Abstract
A complex of five human cytomegalovirus virus (HCMV) proteins, gH, gL, UL128, UL130, and UL131 (gH/gL/UL128-131), is essential for virus entry into epithelial cells. We previously showed that gH/gL/UL128-131 expressed in epithelial cells interferes with subsequent HCMV entry into cells. There was no interference with only gH/gL or gB. We concluded that the expression of gH/gL/UL128-131 causes a mislocalization or downregulation of epithelial cell proteins that HCMV requires for entry. In contrast, gH/gL/UL128-131 expression in fibroblasts did not produce interference, suggesting a different mechanism for entry. Here, we show that the coexpression of another HCMV glycoprotein, gO, with gH/gL in human fibroblasts interferes with HCMV entry into fibroblasts but not epithelial cells. However, the coexpression of gO with gH/gL did not increase the cell surface expression level of gH/gL and did not enhance cell-cell fusion, a process that depends upon cell surface gH/gL. Instead, gO promoted the export of gH/gL from the endoplasmic reticulum (ER) and the accumulation of gH/gL in the trans-Golgi network. Thus, interference with gH/gL or gH/gL/gO, i.e., the mislocalization or blocking of entry mediators, occurs in cytoplasmic membranes and not in cell surface membranes of fibroblasts. Together, the results provide additional support for our hypotheses that epithelial cells express putative gH/gL/UL128-1331 receptors important for HCMV entry and that fibroblasts express distinct gH/gL receptors.
INTRODUCTION
Human cytomegalovirus (HCMV) infects a diverse array of cell types in vivo, including fibroblasts, epithelial and endothelial cells, monocyte-macrophages, leukocytes, neurons, and placental trophoblasts (reviewed in reference 3). One mechanism for this diverse tropism apparently involves the use of different receptor binding proteins to mediate entry into different cell types. Wild-type or clinical HCMV strains all express a complex of five proteins, gH, gL, UL128, UL130, and UL131 (gH/gL/UL128-131), which is necessary for entry into epithelial and endothelial cells, leukocytes, and monocytes (2, 11, 12, 24, 25, 34, 38). Laboratory strains of HCMV, e.g., AD169 and others, were passaged repeatedly in fibroblasts and acquired mutations in the UL128-131 genes so that these viruses cannot assemble gH/gL/UL128-131 and, as a result, cannot infect endothelial and epithelial cells, leukocytes, and monocytes (5, 8, 12). The loss of UL128-131 appears to be an adaptation to growth in fibroblasts, because viruses without UL128-131 apparently possess a selective advantage when growing in these cells. HCMV also expresses another glycoprotein, gO, that forms distinct complexes with gH/gL that are necessary for HCMV entry into human fibroblasts (14, 15, 17, 19, 27, 38, 40). These observations fit with the notion that all herpesviruses rely on gH/gL complexes for entry into cells (31). Other herpesviruses, Epstein-Barr virus (EBV) and human herpesvirus 6, also express different gH/gL complexes that can mediate entry into distinct cell types (reviewed in references 16 and 21).
We described aspects of the HCMV entry pathway into epithelial and endothelial cells, a process that depends upon gH/gL/UL128-131 and endocytosis and low-pH-dependent fusion with endosomes (24). We also provided evidence that gH/gL/UL128-131 functions in epithelial cell entry by binding saturable molecules or receptors present on epithelial cells (26). This evidence involved a process known as interference, in which gH/gL/UL128-131 was expressed in cells by use of nonreplicating adenovirus (Ad) vectors and the cells were then subsequently challenged with HCMV. The expression of gH/gL/UL128-131 efficiently (95%) blocked HCMV entry into epithelial cells. The inhibition involved entry, rather than some other aspect of HCMV replication, because the inhibition could be overcome by use of polyethylene glycol, which chemically fuses the virus into cells. The expression of HCMV gB or gH/gL in epithelial cells did not block HCMV entry. We concluded that the expression of gH/gL/UL128-131 in epithelial cells downregulated or mislocalized saturable molecules that HCMV requires for entry. This concept was based on the example of herpes simplex virus (HSV) gD, which interferes with virus entry when expressed in cells (4, 10, 18). There was little or no interference when HSV gB and gH/gL were expressed in cells. Later, HSV gD receptors were described (reviewed in reference 31), and evidence was presented showing that the expression of gD reduces the availability of these receptors by removing receptors from cell surfaces or blocking receptor availability (32, 33). Thus, our evidence of gH/gL/UL128-131-mediated interference in epithelial cells was consistent with saturable gH/gL/UL128-131 receptors necessary for HCMV entry into these cells. These putative receptors are different from other HCMV receptors that were previously identified (9, 30, 39), because those receptors were defined by using HCMV laboratory strain AD169, which does not express gH/gL/UL128-131.
In contrast to epithelial cells, the expression of gH/gL/UL128-131 in fibroblasts did not block HCMV entry (26). The expression of gH/gL in fibroblasts produced a modest inhibition of HCMV entry. Coupled with observations that gH/gL/UL128-131 is not required for entry into fibroblasts and that HCMV entry into fibroblasts occurs by neutral-pH fusion with the plasma membrane, these results suggested that HCMV entry into fibroblasts does not require the same receptors expressed by epithelial cells (6). Given observations that HCMV mutants lacking gO are defective for replication and spread in fibroblasts (13, 17, 40), the simplest model might be that gH/gL/gO mediates entry into fibroblasts by binding fibroblast receptors. However, the situation is more complex, and two important sets of observations must be considered. First, gO of clinical strain TR was assembled with gH/gL and promoted endoplasmic reticulum (ER) export, but gO was not incorporated into the envelope of extracellular virions and did not attain resistance to endoglycosidase H (27). In addition, an HCMV TR gO-null mutant produced normal quantities of extracellular virus particles, but these particles exhibited highly reduced (90 to 95%) quantities of gH and gL and were highly defective in entering fibroblasts (40). Put together, these data led us to propose that gO acts as a molecular chaperone to promote the incorporation of gH/gL into the virion and that it is gH/gL, and not gH/gL/gO, which mediates entry into fibroblasts.
The robust interference in epithelial cells expressing gH/gL/UL128-131 provided support for saturable gH/gL/UL128-131 receptors. However, in fibroblasts, gH/gL/UL128-131 did not interfere with virus entry. Moreover, there was only a modest interference with gH/gL. Here, we show that the coexpression of gO with gH/gL produced interference in fibroblasts, but this was not the case in epithelial cells. We also made the surprising observation that the expression of gO with gH/gL did not substantially increase the cell surface expression levels of gH/gL. Thus, interference in fibroblasts must involve gH/gL- or gH/gL/gO-mediated effects on putative fibroblast receptors that occur in cytoplasmic membranes and not at the cell surface.
MATERIALS AND METHODS
Cells and viruses.
