Abstract
Sexual transmission of HIV-1 requires virus adsorption to a target cell, typically a CD4+ T lymphocyte residing in the lamina propria, beneath the epithelium. To escape the mucosal clearance system and reach its target cells, HIV-1 has evolved strategies to circumvent deleterious host factors. Galectin-1, a soluble lectin found in the underlayers of the epithelium, increases HIV-1 infectivity by accelerating its binding to susceptible cells. By comparison, galectin-3, a family member expressed by epithelial cells and part of the mucosal clearance system, does not perform similarly. We show here that galectin-1 directly binds to HIV-1 in a β-galactoside-dependent fashion through recognition of clusters of N-linked glycans on the viral envelope gp120. Unexpectedly, this preferential binding of galectin-1 does not rely on the primary sequence of any particular glycans. Instead, glycan clustering arising from the tertiary structure of gp120 hinders its binding by galectin-3. Increased polyvalency of a specific ligand epitope is a common strategy for glycans to increase their avidity for lectins. In this peculiar occurrence, glycan clustering is instead exploited to prevent binding of gp120 by galectin-3, which would lead to a biological dead-end for the virus. Our data also suggest that galectin-1 binds preferentially to CD4, the host receptor for gp120. Together, these results suggest that HIV-1 exploits galectin-1 to enhance gp120-CD4 interactions, thereby promoting virus attachment and infection events. Since viral adhesion is a rate-limiting step for HIV-1 entry, modulation of the gp120 interaction with galectin-1 could thus represent a novel approach for the prevention of HIV-1 transmission.
INTRODUCTION
The frequency of human immunodeficiency virus type 1 (HIV-1) transmission following unprotected sexual intercourse is relatively low compared to other sexually transmitted viruses, such as hepatitis B virus (3, 44, 55). However, once transmission occurs, HIV-1 efficiently replicates and rapidly expands to deplete more than 90% of gut-associated CD4+ T cells within the first few weeks (4, 30, 63). One of the rate-limiting steps of HIV-1 infection involves its early interaction with virus-susceptible cells (62). Prevention of this initial attachment should be exploited to further reduce transmission events, therefore avoiding chronic infection, life-long monitoring, and costly antiretroviral therapies.
The attachment to the surface of the target cell is mediated through binding of the external viral envelope glycoprotein (Env) to the major cellular receptor CD4 (52). This physical contact triggers conformational changes in Env, which leads to fusion of the viral and host plasma membranes. Thus, Env-CD4 interactions are critical to initiate fusion of membranes under optimal conditions. These conditions include high expression levels of surface CD4 and coreceptors (e.g., CCR5 and CXCR4) as well as significant amounts of infectious virus particles. However, such optimal conditions are rarely met under in vivo situations, especially during the initial stages of infection. Even if Env molecules have a relatively high affinity for CD4, the general avidity of Env is unexpectedly low, and it exhibits slow equilibrium binding kinetics at 37°C (14, 36, 37, 51). In addition, only a few Env spikes (i.e., about 20 spikes per virion) are sparsely distributed on the viral surface (65). Thus, during a transmission event, when host cell surface levels of CD4 are far from optimal and when innate mucosal clearance mechanisms are active, the formation of a stable association between Env and CD4 becomes a significant limiting factor to infection (35, 62). Interestingly, HIV-1 has elaborated numerous strategies to compensate for this limiting factor. One of these involves the alteration of the shell-like glycan layer, called the glycocalyx, on the virus surface. This phenomenon results in the formation of interactions between HIV-1 and host lectins expressed as membrane-anchored proteins on the surface of target cells (11, 15, 23, 24, 27, 31, 61).
The glycocalyx of HIV-1 is composed of glycan chains that are covalently linked to host membrane glycoproteins acquired by the virus while emerging from an infected cell (13, 60). In addition, Env itself is densely glycosylated (i.e., from 13 to 33 chains per single molecule). Even though a single virion carries very few Env spikes on its surface (65), Env glycans have been shown to mediate numerous and biologically relevant interactions with host lectins (53). Despite high genetic variability among different groups of HIV-1, the N-glycosylation sites of gp120 are spatially conserved. Two types of N-linked glycans are found on gp120, namely, oligomannose-type (OM) glycans, which are rich in mannose residues, and complex-type (CX) glycans, which carry 2 to 6 β-galactoside residues (i.e., lactosamine residue [LacNAc]) (25, 26, 41, 64). Glycosylation of gp120 exhibits two unique features that distinguish it from glycosylation patterns normally found on host membrane proteins (53). First, gp120 displays unusually high levels of OM glycans, which are considered incomplete processed forms of glycan chains and are therefore rarely found in the extracellular space. Second, OM and CX glycans are spatially distributed on the surface of gp120 to form distinct homogenous patches (53). For example, OM glycans are clustered in an area distal from the CD4 binding site, while CX glycan patches are found proximal to the CD4 binding site. The functionality of OM glycan has already been established, as multiple C-type membrane-anchored lectin receptors (CLRs), such as DC-SIGN, CD206, and DCIR, recognize OM glycans and enhance both HIV-1 attachment and infection (11, 15, 23, 24, 27, 31, 61). In contrast, despite the determination that CX glycan patches are found proximal to the CD4 binding site of gp120, their involvement in HIV-1 pathogenesis remains unclear.
We have recently found that a non-CLR, soluble, host galactoside binding protein, galectin-1 (Gal-1), greatly accelerates (as high as 40-fold) the binding kinetics of HIV-1 to susceptible cells, leading to faster viral entry and a more robust viral replication (33, 40, 59). As of yet, the mechanism as well as potential ligands found on susceptible cells or HIV-1 that would be responsible for this effect of Gal-1 remain unknown. Importantly, this effect was observed with clinical strains of HIV-1 produced in both primary lymphocytes and macrophages (33, 40), suggesting that glycans specific for Gal-1 could be naturally found on the surface of field isolates of HIV-1. Gal-1 belongs to the family of galectins, the most widely expressed class of lectins in multicellular organisms. All members of the galectin family share a primary structure homology in their glycan recognition domains, which have an affinity for glycans containing β-galactosides (2, 21). In humans, Gal-1 and Gal-3 are expressed by various cell types, most of them involved in acquired and innate immunity. Their expression is increased in the context of inflammation or infection and can be found at high concentrations (as high as 10 μM) in lymphoid tissues and their associated mucosa, where active HIV-1 replication has been reported to occur (28, 40, 45, 48–50, 57). Being multivalent, Gal-1 or Gal-3 cross-links at least two different glycans either on the same cell surface or on different constituents. In the latter case, this cross-linking activity confers to galectins the properties of adhesion molecules by directly mediating either cell-to-cell or cell-to-pathogen interactions despite being soluble proteins (28, 45, 48–50). Importantly, even though Gal-1 and Gal-3 often share similar ligands on host cells, only Gal-1, but not Gal-3, can promote binding of HIV-1 to susceptible cells in a β-galactoside-dependent manner (33, 40, 59).
