 gene segment. The σ1 ORF is shown in black, the σ1s ORF in white, and the 5′ and 3′ untranslated regions (UTRs) in gray. The wild-type (upper panel) and σ1s-null (lower panel) S1 gene alleles are shown. (B) rsT3D σ1s-null does not express the σ1s protein. HeLa cells were either mock infected or adsorbed with rsT3D or rsT3D σ1s-null at an MOI of 10 PFU/cell. At 24 h postinfection, cells were fixed and stained with a T3D σ1s-specific monoclonal antibody or reovirus-specific polyclonal antiserum. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI).
To confirm that altering the σ1s translational start site prevents σ1s synthesis, we assessed σ1s expression following infection of HeLa cells with rsT3D or rsT3D σ1s-null by indirect immunofluorescence using T3D σ1s-specific monoclonal antibody 2F4 (Fig. 1B). At 24 h postinfection, σ1s protein was detected in cells infected with wild-type virus but not in those infected with the σ1s-null mutant. In parallel, staining with reovirus-specific polyclonal antiserum showed equivalent levels of infection for both viruses. Thus, rsT3D and rsT3D σ1s-null are isogenic viruses that differ only in σ1s expression.
The σ1s protein is not required for reovirus replication in cell culture.
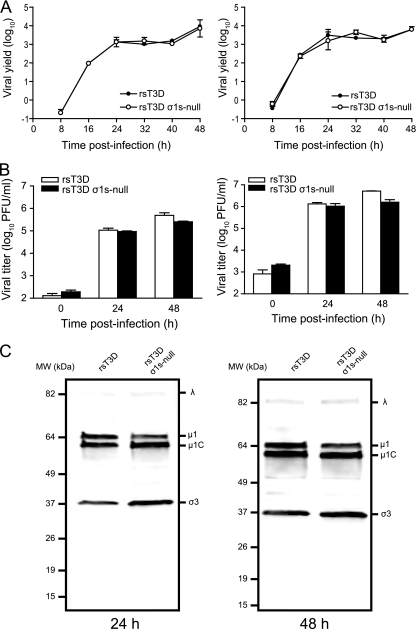
To determine whether σ1s influences reovirus growth in cell culture, we quantified viral yields following infection of mouse L929 cells (Fig. 2A). Cells were infected with rsT3D or rsT3D σ1s-null at MOIs of 0.01 or 1 PFU per cell, and viral titers were determined by plaque assay over a 48-h time course. At each MOI tested, the replication kinetics and yields of viral progeny for the σ1s-null virus were indistinguishable from those of wild-type virus. Furthermore, no differences in replication kinetics or viral yields were observed between wild-type and σ1s-deficient viruses following infection of HeLa cells at an MOI of 0.1 or 1 PFU per cell (Fig. 2B). To determine whether σ1s expression contributes to reovirus protein production, we assessed steady-state viral protein levels by immunoblotting using reovirus-specific antiserum after infection of murine L929 cells with rsT3D or rsT3D σ1s-null (Fig. 2C). At both 24 and 48 h postinfection, no differences in protein levels were observed between the wild-type and mutant viruses. Together, these data are consistent with previous studies showing that σ1s is dispensable for reovirus replication in cultured cells (6, 32).
Fig. 2.
The σ1s protein is dispensable for reovirus growth in cell culture. (A) L cells were adsorbed with rsT3D or rsT3D σ1s-null at MOIs of 0.01 (left) or 1 (right) PFU/cell. (B) HeLa cells were adsorbed with rsT3D or rsT3D σ1s-null at MOIs of 0.1 (left) or 1 (right) PFU/cell. Titers of virus in cell lysates were determined by plaque assay at the indicated times postinfection. Results are expressed as mean viral yield or titers for triplicate samples. Error bars indicate standard deviations. (C) Expression of reovirus proteins by wild-type and σ1s-null viruses. Whole-cell lysates from infected cells were immunoblotted using reovirus-specific antiserum.
The σ1s protein is a determinant of reovirus virulence following intramuscular inoculation.
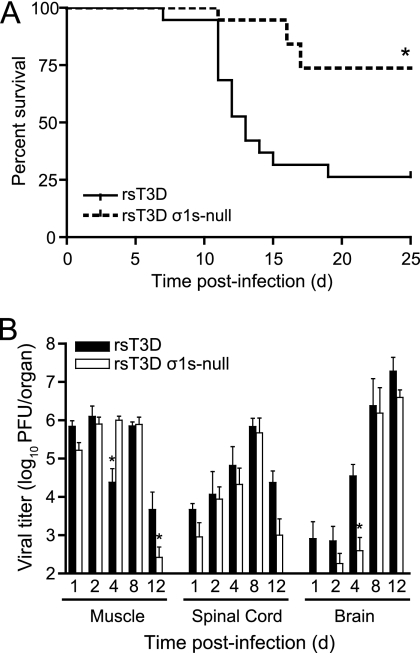
To determine whether σ1s contributes to reovirus virulence, we inoculated newborn C57/BL6 mice intramuscularly with 106 PFU of rsT3D or rsT3D σ1s-null (Fig. 3A). Reovirus normally infects by the oral route, and spread to the CNS is required for reovirus-induced disease. However, reovirus strain T3D replicates poorly in the gastrointestinal tract due to cleavage of its attachment protein by intestinal proteases (4, 5, 23). Inoculation of type 3 reoviruses intramuscularly leads to invasion of the brain by neural routes (41). Infected mice were monitored for signs of disease and euthanized when moribund. Approximately 75% of mice infected with rsT3D succumbed to infection, whereas only 25% of mice died following infection with rsT3D σ1s-null. These data indicate that σ1s influences reovirus virulence following inoculation of the virus at a peripheral site.
