Abstract
Nicotianamine (NA) occurs in all plants and chelates metal cations, including FeII, but reportedly not FeIII. However, a comparison of the FeII and ZnII affinity constants of NA and various FeIII-chelating aminocarboxylates suggested that NA should chelate FeIII. High-voltage electrophoresis of the FeNA complex formed in the presence of FeIII showed that the complex had a net charge of 0, consistent with the hexadentate chelation of FeIII. Measurement of the affinity constant for FeIII yielded a value of 1020.6, which is greater than that for the association of NA with FeII (1012.8). However, capillary electrophoresis showed that in the presence of FeII and FeIII, NA preferentially chelates FeII, indicating that the FeIINA complex is kinetically stable under aerobic conditions. Furthermore, Fe complexes of NA are relatively poor Fenton reagents, as measured by their ability to mediate H2O2-dependent oxidation of deoxyribose. This suggests that NA will have an important role in scavenging Fe and protecting the cell from oxidative damage. The pH dependence of metal ion chelation by NA and a typical phytosiderophore, 2′-deoxymugineic acid, indicated that although both have the ability to chelate Fe, when both are present, 2′-deoxymugineic acid dominates the chelation process at acidic pH values, whereas NA dominates at alkaline pH values. The consequences for the role of NA in the long-distance transport of metals in the xylem and phloem are discussed.
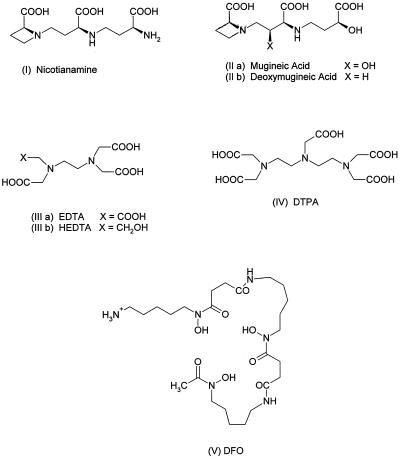
NA, 2(S),3′(S),3"(S)-N-[N-(3-amino-3-carboxypropyl)-3-amino-3-carboxypropyl]-azetidine-2-carboxylic acid (Fig. 1, structure I), and MA, 2(S),2′(S),3′(S),3"(S)-N-[3-carboxy-(3-carboxy-3-hydroxypropylamino)-2-hydroxypropyl]-azetidine-2-carboxylic acid (structure II a) are two structurally similar molecules synthesized by plants. Although both compounds have roles in the acquisition and transport of Fe, their species distribution and physiological functions are distinct. MA and some closely related compounds, termed PS, are made only by graminaceous monocotyledonous plants and are secreted from the roots of Fe-deficient grasses to mobilize Fe from insoluble sources (Takagi, 1976; Sugiura and Nomoto, 1984). These compounds chelate FeIII (Sugiura and Nomoto, 1984), and the FeIIIPS complex is probably the form in which Fe is taken up by the roots of grasses (Römheld and Marschner, 1986; von Wirén et al., 1995).
Figure 1.
The structures of the aminocarboxylate ligands used in the study and of DFO.
By contrast, NA is made by all plants and is present in various plant organs (Stephan et al., 1994; Walter et al., 1995) but is not secreted. Instead, it is thought to have a role in the internal transport of Fe and other metals (Stephan et al., 1994, 1996; Pich and Scholz, 1996). Evidence in support of this role is that the concentrations of NA in the phloem correlate with those of Fe and other metals, and the NA-synthesis-defective tomato mutant chloronerva has a phenotype indicative of Fe deficiency (Pich and Scholz, 1996; Stephan et al., 1996). Despite its structural similarity to MA and other PS, NA is proposed to fulfill its physiological role in Fe trafficking by chelating FeII and not FeIII (Stephan and Scholz, 1993). Although FeIIINA has been demonstrated by electron-spin-resonance spectroscopy (Sugiura and Nomoto, 1984), K for the formation of this complex has not been successfully measured and so it has been assumed to be physiologically unimportant. In contrast, K of NA for FeII has been successfully measured (Beneš et al., 1983; Anderegg and Ripperger, 1989). Thus, it has been concluded that the FeII complex is the only significant one in biological systems, despite the electron-spin-resonance spectroscopy evidence for the formation of an FeIIINA complex.
It is important to establish whether the FeIIINA complex exists under physiological conditions because it could fundamentally affect the conclusions about the forms in which Fe and other metal ions are transported within plants. If NA functions only as an FeII chelator, Fe taken up by grasses as FeIIIPS complexes will need to be reduced before formation of the FeIINA complex for subsequent internal transport to other parts of the plant. However, if NA chelates FeIII, then it may be possible for the Fe to be donated directly to it by the FeIIIPS complex. In addition, if NA binds FeIII more tightly than FeII and other divalent metal ions, this will affect the relative abundance of different NA-metal complexes, possibly altering conclusions about the role of NA in the internal trafficking of these metals (Stephan et al., 1996). Here we provide evidence that NA does indeed form an FeIII complex under physiological conditions, but we also show that the FeIINA complex has an unexpected kinetic stability. The role of NA in protecting cells from oxidative damage through scavenging of Fe and in metal trafficking are considered in relation to these properties.
