Abstract
The experimental infection of newborn calves with bovine norovirus was used as a homologous large animal model to study the pathogenesis of norovirus infection and to determine target cells for viral replication. Six newborn calves were inoculated orally with Jena virus (JV), a bovine norovirus GIII.1 strain, and six calves served as mock-inoculated controls. Following infection, calves were euthanized before the onset of diarrhea (12 h postinoculation [hpi]), shortly after the onset of diarrhea (18 to 21 hpi), and postconvalescence (4 days pi [dpi]). Calves inoculated with JV developed severe watery diarrhea at 14 to 16 hpi, and this symptom lasted for 53.5 to 67.0 h. Intestinal lesions were characterized by severe villus atrophy together with loss and attenuation of villus epithelium. Viral capsid antigen (JV antigen) was detected by immunohistochemistry in the cytoplasm of epithelial cells on villi. In addition, granular material positive for JV antigen was detected in the lamina propria of villi. Lesions first appeared at 12 hpi and were most extensive at 18 to 19 hpi, extending from midjejunum to ileum. The intestinal mucosa had completely recovered at 4 dpi. There was no indication of systemic infection as described for norovirus infection in mice. JV was found in intestinal contents by reverse transcription-PCR (RT-PCR) and enzyme-linked immunosorbent assay (ELISA) as early as 12 hpi. Fecal shedding of the virus started at 13 hpi and stopped at 23 hpi or at necropsy (4 dpi), respectively. Throughout the trial, none of the control calves tested positive for JV by ELISA or RT-PCR.
INTRODUCTION
Noroviruses (NVs) are small nonenveloped viruses approximately 27 to 40 nm in diameter with a positive-sense, single-stranded RNA genome of 7.5 kb containing three open reading frames (9, 10, 21). Based on alignment of the amino acid sequences for the major capsid protein, norovirus strains are currently classified into five genogroups (G) (1, 53). Human noroviruses are found in GI, GII, and GIV, bovine noroviruses belong only to GIII, porcine noroviruses belong to GII, and murine noroviruses are in GV (7, 10, 11, 44). Recently, NVs of GIV were identified in a dead lion cub and a dog (22, 23). Within GIII, two genotypes of bovine norovirus exist (30). These are represented by Jena virus (JV), which was isolated from cattle in Germany (12, 13, 21), and Newbury 2 virus, which was identified in the feces of diarrheic calves in the United Kingdom (52). Recently, noroviruses closely related to the bovine GIII noroviruses were identified in fecal samples from pigs and sheep in New Zealand, possibly representing a third GIII genotype (51).
Norovirus infections are the most frequent cause of nonbacterial diarrheic disease in humans and in several animal species (25, 37, 39). Since noroviruses belonging to GIII have not been found in humans, these viruses do not appear likely to cause human disease (17, 31, 33). However, the recent detection of sequences related to GII.4 human norovirus in swine and cattle in Canada indicates that some noroviruses may cocirculate in human and bovine species (24). The higher seroprevalence for GIII noroviruses in veterinarians compared to the general population in the Netherlands hints at the possibility that some GIII noroviruses might have zoonotic potential (48).
Attempts to study noroviruses have been restricted, because with the exception of murine noroviruses, it has not been possible to propagate these viruses in cell cultures (6, 18, 20, 49). Over the past decade, the systematic application of genome sequencing has contributed to a new era in the study of these viruses, especially the development of new diagnostic procedures (50). However, little progress has been made in studying the biology of infection, since inocula are not readily available and appropriate large animal models are limited, expensive, and technically demanding. In heterologous infection systems, human norovirus was mildly enteropathogenic in gnotobiotic piglets (3) and more pronounced in gnotobiotic calves (42).
Besides humans, only calves infected with bovine noroviruses (37) have been reported to have natural infection with noroviruses causing diarrhea, not pigs (46, 51) or other animal species (38, 51). There are differences in the epidemiological distribution and pathogenicity between bovine noroviruses belonging to GIII genotypes 1 and 2. Bovine norovirus GIII genotype 2, but not genotype 1, was predominantly found in calves in the Netherlands (43), United Kingdom (26), United States (41), South Korea (34), Belgium (25), and Hungary (36). Conventionally kept calves inoculated at 1 to 8 days of age with bovine norovirus GIII genotype 2 (Newbury virus) had little or no diarrhea (52). In gnotobiotic calves, mild diarrhea, transient anorexia, and xylose malabsorption were the common clinical signs (2, 14, 52). Oral inoculation of newborn calves with bovine norovirus GIII genotype 1 (Jena virus) reproducibly induced diarrhea (13; P. H. Otto, unpublished data). Histopathological lesions in calves infected with bovine norovirus of either genotype 1 (JV) or genotype 2 (Newbury) strains were characterized by villus atrophy and crypt hyperplasia in the proximal small intestine (2, 12, 14).
The homologous infection of calves with bovine norovirus genotype 1 is of particular interest and relevance because the clinical signs it induces in calves are comparable to those induced by human norovirus in people. The purpose of this study was to infect calves with bovine norovirus genotype 1 (Jena virus) and evaluate this homologous, matched virus and natural large animal host which may be a useful experimental model for human norovirus infection. We studied the clinical signs, lesions, and distribution of viral antigen in intestinal tissue prior to disease presentation, during severe diarrhea, and following recovery from diarrhea, as well as fecal shedding, during an observation period of 4 days.
MATERIALS AND METHODS
Calves.
A total of 22 clinically healthy, full-term, newborn, male, Jersey-Holstein Frisian crossbred calves were used; 10 for passaging virus for the inocula and 12 for the experimental infections. They were obtained from an enteric-disease-free dairy farm near Jena, Germany. These calves were prevented from nursing and were transported to the animal housing facility at the Friedrich Loeffler Institute immediately following birth. Colostrum from the respective maternal cow was submitted to the study together with each calf.
Inoculum.
Jena virus (JV) isolate 117/80 collected from a diarrheic calf in 1980 was transmitted orally to a further 10 calves as described previously (13). Fecal samples were collected from each calf and examined for JV by transmission electron microscopy (TEM). Fecal samples collected from calf 1481 at the onset of diarrhea contained the most JV; thus, these samples were pooled for the preparation of a standard inoculum. These fecal samples were thoroughly mixed and then divided into aliquots of 5 ml. These aliquots were stored at −80°C until use. To prepare the inoculum, a 1:5 suspension was prepared with phosphate-buffered saline (PBS) (pH 7.4). The suspension was sonicated in an ultrasonic water bath (USS 20; K. W. Meinhardt, Leipzig, Germany). Cell debris and commensal bacteria were removed by low-speed centrifugation at 3,300 × g for 30 min. Supernatants were passed through 0.20-μm-pore-size filters (Sartorius, Göttingen, Germany) and filled to a final volume of 200 ml with PBS. The inocula were screened for viruses by TEM and plated onto blood agar to test for the presence of bacteria.
Experimental procedures (Table 1).
Table 1.
