Abstract
While there can be detrimental consequences of nitric oxide production at pathological concentrations, eukaryotic cells have evolved protective mechanisms to defend themselves against this damage. The unfolded-protein response (UPR), activated by misfolded proteins and oxidative stress, is one adaptive mechanism that is employed to protect cells from stress. Nitric oxide is a potent activator of AMP-activated protein kinase (AMPK), and AMPK participates in the cellular defense against nitric oxide-mediated damage in pancreatic β-cells. In this study, the mechanism of AMPK activation by nitric oxide was explored. The known AMPK kinases LKB1, CaMKK, and TAK1 are not required for the activation of AMPK by nitric oxide. Instead, this activation is dependent on the endoplasmic reticulum (ER) stress-activated protein IRE1. Nitric oxide-induced AMPK phosphorylation and subsequent signaling to AMPK substrates, including Raptor, acetyl coenzyme A carboxylase, and PGC-1α, is attenuated in IRE1α-deficient cells. The endoribonuclease activity of IRE1 appears to be required for AMPK activation in response to nitric oxide. In addition to nitric oxide, stimulation of IRE1 endoribonuclease activity with the flavonol quercetin leads to IRE1-dependent AMPK activation. These findings indicate that the RNase activity of IRE1 participates in AMPK activation and subsequent signaling through multiple AMPK-dependent pathways in response to nitrosative stress.
INTRODUCTION
Nitric oxide, an important mediator of both physiological and pathological processes, has been implicated in the development of a number of inflammatory diseases. When produced at low concentrations, nitric oxide can promote cell growth and survival. At high concentrations, such as those produced during inflammation by inducible nitric oxide synthase (iNOS), nitric oxide induces extensive cellular injury that includes DNA damage, inhibition of oxidative metabolism, and induction of endoplasmic reticulum (ER) stress (5, 12, 39). Pancreatic β-cells are exquisitely sensitive to oxidative damage, as glucose-stimulated insulin secretion requires the oxidation of glucose to CO2, resulting in the accumulation of ATP. Nitric oxide, produced in micromolar concentrations in response to interleukin 1 (IL-1) and gamma interferon (IFN-γ), mediates the damaging effects of these cytokines on β-cell function (3, 33). While nitric oxide stimulates cellular damage, it also activates a number of signaling pathways that limit additional cellular damage and repair existing damage. In pancreatic β-cells, the protective responses activated by nitric oxide include (i) JNK-dependent induction of GADD45α (growth arrest and DNA damage-inducible protein 45α) and DNA repair, (ii) activation of AMP-activated protein kinase (AMPK), resulting in enhanced metabolic recovery, and (iii) activation of the unfolded-protein response (UPR) (25, 34, 38, 54, 57, 61).
AMPK is a conserved heterotrimeric (α, β, and γ subunits) serine/threonine kinase involved in sensing and responding to the energetic demand within eukaryotic cells (15). AMPK is activated by phosphorylation at threonine 172 in the catalytic α subunit (19) in a constitutive fashion by the upstream kinase LKB1; however, this phosphorylation is rapidly removed by a phosphatase to maintain low basal activity (18, 43). AMPK is activated under conditions that decrease cellular ATP levels, such as hypoxia, DNA damage, glucose deprivation, and free radical generation (2, 24, 32, 42). This includes nitric oxide-induced activation of AMPK (2). Activation of AMPK from disruption of energy homeostasis is due to the increased AMP/ATP ratio, leading to binding of AMP to the regulatory γ subunit; this binding of AMP causes a conformational change in the AMPK complex that attenuates dephosphorylation (43). The LKB1-dependent activation of AMPK can also be replicated using AMP mimics such as 5-aminoimidazole-4-carboxyamide ribonucleoside (AICAR) (49). While LKB1 is a dominant AMPK kinase, AMPK can also be phosphorylated and activated independent of the cellular energy status. LKB1-independent activation of AMPK can be mediated by the Ca2+-sensitive calmodulin-dependent protein kinase kinase (CaMKK) (20) and TGFβ-activated kinase-1 (TAK1) (35).
AMPK regulates many cellular processes through the phosphorylation of target substrates. The mammalian target of rapamycin complex 1 (mTORC1) is a multisubunit kinase composed of at least mTOR, FKBP12, mLST8, and Raptor that is negatively regulated by AMPK. Under favorable growth conditions, mTORC1 is active and promotes protein synthesis through an inhibitory phosphorylation of the negative regulator 4E-binding proteins and through an activating phosphorylation of p70 ribosomal S6 kinase 1 (S6K1) (4). Raptor acts as a scaffold to recruit these substrates to the mTOR complex (36, 47). In response to cellular stress, AMPK inhibits mTORC1 signaling, in part through the phosphorylation of Raptor, leading to the dephosphorylation and inactivation of S6K1 (13).
Nitric oxide can cause ER stress and activate the highly conserved UPR (38). The UPR includes three trans-ER membrane proteins—activating transcription factor 6 (ATF6), eukaryotic translation initiation factor 2-alpha kinase 3 (PERK), and inositol-requiring enzyme 1 (IRE1)—which transmit signals from the ER lumen to the cytosol and nucleus (40). ATF6 is a transcription factor that is released from the ER by proteolytic cleavage and translocates to the nucleus to stimulate the expression of UPR-associated genes (51, 59). PERK is a serine/threonine kinase that phosphorylates eukaryotic translation initiation factor 2α (eIF2α) under ER stress conditions. This response attenuates protein synthesis in an effort to reduce the protein burden on the ER (17). IRE1 is both a kinase and an endoribonuclease. In response to ER stress, IRE1 is activated by dimerization and transautophosphorylation and splices the mRNA of XBP1 (6). Active IRE1 can also form a complex with the adaptor protein TRAF2, facilitating the activation of apoptosis signaling kinase 1 (ASK1) and subsequent activation of JNK, thus coupling ER stress to MAPK signaling (23, 50).
We recently reported that IL-1 stimulates AMPK activation in pancreatic islets in a nitric oxide-dependent fashion (34); however, the mechanisms of this activation are currently unknown. The purpose of this study was to identify the mechanisms by which nitric oxide activates AMPK. We show that kinases known to activate AMPK are dispensable for AMPK activation in response to nitric oxide. In contrast, a novel signaling role for the UPR transducer IRE1α in the activation of AMPK in pancreatic β-cells in response to nitric oxide has been identified.
MATERIALS AND METHODS
Materials.
INS832/13 cells were obtained from Chris Newgard (Duke University, Durham, NC), IRE1α-deficient mouse embryo fibroblasts (MEFs) were provided by Fumihiko Urano (University of Massachusetts Medical School), PERK-deficient MEFs were provided by Ronald Wek (University of Indiana), and INS1 cells stably expressing wild-type, kinase-deficient (K599A), or RNase-deficient (K907A) IRE1α mutants under the control of a tetracycline (Tet)-inducible promoter were provided by Feroz Papa (University of California, San Francisco, CA). Plasmids containing inactive mutants of IRE1α and INS832/13 cells expressing RNase-deficient (K907A) and kinase-deficient (K599A) IRE1α have been described previously (37). Dominant negative AMPK adenovirus was provided by Christopher Rhodes (University of Chicago). RPMI 1640 and Dulbecco's modified Eagle medium (DMEM) tissue culture medium, l-glutamine, streptomycin, and penicillin were from Mediatech, Inc. (Manassas, VA). Fetal calf serum and quercetin were from Sigma (St. Louis, MO). (Z)-1(N,N-Diethylamino)diazen-1-ium-1,2-diolate (DEANO), (Z)-1-[N-(3-ammoniopropyl)-N-(n-propyl)amino]diazen-1-ium-1,2-diolate (PAPA NONOate, referred to here as PAPA), thapsigargin, and tunicamycin were purchased from Axxora (San Diego, CA). Antibodies used in this study include those specific for phospho-Thr172-AMPK, phospho-Ser79-ACC, phospho-Ser473-Akt, phospho-Thr308-Akt, phospho-Ser51-eIF2α, total AMPK, phospho-RAPTOR, phospho-p70S6K1, phospho-PERK, IRE1, TAK1, LKB1 (Cell Signaling, Danvers, MA), and GAPDH (Ambion, Foster City, CA). Horseradish peroxidase-conjugated donkey anti-rabbit and donkey anti-mouse immunoglobulins were from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). Predesigned Silencer Select small interfering RNAs (siRNAs) were purchased from Ambion (Austin, TX).