Fig. 3.
(A) The σ1s protein enhances reovirus virulence following intramuscular inoculation. Newborn C57/BL6 mice were inoculated in the left hind limb with 106 PFU of rsT3D or rsT3D σ1s-null. Mice (n = 19 for each virus strain) were monitored for survival for 25 days. *, P < 0.001 as determined by log-rank test in comparison to rsT3D. (B) The σ1s protein is not required for reovirus spread by neural routes. Newborn C57/BL6 mice were inoculated in the left hind limb with 106 PFU of rsT3D or rsT3D σ1s-null. At days 1, 2, 4, 8, and 12 postinoculation, mice were euthanized, hind-limb muscle, spinal cord, and brain were resected, and viral titers were determined by plaque assay. Results are expressed as mean viral titers for 6 to 9 animals for each time point. Error bars indicate standard errors of the means. *, P < 0.05 as determined by Mann-Whitney test in comparison to rsT3D.
The σ1s protein is dispensable for reovirus spread to the CNS by neural routes.
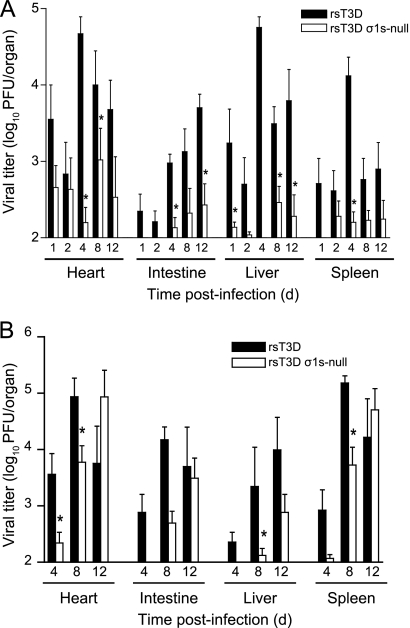
To determine whether σ1s is required for reovirus transmission by neural routes, we quantified viral titers in hind limb muscle, spinal cord, and brain at days 4, 8, and 12 following intramuscular inoculation of 106 PFU of rsT3D or rsT3D σ1s-null virus (Fig. 3B). In the hind-limb muscle, the σ1s-null virus produced higher titers than wild-type virus at day 4. Titers at day 8 were equivalent for the two viruses. At day 12, higher titers of the wild-type virus than of the σ1s-null mutant were detected. These data indicate that the levels of infection were comparable between the wild-type virus and σ1s-null mutant at the site of inoculation.
In the spinal cord, titers of wild-type and σ1s-null viruses at days 4 and 8 were comparable. At day 12, wild-type virus produced higher titers than mutant virus, but this difference was not statistically significant. In the brain, titers of wild-type virus were greater than those of the σ1s-null mutant at days 1, 2, and 4. However, titers of the two viruses were equivalent at days 8 and 12. Both viruses reached a peak titer approaching 107 PFU/brain. Sequence analysis of the S1 gene from 5 viruses recovered from the spinal cord and brain of mice inoculated with rsT3D σ1s-null indicate that the mutant virus retained the σ1s-null mutation. This finding indicates that the σ1s-null virus is stable in the murine CNS. Together, these data suggest that the σ1s protein is not required for reovirus spread by neural routes. Rather, σ1s appears to either facilitate reovirus transport to the brain or enhance viral replication at that site.
The σ1s protein modestly enhances reovirus neurovirulence following intracranial inoculation.
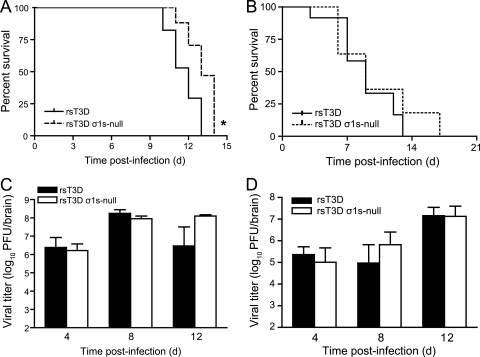
To determine whether σ1s influences reovirus virulence in the murine CNS, we inoculated newborn C57/BL6 mice intracranially with 100 PFU of rsT3D or rsT3D σ1s-null (Fig. 4A). Mice were monitored for signs of disease and euthanized when moribund. Animals infected with either virus developed clinical signs of encephalitis and succumbed to infection. However, mice infected with rsT3D σ1s-null survived approximately 1 day longer than those inoculated with wild-type virus. The average survival times for mice infected with rsT3D or rsT3D σ1s-null were 12 or 13 days, respectively. Similar results were obtained in experiments performed using Swiss Webster mice (Fig. 4B). The average survival time for mice infected with rsT3D σ1s-null was slightly longer than that for rsT3D-infected mice. However, the difference was not statistically significant. Together, these data suggest that σ1s contributes only modestly to reovirus virulence following intracranial inoculation.
Fig. 4.