Calves and experimental study design
| Calf no. | Wt at necropsy (kg) | Age at inoculation (h) | Inoculum | Time of necropsy |
|---|---|---|---|---|
| JV-1 | 48 | 2.50 | JV | 12 hpi |
| JV-2 | 43 | 2.50 | JV | 12 hpi |
| JV-3 | 43 | 2.75 | JV | 18 hpi |
| JV-4 | 53 | 2.75 | JV | 19 hpi |
| JV-5 | 45 | 2.50 | JV | 4 dpi |
| JV-6 | 52 | 3.00 | JV | 4 dpi |
| Co-1 | 50 | 2.25 | PBS | 12 hpi |
| Co-2 | 38 | 3.00 | PBS | 12 hpi |
| Co-3 | 45 | 3.00 | PBS | 21 hpi |
| Co-4 | 54 | 2.20 | PBS | 20 hpi |
| Co-5 | 52 | 3.00 | PBS | 4 dpi |
| Co-6 | 43 | 3.00 | PBS | 4 dpi |
Calves were 2.5 to 3 h old when they arrived at the animal facility. They were each kept in confinement using individual pens. Serum samples were collected upon arrival to evaluate the immunoglobulin status and the presence of bovine NV-specific antibodies. Calves were treated once with 2.5 mg enrofloxacin/kg of body weight (BW) intramuscularly to minimize secondary bacterial infections. Six calves were inoculated with PBS containing the standard JV inoculum (calves JV-1 to JV-6), and six calves were mock inoculated with PBS only (control calves Co-1 to Co-6). Each calf received 200 ml inoculum orally by bottle. Two hours later, calves were fed 2 liters of colostrum followed by normal cows' milk, twice daily. During the incubation period, the animals were checked every hour by a qualified veterinarian for clinical symptoms. Fecal swabs were collected from 5 to 9 h postinoculation (hpi) and from 12 to 16 hpi. After the onset of diarrhea, fecal swabs were collected every 2 h for 24 h and then three times a day. The diarrhea was assessed as no diarrhea (normal) and watery diarrhea (severe diarrhea). Two calves inoculated with JV were euthanized and necropsied at 12 hpi (before the onset of diarrhea), 18 to 21 hpi (within the diarrheic period), and 4 days pi (dpi) when diarrhea had abated. Pairs of control calves were processed at the same time as infected animals.
Necropsy and sampling.
Calves were premedicated with 0.1 mg xylazine hydrochloride/kg of BW and anesthetized with 4 to 8 mg ketamine/kg of BW intravenously. The abdominal cavity was opened and the intestinal convolute exposed. Loops of approximately 10-cm lengths were tied in the duodenum, midjejunum (approximately 10 m distal to the pylorus), distal jejunum (approximately 2 m cranial to the ileocecal junction), jejunum containing a Peyer's patch (JPP), ileum (IPP), and midcolon, and each loop was filled with 4% neutral buffered formalin (NBF). The calves were then euthanized with 40 mg pentobarbital sodium/kg of BW intravenously. Loops and tissue from the cecum and the proximal colon containing lymphoid tissue were collected, opened at the mesenteric attachment, pinned flat on polystyrene, and immersed in NBF for 24 h. The remaining intestines were detached from the mesentery and opened, and the intestinal content and intestinal wall were inspected. A complete necropsy was performed, and samples collected from palatine tonsil, retropharyngeal lymph node, thymus, spleen, cecal lymph node, popliteal lymph node, lung, trachea, heart, liver, kidney, adrenal gland, rumen, abomasum, and pancreas were fixed in NBF. Intestinal contents were sampled from the colon or cecum.
Histological examination.
Tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin (HE) for histological evaluation. The length of the villi and crypts in duodenum, midjejunum, distal jejunum, and ileum were measured by microscopy using a semiautomatic image analysis system (Cell; Olympus, Hamburg, Germany). At each site, the 10 longest villi per section and 10 completely visible crypts were measured and the mean lengths and standard deviations were calculated. A reduction or increase of villus length up to 25% was considered mild, 26 to 50% moderate, and more than 50% severe.
Immunoperoxidase staining.
JV antigen was detected in paraffin sections collected on charged slides by the indirect immunoperoxidase method. The monoclonal antibody (MAb) CM39, which recognizes an epitope on the capsid protein of JV, was used as the primary antibody, and a peroxidase-labeled sheep anti-mouse immunoglobulin (NA931; Amersham, Freiburg, Germany) was used as the secondary antibody. Adjacent sections were incubated with a monoclonal antibody of the same isotype (IgG1) as CM39 recognizing an unrelated antigen (bovine viral diarrhea virus) as an isotype-specific control. Paraffin sections were heated for 20 min in a microwave oven for antigen retrieval. Sections from the duodenum, midjejunum, distal jejunum, JPP, ileum, cecum, proximal colon, midcolon, and cecal lymph node of each calf were examined.
Detection of JV in fecal samples and intestinal contents. RT-PCR and sequencing.
RNA was extracted from 140 μl of fecal suspension using the QIAamp viral RNA minikit (Qiagen, Hilden, Germany) according to the instructions of the manufacturer. The extracted viral RNA was stored at −80°C until analysis. The first amplification using primers JV3 (5′-CGGCTCACAGAGGTCCTGAA-3′, nucleotides [nt] 4606 to 4625) and JV4 (5′-CCAACGCGGCGGTAGAACTT-3′, nt 4913 to 4894) was conducted as a one-step reverse transcription-PCR (RT-PCR) using illustra Ready-To-Go RT-PCR-beads (GE Healthcare Europe GmbH, Freiburg, Germany) yielded an amplicon of 308 bp. Initially, 1 μl of the primer solutions JV1 (5′-TCGAAGTGAAGCAGCAGGTC-3′) and JV2 (5′-GCTGGATCTTGCGGTTAGAG-3′) (100 pmol/μl) was added to 10 μl of the viral RNA. Following denaturation of the RNA at 95°C and rapid freezing in liquid nitrogen, the bead was dissolved in 39 μl of DNase and RNase-free water was added. According to the manufacturer's data sheet, one bead contains 2 U Taq DNA polymerase, 10 mM Tris-HCl, 60 mM KCl, 1.5 mM MgCl2, 0.2 mM each deoxynucleoside triphosphate (dNTP), Moloney murine leukemia virus RT (M-MuLV-RT), RNase inhibitor, and bovine serum albumin. The reaction mix was immediately placed in a thermocycler (MiniCycler; MJ Research Inc., Watertown, MA). The RT-PCR was run according to the following temperature-time profile: 42°C for 30 min, 94°C for 5 min, 35 cycles of 94°C for 30 s, 55°C for 1 min, 72°C for 1 min, and a final extension at 72°C for 10 min. For the second amplification, 1 μl of the 1:100-diluted RT-PCR product was used as a template in a 25-μl reaction mix consisting of 19.0 μl of DNase-and RNase-free water, 0.25 μl of Platinum Taq-DNA polymerase (5 U/μl; Life Technologies, Karlsruhe, Germany), 2.5 μl of 10× Taq-PCR buffer (Life Technologies), 0.75 μl of 50 mM MgCl2, 0.5 μl of 10 mM dNTP mix (Life Technologies), 0.25 μl of 100 pmol of each primer (JV1 and JV2 solution), and 0.5 μl of dimethyl sulfoxide (Sigma, Taufkirchen, Germany). Amplification was carried out using the temperature-time profile as above except that the first step generated a 183-bp product. The PCR products were separated on 1% agarose gels containing 0.5 μg/ml ethidium bromide.
From three fecal samples, inoculum (calf no. 1481), 06V0062 (calf no. Co-5), and 06V0221 (calf no. JV-4), RNA was purified from 150 μl of each fecal filtrate sample by phenol-chloroform extraction followed by precipitation with isopropanol.