Cell culture and treatments.
INS832/13 cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 1 mM sodium pyruvate, 55 μM β-mercaptoethanol, 10 mM HEPES, 100 U/ml penicillin, and 100 μg/ml streptomycin. MEFs were cultured in DMEM supplemented with 10% FBS, 2 mM glutamine, 10 mM HEPES, 100 U/ml penicillin and 100 μg/ml streptomycin. Cells were maintained at 37°C under an atmosphere of 95% air and 5% CO2. For cells treated with nitric oxide donors or AICAR, a stock solution of 10 mM or 50 mM, respectively, was prepared in the appropriate medium immediately before addition to the cells. Mutants of IRE1α were transiently transfected into INS832/13 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Stable Tet-inducible INS-1 cell lines expressing either wild-type (WT) or RNase-deficient (K907A) IRE1α have been described (14). IRE1α expression was induced by the addition of 1 μg/ml doxycycline followed by overnight culture. Treatments were performed 12 to 16 h after induction. Adenoviral transduction was performed as described previously (34).
RNA interference.
Cells were reverse transfected with siRNA and Lipofectamine 2000 in 6-well plates according to the manufacturer's protocol: all transfections contained 100 pmol siRNA. To knock down AMPKα, both isoforms were targeted and cells were cotransfected with 50 pmol of each siRNA. The siRNAs (Silencer Select; Ambion) used were LKB1#1 (S163339), LKB1#2 (S163340), TAK1 (S162205), AMPKα1#1 (S134808), AMPKα2#2 (S134963), IRE1α (S176364), and PKCζ (S130022).
XPB1 splicing.
Total RNA was isolated using an RNeasy kit (Qiagen, Valencia, CA) or TRIzol reagent (Invitrogen). RNA (1 μg) was reverse transcribed to cDNA using oligo(dT) and Moloney murine leukemia virus reverse transcriptase. cDNA was analyzed by quantitative real-time PCR (qRT-PCR) using a Light Cycler 480 (Roche) and primers spanning the splice junctions of XBP-1 (6a). Data were normalized to β-actin. XBP-1 splicing was also analyzed by RT-PCR using following primers: XBP-1 forward, ACACGCTTGGGAATGGACAC; XBP-1 reverse, CCATGGGAAGATGTTCTGGG. PCR products were resolved on a 3% agarose gel stained with ethidium bromide. Quantitative PCR of PGC-1α was performed using a TaqMan gene expression assay according to the manufacturer's instructions in an ABI Prism 7500 analyzer (Applied Biosystems, Foster City, CA). Reactions were carried out in 20 μl and analyzed using the ΔΔCT method.
Immunoblotting.
Cells were washed twice with phosphate-buffered saline (PBS) and then lysed with immunoprecipitation lysis buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 0.5% NP-40, 1 mM sodium orthovanadate, 100 μM phenylmethanesulfonyl fluoride, 50 mM sodium fluoride, and protease inhibitor cocktail; Sigma, St. Louis, MO). The lysates were sonicated and centrifuged at 20,800 × g for 15 min. Protein concentrations were determined by a Bradford assay. Samples were mixed with Laemmli sample buffer (2% sodium dodecyl sulfate [SDS]) and placed in a boiling water bath for 5 min. Proteins were resolved in SDS-polyacrylamide gels and transferred to nitrocellulose, and the membranes were incubated with primary antibody overnight at 4°C. Primary antibodies were used at a 1:1,000 dilution except the GAPDH antibody, which was used at a 1:25,000 dilution. Incubations with horseradish peroxidase-conjugated donkey anti-mouse, donkey anti-rabbit, or donkey anti-rat IgG (1:7,000 dilution) secondary antibodies were performed for 1 h at room temperature, followed by detection with enhanced chemiluminescence.
Aconitase activity.
INS832/13 cells were isolated by centrifugation, and mitochondrial aconitase activity was determined as described previously (34, 45). Briefly, aconitase was assayed at 340 nm in a reaction mixture containing 20 mM citrate, 0.2 mM NADP, 6 mM MnCl2, 50 mM Tris-Cl (pH 7.4), 0.6 U of isocitrate dehydrogenase, and 50 μl of cell extract in a total volume of 200 μl at room temperature. Aconitase activity was quantified as 1 pmol of NADP (reduced) formed per minute per microgram of protein.
Statistical analysis.
Statistical analysis was performed using Student's t test or Student Newman-Keuls post hoc analysis of variance (ANOVA).
RESULTS
Nitric oxide induces AMPK activation and inhibition of mTORC1 signaling.
Treatment of insulinoma INS832/13 cells with the nitric oxide donor (Z)-1(N,N-diethylamino)diazen-1-ium-1,2-diolate (DEANO) for 10 to 60 min results in the rapid activation of AMPK, as measured by the activation-associated phosphorylation of AMPK at threonine 172 and the subsequent phosphorylation of the AMPK substrates acetyl-coenzyme A carboxylase (ACC) and Raptor (Fig. 1A). To verify the regulatory role of AMPK in mTOR signaling, INS832/13 cells were treated with the AMPK activator, AICAR. AICAR enhanced the phosphorylation of AMPK and the AMPK-mediated phosphorylation of Raptor (Fig. 1B). These effects were associated with a concomitant decrease in the phosphorylation of the mTORC1 substrate S6K1. Recent studies have shown that S6K1 phosphorylates the scaffold protein Rictor in the rapamycin-insensitive mTOR complex (TORC2), leading to attenuation of TORC2 substrate phosphorylation (28). One substrate of TORC2 is Akt, which is phosphorylated at serine 473 (44). Consistent with the decrease in S6K1 phosphorylation observed in response to AICAR, there is an increase in the phosphorylation of Akt at serine 473 and threonine 308 (Fig. 1B).
Fig. 1.
Nitric oxide activates AMPK and modifies mTOR signaling. (A) INS832/13 cells were treated with the nitric oxide donor DEANO for 0 to 60 min (B) or the indicated concentrations of AICAR for 1 h. (C) INS832/13 cells were mock transfected or transfected with siRNA for AMPKα1 and AMPKα2 (together) for 48 h followed by treatment with DEANO (1 mM) for the indicated times. (D) INS832/13 cells were transduced with an adenoviral dominant negative AMPK using a multiplicity of infection (MOI) of 50 to 200 for 24 h followed by treatment with 1 mM DEANO for 30 min. Following treatments, the cells were harvested, and lysates were separated by SDS-gel electrophoresis followed by Western blot analysis using the antibodies specific for the indicated target proteins. Results are representative of three independent experiments.
