Abstract
TRIM5α is a natural resistance factor that binds retroviral capsid proteins and restricts virus replication. The B30.2/SPRY domain of TRIM5α is polymorphic in rhesus macaques, and some alleles are associated with reduced simian immunodeficiency virus (SIV) SIVmac251 and SIVsmE543 replication in vivo. We determined the distribution of TRIM5α alleles by PCR and sequence analysis of the B30.2/SPRY domain in a cohort of 82 macaques. Thirty-nine of these macaques were mock vaccinated, 43 were vaccinated with either DNA-SIV/ALVAC-SIV/gp120, ALVAC-SIV/gp120, or gp120 alone, and all were exposed intrarectally to SIVmac251 at one of three doses. We assessed whether the TRIM5α genotype of the macaques affected the replication of challenge virus by studying the number of SIV variants transmitted, the number of exposures required, the SIVmac251 viral level in plasma and tissue, and the CD4+ T-cell counts. Our results demonstrated that TRIM5α alleles, previously identified as restrictive for SIVmac251 replication in vivo following intravenous exposure, did not affect SIVmac251 replication following mucosal exposure, regardless of prior vaccination, challenge dose, or the presence of the protective major histocompatibility complex alleles (MamuA01+, MamuB08+, or MamuB017+). The TRIM5α genotype had no apparent effect on the number of transmitted variants or the number of challenge exposures necessary to infect the animals. DNA sequencing of the SIVmac251 Gag gene of the two stocks used in our study revealed SIVmac239-like sequences that are predicted to be resistant to TRIM5α restriction. Thus, the TRIM5α genotype does not confound results of mucosal infection of rhesus macaques with SIVmac251.
INTRODUCTION
The simian immunodeficiency virus (SIV) SIVmac251 macaque model is widely used to evaluate the relative efficacy of human immunodeficiency virus (HIV) vaccine candidates in macaques. Thus, understanding the natural factors that confer resistance to SIVmac251 replication in rhesus macaques is important in order to minimize the overestimation of vaccine efficacy. HIV-1 does not infect macaques, and the restriction of HIV replication in Old World monkeys occurs at the postentry level (6, 22, 29) and is mediated, in part, by the interaction of TRIM5α and the viral capsid protein (10, 23). TRIM5α is an interferon-inducible gene that is conserved across species and encodes a cytoplasmic (4, 5) protein. Species-specific TRIM5α polymorphisms (22) that affect the efficiency of SIV replication in vitro and in vivo have been characterized in rhesus macaques (30). TRIM5α antiretroviral activity is mediated by the RING domain, which through its E3 ubiquitin ligase activity, polyubiquitinates TRIM5α itself. The polyubiquitinated TRIM5α binds to the viral capsid protein via the B30.2 (SPRY) domain, and the protein complex is degraded by the proteasome (7, 27). However, the disruption of the RING domain, the modulation of the expression of E1 ubiquitin-activating enzyme, or the inhibition of the proteasome activity only partially affects the TRIM5α-mediated antiviral activity (3, 11, 25, 35), suggesting an undefined alternative proteasome-independent mechanism of action.
The B30.2 (SPRY) domain is an important determinant for virus restriction (18, 19, 22), as demonstrated in rhesus macaques, where specific alleles in the B30.2 (SPRY) domain correlated with a decreased level of SIV restriction (19). Based on polymorphisms in the macaque TRIM5α gene, located at nucleic acid positions 997, 1015 to 1020, and 1022, two different groups of alleles can be identified in macaques that differ in terms of restriction activity for SIV. A group of restrictive alleles (TRIMTFP or alleles 1 to 5) (19) and a group of permissive alleles (TRIMQ or alleles 6 to 11) can thus be defined based upon the sequence of the B30.2/SPRY domain. Homozygosity for the restrictive allele (alleles 1 to 5) was associated with lower SIVmac251 replication than observed in macaques homozygous for the permissive alleles (alleles 6 to 11) (19). An intermediate ability to restrict SIV replication was observed in animals heterozygous for alleles 1 to 5 and 6 to 11. A similar, but more pronounced effect, was observed in macaques inoculated with SIVsmE543, apparently due to the lack of adaptation of the capsid of this virus to rhesus TRIM5 (14). An additional chimeric TRIM5-cyclophilin A (CypA) fusion protein, resulting from a G-to-T substitution that alters splicing and replaces the B30.2 domain with CypA, is also observed in rhesus macaques. This gene is restrictive for SIVsmE543 but not for SIVmac239 (14).