Synthesis of cDNA was as follows. Purified RNA (1 μg) was denatured at 65°C for 10 min prior to a thermal cycle of 25°C for 10 min, 55°C for 30 min, and 85°C for 5 min using Transcriptor reverse transcriptase (Roche) according to the manufacturer's instructions. The cDNA was subsequently used as a template in a PCR using primers based on the polymerase-encoding region of the genome. These primers were designed to amplify both JV and NA2 sequences: JV_NA2_Pol F (5′-TCAGCCTGGGACAGCAC-3′) (nt 4267 to 4283) and JV_NA2_Pol R (5′-TGTCGCGACTACCTTCC-3′) (nt 5032 to 5016).
PCR was performed using Phusion polymerase (NEB) with the following cycling conditions: initial denaturation at 98°C for 2 min, followed by 40 cycles of 98°C for 10 s, 55°C for 30 s, and 72°C for 45 s, with a final extension of 72°C for 7 min. These PCR products were gel purified and sequenced using the same primers used to amplify them.
Antigen ELISA.
The JV capsid protein was expressed in insect cells using a baculovirus vector, and the protein self-assembled to form virus-like particles (VLPs) as previously described (4). Purified VLPs were used to make antisera to develop an antigen capture enzyme-linked immunosorbent assay (ELISA), and VLPs were also used as an immunogen for the production of monoclonal antibodies to JV (30). The presence of JV in fecal specimens was measured by antigen capture ELISA.
TEM.
Fecal samples and intestinal contents were examined by negative stain, transmission electron microscopy. Briefly, supernatants of the samples were applied to pioloform-carbon-coated, 400-mesh copper grids (Plano GmbH, Wetzlar, Germany), stained with 2% of aqueous uranyl acetate solution and examined by transmission electron microscopy (TEM) (JEM-1010 JEOL, Tokyo, Japan) at 80 kV acceleration voltage.
Screening for coronavirus, rotavirus, Escherichia coli, and cryptosporidia.
The ELISA Bio K348 digestive kit (BIO X Diagnostics s.p.r.l., Jemelle, Belgium) was used to detect bovine coronavirus, rotavirus, E. coli F5, and cryptosporidia in fecal swabs.
Detection of antibodies against JV.
An ELISA was performed as described previously (30). Briefly, VLPs of Bo/Jena/1980/DE (Jena virus) were used as a test antigen at a concentration of 5 μg/ml. Supernatants from mock-infected Sf9 cells were used as a negative-control antigen. Samples were tested in duplicate at a single dilution of 1:200. The net absorbance for each test sample was determined by subtracting the mean absorbance value of the negative-control antigen from the mean absorbance value of the test antigen. An absorbance difference (ΔE) of 0.2 or more was considered positive.
Statistical analysis.
Statistical analysis was not feasible due to the small number of animals used in the study. However, clear trends were observed, as shown in the figures and described in the text.
RESULTS
Characterization of the inoculum.
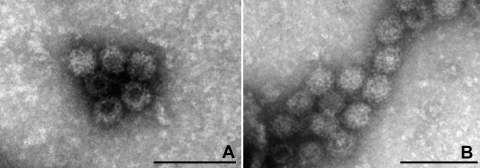
The samples that were pooled to prepare the JV inoculum and the inoculum itself were screened for viruses by TEM. Numerous identical viral particles with the characteristic size (approximately 35 nm) and morphology of noroviruses were observed in the inoculum (Fig. 1 A). The concentration of JV particles was estimated to be ≥108 viral particles/ml. The RT-PCR and sequencing applied to amplicons derived from these samples confirmed the presence of bovine norovirus GIII genotype 1 (JV) (Fig. 2 and data not shown). By TEM, only NV particles were present (Fig. 1A) and no bacteria were detected by routine microbiological screening (data not shown).
Fig. 1.
TEM image of Jena virus (JV) particles, as an aggregated cluster of virions, observed in the inoculum prepared from a fecal sample of calf 1481 (A) and in the fecal sample of calf JV-4 collected 14 hpi (B). Negative staining with uranyl acetate. Bar, 100 nm.
Fig. 2.
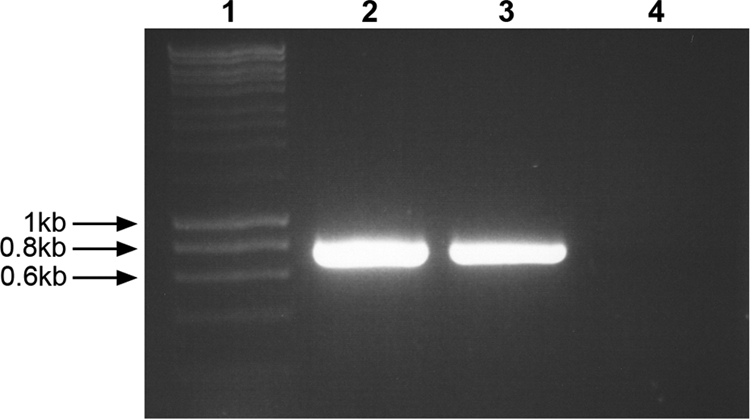
Amplification products of RT-PCR using primers JV_NA2_Pol F/JV_NA2_Pol R for the detection of bovine noroviruses, the inoculum (lane 2), the fecal sample 06V0221 of calf JV-4 (lane 3), and the fecal sample 07V0062 of calf Co-5 (lane 4). A molecular size marker is represented in lane 1.
Clinical signs.
The onset of severe diarrhea was observed for the calves that had received the inoculum containing JV at 14 hpi (calves JV-5 and JV-6), 15 hpi (calf JV-3), or 16 hpi (calf JV-4). Diarrheic feces were yellow or yellow-green, watery, and foul smelling with shreds of mucus. Diarrhea abated after 53.5 or 67.0 hpi, respectively, in the animals JV-5 and JV-6, and the feces were pasty until necropsy at 4 dpi. No clinical signs were observed for the calves necropsied at 12 hpi and for any of the control calves. The body temperature of all calves remained within the physiological range of 37.9 to 39.5°C during the trial.
Macroscopic findings.
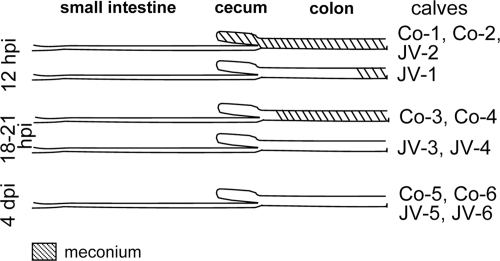
In the control calves, the small intestine contained a small amount of yellow liquid or pasty intestinal contents at 12 hpi and a moderate amount of yellow liquid to semiliquid intestinal contents with small milk clots at 18 to 21 hpi and at 4 dpi (data not shown). The meconium passage should occur within the first 24 to 48 h after birth. The movement of meconium along the intestine after birth reflects the speed of the intestinal passage. In the controls, meconium was found in cecum and colon at 12 hpi and in the colon at 18 to 21 hpi (Fig. 3). At 4 dpi, there was no meconium; however, intestinal contents that became distally increasingly pasty were present in the colon (Fig. 3).
Fig. 3.
Distribution of meconium in the intestinal tract of calves at 12 hpi, 18 to 21 hpi, and 4 dpi.
After inoculation with JV, macroscopic findings at 12 hpi were as in the controls at 12 hpi with the exception of the results for calf JV-2, where cecum and proximal colon were filled with green, semiliquid intestinal contents and meconium was found in the distal colon only (Fig. 3). At 18 to 21 hpi, the small intestine of both calves was filled with a small amount of green or yellow liquid and distended by gas. Yellow, semiliquid intestinal contents were present in the cecum and colon instead of meconium (Fig. 3). This indicates a faster passage of the meconium. Macroscopic findings at 4 dpi were as in the controls at 4 dpi.
