Abstract
Late in infection herpesviruses move DNA-filled capsids from the nucleus to the cytoplasm by enveloping DNA-containing capsids at the inner nuclear membrane (INM) and deenveloping them at the outer nuclear membrane. This process requires two conserved herpesvirus proteins, pUL31 and pUL34. Interaction between pUL34 and pUL31 is essential for targeting both proteins to the nuclear envelope (NE), and sequences that mediate the targeting interaction have been mapped in both proteins. Here, we show that a mutation in the INM-targeting domain of pUL34 fails to support production of infectious virus or plaque formation. The mutation results in multiple defects, including impaired interaction between pUL34 and pUL31, poor NE targeting of pUL34, and misregulated, capsid-independent budding of the NE. The mutant defects in virus production, plaque formation, and pUL31 interaction can be suppressed by other mutations in the INM-targeting domain of pUL31 and by additional mutations in the pUL34 coding sequence.
INTRODUCTION
Efficient nuclear egress of herpesviruses requires formation of a nuclear envelopment complex (NEC) consisting of viral and cellular proteins (24, 26, 34). The NEC contains homologs of the herpes simplex virus (HSV) UL31 and UL34 proteins (called pUL31 and pUL34), and these are critical for nuclear egress in all herpesviruses tested (9, 15, 24, 25, 33). pUL31 and pUL34 homologs interact with each other, and formation of a pUL31/pUL34 complex is required for proper targeting of the complex to the nuclear envelope (NE) (10, 16, 30, 31, 36, 37, 41). The interactions that underlie complex formation are therefore critical for assembly and egress of all herpesviruses.
Despite the conservation of a pUL31-pUL34 interaction, it is not clear that the structural basis for that interaction is completely conserved. The sequence of pUL31 and its homologs can be divided into four conserved regions (CRs) (Fig. 1B) (37). The most N-terminal of these regions (CR1) has been shown to mediate interaction with pUL34 homologs in examples from all herpesvirus subfamilies (19, 37). The situation with pUL34 homologs is less clear. For HSV-1 pUL34, the sequence that interacts with pUL31 and that is required for nuclear envelope targeting was mapped by deletion and domain swapping to amino acids (aa) 137 to 181 (18). This corresponds to the third of three CRs in the pUL34 sequence (Fig. 1A). Consistent with this, a construct containing CR1, CR2, and most of CR3 (aa 1 to 161) of pseudorabies virus (PRV) pUL34 was sufficient to interact with pUL31 in a yeast two-hybrid assay (10). In mouse cytomegalovirus (MCMV), on the other hand, use of small insertions and point mutations implicated a different region of the UL34 homolog, M50, in binding to the UL31 homolog, M53 (4, 19, 28). The interaction region is located in a highly conserved stretch of residues at the N terminus of M50 CR2. Whether the differences between MCMV M50 and HSV pUL34 reflect a very different structural basis for interaction is not yet clear.
Fig. 1.
Schematic diagrams of pUL34 (A) and pUL31 (B) showing the locations of relevant sequence features. Protein sequences are indicated as bars with the N terminus at the left. Sequences in pUL31 and pUL34 that mediate nuclear envelope targeting of the NEC are indicated as stippled regions. Positions of the CL13 charged cluster mutation and of intragenic and extragenic suppressor mutations described in this study are indicated above each of the bars. Positions of conserved regions are indicated immediately below each of the bars. Designation of conserved domains in pUL31 follows the nomenclature proposed by Schnee et al. (37).
At the NE, pUL34 and pUL31 mediate subsequent steps in nuclear egress, including disruption of the nuclear lamina, docking of capsids at the inner nuclear membrane (INM), capsid budding into the INM, and capsid deenvelopment and release to the cytoplasm (3, 17, 22, 23, 26, 29, 33, 38, 39). The capsid budding function also requires interaction between pUL31 and pUL34 (32), but it is not clear whether the same interaction sequences required for NE targeting are required or involved at this stage. It is, however, clear that budding in HSV-1 infection requires additional structural and functional interaction between pUL31 and pUL34. Sequences required for this interaction include, but may not be limited to, CR1 of pUL34 and CR3 and CR4 of pUL31 (32).
Here, we show that an amino acid substitution mutation at a charge cluster within CR3 of HSV-1 pUL34 results in multiple defects during infection. As expected, interaction with pUL31 and NE targeting of pUL34 are impaired. In addition, however, this mutation results in misregulated, capsid-independent vesicularization of the inner nuclear membrane and a specific defect in plaque formation. Extragenic mutations that suppress both the virus growth and UL31 interaction defects map to CR1 of pUL31, suggesting the importance of the pUL31/pUL34 interaction in multiple functions of pUL34.
MATERIALS AND METHODS
Cells and viruses.
Vero cells and cell lines derived from Vero cells were maintained as previously described (33). The properties of HSV-1(F), vRR1072 (TK+), referred to here as UL34-null virus, and the bacterial artificial chromosome (BAC)-derived UL34-null virus have been previously described (8, 32, 33).
Plasmids and cell lines.
pRR1072, pRR1072Rep, and pRR1164, which contains the CL13 charge cluster mutation in UL34 on the pRR1072Rep background, were previously described (2, 33).
The plasmid pRR1369, used for construction of an infection-inducible CL13-expressing cell line, was made by ligation of the CL13 UL34 coding sequence and 5′ promoter/regulatory sequences into the vector pTuner-IRES2 (Clontech), from which the human CMV (HCMV) promoter has been removed. The plasmid pRR1164 was digested with AseI, followed by treatment with Klenow enzyme in the presence of deoxynucleoside triphosphates (dNTPs) to create a blunt end, and then subsequently digested with BspEI. The 1.25-kb fragment containing the UL34 gene was ligated into pTuner-IRES2 that had been digested with AseI, treated with Klenow enzyme in the presence of dNTPs to create a blunt end, and then subsequently digested with XmaI. The resulting plasmid expresses both CL13 pUL34 and enhanced green fluorescent protein (EGFP) from the UL34 promoter/regulatory sequences upon infection with HSV-1. An otherwise identical plasmid for expression of wild-type (wt) pUL34 was constructed using pRR1072Rep as the source of the insert.
The clonal cell line tCL13AP was constructed by transfection of pRR1369 into Vero cells, followed by selection with G418 and isolation of clones by limiting dilution. Candidate-expressing cell clones were identified by their expression of EGFP 16 h after infection with HSV-1(F). Expression of CL13 pUL34 was then confirmed by immunofluorescence assay for pUL34 16 h after infection with UL34-null virus.
The plasmids pRR1370, -1371, -1372, and -1373 were constructed by replacement of the NcoI-AflII fragment of the UL34 gene in pRR1072Rep with the same sequence from PCR-amplified UL34 genes of suppressor viruses CL13RevB, CL13RevC, CL13REevE, and CL13RevG, respectively.