The simultaneous siRNA knockdown of both isoforms (α1 and α2) of the catalytic subunit of AMPKα was used to confirm that under basal (unstimulated) conditions AMPK regulates S6K1 and Akt. As expected, AMPK knockdown increased basal S6K1 phosphorylation and decreased Akt phosphorylation (Fig. 1C, lanes 1 and 2). Under these conditions, the modest level of AMPK knockdown (∼50%) was not sufficient to attenuate the stimulatory effect of nitric oxide on the phosphorylation of Raptor or the subsequent inhibition of S6K1 phosphorylation (Fig. 1C). These findings suggest either that AMPK is present in such a large excess that even a 50% decrease in protein is not sufficient to alter substrate phosphorylation under stimulated conditions or that nitric oxide-induced phosphorylation of Raptor is AMPK independent. To examine these possibilities, INS832/13 cells were transduced with adenovirus expressing a dominant negative mutant of AMPK (DN-AMPK) that has been shown to effectively inhibit AMPK in INS832/13 cells (34). As shown in Fig. 1D, the stimulatory effects of nitric oxide on Raptor phosphorylation are attenuated in cells expressing DN-AMPK. These findings suggest that nitric oxide-induced Raptor phosphorylation is mediated by AMPK.
The mechanism controlling Akt phosphorylation in response to nitric oxide appears to differ from activation induced by pharmacological activation of AMPK. In response to AICAR, the phosphorylation of Akt at 473 and 308 is enhanced (Fig. 1B); however, nitric oxide treatment results in attenuation in the phosphorylation of Akt (Fig. 1C). These findings, which suggest that the regulation of Akt phosphorylation in response to nitric oxide is independent of mTOR, are consistent with previous reports showing that Akt is a direct target for inhibitory S-nitrosation by nitric oxide (58). Overall, these findings confirm that nitric oxide activates AMPK (2, 34, 61) and that AMPK activation correlates with Raptor phosphorylation and the inhibition of mTORC1 signaling (13). These studies also suggest that nitric oxide may alter mTOR signaling in part through AMPK activation.
Nitric oxide-induced AMPK activation is independent of known AMPK kinases.
LKB1, CaMKK, and TAK1 have been shown to function as activators of AMPK (18, 20, 35). LKB1 is a primary kinase responsible for the activating phosphorylation of AMPK (18, 49, 56). To determine if LKB1 is responsible for nitric oxide-stimulated AMPK activation, two distinct siRNAs were used to knock down endogenous LKB1 in INS832/13 cells. These siRNAs reduce steady-state levels of LKB1 protein, and this is associated with attenuation of H2O2-induced AMPK phosphorylation (Fig. 2A). These findings are consistent with previous reports showing that H2O2-induced AMPK activation is LKB1 dependent (49). In contrast, LKB1 knockdown does not influence DEANO-induced AMPK phosphorylation (Fig. 2A). These data suggest that nitric oxide-induced AMPK activation could be independent of LKB1.
Fig. 2.
LKB1, CaMKK, and TAK1 are not essential for nitric oxide-induced activation of AMPK. (A) INS832/13 cells were mock transfected or transfected with two distinct siRNAs for LKB1 for 48 h followed by treatment with 100 μM H2O2 or 1 mM DEANO for 30 min. (B) INS832/13 cells were pretreated for 30 min with 1 to 10 μM concentrations of the CaMKK inhibitor STO-609 followed by treatment with 1 mM DEANO for 30 min. (C) INS832/13 cells were mock transfected or transfected with siRNA for TAK1 for 48 h followed by treatment with 1 mM DEANO for 30 min. (D) INS832/13 cells were transfected with siRNA for LKB1 and/or TAK1 for 48 h or pretreated for 30 min with 10 μM STO-609 as indicated. Cells were then treated with 1 mM DEANO for 30 min, and target protein levels were analyzed by Western blot analysis. (E) Summary of the effects of target molecule inhibition on DEANO-induced AMPK phosphorylation (*, 30 μM PKCζ attenuated AMPK phosphorylation but was toxic to cells, and the loss of AMPK phosphorylation was not confirmed by siRNA for PKCζ). Results are representative of three independent experiments.
In endothelial cells, low concentrations of nitric oxide have been shown to stimulate AMPK activation by a pathway dependent on soluble guanylate cyclase (sGC) and CaMKK (61). To determine if this pathway mediates the activation of AMPK with high levels of nitric oxide, INS832/13 cells were treated with DEANO in the absence or presence of the CaMKK inhibitor STO-609. Inhibition of CaMKK did not influence DEANO-induced activation of AMPK, although basal levels of AMPK phosphorylation were reduced, indicating that the inhibitor was effective (Fig. 2B). Similarly, the sGC inhibitor ODQ did not influence DEANO-induced AMPK phosphorylation (data not shown), nor did the Ca2+ chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrabis(acetoxymethyl ester) (BAPTA-AM) (Fig. 2E). These results suggest that sGC and CaMKK are not involved in the activation of AMPK by nitric oxide in β-cells. However, the ability of STO-609 to attenuate basal AMPK phosphorylation suggests that CaMKK may function as a regulator of basal AMPK activity.
TAK1 has also been reported to phosphorylate AMPK (35) and to influence mTOR signaling through AMPK (22). siRNA knockdown was used to examine the potential role of TAK1 in nitric oxide-induced AMPK phosphorylation. Targeted siRNA effectively reduced TAK1 expression but did not prevent DEANO-induced phosphorylation of AMPK (Fig. 2C). Similar results were obtained using a second distinct siRNA specific to TAK1 (not shown). These findings indicate that TAK1, much like LKB1 and CaMKK, is not responsible for nitric oxide-induced phosphorylation of AMPK. We also explored the possibility that these AMPK kinases function in a compensatory fashion by examining the effects of simultaneous knockdown of LKB1 and TAK1 and inhibition of CaMKK on nitric oxide-induced AMPK and Raptor phosphorylation. The presence of a combination of siRNA against LKB1 and TAK1 and the CaMKK inhibitor STO-609 did not prevent nitric oxide-stimulated AMPK or Raptor phosphorylation (Fig. 2D). These findings indicate that it is unlikely for compensatory AMPK activation by these known AMPK activators to be involved in the regulation of AMPK by nitric oxide. A number of additional potential target molecules were examined in our effort to identify the mechanism of nitric oxide-induced AMPK phosphorylation. The findings from these studies are summarized in Fig. 2E. The sum total of these studies suggests that nitric oxide activates AMPK by a pathway that is independent of known AMPK kinases.
Nitric oxide-induced AMPK activation is IRE1 dependent.
Nitric oxide is known to cause ER stress and activation of the UPR in a number of cell types, including β-cells (12, 38, 53). Consistent with these previous studies, DEANO treatment of INS832/13 cells for 10 to 60 min results in increased phosphorylation of PERK and its substrate eIF2α (Fig. 3A). In response to nitric oxide, UPR activation temporally parallels AMPK activation and the inhibition of mTOR signaling (Fig. 1A). These data raise the possibility of regulatory cross talk between the UPR and AMPK/mTOR signaling pathways in response to nitric oxide.
Fig. 3.
