Abstract
Proapoptotic BH3-interacting death domain agonist (BID) regulates apoptosis and the DNA damage response. Following replicative stress, BID associates with proteins of the DNA damage sensor complex, including replication protein A (RPA), ataxia telangiectasia and Rad3 related (ATR), and ATR-interacting protein (ATRIP), and facilitates an efficient DNA damage response. We have found that BID stimulates the association of RPA with components of the DNA damage sensor complex through interaction with the basic cleft of the N-terminal domain of the RPA70 subunit. Disruption of the BID-RPA interaction impairs the association of ATR-ATRIP with chromatin as well as ATR function, as measured by CHK1 activation and recovery of DNA replication following hydroxyurea (HU). We further demonstrate that the association of BID with RPA stimulates the association of ATR-ATRIP to the DNA damage sensor complex. We propose a model in which BID associates with RPA and stimulates the recruitment and/or stabilization of ATR-ATRIP to the DNA damage sensor complex.
INTRODUCTION
The Bcl-2 family of proteins regulates a mitochondrially directed program of cellular destruction, used by multicellular organisms to dispose of unwanted or damaged cells. BH3-only members such as BH3-interacting death domain agonist (BID) sense cellular stresses and initiate cell death by interacting with multidomain Bcl-2 members (19, 59). Recent data suggest that these BH3-only members may possess additional functions in fundamental cellular processes, placing them in position to sense cellular damage (12, 18, 33, 61).
BID has been demonstrated to play a proapoptotic role in multiple cellular stress-induced responses (11, 35, 38, 39, 47, 50). However, a singular apoptotic function does not account for all of the current data regarding BID's function. BID is highly expressed in hematopoietic cells, and loss of BID impairs cell growth and increases sensitivity to replicative stress, consistent with a survival role to maintain genomic integrity following DNA damage treatments, especially replicative stress (33, 36, 61). Bid−/− mice spontaneously develop chronic myelomonocytic leukemia with significant chromosomal abnormalities (62). Following replicative stress, BID associates with the ataxia telangiectasia and Rad3 related (ATR)–ATR-interacting protein (ATRIP)–replication protein A (RPA) complex (ATR-ATRIP-RPA) through BID helix 4 and the coiled-coil domain of ATRIP (36). BID's association with ATRIP facilitates ATR function as measured by CHK1 phosphorylation and recovery of DNA synthesis following hydroxyurea (HU) (36). Interestingly, the association between ATR-ATRIP and RPA is diminished in Bid−/− cells following replicative stress, suggesting that BID might function to maintain the DNA damage sensor complex containing RPA and ATR-ATRIP (36).
The assembly of the DNA damage sensor complex is a dynamic process involving the ordered recruitment of proteins to the single-stranded DNA (ssDNA) generated by the uncoupled activity of DNA helicases and DNA polymerases (49). RPA, the primary eukaryotic single-strand DNA binding protein, serves as the initial sensor of replicative stress (24). RPA binds and coats the exposed single-stranded DNA, protecting it from nucleolytic damage and inhibiting the formation of secondary structure (24).
The RPA is a modular protein composed of three subunits (RPA70, RPA32, and RPA14) organized into eight domains connected by flexible linkers. Motion between domains allows for optimal association with DNA and associated proteins during DNA processing (2, 8, 24, 42, 48). RPA binds ssDNA using four OB-fold domains that bind sequentially to DNA with a 5′-3′ polarity (20, 28). The binding affinities of each individual domain are weak but together produce a high binding affinity for ssDNA, Kd (dissociation constant) 10−9 to 10−10 M (8). Scanning transmission electron microscopy and gel filtration studies have identified multiple conformations of RPA on DNA, further demonstrating the flexible nature of the protein (9). RPA also physically interacts with DNA processing factors through its protein-protein interaction modules, including RPA70N, RPA70AB, and RPA32C (24). RPA70N is a key protein interaction module for DNA damage response (DDR); a number of proteins involved in DDR have been shown to interact with this domain (54). In one model, RPA70N functions to recruit a series of checkpoint proteins to ssDNA, including RAD9 and ATRIP (24, 54). RPA also maintains genomic stability by interaction with multiple proteins, including RAD51, the MRN complex, and the TIM/TIPIN complex (4, 15, 56), and prevents the generation of excessive ssDNA following replicative stress through recruitment of the annealing helicase SMARCAL1 (HARP) (5, 6, 15, 56–58).
Following replicative stress, proteins of the DNA damage sensor complex are recruited to RPA-coated ssDNA. ATR-ATRIP and the 9-1-1 complexes are independently recruited to RPA-coated ssDNA (63). TopBP1, a key ATR activator, is then recruited to ssDNA by interaction with ATR-ATRIP and Rad9 to facilitate the formation of activated DNA damage sensor complex (21, 34, 44). The activated ATR then phosphorylates numerous substrates required for replicative stress-induced DNA damage response (41). ATR and its effectors maintain genomic integrity by arresting cell cycle, slowing origin firing, stabilizing the replication fork, and facilitating replication fork restart (16).
In our previous study, we demonstrated that BID interacts with the DNA damage sensor complex, including ATR-ATRIP and RPA in vivo and in vitro (36), and facilitates an efficient response to replicative stress through interaction of BID helix 4 with ATRIP. In the present work, we demonstrate that the acidic N-terminal region of BID's helix 5 (named RPA-ID [RPA-interacting domain]) interacts with the basic cleft of RPA70N. In addition, BID stimulates the recruitment of ATR-ATRIP to RPA-coated ssDNA. Interestingly, the association of RPA and PCNA with chromatin is not maintained in the absence of BID. We further demonstrate that the BID-RPA interaction is important for normal ATR function following replicative stress.
MATERIALS AND METHODS
Cell lines and drug treatments.
U2OS cells were cultured in Dulbecco's minimal essential medium (DMEM) (Invitrogen) with 10% fetal bovine serum (FBS), 100 U/ml penicillin-streptomycin, 2 mM glutamine, and 0.1 mM β-mercaptoethanol. Early-passage cells (10 < number of passages < 20) were treated with hydroxyurea (Sigma) as indicated.
RNA interference (RNAi) treatment and stable cell line generation.
To generate U2OS cell lines stably expressing various BID mutants, pMSCVpuro (Clontech) plasmid harboring wild-type (wt) or mutated BID was transfected into 293T cells with packaging vector by Fugene 6 (Roche) to product retrovirus. Silent mutations in the BID small interfering RNA (siRNA)-targeted region (cDNA: G36A/T39C/G42A/C45T/C48T) were introduced in these retrovirus constructs so that the expressed mutated BID would not be knocked down by BID siRNA. Then, U2OS cells were infected twice with retrovirus harboring wild-type or mutated BID and selected by 0.8 to 1 μg/ml puromycin for 48 h. Live cells were cultured in DMEM containing 0.5 μg/ml puromycin.
The control siRNA (1027310; target sequence: AATTCTCCGAACGTGTCACGT) and siRNA targeting human BID (hBID) (SI02662415; target sequence: CAGGGATGAGTGCATCACAAA) were purchased from Qiagen, Inc. Lipofectamine 2000 (Invitrogen) was used to transfect BID siRNA or control siRNA in U2OS cells according to the manufacturer's instructions. Seventy-two hours after transfection, the transfected U2OS cells were treated as indicated in the figure legends.
Immunoprecipitation.
pcDNA3 (Invitrogen) plasmid harboring wild-type or mutated BID was transfected into 293T cells by Fugene 6 (Roche) for 48 h. Cells were lysed in lysis buffer (25 mM HEPES [pH 7.5], 250 mM NaCl, 2 mM EDTA, 10% glycerol, 0.5% NP-40, 1 mM phenylmethylsulfonyl fluoride [PMSF], 4 μg/ml leupeptin-antipain, 0.1 mM orthovanadate, 1 mM NaF). Then, BID was immunoprecipitated by biotinylated anti-hBID/anti-mouse BID (mBID) goat polyclonal antibody (BAF860; R&D Systems) and streptavidin agarose (Novagen). The beads were pelleted, washed, and boiled with 3× Laemmli buffer, and the supernatant was resolved on SDS-PAGE gels.
RPA expression constructs.
RPA70N cDNA harboring R41E/R43E mutant was a generous gift from David Cortez. The expression vector of His-tagged RPA70NAB, AB, N, and 32C domain was a generous gift from Walter Chazin via Ellen Fanning (32).
Protein purification.