Selection of extragenic suppressors.
Twelve-well cultures of tCL13AP cells were each infected with 1 × 107 PFU of UL34-null virus. After 2 h the inoculum was removed, and monolayers were treated with low-pH citrate buffer to inactivate residual virus. One day after infection, virus stocks were prepared from each of the infected cultures by one cycle of freezing and thawing followed by sonication and plated onto fresh cultures of tCL13AP cells. Robust plaques were picked 2 days later and brought through two more rounds of plaque purification on wt pUL34-expressing cells. Four of 10 infected cultures gave rise to viruses that could form robust plaques on tCL13AP cells but not on Vero cells, indicating that they were still UL34 null.
Single-step growth measurement.
Measurement of replication of HSV-1(F), vRR1072(TK+), and CL13Rev viruses on Vero, RepAC, and tCL13AP cells after infection at high multiplicity was performed as previously described (29).
Complementation assays.
Twelve-well cultures of Vero cells containing 400,000 cells were transfected with a total of 650 ng of plasmid mixtures using 5 μl of Lipofectamine according to the manufacturer's instructions. Complementation assays were performed on transfected cultures as previously described (2).
Indirect immunofluorescence.
Immunofluorescence for detection of pUL34 and pUL31-FLAG was performed as previously described (32). All confocal microscopy work was done with a Zeiss 510 confocal microscope. All images shown are representative of experiments performed a minimum of three times.
Immunoblotting.
Nitrocellulose sheets bearing proteins of interest were blocked in 5% nonfat milk plus 0.2% Tween 20 for at least 2 h. The membranes were probed either with a previously described chicken polyclonal antibody directed against pUL34 (1:1,000) (30) and reacted with alkaline phosphatase-conjugated anti-chicken secondary antibody (Aves Laboratories), with mouse monoclonal antibody directed against the HSV-1 scaffolding protein (1:2,000) (Serotec) and reacted with alkaline phosphatase-conjugated anti-mouse secondary antibody (1:1,000) (Sigma), or with anti-FLAG M2 mouse monoclonal antibody (1:1,000) (Sigma) and reacted with alkaline phosphatase-conjugated anti-mouse (1:1,000).
Coimmunoprecipitation.
Transfection of 293T cells and immunoprecipitation with anti-FLAG magnetic beads were performed as previously described (32).
TEM of infected cells.
Confluent monolayers of Vero or tCL13AP cells were infected with vRR1072(TK+) at a multiplicity of 10 for 20 h and then fixed by incubation in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) for 2 h. Cells were postfixed in 1% osmium tetroxide, washed in cacodylate buffer, embedded in Spurr's resin, and cut into 95-nm sections. Sections were mounted on grids, stained with uranyl acetate and lead citrate, and examined with a JEOL 1230 transmission electron microscope (TEM).
BAC construction.
An HSV-1 BAC genome carrying a UL34 deletion and a mutation in UL31 creating an L64I substitution [UL31(L64I)] was engineered using Red recombineering on the background of a UL34-null BAC as previously described (32, 40). The UL34-null BAC was mutagenized at the UL31 locus by insertion and scarless excision of a gentamicin resistance (Gmr) cassette.
The Gm resistance cassette with mutant UL31 flanking sequence was constructed in several steps. First, a Gm resistance cassette containing the Gmr promoter, protein coding sequence, and terminator flanked at the 5′ end with an SceI homing nuclease site was amplified from pFastBac-1 template (Invitrogen) using primers Sce-GmR For and Sce-GmR Rev (Table 1 ). Second, PCR products containing the 5′ and 3′ halves of the Gm resistance gene were amplified from the Gmr cassette template using the primer pair L64I-Gm For and Gm mid Rev and the pair Gm mid For and L64I-Gm Rev, respectively. The two resulting PCR products overlap in the Gm coding sequence. The complete Gm resistance cassette with UL31 flanking sequence was then assembled in a PCR using the overlapping partial genes and the primers L64I unique For and L64I unique Rev (Table 1). The resulting PCR product was recombined into the UL34-null BAC, Gm-resistant recombinants were picked, and genomes were tested for insertion of the Gm cassette by diagnostic PCR using the flanking primers UL31 test Fwd and UL31 test Rev (Table 1). Correct insertion of the Gm cassette was confirmed by direct sequencing of the BAC DNA. Scarless excision of the Gm cassette, leaving an intact UL31 gene carrying the L64I mutation, was carried out as described previously, and Gm-sensitive, kanamycin (Kan)-resistant clones were tested for correct structure at both the UL31 and UL34 loci by diagnostic PCR using the UL34 test For, UL34 test Rev, UL31 test Fwd, and UL31 test Rev primers. Correct structure was confirmed by direct sequencing of the BAC DNA at both loci.
Table 1.
Primers used for construction and analysis of mutant BACs
| Primer name | Primer sequence |
|---|---|
| Sce-GmR Fora | 5′-GACATGGATCCTAGGGATAACAGGGTAATAATTGACATAAGCCTGTTCGGTTCG-3′ |
| Sce-GmR Reva | 5′-GTAGCTCTAGAGGCCGCGGCGTTGTGAC |
| L64I Gm Fwdb | 5′-AACAGGAGCTGTGTTTACACGAGCGCCAGCGCTATCGGGaaTaTTCGCCGCCCTCGCCCAGACGCTCGGATCCTAGGGATAACAGGG-3′ |
| L64I Gm Revb | 5′-TGGCGATCTCCTCGGAGGGCGTCTGGGCGAGGGCGGCGAAtAttCCCCGATAGCGCTGGCGCTCTCTAGAGGCCGCGGCGTTG-3′ |
| Gm mid For | 5′-GGTCGTGAGTTCGGAGACGTAGC-3′ |
| Gm mid Rev | 5′-CACTACGCGGCTGCTCAAACC-3′ |
| L64I unique For | 5′-AACAGGAGCTGTGTTTACACGAGCG-3′ |
| L64I unique Rev | 5′-TGGCGATCTCCTCGGAGGGC-3′ |
| UL31 test Fwd | 5′-TGCCCCTGGTGAAGACCAC-3′ |
| UL31 test Rev | 5′-GCTACGGCGGAGGAAACTCG-3′ |
Underlined sequences have homology to the Gmr cassette.
Underlined sequences have homology to the UL31 gene. Lowercase indicates nucleotides altered to create the L64I mutation and an SspI restriction enzyme cleavage site. Sequences not underlined have homology to the SceI-Gmr cassette.
Viruses were rescued from the wt BAC, UL34-null BAC, and UL31(L64I) BAC by transfection into UL34-expressing complementing cells.