IRE1 participates in the activation of AMPK by nitric oxide. (A) INS832/13 cells were treated with 1 mM DEANO for the indicated times or left untreated, followed by Western blot analysis for phosphorylated PERK and eIF2α. (B) INS832/13 were mock transfected (−) or transfected with siRNA for IRE1α for 48 h, followed by treatment with the indicated concentrations of DEANO for 30 min. The cells were harvested, and AMPK phosphorylation and total IRE1α and AMPK levels were determined by Western blot analysis. (C) The effects of a 30-min treatment with 1 mM DEANO on XBP1 splicing in INS832/13 cells transfected with IRE1α siRNA are shown. Splicing was analyzed by qRT-PCR. (D) INS832/13 cells, mock transfected or transfected with IRE1α siRNA as for panel B, were treated with the indicated concentrations of AICAR for 30 min. The cells were harvested, and levels of phosphorylated AMPK and ACC and total levels of AMPK and IRE1α were determined by Western blot analysis. Results are representative of two or three individual experiments.
Considering the temporal correlation of AMPK and UPR activation in response to nitric oxide, the potential role(s) of the endoribonuclease/kinase IRE1α and the eIF2α kinase PERK in regulating the response of AMPK to nitric oxide was examined. AMPK phosphorylation in response to nitric oxide (DEANO at concentrations from 0.1 to 1 mM) is attenuated in INS832/13 cells depleted of IRE1α by siRNA knockdown (Fig. 3B). Under these conditions, the activity of IRE1α is impaired as the stimulatory effects of nitric oxide on the splicing of XBP1 are attenuated in INS832/13 cells transfected with IRE1α siRNA (Fig. 3C). Importantly, the role of IRE1α in the regulation of AMPK appears to be selective for nitric oxide. IRE1α siRNA knockdown does not influence AICAR-induced AMPK phosphorylation or AMPK-mediated ACC phosphorylation (Fig. 3D). The reduction in nitric oxide-induced AMPK phosphorylation observed in siRNA-treated IRE1α-deficient cells was approximately 40%. While this level of reduction is consistent with a partial knockdown of IRE1α, it is also possible that pathways in addition to IRE1α contribute to AMPK activation in response to nitric oxide. Further, the results presented in Fig. 1 indicate that AMPK is present in excess, and large changes in activity or level are needed to have discernible affects on downstream signaling. Consistent with these findings, the 40% reduction in AMPK phosphorylation in IRE1α knockdown cells (as shown in Fig. 3B) did not alter the phosphorylation of AMPK substrates such as ACC and Raptor in response to nitric oxide (data not shown).
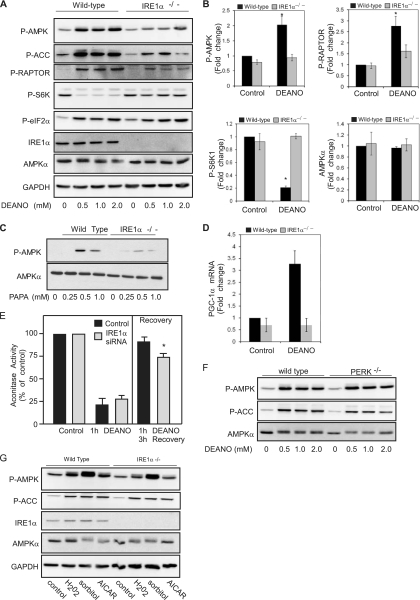
MEFs deficient in IRE1α were used to confirm that IRE1α participates in AMPK activation in response to nitric oxide. Like β-cells, MEFs respond to DEANO with an increase in the phosphorylation of AMPK and Raptor (Fig. 4A). Compared to wild-type cells, there is a marked reduction of nitric oxide-induced AMPK phosphorylation in IRE1α−/− MEFs. At 0.5 mM, DEANO induces maximal AMPK phosphorylation, and there is little further increase in AMPK phosphorylation at concentrations up to 2 mM (Fig. 4A). In IRE1α-deficient MEFs, DEANO-induced AMPK phosphorylation is attenuated at all concentrations examined. Consistent with an inhibition in AMPK activation, DEANO-stimulated phosphorylation of ACC and Raptor is also attenuated in IRE1α−/− cells compared to wild type (Fig. 4A). In concert with Raptor-dependent attenuation of mTORC1 signaling, the phosphorylation of S6K1 was significantly reduced in wild-type MEFs; however, it remained near basal levels in IRE1α−/− cells treated with DEANO (Fig. 4A and B). Even though AMPK activation is attenuated, IRE1α−/− cells are responsive to nitric oxide, as DEANO stimulates the phosphorylation of eIF2α to similar levels in wild-type and IRE1α-deficient MEFs (Fig. 4A). As an additional control, we show that the total levels of AMPKα are indistinguishable in the wild-type and IRE1α−/− cells (Fig. 4A and B), confirming that the defects in AMPK signaling are not associated with reduced levels of this energy sensor. The regulation of AMPK by nitric oxide was confirmed using a second donor, PAPA, which also stimulates the IRE1-dependent phosphorylation of AMPK in MEFs (Fig. 4C).
Fig. 4.
Nitric oxide-induced AMPK activation and signaling is attenuated in IRE1-deficient cells. (A) Wild-type and IREα−/− MEFs were treated with the indicated concentrations of DEANO for 10 min, and the phosphorylation of AMPK, ACC, Raptor, S6K, and eIF2α was determined by Western blot analysis. Total levels of IRE1α confirm its absence in the deficient MEFs, and total levels of AMPK and GAPDH are shown as loading controls. (B) The phosphorylated forms of AMPK, Raptor, and S6K1 and total AMPK in wild-type and IRE1α-deficient MEFs treated with 1 mM DEANO for 10 min were quantified. (C) Wild-type and IREα−/− cells were treated with the indicated concentrations of PAPA for 15 min, and levels of phosphorylated AMPK and total AMPK were determined by Western blot analysis. (D) Wild type and IREα−/− cells were treated with 1 mM DEANO for 3 h and PGC-1α mRNA accumulation was determined by qRT-PCR. (E) INS832/13 cells were transfected with scrambled (control) or IRE1α siRNA for 24 h and then either treated for 1 h with DEANO or treated for 1 h with DEANO, washed, and cultured for 3 additional hours. The cells were isolated, and mitochondrial aconitase activity was measured. (F) Wild-type or PERK−/− MEFs were treated with the indicated concentrations of DEANO for 10 min, and levels of phosphorylated AMPK and ACC and total AMPK were determined by Western blot analysis. (G) Wild-type and IREα−/− MEFs were treated with the AMPK activator H2O2 (100 μM) or sorbitol (0.6 M) for 30 min or AICAR (2 mM) for 2 h. The MEFs were harvested, and levels of phosphorylated AMPK and ACC and total levels of IRE1α, AMPK, and GAPDH were determined by Western blot analysis. Data are means ± standard errors of the means (SEM) (B, D, and E; n = 3 to 4; *, P < 0.05) or are representative of three independent experiments (A, C, F, and G).