The purification of BID was performed as previously described (1). Briefly, Escherichia coli BL21 strain harboring wild-type or mutated BID in the pGEX-6P-1 vector was induced by 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) at 37°C for 4 h. After cells were lysed in lysis buffer (50 mM Tris-HCl, 50 mM NaCl, 5 mM EDTA, 1% Triton X-100, 1 mM dithiothreitol [DTT], 1 mM PMSF; pH 8.0), glutathione S-transferase (GST)-fused BID was purified by glutathione-agarose (Sigma) and BID was released by incubation with PreScission protease (GE Healthcare Bioscience) at 4°C overnight. The expression and purification of His-tagged RPA70NAB, -AB, -N, and -32C constructs have been described previously described (1–3, 36). The construction and purification of His-tagged RPA70-PDI fusion protein were performed as previously described (37).
Protein-protein in vitro interaction.
For the BID-RPA in vitro interaction, 100 pmol of BID and 100 pmol of RPA construct were incubated in binding buffer (20 mM HEPES, 100 mM KCl, 5 mM MgCl2, 0.5 mM EDTA, 0.1% NP-40; pH 7.5) at room temperature for 30 min. Then, BID was immunoprecipitated by biotinylated goat polyclonal anti-human/mouse BID antibody (BAF860; R&D Systems) and streptavidin agarose (Novagen). The beads were pelleted by centrifugation and washed five times with binding buffer. The beads were boiled with 3× Laemmli buffer and the supernatant was resolved on SDS-PAGE gels.
ssDNA pulldown assay was performed as previously described (45). Briefly, 5 pmol of 3′-biotinylated 80-nucleotide DNA oligomer (AGATTCACCAGTCACACGACCAGTAATAAAAGGGACATTCTGGCCAACAGAGATAGAACCCTTCTGACCTGAAAGCGTAA) was incubated with streptavidin agarose (Novagen) in buffer containing 10 mM Tris-HCl (pH 7.5) and 100 mM NaCl at 4°C for 30 min. Then, 1 μg of purified recombinant His-tagged RPA and 80 pmol of precleared wild-type or mutated BID was incubated with beads in binding buffer A (10 mM Tris-HCl, 100 mM NaCl, 10 μg/ml bovine serum albumin [BSA], 0.01% NP-40, 10% glycerol; pH 7.5) at 4°C for 1 h. The beads were pelleted by centrifugation and washed five times with binding buffer A. The beads were resuspended in 3× Laemmli buffer and boiled, and the supernatant was resolved on SDS-PAGE gels.
For the in vitro RPA-ATRIP interaction, 250 pmol of purified recombinant GST–wt-RPA70N or GST-RPA70N-R41E/R43E was incubated with glutathione-agarose (Sigma) in GST-binding buffer (20 mM HEPES, 100 mM KCl, 5 mM MgCl2, 0.5 mM EDTA, 0.2% NP-40, 10% glycerol, 1 mM PMSF; pH 7.5) for 1 h at room temperature. After the agarose beads were pelleted and washed once with GST-binding buffer, 140 μg of nuclear lysate of U2OS cells stably expressing hemagglutinin (HA)-tagged ATRIP (a generous gift from D. Cortez) was added with purified recombinant BID simultaneously. The mixture was incubated for 1 h at room temperature, and then the beads were pelleted by centrifugation and washed five times with GST-binding buffer. The beads were boiled with 3× Laemmli buffer and the supernatant was resolved on SDS-PAGE gels.
NMR analysis.
Nuclear magnetic resonance (NMR) experiments were conducted using Bruker DRX 500-MHz and 600-MHz spectrometers equipped with z-axis gradient TXI cryoprobes. 15N-1H heteronuclear single-quantum correlation (HSQC) spectra were acquired using 75 μM 15N-enriched RPA70N in buffer containing 20 mM Tris (pH 7.0), 50 mM NaCl, and 2 mM DTT. The NMR chemical shift perturbation assays were performed by adding unlabeled protein into the solution of 15N-enriched protein until the molar ratio reached 1:4. Resonance assignments are available for RPA70N (10) and BID (BMRB entry 5340 [14]). All spectra were processed by Topspin v2.0 (Bruker, Billerica, MA) and analyzed with Sparky (University of California, San Francisco, CA).
Docking of RPA70N with hBID.
A model of RPA70N-hBID complex was generated using HADDOCK2 (22, 23). Structures of RPA and hBID were taken from Protein Data Bank (PDB) entries 2B3G and 2BID, respectively. Ambiguous distance restraints (AIRs) were generated based on significant chemical shift perturbations or line broadening and solvent accessibility. Side-chain solvent accessibilities were calculated using NACCESS (27). Residues with a solvent-accessible surface of more than 50% and significant chemical shift perturbations were designated active for docking calculations. The adjacent residues with more than 50% solvent-accessible surface were designated passive residues. An ensemble of 1,000 rigid-body docking models was generated at the first iteration of calculation. From this calculation, the best 200 structures were selected (based on energy), and then a second iteration was performed using a semiflexible simulated annealing protocol, and these structures were further refined in explicit solvent. The final structure solutions were clustered using HADDOCK score and the 10 lowest-energy models were selected from the most populated cluster for structural analysis.
Subcellular fractionation.
Subcellular fractionation of U2OS cells was performed as previously described (36). In brief, 10 × 106 U2OS cells were washed once with phosphate-buffered saline (PBS) and suspended in 400 μl of solution A (10 mM HEPES [pH 7.9], 10 mM KCl, 1.5 mM MgCl2, 0.34 M sucrose, 10% glycerol, 1 mM DTT, 1 mM PMSF, 10 mM NaF, 10 mM β-glycerophosphate, 1 μM microcystin) with 0.1% NP-40 on ice for 5 min. The cytoplasmic and nuclear fractions were collected by centrifugation at 1,300 × g for 4 min at 4°C. The isolated nuclei were washed once with solution A and then lysed in solution B (3 mM EDTA, 0.2 mM EGTA, 1 mM DTT, 1 mM PMSF, 10 mM NaF, 10 mM β-glycerophosphate, 1 μM microcystin). After incubation on ice for 10 min, chromatin fractions were harvested by centrifugation at 1,700 × g for 4 min at 4°C. The chromatin pellet was washed once with solution B and resuspended in 100 μl radioimmunoprecipitation assay (RIPA) buffer. After sonication for 20 seconds, the samples were boiled with 5× Laemmli buffer.
Single-cell gel electrophoresis (Comet) assay.
U2OS cells overexpressing HA-tagged wild-type, RPA-ID-, or IH5-mutated hBID were transfected with BID siRNA for 72 h. Silent mutations were introduced in the BID siRNA-targeted region so that only endogenous BID was knocked down by BID siRNA. Then, cells were treated with hydroxyurea overnight. The untreated and treated cells were collected in ice-cold PBS, and an alkaline comet assay was performed using a CometAssay kit (Trevigen) according to the manufacturer's instructions. Briefly, cells were mixed with molten low-melting-point agarose and pipetted onto CometSlide. After incubation with lysis solution and alkaline solution, slides were placed in Genemate compact gel tank (Bioexpress) and alkaline electrophoresis was performed at 21 V for 30 min. After incubation with 70% ethanol for 5 min, the slides were stained with SYBR green I. Then, samples were examined using a Leica DM IRBE inverted wide-field microscope and analyzed by CometScore program version 1.5.
Antibodies.
The following antibodies were used in this study: anti-BID rabbit polyclonal antibody (52), anti-BID polyclonal antibody (BAF860; R&D Systems), anti-BID polyclonal antibody (FL-195; Santa Cruz), anti-CHK1 monoclonal antibody (G-4; Santa Cruz), anti-phospho-CHK1(S345) polyclonal antibody (#2341; Cell Signaling), antiactin monoclonal antibody (Sigma), anti-histone H3 monoclonal antibody (05-928; Upstate Biotechnology), anti-His monoclonal antibody (#2365; Cell Signaling), anti-HA monoclonal antibody (12CA5; Roche), anti-ATRIP polyclonal antibody (#403) (Cortez et al. [17]), anti-ATR polyclonal antibody (N-19; Santa Cruz), anti-RPA32 monoclonal antibody (#2208; Cell Signaling), anti-RPA70 monoclonal antibody (R3400; US biological), anti-MCL-1 polyclonal antibody (Rockland), anti-BCL-2 monoclonal antibody (Pharmingen), anti-MCM3 polyclonal antibody (A300-192A; Bethyl Laboratories), anti-PCNA monoclonal antibody (555567; BD Pharmingen), and anti-MBP monoclonal antibody (Sigma).
RESULTS
BID interacts with the N-terminal domain of RPA70.
Our previous study demonstrated that BID facilitates an efficient DNA damage response to replicative stress. We demonstrated that BID associates with ATRIP, a member of the DNA damage sensor complex, by coimmunoprecipitation (co-IP) of endogenous proteins. Furthermore, the association of ATRIP and RPA with chromatin is diminished in the absence of BID (36). As RPA plays a key function to sense ssDNA and to recruit the proteins involved in checkpoint response and DNA repair, we asked whether BID might interact with the DNA damage response machinery through association with RPA.