RESULTS
Construction and characterization of a CL13 pUL34-expressing cell line.
The UL34 sequence may be divided into three relatively well-conserved domains, designated CR1, CR2, and CR3, between aa 1 and 190 of HSV pUL34, followed by a relatively poorly conserved region from aa 191 to the transmembrane domain (Fig. 1A) (13). The pUL34 CR3 domain corresponds closely to the pUL34 sequences that are necessary and sufficient for an interaction with pUL31 that leads to NE targeting of the pUL31/pUL34 complex (18). We have generated a charge cluster mutant called CL13 in pUL34 that changed arginine residues at positions 158 and 161 to alanine (2). CL13 mutant UL34 showed little or no ability to complement replication of UL34-null virus in trans, suggesting that an essential function of pUL34 had been disrupted. The CL13 mutation is in the middle of pUL34 CR3 and might therefore be expected to disrupt NE localization of pUL34 and pUL31. However, we previously showed using a transient complementation system that NE localization of CL13 was not completely abrogated since CL13 pUL34 could be tightly localized at the nuclear envelop in some cells (2). The efficiency of NE localization could not be assessed in the previous study because of highly heterogeneous pUL34 expression levels following transient transfection. The failure of CL13 pUL34 to support viral replication despite proper targeting in at least some cells suggested that pUL34 CR3 participates in some critical function(s) in addition to its role in NE targeting.
We used a stable transcomplementation strategy to further characterize both CL13 UL34 localization during infection and any additional functions. This system has the advantage that all cells express relatively uniform levels of mutant pUL34 and so is not prone to overexpression artifacts. Clonal cell lines expressing CL13 pUL34 under the control of its own promoter-regulatory sequences were constructed by stable transfection of Vero cells. Several stable lines were isolated and evaluated for pUL34 expression after infection with UL34-null virus (Fig. 2). One, called tCL13AP (henceforth referred to as CL13 pUL34-expressing cells), was chosen for further study. In these cells CL13 pUL34 expression was not as high as that of wt pUL34 in wild-type virus-infected cells but was higher than that of wt pUL34 expression in wt pUL34-expressing RepAC cells (henceforth referred to as wt pUL34-expressing cells). This allows phenotypic comparison between wt and CL13 pUL34-expressing stable cell lines and ensures that any putative CL13 phenotype observed in comparisons between them could not be ascribed to lower levels of CL13 pUL34 expression.
Fig. 2.

Expression of wild-type and CL13 mutant UL34 by stable cell lines. Digital images of Western blots are shown. Vero cells (lanes 1 and 2), RepAC cells that stably express wt pUL34 (lane 3), or tCL13AP cells that express CL13 pUL34 (lane 4) were infected with wt HSV-1(F) (lane 1) or UL34-null virus (lanes 2 to 4). Blotted infected cell proteins were probed for pUL34. All lanes are from the same blot, but lanes 1 and 2 and lanes 3 and 4 were not adjacent in the blot, as indicated by the vertical black line.
Characterization of the CL13 mutation effect on virus growth and selection of an extragenic suppressor of the CL13 growth phenotype.
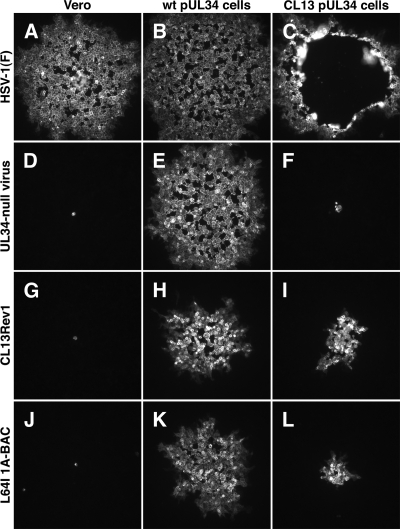
To test the ability of CL13 pUL34-expressing cells to support plaque formation, wt HSV-1(F) and UL34-null virus were plated at low multiplicity of infection on Vero cells, wt pUL34-expressing cells, and CL13 pUL34-expressing cells (Fig. 3). As expected, HSV-1(F) forms robust plaques with similar efficiencies on all three of the cell lines tested (Fig. 3A to C), indicating that CL13 pUL34-expressing cells show no restriction for plaque formation of HSV-1. This is consistent with previous results demonstrating that CL13 pUL34 has no dominant negative phenotype (32). UL34-null virus was unable to form plaques on CL13-expressing cells (Fig. 3F). Infectious foci ranged in size from one to a few infected cells and were only slightly larger than foci formed by UL34-null virus on Vero cells that express no pUL34 (panel D), suggesting that CL13 pUL34 is profoundly impaired in its ability to support virus growth, cell-to-cell spread, or both.
Fig. 3.
Formation of plaques on wt or CL13 mutant pUL34-expressing cell lines. Digital micrographs of immunofluorescently stained infected cell monolayers are shown. Vero cells (A, D, G, and J), wt pUL34-expressing cells (B, E, H, and K), or CL13 pUL34-expressing cells (C, F, I, and L) were infected at low multiplicity with the viruses indicated to the left of each row. Two hours after initiation of infection, monolayers were overlaid with medium containing pooled human immunoglobulin (GamaStan S/D). After 2 days, monolayers were fixed with formaldehyde and immunofluorescently stained using mouse monoclonal antibody directed against gD.
The transcomplementation system used here is well suited to selection for extragenic suppressors of point mutants. Genuine suppressors isolated by passage of UL34-null virus on mutant UL34-expressing cells can only be extragenic suppressors since the virus under selection has no UL34 gene, and thus there is no selection for intragenic suppressors. Four suppressor viruses were isolated from 10 selections performed as described in Materials and Methods, and one of these, called CL13Rev1, was chosen for further characterization. CL13Rev1 could not form plaques in cells that express no pUL34 (Fig. 3G) but formed plaques on both wt and CL13 pUL34-expressing cells (panels H and I, respectively), indicating that the UL34 deficiency in this virus could be complemented by either wt or CL13 mutant UL34. The plaques formed on CL13 pUL34-expressing cells, however, were smaller than those formed on wt pUL34 cells (Fig. 3, compare H and I), indicating that virus production or plaque formation was complemented less efficiently by CL13 pUL34.