(A and B) The σ1s protein modestly enhances reovirus neurovirulence after intracranial inoculation. (A) Newborn C57/BL6 mice were inoculated intracranially with 100 PFU of rsT3D or rsT3D σ1s-null. Mice (n = 17 for each virus strain) were monitored for survival for 14 days. *, P < 0.005 as determined by log-rank test in comparison to rsT3D. (B) Newborn Swiss Webster ND4 mice were inoculated intracranially with 100 PFU of rsT3D or rsT3D σ1s-null. Mice (n = 8 to 12) were monitored for survival for 17 days. (C and D) The σ1s protein is dispensable for reovirus replication in the CNS. (C) Newborn C57/BL6 mice were inoculated intracranially with 100 PFU of rsT3D or rsT3D σ1s-null. At days 4, 8, and 12 postinoculation, viral titers in the brain were determined by plaque assay. Results are expressed as mean viral titers for 5 to 8 animals for each time point. Error bars indicate standard errors of the means. (D) Newborn Swiss Webster ND4 mice were inoculated intracranially with 100 PFU of rsT3D or rsT3D σ1s-null. At days 4, 8, and 12 postinoculation, viral titers in the brain were determined by plaque assay. Results are expressed as mean viral titers for 5 to 8 animals for each time point. Error bars indicate standard errors of the means.
The σ1s protein is not required for reovirus replication in the murine CNS.
To determine whether σ1s expression is required for reovirus replication within the murine CNS, we inoculated newborn C57/BL6 mice intracranially with 100 PFU of rsT3D or rsT3D σ1s-null and quantified viral titers in the brain at days 4, 8, and 12 postinoculation (Fig. 4C). Wild-type and σ1s-null viruses produced equivalent titers at days 4 and 8. At day 12, the σ1s-null mutant produced higher titers than wild-type virus, although the difference was not statistically significant. Similar results were obtained following intracranial inoculation of Swiss Webster mice (Fig. 4D). Titers of wild-type and σ1s-null viruses were equivalent in the brain at each time point assessed. These data indicate that σ1s is dispensable for reovirus replication in the murine CNS.
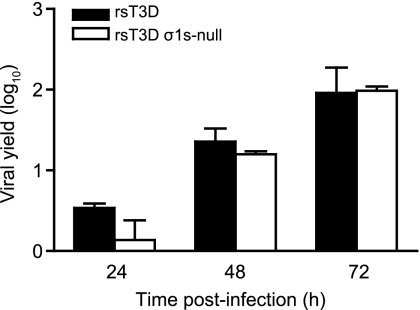
To test directly whether σ1s influences reovirus replication in neurons, we compared replication of wild-type and σ1s-null virus in primary cultures of murine cortical neurons (Fig. 5). Viral yields following infection of primary neuronal cultures were quantified at 24, 48, and 72 h following infection with rsT3D or rsT3D σ1s-null at an MOI of 1 PFU per cell. Wild-type virus produced slightly higher (although not statistically significant) yields than the σ1s-null virus at 24 h postinfection. However, at 48 and 72 h, yields of the σ1s-null virus were indistinguishable from those of wild-type virus. Thus, σ1s is dispensable for reovirus replication in neurons.
Fig. 5.
The σ1s protein is not required for reovirus replication in primary mouse neurons. Cortical neurons were harvested from C57/BL6 mouse embryos at developmental day E15 and cultured for 7 days prior to infection. Neurons were adsorbed with rsT3D or rsT3D σ1s-null at an MOI of 1 PFU/cell. Titers of virus in cell lysates were determined by plaque assay at the indicated times postinfection. Results are expressed as mean viral yield for triplicate samples. Error bars indicate standard deviations.
The σ1s protein enhances reovirus dissemination by hematogenous routes.
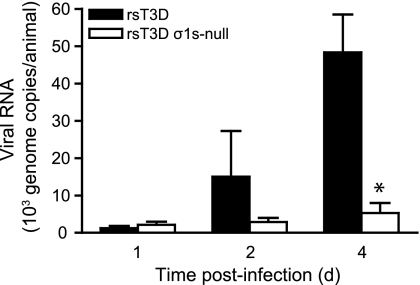
To determine whether σ1s is required for reovirus spread by the bloodstream, newborn C57/BL6 mice were inoculated intramuscularly with 106 PFU of rsT3D or rsT3D σ1s-null, and viral loads in the blood were quantified by RT-qPCR at days 1, 2, and 4 postinoculation (Fig. 6). Low levels of both viruses were detected at day 1. However, although levels of wild-type virus increased substantially by days 2 and 4, loads of the σ1s-null virus in the blood remained low at these time points. By day 4, loads of wild-type virus were approximately 10-fold higher than those of the σ1s-null mutant. These data indicate that σ1s functions to promote the establishment of reovirus viremia.
Fig. 6.
The σ1s protein is required for the establishment of reovirus viremia. Newborn C57/BL6 mice were inoculated intramuscularly with 106 PFU of rsT3D or rsT3D σ1s-null. At days 1, 2, or 4 postinoculation, mice were euthanized, blood was collected, and viral genome copies in blood were determined by RT-qPCR. Results are expressed as mean viral genome copies per animal for 3 to 5 animals at each time point. Error bars indicate standard errors of the means. *, P < 0.05 as determined by Mann-Whitney test in comparison to rsT3D.