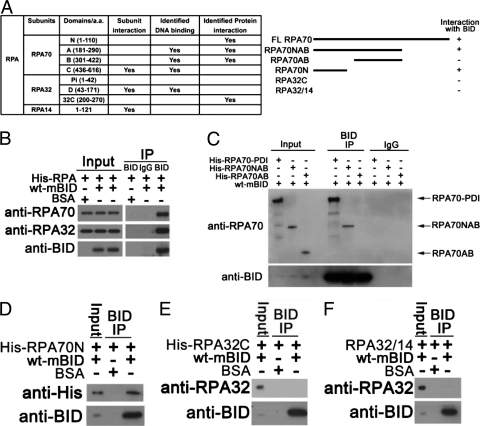
To evaluate the ability of BID to interact with the RPA, the protein was expressed in E. coli and purified (Fig. 1 A) (24). The purified RPA was incubated with purified recombinant mouse BID and immunoprecipitated with anti-BID antibody. The immunoprecipitated product was resolved on SDS-PAGE gels and immunoblotted with the indicated antibodies. Our experiments showed that BID associates with RPA in vitro (Fig. 1B). As noted above, RPA70N, RPA70AB, and RPA32C are the protein interaction modules of RPA (Fig. 1A) (24). To identify the domain(s) that associates with BID, various RPA domain constructs were overexpressed in E. coli, purified, and then evaluated for association with BID by immunoprecipitation. As the expression of the RPA70 subunit of RPA in E. coli is completely insoluble (26), we used the PDI fusion system to express full-length RPA70 subunit and successfully purified soluble RPA70 in E. coli. To assess BID-RPA70 interaction, wild-type BID was incubated with His-tagged RPA70-PDI fusion protein, His-tagged RPA70NAB, His-tagged RPA70AB, or His-tagged RPA70N. After BID was immunoprecipitated by anti-BID antibody, the RPA70-PDI fusion, RPA70NAB, and RPA70N were detected in the immunoprecipitated products, but RPA70AB was not (Fig. 1C and D). These results suggest that BID interacts with the RPA70N domain. To evaluate whether BID may also associate with RPA32C, wild-type BID was incubated with purified His-tagged RPA32C or RPA32/RPA14. After BID was immunoprecipitated by anti-BID antibody, no significant RPA32C or RPA32/RPA14 was found in the immunoprecipitated product (Fig. 1E and F), suggesting that BID does not interact with RPA32C. As controls, we established that when BID was incubated with His-tagged PDI or His-tagged MBP, followed by immunoprecipitation with anti-BID antibody, no significant His-tagged PDI or His-tagged MBP (unpublished data) was detected in the immunoprecipitated product. Accordingly, we conclude that BID specifically interacts with the RPA70N domain.
Fig. 1.
BID interacts with the N-terminal domain of RPA70 in vitro. (A) Schematic diagram of the functional domains of RPA. The interactions between BID and various RPA domains are summarized. (B) BID interacts with RPA. Purified recombinant mouse BID and His-tagged RPA were incubated in binding buffer at room temperature for 30 min. BID was immunoprecipitated using anti-BID antibody, and the immunoprecipitated proteins were resolved on SDS-PAGE gels and immunoblotted with the indicated antibodies. (C) BID interacts with RPA70. Purified recombinant mouse BID was incubated with His-tagged RPA70-PDI fusion protein, His-tagged RAP70NAB domain, or His-tagged RPA70AB domain as in panel B. BID was immunoprecipitated using anti-BID antibody, and the immunoprecipitated proteins were resolved on SDS-PAGE gels and immunoblotted with the indicated antibodies. (D) BID interacts with the N domain of RPA70. Purified recombinant mouse BID and His-tagged RPA70N domain (amino acids [aa] 1 to 168) were incubated as in panel B. BID was immunoprecipitated using anti-BID antibody, and the immunoprecipitated proteins were resolved on SDS-PAGE gels and immunoblotted with the indicated antibodies. (E) BID does not interact with the C domain of RPA32. Purified recombinant mouse BID and His-tagged RPA32C domain were incubated as in panel B. BID was immunoprecipitated using anti-BID antibody, and the immunoprecipitated proteins were resolved on SDS-PAGE gels and immunoblotted with the indicated antibodies. (F) BID does not interact with RPA32/RPA14 domains. Purified recombinant mouse BID and RPA32/RPA14 subunits were incubated as in panel B. BID was immunoprecipitated using anti-BID antibody, and the immunoprecipitated proteins were resolved on SDS-PAGE gels and immunoblotted with the indicated antibodies.
The acidic N-terminal region of helix 5 of BID interacts with the basic cleft of RPA70N.
RPA70N has been demonstrated to play an important role in sensing and recruiting various DNA damage response factors to ssDNA following replicative stress. The basic cleft of RPA70N serves as a docking surface for a series of checkpoint proteins, including RAD9 and ATRIP, which interact primarily through charge-charge interactions (54). To further characterize the interaction between BID and RPA70N, 15N-1H heteronuclear single quantum correlation (HSQC) NMR was employed to map the interaction surfaces on the two proteins. This approach has been used extensively in the case of RPA to characterize binding interactions (1–3, 43, 54). In these experiments, one of the two binding partners is produced with enrichment in the NMR-active 15N isotope, and 15N-1H-HSQC NMR spectra are acquired as the unlabeled binding partner is titrated into the solution. The assay is effective because the NMR chemical shift is extremely sensitive to electronic environment, so when a molecule that interacts with the labeled protein is added to the solution the chemical shifts of signals from residues at the binding interface are perturbed. It should be noted that additional chemical shift perturbations may arise as a result of changes in the structure of the labeled protein that are induced by the interactions with the binding partner.
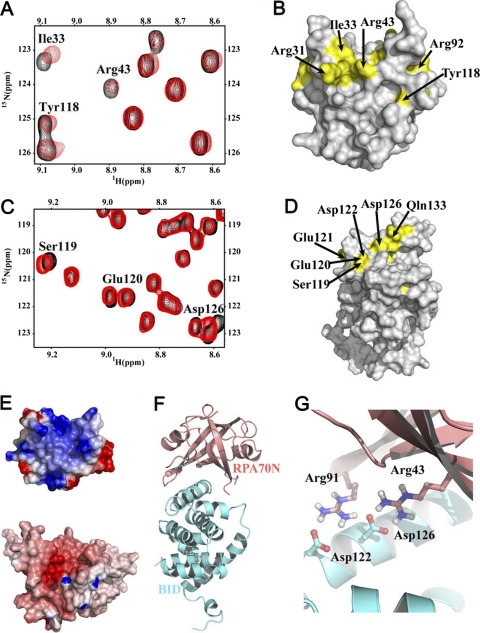
Titration of 15N-labled RPA70N with BID gave rise to a select set of chemical shift perturbations in 15N-1H HSQC NMR spectra (Fig. 2 A), which indicated a specific binding interaction. The effects were within the fast-exchange regime on the NMR timescale, which meant that chemical shift assignments could be traced from the continuous shifting of signals. The RPA70N residues most affected were mapped onto the structure of RPA70N (Fig. 2B) and included Arg31, Ile33, Arg43, Leu44, Arg92, and Tyr118. These residues are all located in the basic cleft of RPA70N, indicating that the binding site for BID on RPA70N is similar to that of ATRIP, MRE11, and RAD9 (1–3, 54). A reciprocal titration of RPA70N into 15N-labeled BID was also performed. The residues with the most significant chemical shift perturbations were located in helices 4 and 5 and the loop between them, including Arg118, Ser119, Glu120, Glu121, Asp 122, Asp126, Leu127, Thr129, Ala130, and Gln133 (Fig. 2C and D). This region is termed the RPA-interacting domain (RPA-ID) of BID.
Fig. 2.
NMR analysis and model of the interaction of RPA70N and BID. (A) Identification of the RPA70N residues in the BID binding site. Overlaid 15N-1H HSQC spectra of 15N-labled RPA70N (aa 1 to 120) in the absence (black) and presence (red) of BID. (B) Map of RPA70N residues (yellow) perturbed upon the addition of BID on the structure of RPA70N. (C) Identification of the BID residues in the RPA70N interaction domain. Binding of RPA70N with BID monitored on the 15N-1H HSQC spectra of 15N-labled BID in the absence (black) and presence (red) of RPA70N. (D) Map of BID residues (yellow) perturbed upon the addition of RPA70N on the structure of BID. (F) Electrostatic complementarity of the RPA70N (top) and BID (bottom) binding sites. (E) NMR-based model of the complex of BID with RPA70N. Ribbon representations of PA70N and BID are colored in salmon red and cyan, respectively. (G) A close-up view of the model of the BID-RPA70N complex.