The single-step growth kinetics of HSV-1(F), UL34-null virus, and CL13Rev1 were measured on Vero cells, wt pUL34-expressing cells, and CL13 pUL34-expressing cells (Fig. 4). As expected, neither the UL34-null nor CL13Rev1 virus replicates well on Vero cells. All three tested viruses replicated efficiently on wt UL34-expressing cells, indicating that both UL34-null viruses can be complemented by wt pUL34. Both UL34-null viruses produce about 10-fold less virus than wt HSV-1(F), however, probably because of the low level of wt pUL34 expressed by the complementing cells. CL13Rev1 replicates efficiently on CL13 pUL34-expressing cells, achieving a peak titer slightly better than that obtained on wt pUL34-expressing cells and only 5-fold lower than that attained by wt virus, whereas UL34-null virus achieves a peak titer about 100-fold less than that of wt virus. This result indicates that CL13Rev1 replication can be efficiently complemented by CL13 pUL34 while its parent cannot.
Fig. 4.
Single step growth of wild-type and mutant viruses on complementing and noncomplementing cells. Replicate cultures of Vero, wt pUL34-expressing, or CL13 pUL34-expressing cells were infected at an MOI of 5 with HSV-1(F), the UL34-null virus, or CL13Rev1. Residual virus was removed or inactivated with a low-pH wash, and at the indicated times total culture virus was titrated on wt UL34-expressing cells. Virus yields are expressed as the number of PFU per milliliter on a logarithmic scale. Each data point represents the mean of three independent experiments. Error bars indicate the range of values.
The growth defect observed with CL13 pUL34 was not due to a deficiency in a step that precedes nuclear egress since similar amounts of DNA-containing capsids accumulated in the nuclei of infected wt pUL34-expressing and CL13 pUL34-expressing cells (data not shown).
An extragenic suppressor of the CL13 mutation maps to UL31.
The CL13 mutation in pUL34 is in the middle of pUL34 CR3 (Fig. 1), which was previously shown to interact with the CR1 region of pUL31 in order to mediate targeting of both proteins to the nuclear envelope (18, 19, 37). The isolation of extragenic suppressors of the CL13 mutation strongly suggested that the function(s) disrupted by the CL13 mutation is mediated by interaction with another viral protein, perhaps pUL31. Sequencing of the UL31 gene from the suppressor virus revealed a single nucleotide substitution that results in the change of the leucine residue at position 64 to isoleucine (L64I). This change is in the UL31 CR1 that has been shown to interact with pUL34 CR3. To test the hypothesis that the L64I substitution in pUL31 is sufficient to suppress the CL13 mutant phenotype, we tested the phenotype of a recombinant virus rescued from a BAC that was UL34 null and carried UL31(L64I) [UL34-null/UL31(L64I)]. Starting with a previously described UL34-null BAC, we performed point mutagenesis of the UL31 gene as previously described (32). Wild-type, UL34-null, and UL34-null/UL31(L64I) viruses were rescued by transfection of the BAC clones into wt pUL34-expressing complementing cells. Introduction of the L64I mutation into the UL31 locus was confirmed by sequencing of the UL31 gene in the context of the intact BAC and by sequencing of the UL31 gene PCR amplified from the rescued UL34-null/UL31(L64I) virus.
To determine whether the presence of the L64I mutation is sufficient for suppression of the CL13 phenotype, Vero, wild-type pUL34-expressing, and CL13 pUL34-expressing cells were infected at low multiplicity with BAC-derived wild type, UL34-null, and UL34-null/UL31(L64I) viruses. After 2 days, plaques were detected by indirect immunofluorescence using primary antibody directed against glycoprotein D (Fig. 3). BAC-derived wild-type and UL34-null viruses formed plaques indistinguishable from the non-BAC-derived viruses on all three cell lines (data not shown). The UL34-null/UL31(L64I) BAC-derived virus was unable to form plaques on Vero cells (Fig. 3J) but formed plaques on CL13 UL34-expressing cells comparable in size to those formed by CL13Rev1 virus (Fig. 3L and I, respectively), suggesting that in the context of a recombinant virus, the L64I mutation is sufficient to allow suppression of the CL13 plaque formation phenotype.
Intragenic suppressors of the CL13 mutation implicate pUL34 CR1 and CR3 in interaction with UL31.
Whereas extragenic suppressors provide information about other proteins that cooperate with pUL34, intragenic suppressors (mutations within UL34) provide information about other residues and sequence regions of UL34 that contribute to the wild-type phenotype. Our transcomplementation system does not allow for selection of intragenic suppressors. Therefore, we attempted to isolate a recombinant virus carrying CL13 UL34 that would serve as a parent for selection of intragenic suppressors. Multiple attempts were made to isolate and amplify on complementing cells a recombinant HSV-1 that carries a CL13 pUL34 gene. These attempts invariably resulted in isolation of viruses that formed plaques on Vero cells that expressed no pUL34, suggesting that the amplified viruses had either undergone homologous repair of the UL34 locus or acquired additional mutations that suppressed the CL13 phenotype. The UL31 and UL34 genes were PCR amplified and sequenced from these viruses. Interestingly, all of the viruses isolated still carried the CL13 mutation at the UL34 locus and had additional mutations in either UL31 or UL34 (Table 2). The single mutation found in the UL31 gene in CL13RevI is of the same type as that found in the extragenic suppressor virus CL13Rev1 (i.e., substitution of one bulky hydrophobic residue with another) and very close to the L64I mutation, suggesting that it, too, is an extragenic suppressor of the CL13 pUL34 phenotype. The remaining viruses all had putative intragenic suppressor mutations within the UL34 gene itself.
Table 2.
UL31 and UL34 putative suppressor mutations in CL13 recombinant viruses
| CL13Rev isolate | UL31 mutation |
UL34 mutation |
||
|---|---|---|---|---|
| SNPa | Amino acid change | SNPa | Amino acid change | |
| A, B, D, H | None | None | A→C at nt 460 | S154R |
| C | None | None | A→C at nt 233 | E78G |
| A→C at nt 460 | S154R | |||
| E | None | None | C→T at nt 530 | T177M |
| G | None | None | T→G at nt 74 | L25R |
| I | A→T at nt 232 | I78F | None | None |
Positions of nucleotide (nt) substitutions are numbered from the beginning of the protein coding sequence. SNP, single nucleotide polymorphism.
To determine whether the mutations in the UL34 gene were sufficient to suppress the CL13 mutant phenotype, the UL34 genes from isolates CL13RevB, -C, -E, and -G that contain both the CL13 mutation and the putative intragenic suppressor mutations were cloned into plasmids and tested for their ability to complement replication of UL34-null virus in trans (Fig. 5A). All of the plasmids containing the CL13 mutation and a putative intragenic suppressor were found to complement UL34-null virus replication to a degree similar to that of the wild-type UL34 plasmid. There were, however, small but significant differences among the suppressor plasmids in their complementation activity. Plasmids encoding CL13RevB and CL13RevG were not significantly different from wt UL34, but both CL13RevC and CL13RevE complemented only about half as well as CL13RevB (P = 0.022 and P = 0.001, respectively, in a two-tailed, paired Student t test). The CL13RevC has the same S154R substitution found in CL13RevB plus another substitution nearer the N terminus. Despite the additional mutation, CL13RevC does not complement as well as CL13RevB, suggesting that the additional substitution does not contribute to the suppressor phenotype and may, in fact, detract from it.