Neonatal normal human foreskin fibroblasts (HFFs) were obtained from Invitrogen and grown in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 12% fetal bovine serum (FBS) (HyClone). Human normal lung fibroblast (MRC-5) cells were provided by David Andrews from McMaster University (Hamilton, Ontario, Canada) and were maintained in DMEM plus 10% FBS. Human retinal pigmented epithelial (ARPE-19) cells were obtained from the ATCC and grown in DMEM-F12 medium plus 10% FBS. HCMV TR is a clinical HCMV strain that was derived from an ocular vitreous fluid sample from a patient with HIV disease and was cloned into a bacterial artificial chromosome (BAC) after limited passage in fibroblasts (22, 29). HCMV stocks were produced by infecting NHDFs using 0.1 PFU per cell for 10 to 16 days. Viral particles were purified and concentrated from culture supernatants by centrifugation through a cushion of 20% sorbitol in phosphate-buffered saline (PBS) at 50,000 × g for 1 h. Pellets were resuspended in DMEM plus 10% FBS and frozen at −70°C. The number of PFU was determined by plaque assays on replicate NHDF cultures.
Replication-defective Ad vectors.
Nonreplicating (E1−) adenovirus (Ad) vectors that express HCMV TR gH, gL, UL128, UL130, UL131, gB, and gO were described previously (25, 27, 37). Ad vector stocks were generated by infecting 293 M cells (Microbix) at 0.1 PFU/cell. Cells were harvested 6 to 10 days after infection and centrifuged at 800 × g for 5 min. Cell pellets were suspended in DMEM plus 10% FBS and sonicated to release cell-associated virus, followed by centrifugation at 3,000 × g for 5 min to remove large cellular debris. Virus-containing cell lysates were stored at −80°C. Ad stock titers were determined by plaque assays on 293 M cells.
Interference assays.
Interference assays were performed as previously described (26). Briefly, HFFs, MRC-5 fibroblasts, or ARPE-19 epithelial cells seeded into 24-well culture dishes were transduced with nonreplicating Ad vectors expressing selected HCMV glycoproteins or a control Ad vector that expresses green fluorescent protein (Ad-GFP). Under each condition, Ad-GFP was added at different amounts so that the total number of PFU for ARPE-19 cells was 60 and the total number of PFU for fibroblast cells was 540. Under conditions of expression of gH/gL/UL128-131, no Ad-GFP was included. After transduction, cells were incubated for 24 h to allow adequate protein expression and then infected with HCMV TR at 2 PFU per cell. At 48 h after infection with HCMV, entry was analyzed by immunofluorescence staining of the HCMV immediate-early protein IE-86 (24).
Cell-cell fusion assays.
Cell-cell fusion assays were performed by directly transducing adherent cells seeded into 60-mm culture dishes. For ARPE-19 cells, Ad vectors expressing tet-trans, gB, gH, and gL were used at 75 PFU/cell, and an Ad vector expressing gO was used at 3 PFU/cell. For HFFs and MRC-5 fibroblasts, Ad vectors expressing the tetracycline transactivator (tet-trans), gB, gH, and gL were used at 90 PFU/cell, and an Ad vector expressing gO was used at 3 PFU/cell. At 12 h posttransduction, the cell monolayers were washed with PBS and incubated in growth medium for an additional 48 h. For quantitative analysis of cell-cell fusion, cell monolayers were fixed in 2% paraformaldehyde and analyzed under a bright-field microscope. The level of fusion was quantified by counting the total number of cell nuclei involved in syncytium formation divided by the total number of cells in the same field and was expressed as the percentage of cells that were fused. Typically, each field contained about 200 cells, three separate fields were analyzed under each condition, and only syncytia containing at least five nuclei were scored.
FACS analysis of gH/gL cell surface expression.
HFFs or MRC-5 fibroblasts were transduced with Ad vectors similar to those described above for interference assays. At 48 h posttransduction, the cells were removed from dishes by incubation with PBS containing 0.53 mM EDTA and 0.25% (wt/vol) trypsin. The cells were washed twice with fluorescence-activated cell sorting (FACS) buffer (PBS containing 2% horse serum and 0.1 mM NaN3) and stained with anti-gH monoclonal antibody (MAb) 14-4b for 45 to 60 min on ice. The cells were washed three times in FACS buffer and then stained with secondary R-phycoerythrin-conjugated goat anti-mouse IgG for 45 to 60 min on ice (Molecular Probes, Eugene, OR). The cells were then washed again 3 times in FACS buffer, fixed with 1% paraformaldehyde for 30 min on ice, and analyzed with a Becton Dickinson FACSCalibur flow cytometer.
Radiolabeling of cells and immunoprecipitation of gH.
To metabolically label cells, cell monolayers were washed extensively with labeling medium lacking methionine and cysteine and then incubated in this medium for 30 min at 37°C. The cells were then incubated in labeling medium supplemented with [35S]methionine-cysteine (50 to 200 μCi/ml; Amersham) and incubated at 37°C for 4 h. For pulse-chase experiments, cells were washed extensively in labeling medium (DMEM lacking methionine and cysteine) and then incubated in this medium for 30 min at 37°C. Cells were then incubated for 1 h in labeling medium supplemented with [35S]methionine-cysteine (100 to 500 μCi/ml; Amersham) and then washed and incubated for 6 h in DMEM containing a 10-fold excess of nonradioactive methionine and cysteine. Extracts of labeled cells were made by using 1% Triton X-100 in Tris-buffered saline (TBS) supplemented with 1 mg/ml bovine serum albumin and 1 mM phenylmethylsulfonyl fluoride. Extracts were then precleared by incubation with protein A-agarose beads for 30 min, and the protein A-agarose was then removed by low-speed centrifugation. To immunoprecipitate labeled proteins, 40 μl of 14-4b hybridoma supernatant, 3 μl MAb 27-156 purified from hybridoma supernatant, and 10 μl rabbit serum gO-254 were added to immunoprecipitate gH/gL, gB, and gO, respectively. Lysates were incubated with antibodies for 2 h, followed by protein A-agarose (50 μl) for an additional 2 h. The agarose beads were centrifuged at low speed, washed three times in 1% Triton X-100 in TBS, eluted from the protein A-agarose by boiling in 2% SDS and 2% β-mercaptoethanol, and separated by electrophoresis using a 10% SDS-polyacrylamide gel. In the case of gO immunoprecipitates, the protein A-agarose beads were treated with endo-β-N-acetylglucosaminidase H (endo H) treatment as described previously (25).
Wide-field deconvolution microscopy.