Histological findings.
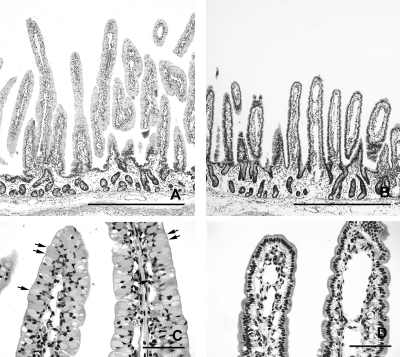
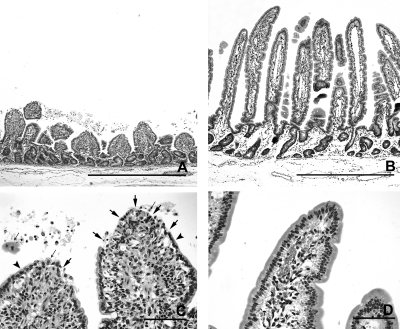
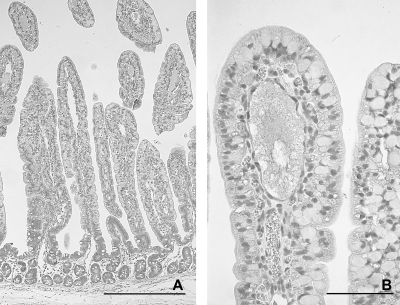
In the control calves, the length of the intestinal villi differed along the small intestine and changed with age. The duodenum had plump and irregular villi that were shorter than the villi in other sites of the small intestine (Fig. 4 A, B, and C). The longest villi were seen in midjejunum at 12 hpi (Fig. 4A). There was a marked reduction in length of the villi in jejunum and ileum from 18 to 21 hpi to 4 dpi (Fig. 4B, 4C, 5A, and 5B), but the villi remained slender. At 12 hpi and 18 to 21 hpi, villi were predominantly covered by vacuolated columnar epithelial cells filled with globular eosinophilic material that represents absorbed colostrum droplets (Fig. 5 C). At 4 dpi, epithelial cells filled with colostrum droplets had been replaced by cuboidal to columnar enteroabsorptive cells (Fig. 5D). These changes have been described as the adaptation process and intestinal closure in healthy calves after birth.
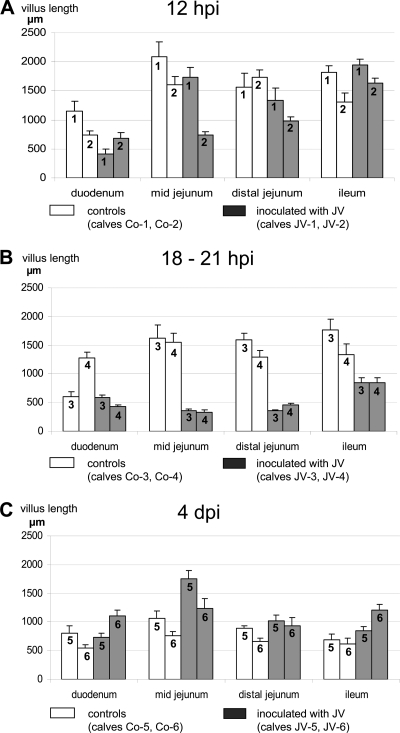
Fig. 4.
Villus length (mean and standard deviation) in the duodenum, midjejunum, distal jejunum, and ileum of control calves and calves infected with JV at 12 hpi (A), 18 to 21 hpi (B), and 4 dpi (C). Each bar represents an individual calf with calf numbers indicated on the histogram. Statistical significance was omitted due to the small number of animals.
Fig. 5.
Morphology of small intestinal mucosa in control calves at 18 to 21 hpi (calf Co-3) (A and C) and at 4 dpi (calf Co-5) (B and D), with HE stain. (A) Very long, slender villi, characteristic of newborn calves in the midjejunum at 18 to 21 hpi. Bar, 1 mm. (B) Villi are long and slender, but shorter than those found in newborn calves in the midjejunum at 4 dpi. Bar, 1 mm. (C) Higher magnification of panel A. Epithelial cells, located at the upper part of the villi, are filled with colostrum droplets (arrows, examples). Bar, 100 μm. (D) Higher magnification of panel B. Prismatic and columnar enteroabsorptive cells without colostrum droplets are lining the villi. Bar, 100 μm.
After inoculation with JV, severe shortening of intestinal villi was seen in midjejunum and moderate shortening in distal jejunum of one calf (JV-2) at 12 hpi already (Fig. 4A). At 18 to 21 hpi, shortening of villi was severe in mid- and distal jejunum of both calves (JV-3 and JV-4) (Fig. 4B and 6A). The plump, shortened villi were not covered by vacuolated epithelial cells but by irregular, attenuated epithelium, and the tips of the stunted villi were often denuded from epithelial cells (Fig. 6 C). In the ileum, villus length was moderately reduced and vacuolated columnar epithelial cells filled with colostrum droplets predominated as in the controls. At 4 dpi, the mucosa had recovered and formed long slender villi (Fig. 6B). Compared to those of the control calves, villus length was moderately to severely increased in midjejunum and mildly to moderately increased in distal jejunum and ileum (Fig. 4C). The epithelial morphology was comparable to those observed in the age-matched control calves (Fig. 6D).
Fig. 6.
Morphology of small intestinal mucosa in calves inoculated with JV at 18 to 21 hpi (calf JV-4) (A and C) and at 4 dpi (calf JV-5) (B and D), with HE stain. (A) Severe shortening and thickening of the villi in the distal jejunum of a calf at 18 to 21 hpi. Bar, 1 mm. (B) Mucosa has recovered. Villi are long and slender in the distal jejunum of a calf at 4 dpi. Bar, 1 mm. (C) Higher magnification of panel A. The tips of the stunted villi are partly to extensively denuded (arrows). The remaining epithelial cells are flattened (arrowheads) or rounded and detached (thin arrows). Bar, 100 μm. (D) Higher magnification of panel B. Villi are covered by columnar enteroabsorptive cells without colostrum droplets as in the controls. Bar, 100 μm.
In all calves, Peyer's patches in the jejunum (JPP) and ileum (IPP) were small and inactive (data not shown). No lesions were seen in the cecum and large intestine. Cecal lymph nodes, tonsil, spleen, and popliteal lymph node were small and inactive.
Tissue distribution of JV antigen.
At 12 hpi, JV capsid antigen (here called JV antigen) was detected in the jejunum and ileum of both calves that had been inoculated with JV (Table 2). Epithelial cells located on the villi of mid- and distal jejunum were positive for JV antigen. Positively stained epithelial cells were found along the entire length of the long villi and were particularly frequent along the sides of villi (Fig. 7 A). Viral antigen presented as fine to coarse granular staining within the cytoplasm of epithelial cells (Fig. 7B). Epithelial cells at the tip of stunted villi were also positive for JV antigen in calf JV-2. In the distal jejunum, viral antigen was detected within the cytoplasm of epithelial cells at the villus base (data not shown). A few JV antigen-positive cells were found at the tips of the villi in the ileum.
Table 2.