Fig. 5.
Suppressor function of pUL34 mutants. (A) Transcomplementation of single-step UL34-null replication. Vero cells were transfected with plasmids that express either wt pUL34 (pRR1072Rep) or mutant pUL34 encoding the CL13 mutation and putative intragenic suppressor mutations under the control of the UL34 promoter/regulatory sequences (pRR1370-pRR1373) or with a plasmid (pRR1072) that expresses EGFP under the control of pUL34 promoter/regulatory sequences. Cells were also transfected with a beta-galactosidase-expressing plasmid to allow normalization for transfection efficiency. Transfected cells were subsequently infected with UL34-null virus, and at 18 h after infection the amount of infectious virus produced was determined by plaque assay on wt UL34-complementing cells. Virus production was normalized for transfection efficiency, and the complementation index was calculated as the ratio of virus produced by the test plasmid to that produced by the plasmid that encodes wt pUL34. Data are graphed on a logarithmic scale, and each point represents the mean of three independent experiments. Error bars represent the range of values. (B) Sizes of plaques formed by intragenic suppressor viruses. Histograms of mean plaque sizes on Vero and wt pUL34-expressing RepAC cells are shown. Immunofluorescently stained plaques were photographed, and plaque areas in image pixels were determined using ImageJ. The asterisk indicates that only single cells or small clusters of a few cells were formed by UL34-null virus on Vero cells.
Interestingly, despite the observation that all of the mutant UL34 genes had function similar to that of the wild type in the complementation assay, the viruses from which the mutant genes were derived formed significantly smaller plaques on both Vero cells and on wt pUL34-expressing cells than wt virus (Fig. 5B), suggesting that the CL13 mutation also affects the cell-to-cell spread function of pUL34 (13) and that this phenotype is not efficiently suppressed by the intragenic mutations.
Localization of CL13 UL34 in infected and transfected cells.
To determine whether proper localization of pUL34 was maintained in the stable CL13-expressing cell line, Vero, wt pUL34-expressing, and CL13 pUL34-expressing cells were plated onto coverslips and then infected for 16 h with HSV-1(F), UL34-null virus, or CL13Rev1. pUL34 was detected by immunofluorescence (Fig. 6). pUL34 from wt virus infection showed tight localization at the nuclear envelope, as expected (Fig. 6A and B), indicating that neither of the cell lines has a nonspecific defect in pUL34 localization. Wild-type pUL34 expressed from the cellular genome also showed wild-type localization at the NE (Fig. 6C). CL13 pUL34, in contrast, showed a range of localization phenotypes (panels D to F). In a small minority of cells (less than 10%) CL13 pUL34 showed typical wild-type localization (Fig. 6D). In most cells, however, it was less tightly localized at the NE (panels E and F), with some or most of the pUL34 found in the cytoplasm. The CL13 defect in NE localization was not suppressed during infection with CL13Rev1 since most CL13 pUL34 was still found away from the nuclear rim in most cells (Fig. 6G).
Fig. 6.
Localization of wt pUL34 and CL13 mutant pUL34 in infected cells. Digital confocal images are shown of wt pUL34-expressing cells (A and C) or CL13 pUL34-expressing cells (B and D to G) subsequently infected with either HSV-1(F) (A and B), UL34-null virus (C to F), or the UL34-null suppressor virus CL13Rev1 (G). Note that the background is much higher for panels C to G because of the relatively low level of pUL34 expression from the cellular genome in these cell lines.
The abnormal localization of pUL34 in infected cells suggested the possibility that interaction between pUL34 and pUL31 might be impaired by the CL13 mutation. To test this hypothesis, we cotransfected plasmids that express wild-type and mutant pUL34 and pUL31 into Vero cells and assessed colocalization of the two proteins (Fig. 7). pUL31 expressed alone localized diffusely within the nucleoplasm, and pUL34 expressed alone localized on cytoplasmic membranes, as previously reported (Fig. 7A and B). Localization of the CL13 mutant pUL34 and of the L64I mutant pUL31 expressed alone was exactly the same as that of their wild-type counterparts (data not shown). As expected, wt pUL34 and wt pUL31 completely colocalized and were found in part at the nuclear rim in small puncta (panel C). We also observed small aggregations of colocalized pUL34 and pUL31 in the nucleus. Coexpression of CL13 pUL34 and wt pUL31 resulted in poor colocalization, with many cells showing no colocalization and no recruitment of either protein to the nuclear envelope (Fig. 7D). Expression of L64I UL31 with CL13 pUL34 resulted in partial restoration of the phenotype. In the vast majority of cells, the suppressor mutant UL31 completely colocalized with pUL34, but not vice versa (Fig. 7E). Also, the colocalized pUL31 and pUL34 were not recruited to the nuclear rim. Rather, they were found solely in intranuclear aggregates. The same phenotype was observed when UL34 genes carrying any of the intragenic suppressor mutations were coexpressed with wt pUL31 (CL13RevB is shown as an example in Fig. 7F).
Fig. 7.
Interaction of wild-type and mutant pUL34 and pUL31 in transfected cells. (A to F) Digital confocal images of Vero cells transfected with pUL31-FLAG and pUL34 expression constructs are shown. At 48 h after transfection, cells were fixed and immunofluorescently stained for pUL34 using anti-UL34 antibody (green) or for pUL31-FLAG using anti-FLAG antibody (red). The constructs trans- fected for each image are indicated to the left of the panel. (G) Quantitation of pUL31 colocalization with pUL34. Under each transfection condition, 50 randomly chosen cells were scored for the amount of pUL31 colocalized with pUL34. Note that while pUL31 might be completely colocalized with pUL34, the reverse was rarely observed (i.e., there was almost always pUL34 that was not colocalized with pUL31).
The results of colocalization experiments suggested that the CL13 mutation partially disrupts both the interaction between pUL34 and pUL31 and their recruitment to the nuclear rim. The results further suggest that the intragenic and extragenic suppressor mutations may restore the interaction but not recruitment. To confirm the effect on interaction, we determined whether CL13 pUL34 would coimmunoprecipitate with the wild-type or suppressor mutant pUL31 (Fig. 8). As expected, wt pUL34 could be efficiently coprecipitated with wt pUL31. The amount of coprecipitated CL13 pUL34 was diminished compared to the amount of wt pUL34. Efficient coprecipitation was restored with the L64I mutation in pUL31 or by any of the intragenic suppressor mutations in pUL34.
Fig. 8.