MRC-5 fibroblasts seeded onto glass coverslips were transduced with Ad vectors expressing HCMV glycoproteins under conditions similar to those described above for interference assays. At 48 h posttransduction, the cells were fixed with 4% paraformaldehyde for 30 min, permeabilized with PBS containing 0.2% Triton X-100, and then washed with PBS-Tween (PBS-T). To detect gH, cells were blocked with 2% serum in PBS-T and then incubated with MAb 14-4b for 2 h, washed, and incubated with Dylight 594-conjugated goat anti-mouse IgG. To detect calnexin and TGN-46, replicate coverslips were prepared as described above and then stained with a rabbit polyclonal antibody to calnexin (Stress-gen) or a sheep polyclonal antibody to TGN-46 (Sero-tech). Cells were washed with PBS-T and then stained with Dylight 594-congugated goat anti-mouse IgG or Alexa Fluor 488-congugated goat anti-sheep IgG, respectively. Coverslips were mounted onto glass slides with Fluoromount-G (SouthernBiotech). Immunofluorescence microscopy was performed with a Nikon TE 200-based Applied Precision Instruments (API) Deltavision image restoration system. Captured images were deconvolved by use of SoftWorx software.
RESULTS
HCMV glycoproteins gH/gL and gO cause interference of HCMV on human fibroblasts.
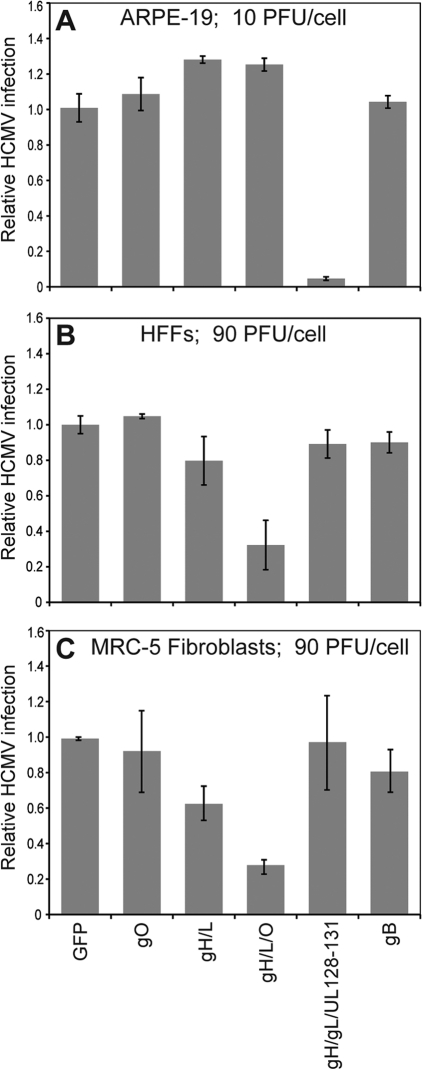
To test whether the coexpression of gO with gH/gL might increase interference, ARPE-19 epithelial cells, human foreskin fibroblasts (HFFs), and MRC-5 fibroblasts were transduced with Ad vectors expressing gO, gH/gL, gH/gL/gO, gH/gL/UL128-131, gB, or GFP as previously described (26, 27). We had previously shown that with HFFs and MRC-5 cells, it was necessary to use higher doses of Ad vectors expressing gH, gL, UL128, UL130, and UL131 (90 PFU/cell of each vector) in order to match the levels of gH/gL expression to those in ARPE-19 cells (10 PFU/cell) (26). The Ad vector that expresses TRgO(co) was codon optimized and expresses very high levels of gO and was used at 3 PFU/cell based on previous analyses of expression, again matching gO expression to that of gH/gL and UL128-131 (27). Following 24 h of transduction with Ad vectors, cells were infected with HCMV TR (2 PFU/cell) for an additional 48 h, and the cells were then stained for the immediate-early protein IE-86 as described previously (26). As in previously reported experiments (26), the expression of gH/gL/UL128-131 effectively inhibited (>95%) HCMV entry into epithelial cells (Fig. 1A). The expression of gH/gL/gO in ARPE-19 epithelial cells had no effect on HCMV entry. In contrast, the expression of gH/gL/gO in HFFs and MRC-5 fibroblasts reduced the number of HCMV-infected cells by 68% and 70%, respectively (Fig. 1B and C). Again, there was some inhibition in both sets of fibroblasts with gH/gL alone, but the expression of gO substantially boosted interference. The expression of gB or gO alone or the gH/gL/UL128-131 complex had no significant effect on HCMV infection of fibroblasts (Fig. 1B and C). Importantly, in no case did we observe cytopathic effects of these nonreplicating Ad vectors, and Ad vectors expressing all of the proteins gH, gL, UL128, UL130, and UL131 did not inhibit HCMV entry into fibroblasts. Moreover, in previous experiments, we showed that the entry of HSV into cells was not reduced by these Ad vectors (26). In addition, we showed that the inhibition of HCMV IE-86 expression mediated in fibroblasts expressing gH/gL/gO is at the level of entry, because a 30-s treatment with the chemical fusogen polyethylene glycol (PEG) (44%) overcame the effects of gH/gL/gO (not shown), as described previously (26). Therefore, we concluded that the coexpression of gO with gH/gL substantially increases interference in human fibroblasts compared with gH/gL alone and that gH/gL/gO does not interfere in epithelial cells.
Fig. 1.
Interference mediated by HCMV glycoproteins gH/gL/gO and gH/gL/UL128-131 in epithelial cells and fibroblasts. ARPE-19 epithelial cells (A), normal human foreskin fibroblasts (HFFs) (B), or MRC-5 fibroblasts (C) were transduced for 24 h with the indicated combinations of Ad vectors expressing HCMV glycoproteins, as previously described (26). The cells were then infected with HCMV strain TR (2 PFU per cell) for an additional 48 h and stained for the HCMV IE-86 protein as described previously (26). Under each condition, at least 350 cells were characterized for IE-86 expression in each of three random fields. Relative HCMV infection refers to the average number of IE-86-positive cells normalized to the average number of infected cells observed in monolayers infected with a control Ad vector expressing GFP (set at 1.0). HCMV infected 50 to 60% of ARPE-19 cells and 90 to 100% of the HFFs and MRC-5 cells transduced with Ad-GFP. Error bars indicate the standard deviations from the means. Results shown are representative of three separate experiments.
HCMV gO does not increase cell-cell fusion of fibroblast or epithelial cells.