MATERIALS AND METHODS
Chemicals
NA (structure I), MA (structure II a), and DMA (structure II b) were chemically synthesized as previously described and were at least 95% pure as judged by 1H-NMR (Shioiri et al., 1995). EDTA (structure III a, >99%), HEDTA (structure III b, >98%), and DTPA (structure IV, >98%) were purchased from Sigma. DFO (structure V, >90%) was a gift from Novartis (Basel, Switzerland). All other chemicals were from Aldrich. For the preparation of labeled chelates an aliquot of 10 mm Fe(NO3)3 containing 20 kBq 59FeCl3 (Amersham) was added to 10 μL of 12 mm chelator solution and adjusted to pH 7.0 with 100 mm Mops-KOH.
High-Voltage Electrophoresis
A sheet of filter paper (grade 1F, Munktell, Stockholm, Sweden) was placed on a cooling plate covered by acetate foil in an electrophoresis cuvette (Multiphor II, Pharmacia). The filter paper was positioned so that both ends dipped in buffer solution (0.1 m Mops-KOH, pH 7.0) and the paper was pre-run at 400 V for 20 min. Small pieces of filter paper (10 × 4 mm) were loaded with approximately 10 μL of 1 mm 59Fe-chelate solution (2 kBq) and were then placed on a start line in the middle of the paper sheet. Separation was achieved in the dark at a constant 400 V at 10°C. After 60 min, electrophoresis was stopped, and the paper sheet was immediately dried with a hair drier and cut into 8-mm-wide strips parallel to the start line. The amount of radioactivity on the paper strips and on the deposit paper was determined in a γ-counter (LKB, Bromma, Sweden) by dry Cerenkov counting. For a comparison of migration distances, results were expressed in counts per minute per strip. The net charge was calculated from the migration distances using the 59FeIII and 65ZnII complexes of EDTA, HEDTA, and DTPA as the standards.
pKa Determination
Equilibrium constants of protonated ligands were determined using an automated, computerized system capable of simultaneously analyzing spectrophotometric and potentiometric measurements. A blank titration of 0.1 m KCl was performed to determine the electrode zero using Gran's plot method (Gran, 1952). A combined pH electrode (Sirius Analytical Instruments Ltd., Forest Row, East Sussex, UK) was used to calibrate the electrode zero. The solution (0.1 m KCl, 25 mL), contained in a jacketed titration cell to maintain the temperature at 25°C ± 0.5°C, was under an argon atmosphere and was acidified with 0.15 mL of 0.2 m HCl. Titrations were carried out against 0.2 m KOH added in 0.01-mL increments dispensed from a Metrohm 665 dosimat (Metrohm Ltd., Buckingham, UK). The titration was repeated in the presence of ligand. The data obtained from the titrations were analyzed by the TITRFIT program, a modified version of NONLIN (Taylor et al., 1988).
Determination of K
The K for the FeIII-ligand interaction was determined by a spectrophotometric competition study of ligand-FeIII-maltol using the automated system described above. The FeIII complexes of the ligand were prepared in a 10:1 ligand:Fe molar ratio (total Fe concentration = 4.4 × 10−5 m) in 0.1 m Mops-KOH buffer, pH 7.4. This solution was then titrated against maltol (3-hydroxy-2-methylpyran-4-one), resulting in the dissociation of FeIIINA and the formation of the orange FeIIImaltol complex. The resulting spectrophotometric data were inserted into the COMPT1 program (H. Khodr, unpublished data) to evaluate K of the complex. Speciation and pM plots were obtained by running the program SPECIAZ1 (H. Khodr, unpublished data). These programs require concentrations of metal and ligand, K(FeIII) values of complexes, K values for FeIII-OH interactions, and pKa values of the ligand. Between pH 1.0 and 9.0, the dominant FeIII-OH species are [FeIII(OH)]2+, [FeIII(OH)2]+, [FeIII(OH)3], and [FeIII(OH)4]−.
CE
Separations were performed at 25°C in fused silica capillaries (37 cm long x 75 μm i.d.; Metal Services Ltd., Worcester, UK) on a PACE 5510 apparatus (Beckman) equipped with a photodiode array detector and using Nouveau Gold software (Beckman) for data acquisition. The capillary was rinsed with 0.1 mm NaOH and then with water for 5 min before equilibration for 20 min with the carrier electrolyte. These procedures were repeated but for only 2 min each between each separation. The solutes were injected in the hydrodynamic mode by overpressure (3.45 kPa). Electroosmotic flow was evaluated from the migration time of formamide. For the separation of NA-metal complexes, the concentration of NA was 2.5 mm and that of the metal ion was 3 mm. Borate buffer (50 mm, pH 9.2) was the carrier electrolyte and separation was for 12 s at 667 V cm−1. For the competition studies between NA and citrate, the conditions were the same, except 25 mm Na citrate was added and the carrier electrolyte was 50 mm phosphate buffer, pH 5.5.
Measurement of Fenton Activity of FeNA complexes
Fenton activity was determined in the presence of 1.5 mm H2O2 and 2.8 mm deoxyribose by incubation of various FeNA complexes (total Fe concentration = 20 μm, total NA concentration = 100 μm) in 50 mm phosphate buffer, pH 7.4, at 37°C. (Halliwell et al., 1987). The formation of thiobarbituric acid-reactive substances from deoxyribose was quantified by measurement of A532 (Halliwell and Gutteridge, 1981). Results are the mean of five determinations and are corrected for absorbance in the absence of deoxyribose. When present, ascorbic acid was used at 100 μm.