Distribution of bovine norovirus (JV) antigen in intestinal epithelium
| Time | Calf no. | Presence of antigena in: |
|||
|---|---|---|---|---|---|
| Duodenum | Midjejunum | Distal jejunum | Ileum | ||
| 12 hpi | Co-1 | − | − | − | − |
| Co-2 | − | − | − | − | |
| JV-1 | − | ++ | ++ | (+) | |
| JV-2 | − | ++ | ++ | (+) | |
| 18-21 hpi | Co-3 | − | − | − | − |
| Co-4 | − | − | − | − | |
| JV-3 | − | + | + | (+) | |
| JV-4 | − | ++ | +++ | + | |
| 4 dpi | Co-5 | − | − | − | − |
| Co-6 | − | − | − | − | |
| JV-5 | − | − | − | − | |
| JV-6 | − | − | − | − | |
−, no JV antigen detected in epithelial cells; (+), single epithelial cells on villi positive for JV antigen; +, single cells and small groups of epithelial cells on villi positive for JV; ++, many groups of epithelial cells on villi positive for JV antigen; +++, most epithelial cells on villi positive for JV antigen.
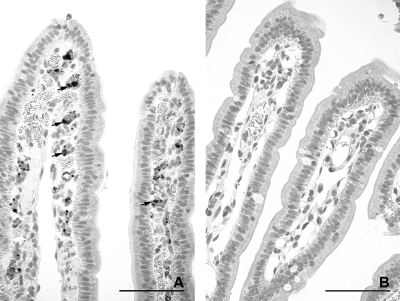
Fig. 7.
Distribution of JV antigen in the midjejunum at 12 hpi with JV (calf JV-1), as shown by immunohistology. (A) Single or groups of epithelial cells along the entire length of the villi have stained for JV antigen (arrows, examples). Villi are very long and slender in spite of the presence of JV antigen. Bar, 500 μm. (B) Higher magnification of panel A. JV is present in the cytoplasm of epithelial cells (arrows, examples). Bar, 100 μm.
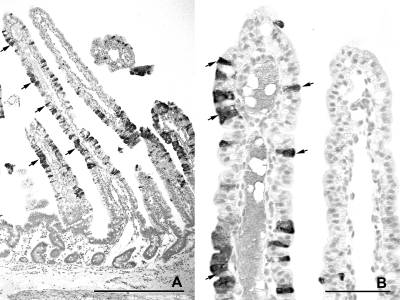
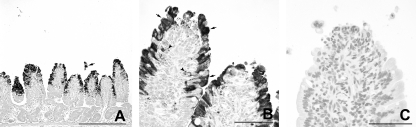
At 18 to 21 hpi, the numbers of infected epithelial cells on villi varied between the two calves (Table 2). In calf JV-3, individual cells and small groups of positively stained epithelial cells were present on the jejuna and ileal villi. Rounded, dislodged epithelial cells containing JV antigen were frequently observed within the intestinal lumen. In calf JV-4, groups of epithelial cells were positive for JV antigen in the midjejunum and most villus epithelial cells in the distal jejunum (Fig. 8 A and B). In the ileum, small groups of vacuolated epithelial cells on the tips of villi stained positive for JV antigen (Fig. 9 A and B). In addition, pronounced, diffuse staining of the microvillus border of the epithelial cells was seen multifocally (Fig. 9B). JV antigen was also found in cells of the follicle-associated epithelium covering domes of a JPP (data not shown). In both calves, JV antigen-positive granular material was seen in macrophage-like cells in the lamina propria (Fig. 8B). No staining was observed in sections incubated with an unrelated antibody of the same isotype (Fig. 8C and 9C). The control calves were negative for JV (Table 2; Fig. 10 A and B).
Fig. 8.
Distribution of JV antigen in the distal jejunum at 18 to 21 hpi with JV (calf JV-4), as shown by immunohistology. (A) Numerous epithelial cells lining the stunted villi are stained for JV antigen. A group of detached JV-antigen positive epithelial cells is present in the intestinal lumen (arrow). Bar, 500 μm. (B) Higher magnification of panel A. JV is present in the cytoplasm of rounded and attenuated epithelial cells (arrows, examples). Antigen-positive granular material is present in the lamina propria (arrowheads). Bar, 100 μm. (C) Higher magnification of panel A. No staining is seen after incubation with an unrelated antibody of the same isotype. Bar, 100 μm.
Fig. 9.
Distribution of JV antigen in the ileum at 18 to 21 hpi with JV (JV-4), as shown by immunohistology. (A) A few epithelial cells on the tips of villi are positive for JV. The ingesta (star) is also positive for JV. Bar, 500 μm. (B) Higher magnification of panel A. JV (arrows, examples) is present in the cytoplasm of epithelial cells containing large colostrum droplets (stars, examples). There is a strong diffuse staining of the microvillus border of some epithelial cells for JV (arrowheads). Bar, 100 μm. (C) Higher magnification of panel A. No staining is seen after incubation with an unrelated antibody of the same isotype. Bar, 100 μm.
Fig. 10.
Staining for JV antigen in the midjejunum of a control calf (calf Co-3) at 18 to 21 hpi. No staining is seen at either low (A; bar, 500 μm) or high (B; bar, 100 μm) magnification by immunohistology.
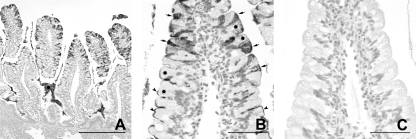
At 4 dpi, JV antigen was not detected within epithelial cells, but small amounts of granular JV antigen-positive material were found in macrophage-like cells in the lamina propria of villi in jejunum and ileum of the calves that had been inoculated with JV (Fig. 11 A, Table 2). No staining was observed in sections incubated with an unrelated antibody of the same isotype (Fig. 11B). The control calves were negative for JV in the epithelium. In calf Co-5, a small amount of granular JV antigen-positive material was present in the lamina propria.
Fig. 11.
Distribution of JV antigen in the midjejunum at 4 dpi with JV (calf JV-6), as shown by immunohistology. (A) Epithelial cells are negative for JV after recovery. Granular JV-positive material (arrows, example) is retained in the lamina propria. Bar, 100 μm. (B) No staining is seen after incubation with an unrelated antibody of the same isotype. Bar, 100 μm.
JV antigen was not found in the duodenum, cecum, colon, and cecal lymph node at any time. However, the intestinal contents in the intestinal lumen of the cecum and colon were positive by immunohistology at 18 and 19 hpi in the calves inoculated with JV.
Viral shedding and antibodies.
In all virus-infected calves JV was detected by RT-PCR and/or ELISA in intestinal contents and/or feces (Table 3). Of 25 fecal samples and intestinal contents, 8 were positive by both RT-PCR and ELISA. The other samples were positive by either RT-PCR (10 samples) or ELISA (7 samples). Therefore, samples were considered positive if JV was detected by either RT-PCR or ELISA.
Table 3.