A second method for defining how HCMV glycoproteins function in entry involves cell-cell fusion assays. Our previous studies showed that the expression of gB with gH/gL in ARPE-19 epithelial cells produced extensive cell-cell fusion; i.e., 50% of the cells fused (37). This result was surprising, given that gH/gL is not sufficient for entry; i.e., gH/gL/UL128-131 is necessary in epithelial cells. However, we suggested that gH/gL and gB act as the core fusion machinery, while UL128-131 is needed for some other aspect of entry, e.g., events that lead to endocytosis or taking place inside endosomes during entry into epithelial cells (24). MRC-5 cells expressing gB and gH/gL also extensively fused, but no fusion was observed with HFFs (37). The latter result suggested the need for gO. To determine whether the coexpression of gO along with gH/gL and gB increased fusion, MRC-5 cells, HFFs, and ARPE-19 epithelial cells were transduced with Ad vectors expressing various glycoproteins for 60 h, and cell-cell fusion was then measured by counting syncytia containing 5 or more fused cells, as previously described (37). The expression of gB and gH/gL in ARPE-19 epithelial cells caused nearly 40% of the cells to fuse, and the coexpression of gO with gH/gL and gB did not significantly increase or decrease cell-cell fusion (Fig. 2A). As before, the level of fusion of HFFs with gH/gL and gB was very low (1 to 4%) and did not increase when gO was coexpressed with these glycoproteins (Fig. 2B). With MRC-5 fibroblasts, there was extensive (75%) fusion when the cells were transduced with gB and gH/gL (Fig. 2C). However, again, the coexpression of gO with gH/gL and gB in MRC-5 cells did not affect the level of cell-cell fusion. To ensure that we obtained the expression of the intended HCMV proteins, ARPE-19 and HFF cells transduced with either Ad-tet-trans or Ad vectors expressing gB, gH/gL, and gO were metabolically labeled, and the target proteins were immunoprecipitated from the cell lysates. As expected, proteins of the expected size for gB, gH/gL, and gO were immunoprecipitated from lysates of cells expressing gB, gH/gL, and gO but not from lysates of cells expressing tet-trans (Fig. 2D and E). From these results, we concluded that the expression of gO does no enhance cell-cell fusion in any of these cells.
Fig. 2.
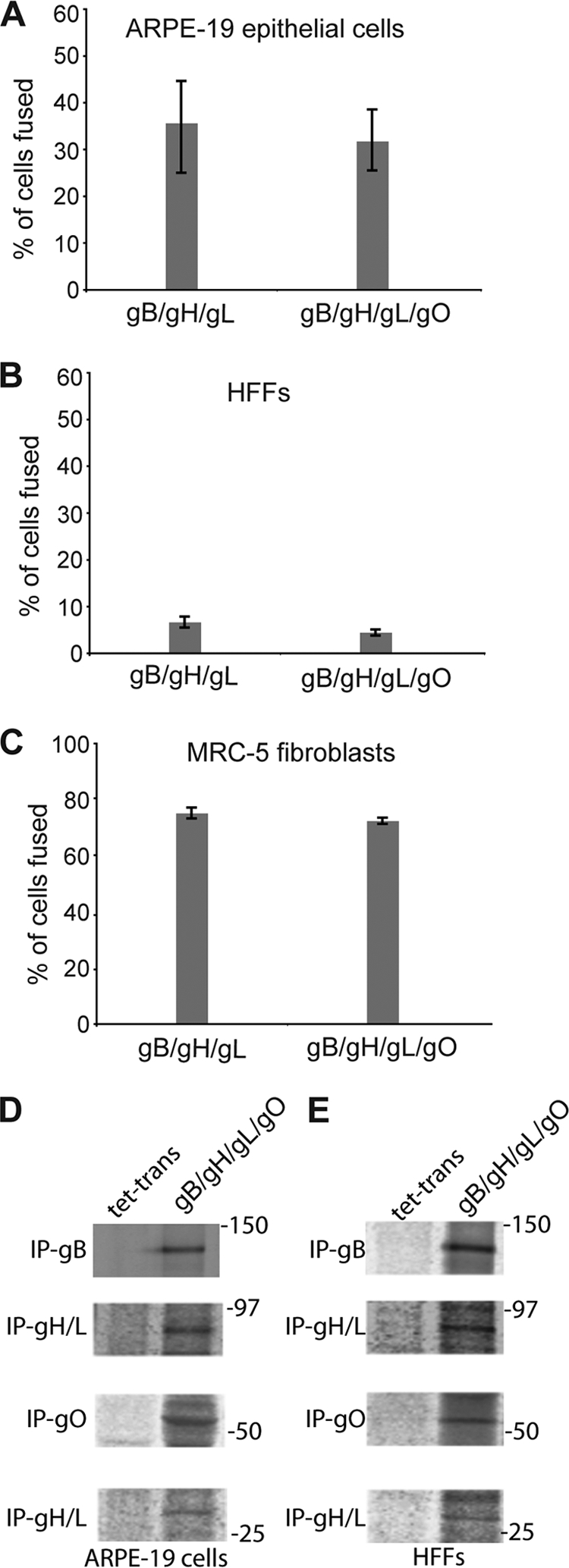
Cell-cell fusion of epithelial and fibroblast cells expressing HCMV glycoproteins. (A to C) ARPE-19 epithelial cells (A), HFFs (B), or MRC-5 fibroblasts (C) were transduced with Ad vectors expressing gB and gH/gL (gB/gH/gL) or gB, gH/gL, and gO (gB/gH/gL/gO) for 60 h, as described in Materials and Methods. Cell-cell fusion was quantified from micrographs by counting the number of syncytia containing at least 5 nuclei and then dividing this number by the total number of nuclei in each field and expressing the data as a percentage of cells fused. Values represent the averages derived from three separate fields, each including >200 cells. Error bars represent standard deviations. (D and E) To analyze the expression of HCMV gB, gH/gL, and gO, ARPE-19 (D) and HFF (E) cells were transduced with Ad vectors expressing Ad-tet-trans (tet-trans) alone or Ad vectors expressing gB, gH/gL, and gO (gB/gH/gL/gO) at the same multiplicities of infection (MOIs) as those used for the fusion assays, and at 24 h posttransduction, the cells were radiolabeled with [35S]methionine-cysteine, and gB, gH/gL, and gO were immunoprecipitated from the cell extracts and analyzed by SDS-PAGE. The antibodies used for the immunoprecipitation (IP) are indicated to the left of the panels, and the positions of the molecular weight standards (in thousands) are indicated to the right of the panels.
Coexpression of HCMV gO does not increase gH/L surface expression.