RESULTS AND DISCUSSION
Both NA and MA have six ligands for Fe complexation, and the distances between the groups facilitate octahedral coordination of Fe and the formation of three 5-membered and two 6-membered chelate rings (Ripperger and Schreiber, 1982). The only differences between NA and MA are the hydroxyl/amino substitution on C3" and the presence of a hydroxyl group on C2′ of MA (Fig. 1). Two observations led us to query the conclusion that there is no interaction of NA with FeIII.
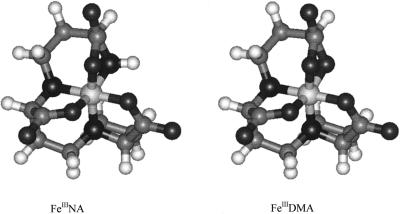
The first was a comparison of NA's K for FeII and ZnII (Anderegg and Ripperger, 1989) with those of a closely related group of oligodentate aminocarboxylate ligands, including MA and DMA, which are known to chelate FeIII. The ratios of the affinities of these ligands for ZnII and FeII are similar to that for NA and all fall into a tight range, 1.12 to 1.26 (Table I). The value of this ratio for NA (1.20) is close to the mean for the group (1.19). Similarly, the ratios of affinities for FeIII and FeII fall between 1.62 and 1.77. Using these ratios to predict the K for the interaction between FeIII and NA suggests a value that falls in the range of 1020 to 1022. This estimated value is much higher than that reported for the FeII-NA interaction (1012.1–1012.8; Beneš et al., 1983; Anderegg and Ripperger, 1989) or for the FeIII-MA interaction (1018.2; Sugiura and Nomoto, 1984). Furthermore, molecular modeling of a putative FeIIINA complex and comparison with that for FeIIIDMA showed that the two structures are very similar, with no obvious strain in the FeIIINA complex (Fig. 2).
Table I.
Comparison of the affinity constants of EDTA, HEDTA, DPTA, MA, DMA, and NA for ZnII, FeII, and FeIII
| Chelator | Log K for Metal Ion
|
||||
|---|---|---|---|---|---|
| ZnII | FeII | FeIII | ZnII/FeII | FeIII/FeII | |
| EDTAa | 16.0 | 14.3 | 25.1 | 1.12 | 1.76 |
| HEDTAa | 14.6 | 12.2 | 19.8 | 1.20 | 1.62 |
| DTPAa | 18.3 | 16.4 | 28.0 | 1.12 | 1.71 |
| MAb | 12.7 | 10.1 | 17.7 | 1.26 | 1.75 |
| DMAb | 12.8 | 10.4 | 18.4 | 1.23 | 1.77 |
| NAc | 15.4 | 12.8 | n.r.d | 1.20 | n.r. |
Data are from Smith and Martell (1989).
Data are from Murakami et al. (1989).
Data are from Anderegg and Ripperger (1989).
n.r., Not reported in the literature.
Figure 2.
Computer-generated models of the FeIII complexes of NA and DMA. The coordinates are based on the CoIII complex of MA (Sugiura et al., 1981) and the ionization state demonstrated by the high-voltage electrophoresis data in Figure 3.
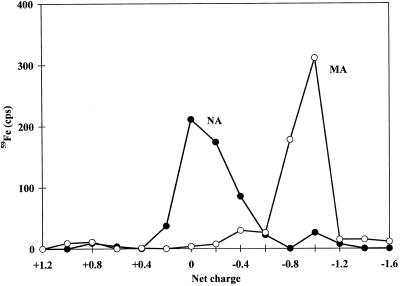
Second, using high-voltage electrophoresis of NA and MA in the presence of 59FeIII at pH 7.0 under aerobic conditions in the dark (so FeII would not be present), we measured net charges of 0 and −1.0 for the FeNA and FeMA complexes, respectively (Fig. 3). These values are entirely consistent with a hexadentate structure for both molecules chelating FeIII (Fig. 2). The lack of charge on the NA complex is due to the compensation of the negative charges of the carboxylate groups by the FeIII. In the case of MA, the extra negative charge is associated with the deprotonation of the terminal OH function in the presence of FeIII (Sugiura et al., 1981). These net charges specifically exclude pentadentate (terminal amino function not involved in coordination) and tetradentate (α-amino acid functions not involved in coordination) structures, which for NA would both have net charges of +1. The FeIII complex of DMA also possesses a net charge of −1.0 at pH 7.0 (data not shown).
Figure 3.
Net charge of FeIII complexes of NA (•) and MA (○) as determined by high-voltage paper electrophoresis at pH 7.0 under aerobic conditions in the dark.