Having used NMR to map the residues involved in the interaction between BID and RPA70N, we turned to computational docking using these data to guide the generation of a structural model of the complex. The program HADDOCK (high ambiguity driven biomolecular docking) was used for this purpose (22, 23). Interface restraints were generated for residues that exhibited significant chemical shift perturbations or broadening and also had side chains with >50% solvent accessibility. An overview of the model of the BID-RPA70N complex is shown in Fig. 2E to G. A comparison of the electrostatic surfaces in the binding regions of the two proteins shows there is a significant electrostatic complementarity (Fig. 2F). For example, there are a number of specific salt bridges in the model, in particular from acidic residues in RPA-ID of BID (e.g., Glu121, Asp122, Asp126) and basic residues in the basic cleft region of RPA70N (e.g., Arg43, Arg91) (Fig. 2G).
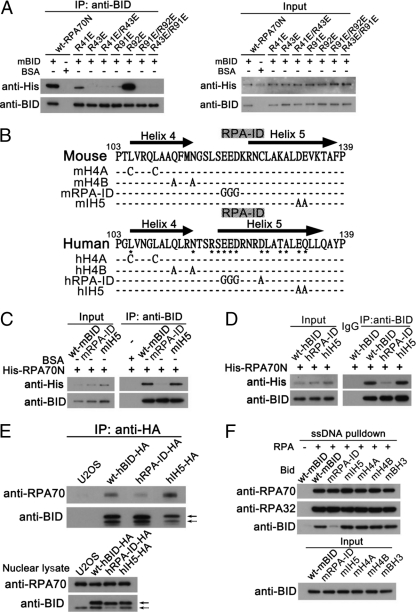
The structural model suggests that key amino acids in the basic cleft region of RPA70N interact with acidic residues in the RPA-ID of BID. To test these predictions, a series of RPA70N and BID mutations were prepared and interactions were tested by coimmunoprecipitation experiments. Introduction of mutations in RPA70N basic residues Arg43 and Arg91 significantly impaired the BID-RPA70N interaction (Fig. 3 A). Interestingly, although NMR chemical shift perturbations are observed for the Arg41 and Arg92 residues, mutation of these residues results in little to no effect on the BID-RPA70N interaction (Fig. 3A). These observations confirm the validity of the model, as residues 41 and 92 do not face directly into the BID interaction interface and chemical shift perturbations can be attributed to changes induced by the binding of adjacent residues to BID. We note that some chemical shift perturbations in BID residues were observed for residues outside of the RPA-ID, particularly in helix 4 and helix 5. These effects are also attributable to a secondary effect induced by the binding of RPA70N to the BID RPA-ID. To test the involvement of the RPA-ID of BID in the BID-RPA70N interaction, acidic residues in the RPA-ID of BID were mutated. As a control, acidic residues inside of helix 5 (IH5) (Fig. 3B) were also tested. In the BID-RPA70N co-IP experiments, wild-type BID and IH5 mutants were found to interact with RPA70N, but RPA-ID-mutated human or mouse BID did not (Fig. 3B to D). These results further support the BID-RPA70N structural model in which the acidic RPA-ID primarily contacts the basic cleft region of RPA70N.
Fig. 3.
Mutations in the RPA-ID of BID or the basic cleft region of RPA70N impair BID-RPA70N interaction. (A) BID interacts with the basic cleft of RPA70N. Purified recombinant His-tagged wild-type RPA70N or RPA70N mutated at residues 41, 43, 91, and 92, charged residues located around the RPA basic cleft region, was incubated with BID in binding buffer at room temperature for 30 min. BID was immunoprecipitated using anti-BID antibody, and the immunoprecipitated proteins were resolved on SDS-PAGE gels and immunoblotted with the indicated antibodies. (B) Schematic diagram of the RPA-ID and IH5-mutated BID. Glu121, Asp122, and Asp126 in the RPA-ID region of BID are marked. (C) The BID RPA-ID region interacts with RPA70N. Purified recombinant His-tagged wild-type RPA70N was incubated with wild-type, RPA-ID-, or IH5-mutated mouse BID in binding buffer at room temperature for 30 min. BID was immunoprecipitated using anti-BID antibody, and the immunoprecipitated proteins were resolved on SDS-PAGE gels and immunoblotted with the indicated antibodies. (D) Purified recombinant His-tagged wild-type RPA70N was incubated with wild-type, RPA-ID-, or IH5-mutated human BID in binding buffer at room temperature for 30 min. BID was immunoprecipitated using anti-BID antibody, and the immunoprecipitated proteins were resolved on SDS-PAGE gels and immunoblotted with the indicated antibodies. (E) Mutation in the RPA-ID region of BID impairs BID-RPA interaction in U2OS cells. U2OS cells harboring stably expressed HA-tagged wild-type hBID or mutated hBID in RPA-ID or the IH5 region were treated with 10 mM hydroxyurea for 5 h. Then, the expressed HA-tagged BID was immunoprecipitated from purified nuclear extracts by anti-HA antibody and RPA70 was detected in the immunoprecipitated products. A solid arrow denotes HA-tagged BID. A dashed arrow denotes endogenous BID. (F) Biotinylated ssDNA was incubated with streptavidin beads for 30 min at 4°C. Then, the pulldown products were incubated with His-tagged RPA in the presence of wild-type or mutated mouse BID for 1 h at 4°C and the pulldown products were resolved on SDS-PAGE gels and immunoblotted with the indicated antibodies.
To investigate whether mutation in the RPA-ID region of BID impairs BID-RPA interaction when expressed at endogenous levels, U2OS cells harboring HA-tagged wild-type hBID or mutated hBID in RPA-ID or the IH5 region were treated with hydroxyurea to induce replicative stress. Then, the expressed HA-tagged BID was immunoprecipitated from purified nuclear extracts by anti-HA antibody and RPA70 was detected in the immunoprecipitated products. RPA-ID-mutated BID significantly deceased BID-RPA interaction, while wild-type and IH5-mutated BID show an intact BID-RPA interaction following replicative stress (Fig. 3E).
We further evaluated the ability of wild-type BID and BID mutants in the RPA-ID or IH5 to associate with RPA bound to ssDNA. An 80-nucleotide 3′-biotinylated DNA oligomer was incubated with streptavidin agarose and then coated with His-tagged RPA. Wild-type BID or BID mutated in RPA-ID or IH5 was incubated with the RPA-coated ssDNA and pulled down by streptavidin agarose. Wild-type BID and IH5-mutated BID but not the RPA-ID mutant was found in the pulldowns (Fig. 3F). Additional controls were performed on mutants in other functional domains of BID (i.e., BH3 domain and helix 4 domain), and these also did not significantly impair the BID-RPA70N interaction (unpublished). The above results confirm the importance of the BID RPA-ID for the interaction of RPA70N with BID.
The RPA-ID of BID is important for normal ATR function following replicative stress.
The N-terminal domain of RPA70 has been demonstrated to play a crucial role in the ATR-mediated DNA damage response (64). Cells harboring RPA70-R41E/R43E exhibit significantly diminished ATR-mediated CHK1 phosphorylation following UV treatment (54). To test whether the BID-RPA interaction is important for normal ATR function, wild-type BID or an RPA-ID mutant BID was reintroduced into BID-deficient U2OS cells. Compared with that of mouse BID, the RPA-ID region of human BID harbors an additional acidic amino acid (D126), which displays an obvious chemical shift perturbation in NMR HSQC spectrum upon adding RPA70N. Therefore, we also mutated Asp 126 to Ala in human RPA-ID-mutated BID (Fig. 2B).
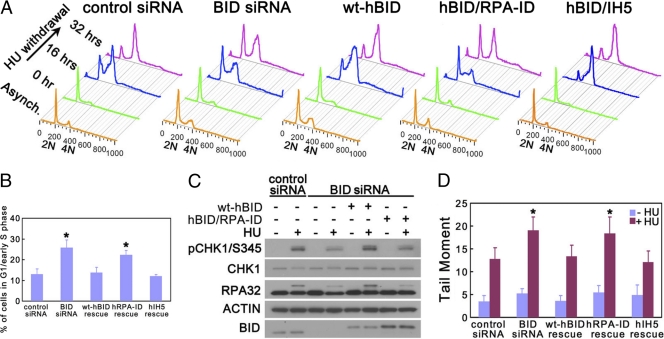
One unique ATR function is to facilitate cell cycle reentry after release from replicative stress (16). U2OS cells harboring wild-type, RPA-ID-, or IH5-mutated human BID were transfected with BID-targeted siRNA to knock down endogenous BID. Then, cells were arrested in early S phase by 10 mM HU overnight and released into fresh medium with nocodazole to prevent cell division. BID-deficient U2OS cells demonstrated impaired DNA recovery of replication and progression through S phase (Fig. 4 A). Expression of wild-type and IH5-mutated BID but not RPA-ID-mutated BID rescued the recovery and completion of DNA synthesis in BID-deficient U2OS cells (Fig. 4A and B), suggesting that the BID-RPA association is important for normal ATR function following replicative stress.