We previously observed that the coexpression of either UL128-131 or gO increases the ER export of gH/gL (25, 27). Given that gO also increased interference in fibroblasts, it seemed likely that there would be increased cell surface levels of gH/gL. To test this, we quantified the level of gH/gL on the surfaces of fibroblasts by using fluorescence-activated cell sorting (FACS). HFFs and MRC-5 cells were transduced with gH/gL, gH/gL/gO, gH/gL/UL128-131, or a negative-control vector, Ad tet-trans. After 48 h, the cells were stained with anti-gH MAb 14-4b and fluorescent secondary antibodies and analyzed by FACS (23). The expression of gH/gL alone in HFFs produced some cell surface staining in HFFs (Fig. 3A and B). The coexpression of gH/gL with UL128-131 increased surface gH/gL levels by 720% (Fig. 3A and B). In contrast, the coexpression of gO with gH/gL in HFFs had little effect on the cell surface expression of gH (Fig. 3A and B). In MRC-5 fibroblasts, UL128-131 increased surface gH/gL levels by 70%, and the coexpression of gO with gH/gL reduced cell surface gH/gL levels (Fig. 3D and E). To rule out the possibility that the FACS results were due to differences in the levels of gH/gL expression under different conditions, cells were transduced exactly as was done for FACS analyses and then radiolabeled with [35S]methionine-cysteine using a pulse-chase format, and the gH immunoprecipitated. The expression levels of gH were similar in cells expressing gH/gL, gH/gL/gO, and gH/gL/UL128-131 (Fig. 3C and F). We noted that the electrophoretic migration of gH labeled in both cell types expressing gH/gL/UL128-131 was slower and more diffuse, consistent with the addition of sialic acid residues to oligosaccharides and intracellular transport to the trans-Golgi apparatus and cell surfaces (25). Importantly, this change in electrophoretic mobility was not observed with gH immunoprecipitated from cells transduced with gH/gL/gO (Fig. 3C and F). Therefore, the coexpression of gO with gH/gL does not increase cell surface gH/gL levels.
Fig. 3.
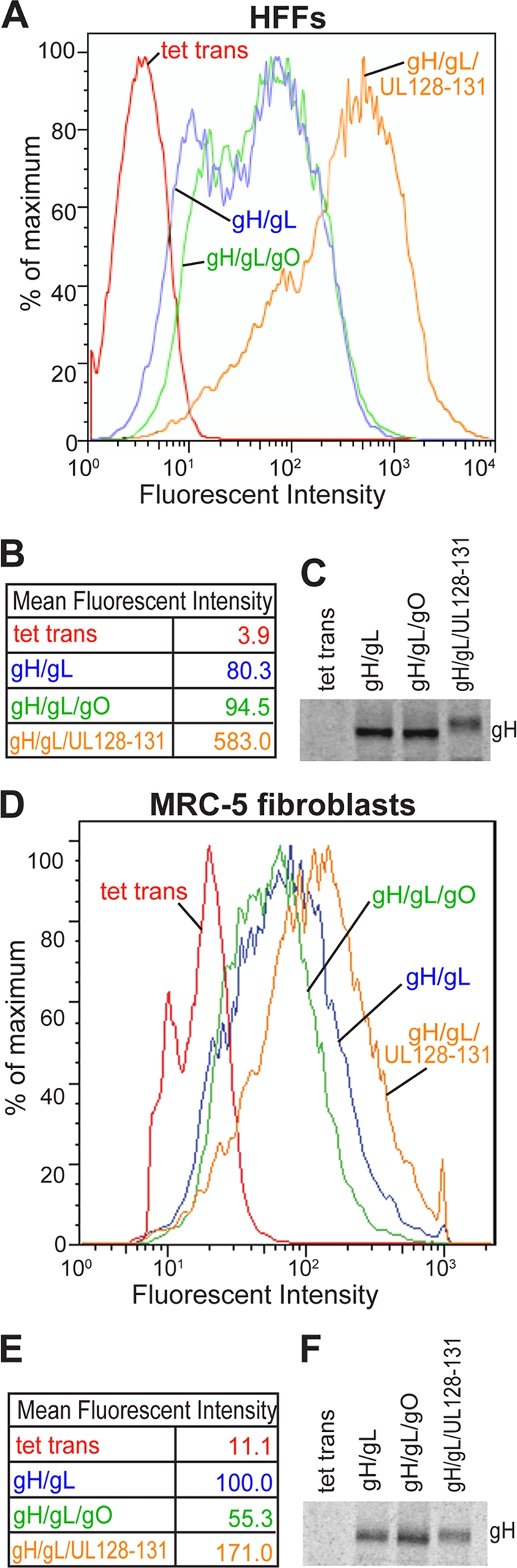
Cell surface expression of gH/gL in fibroblasts coexpressing gO or UL128-131. HFF cells (A to C) or MRC-5 cells (D to F) were transduced with a control Ad vector expressing tet-trans or with Ad vectors expressing gH/gL, gH/gL/gO, or gH/gL/UL128-131. At 48 h posttransduction, the cells were stained with anti-gH MAb 14-4b followed by goat anti-mouse IgG antibodies conjugated with R-phycoerythrin (Molecular Probes) and then analyzed by FACS. (A and D) FACS data are shown in a histogram format. (B and E) Mean fluorescent intensities were calculated from the data in panels A and D and are shown in table form. (C and F) HFFs or MRC-5 cells were transduced with Ad vectors as described above for 48 h, the cells were radiolabeled with [35S]methionine-cysteine for 1 h, and the cells were then washed free of radioactivity and label chased for 6 h. gH was immunoprecipitated from cell extracts using anti-gH MAb 14-4b, and immunoprecipitated proteins were analyzed by SDS-PAGE.
HCMV gO and UL128-131 facilitate transport of gH/gL to the trans-Golgi network.
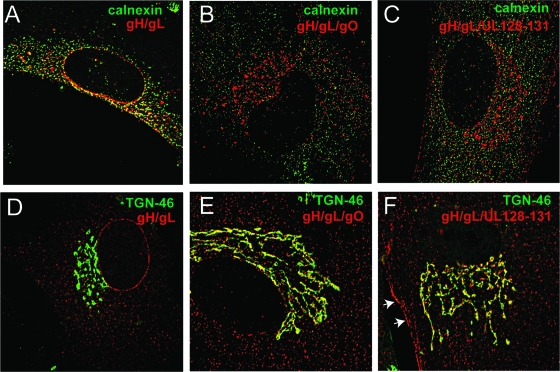
Observations that gO did not increase cell surface expression levels required further investigation. This was different from observations that UL128-131 increased levels of surface expression of gH/gL (Fig. 3) and secretion of a soluble gH/gL (27). To characterize this further, MRC-5 fibroblasts were transduced with Ad vectors expressing gH/gL, gH/gL/gO, or gH/gL/UL128-131 for 48 h, and the cells were then fixed, permeabilized, and stained with anti-gH MAb and simultaneously stained with antibodies specific for calnexin or TGN-46. Wide-field deconvolution microscopy was performed in order to determine whether gH/gL was found associated with calnexin, which remains primarily in the ER, or with TGN-46, a protein found in the trans-Golgi network (TGN). In cells that expressed gH/gL alone, the gH-specific staining overlapped extensively with calnexin (Fig. 4A) and not with TGN-46 (Fig. 4D). As in previous experiments in which gH/gL was expressed without UL128-131 or gO and remained sensitive to endoglycosidase H (25, 27), this suggested that gH/gL is retained in the ER. The coexpression of gO with gH/gL reduced the quantities of gH/gL that colocalized with calnexin (Fig. 4B) and dramatically increased colocalization with TGN-46 (Fig. 4E). Similarly, the coexpression of UL128-131 with gH/gL decreased colocalization with calnexin and increased colocalization with TGN-46 (Fig. 4C and F). However, there was also an obvious expression of gH/gL/UL128-131 on cell surfaces (Fig. 4F, arrows). This was not observed for cells transduced with gH/gL/gO. Together, these results show that both gO and UL128-131 increase the ER export of gH/gL, but in the case of gO, the coexpression of most of the gH/gL accumulates in the TGN, whereas with UL128-131 coexpression, there is both TGN and cell surface localizations of gH/gL.