Detection of JV by ELISA and RT-PCR in fecal swabs or intestinal contents of calvesa
| Necropsy time | Calf no. | FS/IC | Time(s) of sampling (hpi) | Result |
|
|---|---|---|---|---|---|
| ELISA | RT-PCR | ||||
| 12 hpi | JV-1 | FS | 6.0, 12.0 | 0 | 0 |
| IC | 12.0 | 0 | + | ||
| JV-2 | FS | 6.0 | 0 | 0 | |
| IC | 12.0 | + | 0 | ||
| Co-1 | FS | NT | NT | NT | |
| IC | 12.5 | 0 | 0 | ||
| Co-2 | FS | 7.0 | 0 | 0 | |
| IC | 13.0 | 0 | 0 | ||
| 18-21 hpi | JV-3 | FS | 6.0 | 0 | 0 |
| FS | 13.0 | + | + | ||
| FS | 15.0 | + | 0 | ||
| IC | NT | NT | NT | ||
| JV-4 | FS | 5.0 | 0 | 0 | |
| FS | 16.0 | 0 | + | ||
| FS | 17.0, 17.5, 18.5 | + | + | ||
| IC | 19,0 | + | 0 | ||
| Co-3 | FS | 7.0 | 0 | 0 | |
| Co-4 | IC | 20,5 | 0 | 0 | |
| 4 dpi | JV-5 | FS | 9.0 | 0 | 0 |
| FS | 14.0, 19.0, 21.0, 23.0 | + | 0 | ||
| FS | 25.0, 27.0, 33.0, 43.0, 47.0, 49.0, 54.0, 57.0, 67.0, 79.0 | 0 | 0 | ||
| IC | 90.0 | 0 | 0 | ||
| JV-6 | FS | 12.0 | 0 | + | |
| FS | 14.0, 16.0, 18.0, 20.0 | + | + | ||
| FS | 32.0, 46.0, 50.0, 56.0, 68.0, 80.0 | 0 | + | ||
| IC | 92.0 | 0 | + | ||
| Co-5 | FS | 9.5, 19.5, 43.5, 92.0 | 0 | 0 | |
| IC | 92.5 | 0 | 0 | ||
| Co-6 | FS | 19.5, 43.5, 67.5, 91.5 | 0 | 0 | |
| IC | 95.5 | 0 | 0 | ||
FS, fecal swabs; IC, intestinal contents; NT, not tested; +, JV positive; 0, JV negative.
JV was found in intestinal content samples collected at necropsy in four of the six calves inoculated with JV (JV-1, JV-2, JV-4, and JV-6). The two calves euthanized at 12 hpi (JV-1, JV-2) did not shed virus in the feces. The earliest fecal shedding occurred at 12, 13, 14, and 16 hpi in calves JV-6, JV-3, JV-5 and JV-4, respectively (Fig. 1B, Table 3, and data not shown). In the calves observed until 4 dpi, shedding ended in one calf (JV-5) at 25 hpi, while it continued in another calf (JV-6) until necropsy at 4 dpi (Table 3). All samples of intestinal content and feces collected from the mock-inoculated calves were negative for JV (data not shown).
The blood samples from all calves were serologically negative for JV by ELISA (ΔE ≤ 0.17) with the exception of calf JV-5, which had specific antibodies to JV (ΔE = 0.64).
Differential diagnoses.
The antigen ELISAs for common endemic pathogens revealed no bovine coronavirus, group A rotavirus, E. coli F5, or cryptosporidia in any of the fecal swabs from both control calves and calves inoculated with JV (data not shown).
DISCUSSION
Infection with JV was successfully established in six of six calves (100%) by oral inoculation using sterile filtrates of pooled fecal material from infected calves. Coinfections with other common enteric viral, bacterial, or parasitic pathogens (bovine coronavirus, rotavirus, E. coli F5, and cryptosporidia) were not detected. Since there is a high seroprevalence for bovine norovirus in Germany, colostral antibodies may have prevented infection by JV (32); therefore, calves were initially deprived of colostrum and were allowed to receive it only 2 h after inoculation. This delay was sufficient for JV to establish infection in all of the inoculated calves.
The effect of the colostrum on the course of infection is unclear. The presence of JV-specific antibodies in the serum of the calf JV-5 revealed that this animal had already received colostrum before the time of infection. Although the detection rate for JV in the fecal samples of this calf was reduced, the animal was not completely protected from the experimental JV infection.
The protein droplets found in vacuolated villus epithelial cells of infected and control calves at 12 hpi and 18 to 21 hpi indicate that colostrum uptake occurred as described in healthy newborn calves (19). Although colostrum did not prevent infection, the finding of only a few vacuolated cells that were positive for JV antigen might indicate a low infection rate of cells loaded with colostral antibodies. This may have limited the spread of infection and thus reduced the severity and duration of diarrhea under the experimental conditions used.
The first signs of diarrhea were observed at 14 to 16 hpi, indicating a very short incubation period. Thus, diarrhea might be seen under field conditions in 1- or 2-day-old calves. The diarrhea was severe and lasted for about 3 days. This is identical to the mean duration seen after inoculation of gnotobiotic calves with the human NV strain HS66 (42). Under field conditions, the course of disease might be more protracted due to secondary infections, since mixed infections are frequent in neonatal calf diarrhea (15). In the current investigation, secondary infections were avoided by the experimental settings, including single confinement of calves, high hygienic standards, application of colostrum, and early antibiotic treatment.
The onset and severity of diarrhea were identical to the findings reported for the initial experiments with JV (12). The symptoms were more severe than those reported for bovine norovirus GIII genotype 2 and Newbury virus 1 and 2 (2, 14). Infection of calves with Nebraska virus, another enteropathogenic calicivirus in calves, resulted in diarrhea of a longer duration after an incubation time of 3 to 4 days (16, 40). Calves are also susceptible to human norovirus infection, and inoculation of gnotobiotic calves with human norovirus GII.4 resulted in diarrhea from 2 to 6 dpi in five of five calves (42).
In all calves infected with JV, detection of JV by ELISA or RT-PCR in fecal samples or intestinal contents was successful. The higher number of JV-positive results by RT-PCR indicates a higher sensitivity of this method in comparison with the ELISA. This was also observed after infection of calves with human NV strain HS66 (42). The relatively high number of samples which were JV negative by RT-PCR could be explained by inhibitory factors in the fecal samples which may have been caused by the high levels of colostrum. In calves inoculated with human NV strain HS66, the intestinal contents of only one of two calves were positive at 3 dpi (42).
The course of infection can be deduced from the sequential findings in the intestinal mucosa before the onset of diarrhea, during diarrhea, and after recovery from diarrhea. JV antigen was detected by immunohistochemistry in villus epithelial cells in both calves at 12 hpi, before the onset of diarrhea. One of the calves at 12 hpi had long villi with numerous epithelial cells at all levels of the villi positive for JV antigen. This indicates that JV infects all enteroabsorptive cells and has no special tropism for cells on the tips of villi, as does bovine rotavirus, or on the bases of villi, as does bovine torovirus (29). No changes of epithelial cell morphology were detected at this stage of infection by light microscopy. The continued replication of JV in the epithelial cells most likely resulted in damage and detachment of infected cells, as has been described for infections with enteropathogenic viruses (28). The loss of infected epithelial cells was abrupt, since in the second calf at 12 hpi, moderately to severely stunted villi and no intermediate lesions were observed.
In both calves examined 18 to 21 hpi, shortly after the onset of diarrhea, a severe reduction of villus length in the jejunum was observed. This is functionally reflected in a severe loss of absorptive and digestive capacity, causing diarrhea (28). The infection also accelerated the passage of the meconium, which had been completely passed in the infected calves at 18 and 19 hpi but filled the large intestine in control calves at 20 and 21 hpi. The first diarrheic feces were passed after the last of the meconium had been excreted, at 14 to 16 hpi, and contained the largest amount of JV. By immunohistology, numerous JV antigen-positive epithelial cells were present in one calf. In the other calf, the stunted villi were more extensively denuded and thus the number of JV positive epithelial cells was lower. The severe villus atrophy and loss of mature enterocytes reduces the number of cells susceptible to JV infection and thus may limit the duration of infection.