Upon transmission, HIV-1 must penetrate across an epithelium that is covered with heavily glycosylated mucins. Gal-3 is abundantly expressed at the apical surface of these mucosal membranes (38, 39) and is believed to be an active participant of the mucosal clearance system (1). Thus, mucins and Gal-3 are most likely among the first molecules that HIV-1 encounters, and they would severely reduce its penetration efficiency. In contrast to Gal-3, Gal-1 is highly expressed in the lamina muscularis mucosae, just beneath the epithelium and its lamina propria, where susceptible CD4+ T cells can be found (12, 39, 56). Both of these galectins normally exhibit similar glycan binding properties, but our results suggest that they nonetheless differentially affect HIV-1 binding to susceptible cells (33, 40, 59). Considering the clearance role of Gal-3 and the positive influence of Gal-1 for viral attachment and entry, this differential binding could be advantageous for efficient viral transmission and early viral replication. The molecular mechanisms responsible for this differential specificity are currently unknown, but their elucidation could pave the way for the development of new therapies seeking to undermine the ability of HIV-1 to infect founder cells during transmission events. We now report here for the first time that both gp120 and CD4 are specifically bound by Gal-1. We provide evidence that Gal-1 binds preferentially to clustered CX glycans of gp120 on HIV-1 and to CX glycans of CD4 to drastically increase the avidity of HIV-1 for susceptible CD4-expressing T cells, therefore enhancing virus infectivity. Our results also suggest that clustering of CX glycans, resulting from structural constraints of the peptide backbone of gp120, effectively prevents Gal-3 binding. Thus, the differential binding of HIV-1 gp120 to Gal-1 and Gal-3 does not rely on the presence of any particular CX glycan sequence (primary structure), as is normally the case for most lectins, but relies instead on the peculiar arrangement and clustering of CX glycans attached to gp120 (tertiary structure).
MATERIALS AND METHODS
Reagents.
Chemicals and other reagents were obtained from Sigma Chemical Co. (St. Louis, MO). Recombinant HIV-1 Bal gp120 (R5), 96ZM651 gp120 (X4), recombinant soluble human CD4 (produced by a mammalian [CHO cell] expression system), interleukin-2, and antiserum to gp120 were provided by the NIH AIDS Research and Reference Reagent Program (Germantown, MD). A polyclonal antibody against human gp120 was purified from sheep antiserum (18). Peroxidase-AffiniPure rabbit anti-sheep IgG was obtained from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA).
Recombinant galectins were produced and purified as previously described (40, 59). In some experiments, Gal-1 was S-carboxyamidomethylated with iodoacetamide (58, 59) to prevent its oxidation. This stabilized form of Gal-1 exhibits similar activity for HIV-1 binding and infection (59). The stabilized Gal-1 was used for Gal-1-agarose, while all Gal-1 column chromatographies were performed with both native and stabilized Gal-1, and both Gal-1 forms gave similar results. Alexa 488-labeled Gal-1 and Gal-3 were prepared following the manufacturer's instructions (Molecular Probes, Eugene, OR) with slight modifications (42, 43).
Viral preparations and binding and infection assays.
Virus particles were prepared from the culture medium of HEK 293T cells that were transiently transfected with the molecular clones pNL4-3, pNL4-3Balenv, or pNL4-3Δenv as previously described (59). Alternatively HIV-1 (X4R5 or R5) was purified from the medium of peripheral blood mononuclear cells (PBMCs) infected with HIV-1 as previously described (59). For lectin affinity chromatography, virus particles were inactivated either by a 30-min incubation with 0.5% Triton X-100 (for virus lysates) or by overnight incubation with 2-aldrithiol (AT-2; 2,2′-dithiodipyridine; for whole virus particles) (9, 46). HIV-1 binding and infection experiments were carried out as previously published (59). Briefly, for viral binding, activated CD4+ T lymphocytes were first incubated with HIV-1 in the presence or absence of Gal-1 or -3 for 1 h. Cells were then washed with phosphate-buffered saline (PBS) to remove unbound HIV-1 and lysed with a disruption buffer (PBS [pH 7.4] and Triton X-100 at 0.5%). Levels of HIV-1 binding were estimated by a double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) directed against p24. For Raji cells, either a Raji cell line or Raji derivative stably expressing CD4 (6) was incubated with HIV-1 (10 ng of p24 per 1 × 105 cells) for 10 min at room temperature. For infection studies, target cells were incubated with HIV-1 (10 ng of p24 per 1 × 105 cells) for 3 days in the presence or absence of Gal-1, and HIV-1 production was estimated by measuring p24 levels in cell-free supernatants.
Microscale affinity chromatography.
Gal-1, Gal-3, and CD4-agarose columns were prepared using AminoLink Plus coupling resin (Pierce Biotechnology, Rockford, IL) with some modifications (43). Briefly, 1 to 2 mg/ml of purified protein was coupled to AminoLink Plus coupling gel through a 4-h incubation at room temperature. For galectin-agarose, lactose was added when immobilized to protect the glycan binding domains. AT-2-treated virus particles (containing 1 to 1.5 μg of p24), viral lysates prepared from HIV-1 (p24, 500 ng) treated with 0.5% Triton X-100-Tris-HCl (pH 7.2)-0.12 M NaCl, gp120 (2 μg), or labeled glycans were applied to the galectin column (0.5-ml bed volume). For viral lysates, the fractionation was done in the presence of 0.5% Triton X-100. For whole virus particles, the fractionation was done in the absence of such detergent. Columns were then washed with PBS (0.5 ml, 3 fractions), followed by elution with 100 mM mannose (Man; 0.5 ml, 3 fractions) and finally with 100 mM lactose (Lac; 0.3 ml, 6 fractions). Levels of HIV-1, gp120, and 2-aminobenzamide (2-AB)-labeled glycans in the fractions were estimated by using p24 ELISA, Western blotting with anti-gp120 antibody, and 2-AB fluorescence, respectively. In the case of CD4, galectins were applied to a CD4-agarose column (25-μl bed volume), and the presence of galectins in the eluted fractions was estimated by the level of Alexa 488 fluorescence associated with galectins. For the binding of gp120 to CD4, gp120 (R5) in the presence or absence of Gal-1 was incubated with CD4-agarose (25-μl bed volume) for 15 min with or without Lac (100 mM) at room temperature. After unbound material was removed by two washes with 100 μl of Tris-buffered saline (TBS)-0.1% Triton X-100, beads were treated with SDS-PAGE sample buffer at 65°C for 5 min to recover bound material, which was subjected to Western blotting with anti-gp120 antibody.
Western blotting.
To detect gp120, samples were subjected to a 7.5% polyacrylamide gel followed by electrotransfer on a 0.45 μm polyvinylidene difluoride membrane. Membranes were then blocked overnight at 37°C with 5% gelatin in TBS-1% Tween 20 (TBST) and incubated with a sheep IgG anti-gp120 (1:1,000) for 4 h at room temperature in 0.5% gelatin-TBST. After incubation with peroxidase-labeled rabbit anti-sheep IgG (1:10,000) for 2 h at room temperature, gp120 bands were detected by using the Western lighting chemiluminescence reagent (Perkin-Elmer LAS, Inc.).
Glycan purification and labeling.
N-linked glycans attached to recombinant gp120 (X4 and R5) were removed either by PNGase F (glycerol free; New England BioLabs, Pickering, ON, Canada) or by hydrazinolysis as previously described (29). Recovered glycans were purified with LudgerClean E cartridges (QA-bio, San Mateo, CA) and then dried and labeled with 2-AB using the LudgerTag 2-AB labeling kit (QA-bio). Free dyes were removed using LudgerClean S (QA-bio).
Glycan structure analyses.
As previously described, 2-AB-labeled glycans were analyzed by anion-exchange chromatography, normal-phase high-performance liquid chromatography, and sequential glycosidase digestion (29).
RESULTS
Gal-1, but not Gal-3, binds to HIV-1 in a β-galactoside-dependent manner.