Fig. 4.
Subcellular distribution of gH/gL in cells coexpressing gO or UL128-131. MRC-5 fibroblasts on glass coverslips were transduced with Ad vectors expressing gH/gL (A and D), gH/gL/gO (B and E), or gH/gL/UL128-131 (C and F). At 48 h posttransduction, the cells were fixed and permeabilized with 0.2% Triton X-100. In panels A to C, the cells were stained with mouse monoclonal antibody 14-4b to gH and rabbit anti-calnexin polyclonal antibodies and then stained with the following secondary antibodies: Dylight 594-conjugated goat anti-mouse IgG and Dylight 488-congugated goat anti-rabbit IgG. In panels D to F, cells were stained with monoclonal antibody 14-4b to gH and a sheep TGN-46 polyclonal antibodies and then stained with the following secondary antibodies: Dylight 594-congugated goat anti-mouse IgG and Alexa Fluor 488-congugated goat anti-sheep IgG. All images captured are presented with the red (gH) and green (calnexin/TGN-46) channels superimposed. The arrows in panel F highlight the localization of gH at the plasma membrane.
DISCUSSION
Our previous interference data involving gH/gL/UL128-131 in epithelial cells suggested the hypothesis that gH/gL/UL128-131 binds to molecules that facilitate HCMV entry into these cells and likely causes one of two conditions: (i) a mislocalization of the cellular molecules or (ii) a blocking of these putative receptors so that HCMV entry is inhibited (26). That these putative epithelial cell receptors were not utilized by HCMV for entry into fibroblasts was suggested by observations that gH/gL/UL128-131 did not interfere in fibroblasts. Here, we report that the coexpression of gO with gH/gL in fibroblasts caused substantial interference, up to 70% in HFFs and MRC-5 cells, yet no interference was observed when gH/gL/gO was expressed in epithelial cells. This provides further support for our model of distinct receptors in epithelial cells versus fibroblasts. However, until these putative receptors are identified and characterized, the notion of their existence relies entirely on the observations that different gH/gL complexes caused interference and that gB does not. Certainly, the experience with HSV interference (4, 18, 32, 33) and retrovirus interference (1, 20, 35, 36) has clearly demonstrated that viral receptor binding proteins expressed in trans can block or downregulate receptors.
Surprisingly, the coexpression of gO with gH/gL did not increase cell surface expression levels of gH/gL in any cell type investigated. This was different from the coexpression of UL128-131 with gH/gL, which markedly increased the surface expression levels of gH/gL, compared with gH/gL alone. The effects of the coexpression of gO with gH/gL in fibroblasts were explained by our immunofluorescence imaging studies. gH/gL expressed without either gO or UL128-131 accumulated principally in the ER. This fits well with previously reported observations that gH/gL remains sensitive to endoglycosidase H when gO or UL128-131 is absent (25, 27). In contrast, gH/gL coexpressed with gO attained endoglycosidase H resistance and accumulated in the TGN. gO itself remained sensitive to endoglycosidase H, consistent with the notion that gO does not reach the trans-Golgi apparatus (27). Moreover, gO was not found in the envelope of mature, extracellular TR virions both in Western blots and in pulse-chase labeling experiments (40). Therefore, gO promotes the ER export of gH/gL but dissociates from gH/gL before the complex reaches the TGN, likely in the endoplasmic reticulum-Golgi-intermediate compartment (ERGIC) or medial Golgi apparatus. gO can be considered a molecular chaperone based on the dissociation from gH/gL, and it is gH/gL, and not gH/gL/gO, that is incorporated into the TR virion envelope. Importantly, gH/gL coexpressed with gO accumulated primarily in the TGN, and there were not large amounts of gH/gL on the surfaces of cells.
Like gO, UL128-131 complexes with gH/gL in the ER, and gH/gL/UL128-131 is exported from the ER to the Golgi apparatus and TGN (25). Here, our immunofluorescence studies showed that a substantial fraction of gH/gL expressed with UL128-131 accumulated in the TGN and that another, roughly equal fraction reached cell surfaces. This finding was consistent with previously reported observations involving pulse-chase labeling of cells expressing soluble gH/gL/UL128-131 that showed that about half of the protein complex was secreted (25). Here, we observed that the gH polypeptide coexpressed with gL and UL128-131 exhibited reduced electrophoretic mobility, probably related to the addition of terminal sialic acid residues on oligosaccharides, compared with gH coexpressed with gL and gO. We concluded that gH/gL/UL128-131 moves not only to the TGN but also to the plasma membrane. Unlike gO, gH/gL/UL128-131 is found in the envelope of HCMV TR extracellular virus particles, apparently joining gH/gL (27, 40). One hypothesis suggests that gO protects a fraction of the gH/gL that would normally bind UL128-131, providing a pool of gH/gL that is incorporated into the virion envelope. The passage of HCMV leads to a loss of UL128-131, favoring the expression of gH/gL/gO that is important in fibroblasts. It also makes ample sense that both gH/gL and gH/gL/UL128-131 are not transported efficiently to the plasma membrane (when expressed using Ad vectors), because in infected cells, these glycoproteins are incorporated into the virion envelope in the Golgi apparatus or TGN (7, 28).