We also examined whether AMPK-dependent gene expression is negatively affected by loss of IRE1α. PGC-1α expression is increased in response to nitric oxide in an AMPK-dependent fashion (30). Following exposure to exogenous nitric oxide, PGC-1α is increased in wild-type (WT) MEFs, while nitric oxide-induced expression of PGC-1α is absent in IRE1α−/− cells (Fig. 4D). These data indicate that IRE1 participates in the regulation of nitric oxide-induced AMPK activation and subsequent signaling to targets, such as PGC-1α, that participate in regulation of mitochondrial function and biogenesis. Since AMPK participates in the recovery of mitochondrial function following nitric oxide-mediated damage (34), the effects of IRE1 depletion on the recovery of mitochondrial aconitase activity were evaluated. INS832/13 or INS832/13 cells depleted of IRE1 using siRNA were treated for 1 h with DEANO or were treated for 1 h with DEANO, washed, and then cultured for 3 additional hours, at which time mitochondrial aconitase activity was examined. One hour of incubation with DEANO results in ∼75% reduction in aconitase activity (Fig. 4E). Removal of the nitric oxide donor by washing and continued culture for 3 h results in a nearly complete recovery of aconitase activity in control cells, while the recovery of aconitase activity is attenuated (by 20%) in cells deficient in IRE1α (siRNA knockdown resulted in a 70% loss of IRE1, as determined by Western blot analysis [data not shown]). The depletion of IRE1 attenuates the recovery of aconitase activity (Fig. 4E) to levels similar to those observed under conditions in which AMPK is inhibited (34). These findings indicate that the stimulatory effects of nitric oxide on PGC-1α expression and the recovery of mitochondrial aconitase activity following nitric oxide-induced damage are regulated, at least in part, by the IRE-1α-dependent activation of AMPK.
To determine if IRE1 deficiency causes a general defect in signaling to AMPK, the effects of activators (H2O2 and sorbitol) that are known to enhance AMPK activity in an LKB1-dependent fashion (49) were examined. The expression of LKB1 (data not shown) and the phosphorylation of AMPK and its downstream substrate ACC are similar in wild-type and IRE1α−/− cells treated with H2O2 (change [fold] between phosphorylated AMPK and AMPK, 1.86 ± 0.25 in WT versus 2.5 ± 0.76 in IRE1−/− cells; n = 3; not significant [NS]) and sorbitol (change [fold] between phosphorylated AMPK and AMPK, 5.2 ± 1.3 in WT versus 6.1 ± 3.3 in IRE1−/− cells; n = 3; NS). For these studies, the AMPK activator AICAR was used as a positive control (Fig. 4G). To explore the potential influence of additional UPR transducers, AMPK activation in response to nitric oxide was also examined using PERK-deficient MEFs. PERK−/− and wild-type MEFs respond to DEANO in a similar manner, with an increase in the phosphorylation of AMPK and ACC (Fig. 4F), suggesting that PERK does not influence AMPK activation in response to nitric oxide. Together, these data support the identification of a novel role for IRE1α in a pathway leading to the activation of AMPK in response to nitric oxide. This pathway now provides a mechanistic association of one component of the UPR with the mTOR signaling pathway and induction of PGC-1α expression in response to nitric oxide.
ER stress alone is insufficient to activate AMPK.
To determine if IRE1α-dependent activation of AMPK is a general feature of ER stress, the phosphorylation of AMPK, Raptor, and eIF2α was examined in wild-type MEFs and INS832/13 cells treated with UPR activators. While the glycosylation inhibitor tunicamycin and nitric oxide both stimulate ER stress, as indicated by increased XBP-1 splicing (Fig. 5A) and phosphorylation of eIF2α (Fig. 5B), tunicamycin does not increase the phosphorylation of AMPK or Raptor (Fig. 5B). In contrast, DEANO stimulates AMPK and Raptor phosphorylation in wild-type MEFs (Fig. 5B). As with MEFs, treatment of INS832/13 cells with the Ca2+ ATPase inhibitor thapsigargin, or the reducing agent dithiothreitol (DTT), results in the induction of ER stress, as evidenced by the increased phosphorylation of eIF2α. Under these conditions, thapsigargin and DTT failed to increase AMPK phosphorylation (Fig. 5C). In contrast, DEANO stimulates both AMPK and eIF2α phosphorylation following a 30-min incubation (Fig. 5C). These data suggest that the presence of IRE1 is required for nitric oxide-induced AMPK activation; however, the induction of general ER stress using classical activators is not sufficient to induce AMPK phosphorylation.
Fig. 5.
IRE1α RNase but not ER stress activates AMPK. (A) Wild-type MEFs were treated with tunicamycin (5 μM) or DEANO (1 mM) for the indicated times, and XBP-1 splicing was measured by RT-PCR. (B) Wild-type MEFs were treated with 5 μM tunicamycin for the indicated times or left untreated, and phosphorylated AMPK, Raptor, and eIF2α were examined by Western blot analysis. Wild-type MEFs treated with DEANO were used as a positive control. The asterisk indicates a variable cross-reactive band. (C) INS832/13 cells were treated with thapsigargin (1 μM) or DTT (2 mM) followed by examination of eIF2α phosphorylation; DEANO was used as a positive control. (D and E) INS832/13 cells transfected with wild-type IRE1α or with IRE1α-K599A (kinase deficient) or IRE1α-K907A (RNase deficient) mutants were treated with DEANO (1 mM) for 30 min, and AMPK phosphorylation was examined by Western blot. (F) Stable INS1 cell lines were treated for 16 h with doxycycline (1 μg/ml) to induce WT or K907A-IRE1α-myc, followed by treatment with IL-1β (10 U/ml) for 16 h. The level of phosphorylated AMPK was quantified. Data are means ± SEM; n = 3; *, P < 0.05 versus WT control.
The RNase activity of IRE1α is required for nitric oxide-induced AMPK activation.
Mutations in the kinase and RNase domains of IRE1α were used to determine the mechanisms by which IRE1α regulates AMPK. INS832/13 cells expressing WT IRE1α, IRE1α-K599A (kinase deficient), or IRE1α-K907A (RNase deficient) were treated with nitric oxide for 30 min, and AMPK activation was examined. Mutation of the kinase activity of IRE1α did not prevent but rather enhanced nitric oxide-induced AMPK phosphorylation (Fig. 5D and E). In contrast, mutation of the RNase activity of IRE1α significantly attenuated nitric oxide-induced AMPK phosphorylation (Fig. 5D and E). IL-1β induces nitric oxide-dependent activation of AMPK (34). Consistent with the previous results, mutation of the RNase activity of IRE1α significantly attenuated IL-1β-induced AMPK phosphorylation (Fig. 5F). These data suggest that exogenously supplied and endogenously produced nitric oxide stimulate the IRE1α RNase-dependent activation of AMPK.
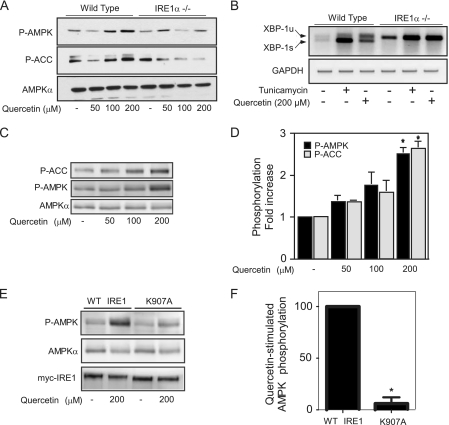
Recently, flavonols such as quercetin have been identified as IRE1 ligands that, in addition to other biological actions, stimulate IRE1 RNase activity independent of kinase activation (55). Additionally, quercetin has been shown to activate AMPK (1, 21). Therefore, to independently verify that IRE1α RNase activation stimulates AMPK phosphorylation, the effects of quercetin on AMPK phosphorylation were examined. While the phosphorylation of AMPK and ACC is stimulated in a concentration-dependent manner by quercetin in WT MEFs (Fig. 6A), this stimulatory effect is attenuated in IRE1α-deficient cells (Fig. 6A). The effects of tunicamycin and quercetin on IRE1α-dependent splicing of XBP1 were used to confirm that quercetin stimulates IRE1 RNase activation (Fig. 6B). As with the MEFs, quercetin stimulated the concentration-dependent phosphorylation of AMPK and ACC in INS832/13 cells (Fig. 6C and D). To confirm that quercetin stimulates IRE1α RNase-dependent activation of AMPK, INS1 cells stably expressing a Tet-inducible WT or RNase-deficient mutant (K907A IRE1α-myc) were examined. Quercetin stimulated AMPK phosphorylation in cells expressing WT IRE1α-myc, and this phosphorylation was significantly attenuated in cells expressing the RNase-deficient mutant K907A-IRE1α-myc (Fig. 6E and F). These data indicate that nitric oxide and the flavonol quercetin stimulate AMPK activation through IRE1α RNase activation.