Fig. 4.
RPA-ID of BID is important for normal ATR function following replicative stress. (A) An intact RPA-ID region of BID is required for normal recovery of DNA synthesis following hydroxyurea withdrawal. U2OS cells overexpressing HA-tagged wild-type, RPA-ID-, or IH5-mutated human BID were transfected with BID siRNA for 72 h. Then, cells were treated with 10 mM hydroxyurea overnight and released into fresh medium containing 1 μg/ml nocodazole for the indicated times. Cells were fixed and stained with propidium iodide. Live cells were analyzed by flow cytometry gated on forward- and side-scatter channels (FSC/SSC). The quantitative analysis of the arrested G1-/early-S-phase cells following a 32-h HU withdrawal is shown in panel B. *, P < 0.05. (C) An intact RPA-ID region of BID is important for normal ATR substrate phosphorylation following hydroxyurea treatment. U2OS cells overexpressing HA-tagged wild-type BID or RPA-ID-mutated human BID were transfected with BID siRNA for 72 h. Silent mutations were introduced into the BID siRNA-targeted region so that only endogenous BID was knocked down by BID siRNA. Then, cells were treated with 10 mM hydroxyurea for 5 h, and protein extracts were resolved on SDS-PAGE gels and immunoblotted with anti-phospho-CHK1 and anti-RPA32. (D) U2OS cells overexpressing HA-tagged wild-type, RPA-ID, or IH5 RPA-ID-mutated human BID were transfected with BID siRNA for 72 h. Silent mutations were introduced into the BID siRNA-targeted region so that only endogenous BID was knocked down by BID siRNA. Then, cells were treated with 10 mM hydroxyurea overnight. The untreated and treated cells were collected in ice-cold PBS and detected in an alkaline comet assay (Trevigen). The samples were run in alkaline electrophoresis solution at 21 V for 30 min. At least 60 randomly chosen comets per sample were analyzed by CometScore program version 1.5. *, P < 0.05.
ATR signals to downstream effectors in the DDR through phosphorylation of key substrates. Following HU-induced replicative stress, the phosphorylation of ATR substrates (i.e., CHK1 and RPA32) was diminished in BID-deficient U2OS cells (36) (Fig. 4C), suggesting that ATR kinase activity is limited in the absence of BID. Reintroduction of wild-type but not the RPA-ID-mutated BID rescued the defects of ATR substrate phosphorylation (Fig. 4C), suggesting that the RPA-ID of BID is important for normal ATR activity in the cellular response to replicative stress.
Following replicative stress, ATR phosphorylates numerous substrates to maintain the stalled replication fork and genomic integrity. To detect the DNA damage level following replicative stress, U2OS cells harboring wild-type or RPA-ID-mutated human BID were transfected with BID-targeted siRNA to knock down endogenous BID. Then, cells were treated with HU overnight. In the BID-deficient U2OS cells, the DNA damage level is significantly increased following HU treatment (Fig. 4D). Reintroduction of wild-type but not the RPA-ID-mutated BID rescued the increased DNA damage level (Fig. 4D), suggesting that the RPA-ID of BID is important to maintain genomic integrity following replicative stress.
The helix 4 domain interacts with the coiled-coil region of ATRIP following replicative stress, and the reintroduction of helix 4-mutated BID into BID-deficient cells cannot rescue the defects in cell cycle reentry ability, ATR substrate phosphorylation, and DNA damage level (36).These results suggest that both the BID-ATRIP and the BID-RPA interactions are important for BID's function in the ATR-mediated DNA damage-signaling pathways.
RPA-ID-mutated BID maintains proapoptotic function.
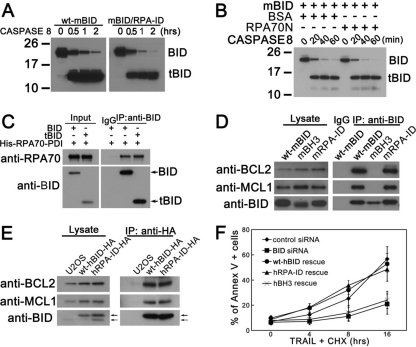
To investigate whether mutations in RPA-ID affect BID's cell death function, purified wild-type BID or RPA-ID mutant was incubated with purified caspase 8, the initiator caspase responsible for cleaving BID following death receptor activation. Purified recombinant wild-type BID and the RPA-ID mutant show similar sensitivities to caspase 8 in vitro (Fig. 5 A). Furthermore, incubation of BID with RPA70N did not change the sensitivity of BID to caspase 8 (Fig. 5B) in this in vitro assay. In addition, truncated BID showed similar interaction with RPA70 as full-length BID (Fig. 5C), suggesting that cleavage of BID by caspase 8 does not impair BID-RPA interaction in vitro. These results are consistent with a distinct separation of the RPA-ID and caspase cleavage site of BID. To determine if mutation in RPA-ID resulted in loss of interaction with other apoptosis factors, we evaluated the ability of the RPA-ID mutant to associate with BCL-2 and MCL-1 (Fig. 5D and E). Wild-type BID or RPA-ID-mutated mouse BID was expressed in 293T cells and immunoprecipitated with anti-BID antibody. The immunoprecipitated product was resolved on SDS-PAGE gels and immunoblotted with anti-BCL-2 and anti-MCL-1. We found that the RPA-ID mutant associates with both BCL-2 and MCL-1 (Fig. 5D), suggesting that mutation does not disrupt the global structure of BID but maintains the ability to bind other BCL-2 family members. Similar results were also observed in U2OS cells stably expressing wild-type or RPA-ID-mutated human BID (Fig. 5E). In addition, TRAIL-induced cell death was restored by reintroduction of wild-type and RPA-ID-mutated human BID but not BH3 domain-mutated human BID (Fig. 5F), suggesting that RPA-ID-mutated BID maintains its proapoptotic function. The above results are consistent with biological and biochemical separation of the functions of BID in cell death and the DNA damage response.
Fig. 5.
Mutation in the RPA-ID region of BID does not significantly alter BID's apoptotic function in the extrinsic cell death pathway. (A) Caspase 8 cleaves RPA-ID-mutated BID. Purified wild-type and RPA-ID-mutated mouse BID were incubated with active caspase 8 (Millipore) in vitro for 0.5, 1, and 2 h. The reaction products were resolved by SDS-PAGE and immunoblotted with anti-BID antibody. tBID, caspase-cleaved BID. (B) Incubation with RPA does not affect BID's sensitivity to caspase 8 in vitro. Purified wild-type mouse BID was incubated with active caspase 8 in the presence of RPA or BSA for 20, 40, and 60 min. The reaction products were resolved by SDS-PAGE and immunoblotted with anti-BID antibody. (C) Caspase-cleaved BID (tBID) binds with RPA in vitro. Purified recombinant mouse BID was first incubated with activated caspase 8 for 4 h at 37°C. Then, tBID was incubated with purified recombinant His-tagged RPA70-PDI fusion protein, and in vitro co-IP assay was performed. (D) RPA-ID-mutated BID binds to other BCL-2 family proteins. 293T cells were transfected with wild-type BID or RPA-ID-mutated mouse BID for 48 h. Then, BID was immunoprecipitated from whole-cell extracts with anti-BID antibody. The immunoprecipitated products were resolved by SDS-PAGE followed by immunoblotting with anti-BID, anti-MCL1, and anti-BCL2 antibodies. (E) U2OS cells overexpressing HA-tagged wild-type or RPA-ID-mutated human BID were lysed, and HA-tagged BID was immunoprecipitated from whole-cell extracts with anti-HA antibody. The immunoprecipitated products were resolved by SDS-PAGE followed by immunoblotting with anti-BID, anti-MCL1, and anti-BCL2 antibodies. A solid arrow denotes HA-BID, and a dashed arrow denotes endogenous BID. (F) Cells harboring RPA-ID-mutated BID show similar sensitivities to TRAIL/CHX treatment. U2OS cells overexpressing HA-tagged wild-type or RPA-ID-mutated human BID were transfected with BID siRNA for 72 h. Then, cells were treated with 50 ng/ml TRAIL and 5 μg/ml CHX for the indicated times. The apoptotic cells were detected using the annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (BioVision) according to the manufacturer's instructions.