Coimmunoprecipitation of wild-type and mutant pUL34 and pUL31 from transfected cells. Digital images of Western blots (WB) are shown. 293T cells were transfected with the UL34 or UL31-FLAG constructs indicated above each lane, and lysates were prepared. Lysates were assayed for pUL31 and pUL34 expression by Western blotting (data not shown). All lysates from UL34-transfected cells expressed similar levels of pUL34. Samples containing equivalent amounts of pUL31-FLAG were prepared and immunoprecipitated (IP) with anti-FLAG magnetic beads. Immunoprecipitated samples were split in half, separated by SDS-PAGE, blotted to nitrocellulose, and probed with anti-FLAG antibody to detect pUL31-FLAG (top panel) or with anti-UL34 antibody to detect pUL34 (bottom panel).
CL13 pUL34 mutation confers a defect in regulation of nuclear membrane budding.
In order to characterize the defect in CL13 pUL34, Vero cells or CL13 pUL34-expressing cells were infected with HSV-1(F) or UL34-null mutant virus at an MOI of 10 for 20 h and then prepared for analysis by transmission electron microscopy (Fig. 9). Vero cells infected with HSV-1(F) (Fig. 9A) produce abundant cell surface virus particles (a few of many examples are indicated with small arrowheads) and nuclear and cytoplasmic egress intermediates, including perinuclear virions found between the inner and outer nuclear membranes (panel C). As shown previously, Vero cells infected with UL34-null virus do not produce cell surface or cytoplasmic virions or capsids even though nuclear empty and DNA-containing capsids are present (data not shown). CL13 pUL34-expressing cells infected with UL34-null virus also produce neither cell surface nor cytoplasmic virions or capsids but differed from normal Vero cells in that about half of cell sections (11 of 20 randomly selected sections) showed the presence of nuclear inclusions containing invaginated or invaginating vesicles and tubules (Fig. 9B). Given that each section samples only a small fraction of the volume of the nucleus, the appearance of these structures in 50% of the sections suggests that these structures may be present in most or all infected nuclei. The vesicles were generally somewhat larger than primary virions (compare primary virion in Fig. 9C with vesicles in D) and in most cases did not contain viral capsids. Those that did contain capsids differed from normal primary virions in that the membrane was not closely and uniformly apposed to the capsid (compare the example indicated by the arrowhead in Fig. 9D with the typical primary virion in C). In many sections connections between these intranuclear inclusions and the inner nuclear membrane were observed (Fig. 9E), suggesting that these inclusions are derived from the inner nuclear membrane.
Fig. 9.
Transmission EM analysis of nuclear egress from cells that express CL13 pUL34. Digital micrographs show CL13 pUL34-expressing cells infected with the HSV-1(F) (A and C) or UL34-null virus (B, D, and E) for 20 h. Small black arrowheads in panel A point to examples of extracellular mature viruses. Large black arrowheads in panel B point to examples of intranuclear inclusions typical of UL34-null infection of CL13 pUL34-expressing cells. The black arrowhead in panel C points to a typical perinuclear virion formed in wild-type infection. The arrowhead in panel D points to a capsid-containing vesicle. Nucleus (Nuc) and cytoplasm (Cyt) are indicated in panels C to E. Scale bars are shown at the lower left of each panel.
DISCUSSION
pUL34 participates in multiple functions during virus egress, including disruption of the nuclear lamina, docking of capsids, and budding of the INM around the capsid during nuclear egress (3, 17, 22, 23, 26, 29, 33, 38, 39). Following nuclear egress, pUL34 is also required for efficient cell-to-cell spread (13). One of the advantages of detailed analysis of point mutations is that it can reveal defects in discrete steps along the egress pathway and assign functional roles to specific sequences in the protein. Analysis of the CL13 mutation confirms the importance of pUL34 CR3 sequences for formation and NE targeting of the pUL31/pUL34 complex but also indicates a previously unsuspected role for sequences near the N terminus of pUL34 in formation of the NEC. Our results also indicate a role for sequences in pUL34 CR3 in cell-to-cell spread and demonstrate a novel function for pUL34 in regulation of INM budding.
CL13 and pUL31-pUL34 interaction and NE targeting.
The CL13 mutation disrupts sequences in the CR3 region of pUL34 that were previously shown to be required for interaction with pUL31 and for NE targeting (18). The CL13 pUL34 colocalizes and coimmunoprecipitates with wt pUL31 poorly in cotransfected cells and shows poor localization to the nuclear envelope in infected cells. This confirms the importance of pUL34 CR3 in mediating stable interaction with pUL31 and in targeting of the complex to the NE. Interestingly, mutations in either pUL34 or pUL31 that suppress the CL13 virus replication defect restore the interaction between pUL34 and pUL31. The mutations in the UL31 gene that suppress CL13 replication and pUL34-pUL31 interaction defects cause changes in the amino acid sequence in CR1 of pUL31. This result is consistent with the observations of Schnee et al. that showed pUL31 CR1 to mediate interaction with pUL34 (37). Two of the three intragenic suppressor mutations in UL34 are also unsurprising since they are located in pUL34 CR3 and are relatively close to the original CL13 mutation. These mutations support the importance of CR3 sequences in interaction with pUL31. The CL13RevG mutation, however, is in pUL34 CR1 and is consistent with the idea that this region also plays a role in UL31 interaction during formation and targeting of the NEC.
CL13 and regulation of nuclear membrane budding.
None of the suppressor mutations characterized here could restore normal UL34 targeting in transfected or infected cells despite restoring the pUL31-pUL34 interaction. Surprisingly, they all nonetheless restored efficient virus replication, suggesting that even relatively poor NE localization may still support efficient nuclear egress. This further suggests that the poor NE localization of CL13 pUL34 is only partly responsible for the defects in replication and nuclear egress and that the CL13 mutation may lead to loss of some other critical function that depends on pUL31-pUL34 interaction. This other function may be regulation of INM budding.
Functional interaction between pUL31 and pUL34 is required to drive budding of the nuclear membrane in infection, and their expression and interaction are sufficient to drive budding of the nuclear membrane when they are expressed in the absence of other viral proteins (14, 32). While the CL13 mutation does not support efficient nuclear egress, it does support budding of vesicles into the inner nuclear membrane. The budding mediated by CL13 pUL34 is, however, misregulated. Budding at the inner nuclear membrane is normally regulated such that it rarely occurs in the absence of a capsid. Empty vesicles derived from the inner nuclear membrane are not a common feature of wild-type infections. Additionally, nuclear envelopment shows a preference for DNA-containing capsids, suggesting that interaction with some component of a mature capsid may be required to trigger the membrane curvature function of the NEC (1, 5, 7, 12, 21, 27, 35). Klupp et al. have shown that this restriction can be overcome by overexpression of pUL31 and pUL34 in the absence of other viral proteins (14). Our observations show that the restriction in infected cells is dependent on correct function of the pUL31/pUL34 complex and can be abrogated by the CL13 mutation in pUL34. This suggests that one function of wt pUL34 is to ensure that membrane budding events are properly regulated and occur only when triggered by a capsid.