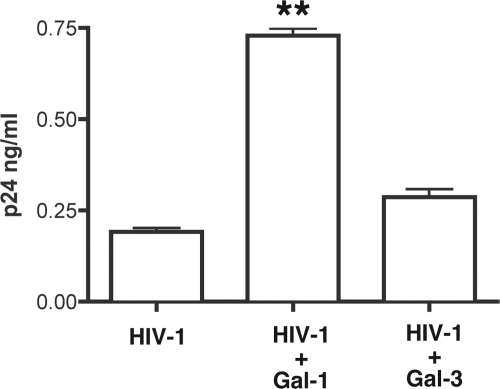
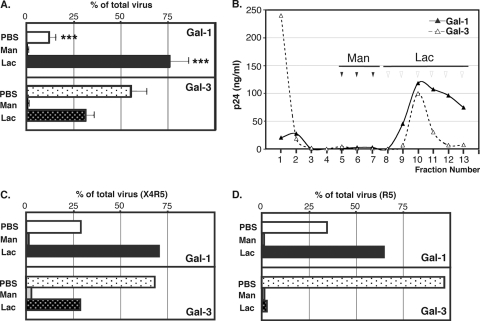
Our previous studies suggest that Gal-1 accelerates the kinetics of HIV-1 attachment to the target cell surface, and this effect is dependent on its β-galactoside binding activity, since the galectin antagonist lactose efficiently inhibits virus binding (33, 40, 59). In contrast to Gal-1, Gal-3 does not enhance HIV-1 infection (X4, R5, or X4R5 variants) in host cells. Since both galectins exhibit relatively similar glycan binding specificity, the molecular mechanism for this Gal-1 specificity in the context of HIV-1 infection remains unknown. As shown in Fig. 1, when HIV-1 was exposed to CD4+ T cells in the presence or absence of the studied galectins, Gal-1, but not Gal-3, significantly enhanced HIV-1 binding to susceptible cells (Fig. 1), suggesting that Gal-1-specific HIV-1 binding contributes to the increase of virus infection. Therefore, even though HIV-1 virus particles are covered with a thick glycocalyx (53), only Gal-1 participates in increasing virus attachment to susceptible cells. To shed light on the precise mechanism(s) by which Gal-1 increases virus adsorption, intact infectious HIV-1 particles produced by HEK cells were subjected to galectin affinity chromatography to determine whether Gal-1 directly binds to HIV-1 or not (Fig. 2A and B). After removing unbound virus, a first step of elution of galectin-associated virus was performed with mannose (Man). This first elution step thus represented a specificity control for β-galactoside-dependent binding of Gal-1 to HIV-1, since Man does not inhibit the Gal-1-mediated enhancement of HIV-1 infectivity (40, 59). A second elution step was then performed with Lac, which binds to galectins and competes with viral particles associated with Gal-1 in a β-galactoside-dependent manner. As shown in Fig. 2A and B, as much as 75% of HIV-1 was eluted from the Gal-1 column by Lac, suggesting that Gal-1 directly binds to HIV-1 particles through β-galactoside-dependent interactions. No significant level of virus was eluted by Man, confirming that Gal-1 does not bind to HIV-1 through OM glycans. In contrast, only 30% of HIV-1 applied to the Gal-3 column was eluted by Lac, and the majority of virus particles were found in the washing steps, prior to Man elution. Since some peripheral modification of glycans can be altered depending on cell type or lineage, it is possible that HIV-1 produced by primary cells displays a different pattern of surface protein glycosylation from those glycans that arise from established cell lines. Such a difference might have an impact on Gal-1-specific recognition of HIV-1. To address this possibility, we next investigated binding of Gal-1 to X4R5 and R5 virus particles that were prepared from HIV-1-infected PBMCs. As shown in Fig. 2C, X4R5 HIV-1 preferentially binds to Gal-1 (69.8% of total input virus) compared to Gal-3 (28.7%). This trend was more evident with R5 virus from infected PBMCs, since as little as 5% of HIV-1 bound to Gal-3 while Gal-1 captured close to 69% of input virus (Fig. 2D). Thus, these data suggest that Gal-1-specific binding is consistent whether HIV-1 is produced by PBMCs or HEK cells.
Fig. 1.
Gal-1 but not Gal-3 facilitates HIV-1 adsorption to CD4+ T cells. Activated CD4+ T cells were incubated with NL4-3 HIV-1 with or without Gal-1 or Gal-3 (2 μM) for 1 h at 4°C. After unbound virions were removed, cells were lysed and HIV-1 binding was estimated by measuring the levels of p24 associated with cells in an ELISA. Data shown represent the means ± standard errors of the means of four determinations. The representative results from three different experiments are shown. P values were determined from comparisons to HIV-1 binding in the absence of galectin. **, P < 0.01.
Fig. 2.
Gal-1 but not Gal-3 binds to HIV-1 in a β-galactoside-dependent manner. (A) AT-2-treated HIV-1 virus particles were applied to a Gal-1- or Gal-3-agarose column (0.5-ml bed volume), and each column was first washed with PBS (0.5 ml, 3 fractions), followed by Man (0.5 ml, 3 fractions) and Lac (0.3 ml, 6 fractions) to elute bound HIV-1. The levels of virus in each fraction were estimated by p24 ELISA. Data shown represent the means ± standard errors of the means of three determinations. The representative results from two different experiments are shown. P values were determined by comparison of the percentages of virus in each fraction collected from the galectin-1 column to those collected on the galectin-3 column. ***, P < 0.005. (B) Elution profile of HIV-1 by galectin affinity chromatography. Representative results from two different experiments are shown. (C) HIV-1 virus (X4R5 and R5), which were prepared from HIV-infected PBMC, were applied to a Gal-1- or Gal-3-agarose column as described for panel A.
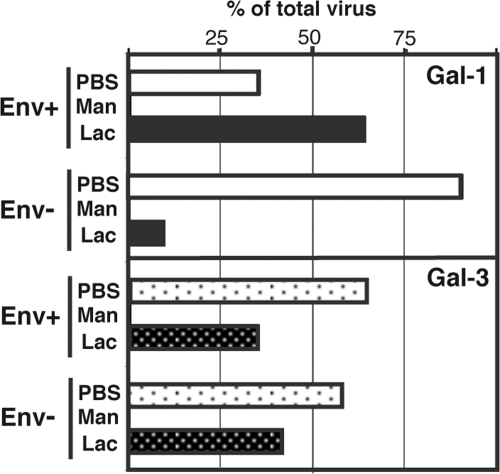
HIV-1 is an enveloped virus that acquires both host glycoproteins and virus-encoded Env glycoproteins during its budding from the infected cell. Since glycans can be found on all of these glycoproteins (7, 13), it is not clear whether such differences in retention of the virus by galectins are due to specific recognition of Env or host-derived glycoprotein incorporated within virions. Thus, the affinities of virus particles either lacking (i.e., NL4-3Δenv) or bearing Env (i.e., NL4-3) for Gal-1 and Gal-3 were next compared (Fig. 3). Regardless of the presence or absence of Env, comparable amounts of virus particles were retained by the Gal-3 column, suggesting that Gal-3 binds to virus-associated host glycoproteins rather than Env. In contrast to Gal-3, elimination of Env from the virion dramatically reduced its binding to the Gal-1 column, therefore indicating that Gal-1 uniquely recognizes the presence of Env on HIV-1.
Fig. 3.
Gal-1 binds specifically to Env-bearing HIV-1. AT-2-treated virions either bearing or lacking Env were applied to Gal-1- or Gal-3-agarose columns. The representative results from two different experiments are shown.
Viral gp120 of both X4 and R5 is recognized by Gal-1.