A model for how gH/gL/gO and gH/gL/UL128-131 interfere with HCMV entry is described, as follows. The gH/gL/UL128-131 complex is formed in the ER and is transported to the Golgi apparatus and TGN. A substantial fraction of gH/gL/UL128-131 appears to be transported to the cell surface; thus, the simplest interpretation of the gH/gL/Ul128-131 interference data is that gH/gL/UL128-131 interacts with putative cell surface epithelial cell receptors, blocking the binding of HCMV or causing the internalization of the receptors so that they are not available to HCMV. This is similar to the interference mediated by HSV gD, which involves binding to cell surface nectin-1 or herpesvirus entry mediator (HVEM), which is then rapidly internalized or blocked so that the gD in the envelope of incoming virus particles cannot bind (32, 33). In contrast, the HCMV interference observed for fibroblasts expressing gH/gL/gO appears to be different. As noted above, we propose a role whereby gO acts as a chaperone to promote the ER export of gH/gL, and it is gH/gL, and not gH/gL/gO, that reaches the TGN. Unlike the expression of gH/gL with UL128-131, gH/gL expressed with gO did not lead to increased surface expression levels of gH/gL. Therefore, interference mediated in fibroblasts coexpressing gH/gL and gO must involve interactions between gH/gL and putative fibroblast receptors in cytoplasmic membranes, i.e., the ERGIC, the Golgi apparatus, or the TGN. Likely, putative fibroblast receptors are bound by gH/gL and mislocalized, e.g., retained in an intracellular compartment rather than reaching the cell surface or targeted to lysosomes. It is unclear if the enhanced ability of gH/gL to bind putative fibroblast receptors in cytoplasmic membranes is due solely to the ability of gO to promote the trafficking of gH/gL to the TGN or because gO has the ability to promote a conformation change in gH/gL that allows gH/gL to interact with cellular molecules and promote interference. Regardless, this is the first example of herpesvirus interference that occurs within cytoplasmic membranes. It should be noted that a fraction of gH/gL/UL128-131 also accumulates in the TGN. Therefore, it is also possible that gH/gL/UL128-31 interacts with and mislocalizes putative receptors in cytoplasmic membranes. Related observations were made previously with EBV gp42, which binds to major histocompatibility complex (MHC) class II proteins, causing gp42 to traffic into the acidic class II pathway, leading to the degradation of gp42 (16).
Paralleling our observations that the cell surface expression level of gH/gL was not increased when gO was coexpressed in MRC-5 cells and HFFs, the level of cell-cell fusion was also not increased. Cell-cell fusion depends upon cell surface gH/gL and gB (37). In terms of an understanding of the cell-cell fusion results, it is important to recognize that the effects of gO and UL128-131 on the intracellular traffic of gH/gL were not absolute. There were smaller quantities of gH/gL (expressed without gO or UL128-131) that escaped from the ER, reaching the TGN and cell surfaces in these Ad-transduced fibroblasts. Obviously, these smaller quantities of gH/gL were sufficient for cell-cell fusion. MRC-5 cells expressing gB and gH/gL fused extensively whether gO was expressed or not. Thus, fusion was already very efficient with the small fraction of cell surface gH/gL that reached cell surfaces without gO. There was a 45% reduction in the levels of cell surface gH/gL when gO was coexpressed in MRC-5 cells, yet cell-cell fusion was not diminished significantly. This is best explained by the suggestion that gH/gL levels remained sufficient for efficient cell-cell fusion. It should be considered that cell-cell fusion analyses frequently involve transfection to express herpesvirus proteins. Our Ad vectors express herpesvirus glycoproteins more effectively and over a shorter period of time than with transfection so that the minimal levels required for cell-cell fusion are attained more readily. HFFs differed dramatically from MRC-5 cells: only 1 to 4% of HFFs fused when gH/gL and gB were coexpressed in the cells, and gO did not alter this. It is unclear why HFFs are relatively resistant to cell-cell fusion, but this may be related to differences in cell surface proteoglycans or lipids. Alternatively, the relatively low levels of gH/gL that reach the surfaces of HFFs, with or without gO, may be insufficient for cell-cell fusion.
Observations that gO and UL128-131 did not boost cell-cell fusion continue to support the hypothesis that gB and gH/gL represent the core fusion machinery of HCMV (37). Cell-cell fusion and virus entry differ in important respects. It appears that cell-cell fusion is relatively slow, requiring hours of expression of herpesvirus proteins. Moreover, only a small segment of the entire plasma membrane of one cell needs to fuse with a segment of an adjacent cell to produce syncytia, so relatively low local concentrations of gH/gL and gB are necessary. In contrast, HCMV glycoproteins are highly concentrated in the virion envelope, and once triggered, virion-cell fusion is rapid. In addition, HCMV enters epithelial cells by fusing with endosomes in a process that requires gH/gL/UL128-131. In contrast, the fusion of ARPE-19 epithelial cells occurs at a neutral pH, without endocytosis, and without UL128-131. One possibility is that UL128-131 proteins might act downstream of endocytosis but before membrane fusion. For example, UL128-131 might interact with cellular molecules that promote HCMV endocytosis, or UL128-131 could function to block fusion until the virus reaches the low-pH environment of endosomes. Supporting the latter possibility, UL128-131 reduces cell-cell fusion when coexpressed with gH/gL and gB (37). It is more obvious why gO is not required for cell-cell fusion, as gO acts as a chaperone without increasing cell surface gH/gL levels, and gO is not found in the virion envelope during entry.
In summary, our observations that the coexpression of gO with gH/gL significantly increases the interference of HCMV entry into fibroblasts provide further evidence for fibroblast molecules that are mislocalized or blocked by the expression of gH/gL or gH/gL/gO. These putative fibroblast receptors appear to be different from those expressed by epithelial cells, which express molecules blocked by gH/gL/UL128-131. When gO was coexpressed with gH/gL in fibroblasts, the level of gH/gL was not increased on cell surfaces, yet increased interference was observed. This finding supports the novel hypothesis that interference with putative HCMV gH/gL fibroblast receptors involves alterations in the cytoplasmic trafficking of these molecules, e.g., the ERGIC, the Golgi apparatus, or the TGN.
ACKNOWLEDGMENTS
This work was supported by grant R01AI081517 from the National Institutes of Health to D.C.J. and by a postdoctoral fellowship from the American Heart Association, 09POST2090081, to A.L.V.
We are grateful to William J. Britt (University of Alabama—Birmingham) and Teresa Compton (Novartis) for supplying important antibodies. Tiffani Howard prepared the graphics. Finally, we appreciate advice and support from Paul Wille, Brent Ryckman, and members of the Johnson laboratory.
Footnotes
Published ahead of print on 31 August 2011.