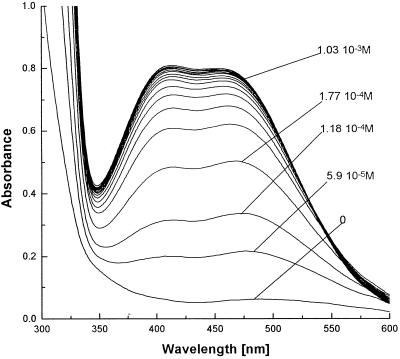
In view of these strong indications that NA can bind FeIII, a spectrophotometric titration procedure was used to determine directly both the pKa values and the K for FeIIINA by measuring the ability of NA to compete with maltol for FeIII. Titration data for NA are shown in Figure 4. Maltol is a powerful FeIII chelator that generates an orangecolor on complexation with FeIII (Hider and Hall, 1991). This method avoids the difficulty of Fe(OH)3 precipitation reported by Beneš et al. (1983). To verify the accuracy of the method, pKa and FeIII K were measured for DMA and EDTA. The measured values agreed well with those from the literature (Table II). The method gave a value of 1020.6 for the K for the interaction of NA with FeIII (Table II). This is in agreement with the predictions from the data from Table I, providing a ratio for the K for FeIII and FeII of 1.61, and indicates that, contrary to current dogma, NA binds FeIII with an affinity 108 times greater than that with which it binds FeII. This finding also offers an explanation for the reported redox potentials of the Fe complexes of MA and NA (Table III; Sugiura and Nomoto, 1984). More negative redox potentials indicate selectivity for FeIII over FeII as exemplified by DFO, a highly specific FeIII-chelating bacterial trishydroxamate siderophore (KFeIII = 30.6, KFeII = 7.2; Anderegg et al., 1963). The redox potential of DFO is significantly more negative than that of NA or MA, consistent with DFO's relative inability to chelate FeII.
Figure 4.
Spectrophotometric titration against maltol of a 43.5 μm solution of FeIIINA complex. FeIIINA dissociates with increasing concentrations of maltol, leading to the formation of the orange FeIIImaltol complex. The concentrations of maltol used are indicated.
Table II.
Measured and published pKa values and K for FeIII complexes of NA, DMA, and EDTA
| Chelator | pK1 | pK2 | pK3 | pK4 | KFeIII |
|---|---|---|---|---|---|
| EDTA | 9.85 (10.17) | 6.01 (6.11) | 2.93 (2.68) | 2.24 (2.00) | 25.2 (25.0) |
| DMA | 9.55 (10.00) | 7.78 (8.25) | 3.40 (3.19) | 2.72 | 18.1 (18.38) |
| NA | 10.09 (10.17) | 9.14 (9.14) | 6.92 (7.01) | 2.86 (2.20) | 20.6 |
Published values (where available) are in parentheses: Smith and Martell (1989) (DMA), Murakami et al. (1989) (NA), and Anderegg and Ripperger (1989) (EDTA).
Table III.
The redox potentials of the FeIII complexes of EDTA, MA, NA, and DFO
| Complex | Redox Potential |
|---|---|
| V versus NHEa | |
| FeIIIEDTA | +0.12b |
| FeIIIMA | −0.10c |
| FeIIINA | −0.18c |
| FeIIIDFO | −0.47d |
NHE, Normal hydrogen electrode.
Data are from Smith and Martell (1989).
Data are from Sugiura and Nomoto (1984).
Data are from Anderegg et al. (1963).
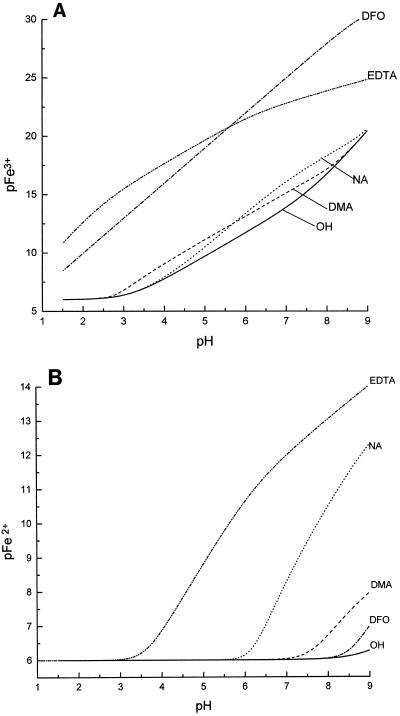
The efficiency of chelation by different ligands is best compared using pM values, which provide a measure of the free metal ion concentration in the presence of ligand (Hider, 1995). The higher the pM value, the more powerful the chelator. The influence of pH on the pFe3+ values for NA, DMA, EDTA, and DFO is presented in Figure 5A. It is clear that NA and DMA are both markedly weaker chelators of FeIII than either EDTA or DFO. They are just able to compete with the hydroxide anion over the pH range of 3.0 to 8.0. In contrast, although the K values of the FeII complexes of NA and DMA are much lower than those of the corresponding FeIII complexes (Tables I and II), the pFe2+ values indicate that NA is capable of efficiently competing with hydroxide at pH values greater than 6.5 (Fig. 5B). DMA is an inferior ligand for FeII, only beginning to compete effectively with the hydroxide anion at pH values greater than 7.0. The plot also indicates the relative inability of DFO to complex FeII. In summary, in aqueous solution NA binds FeIII over the pH range of 4.0 to 9.0 and binds FeII over the pH range of 6.0 to 9.0, whereas in general, DMA and PS bind FeIII over the pH range of 2.5 to 8.5 and bind FeII over the pH range of 7.5 to 9.0.
Figure 5.
The influence of pH on pFe3+ (A) and pFe2+ (B) values for hydroxide, NA, DMA, EDTA, and DFO. The total Fe concentration was 1 μm and the total ligand concentration was 10 μm. The higher the pM value, the more effective the ligand.