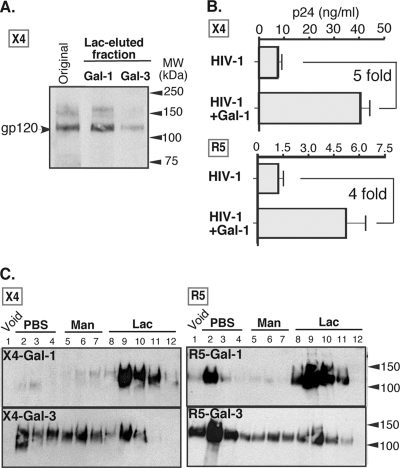
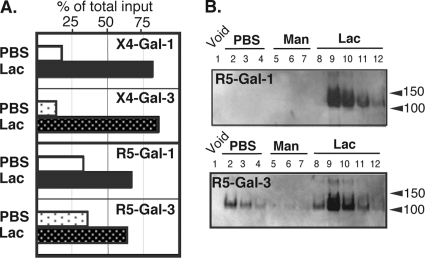
To further elucidate Gal-1-specific recognition of Env, a detergent-soluble lysate of X4-tropic HIV-1 particles was applied to a galectin-agarose column. Significant levels of HIV-1 gp120 were retained by the Gal-1 column and recovered in the Lac fractions, while very low levels, if any, could be recovered from the Gal-3 column (Fig. 4A). These results demonstrate that viral gp120 indeed binds to Gal-1 and also that specific binding of gp120 by Gal-1 is not likely due to an architectural presentation of gp120 on the virus surface. Since the above-mentioned experiments were performed with an X4 strain of HIV-1, we verified whether this Gal-1-specific recognition and enhancement of HIV-1 infectivity could also be observed with an R5-tropic variant. As shown in Fig. 4B, Gal-1 enhanced HIV-1 infectivity of both X4- and R5-using strains following acute infection of primary human CD4+ T cells. Furthermore, consistent with the results of HIV-1 lysates, the binding preference of Gal-1 for gp120 was also observed when recombinant gp120 from either X4 and R5 variants was used for galectin affinity chromatography (Fig. 4C). In contrast, recombinant gp120 molecules failed to be retained by a Gal-3 column and their retention was rather retarded throughout elution without forming any significant peaks when lactose was introduced. Together, these data suggest that Gal-1, but not Gal-3, specifically recognizes both soluble and virus-bound gp120.
Fig. 4.
Viral gp120s of both the X4 and R5 variants are recognized by Gal-1. (A) HIV-1 lysate was applied to a Gal-1- or Gal-3-agarose column, and PBS-, Man-, and Lac-eluted fractions were collected. Levels of gp120 in Lac-eluted fractions were detected with anti-gp120 antibody. Representative results from three different experiments are shown. (B) Activated CD4+ T cells were infected with either X4 or R5 HIV-1 with or without Gal-1 (2 μM) for 1 h. After unbound virions were removed, cells were further incubated for 48 h. The levels of p24 in the cell-free medium were used to estimate virus infection. Representative results from two different experiments are shown. (C) Recombinant gp120 (X4 or R5) was subjected to galectin affinity chromatography as described for Fig. 1. The presence of gp120 in each fraction was revealed by using anti-gp120 antibody. Results shown are representative of three different experiments.
Structural analysis of N-linked glycans of gp120.
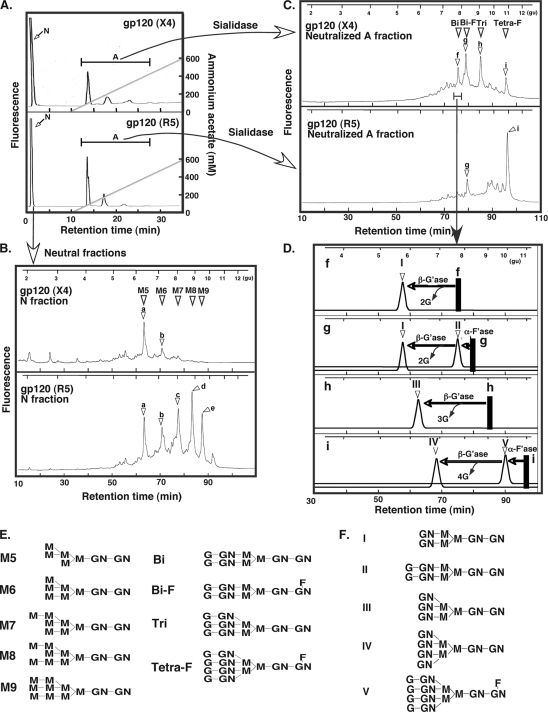
Previous studies on the structure of the glycans of gp120 from various sources suggest that it contains bi-, tri-, and tetra-antennary CX glycans, which carry 2, 3, and 4 LacNAc groups, respectively (16, 34, 41). Separate studies on the binding specificities of galectins have established that Gal-3 displays a higher affinity and robust avidity for CX glycans carrying a higher number of LacNAc groups than does Gal-1 (8, 20, 47, 58). Thus, the results presented so far are not easily reconciled with the established glycan specificities of Gal-1 and Gal-3. In order to further study this specific recognition of gp120 by Gal-1, N-linked glycan structures of the X4 and R5 gp120s were characterized in details. N-linked glycans were released from gp120 by hydrazinolysis as previously described (29). These glycans were first separated by anion-exchange (MonoQ) column chromatography into neutral (N) and acidic (A) fractions, and the ratio of N to A was approximately 7 to 3 (Fig. 5A). When subjected to normal-phase (GlycoSepN) column chromatography, the majority of the neutral fractions were eluted at positions corresponding to the 2-AB-labeled authentic OM glycans (Fig. 5B, triangles M5, M6, M7, M8, and M9; their structures are shown in E). These data suggest that, irrespective of viral tropism, 70% of gp120 glycans are OM glycans (Table 1). The acidic fractions were further studied by treating the acidic glycans with sialidase to remove sialic acid residues before being run on a GlycoSepN column. Most of the glycans were eluted at positions that corresponded to the authentic CX glycan standards (Fig. 5C, triangles Bi, Bi-F, Tri, and Tetra-F; their structures are shown in E). Further confirmation of these glycan structures (fractions f, g, h, and i) were performed as shown in Fig. 5D. Fractions f and h, which were eluted at positions corresponding to authentic biantennary and triantennary CX glycans, respectively, were incubated with β-galactosidase. This digestion removed 2 and 3 galactose residues from fractions f and h, respectively, as indicated by the position at which they were eluted from the GlycoSepN column. In addition, the digested glycans were eluted at positions corresponding to the authentic bi- and triantennary glycan standards that lack terminal galactose residues (Fig. 5F, structures I and III), respectively. Together, these analyses suggest that the glycan structures in fractions f and h were bi- and triantennary CX glycans, respectively (Fig. 5E, Bi and Tri). As fractions g and i eluted at the positions of monofucosylated bi- and tetra-antennary glycans, these fractions were first treated with α-fucosidase. Once digested, glycans were eluted at positions representing one less monosaccharide than their original glycan size (Fig. 5D, peaks II and V) and also corresponded to the defucosylated bi- and tetra-antennary chain (Fig. 5F, structures II and V). Those defucosylated glycans were further incubated with β-galactosidase, resulting in a reduction of 2 and 4 monosaccharide residues, and they eluted at the positions of standard structures I and IV of Fig. 5F. Thus, the glycan structures of fractions g and i are monofucosylated bi- and tetra-antennary glycans, respectively. Structures and their percent ratios of N-linked gylcans from gp120 are summarized in Table 1. Together, detailed analysis of the glycan structure of the recombinant gp120 proteins used in the previous section showed that 30% of glycans of both X4 and R5 gp120 contain CX glycans (Fig. 5 and Table 1).