To determine whether σ1s is required for hematogenous spread of type 3 reovirus to tissues that support secondary rounds of viral replication, we inoculated newborn C57/BL6 mice intramuscularly with 106 PFU of rsT3D or rsT3D σ1s-null virus (Fig. 7A). Viral titers were quantified in peripheral organs that reovirus infects via the bloodstream, including heart, liver, intestine, and spleen. At each time point assessed, titers of wild-type virus in the organs assayed were greater than those of the σ1s-null virus. To determine whether σ1s is required for hematogenous reovirus spread following intracranial inoculation, we inoculated newborn C57/BL6 mice intracranially with 100 PFU of rsT3D or rsT3D σ1s-null and determined titers in the heart, intestine, liver, and spleen at days 4, 8, and 12 postinoculation (Fig. 7B). Again, titers of wild-type virus were higher than those of σ1s-deficient virus in each of the target organs tested at days 4 and 8. Only at day 12 did titers of the σ1s-null mutant approximate, or in some cases exceed, those of wild-type virus. These data suggest that σ1s is required for efficient reovirus spread by hematogenous routes, regardless of the site of inoculation.
Fig. 7.
(A) The σ1s protein is required for hematogenous reovirus dissemination following intramuscular inoculation. Newborn C57/BL6 mice were inoculated in the left hind limb with 106 PFU of rsT3D or rsT3D σ1s-null. At days 1, 2, 4, 8, and 12 postinoculation, mice were euthanized, the organs shown were resected, and viral titers were determined by plaque assay. Results are expressed as mean viral titers for 6 to 9 animals for each time point. Error bars indicate standard errors of the means. *, P < 0.05 as determined by Mann-Whitney test in comparison to rsT3D. (B) The σ1s protein enhances reovirus dissemination following intracranial inoculation. Newborn C57/BL6 mice were inoculated intracranially with 100 PFU of rsT3D or rsT3D σ1s-null. At days 4, 8, and 12 postinoculation, mice were euthanized, the organs shown were resected, and viral titers were determined by plaque assay. Results are expressed as mean viral titers for 5 to 8 animals for each time point. Error bars indicate SEM. *, P < 0.05 as determined by Mann-Whitney test in comparison to rsT3D.
Analysis of S1 gene sequences of virus isolates from organs containing disseminated σ1s-null virus following intracranial inoculation revealed a reversion to wild-type sequence in 20 of 47 viruses tested (43%). These data provide further support for the contention that σ1s acts to facilitate hematogenous reovirus dissemination.
Reovirus disseminates to the brain by hematogenous and neural routes.
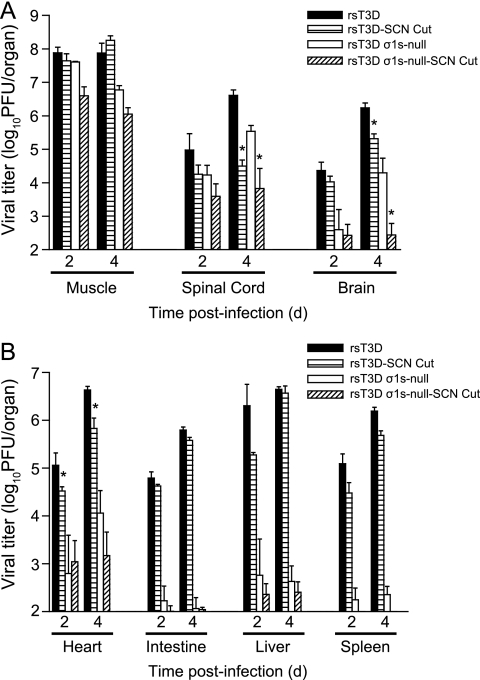
To determine whether type 3 reovirus can disseminate to the brain using hematogenous pathways, we transected the sciatic nerve prior to hind limb inoculation. The sciatic nerve is the principal neural conduit by which reovirus spreads from the hind limb to the spinal cord (41). Sectioning the sciatic nerve should inhibit virus transmission to the CNS by neural routes but not affect spread by hematogenous pathways (41). The left sciatic nerve of two-day-old C57/BL6 mice was transected prior to inoculation in the left hind limb muscle with 106 PFU of rsT3D or rsT3D σ1s-null virus. In parallel, mice that were not subjected to sciatic nerve section were inoculated intramuscularly with 106 PFU of rsT3D or rsT3D σ1s-null virus. At 2 and 4 days postinoculation, viral titers were quantified in the hind limb muscle, spinal cord, brain, heart, intestine, liver, and spleen (Fig. 8). Titers of wild-type and σ1s-null virus at the site of inoculation were unaffected by the neurectomy (Fig. 8A). However, sciatic nerve sectioning resulted in a modest decrease in spinal cord titers of both viruses at day 2 and a 100-fold decrease at day 4 in comparison to those in mice with intact sciatic nerves. This finding suggests that viral spread to the spinal cord by neural pathways was successfully interrupted by sciatic nerve transection. The residual virus in the spinal cord likely results from spread via the femoral nerve, which enervates the quadriceps muscle that opposes the hamstring muscle into which virus was inoculated.
Fig. 8.