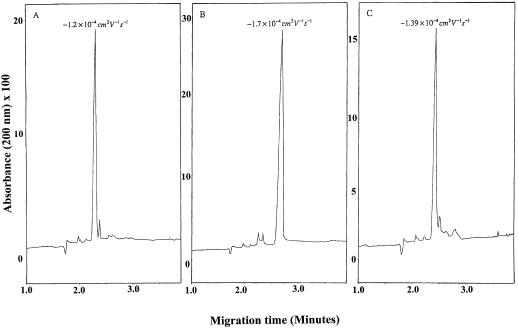
Although EDTA forms a complex with FeII, this rapidly autoxidizes in air and thus the FeIII complex always dominates under aerobic conditions (Zang and van Eldik, 1990). By virtue of the high affinity of NA for FeIII, the FeIINA complex would be expected to behave similarly, which would mean that the complex would be unlikely to have a transport function in aerobic plant tissues. Therefore, the stability of the FeIINA complex was investigated using CE. Unlike the FeII complexes of EDTA and DTPA, the FeIINA complex was found to be quite stable, possessing a running time similar to that of CuIINA and quite distinct from that of the FeIIINA complex (Fig. 6). There was no evidence of autoxidation of the FeIINA complex, even when O2 was bubbled through the solution. This is surprising in view of the values of the stability constants of the FeII and FeIII complexes (see above) and indicates that, once formed, the FeIINA complex is kinetically stable. To test this, equimolar amounts of FeII and FeIII were added to NA at pH 7.0 (final ratio of total Fe:NA was 2:1) and the complexes separated by CE. Only the FeII complex was detected (not shown). Thus, at pH 7.0, NA will preferentially scavenge FeII even in the presence of FeIII, and the FeIINA complex will persist by virtue of its kinetic stability. Consistent with this finding, measurements of the potency of FeNA complexes as Fenton reagents, determined from their ability to mediate H2O2-dependent oxidation of deoxyribose, showed that they are not highly active Fenton reagents (Table IV) and do not redox cycle efficiently. These physicochemical properties will allow NA to scavenge FeII, preventing FeII-mediated production of hydroxyl radicals from H2O2 via the Fenton reaction (Guerinot and Yi, 1994; Henle and Linn, 1997) and protecting the cell from Fe-induced oxidative damage.
Figure 6.
Migration times of NA and its Fe complexes as measured by CE. A, NA alone; B, FeIINA; C, FeIIINA.
Table IV.
Fenton activities of Fe complexes of NA in comparison with those of Fe-EDTA and Fe-phosphate
| Fe Oxidation State | Fenton Activity of Salt or Complex
|
||
|---|---|---|---|
| Phosphate | EDTA | NA | |
| absorbance | |||
| FeII | 0.11 ± 0.02 | 0.24 ± 0.02 | 0.13 ± 0.03 |
| FeIII + ascorbic acid | 0.29 ± 0.01 | 0.39 ± 0.01 | 0.18 ± 0.03 |
Values are means ± se of five separate determinations.
Physiological Implications
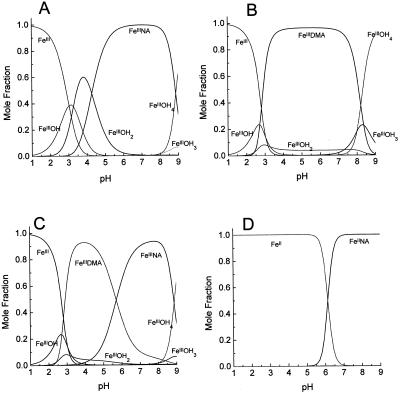
To determine the possible physiological consequences of these observations for metal trafficking in plants, we used the pKa and measured K of NA to calculate the concentrations of different complexes under different conditions and compared these with other physiologically important FeIII chelators, such as PS and citrate. First, we examined the effect of pH on the relative abilities of NA and DMA to chelate FeIII to determine whether the hydroxyl/amino substitution at C3" has any major significance that could explain the different physiological roles that these molecules play in Fe acquisition and trafficking (see the introduction). Although both compounds chelate FeIII over a broad pH range, FeIIINA is more acid labile than DMA and, conversely, is more stable at alkaline pH values (Fig. 7, A and B). As a result, in an equilibrium mixture FeIIIDMA will dominate at pH 3.0 to 6.0 and FeIIINA at pH 7.0 to 9.0 (Fig. 7C); this agrees with the data presented in Figure 5A. The FeIINA complex is also acid labile (Fig. 7D; Stephan et al., 1996). These observations are consistent with the proposed physiological roles of NA and PS. In graminaceous plants PS need to be able to chelate FeIII over a wide pH range, from alkaline values in calcareous soils where Fe deficiency is most common (Guerinot and Yi, 1994) to slightly acidic values in the root apoplast where the FeIIIPS complex is transported across the root plasma membrane. This behavior is supported by the observation that PS can mobilize Fe and mediate its uptake over this pH range (Römheld and Marschner, 1986; Treeby et al., 1989). Once the FeIIIPS complex has been absorbed, the greater relative stability of the NA complex at cytosolic pH values (7.2–7.5) means that NA will more efficiently chelate Fe in this compartment. Any metabolism or reduction of the FeIIIPS complex that occurs to enhance Fe release will facilitate the rate of transfer, and the ability of NA to chelate both FeII and FeIII will result in Fe being scavenged rapidly irrespective of which form becomes available.