Fig. 5.
Characterization of N-linked glycans of gp120. (A) 2-AB-labeled N-linked glycan of gp120 was first separated into neutral and acidic fractions by using anion-exchange chromatography (MonoQ HR5/5). N, neutral fraction; A, acidic fraction. (B) Neutral fractions were further subjected to normal-phase column chromatography by using a GlycoSep N column. The open triangles (M5 to M9, peaks a, b, c, and d, respectively) and their structures are shown in panel E and indicate the elution positions of the 2AB-labeled authentic OM N-glycan standards. The 2-AB-labeled glucose oligomer ladder (gu) was used to estimate the size change of glycans after glycosidase digestion and was shown at the top. The molar ratios of each component are summarized in Table 1. (C) The acidic fraction from panel A was first treated with sialidase to remove sialic acid residues and then subjected to normal-phase column chromatography (GlycoSep N column). The open triangles indicate the elution positions of the 2-AB-labeled authentic CX N-linked glycan standards, as shown in panel F. The structures and molar ratios of each component are summarized in Table 1. (D) Sequential glycosidase digestion of each component in the acidic fraction. Each component (f to i) in panel C was fractionated as a single peak by reverse-phase and normal-phase column chromatography. After digestion by a particular glycosidase, each component was then analyzed and recovered by reverse-phase and normal-phase column chromatography. The displacements of the elution positions on the normal-phase column by sequential glycosidase digestion are shown. The arrows indicate displacements of elution positions by digestion with the following glycosidases: β-G'ase, β-galactosidase (Streptococcus 6646K); α-F'ase, α-fucosidase (bovine kidney). The open triangles indicate the elution positions of the following 2AB-labeled authentic N-glycan standards as shown in panel E. (E) Glycan structures of gp120 glycans. (F) Glycan structures of the authentic 2-AB-labeled glycan standards. M, mannose; GN, N-acetylglucosamine; G, galactose; F, fucose; Bi, biantennary CX glycan; Bi-F, fucosylated biantennary glycan; Tri, triantennary glycan; Tetra-F, fucosylated triantennary glycan; gu, glucose unit.
Table 1.
Percent molar ratios of N-linked glycans attached to gp120 of HIV-1a
| Fraction | Elution position | Chromatography peak | HIV-1 gp120 molar ratio (%) |
|
|---|---|---|---|---|
| X4 | R5 | |||
| Neutral, OM type | M5 | a | 48 | 10 |
| M6 | b | 22 | 14 | |
| M7 | c | 0 | 18 | |
| M8 | d | 0 | 18 | |
| M9 | e | 0 | 10 | |
| Acidic, CX type | Bi | f | 6 | 0 |
| Bi-F | g | 10 | 7 | |
| Tri | h | 10 | 0 | |
| Tetra-F | i | 0 | 18 | |
Structures and chromatography peaks for these data are provided in Fig. 5.
Gal-1, but not Gal-3, recognizes clustered CX glycan patches of gp120.
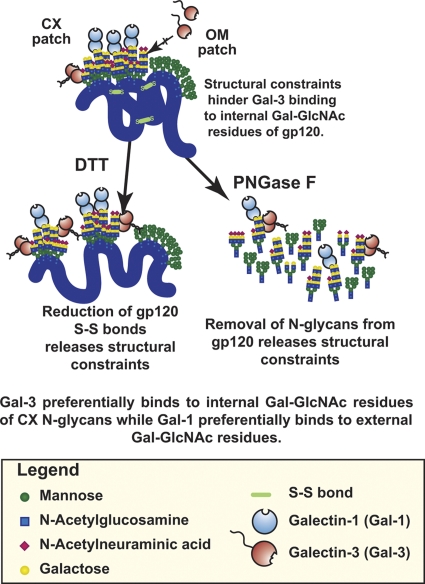
Data from previous glycan binding specificity studies indicated that such glycans display similar affinity for both Gal-1 and Gal-3. For example, Gal-3 has a higher affinity (at least 3- to 5-fold-higher Kd) for tri- and tetra-antennary CX glycans, while biantennary CX glycans show a persistent avidity for Gal-1 under low concentrations (8, 20, 47, 58). This glycan analysis thus suggests that the observed preference of Gal-1 for gp120, compared to that of Gal-3, is not likely to be due to a de facto preference of one galectin toward specific glycans displayed by gp120. Indeed, when glycans released from both X4 and R5 recombinant gp120 were subjected to galectin affinity chromatography, both the Gal-1 and Gal-3 columns captured similar proportions of gp120-derived glycans (Fig. 6A). Thus, the released glycans did not show any binding preference for Gal-1 over Gal-3, which is consistent with the established glycan binding specificity of Gal-1 and Gal-3. Thus, while Gal-3 can recognize the free glycans of gp120, it is unable to do so when these same glycans are attached to gp120. We next studied whether or not the CX glycan clusters are involved in the inability of Gal-3 to recognize these glycans in the specific context of an intact gp120 glycoprotein tertiary structure. Thus, gp120 disulfide bonds were reduced by dithiothreitol (DTT) treatment, and the reduced protein was applied to Gal-1 and Gal-3 columns. As shown in Fig. 6B, the reduced form of gp120 was retained efficiently by the Gal-3 column, and its elution pattern became similar to that of the Gal-1 column. Together, these results suggest that the spatial organization of CX glycans on gp120, rather than their composition, contributes mostly to its specific recognition by Gal-1 and the repulsion of Gal-3.
Fig. 6.
Both Gal-1 and Gal-3 recognize glycans of gp120 once they are released from glycan patches or gp120 is partially denatured. (A) N-linked glycans were first released from gp120 and then labeled with 2-AB. The glycans were subjected to galectin affinity chromatography. Representative results from three different experiments are shown. (B) DTT-treated (overnight, 1 mM) gp120 (R5) was applied to galectin columns. Results shown are representative of three different experiments.
CD4 is a ligand for Gal-1.
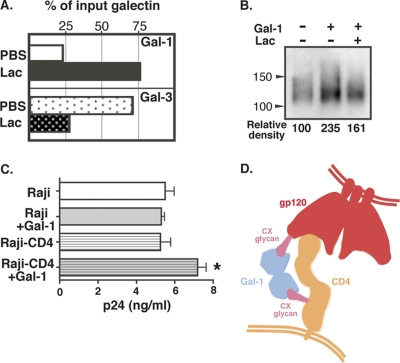
The above data suggest that Gal-1 binds to CX glycans found on native gp120, which are adjacent to the CD4 binding pocket. Gal-1 is a homodimer and thus harbors two glycan binding sites. Since Gal-1 rapidly stabilizes HIV-1 attachment onto susceptible target cells (40), it is possible that Gal-1 also binds to glycans attached to the primary cellular receptor of gp120, the CD4 molecule. CD4 carries two N-linked glycans, both of which are CX biantennary chains (64). Thus, to test this possibility, Gal-1 or Gal-3 protein was subjected to CD4 affinity chromatography. As shown in Fig. 7A, Gal-1, but not Gal-3, bound to CD4 in a β-galactoside-dependent manner. Furthermore, in the presence of Gal-1, the amount of gp120 retained by the CD4 column was higher and was specifically inhibited by Lac (Fig. 7B), suggesting that Gal-1 stabilizes the interaction between gp120 and CD4. To confirm the ability of Gal-1 to cross-link virus-associated gp120 and cell surface CD4, experiments were performed with a CD4-negative Raji cell line and a Raji derivative stably expressing CD4 (Raji-CD4). Results depicted in Fig. 7C demonstrate that binding of HIV-1 was significantly enhanced in Raji-CD4 cells in the presence of Gal-1, in contrast to what was seen in Raji cells (CD4 negative), for which virus adsorption was not affected by the lectin. Altogether, these data suggest that Gal-1 can cross-link gp120 and CD4 (Fig. 7D). Therefore, the results raise the possibility that CD4 represents one of the ligands targeted by Gal-1 to promote HIV-1 attachment on the cell surface.