Fig. 6.
Quercetin stimulates IRE1α RNase-dependent AMPK activation. (A) Wild-type and IREα−/− MEFs were treated with the indicated concentrations of quercetin, and phosphorylated AMPK and ACC were measured by Western blotting. (B) Wild-type and IREα−/− MEFs were treated with tunicamycin (5 μM) or quercetin (200 μM) for 2 h, and XBP-1 splicing was measured by RT-PCR. XBP-1u and XBP-1s indicate unspliced and spliced XBP-1, respectively. (C and D) INS832/13 cells were treated with the indicated concentrations of quercetin, and phosphorylated AMPK and ACC were measured by Western blotting. (E and F) Stable INS1 cell lines were treated for 16 h with doxycycline (1 μg/ml) to induce WT or K907A-IRE1α-myc, followed by treatment with quercetin for 1 h. The levels of phosphorylated AMPK and total AMPK and IRE1α-myc were examined. Data are means ± SEM (D and F; n = 3; *, P < 0.05 versus control) or are representative of three independent experiments (A, B, C, and E).
DISCUSSION
Nitric oxide, produced in response to inflammatory cytokines or supplied exogenously, causes widespread cellular damage. Nonetheless, β cells can survive this onslaught and repair the damage if nitric oxide is removed from the cells before a critical threshold of damage is reached (8, 25, 45). The mechanisms and participants responsible for this recovery process are only beginning to be elucidated. We showed previously that AMPK is transiently activated in a nitric oxide-dependent fashion in response to IL-1β or by the exogenous addition of nitric oxide using donors, and this activation is associated with improved metabolic function and an attenuation of β-cell death following nitric oxide treatment (34). This is particularly important for β cells because proper metabolic function (oxidation of glucose and production of ATP) is essential for glucose-stimulated insulin secretion. While these previous studies outlined an important protective action of AMPK, the mechanisms responsible for nitric oxide-mediated AMPK activation in β cells were unknown.
To explore the mechanisms by which nitric oxide modifies AMPK signaling, we initially evaluated the effects of inhibitors and siRNA knockdown for each of the known AMPK kinases, LKB1, CaMKK, and TAK1. In all cases, inhibition or siRNA knockdown did not inhibit nitric oxide-stimulated AMPK activation, suggesting that each of these known AMPK kinases is dispensable (Fig. 2) for the stimulatory actions of nitric oxide on AMPK. Nitric oxide has also been shown to activate ER stress responses in β cells, and the temporal nature of this activation correlates with the ability of nitric oxide to activate AMPK. Specifically, nitric oxide stimulation of AMPK phosphorylation correlates temporally with PERK and eIF2α phosphorylation, suggesting that ER stress may influence the activation of AMPK in response to nitric oxide. In examining this hypothesis, we made the unexpected observation that the IRE1 pathway regulates AMPK activation in response to nitric oxide. This signaling through AMPK leads to IRE1-dependent regulation of mTOR. Interestingly, this appears to be selective for nitric oxide, as cells lacking IRE1 respond normally to LKB1-mediated activation of AMPK (e.g., H2O2) (Fig. 4G). PERK, an eIF2α kinase, does not participate in the regulation of AMPK in response to nitric oxide, nor does the general induction of ER stress using classical activators tunicamycin, DTT, or thapsigargin (Fig. 5). These findings suggest a novel signaling role for IRE1 in the activation of AMPK in response to nitric oxide (Fig. 7). This signaling pathway is selective for nitric oxide, as classical activators of ER stress do not stimulate AMPK activation, and IRE1 deficiency does not lead to a general loss of AMPK activation (Fig. 7).
Fig. 7.
Schematic diagram of nitric oxide-activated AMPK activation in β cells. Nitric oxide induces IRE1-dependent phosphorylation of AMPK and subsequent AMPK-dependent phosphorylation of Raptor and ACC. The AMPK signaling node connects IRE1 to mTORC1 in response to nitric oxide (black arrows) and is independent of the currently known AMPK kinases (gray arrows, indicating that they do not participate in this pathway). The direct effects of nitric oxide on IRE1 are currently unknown.
Downstream of the IRE1-dependent activation of AMPK is the attenuation of mTOR signaling through the phosphorylation of Raptor. The ability of nitric oxide to modify the phosphorylation status of Raptor is mediated by AMPK, as adenovirus-mediated expression of a dominant negative mutant of AMPK attenuates nitric oxide-induced Raptor phosphorylation (Fig. 1D), consistent with previous findings (13). In response to nitric oxide, AMPK, in an IRE1-dependent fashion, inhibits mTORC1 signaling, in part, through the phosphorylation of Raptor leading to the dephosphorylation and inactivation of S6K1. These results are consistent with the effects of AMPK activation and inhibition of mTORC1 signaling, in part through the phosphorylation of Raptor, leading to the dephosphorylation and inactivation of S6K1 in response to cell stress (13). While we have focused on delineating the mechanism of AMPK activation in response to nitric oxide and the downstream influence on mTOR signaling in the context of Raptor, this is only one aspect of a complex signaling network altered by nitric oxide that influences mTOR signaling. In Fig. 1C, we show that nitric oxide reduces the activation-associated phosphorylation of Akt. When active, Akt inhibits the negative regulator tuberous sclerosis complex 2 (TSC2), and the inhibition of TSC2 promotes the activation of mTOR (26, 31). Thus, the ability of nitric oxide to remove the positive input from Akt would serve to further reduce mTOR activity. Additionally, Akt is a negative regulator, through inhibitory phosphorylation, of glycogen synthase kinase 3 (GSK3) (9). GSK3 also phosphorylates TSC2, and this serves to inhibit mTOR; however, this requires a priming phosphorylation by AMPK on TSC2 (27). Therefore, following exposure to nitric oxide, IRE1 may also signal to TSC2 through AMPK to reduce mTOR activity. One consequence of this signaling would be the inhibition of protein synthesis, and it is well known that nitric oxide is an effective inhibitor of protein synthesis in many cell types, including β cells (10, 11). Additional studies will clarify the role for each of these pathways in response to nitric oxide and the role of this regulation in cellular recovery and survival following nitric oxide-mediated damage.
The kinase(s) responsible for the direct phosphorylation of AMPK in response to nitric oxide has yet to be identified. The expression of a kinase-deficient IRE1α mutant in insulinoma cells did not prevent nitric oxide-induced AMPK activation, indicating that IRE1 does not directly phosphorylate AMPK. It is also unlikely that JNK phosphorylates AMPK under these conditions, as the knockout of IRE1α does not prevent nitric oxide-induced JNK phosphorylation (data not shown), consistent with nitric oxide-induced JNK activation's being dependent on sGC (46). Our inhibitor and siRNA studies, summarized in Fig. 2, identified a number of broad signaling pathways that were not responsible for AMPK phosphorylation, including pathways dependent on calcium, mitochondrion permeability transition, ubiquitin, phosphatidylinositol-3-kinase (PI3K), HSP90, and others. Additionally, it is possible that nitric oxide-induced inhibition of metabolic enzymes such as cytochrome c oxidase (7) is necessary to elevate AMP levels and promote the AMP-bound and phosphatase-resistant AMPK conformation (43).