Reovirus disseminates to the CNS by hematogenous and neural routes. The left sciatic nerve of newborn C57/BL6 mice was sectioned prior to inoculation in the left hind limb with 106 PFU of rsT3D or rsT3D σ1s-null. In parallel, mice in which the left sciatic nerve was not sectioned were inoculated in the left hind limb with 106 PFU of rsT3D or rsT3D σ1s-null. At days 2 and 4 postinoculation, mice were euthanized, hind limb muscle, spinal cord, and brain (A) and heart, intestine, liver, and spleen (B) were resected, and viral titers were determined by plaque assay. Results are expressed as mean viral titers for 6 animals for each time point. Error bars indicate standard errors of the means. *, P < 0.05 as determined by Mann-Whitney test in comparison to animals in which the sciatic nerve was not sectioned.
Following disruption of neural transmission by sciatic nerve section, rsT3D was still detected in the brain (Fig. 8A). Although titers of wild-type virus in the spinal cord were reduced 100-fold compared to those in controls following sciatic nerve transection, neurectomy reduced viral titers in the brain less than 10-fold. In addition, viral titers in organs that reovirus infects via the blood, including the heart, intestine, liver, and spleen, were largely unaffected by sciatic nerve transection. These findings confirm that spread to these organs is achieved by hematogenous routes and indicate that hematogenous pathways disseminate type 3 reovirus to every organ system in the animal, including the brain. Most strikingly, titers of the σ1s-null virus in the brain were markedly reduced following sciatic nerve section. Thus, type 3 reovirus uses both neural and hematogenous routes to spread to the brain, and σ1s is required for dissemination only by the hematogenous pathway.
DISCUSSION
In this study, we found that nonstructural protein σ1s is a critical determinant of type 3 reovirus dissemination within its host by hematogenous routes but is dispensable for spread by neural pathways. Following intramuscular inoculation of newborn mice, viral loads were markedly higher in the blood of animals infected with wild-type virus than in those inoculated with σ1s-null virus. Concordantly, wild-type virus produced higher titers in organs that reovirus targets via the bloodstream, including the heart, intestine, liver, and spleen. In contrast, wild-type and σ1s-null viruses traffic to the spinal cord with equivalent efficiency. Together, these data support a role for σ1s in promoting reovirus spread through the bloodstream and indicate that σ1s is dispensable for reovirus neurotransmission.
Type 3 reoviruses disseminate within their hosts by hematogenous (1) and neural (41) pathways. Severing the sciatic nerve prior to hind-limb inoculation blocks virus spread to the spinal cord (41). This finding indicates that neural, but not hematogenous, pathways are important for delivering reovirus to the CNS. Consistent with the hypothesis that type 3 reoviruses invade the CNS by neural routes, we found that titers of wild-type and σ1s-null viruses were equivalent in the spinal cord at each time point examined (Fig. 3B). In contrast, viral titers in the brain were substantially higher at day 4 for mice inoculated with wild-type virus in comparison to those infected with the σ1s-null mutant (Fig. 3B). These data suggest that invasion of the brain by type 3 reovirus by neural pathways is preceded by virus delivered hematogenously. At days 8 and 12 postinoculation, titers in the brain were equivalent for wild-type and σ1s-null virus (Fig. 3B), suggesting that the majority of reovirus transport to the brain by neural pathways occurs subsequent to delivery of virus to the brain via the bloodstream.
To determine whether hematogenous mechanisms can disseminate type 3 reovirus to the brain, we interrupted virus transmission via neural routes by sectioning the sciatic nerve prior to hind limb inoculation. Consistent with previous work (41), we found that sciatic nerve transection markedly reduced viral titers in the spinal cord (Fig. 8A), presumably as a consequence of limited neural transmission. However, although brain titers were diminished by sciatic nerve section, significant levels of wild-type virus were detected at this site. In addition, section of the sciatic nerve prior to inoculation with the σ1s-null virus almost completely eliminated its spread to the brain. These results indicate that both hematogenous and neural pathways function to disseminate type 3 reovirus to the brain.
How reovirus accesses the bloodstream after intramuscular inoculation is not known. It also has not been determined whether reovirus disseminates hematogenously using cell-free or cell-associated mechanisms. Following viral replication in the hind limb muscle (12), virus may reach the blood by infecting endothelial cells that form the lymphatic and blood vessels that supply muscle tissue. Alternatively, virus may infect phagocytic cells that subsequently migrate to lymphatic channels and eventually the bloodstream. Based on these possibilities, we think that there are three mechanisms by which σ1s may promote reovirus dissemination following intramuscular inoculation. First, σ1s may be essential for replication in a cell type that is required for hematogenous spread, such as lymphocytes or mononuclear cells. Second, σ1s may trigger apoptosis in cells at the site of viral inoculation (16), which is required for viral release and subsequent bloodstream invasion. Third, σ1s may inhibit some component of the host antiviral response to reovirus infection. It is possible that σ1s disrupts innate or adaptive immune mechanisms that allow reovirus to evade immune detection. In this scenario, the absence of σ1s would lead to more efficient reovirus clearance.