Fig. 7.
CD4 is a ligand for Gal-1. (A) Gal-1 was applied to a CD4-agarose column, and bound lectin was eluted with Lac as described for Fig. 1. Results shown are representative of three different experiments. (B) In the presence or absence of Gal-1, gp120 was incubated with CD4-agarose. After unbound materials were removed by a series of PBS washes, gp120 that was bound to CD4 was released by adding SDS-PAGE sample buffer and detected by anti-gp120 antibody. Representative results from two different experiments are shown. (C) HIV-1 (10 ng of p24) was incubated with Raji and Raji-CD4 cells at room temperature for 10 min in the absence or presence of Gal-1 (2 μM). Controls consisted of cells incubated with HIV-1 in the absence of Gal-1. The representative results from two different experiments are shown. P values were created by comparison to HIV-1 binding in the absence of galectin 1. *, P < 0.05. (D) Proposed model of the interaction of gp120 and CD4 together with Gal-1.
DISCUSSION
Sexual transmission of HIV-1 is severely limited by multiple anatomical, chemical, and immunological barriers so that, when it occurs, only a small number of infectious viral particles successfully reach the underlying mucosa to create a founder cell population that rapidly expands and establishes a self-propagating infection (17, 19, 22, 32, 54). Mucosa-associated CD4+ T cells found just beneath the vaginal and cervical epithelium are known to be somewhat less competent than fully activated peripheral blood CD4+ T cells (17, 22, 32). Their efficient infection is further complicated by the fact that HIV-1 must compensate in some way for the paucity of functional Env spikes on its surface, which results in a low avidity for its cellular receptor, CD4. HIV-1 must therefore evade and/or exploit host mucosal immune defenses, such as glycan-lectin interactions, to facilitate its specific interaction with susceptible cells in the underlying lamina propria. These initial steps thus represent a significant bottleneck of HIV-1 infection that remains to be exploited in order to further reduce the frequency of transmission events.
Glycosylation of gp120 has been the subject of intense studies since the discovery of HIV-1 as the etiologic agent of AIDS. This interest arises from the large amount of glycans attached to gp120 as well as from their unusual composition and their peculiar arrangement in specific clusters. Three clusters of glycans are found on the surface of gp120: one is formed from OM glycans, and two are formed from CX glycans (53). HIV-1 uses the host's own glycosylation machinery for glycosylation of gp120. As such, the arrangement of glycans on gp120 is considered unique, since glycans attached to host glycoproteins normally exhibit considerable conformational freedom because of their highly hydrophilic nature. Recognition of OM glycans of gp120 by CLRs of immune cells is exploited by HIV-1 to enhance infection of susceptible cells (11, 15, 23, 24, 27, 31, 61). The present study now suggests a role for the CX glycans of gp120. Recognition of gp120 CX patches by non-CLR soluble lectin, Gal-1, but not Gal-3, explains the specific enhancement of HIV-1 binding to susceptible cells mediated by the former and not the latter. While the recent report by Doores and colleagues suggested a relatively low abundance of CX glycans on native HIV-1 (10), our previously published work and the present study demonstrate that Gal-1 promotes attachment of native HIV-1 produced by both lymphocytes and macrophages through a specific interaction with CX glycans (33, 40). Thus, regardless of levels of CX glycans attached to native virus, Gal-1, but not Gal-3, binds to the CX glycans, facilitating HIV-1 infection. It is likely that the anatomical localization of cells expressing each galectin could explain how HIV-1 has evolved its glycan shield to recruit Gal-1 and not Gal-3. Indeed, Gal-3 is highly expressed at the apical surface of mucosal membranes, while Gal-1 is highly expressed in the lamina muscularis mucosae, just beneath the epithelia and its lamina propria, where abundant susceptible CD4+ T cells can be found (12, 39, 56).
Recognition of HIV-1 by Gal-1 but not Gal-3 may arise from a unique binding preference (or a positional selection) of each galectin for the position of LacNAc displayed by CX glycans. Loss of differential binding to each galectin following enzymatic cleavage of gp120 glycans or partially denatured gp120 suggest that specific binding of Gal-1 to gp120 largely depends on the peculiar presentation of CX glycans by the peptide backbone itself. Indeed, those glycans are constrained within tight clusters on gp120, where sugar-sugar interactions are formed between neighboring glycans (53). Thus, for a lectin such as Gal-3, which has been shown to bind to internal LacNAc residues (8, 20, 47, 58), tightly clustered glycans may prevent efficient binding. In contrast, Gal-1 preferentially binds to the peripheral LacNAc of CX glycans (8, 20, 58), and it is thus possible that Gal-1 efficiently recognizes LacNAc residues of even densely packed glycans, as indicated by this study (Fig. 8). Such glycan clustering-dependent exclusion of lectin binding, for which the biological significance had not been recognized previously, may also be beneficial for HIV-1 to escape from being captured by Gal-3 at the surface of mucosal membranes, where no susceptible cells can be found and where multiple mucosal clearance mechanisms are active. Conversely, HIV-1 binding by Gal-1, which is found proximal to the lamina propria, may allow rapid and efficient attachment of HIV-1 to susceptible founder cells and prevent entrapment and dilution of the initial viral inoculum. Together with its localization and its potential to accelerate viral attachment and entry, the soluble lectin Gal-1 can be proposed as a host immune factor exploited by HIV-1 for the eclipse and initial phase of infection.
Fig. 8.
Glycan density-dependent gp120 recognition by Gal-1.
Our previous studies suggested that Gal-1 facilitates viral infection even under suboptimal conditions, such as in the presence of anti-CD4 antibody or fusion inhibitors, although virus entry remains entirely dependent on CD4-gp120 interactions. Thus, it seems that it is the ability of Gal-1 to greatly accelerate the binding kinetics of HIV-1 on susceptible cells that severely cripples the efficiency of entry inhibitors. Gal-1 is a homodimer, and its glycan binding domain is of a size that accommodates only one LacNAc of a glycan. Thus, when Gal-1 cross-links a virus particle to a susceptible cell, at least one LacNAc of a glycan of gp120 and one LacNAc of a glycan of the cell are expected to simultaneously bind to one dimer of Gal-1 (Fig. 7D). This rapid HIV-1 fixation on the surface of cells leads to a significant increase in HIV-1 infectivity, suggesting that Gal-1 could help the viral particle via efficient initiation of fusion. Indeed, our present data suggest that Gal-1 recognizes CD4 and increases the interaction between CD4 and gp120. Together, the present data indicate that the peculiar spatial arrangement of CX glycans of gp120 would allow its cross-linking with a cellular ligand, such as CD4, by dimeric Gal-1 molecules without interfering with the gp120/CD4 binding site and later fusion events (Fig. 7D). Although Gal-1 could bind to other cellular ligands (5), CD4 can be considered an important ligand in the context of HIV-1 infection. These results further suggest that HIV-1 exploits the Gal-1 cross-linking ability not only to increase its avidity toward highly susceptible CD4-expressing cells but also to directly target its gp120 spikes toward its cognate cellular receptor.