Cells may modify the mechanism of AMPK activation depending on the level of nitric oxide and/or cell type. At low concentrations of nitric oxide, replicating those produced by endothelial NOS (eNOS), AMPK is activated by CaMKK in a guanylate cyclase-dependent fashion in endothelial cells (61). However, in response to high levels of nitric oxide, replicating those produced by iNOS, AMPK activation is unaffected by CaMKK inhibition with STO-609, guanylate cyclase inhibition with ODQ, and calcium chelation with BAPTA-AM (Fig. 2). While these findings suggest concentration-dependent mechanisms of action of nitric oxide, lower concentrations of nitric oxide (DEANO, 100 μM) also stimulate AMPK phosphorylation in β cells in an IRE1-dependent fashion (Fig. 3B). These findings suggest that the role of IRE1α in the regulation of AMPK by nitric oxide may be cell type dependent. Somewhat unique to pancreatic β cells is the efficient folding, trafficking, and secretory capacity that is needed for the synthesis and secretion of insulin. In addition, there is a strict dependence of β cells on oxidative metabolism (glucose to CO2) for function (insulin secretion) and, because of this secretory demand, β cells have an adaptive response to oxidative stress that is associated with UPR induction (48). Thus, it seems physiologically plausible to couple the induction of protective responses to cellular stress (IRE1α and the UPR) with regulators of oxidative capacity (AMPK) to provide a mechanism by which β cells coordinate the regulation of oxidative metabolism with the response to cellular stress as mechanisms to afford protection from nitric oxide. In addition, the concentration- and cell type-selective nature of these actions could also suggest that the mechanism of nitric oxide-induced AMPK activation is dependent on the physiological context, e.g., regulation of vascular relaxation (eNOS) versus cell defense in response to inflammation (iNOS) and injury.
The finding that ER stress alone is not sufficient to stimulate AMPK activation (Fig. 5) suggests that IRE1 may be differentially activated in response to nitric oxide compared to ER stress-inducing agents such as tunicamycin and thapsigargin. Whereas tunicamycin stimulates both kinase and RNase activation of IRE1 (60), stimuli such as nitric oxide and quercetin may preferentially activate IRE1 RNase activity. Quercetin has been shown to activate IRE1 RNase activity independent of kinase activation (55), while nitric oxide induces RNase activation, but the impact on kinase activation is unknown and requires further investigation. Our findings that quercetin induces IRE1-dependent activation of AMPK and that cells expressing kinase-deficient IRE1 have enhanced nitric oxide-induced AMPK activation suggest that IRE1 RNase activation in response to these agents participates in a pathway leading to AMPK phosphorylation and that this response may be attenuated by IRE1 kinase activation. This is in line with the recent finding that the kinase and RNase activities of IRE1 can differentially impact cell fate (14). Thus, the RNase-dependent activation of AMPK may promote adaptive responses through upregulation of genes such as PGC-1α, while IRE1 kinase activation may suppress this response to promote elimination of irreparably damaged cells.
From an evolutionary standpoint, it is logical for IRE1 to play a regulatory role in the activation of AMPK signaling pathways. Both of these highly conserved proteins are responsive to cellular stress, and once active, they promote the restoration of cellular homeostasis and adaptation to environmental changes (15, 29). Additionally, ER stress has newly found roles in the regulation of lipid metabolism and gluconeogenesis (41, 52) pathways that have long been known to be regulated by AMPK (16). The IRE1-AMPK pathway could also provide a mechanism to couple the ER stress pathway with the regulation of metabolism. This form of regulation may be critical for cell survival or the loss of cell viability in response to stress. By coupling these pathways, β cells would have an exquisite level of control over rapid posttranslational modifications and long-term adaptive responses through regulation of gene expression.
ACKNOWLEDGMENTS
This work was supported by grants from the NIH (F32 DK084645 to G.P.M. and DK052194 and AI-44458 to J.A.C.) and a gift from the Forest County Potawatomi Foundation.
Footnotes
Published ahead of print on 6 September 2011.
REFERENCES
- 1. Ahn J., Lee H., Kim S., Park J., Ha T. 2008. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem. Biophys. Res. Commun. 373:545–549 [DOI] [PubMed] [Google Scholar]
- 2. Almeida A., Moncada S., Bolaños J. 2004. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat. Cell Biol. 6:45–51 [DOI] [PubMed] [Google Scholar]
- 3. Arnush M., Scarim A., Heitmeier M., Kelly C., Corbett J. 1998. Potential role of resident islet macrophage activation in the initiation of autoimmune diabetes. J. Immunol. 160:2684–2691 [PubMed] [Google Scholar]
- 4. Bhaskar P. T., Hay N. 2007. The two TORCs and Akt. Dev. Cell 12:487–502 [DOI] [PubMed] [Google Scholar]
- 5. Calabrese V., et al. 2009. Nitric oxide in cell survival: a janus molecule. Antioxid. Redox. Signal. 11:2717–2739 [DOI] [PubMed] [Google Scholar]
- 6. Calfon M., et al. 2002. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415:92–96 [DOI] [PubMed] [Google Scholar]
- 6a. Chambers K. T., et al. 2008. The role of nitric oxide and the unfolded protein response in cytokine-induced β-cell death. Diabetes 57:124–132 [DOI] [PubMed] [Google Scholar]
- 7. Cleeter M. W., Cooper J. M., Darley-Usmar V. M., Moncada S., Schapira A. H. 1994. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 345:50–54 [DOI] [PubMed] [Google Scholar]
- 8. Corbett J., McDaniel M. 1994. Reversibility of interleukin-1 beta-induced islet destruction and dysfunction by the inhibition of nitric oxide synthase. Biochem. J. 299(Pt. 3):719–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cross D. A., Alessi D. R., Cohen P., Andjelkovich M., Hemmings B. A. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785–789 [DOI] [PubMed] [Google Scholar]
- 10. Curran R. D., Billiar T. R., Stuehr D. J., Hofmann K., Simmons R. L. 1989. Hepatocytes produce nitrogen oxides from L-arginine in response to inflammatory products of Kupffer cells. J. Exp. Med. 170:1769–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Di Matteo M. A., et al. 1997. Superoxide, nitric oxide, peroxynitrite and cytokine combinations all cause functional impairment and morphological changes in rat islets of Langerhans and insulin secreting cell lines, but dictate cell death by different mechanisms. Apoptosis 2:164–177 [DOI] [PubMed] [Google Scholar]
- 12. Gotoh T., Mori M. 2006. Nitric oxide and endoplasmic reticulum stress. Arterioscler. Thromb Vasc Biol. 26:1439–1446 [DOI] [PubMed] [Google Scholar]
- 13. Gwinn D., et al. 2008. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 30:214–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Han D., et al. 2009. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell 138:562–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hardie D. 2007. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 8:774–785 [DOI] [PubMed] [Google Scholar]
- 16. Hardie D. 2004. The AMP-activated protein kinase pathway—new players upstream and downstream. J. Cell Sci. 117:5479–5487 [DOI] [PubMed] [Google Scholar]
- 17. Harding H., Zhang Y., Bertolotti A., Zeng H., Ron D. 2000. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell 5:897–904 [DOI] [PubMed] [Google Scholar]