Although titers of wild-type and σ1s-null virus in the brain are equivalent at day 4 following intracranial inoculation, only a small fraction of infected animals contain σ1s-null virus in other organs in comparison to those infected with wild-type virus (Fig. 7B). The percentage of organs with disseminated σ1s-null virus increases at day 8, and by day 12, titers of the σ1s-null virus approximate those of wild-type virus. We envision three possibilities to explain the kinetics of reovirus dissemination from the brain following intracranial inoculation. First, the block to hematogenous dissemination for the σ1s-null virus may not be absolute, and a small percentage of the virus may reach the bloodstream even in the absence of σ1s expression. Thus, the low titers of σ1s-null virus in peripheral organs results from this limited level of disseminated virus that is capable of seeding organs such as the heart, liver, and spleen. Second, virus might spread from the brain by neural pathways and thus not require functional σ1s for this mode of transit. Much like the vasculature, neuronal pathways traverse every organ in the animal and provide a direct conduit for virus to access peripheral sites. The delayed kinetics with which the σ1s-null virus spreads to peripheral organs would be consistent with the idea that bloodstream dissemination is more rapid than neural dissemination. Third, hematogenous spread of the σ1s-null virus may result from reversion of the σ1s-null mutation to the wild type. In our experiments, a substantial portion of the disseminated σ1s-null virus had reverted to the wild type, supporting this possibility and further underscoring the importance of σ1s in promoting viremic spread. These mechanisms are not mutually exclusive, and it is possible that reovirus disseminates systemically from the brain following intracranial inoculation by a combination of neural and hematogenous pathways.
The means by which reovirus traffics within neurons is not known. The administration of colchicine to inhibit fast axonal transport (18, 35) prior to intramuscular inoculation inhibits spread of virus to the spinal cord (41). In contrast, transport to the spinal cord is not affected by treatment with β-β′-iminodipropronitrile (41), which selectively inhibits slow axonal transport (14). These data indicate that fast axonal transport pathways facilitate virus spread from the hind-limb muscle through the sciatic nerve to the spinal cord and presumably to the brain. However, the delay in invasion of the brain by the σ1s-null virus suggests that neural transmission of virus from the spinal cord to the brain is not rapid. Other viruses that traffic by neural routes from peripheral sites to the brain using fast axonal transport reach the brain with rapid kinetics (25, 48). For example, poliovirus is detected in the spinal cord within 2 h and reaches the brain by 24 h following intramuscular inoculation (21). Thus, it is possible that a mechanism other than fast axonal transport mediates transit of reovirus from the spinal cord to the brain.
Reovirus serotypes differ in tropism within the murine CNS (22, 45, 46). These differences segregate with the S1 gene (10, 38), which encodes attachment protein σ1 and nonstructural protein σ1s (33, 44). We previously demonstrated that type 1 reovirus σ1s protein is not required for viral infection and replication in the mouse brain (6). In this study, we found that a type 3 σ1s-null virus replicates in the murine CNS as efficiently as wild-type virus (Fig. 4B and C). Moreover, identical cell types and brain regions are infected by wild-type and σ1s-null viruses (data not shown). The σ1s protein also is dispensable for replication in primary cultures of neurons (Fig. 5). Collectively, these findings demonstrate that σ1s is not a determinant of viral tropism or replication in the brain. Instead, σ1s mediates hematogenous spread within the infected host. Differences in viral tropism in the murine CNS appear to be governed by attachment protein σ1. It remains unclear why infection with type 1 and type 3 reoviruses differs in the mouse brain. However, our work clearly indicates that σ1s does not influence this difference; rather, the basis for this distinction is likely differential binding to host cell receptors by the σ1 protein.
Following intramuscular inoculation, the survival of mice infected with the σ1s-null mutant was markedly increased compared to that of mice infected with wild-type virus (Fig. 3A). It is possible that systemic infection, leading to disease in multiple organ systems, is a major contributor to reovirus-induced mortality. In experimental mouse models, reovirus can cause a variety of diseases, including myocarditis (34), hepatitis (17, 26, 27), and biliary obstruction (2). Following intramuscular inoculation, higher titers of wild-type virus than of the σ1s-null mutant at sites of secondary replication may cause increased injury to other organ systems that leads to the dramatic differences in survival. The kinetics of reovirus delivery to the CNS also may be critical for the onset of encephalitis. Susceptibility to reovirus encephalitis is age dependent, and mice are refractory to reovirus disease when inoculated later than 8 days of age (37). It is possible that titers of the σ1s-null mutant in the brain do not reach the threshold required to cause disease within the necessary time frame.
We previously showed that the σ1s protein is required for hematogenous spread of type 1 reoviruses (6), which disseminate within the host by strictly blood-borne mechanisms. A type 1 σ1s-null virus replicates equivalently to wild-type virus in the intestine following peroral inoculation. Although both viruses are taken up by Peyer's patches, only the wild-type virus spreads to the mesenteric lymph node and bloodstream. These findings suggest that σ1s is essential for reovirus spread from intestinal lymphatics to the circulation, thereby allowing the establishment of viremia and dissemination to sites of secondary replication. Here, we demonstrate that type 3 reovirus σ1s promotes viral bloodstream dissemination but is dispensable for transmission by neural routes. Collectively, these studies suggest that following replication at a site of inoculation, reoviruses use a serotype-independent pathway that requires σ1s to disseminate through the lymphatic-hematogenous system to organs that support secondary viral replication. This work reveals how different mechanisms of dissemination contribute to reovirus pathogenesis and provides insight into the events at the pathogen-host interface that lead to systemic infection and disease.
ACKNOWLEDGMENTS
We thank members of our laboratory for many useful discussions and Pranav Danthi, Geoff Holm, and Bernardo Mainou for reviews of the manuscript. We thank Laura Ooms for assistance with quantitative RT-PCR. We are grateful to Patty Chen and Ken Tyler for assistance in developing the sciatic nerve section protocol.