In summary, our results provide evidence that Gal-1 is the first soluble host lectin to be exploited by HIV-1 to improve its chances of being efficiently transmitted through its glycan structures. Our study has unveiled the unexpected role of clustered CX glycans in the specific recognition of gp120 by Gal-1. Furthermore, we have demonstrated specific binding of CD4 by Gal-1, raising the possibility that the Gal-1-mediated cross-linking ability could direct viral spikes to their cognate receptors. Modulation of Gal-1 expression or activity at mucosal sites where susceptible cells initially encounter HIV-1 thus represents an interesting therapeutic avenue to decrease the likelihood of sexual transmission of HIV-1.
ACKNOWLEDGEMENTS
We acknowledge Isabelle Pelletier for her preliminary results of Gal-1 binding to HIV-1, Sean Stowell for the method of iodoacetamidation of Gal-1, Yukiko Sato for purification of galectins, and Julie Nieminen for her editorial work on the manuscript.
This work was supported by an operating grant from the Canadian Institutes of Health Research (CIHR) to S.S. and M.J.T. (MOP-89743). C.S.-P. holds a Graduate Scholarship Award from CIHR, S.S. holds a Scholarship Award (senior level) from the Fonds de la Recherche en Santé du Québec, and M.J.T. is the recipient of a Tier 1 Canada Research Chair in Human Immuno-Retrovirology.
Footnotes
Published ahead of print on 31 August 2011.
REFERENCES
- 1. Argueso P., et al. 2009. Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. J. Biol. Chem. 284: 23037–23045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barondes S. H., et al. 1994. Galectins: a family of animal beta-galactoside-binding lectins. Cell 76: 597–598 [DOI] [PubMed] [Google Scholar]
- 3. Boily M. C., et al. 2009. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect. Dis. 9: 118–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brenchley J. M., et al. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200: 749–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Camby I., Le Mercier M., Lefranc F., Kiss R. 2006. Galectin-1: a small protein with major functions. Glycobiology 16: 137R–157R [DOI] [PubMed] [Google Scholar]
- 6. Cantin R., Fortin J. F., Lamontagne G., Tremblay M. 1997. The acquisition of host-derived major histocompatibility complex class II glycoproteins by human immunodeficiency virus type 1 accelerates the process of virus entry and infection in human T-lymphoid cells. Blood 90: 1091–1100 [PubMed] [Google Scholar]
- 7. Cantin R., Methot S., Tremblay M. J. 2005. Plunder and stowaways: incorporation of cellular proteins by enveloped viruses. J. Virol. 79: 6577–6587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cerra R. F., Gitt M. A., Barondes S. H. 1985. Three soluble rat beta-galactoside-binding lectins. J. Biol. Chem. 260: 10474–10477 [PubMed] [Google Scholar]
- 9. Chertova E., et al. 2003. Sites, mechanism of action and lack of reversibility of primate lentivirus inactivation by preferential covalent modification of virion internal proteins. Curr. Mol. Med. 3: 265–272 [DOI] [PubMed] [Google Scholar]
- 10. Doores K. J., et al. 2010. Envelope glycans of immunodeficiency virions are almost entirely oligomannose antigens. Proc. Natl. Acad. Sci. U. S. A. 107: 13800–13805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feinberg H., Mitchell D. A., Drickamer K., Weis W. I. 2001. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294: 2163–2166 [DOI] [PubMed] [Google Scholar]
- 12. Fichorova R. N. 2009. Impact of T. vaginalis infection on innate immune responses and reproductive outcome. J. Reprod. Immunol. 83: 185–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fortin J. F., Cantin R., Lamontagne G., Tremblay M. 1997. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J. Virol. 71: 3588–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fouts T. R., Binley J. M., Trkola A., Robinson J. E., Moore J. P. 1997. Neutralization of the human immunodeficiency virus type 1 primary isolate JR-FL by human monoclonal antibodies correlates with antibody binding to the oligomeric form of the envelope glycoprotein complex. J. Virol. 71: 2779–2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Geijtenbeek T. B., et al. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100: 587–597 [DOI] [PubMed] [Google Scholar]
- 16. Geyer H., Holschbach C., Hunsmann G., Schneider J. 1988. Carbohydrates of human immunodeficiency virus. Structures of oligosaccharides linked to the envelope glycoprotein 120. J. Biol. Chem. 263: 11760–11767 [PubMed] [Google Scholar]
- 17. Haase A. T. 2005. Perils at mucosal front lines for HIV and SIV and their hosts. Nat. Rev. Immunol. 5: 783–792 [DOI] [PubMed] [Google Scholar]
- 18. Hatch W. C., et al. 1992. Persistent productive HIV-1 infection of a CD4− human fetal thymocyte line. J. Immunol. 148: 3055–3061 [PubMed] [Google Scholar]
- 19. Hel Z., McGhee J. R., Mestecky J. 2006. HIV infection: first battle decides the war. Trends Immunol. 27: 274–281 [DOI] [PubMed] [Google Scholar]
- 20. Hirabayashi J., et al. 2002. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim. Biophys. Acta 1572: 232–254 [DOI] [PubMed] [Google Scholar]
- 21. Hirabayashi J., Kasai K. 1993. The family of metazoan metal-independent beta-galactoside-binding lectins: structure, function and molecular evolution. Glycobiology 3: 297–304 [DOI] [PubMed] [Google Scholar]
- 22. Karlsson Hedestam G. B., et al. 2008. The challenges of eliciting neutralizing antibodies to HIV-1 and to influenza virus. Nat. Rev. Microbiol. 6: 143–155 [DOI] [PubMed] [Google Scholar]
- 23. Kwon D. S., Gregorio G., Bitton N., Hendrickson W. A., Littman D. R. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 16: 135–144 [DOI] [PubMed] [Google Scholar]
- 24. Lambert A. A., Gilbert C., Richard M., Beaulieu A. D., Tremblay M. J. 2008. The C-type lectin surface receptor DCIR acts as a new attachment factor for HIV-1 in dendritic cells and contributes to trans- and cis-infection pathways. Blood 112: 1299–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leonard C. K., et al. 1990. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J. Biol. Chem. 265: 10373–10382 [PubMed] [Google Scholar]
- 26. Li H., et al. 2009. Proximal glycans outside of the epitopes regulate the presentation of HIV-1 envelope gp120 helper epitopes. J. Immunol. 182: 6369–6378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin G., et al. 2003. Differential N-linked glycosylation of human immunodeficiency virus and Ebola virus envelope glycoproteins modulates interactions with DC-SIGN and DC-SIGNR. J. Virol. 77: 1337–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu F. T. 2005. Regulatory roles of galectins in the immune response. Int. Arch. Allergy Immunol. 136: 385–400 [DOI] [PubMed] [Google Scholar]
- 29. Manya H., et al. 2000. Comparative study of the asparagine-linked sugar chains of human lipocalin-type prostaglandin D synthase purified from urine and amniotic fluid, and recombinantly expressed in Chinese hamster ovary cells. J. Biochem. 127: 1001–1011 [DOI] [PubMed] [Google Scholar]
- 30. Mattapallil J. J., et al. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434: 1093–1097 [DOI] [PubMed] [Google Scholar]