- 18. Hawley S., et al. 2003. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hawley S., et al. 1996. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J. Biol. Chem. 271:27879–27887 [DOI] [PubMed] [Google Scholar]
- 20. Hawley S., et al. 2005. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2:9–19 [DOI] [PubMed] [Google Scholar]
- 21. Hawley S. A., et al. 2010. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 11:554–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herrero-Martín G., et al. 2009. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J. 28:677–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hetz C., Glimcher L. H. 2009. Fine-tuning of the unfolded protein response: assembling the IRE1alpha interactome. Mol. Cell 35:551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang Q., Wu Y., Tan H., Ong C., Shen H. 2009. A novel function of poly(ADP-ribose) polymerase-1 in modulation of autophagy and necrosis under oxidative stress. Cell Death Differ. 16:264–277 [DOI] [PubMed] [Google Scholar]
- 25. Hughes K., Meares G., Chambers K., Corbett J. 2009. Repair of nitric oxide-damaged DNA in beta-cells requires JNK-dependent GADD45α expression. J. Biol. Chem. 284:27402–27408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Inoki K., Li Y., Zhu T., Wu J., Guan K. 2002. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4:648–657 [DOI] [PubMed] [Google Scholar]
- 27. Inoki K., et al. 2006. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126:955–968 [DOI] [PubMed] [Google Scholar]
- 28. Julien L. A., Carriere A., Moreau J., Roux P. P. 2010. mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling. Mol. Cell. Biol. 30:908–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaufman R. J., Back S. H., Song B., Han J., Hassler J. 2010. The unfolded protein response is required to maintain the integrity of the endoplasmic reticulum, prevent oxidative stress and preserve differentiation in beta-cells. Diabetes Obes. Metab. 12(Suppl. 2):99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lira V. A., et al. 2010. Nitric oxide and AMPK cooperatively regulate PGC-1 in skeletal muscle cells. J. Physiol. 588:3551–3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Manning B., Tee A., Logsdon M., Blenis J., Cantley L. 2002. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol. Cell 10:151–162 [DOI] [PubMed] [Google Scholar]
- 32. Marsin A., Bouzin C., Bertrand L., Hue L. 2002. The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase. J. Biol. Chem. 277:30778–30783 [DOI] [PubMed] [Google Scholar]
- 33. McDaniel M., Kwon G., Hill J., Marshall C., Corbett J. 1996. Cytokines and nitric oxide in islet inflammation and diabetes. Proc. Soc. Exp. Biol. Med. 211:24–32 [DOI] [PubMed] [Google Scholar]
- 34. Meares G. P., et al. 2010. AMP-activated protein kinase attenuates nitric oxide-induced beta-cell death. J. Biol. Chem. 285:3191–3200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Momcilovic M., Hong S. P., Carlson M. 2006. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J. Biol. Chem. 281:25336–25343 [DOI] [PubMed] [Google Scholar]
- 36. Nojima H., et al. 2003. The mammalian target of rapamycin (mTOR) partner, raptor, binds the mTOR substrates p70 S6 kinase and 4E-BP1 through their TOR signaling (TOS) motif. J. Biol. Chem. 278:15461–15464 [DOI] [PubMed] [Google Scholar]
- 37. Novoa I., Zeng H., Harding H., Ron D. 2001. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J. Cell Biol. 153:1011–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oyadomari S., et al. 2001. Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proc. Natl. Acad. Sci. U. S. A. 98:10845–10850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rabinovitch A., Suarez-Pinzon W. L. 1998. Cytokines and their roles in pancreatic islet beta-cell destruction and insulin-dependent diabetes mellitus. Biochem. Pharmacol. 55:1139–1149 [DOI] [PubMed] [Google Scholar]
- 40. Ron D., Walter P. 2007. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8:519–529 [DOI] [PubMed] [Google Scholar]
- 41. Rutkowski D. T., et al. 2008. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev. Cell 15:829–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Salt I., Johnson G., Ashcroft S., Hardie D. 1998. AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic beta cells, and may regulate insulin release. Biochem. J. 335(Pt. 3):533–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sanders M., Grondin P., Hegarty B., Snowden M., Carling D. 2007. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem. J. 403:139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307:1098–1101 [DOI] [PubMed] [Google Scholar]
- 45. Scarim A., Heitmeier M., Corbett J. 1997. Irreversible inhibition of metabolic function and islet destruction after a 36-hour exposure to interleukin-1β. Endocrinology 138:5301–5307 [DOI] [PubMed] [Google Scholar]
- 46. Scarim A., Nishimoto S., Weber S., Corbett J. 2003. Role for c-Jun N-terminal kinase in beta-cell recovery from nitric oxide-mediated damage. Endocrinology 144:3415–3422 [DOI] [PubMed] [Google Scholar]
- 47. Schalm S., Fingar D., Sabatini D., Blenis J. 2003. TOS motif-mediated raptor binding regulates 4E-BP1 multisite phosphorylation and function. Curr. Biol. 13:797–806 [DOI] [PubMed] [Google Scholar]
- 48. Scheuner D., Kaufman R. J. 2008. The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr. Rev. 29:317–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shaw R., et al. 2004. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. U. S. A. 101:3329–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Urano F., et al. 2000. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287:664–666 [DOI] [PubMed] [Google Scholar]
- 51. Wang Y., et al. 2000. Activation of ATF6 and an ATF6 DNA binding site by the endoplasmic reticulum stress response. J. Biol. Chem. 275:27013–27020 [DOI] [PubMed] [Google Scholar]
- 52. Wang Y., Vera L., Fischer W. H., Montminy M. 2009. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature 460:534–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Weber S., Chambers K., Bensch K., Scarim A., Corbett J. 2004. PPARγ ligands induce ER stress in pancreatic beta-cells: ER stress activation results in attenuation of cytokine signaling. Am. J. Physiol. Endocrinol. Metab. 287:E1171–E1177 [DOI] [PubMed] [Google Scholar]
- 54. Weber S., Scarim A., Corbett J. 2004. PPARγ is not required for the inhibitory actions of PGJ2 on cytokine signaling in pancreatic beta-cells. Am. J. Physiol. Endocrinol. Metab. 286:E329–E336 [DOI] [PubMed] [Google Scholar]
- 55. Wiseman R. L., et al. 2010. Flavonol activation defines an unanticipated ligand-binding site in the kinase-RNase domain of IRE1. Mol. Cell 38:291–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Woods A., et al. 2003. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 13:2004–2008 [DOI] [PubMed] [Google Scholar]
- 57. Xu W., Liu L., Charles I., Moncada S. 2004. Nitric oxide induces coupling of mitochondrial signalling with the endoplasmic reticulum stress response. Nat. Cell Biol. 6:1129–1134 [DOI] [PubMed] [Google Scholar]
- 58. Yasukawa T., et al. 2005. S-nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. J. Biol. Chem. 280:7511–7518 [DOI] [PubMed] [Google Scholar]
- 59. Ye J., et al. 2000. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 6:1355–1364 [DOI] [PubMed] [Google Scholar]
- 60. Yoshida H., Matsui T., Yamamoto A., Okada T., Mori K. 2001. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107:881–891 [DOI] [PubMed] [Google Scholar]
- 61. Zhang J., et al. 2008. Identification of nitric oxide as an endogenous activator of the AMP-activated protein kinase in vascular endothelial cells. J. Biol. Chem. 283:27452–27461 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]