RPA and PCNA are not maintained at the stalled replication fork in Bid-deficient cells.
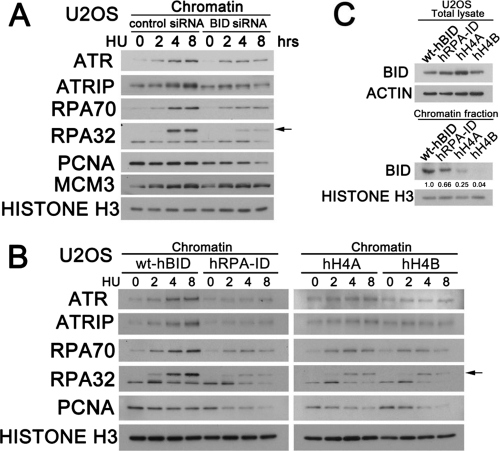
Following replicative stress, the ATR-mediated DNA damage response stabilizes stalled replication forks and facilitates replication reentry. In our previous study, Bid-deficient cells demonstrated limited replication reentry following replicative stress and decreased association of ATR-ATRIP and RPA, suggesting the possibility that BID may be involved at the replication fork. To evaluate the role of BID on the association of replication fork proteins with chromatin following replicative stress, U2OS cells transfected with control siRNA or BID-targeted siRNA were treated with HU. Cells were then harvested at the indicated time points, and chromatin fractions were isolated and resolved on SDS-PAGE gels and immunoblotted with the indicated antibodies detecting factors involved in the replication fork. Consistent with our previous result, replicative stress induced an accumulation of ATR-ATRIP on the chromatin fraction in control knockdown (KD) but not BID KD U2OS cells (Fig. 6 A). As a key ssDNA sensor, RPA accumulated in chromatin fractions in both control KD and BID KD U2OS cells. However, following treatment with HU, the accumulation of RPA on chromatin was significantly diminished in BID-deficient U2OS cells, most notably at 4 and 8 h of HU treatment (Fig. 6A).
Fig. 6.
Stability of the replication fork is diminished in BID-deficient cells following replicative stress. (A) U2OS cells were transfected with control siRNA or BID siRNA for 72 h. Then, cells were treated with 10 mM hydroxyurea over time, and the chromatin fraction was purified and resolved on SDS-PAGE gels and immunoblotted with the indicated antibodies to various factors involved in the replication fork. (B) U2OS cells overexpressing HA-tagged wild-type hBID, RPA-ID-mutated hBID, or helix 4-mutated hBID were transfected with BID siRNA for 72 h. Overexpressed BID and BID mutants harbor silent mutations in the BID siRNA-targeted region to evade siRNA knockdown, and thus only endogenous BID was knocked down by BID siRNA. Then, cells were treated with 10 mM hydroxyurea over time, and the chromatin fraction was purified, resolved on SDS-PAGE gels, and immunoblotted with the indicated antibodies. (C) U2OS cells used in B, overexpressing HA-tagged wild-type hBID, RPA-ID-mutated hBID, or helix 4-mutated hBID harboring silent mutations to evade siRNA knockdown, were transfected with BID siRNA for 72 h. Then, cells were treated with 10 mM hydroxyurea for 5 h, and the chromatin fraction was purified, resolved on SDS-PAGE gels, and immunoblotted with anti-BID antibody.
Following replicative stress, BID-deficient cells demonstrated increased DNA damage level (Fig. 4D). As the ATR signal contributes to genomic integrity by stabilization of stalled replication fork (17), we asked whether the replication fork is stable in the absence of BID. Strikingly, the maintenance of PCNA, a key cofactor of DNA polymerase delta in the replication fork, was significantly reduced in the BID-deficient chromatin fraction at 8 h after HU treatment, while PCNA was well maintained in control KD chromatin after HU treatment (Fig. 6A). Of note is that the total level of PCNA in BID-deficient cells was not significantly changed following HU treatment over time (unpublished). Interestingly, chromatin-bound minichromosome maintenance complex 3 (MCM3) was maintained normally in BID-deficient cells following HU treatment, indicating that BID plays a minimal role to maintain the helicase on chromatin. This defect can be rescued by the reintroduction of wild-type BID but not by the RPA-ID-mutated or helix 4-mutated BID (Fig. 6B). In addition, the chromatin-bound RPA-ID or helix 4-mutated BID was significantly diminished following replicative stress (Fig. 6C). These results are consistent with the decreased stability of the protein complex involved in the DNA damage response at stalled replication forks in the absence of BID. Furthermore, both the BID-ATRIP and BID-RPA interactions are important for BID's function to maintain the DNA damage sensor complex.
BID facilitates the RPA-ATRIP interaction in vitro in a dose-dependent manner.
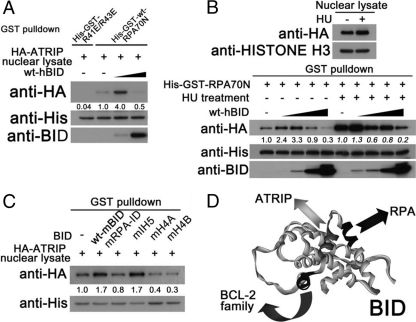
Following replicative stress, BID associates with the ATR-ATRIP-RPA complex. The association between ATR-ATRIP and RPA is diminished in Bid−/− cells (36). To investigate the mechanism of BID's regulation of the ATR-ATRIP-RPA complex, the ATRIP-RPA interaction was evaluated in the absence or presence of purified recombinant BID. Interestingly, low concentrations of BID stimulate binding of HA-ATRIP in the nuclear lysate to GST-RPA70N, while high concentrations of BID compete with HA-ATRIP binding to GST-RPA70N (Fig. 7 A). In addition, the induction of HA-ATRIP bound to GST-RPA70N was also observed in HU-treated nuclear lysate, although to a lesser extent (Fig. 7B). It is worthwhile to note that HU treatment dramatically increases the binding ability of HA-ATRIP to GST-RPA70N (Fig. 7B), suggesting that a more stable ATR-ATRIP-RPA complex is formed following replicative stress. Mutations in either the RPA-ID or the helix 4 region of BID impair BID's ability to facilitate the RPA-ATRIP interaction in this in vitro system (Fig. 7C), which is consistent with our finding that BID facilitates formation of the DNA damage sensor complex in vivo, through both the BID-ATRIP interaction and the BID-RPA interaction. BID thus interacts with proteins in the DNA damage sensor complex through a novel functional domain of the protein that is distinct from the BH3 domain, used to interact with other BCL-2 family members (Fig. 7D). Although BID is poised at the interface between the sensor complex of DNA damage response and apoptosis, further studies will be necessary to elucidate how these two BID functions are balanced and coordinated.
Fig. 7.
BID facilitates the RPA-ATRIP interaction in vitro in a dose-dependent manner. (A) His-GST fused wild-type RPA70N or RPA70N harboring R41E/R43E mutations (250 pmol) was bound to glutathione-agarose beads. Then, the nuclear fraction was purified from U2OS cells stably expressing HA-tagged ATRIP. The nuclear lysate was incubated with the RPA70N or RPA70N-R41E/R43E beads in the presence of 50 or 1,250 pmol of purified human BID at room temperature for 1 h. The GST pulldown product was resolved on SDS-PAGE gels and immunoblotted with the indicated antibodies. (B) His-GST fused wild-type RPA70N (250 pmol) was bound to glutathione-agarose beads. Then, U2OS cells stably expressing HA-tagged ATRIP were left untreated or were treated with 10 mM HU for 5 h, and the nuclear fraction was purified. The nuclear lysate was incubated with the RPA70N glutathione-agarose beads in the presence of 10, 50, 250, and 1,250 pmol of purified human BID at room temperature for 1 h. The GST pulldown product was resolved on SDS-PAGE gels and immunoblotted with the indicated antibodies. (C) His-GST fused wild-type RPA70N (250 pmol) was bound to glutathione-agarose beads. Then, the nuclear fraction was purified from U2OS cells stably expressing HA-tagged ATRIP. The nuclear lysate was incubated with the RPA70N glutathione-agarose beads in the presence of 10 pmol of purified wild-type mouse BID or BID mutated in the RPA-ID, IH5, H4A (L105C/L109C), and H4B (Q112A/N115A) regions at room temperature for 1 h. The GST pulldown product was resolved on SDS-PAGE gels and immunoblotted with the indicated antibodies. (D) A model of representing the regions of BID that interact with proteins in the DNA damage sensor complex to mediate DNA damage response and the region of BID that interacts with proteins in the BCL-2 family to mediate apoptosis.