Figure 7.
Computer simulations of the pH dependence of the Fe complexes of NA and DMA. A, FeIII and NA; B, FeIII and DMA; C, competition for FeIII between NA and DMA; D, FeII and NA. In all cases, the total Fe concentration is 1 μm, and NA or DMA is present at 10 μm.
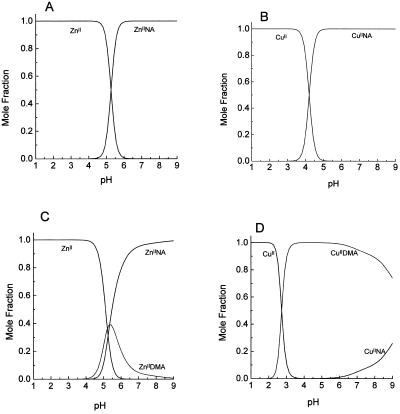
PS also facilitate both the mobilization of Zn2+ and Cu2+ (Treeby et al., 1989) and the uptake of Zn2+ (von Wirén et al., 1996), and NA is proposed to traffic these metals within the plant (Stephan et al., 1996). Thus, considerations similar to those mentioned above occur in relation to the relative stability of the PS and NA complexes of these metals. As with Fe, the NA complexes with ZnII and CuII are acid labile, with the CuII complex being stable to slightly lower pH values than the ZnII complex (Fig. 8, A and B; Stephan et al., 1996). However, when DMA is also present, the equilibrium metal distribution is different for the two metals. For ZnII, the NA complex dominates virtually over the entire physiologically relevant pH range (Fig. 8C), whereas the DMA complex dominates for CuII (Fig. 8D).
Figure 8.
Computer simulations of the pH dependence of the Zn and Cu complexes of NA and MA. A, ZnII and NA; B, CuII and NA; C, competition for ZnII between NA and DMA; D, competition for CuII between NA and DMA. The total concentration of ZnII or CuII is 1 μm, and NA or DMA is present at 10 μm.
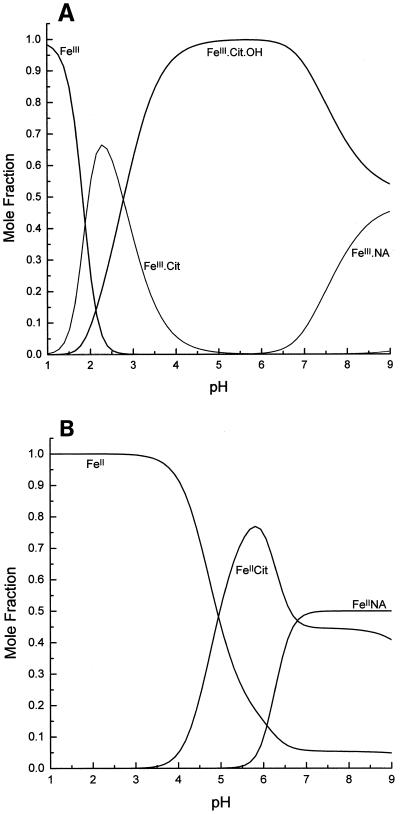
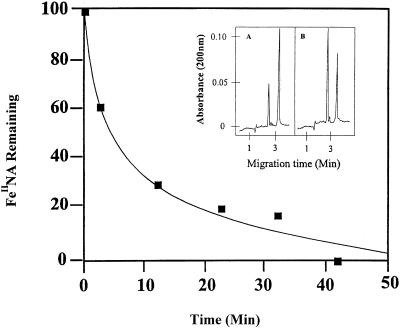
The major role that has been proposed for NA is as a facilitator of the long-distance transport of a number of metals in the phloem (Stephan and Scholz, 1993; Schmidke and Stephan, 1995; Stephan et al., 1996) and of CuII in the xylem (Pich et al., 1994; Pich and Scholz, 1996). Some of these conclusions have been based on the assumption that NA chelates FeII but not FeIII, and so it is important to consider whether these general conclusions would change with the knowledge that NA binds FeIII with high affinity. Therefore, we calculated the equilibrium concentrations of different metal complexes in conditions approximating those in the xylem and phloem. Calculations for the xylem (NA = 20 μm, citrate = 150 μm, Fe = 40 μm, Zn = 5 μm, and Cu = 5 μm, pH 5.5; Pich and Scholz, 1996; A. Pich, personal communication) showed that all Fe is complexed with citrate. This is expected on the basis of the speciation plots, which show that for both FeIII and FeII, citrate complexes totally dominate at pH 5.5 (Fig. 9). However, unlike FeIINA, the FeIIcitrate complex rapidly autoxidizes (Harris and Aisen, 1973). We have confirmed with CE that citrate removes Fe from FeIINA at pH 5.5 (Fig. 10). Under these conditions Fe is removed from NA with an estimated half-life of less than 5 min and thus must be converted to FeIIIcitrate; hence, this complex will dominate even if FeII is the major form in which Fe is loaded into the xylem. As there are reports of citrate concentrations much higher than 150 μm in the xylem (e.g. up to 1700 μm; Tiffin, 1996; White et al., 1981), the speciation plots were also calculated with a citrate concentration of 1500 μm. This made no difference to the conclusions about the complexation of Fe at pH 5.5 but slightly increased the preponderance of Fe citrate complexes at alkaline pH values (not shown). In contrast to Fe, NA was found to be more important than citrate in the chelation of both ZnII and CuII. With the metal and ligand concentrations given above and with citrate at 150 μm, the calculations showed that NA chelates 50% of the ZnII and all of the CuII.