In one of the calves observed until convalescence, virus shedding ended at 25 hpi and diarrhea abated about 1 day later. This correlates well with the time needed for replacement of villus epithelium by crypt epithelial cells to restore the absorptive capacity of villi (27). The loss of epithelial cells from the villi will induce an increased replication of epithelial cells in the crypts. This may be the cause for the increased villus length seen at 4 dpi in the calves inoculated with JV compared to the controls.
Lesions were significant in mid- and distal jejunum and mild in the ileum. This indicates that the tropism of JV is not limited to the proximal jejunum as reported (12, 14). Lesions and JV-positive cells were not seen in the duodenum, which is in contrast to the findings after infection of calves with human NV strain HS66 (42). The increased number of JV-positive cells in the distal jejunum and ileum of one of the calves at 18 hpi might indicate a spread of the infection along the intestine. However, it is very clear that infection by JV, like that of bovine rotavirus and human NV strain HS66, is limited to the villi (27, 42). There is no tropism for crypt epithelial cells as has been reported for corona- and torovirus infections in calves (5, 35). This may accelerate recovery of the mucosa after JV infection. There was no indication of systemic infection as described for norovirus infection in mice (47).
Infection also involved the follicle-associated epithelium (FAE) of Peyer's patches in the jejunum. This specialized epithelium is crucial for antigen sampling and for maintaining appropriate immune responses at intestinal mucosal surfaces (8). Damage of FAE by JV infection might thus, at least transiently, interfere with antigen uptake and the initiation of immune responses.
JV antigen was seen not only in the cytoplasm of villus epithelial cells but also as granular deposits in macrophage-like cells in the lamina propria and domes, which were not further characterized; this finding has also been reported after infection of calves with human norovirus (42). Some of the damaged infected epithelial cells may have been phagocytosed by macrophages in the lamina propria. On the other hand, an uptake of macromolecules through intact intestinal epithelium has been reported (45) and the material might represent NV from the infected intestinal contents.
At 4 dpi, both calves had recovered from diarrhea and had normal intestinal mucosa, although one of the calves continued to shed JV in the feces. Mucosal morphology had acquired adult characteristics, with slender villi that were shorter than those in newborn calves (27). No vacuolated epithelial cells containing droplets of colostrum were observed. JV antigen was not detected in epithelial cells but only as granular material in macrophage-like cells in the lamina propria. If this material represents infectious JV, it might cause reinfection of villus epithelial cells. On the other hand, an extended presence of viral antigen may be important for the interaction with the local immune system.
This homologous infection system in calves resembles in many aspects the disease observed after norovirus infection of humans. It has the advantage of a normal development of the immune system in contrast to the models using gnotobiotic calves. Thus, it will allow a detailed investigation of the immune responses after natural infection or vaccination and protection against repeated infections.
ACKNOWLEDGMENTS
We are very grateful to Sylke Stahlberg and the technical staff of the animal house for their skillful assistance during the study. We acknowledge the outstanding work of Lesley Cutcliffe, Renate Danner, Monika Godat, Sabine Lied, Wolfram Maginot, Petra Sippach, and Maria-Margarida Vargas.
We acknowledge support of a Wellcome Trust program grant, number 086112, to I.N.C. and P.R.L.
Footnotes
Published ahead of print on 31 August 2011.
REFERENCES
- 1. Ando T., Noel J. S., Fankhauser R. L. 2000. Genetic classification of Norwalk-like viruses. J. Infect. Dis. 182(Suppl. 2): S336–S348 [DOI] [PubMed] [Google Scholar]
- 2. Bridger J. C., Hall G. A., Brown J. F. 1984. Characterization of a calici-like virus (Newbury agent) found in association with astrovirus in bovine diarrhea. Infect. Immun. 43: 133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheetham S., et al. 2006. Pathogenesis of a genogroup II human norovirus in gnotobiotic pigs. J. Virol. 80: 10372–10381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deng Y., et al. 2003. Studies of epidemiology and seroprevalence of bovine noroviruses in Germany. J. Clin. Microbiol. 41: 2300–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doughri A. M., Storz J. 1977. Light and ultrastructural pathologic changes in intestinal coronavirus infection of newborn calves. Zentralbl. Veterinarmed. B 24: 367–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duizer E., Schwab K. J., Neill F. H., Atmar R. L. 2004. Laboratory efforts to cultivate noroviruses. J. Gen. Virol. 85: 79–87 [DOI] [PubMed] [Google Scholar]
- 7. Fankhauser R. L., et al. 2002. Epidemiologic and molecular trends of “Norwalk-like viruses” associated with outbreaks of gastroenteritis in the United States. J. Infect. Dis. 186: 1–7 [DOI] [PubMed] [Google Scholar]
- 8. Gebert A., Rothkötter H. J., Pabst R. 1996. M cells in Peyer's patches of the intestine. Int. Rev. Cytol. 167: 91–159 [DOI] [PubMed] [Google Scholar]
- 9. Green K. Y. 2007. Caliciviridae: the noroviruses, p. 949–979 In Knipe D. M., et al. (ed.), Fields virology, 5th ed. Lippincott, Williams and Wilkins, Philadelphia, PA [Google Scholar]
- 10. Green K. Y., et al. 2000. Taxonomy of the caliciviruses. J. Infect. Dis. 181(Suppl. 2): S322–S330 [DOI] [PubMed] [Google Scholar]
- 11. Greening G. E., Wolf S. 2010. Calicivirus environmental contamination, p. 25–44 In Hansman G. S., Jiang X. J., Green K. Y.(ed.), Caliciviruses. Molecular and cellular virology. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 12. Günther H., Otto P. 1987. Diarrhea in young calves. 7. “Zackenvirus” (Jena agent 117/80)—a new diarrhea pathogen in calves. Arch. Exp. Vet. Med. 41: 934–938 (In German.) [PubMed] [Google Scholar]
- 13. Günther H., Otto P., Heilmann P. 1984. Diarrhea in young calves. 6. Determination of the pathogenicity of a bovine coronavirus and an unidentified icosahedral virus. Arch. Exp. Veterinarmed. 38: 781–792 (In German.) [PubMed] [Google Scholar]
- 14. Hall G. A., Bridger J. C., Brooker B. E., Parsons K. R., Ormerod E. 1984. Lesions of gnotobiotic calves experimentally infected with a calicivirus-like (Newbury) agent. Vet. Pathol. 21: 208–215 [DOI] [PubMed] [Google Scholar]
- 15. Hall G. A., Reynolds D. J., Parsons K. R., Bland A. P., Morgan J. H. 1988. Pathology of calves with diarrhoea in southern Britain. Res. Vet. Sci. 45: 240–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Han M. G., Cheetham S., Azevedo M., Thomas C., Saif L. J. 2006. Immune responses to bovine norovirus-like particles with various adjuvants and analysis of protection in gnotobiotic calves. Vaccine 24: 317–326 [DOI] [PubMed] [Google Scholar]