REFERENCES
- 1. Adkins H. B., et al. 1997. Identification of a cellular receptor for subgroup E avian leukosis virus. Proc. Natl. Acad. Sci. U. S. A. 94: 11617–11622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adler B., et al. 2006. Role of human cytomegalovirus UL131A in cell type-specific virus entry and release. J. Gen. Virol. 87: 2451–2460 [DOI] [PubMed] [Google Scholar]
- 3. Britt W. J. 2006. Human cytomegalovirus infections and mechanisms of disease, p. 1–28 In Reddehase M. J. (ed.), Cytomegaloviruses: biology and immunology. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 4. Campadelli-Fiume G., Arsenakis M., Farabegoli F., Roizman B. 1988. Entry of herpes simplex virus 1 in BJ cells that constitutively express viral glycoprotein D is by endocytosis and results in degradation of the virus. J. Virol. 62: 159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cha T. A., et al. 1996. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J. Virol. 70: 78–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Compton T., Nepomuceno R. R., Nowlin D. M. 1992. Human cytomegalovirus penetrates host cells by pH-independent fusion at the cell surface. Virology 191: 387–395 [DOI] [PubMed] [Google Scholar]
- 7. Das S., Vasanji A., Pellett P. E. 2007. Three-dimensional structure of the human cytomegalovirus cytoplasmic virion assembly complex includes a reoriented secretory apparatus. J. Virol. 81: 11861–11869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dolan A., et al. 2004. Genetic content of wild-type human cytomegalovirus. J. Gen. Virol. 85: 1301–1312 [DOI] [PubMed] [Google Scholar]
- 9. Feire A. L., Koss H., Compton T. 2004. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc. Natl. Acad. Sci. U. S. A. 101: 15470–15475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Geraghty R. J., Jogger C. R., Spear P. G. 2000. Cellular expression of alphaherpesvirus gD interferes with entry of homologous and heterologous alphaherpesviruses by blocking access to a shared gD receptor. Virology 268: 147–158 [DOI] [PubMed] [Google Scholar]
- 11. Gerna G., et al. 2005. Dendritic-cell infection by human cytomegalovirus is restricted to strains carrying functional UL131-128 genes and mediates efficient viral antigen presentation to CD8+ T cells. J. Gen. Virol. 86: 275–284 [DOI] [PubMed] [Google Scholar]
- 12. Hahn G., et al. 2004. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J. Virol. 78: 10023–10033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hobom U., Brune W., Messerle M., Hahn G., Koszinowski U. H. 2000. Fast screening procedures for random transposon libraries of cloned herpesvirus genomes: mutational analysis of human cytomegalovirus envelope glycoprotein genes. J. Virol. 74: 7720–7729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huber M. T., Compton T. 1998. The human cytomegalovirus UL74 gene encodes the third component of the glycoprotein H-glycoprotein L-containing envelope complex. J. Virol. 72: 8191–8197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huber M. T., Compton T. 1999. Intracellular formation and processing of the heterotrimeric gH-gL-gO (gCIII) glycoprotein envelope complex of human cytomegalovirus. J. Virol. 73: 3886–3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hutt-Fletcher L. M. 2007. Epstein-Barr virus entry. J. Virol. 81: 7825–7832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jiang X. J., et al. 2008. UL74 of human cytomegalovirus contributes to virus release by promoting secondary envelopment of virions. J. Virol. 82: 2802–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson R. M., Spear P. G. 1989. Herpes simplex virus glycoprotein D mediates interference with herpes simplex virus infection. J. Virol. 63: 819–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kinzler E. R., Theiler R. N., Compton T. 2002. Expression and reconstitution of the gH/gL/gO complex of human cytomegalovirus. J. Clin. Virol. 25(Suppl. 2): S87–S95 [DOI] [PubMed] [Google Scholar]
- 20. Miller D. G., Miller A. D. 1994. A family of retroviruses that utilize related phosphate transporters for cell entry. J. Virol. 68: 8270–8276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mori Y. 2009. Recent topics related to human herpesvirus 6 cell tropism. Cell. Microbiol. 11: 1001–1006 [DOI] [PubMed] [Google Scholar]
- 22. Murphy E., et al. 2003. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc. Natl. Acad. Sci. U. S. A. 100: 14976–14981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Polcicova K., Goldsmith K., Rainish B. L., Wisner T. W., Johnson D. C. 2005. The extracellular domain of herpes simplex virus gE is indispensable for efficient cell-to-cell spread: evidence for gE/gI receptors. J. Virol. 79: 11990–12001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ryckman B. J., Jarvis M. A., Drummond D. D., Nelson J. A., Johnson D. C. 2006. Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion. J. Virol. 80: 710–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ryckman B. J., et al. 2008. Characterization of the human cytomegalovirus gH/gL/UL128-131 complex that mediates entry into epithelial and endothelial cells. J. Virol. 82: 60–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ryckman B. J., Chase M. C., Johnson D. C. 2008. HCMV gH/gL/UL128-131 interferes with virus entry into epithelial cells: evidence for cell type-specific receptors. Proc. Natl. Acad. Sci. U. S. A. 105: 14118–14123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ryckman B. J., Chase M. C., Johnson D. C. 2010. Human cytomegalovirus TR strain glycoprotein O acts as a chaperone promoting gH/gL incorporation into virions but is not present in virions. J. Virol. 84: 2597–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sanchez V., Greis K. D., Sztul E., Britt W. J. 2000. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J. Virol. 74: 975–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smith I. L., et al. 1998. Clinical failure of CMV retinitis with intravitreal cidofovir is associated with antiviral resistance. Arch. Ophthalmol. 116: 178–185 [DOI] [PubMed] [Google Scholar]
- 30. Soroceanu L., Akhavan A., Cobbs C. S. 2008. Platelet-derived growth factor-alpha receptor activation is required for human cytomegalovirus infection. Nature 455: 391–395 [DOI] [PubMed] [Google Scholar]
- 31. Spear P. G., Longnecker R. 2003. Herpesvirus entry: an update. J. Virol. 77: 10179–10185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stiles K. M., Krummenacher C. 2010. Glycoprotein D actively induces rapid internalization of two nectin-1 isoforms during herpes simplex virus entry. Virology 399: 109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stiles K. M., et al. 2010. Herpes simplex virus glycoprotein D interferes with binding of herpesvirus entry mediator to its ligands through downregulation and direct competition. J. Virol. 84: 11646–11660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Straschewski S., et al. 2011. Protein pUL128 of human cytomegalovirus is necessary for monocyte infection and blocking of migration. J. Virol. 85: 5150–5158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tailor C. S., Nouri A., Zhao Y., Takeuchi Y., Kabat D. 1999. A sodium-dependent neutral-amino-acid transporter mediates infections of feline and baboon endogenous retroviruses and simian type D retroviruses. J. Virol. 73: 4470–4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tailor C. S., Willett B. J., Kabat D. 1999. A putative cell surface receptor for anemia-inducing feline leukemia virus subgroup C is a member of a transporter superfamily. J. Virol. 73: 6500–6505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vanarsdall A. L., Ryckman B. J., Chase M. C., Johnson D. C. 2008. Human cytomegalovirus glycoproteins gB and gH/gL mediate epithelial cell-cell fusion when expressed either in cis or in trans. J. Virol. 82: 11837–11850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang D., Shenk T. 2005. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc. Natl. Acad. Sci. U. S. A. 102: 18153–18158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang X., Huong S. M., Chiu M. L., Raab-Traub N., Huang E. S. 2003. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 424: 456–461 [DOI] [PubMed] [Google Scholar]
- 40. Wille P. T., Knoche A. J., Nelson J. A., Jarvis M. A., Johnson D. C. 2010. A human cytomegalovirus gO-null mutant fails to incorporate gH/gL into the virion envelope and is unable to enter fibroblasts and epithelial and endothelial cells. J. Virol. 84: 2585–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]