Figure 9.
Computer simulations of the competition between citrate and NA for FeIII (A) and FeII (B). Metal and ligand concentrations are those estimated for the xylem: total Fe = 40 μm, citrate (Cit) = 150 μm, and NA = 20 μm.
Figure 10.
Competition for FeII between NA and citrate at pH 5.5, as monitored by CE. Citrate was added at 0 min and the amount of FeIINA was measured by CE at the times indicated. The inset shows traces from the CE at 2 min (A) and 12 min (B). In each trace, the left peak is NA and the right peak is FeIINA.
Calculations for the phloem (NA = 130 μm, citrate = 1500 μm, Fe = 45 μm, Zn = 30 μm, and Cu = 15 μm, pH 8.0; Schmidke and Stephan, 1995; value for citrate from Jeschke et al., 1986) indicated that all of the metal ions are present as their NA complexes; citrate complexes are predicted to play no role in this pathway. Whether FeIINA or FeIIINA is the major Fe complex in the phloem is unclear, but if Fe is loaded into the phloem as either FeIINA or FeII, then the kinetic stability of the FeIINA complex will probably ensure that this complex dominates.
CONCLUSIONS
It is now clear that studies of the physiological role of NA in plants have wrongly assumed that NA does not chelate FeIII. The studies in this paper show that NA is an effective chelator of FeIII, with an K appreciably higher than that of the chemically related PS, which are established FeIII chelators (Sugiura and Nomoto, 1984). However, the FeIINA complex possesses an unusual kinetic stability and does not rapidly autoxidize to FeIIINA at physiological pH values. Clearly this is an important property of the complex governing the evolution of this molecule as an Fe chelator in plants; the significance of this property requires further investigation. By demonstrating the existence of the FeIIINA complex and analyzing the pH dependence of metal-NA complexes, this study has provided a firmer physicochemical basis for further physiological investigations of the role of NA in chelation of Fe and other metals in plants. It has also shown that scavenging of Fe by NA may be important in protecting the cell from oxidative damage resulting from the Fenton reaction.
ACKNOWLEDGMENTS
We thank Dr. P. Evans (King's College, London, UK) for making the measurements of Fenton activities and Novartis for the supply of DFO.
Abbreviations:
- CE
capillary electrophoresis
- DFO
desferrioxamine
- DMA
deoxymugineic acid
- DTPA
diethylenetriaminepentaacetic acid
- HEDTA
N-hydroxyethylethylenediaminetriacetic acid
- K
affinity constant(s)
- MA
mugineic acid
- NA
nicotianamine
- pM
−log10 free metal ion concentration
- PS
phytosiderophore(s)
Footnotes
The work was supported by a grant from the Biotechnology and Biological Sciences Research Council (BBSRC) of the United Kingdom and by a short-term fellowship to N.v.W. from the joint BBSRC/Institut National de la Recherche Agronomique collaboration scheme. IACR is grant-aided by the BBSRC.
LITERATURE CITED
- Anderegg G, L'Eplattenier F, Schwarzenbach G. Hydroxamatkomplexe III. Eisen(III)-austansch zwischen sideraminen und komplexen. Discussion der bildungskonstanten der hydroxamatkomplexe. Helv Chim Acta. 1963;46:1409–1422. [Google Scholar]
- Anderegg G, Ripperger H (1989) Correlation between metal complex formation and biological activity of nicotianamine analogues. J Chem Soc Chem Commun 647–650
- Beneš I, Schreiber K, Ripperger H, Kircheiss A. Metal complex formation by nicotianamine, a possible phytosiderophore. Experientia. 1983;39:261–262. [Google Scholar]
- Gran G. Determination of equivalence point in potentiometric titrations: part II. Analyst. 1952;77:661–671. [Google Scholar]
- Guerinot ML, Yi Y. Iron. Nutritious, noxious, and not readily available. Plant Physiol. 1994;104:815–820. doi: 10.1104/pp.104.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Formation of a thiobarbituric acid-reactive substance from deoxyribose in the presence of iron salts. FEBS Lett. 1981;128:347–352. doi: 10.1016/0014-5793(81)80114-7. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC, Aruoma OI. The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reaction of hydroxyl groups. Anal Biochem. 1987;165:215–219. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- Harris DC, Aisen P. Facilitation of Fe(II) autoxidation by Fe(III) complexing agents. Biochim Biophys Acta. 1973;329:156–158. doi: 10.1016/0304-4165(73)90019-6. [DOI] [PubMed] [Google Scholar]
- Henle ES, Linn S. Formation, prevention and repair of DNA damage by iron/hydrogen peroxide. J Biol Chem. 1997;272:19095–19098. doi: 10.1074/jbc.272.31.19095. [DOI] [PubMed] [Google Scholar]
- Hider RC. Potential protection from toxicity by oral iron chelators. Toxicol Lett. 1995;82/83:961–967. doi: 10.1016/0378-4274(95)03606-7. [DOI] [PubMed] [Google Scholar]