This research was supported by Public Health Service awards T32 CA09385 (K.W.B.), F32 AI075776 (K.W.B.), T32 AI07611 (J.M.F.), T32 CA09385 (J.L.K.), F32 AI081486 (J.L.K.), and R37 AI38296 (T.S.D.) and the Elizabeth B. Lamb Center for Pediatric Research. Additional support was provided by Public Health Service awards CA68485 for the Vanderbilt-Ingram Cancer Center and DK20593 for the Vanderbilt Diabetes Research and Training Center.
Footnotes
Published ahead of print on 14 September 2011.
REFERENCES
- 1. Antar A. A. R., et al. 2009. Junctional adhesion molecule-A is required for hematogenous dissemination of reovirus. Cell Host Microbe 5: 59–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barton E. S., et al. 2003. Utilization of sialic acid as a coreceptor is required for reovirus-induced biliary disease. J. Clin. Invest. 111: 1823–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bijlenga G. 1978. A potency test which simulates natural exposure for measuring post-exposure activity of rabies vaccines. A proposal for preparing a relevant international reference preparation. Dev. Biol. Stand. 40: 203–208 [PubMed] [Google Scholar]
- 4. Bodkin D. K., Fields B. N. 1989. Growth and survival of reovirus in intestinal tissue: role of the L2 and S1 genes. J. Virol. 63: 1188–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bodkin D. K., Nibert M. L., Fields B. N. 1989. Proteolytic digestion of reovirus in the intestinal lumens of neonatal mice. J. Virol. 63: 4676–4681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boehme K. W., Guglielmi K. M., Dermody T. S. 2009. Reovirus nonstructural protein σ1s is required for establishment of viremia and systemic dissemination. Proc. Natl. Acad. Sci. U. S. A. 106: 19986–19991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cashdollar L. W., Chmelo R. A., Wiener J. R., Joklik W. K. 1985. Sequences of the S1 genes of the three serotypes of reovirus. Proc. Natl. Acad. Sci. U. S. A. 82: 24–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cenatiempo Y., et al. 1984. Two initiation sites detected in the small s1 species of reovirus mRNA by dipeptide synthesis in vitro. Proc. Natl. Acad. Sci. U. S. A. 81: 1084–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dermody T. S., Nibert M. L., Bassel-Duby R., Fields B. N. 1990. Sequence diversity in S1 genes and S1 translation products of 11 serotype 3 reovirus strains. J. Virol. 64: 4842–4850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dichter M. A., Weiner H. L. 1984. Infection of neuronal cell cultures with reovirus mimics in vitro patterns of neurotropism. Ann. Neurol. 16: 603–610 [DOI] [PubMed] [Google Scholar]
- 11. Ernst H., Shatkin A. J. 1985. Reovirus hemagglutinin mRNA codes for two polypeptides in overlapping reading frames. Proc. Natl. Acad. Sci. U. S. A. 82: 48–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Flamand A., Gagner J. P., Morrison L. A., Fields B. N. 1991. Penetration of the nervous systems of suckling mice by mammalian reoviruses. J. Virol. 65: 123–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Furlong D. B., Nibert M. L., Fields B. N. 1988. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J. Virol. 62: 246–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hansson G., Kristensson K., Olsson Y., Sjostrand J. 1971. Embryonal and postnatal development of mast cells in rat peripheral nerve. Acta Neuropathol. 17: 139–149 [DOI] [PubMed] [Google Scholar]
- 15. Hoyt C. C., Bouchard R. J., Tyler K. L. 2004. Novel nuclear herniations induced by nuclear localization of a viral protein. J. Virol. 78: 6360–6369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hoyt C. C., et al. 2005. Nonstructural protein σ1s is a determinant of reovirus virulence and influences the kinetics and severity of apoptosis induction in the heart and central nervous system. J. Virol. 79: 2743–2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnson E. M., et al. 2009. Genetic and pharmacologic alteration of cathepsin expression influences reovirus pathogenesis. J. Virol. 83: 9630–9640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karlsson J. O., Sjostrand J. 1969. The effect of colchicine on the axonal transport of protein in the optic nerve and tract of the rabbit. Brain Res. 13: 617–619 [DOI] [PubMed] [Google Scholar]
- 19. Kobayashi T., et al. 2007. A plasmid-based reverse genetics system for animal double-stranded RNA viruses. Cell Host Microbe 1: 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kristensson K., Lycke E., Sjostrand J. 1971. Spread of herpes simplex virus in peripheral nerves. Acta Neuropathol. 17: 44–53 [DOI] [PubMed] [Google Scholar]