The vesicles produced in the intranuclear inclusions of CL13 pUL34-expressing cells are of heterogeneous size and generally larger than primary virions. This suggests that virion size is not fully determined by the interactions within the pUL34/pUL31 complex in the nuclear membrane. Heterogeneous vesicle size was also observed in coexpression of pUL31 and pUL34 alone (14). These observations suggest that the virus capsid may play a scaffolding role in membrane curvature and that interactions between the NEC and the exterior of the capsid are likely to be involved in determining the correct degree of curvature. A scaffold function for capsid proteins in budding has precedent in the assembly of alphaviruses, where interactions between the icosahedrally ordered capsid and the internal tails of envelope proteins impose icosahedral symmetry on the envelope proteins (6, 11, 20).
We have previously provided genetic evidence that an interaction involving sequences near the N terminus of pUL34 and near the C-terminal end of pUL31 CR3 is required for nuclear membrane budding around the capsid (32). A physical interaction mediated by aa 1 to 90 of pUL34 (a region that includes CR1 and the site of the CL13RevG mutation) and aa 126 to 305 of pUL31 (including UL31 CR2, -3, and -4) can also be demonstrated (32). Interestingly, when pUL31 and pUL34 sequences are expressed in the absence of other viral proteins, this physical interaction requires that the N-terminal 125 aa of pUL31 (including CR1) be absent. These observations can be explained in a number of ways, but our results suggest that some interactions help form and stabilize the NEC and that others that occur only after conformational changes mediate and regulate membrane budding by the NEC. The interactions that help form and stabilize the NEC include those previously documented between UL34 CR3 and UL31 CR1 but are also likely to involve sequences near the N terminus of pUL34, as indicated by the location of the CL13RevG intragenic suppressor. During wild-type infection, docking of a capsid might initiate a conformational change that allows pUL34 N-terminal sequences to interact with the pUL31 C-terminal region, resulting in clustering/oligomerization of the NEC and budding vesicle formation. The presence of the CL13 mutation apparently destabilizes some pUL31-pUL34 interaction with two effects: inhibited NE localization and inappropriate, capsid-independent formation of interactions that mediate budding. Fewer pUL31/CL13 pUL34 complexes would arrive at the nuclear envelope, but those that did would mediate unregulated membrane budding. In this model, suppressor mutations in any of pUL31 CR1, pUL34 CR3, or pUL34 CR1 might stabilize the pUL34/pUL31 complex, restoring stable pUL31/pUL34 and perhaps proper regulation of budding. Involvement of the UL34 N terminus in formation and targeting of the NEC to the NE has not previously been reported for HSV UL34 but has been seen with the mouse cytomegalovirus (MCMV) homolog M50, where mutation of the tyrosine at position 57 causes failure of NE localization (4, 28).
CL13 and cell-cell spread.
pUL34 is required for efficient cell-cell spread, but the sequences required for this activity have not been defined (13). Mutation of Y68 of pUL34 (the residue homologous to Y57 of MCMV M50) causes a profound defect in cell-to-cell spread (13). The different behaviors of the suppressor viruses characterized here with respect to replication and plaque formation suggest that the residues mutated in CL13 pUL34 may also be important for cell-cell spread of HSV. The observation that CL13Rev1 replication on CL13 pUL34-expressing cells is as good as or better than that on wt pUL34-expressing cells while plaque formation is poorer (Fig. 3) suggests that the CL13 mutation negatively affects both replication and cell-cell spread and that CL13Rev1 does not suppress these two defects equally well. Similarly, the intragenic suppressor mutant pUL34 proteins support efficient viral replication in a transient complementation assay (Fig. 5A), but the viruses are severely impaired in plaque formation (Fig. 5C). These suppressor viruses that combine efficient viral replication with significant spread defects represent powerful tools for exploring the mechanism of pUL34 function in cell-cell spread.
ACKNOWLEDGMENTS
We thank the staff of the Central Microscopy Research Facility of the University of Iowa and especially Jean Ross for expertise and help with TEM analysis. We are grateful to Martina Maric for critical reading of the manuscript.
These studies were supported by the University of Iowa and Public Health Service award AI 41478. N.K. was supported by NSF REU site grant DBI-0097361.
Footnotes
Published ahead of print on 7 September 2011.
REFERENCES
- 1. al-Kobaisi M. F., Rixon F. J., McDougall I., Preston V. G. 1991. The herpes simplex virus UL33 gene product is required for the assembly of full capsids. Virology 180: 380–388 [DOI] [PubMed] [Google Scholar]
- 2. Bjerke S. L., et al. 2003. Effects of charged cluster mutations on the function of herpes simplex virus type 1 UL34 protein. J. Virol. 77: 7601–7610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bjerke S. L., Roller R. 2006. Roles for herpes simplex type 1 UL34 and US3 proteins in disrupting the nuclear lamina during herpes simplex virus type 1 egress. Virology 347: 261–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bubeck A., et al. 2004. Comprehensive mutational analysis of a herpesvirus gene in the viral genome context reveals a region essential for virus replication. J. Virol. 78: 8026–8035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang Y. E. V. S., Krug C., Sears P. W., Roizman A. E. B. 1997. The null mutant of the UL31 gene of herpes simplex virus 1: construction and phenotype in infected cells. J. Virol. 71: 8307–8315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng R. H., et al. 1995. Nucleocapsid and glycoprotein organization in an enveloped virus. Cell 80: 621–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Church G. A., Wilson D. W. 1997. Study of herpes simplex virus maturation during a synchronous wave of assembly. J. Virol. 71: 3603–3612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ejercito P. M., Kieff E. D., Roizman B. 1968. Characteristics of herpes simplex virus strains differing in their effect on social behavior of infected cells. J. Gen. Virol. 2: 357–364 [DOI] [PubMed] [Google Scholar]
- 9. Farina A., et al. 2005. BFRF1 of Epstein-Barr virus is essential for efficient primary viral envelopment and egress. J. Virol. 79: 3703–3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fuchs W., Klupp B. G., Granzow H., Osterrieder N., Mettenleiter T. C. 2002. The interacting UL31 and UL34 gene products of pseudorabies virus are involved in egress from the host-cell nucleus and represent components of primary enveloped but not mature virions. J. Virol. 76: 364–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fuller S. D. 1987. The T=4 envelope of Sindbis virus is organized by interactions with a complementary T=3 capsid. Cell 48: 923–934 [DOI] [PubMed] [Google Scholar]