- Hider RC, Hall RD. Clinically useful chelators of rsi positive elements. Prog Med Chem. 1991;28:41–173. doi: 10.1016/s0079-6468(08)70363-1. [DOI] [PubMed] [Google Scholar]
- Jeschke WD, Pate JS, Atkins CA. Ion circulation via phloem and xylem between roots and shoot of nodulated white lupin. J Plant Physiol. 1986;117:319–330. doi: 10.1016/S0176-1617(85)80068-7. [DOI] [PubMed] [Google Scholar]
- Murakami T, Ise K, Hayakawa M, Kamei S, Takagi S (1989) Stabilities of metal complexes of mugineic acid and their specific affinities for iron(III). Chem Lett 2137–2140
- Pich A, Scholz G, Stephan UW. Iron-dependent changes of heavy metals, nicotianamine, and citrate in different plant organs and in the xylem of two tomato genotypes: nicotianamine as possible copper translocator. Plant Soil. 1994;165:189–196. [Google Scholar]
- Pich A, Scholz I. Translocation of copper and other micronutrients in tomato plants (Lycopersicon esculentum Mill.): nicotianamine-stimulated copper transport in the xylem. J Exp Bot. 1996;47:41–47. [Google Scholar]
- Ripperger H, Schreiber K. Nicotianamine and analogous amino acids, endogenous iron carriers in plants. Heterocycles. 1982;17:447–461. [Google Scholar]
- Römheld V, Marschner H. Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiol. 1986;80:175–180. doi: 10.1104/pp.80.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidke I, Stephan UW. Transport of metal micronutrients in the phloem of castor bean (Ricinus communis) seedlings. Physiol Plant. 1995;95:147–153. [Google Scholar]
- Shioiri T, Hamada Y, Matsuura F. Total synthesis of phytosiderophores. Tetrahedron. 1995;51:3939–3958. [Google Scholar]
- Smith RM, Martell AE (1989) Critical Stability Constants, Vols 1–6. Plenum Press, New York
- Stephan UW, Schmidke I, Pich A. Phloem translocation of Fe, Cu, Mn, and Zn in Ricinus seedlings in relation to the concentrations of nicotianamine, an endogenous chelator of divalent metal ions, in different seedling parts. Plant Soil. 1994;165:181–188. [Google Scholar]
- Stephan UW, Schmidke I, Stephan VW, Scholz G. The nicotianamine molecule is made-to-measure for complexation of metal micronutrients in plants. Biometals. 1996;9:84–90. [Google Scholar]
- Stephan UW, Scholz G. Nicotianamine: mediator of transport of iron and heavy metals in the phloem? Physiol Plant. 1993;88:522–529. [Google Scholar]
- Sugiura Y, Nomoto K. Phytosiderophores: structures and properties of mugineic acids and their metal complexes. Struct Bonding. 1984;58:107–135. [Google Scholar]
- Sugiura Y, Tanaka H, Mino Y, Ishida T, Ota N, Inoue M, Nomoto K, Yoshioka H, Takemoto T. Structure, properties and transport mechanism of iron(III) complex of mugineic acid, a possible phytosiderophore. J Am Chem Soc. 1981;103:6979–6982. [Google Scholar]
- Takagi S. Naturally occurring iron-chelating compounds in oat- and rice-root washings. I. Activity measurement and preliminary characterization. Soil Sci Plant Nutr. 1976;22:423–433. [Google Scholar]
- Taylor PD, Morrison IEG, Hider RC. Microcomputer application of non-linear regression analysis to metal-ligand equilibrium. Talanta. 1988;35:507–512. doi: 10.1016/0039-9140(88)80123-1. [DOI] [PubMed] [Google Scholar]
- Tiffin LO. Iron translocation. I. Plant culture, exudate sampling, iron-citrate analysis. Plant Physiol. 1966;41:510–514. doi: 10.1104/pp.41.3.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treeby M, Marschner H, Römheld V. Mobilization of iron and other micronutrient cations from a calcareous soil by plant-borne, microbial, and synthetic metal chelators. Plant Soil. 1989;114:217–226. [Google Scholar]
- von Wirén N, Marschner H, Römheld V. Uptake kinetics of iron-phytosiderophores in two maize genotypes differing in iron efficiency. Physiol Plant. 1995;93:611–616. [Google Scholar]
- von Wirén N, Marschner H, Römheld V. Roots of iron-efficient maize also absorb phytosiderophore-chelated zinc. Plant Physiol. 1996;111:1119–1125. doi: 10.1104/pp.111.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter A, Pich A, Scholz G, Marschner H, Römheld V. Effects of iron nutritional status and time of day on concentrations of phytosiderophores and nicotianamine in different root and shoot zones of barley. J Plant Nutr. 1995;18:1577–1593. [Google Scholar]
- White MC, Decker AM, Chaney RL. Metal complexation in xylem fluid. I. Chemical composition of tomato and soybean stem exudate. Plant Physiol. 1981;67:292–300. doi: 10.1104/pp.67.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang V, van Eldik R. Kinetics and mechanism of the autoxidation of iron(II) induced through chelation by ethylenediaminetetraacetate and related ligands. Inorg Chem. 1990;29:1705–1711. [Google Scholar]