DISCUSSION
Although the BCL-2 family of proteins was first characterized as sensors and/or transducers that function at the mitochondria to activate the intrinsic apoptosis pathway, accumulating data have implicated increasing numbers of BCL-2 family members that function in the nucleus following DNA damage. BCL-2 plays a role to suppress DNA double-strand break repair and V(D)J recombination by interaction with Ku70 and Ku86 via its BH1 and BH4 domains (53). Following etoposide treatment, MCL-1 is found in the DNA damage complex containing NBS1 and γH2A.X to facilitate ATR-dependent CHK1 phosphorylation (29–31). The accumulation of nuclear Mcl-1 in response to DNA damage is mediated by interaction with the IEX-1 protein (46). Aven, a BCL-XL-interacting protein, functions as an ATM activator to inhibit G2/M progression (25). In addition to these antiapoptotic members, we have shown that BID, a proapoptotic BH3-only protein, associates with the DNA damage sensor complex (36). Interestingly, following replicative stress, BID interacts with the DNA damage sensor complex (i.e., ATRIP and RPA) by its unique helix 4 and RPA-ID regions, which are not shared by any other BCL-2 family members, supporting the notion that this function in the cellular response to replicative stress is unique to BID.
As the direct sensor of single-stranded DNA, RPA plays a crucial role in ATR-mediated DNA damage response to replicative stress (64). RPA binds to exposed ssDNA and recruits other checkpoint proteins to form an activated DNA damage sensor complex. It is important to note that although we find a primary interaction of BID with RPA70N, it is also possible that other regions of RPA may participate in the interaction with BID. Following replicative stress, the ATR-ATRIP complex and the RAD9-HUS1-RAD1 complex (9-1-1) are recruited independently to RPA-coated ssDNA (3, 7, 63). The function of RPA in the ATR recruitment/activation process is predominantly mediated by its RPA70N domain, and RAD9 and ATRIP compete for the same basic cleft region of RPA70N in vitro (54). However, both RAD9 and ATRIP are essential positive regulators for normal ATR-mediated response in vivo (17, 54) and are required to initiate and maintain the activated DNA damage sensor complex on chromatin. Both the length of ssDNA and the structure of the damaged DNA have also been reported to be important factors to initiate the ATR-mediated checkpoint response (13, 40, 51). Together, these results suggest that a higher-order structure is required for a robust checkpoint response. In our studies, the association between ATR-ATRIP and RPA is diminished in Bid−/− cells following replicative stress (36), and BID stimulates the association of ATRIP with RPA on chromatin in vitro, suggesting that BID plays a role in vivo to maintain the high-order structure of the DNA damage sensor complex (Fig. 7D).
Our studies also showed that chromatin-bound PCNA is significantly diminished in BID-deficient cells following replicative stress (Fig. 6). Although PCNA is prone to release from stalled replication forks in the absence of BID, the chromatin-bound MCM3 is maintained normally in BID-deficient cells (Fig. 6). These results are consistent with the observation that although Bid−/− cells double more slowly, DNA replication can function, and Bid−/− mice are viable (36, 55). Our results are most consistent with a role for BID in the response to replicative stress, functioning as a scaffolding protein to help coordinate an efficient response to this cellular damage.
It is possible that BID's function in the DNA damage response to replicative stress plays a context-dependent role in settings such as increased replication of hematopoietic progenitor cells to replace hematopoietic cells depleted by genotoxic bone marrow damage. Indeed, we have found that following long-term HU treatment, the hematopoietic stem cell-enriched (HSC-enriched) LSK and myeloid progenitor cell populations are significantly diminished in Bid−/− mice and γH2A.X-positive cells are significantly increased in Bid−/− bone marrow. In addition, HSC function is diminished in Bid−/− bone marrow (Y. Liu et al., submitted for publication). Our studies are most consistent with BID facilitating normal ATR function to maintain replication fork and genomic integrity in the cycling bone marrow progenitor cells following replicative stress, which prevents excessive mobilization of HSCs and resultant depletion of HSC function.
ACKNOWLEDGMENTS
We thank Ellen Fanning, David Cortez, Jennifer Pietenpol, Scott Hiebert, Elizabeth Yang, Laurie Lee, and Kathy Gould for many helpful discussions. We thank David Cortez for the anti-ATRIP antibody, the U2OS cells expressing HA-ATRIP, and the RPA70N construct harboring R41E/R43E mutation. We thank James Chou and Gerhard Wagner for the human BID NMR assignments.
This work was supported by funds from NIH R01 HL088347, a Gabrelle's Angel Foundation Scholar award, a Kimmel Scholar award, and ACS IRG58-009-47 to S.S.Z., NIH R01 GM65484 to W.J.C., and support for use of facilities from the Vanderbilt-Ingram Cancer Center (NIH 2P30CA068485) and the Vanderbilt Center in Molecular Toxicology (NIH 5P30ES000267). Cell imaging experiments were performed in the VUMC Cell Imaging Shared Resource (NIH grants CA68485, DK20593, DK58404, HD15052, DK59637, and EY08126).
Footnotes
Published ahead of print on 22 August 2011.
REFERENCES
- 1. Arunkumar A. I., et al. 2005. Insights into hRPA32 C-terminal domain–mediated assembly of the simian virus 40 replisome. Nat. Struct. Mol. Biol. 12:332–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arunkumar A. I., Stauffer M. E., Bochkareva E., Bochkarev A., Chazin W. J. 2003. Independent and coordinated functions of replication protein A tandem high affinity single-stranded DNA binding domains. J. Biol. Chem. 278:41077–41082 [DOI] [PubMed] [Google Scholar]
- 3. Ball H. L., et al. 2007. Function of a conserved checkpoint recruitment domain in ATRIP proteins. Mol. Cell. Biol. 27:3367–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ball H. L., Myers J. S., Cortez D. 2005. ATRIP binding to replication protein A-single-stranded DNA promotes ATR-ATRIP localization but is dispensable for Chk1 phosphorylation. Mol. Biol. Cell 16:2372–2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bansbach C. E., Betous R., Lovejoy C. A., Glick G. G., Cortez D. 2009. The annealing helicase SMARCAL1 maintains genome integrity at stalled replication forks. Genes Dev. 23:2405–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bansbach C. E., Boerkoel C. F., Cortez D. 2010. SMARCAL1 and replication stress: an explanation for SIOD? Nucleus 1:245–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bermudez V. P., et al. 2003. Loading of the human 9-1-1 checkpoint complex onto DNA by the checkpoint clamp loader hRad17-replication factor C complex in vitro. Proc. Natl. Acad. Sci. U. S. A. 100:1633–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhattacharya S., et al. 2002. Characterization of binding-induced changes in dynamics suggests a model for sequence-nonspecific binding of ssDNA by replication protein A. Protein Sci. 11:2316–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blackwell L. J., Borowiec J. A., Mastrangelo I. A. 1996. Single-stranded-DNA binding alters human replication protein A structure and facilitates interaction with DNA-dependent protein kinase. Mol. Cell. Biol. 16:4798–4807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bochkareva E., et al. 2005. Single-stranded DNA mimicry in the p53 transactivation domain interaction with replication protein A. Proc. Natl. Acad. Sci. U. S. A. 102:15412–15417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bonzon C., Bouchier-Hayes L., Pagliari L. J., Green D. R., Newmeyer D. D. 2006. Caspase-2-induced apoptosis requires bid cleavage: a physiological role for bid in heat shock-induced death. Mol. Biol. Cell 17:2150–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheng W. C., et al. 2006. Mitochondrial factors with dual roles in death and survival. Oncogene 25:4697–4705 [DOI] [PubMed] [Google Scholar]
- 13. Choi J. H., et al. 2010. Reconstitution of RPA-covered single-stranded DNA-activated ATR-Chk1 signaling. Proc. Natl. Acad. Sci. U. S. A. 107:13660–13665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chou J. J., Li H., Salvesen G. S., Yuan J., Wagner G. 1999. Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell 96:615–624 [DOI] [PubMed] [Google Scholar]
- 15. Ciccia A., et al. 2009. The SIOD disorder protein SMARCAL1 is an RPA-interacting protein involved in replication fork restart. Genes Dev. 23:2415–2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cimprich K. A., Cortez D. 2008. ATR: an essential regulator of genome integrity. Nat. Rev. Mol. Cell Biol. 9:616–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cortez D., Guntuku S., Qin J., Elledge S. J. 2001. ATR and ATRIP: partners in checkpoint signaling. Science 294:1713–1716 [DOI] [PubMed] [Google Scholar]