- 21. Lancaster K. Z., Pfeiffer J. K. 2010. Limited trafficking of a neurotropic virus through inefficient retrograde axonal transport and the type I interferon response. PLoS Pathog. 6: e1000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morrison L. A., Sidman R. L., Fields B. N. 1991. Direct spread of reovirus from the intestinal lumen to the central nervous system through vagal autonomic nerve fibers. Proc. Natl. Acad. Sci. U. S. A. 88: 3852–3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nibert M. L., Chappell J. D., Dermody T. S. 1995. Infectious subvirion particles of reovirus type 3 Dearing exhibit a loss in infectivity and contain a cleaved σ1 protein. J. Virol. 69: 5057–5067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nibert M. L., Schiff L. A. 2001. Reoviruses and their replication, p. 1679–1728. In Knipe D. M., Howley P. M. (ed.), Fields virology, fourth ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 25. Ohka S., Yang W. X., Terada E., Iwasaki K., Nomoto A. 1998. Retrograde transport of intact poliovirus through the axon via the fast transport system. Virology 250: 67–75 [DOI] [PubMed] [Google Scholar]
- 26. Papadimitriou J. M. 1965. Electron micrographic features of acute murine reovirus hepatitis. Am. J. Pathol. 47: 565–585 [PMC free article] [PubMed] [Google Scholar]
- 27. Papadimitriou J. M. 1966. Ultrastructural features of chronic murine hepatitis after reovirus type 3 infection. Br. J. Exp. Pathol. 47: 624–631 [PMC free article] [PubMed] [Google Scholar]
- 28. Penfold M. E., Armati P., Cunningham A. L. 1994. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc. Natl. Acad. Sci. U. S. A. 91: 6529–6533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Poggioli G. J., Dermody T. S., Tyler K. L. 2001. Reovirus-induced 1s-dependent G2/M cell cycle arrest results from inhibition of p34cdc2. J. Virol. 75: 7429–7434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Poggioli G. J., Keefer C. J., Connolly J. L., Dermody T. S., Tyler K. L. 2000. Reovirus-induced G2/M cell cycle arrest requires σ1s and occurs in the absence of apoptosis. J. Virol. 74: 9562–9570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Richardson B. A., Overbaugh J. 2005. Basic statistical considerations in virological experiments. J. Virol. 79: 669–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rodgers S. E., Connolly J. L., Chappell J. D., Dermody T. S. 1998. Reovirus growth in cell culture does not require the full complement of viral proteins: identification of a σ1s-null mutant. J. Virol. 72: 8597–8604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sarkar G., et al. 1985. Identification of a new polypeptide coded by reovirus gene S1. J. Virol. 54: 720–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sherry B. 1998. Pathogenesis of reovirus myocarditis. Curr. Top. Microbiol. Immunol. 233(Pt 2): 51–66 [DOI] [PubMed] [Google Scholar]
- 35. Sjostrand J., Karlsson J. O. 1969. Axoplasmic transport in the optic nerve and tract of the rabbit: a biochemical and radioautographic study. J. Neurochem. 16: 833–844 [DOI] [PubMed] [Google Scholar]
- 36. Smith R. E., Zweerink H. J., Joklik W. K. 1969. Polypeptide components of virions, top component and cores of reovirus type 3. Virology 39: 791–810 [DOI] [PubMed] [Google Scholar]
- 37. Tardieu M., Powers M. L., Weiner H. L. 1983. Age-dependent susceptibility to reovirus type 3 encephalitis: role of viral and host factors. Ann. Neurol. 13: 602–607 [DOI] [PubMed] [Google Scholar]
- 38. Tardieu M., Weiner H. L. 1982. Viral receptors on isolated murine and human ependymal cells. Science 215: 419–421 [DOI] [PubMed] [Google Scholar]
- 39. Tsiang H. 1979. Evidence for intraaxonal transport of fixed and street rabies virus. J. Neuropathol. Exp. Neurol. 38: 286–297 [DOI] [PubMed] [Google Scholar]
- 40. Tyler K. L., Bronson R. T., Byers K. B., Fields B. N. 1985. Molecular basis of viral neurotropism: experimental reovirus infection. Neurology 35: 88–92 [DOI] [PubMed] [Google Scholar]
- 41. Tyler K. L., McPhee D. A., Fields B. N. 1986. Distinct pathways of viral spread in the host determined by reovirus S1 gene segment. Science 233: 770–774 [DOI] [PubMed] [Google Scholar]
- 42. van't Wout A. B., et al. 1994. Macrophage-tropic variants initiate human immunodeficiency virus type 1 infection after sexual, parenteral, and vertical transmission. J. Clin. Invest. 94: 2060–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Virgin H. W., Bassel-Duby IV. R., Fields B. N., Tyler K. L. 1988. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing). J. Virol. 62: 4594–4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weiner H. L., Ault K. A., Fields B. N. 1980. Interaction of reovirus with cell surface receptors. I. Murine and human lymphocytes have a receptor for the hemagglutinin of reovirus type 3. J. Immunol. 124: 2143–2148 [PubMed] [Google Scholar]
- 45. Weiner H. L., Drayna D., Averill D. R., Jr., Fields B. N. 1977. Molecular basis of reovirus virulence: role of the S1 gene. Proc. Natl. Acad. Sci. U. S. A. 74: 5744–5748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weiner H. L., Powers M. L., Fields B. N. 1980. Absolute linkage of virulence and central nervous system tropism of reoviruses to viral hemagglutinin. J. Infect. Dis. 141: 609–616 [DOI] [PubMed] [Google Scholar]
- 47. Yanagi Y., Takeda M., Ohno S. 2006. Measles virus: cellular receptors, tropism and pathogenesis. J. Gen. Virol. 87: 2767–2779 [DOI] [PubMed] [Google Scholar]
- 48. Yang W. X., et al. 1997. Efficient delivery of circulating poliovirus to the central nervous system independently of poliovirus receptor. Virology 229: 421–428 [DOI] [PubMed] [Google Scholar]