- 17. Han M. G., Smiley J. R., Thomas C., Saif L. J. 2004. Genetic recombination between two genotypes of genogroup III bovine noroviruses (BoNVs) and capsid sequence diversity among BoNVs and Nebraska-like bovine enteric caliciviruses. J. Clin. Microbiol. 42: 5214–5224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herbst-Kralovetz M., et al. 2009. Lack of success in culturing noroviruses in 3-D cell culture systems. 5th International Conference on Vaccines for Enteric Diseases, Malaga, Spain [Google Scholar]
- 19. Jochims K., Kaup F. J., Drommer W., Pickel M. 1994. An immunoelectron microscopic investigation of colostral IgG absorption across the intestine of newborn calves. Res. Vet. Sci. 57: 75–80 [DOI] [PubMed] [Google Scholar]
- 20. Lay M. K., et al. 2010. Norwalk virus does not replicate in human macrophages or dendritic cells derived from the peripheral blood of susceptible humans. Virology 406: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu B. L., et al. 1999. Molecular characterization of a bovine enteric calicivirus: relationship to the Norwalk-like viruses. J. Virol. 73: 819–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martella V., Lorusso E., Decaro N., Elia G., Radogna A. 2008. Detection and molecular characterization of a canine norovirus. Emerg. Infect. Dis. 14: 1306–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martella V., Campolo M., Lorusso E., Cavicchio P., Cameron M. 2007. Norovirus in captive lion cub (Panther oleo). Emerg. Infect. Dis. 13: 1071–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mattison K., et al. 2007. Human noroviruses in swine and cattle. Emerg. Infect. Dis. 13: 1184–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mauroy A., et al. 2009. Epidemiological study of bovine norovirus infection by RT-PCR and a VLP-based antibody ELISA. Vet. Microbiol. 137: 243–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Milnes A. S., Binns S. H., Oliver S. L., Bridger J. C. 2007. Retrospective study of noroviruses in samples of diarrhoea from cattle, using the Veterinary Laboratories Agency's Farmlife database. Vet. Rec. 160: 326–330 [DOI] [PubMed] [Google Scholar]
- 27. Moon H. W. 1983. Intestine, p. 503–529 In Cheville N. F.(ed.), Cell pathology, 2nd ed. Iowa State Press, Ames, IA [Google Scholar]
- 28. Moon H. W. 1994. Pathophysiology of viral diarrhea. p. 31–45 In Kapikian A. Z. (ed.) Viral infections of the gastrointestinal tract. Marcel Dekker, New York, NY [Google Scholar]
- 29. Moon H. W. 1997. Comparative histopathology of intestinal infections. Adv. Exp. Med. Biol. 412: 1–19 [DOI] [PubMed] [Google Scholar]
- 30. Oliver S. L., et al. 2006. Genotype 1 and genotype 2 bovine noroviruses are antigenically distinct but share a cross-reactive epitope with human noroviruses. J. Clin. Microbiol. 44: 992–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oliver S. L., et al. 2003. Molecular characterization of bovine enteric caliciviruses: a distinct third genogroup of noroviruses (Norwalk-like viruses) unlikely to be of risk to humans. J. Virol. 77: 2789–2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oliver S. L., et al. 2007. Serotype 1 and serotype 2 bovine noroviruses are endemic in cattle in the United Kingdom and Germany. J. Clin. Microbiol. 45: 3050–3051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palmer S., Brown D., Morgan D. 2005. Early qualitative risk assessment of the emerging zoonotic potential of animal diseases. BMJ 331: 1256–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Park S. I., et al. 2007. Molecular epidemiology of bovine noroviruses in South Korea. Vet. Microbiol. 124: 125–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pohlenz J. F., Cheville N. F., Woode G. N., Mokresh A. H. 1982. Cellular lesions in intestinal mucosa of gnotobiotic calves experimentally infected with a new unclassified bovine virus (Breda virus). Vet. Pathol. 21: 407–417 [DOI] [PubMed] [Google Scholar]
- 36. Reuter G., Pankovics P., Egyed L. 2009. Detection of genotype 1 and 2 bovine noroviruses in Hungary. Vet. Rec. 165: 537–538 [DOI] [PubMed] [Google Scholar]
- 37. Scipioni A., et al. 2008a. Detection and quantification of human and bovine noroviruses by a TaqMan RT-PCR assay with a control for inhibition. Mol. Cell. Probes 22: 215–222 [DOI] [PubMed] [Google Scholar]
- 38. Scipioni A., Mauroy A., Vinje J., Thiry E. 2008. Animal noroviruses. Vet. J. 178: 32–45 [DOI] [PubMed] [Google Scholar]
- 39. Siebenga J. J., Duizer E., Koopmans M. P. G. 2010. Norovirus epidemiology, p. 1-24. In Hansman G. S., Jiang X. J., Green K. Y.(ed.), Caliciviruses. Molecular and cellular virology. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 40. Smiley J. R., Chang K. O., Hayes J., Vinjé J., Saif L. J. 2002. Characterization of an enteropathogenic bovine calicivirus representing a potentially new calicivirus genus. J. Virol. 76: 10089–10098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smiley J. R., Hoet A. E., Traven M., Tsunemitsu H., Saif L. J. 2003. Reverse transcription-PCR assay for detection of bovine enteric caliciviruses (BEC) and analysis of the genetic relationship among BEC and human caliciviruses. J. Clin. Microbiol. 41: 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Souza M., Azevedo M. S. P., Jung K., Cheetham S., Saif L. J. 2008. Pathogenesis and immune responses in gnotobiotic calves after infection with the genogroup II. 4-HS66 strain of human norovirus. J. Virol. 82: 1777–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Van der Poel W. H., et al. 2003. Epidemiology of Norwalk-like virus infections in cattle in The Netherlands. Vet. Microbiol. 92: 297–309 [DOI] [PubMed] [Google Scholar]
- 44. Vinjé J., Koopmans M. P. 2000. Simultaneous detection and genotyping of “Norwalk-like viruses” by oligonucleotide array in a reverse line blot hybridization format. J. Clin. Microbiol. 38: 2595–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Volkheimer G., Schulz F. H. 1968. The phenomenon of persorption. Digestion 1: 213–218 [DOI] [PubMed] [Google Scholar]
- 46. Wang Q. H., et al. 2005. Porcine noroviruses related to human noroviruses. Emerg. Infect. Dis. 11: 1874–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ward J. M., et al. 2006. Pathology of immunodeficient mice with naturally occurring murine norovirus infection. Toxicol. Pathol. 34: 708–715 [DOI] [PubMed] [Google Scholar]
- 48. Widdowson M. A., et al. 2005. Detection of serum antibodies to bovine norovirus in veterinarians and the general population in the Netherlands. J. Med. Virol. 76: 119–128 [DOI] [PubMed] [Google Scholar]
- 49. Wobus C. E., et al. 2004. Replication of norovirus in cell culture reveals a tropism for dentritic cells and macrophages. PLoS Biol. 2: e432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wolf S., et al. 2007. Sensitive multiplex real-time reverse transcription-PCR assay for the detection of human and animal noroviruses in clinical and environmental samples. Appl. Environ. Microbiol. 73: 5464–5470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wolf S., et al. 2009. Molecular detection of norovirus in sheep and pigs in New Zealand farms. Vet. Microbiol. 133: 184–189 [DOI] [PubMed] [Google Scholar]
- 52. Woode G. N., Bridger J. C. 1978. Isolation of small viruses resembling astroviruses and caliciviruses from acute enteritis of calves. J. Med. Microbiol. 11: 441–452 [DOI] [PubMed] [Google Scholar]
- 53. Zheng D. P., et al. 2006. Norovirus classification and proposed strain nomenclature. Virology 346: 312–323 [DOI] [PubMed] [Google Scholar]