- 12. Granzow H., et al. 2001. Egress of alphaherpesviruses: comparative ultrastructural study. J. Virol. 75: 3675–3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haugo A. C., Szpara M. L., Parsons L., Enquist L. W., Roller R. J. 2011. Herpes simplex virus type 1 pUL34 plays a critical role in cell-to-cell spread of virus in addition to its role in virus replication. J. Virol. 85: 7203–7215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klupp B. G., et al. 2007. Vesicle formation from the nuclear membrane is induced by coexpression of two conserved herpesvirus proteins. Proc. Natl. Acad. Sci. U. S. A. 104: 7241–7246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klupp B. G., Granzow H., Mettenleiter T. C. 2000. Primary envelopment of pseudorabies virus at the nuclear membrane requires the UL34 gene product. J. Virol. 74: 10063–10073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lake C. M., Hutt-Fletcher L. M. 2004. The Epstein-Barr virus BFRF1 and BFLF2 proteins interact and coexpression alters their cellular localization. Virology 320: 99–106 [DOI] [PubMed] [Google Scholar]
- 17. Leach N., et al. 2007. Emerin is hyperphosphorylated and redistributed in herpes simplex virus type 1-infected cells in a manner dependent on both UL34 and US3. J. Virol. 81: 10792–10803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liang L., Baines J. D. 2005. Identification of an essential domain in the herpes simplex virus 1 UL34 protein that is necessary and sufficient to interact with UL31 protein. J. Virol. 79: 3797–3806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lötzerich M., Ruzsics Z., Koszinowski U. H. 2006. Functional domains of murine cytomegalovirus nuclear egress protein M53/p38. J. Virol. 80: 73–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mancini E. J., Clarke M., Gowen B. E., Rutten T., Fuller S. D. 2000. Cryo-electron microscopy reveals the functional organization of an enveloped virus, Semliki Forest virus. Mol. Cell 5: 255–266 [DOI] [PubMed] [Google Scholar]
- 21. McNab A. R. D., et al. 1998. The product of the herpes simplex virus type 1 UL25 gene is required for encapsidation but not for cleavage of replicated viral DNA. J. Virol. 72: 1060–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morris J. B., Hofemeister H., O'Hare P. 2007. Herpes simplex virus infection induces phosphorylation and delocalization of emerin, a key inner nuclear membrane protein. J. Virol. 81: 4429–4437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mou F., Forest T., Baines J. D. 2007. Us3 of Herpes simplex type 1 encodes a promiscuous protein kinase that phosphorylates and alters localization of lamin A/C in infected cells. J. Virol. 81: 6459–6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muranyi W., Haas J., Wagner M., Krohne G., Koszinowski U. H. 2002. Cytomegalovirus recruitment of cellular kinases to dissolve the nuclear lamina. Science 297: 854–857 [DOI] [PubMed] [Google Scholar]
- 25. Neubauer A., Rudolph J., Brandmuller C., Just F. T., Osterrieder N. 2002. The equine herpesvirus 1 UL34 gene product is involved in an early step in virus egress and can be efficiently replaced by a UL34-GFP fusion protein. Virology 300: 189–204 [DOI] [PubMed] [Google Scholar]
- 26. Park R., Baines J. 2006. Herpes simplex virus type 1 infection induces activation and recruitment of protein kinase C to the nuclear membrane and increased phosphorylation of lamin B. J. Virol. 80: 494–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Poon A. P., Roizman B. 1993. Characterization of a temperature-sensitive mutant of the UL15 open reading frame of herpes simplex virus 1. J. Virol. 67: 4497–5503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Popa M., et al. 2010. Dominant negative mutants of the murine cytomegalovirus M53 gene block nuclear egress and inhibit capsid maturation. J. Virol. 84: 9035–9046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reynolds A. E., Liang L., Baines J. D. 2004. Conformational changes in the nuclear lamina induced by herpes simplex virus type 1 require genes UL31 and UL34. J. Virol. 78: 5564–5575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reynolds A. E., et al. 2001. UL31 and UL34 proteins of herpes simplex virus type 1 form a complex that accumulates at the nuclear rim and is required for envelopment of nucleocapsids. J. Virol. 75: 8803–8817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reynolds A. E., Wills E. G., Roller R. J., Ryckman B. J., Baines J. D. 2002. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J. Virol. 76: 8939–8952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roller R. J., Bjerke S. L., Haugo A. C., Hanson S. 2010. Analysis of a charge cluster mutation of herpes simplex virus type 1 UL34 and its extragenic suppressor suggests a novel interaction between pUL34 and pUL31 that Is necessary for membrane curvature around capsids. J. Virol. 84: 3921–3934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roller R. J., Zhou Y., Schnetzer R., Ferguson J., DeSalvo D. 2000. Herpes simplex virus type 1 UL34 gene product is required for viral envelopment. J. Virol. 74: 117–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ryckman B. J., Roller R. J. 2004. Herpes simplex virus type 1 primary envelopment: UL34 protein modification and the US3-UL34 catalytic relationship. J. Virol. 78: 399–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Salmon B., Cunningham C., Davison A. J., Harris W. J., Baines J. D. 1998. The herpes simplex virus type 1 UL17 gene encodes virion tegument proteins that are required for cleavage and packaging of viral DNA. J. Virol. 72: 3779–3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Santarelli R., et al. 2008. Identification and characterization of the product encoded by ORF69 of Kaposi's sarcoma-associated herpesvirus. J. Virol. 82: 4562–4572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schnee M., Ruzsics Z., Bubeck A., Koszinowski U. H. 2006. Common and specific properties of herpesvirus UL34/UL31 protein family members revealed by protein complementation assay. J. Virol. 80: 11658–11666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scott E. S., O'Hare P. 2001. Fate of the inner nuclear membrane protein lamin B receptor and nuclear lamins in herpes simplex virus type 1 infection. J. Virol. 75: 8818–8830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Simpson-Holley M., Colgrove R. C., Nalepa G., Harper J. W., Knipe D. M. 2005. Identification and functional evaluation of cellular and viral factors involved in the alteration of nuclear architecture during herpes simplex virus 1 infection. J. Virol. 79: 12840–12851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tischer B. K., von Einem J., Kaufer B., Osterrieder N. 2006. Two-step red-mediated recombination for versatile high-efficiency markerless DNA manipulation in Escherichia coli. Biotechniques 40: 191–197 [DOI] [PubMed] [Google Scholar]
- 41. Yamauchi Y., et al. 2001. Herpes simplex virus type 2 UL34 protein requires UL31 protein for its relocation to the internal nuclear membrane in transfected cells. J. Gen. Virol. 82: 1423–1428 [DOI] [PubMed] [Google Scholar]