- 31. McDonald D., et al. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300: 1295–1297 [DOI] [PubMed] [Google Scholar]
- 32. McMichael A. J., Borrow P., Tomaras G. D., Goonetilleke N., Haynes B. F. 2010. The immune response during acute HIV-1 infection: clues for vaccine development. Nat. Rev. Immunol. 10: 11–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mercier S., et al. 2008. Galectin-1 promotes HIV-1 infectivity in macrophages through stabilization of viral adsorption. Virology 371: 121–129 [DOI] [PubMed] [Google Scholar]
- 34. Mizuochi T., et al. 1990. Diversity of oligosaccharide structures on the envelope glycoprotein gp120 of human immunodeficiency virus 1 from the lymphoblastoid cell line H9. Presence of complex-type oligosaccharides with bisecting N-acetylglucosamine residues. J. Biol. Chem. 265: 8519–8524 [PubMed] [Google Scholar]
- 35. Mondor I., Ugolini S., Sattentau Q. J. 1998. Human immunodeficiency virus type 1 attachment to HeLa CD4 cells is CD4 independent and gp120 dependent and requires cell surface heparans. J. Virol. 72: 3623–3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moore J. P., McKeating J. A., Huang Y. X., Ashkenazi A., Ho D. D. 1992. Virions of primary human immunodeficiency virus type 1 isolates resistant to soluble CD4 (sCD4) neutralization differ in sCD4 binding and glycoprotein gp120 retention from sCD4-sensitive isolates. J. Virol. 66: 235–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moore J. P., Sweet R. W. 1993. The HIV gp120-CD4 interaction: a target for pharmacological or immunological intervention? Perspect. Drug Disc. Des. 1: 235–250 [Google Scholar]
- 38. Nio-Kobayashi J., Iwanaga T. 2010. Immunohistochemical localization of galectins in mouse genital tract. Kaibougaku Zasshi (Tokyo) 85: 58(In Japanese with English abstract.) [Google Scholar]
- 39. Nio-Kobayashi J., Takahashi-Iwanaga H., Iwanaga T. 2009. Immunohistochemical localization of six galectin subtypes in the mouse digestive tract. J. Histochem. Cytochem. 57: 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ouellet M., et al. 2005. Galectin-1 acts as a soluble host factor that promotes HIV-1 infectivity through stabilization of virus attachment to host cells. J. Immunol. 174: 4120–4126 [DOI] [PubMed] [Google Scholar]
- 41. Pang P. C., Morris H. R., Haslam S. M., Clark G. F., Dell A. 2007. Glycomics analysis of HIV-1 gp120 glycoforms, abstr. 275. Glycobiology 17: 1274 [Google Scholar]
- 42. Pelletier I., et al. 2003. Specific recognition of Leishmania major poly-beta-galactosyl epitopes by galectin-9: possible implication of galectin-9 in interaction between L. major and host cells. J. Biol. Chem. 278: 22223–22230 [DOI] [PubMed] [Google Scholar]
- 43. Pelletier I., Sato S. 2002. Specific recognition and cleavage of galectin-3 by Leishmania major through species-specific polygalactose epitope. J. Biol. Chem. 277: 17663–17670 [DOI] [PubMed] [Google Scholar]
- 44. Piot P., Goilav C., Kegels E. 1990. Hepatitis B: transmission by sexual contact and needle sharing. Vaccine 8(Suppl.): S37–S40 [DOI] [PubMed] [Google Scholar]
- 45. Rabinovich G. A., Liu F. T., Hirashima M., Anderson A. 2007. An emerging role for galectins in tuning the immune response: lessons from experimental models of inflammatory disease, autoimmunity and cancer. Scand. J. Immunol. 66: 143–158 [DOI] [PubMed] [Google Scholar]
- 46. Rossio J. L., et al. 1998. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J. Virol. 72: 7992–8001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sato S., Hughes R. C. 1992. Binding specificity of a baby hamster kidney lectin for H type I and II chains, polylactosamine glycans, and appropriately glycosylated forms of laminin and fibronectin. J. Biol. Chem. 267: 6983–6990 [PubMed] [Google Scholar]
- 48. Sato S., Nieminen J. 2004. Seeing strangers or announcing “danger”: galectin-3 in two models of innate immunity. Glycoconj. J. 19: 583–591 [DOI] [PubMed] [Google Scholar]
- 49. Sato S., Rabinovich G. A. 2008. Galectins as danger signals in host-pathogen and host-tumor interactions: new members of the growing group of “alarmins”?, p. 115–146In Klysov A. A., Witczak Z. J., Platt D.(ed.), Galectins. John Wiley & Sons, Inc., New York, NY [Google Scholar]
- 50. Sato S., St.-Pierre C., Bhaumik P., Nieminen J. 2009. Galectins in innate immunity: dual functions of host soluble beta-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs). Immunol. Rev. 230: 172–187 [DOI] [PubMed] [Google Scholar]
- 51. Sattentau Q. J., Moore J. P. 1995. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J. Exp. Med. 182: 185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sattentau Q. J., Weiss R. A. 1988. The CD4 antigen: physiological ligand and HIV receptor. Cell 52: 631–633 [DOI] [PubMed] [Google Scholar]
- 53. Scanlan C. N., Offer J., Zitzmann N., Dwek R. A. 2007. Exploiting the defensive sugars of HIV-1 for drug and vaccine design. Nature 446: 1038–1045 [DOI] [PubMed] [Google Scholar]
- 54. Shattock R. J., Moore J. P. 2003. Inhibiting sexual transmission of HIV-1 infection. Nat. Rev. Microbiol. 1: 25–34 [DOI] [PubMed] [Google Scholar]
- 55. Shepard C. W., Simard E. P., Finelli L., Fiore A. E., Bell B. P. 2006. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol. Rev. 28: 112–125 [DOI] [PubMed] [Google Scholar]
- 56. Singh B. N., et al. 2009. Structural details and composition of Trichomonas vaginalis lipophosphoglycan in relevance to the epithelial immune function. Glycoconj. J. 26: 3–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stillman B. N., et al. 2006. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J. Immunol. 176: 778–789 [DOI] [PubMed] [Google Scholar]
- 58. Stowell S. R., et al. 2008. Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J. Biol. Chem. 283: 10109–10123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. St.-Pierre C., Ouellet M., Tremblay M. J., Sato S. 2010. Galectin-1 and HIV-1 infection. Methods Enzymol. 480: 267–294 [DOI] [PubMed] [Google Scholar]
- 60. Tardif M. R., Tremblay M. J. 2003. Presence of host ICAM-1 in human immunodeficiency virus type 1 virions increases productive infection of CD4+ T lymphocytes by favoring cytosolic delivery of viral material. J. Virol. 77: 12299–12309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Turville S., Wilkinson J., Cameron P., Dable J., Cunningham A. L. 2003. The role of dendritic cell C-type lectin receptors in HIV pathogenesis. J. Leukoc. Biol. 74: 710–718 [DOI] [PubMed] [Google Scholar]
- 62. Ugolini S., Mondor I., Sattentau Q. J. 1999. HIV-1 attachment: another look. Trends Microbiol. 7: 144–149 [DOI] [PubMed] [Google Scholar]
- 63. Veazey R. S., et al. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280: 427–431 [DOI] [PubMed] [Google Scholar]
- 64. Yuen C. T., Carr S. A., Feizi T. 1990. The spectrum of N-linked oligosaccharide structures detected by enzymic microsequencing on a recombinant soluble CD4 glycoprotein from Chinese hamster ovary cells. Eur. J. Biochem. 192: 523–528 [DOI] [PubMed] [Google Scholar]
- 65. Zhu P., et al. 2006. Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature 441: 847–852 [DOI] [PubMed] [Google Scholar]