- 18. Danial N. N. 2008. BAD: undertaker by night, candyman by day. Oncogene 27(Suppl. 1):S53–S70 [DOI] [PubMed] [Google Scholar]
- 19. Danial N. N., Korsmeyer S. J. 2004. Cell death: critical control points. Cell 116:205–219 [DOI] [PubMed] [Google Scholar]
- 20. de Laat W. L., et al. 1998. DNA-binding polarity of human replication protein A positions nucleases in nucleotide excision repair. Genes Dev. 12:2598–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Delacroix S., Wagner J. M., Kobayashi M., Yamamoto K., Karnitz L. M. 2007. The Rad9-Hus1-Rad1 (9-1-1) clamp activates checkpoint signaling via TopBP1. Genes Dev. 21:1472–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Vries S. J., et al. 2007. HADDOCK versus HADDOCK: new features and performance of HADDOCK2.0 on the CAPRI targets. Proteins 69:726–733 [DOI] [PubMed] [Google Scholar]
- 23. Dominguez C., Boelens R., Bonvin A. M. 2003. HADDOCK: a protein-protein docking approach based on biochemical or biophysical information. J. Am. Chem. Soc. 125:1731–1737 [DOI] [PubMed] [Google Scholar]
- 24. Fanning E., Klimovich V., Nager A. R. 2006. A dynamic model for replication protein A (RPA) function in DNA processing pathways. Nucleic Acids Res. 34:4126–4137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guo J. Y., et al. 2008. Aven-dependent activation of ATM following DNA damage. Curr. Biol. 18:933–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Henricksen L. A., Umbricht C. B., Wold M. S. 1994. Recombinant replication protein A: expression, complex formation, and functional characterization. J. Biol. Chem. 269:11121–11132 [PubMed] [Google Scholar]
- 27. Hubbard S. J. T., Thornton J. M. 1993. NACCESS, computer program. Department of Biochemistry and Molecular Biology, University College, London [Google Scholar]
- 28. Iftode C., Borowiec J. A. 2000. 5′ → 3′ molecular polarity of human replication protein A (hRPA) binding to pseudo-origin DNA substrates. Biochemistry 39:11970–11981 [DOI] [PubMed] [Google Scholar]
- 29. Jamil S., Mojtabavi S., Hojabrpour P., Cheah S., Duronio V. 2008. An essential role for MCL-1 in ATR-mediated CHK1 phosphorylation. Mol. Biol. Cell 19:3212–3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jamil S., et al. 2005. A proteolytic fragment of Mcl-1 exhibits nuclear localization and regulates cell growth by interaction with Cdk1. Biochem. J. 387:659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jamil S., Stoica C., Hackett T. L., Duronio V. 2010. MCL-1 localizes to sites of DNA damage and regulates DNA damage response. Cell Cycle 9:2843–2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jiang X., et al. 2006. Structural mechanism of RPA loading on DNA during activation of a simple pre-replication complex. EMBO J. 25:5516–5526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kamer I., et al. 2005. Proapoptotic BID is an ATM effector in the DNA-damage response. Cell 122:593–603 [DOI] [PubMed] [Google Scholar]
- 34. Kumagai A., Lee J., Yoo H. Y., Dunphy W. G. 2006. TopBP1 activates the ATR-ATRIP complex. Cell 124:943–955 [DOI] [PubMed] [Google Scholar]
- 35. Li H., Zhu H., Xu C. J., Yuan J. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491–501 [DOI] [PubMed] [Google Scholar]
- 36. Liu Y., Bertram C. C., Shi Q., Zinkel S. S. 2011. Proapoptotic Bid mediates the Atr-directed DNA damage response to replicative stress. Cell Death Differ. 18:841–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu Y., Zhao T. J., Yan Y. B., Zhou H. M. 2005. Increase of soluble expression in Escherichia coli cytoplasm by a protein disulfide isomerase gene fusion system. Protein Expr. Purif. 44:155–161 [DOI] [PubMed] [Google Scholar]
- 38. Luo X., Budihardjo I., Zou H., Slaughter C., Wang X. 1998. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481–490 [DOI] [PubMed] [Google Scholar]
- 39. Maas C., de Vries E., Tait S. W., Borst J. 2011. Bid can mediate a pro-apoptotic response to etoposide and ionizing radiation without cleavage in its unstructured loop and in the absence of p53. Oncogene 30:3636–3647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. MacDougall C. A., Byun T. S., Van C., Yee M. C., Cimprich K. A. 2007. The structural determinants of checkpoint activation. Genes Dev. 21:898–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Matsuoka S., et al. 2007. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 316:1160–1166 [DOI] [PubMed] [Google Scholar]
- 42. Mer G., Bochkarev A., Chazin W. J., Edwards A. M. 2000. Three-dimensional structure and function of replication protein A. Cold Spring Harbor Symp. Quant. Biol. 65:193–200 [DOI] [PubMed] [Google Scholar]
- 43. Mer G., et al. 2000. Structural basis for the recognition of DNA repair proteins UNG2, XPA, and RAD52 by replication factor RPA. Cell 103:449–456 [DOI] [PubMed] [Google Scholar]
- 44. Mordes D. A., Glick G. G., Zhao R., Cortez D. 2008. TopBP1 activates ATR through ATRIP and a PIKK regulatory domain. Genes Dev. 22:1478–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Namiki Y., Zou L. 2006. ATRIP associates with replication protein A-coated ssDNA through multiple interactions. Proc. Natl. Acad. Sci. U. S. A. 103:580–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pawlikowska P., et al. 2010. ATM-dependent expression of IEX-1 controls nuclear accumulation of Mcl-1 and the DNA damage response. Cell Death Differ. 17:1739–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Plesnila N., et al. 2001. BID mediates neuronal cell death after oxygen/ glucose deprivation and focal cerebral ischemia. Proc. Natl. Acad. Sci. U. S. A. 98:15318–15323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pretto D. I., et al. 2010. Structural dynamics and single-stranded DNA binding activity of the three N-terminal domains of the large subunit of replication protein A from small angle X-ray scattering. Biochemistry 49:2880–2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Segurado M., Tercero J. A. 2009. The S-phase checkpoint: targeting the replication fork. Biol. Cell 101:617–627 [DOI] [PubMed] [Google Scholar]
- 50. Upton J. P., et al. 2008. Caspase-2 cleavage of BID is a critical apoptotic signal downstream of endoplasmic reticulum stress. Mol. Cell. Biol. 28:3943–3951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Van C., Yan S., Michael W. M., Waga S., Cimprich K. A. 2010. Continued primer synthesis at stalled replication forks contributes to checkpoint activation. J. Cell Biol. 189:233–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang K., Yin X. M., Chao D. T., Milliman C. L., Korsmeyer S. J. 1996. BID: a novel BH3 domain-only death agonist. Genes Dev. 10:2859–2869 [DOI] [PubMed] [Google Scholar]
- 53. Wang Q., et al. 2008. Bcl2 negatively regulates DNA double-strand-break repair through a nonhomologous end-joining pathway. Mol. Cell 29:488–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xu X., et al. 2008. The basic cleft of RPA70N binds multiple checkpoint proteins, including RAD9, to regulate ATR signaling. Mol. Cell. Biol. 28:7345–7353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yin X. M., et al. 1999. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400:886–891 [DOI] [PubMed] [Google Scholar]
- 56. Yuan J., Ghosal G., Chen J. 2009. The annealing helicase HARP protects stalled replication forks. Genes Dev. 23:2394–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yusufzai T., Kadonaga J. T. 2008. HARP is an ATP-driven annealing helicase. Science 322:748–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yusufzai T., Kong X., Yokomori K., Kadonaga J. T. 2009. The annealing helicase HARP is recruited to DNA repair sites via an interaction with RPA. Genes Dev. 23:2400–2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zinkel S., Gross A., Yang E. 2006. BCL2 family in DNA damage and cell cycle control. Cell Death Differ. 13:1351–1359 [DOI] [PubMed] [Google Scholar]
- 60. Zinkel S. S., Hurov K. E., Gross A. 2007. Bid plays a role in the DNA damage response. Cell 130:9–10(Author's reply, 130:10–11.) [DOI] [PubMed] [Google Scholar]
- 61. Zinkel S. S., et al. 2005. A role for proapoptotic BID in the DNA-damage response. Cell 122:579–591 [DOI] [PubMed] [Google Scholar]
- 62. Zinkel S. S., et al. 2003. Proapoptotic BID is required for myeloid homeostasis and tumor suppression. Genes Dev. 17:229–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zou L., Cortez D., Elledge S. J. 2002. Regulation of ATR substrate selection by Rad17-dependent loading of Rad9 complexes onto chromatin. Genes Dev. 16:198–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zou L., Elledge S. J. 2003. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 300:1542–1548 [DOI] [PubMed] [Google Scholar]