TRIM5α restriction in vitro depends on the dose of SIV used (19), suggesting the importance of the stoichiometry between the capsid and the TRIM5α proteins. Whether the effect of TRIM5α in vivo is also dose dependent in in vivo challenge experiments has not been evaluated. Since there is a growing use of repeated low doses of SIV strains by mucosal routes of transmission for the evaluation of the efficacy of HIV vaccine candidates in macaques, we assessed here whether either the dose of the SIVmac251 challenge or the prior vaccination contributed to the ability of certain TRIM5α polymorphisms to restrict SIVmac251 replication. Surprisingly, our results on a cohort of 82 macaques, of which 43 were vaccinated and 39 were not, demonstrated that the presence of certain TRIM5 alleles shown to restrict SIV mac251 replication following intravenous exposure was not associated with restriction following mucosal exposure, regardless of the dose of challenge virus, prior vaccination, and/or the presence of protective major histocompatibility complex class I (MHC-I) alleles.
MATERIALS AND METHODS
Animals and study design.
We used 82 colony-bred Indian rhesus macaques (Macaca mulatta), obtained from Covance Research Products (Alice, TX). The animals were housed and maintained in accordance with the standards of the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All rhesus macaques were tested for simian retrovirus, simian T cell leukemia virus type 1, and herpesvirus B before the study. The major histocompatibility status was determined, as previously described (15). Thirty-nine macaques were mock vaccinated or naive; eight of them were intrarectally challenged with a single dose of 6,100 50% tissue culture infective doses (TCID50) of SIVmac251, 12 were exposed twice to 470 TCID50 of SIVmac251, and 19 were challenged with repeated low doses of 120 TCID50 of SIVmac251 until all of the animals were infected. All of these animals, except for eight from the group challenged with 120 TCID50 of SIVmac251, were given alum. Of the remaining eight, five had received mucosal immunization with Ad5 host-range mutant (Ad5h)-control vector + Ad5h-green fluorescent protein, followed by two administrations of MPL-SE adjuvant intramuscularly. The other three macaques were naive. Forty-three macaques were vaccinated; of these, 20 macaques were immunized with DNA-SIV-gag, pol, and env and then boosted with the recombinant avian canarypox virus ALVAC-SIVgpe expressing SIV gag, pol, and env genes and SIVmac251 gp120 protein, eight of these were then challenged intrarectally with a single dose of 6,100 TCID50 of SIVmac251 and 12 were challenged twice, with doses of 470 TCID50 of SIVmac251. The other 23 animals were immunized with ALVAC-SIVgpe/gp120 (11 animals) or with gp120 alone (12 animals). All of them were exposed to a repeated low dose of 120 SIVmac251. Immunization with 108 PFU of ALVAC-SIVgpe (24) was performed at weeks 0, 4, 12, and 24 in the thigh, and the gp120 immunization (200 mg of SIV-gp120 protein formulated in alum) was administered intramuscularly in the contralateral thigh at weeks 12 and 24. The control group received alum at weeks 12 and 24. All of the animals were then challenged with SIVmac251 starting at week 28. SIVmac251 was primarily isolated from an infected macaque. A SIVmac251 stock propagated in human cells was utilized in the 6,100- and 470-TCID50 challenges (17). SIVmac251 propagated in rhesus PBMC was utilized in the 120-TCID50 challenge (see Table 2). Either way, the initial number of variant was higher than 10.
Table 2.
SIVmac251 doses used in challenge experiments
| Expt | No. of animals | SIV mac251 (TCID50)a | Treatment |
|---|---|---|---|
| 1 | 8 | 6,100* | DNA/ALVAC/gp120 |
| 2 | 8 | 6,100 | Alum |
| 3 | 12 | 470* | DNA/ALVAC/gp120 |
| 4 | 12 | 470 | Alum |
| 5 | 11 | 120† | ALVAC/gp120 |
| 6 | 12 | 120 | gp120 |
| 7 | 11 | 120 | Alum |
| 8 | 3 | 120 | |
| 9 | 5 | 120 | Ad5 h-GFP or empty/MPL-SE |
*, SIVmac251 propagated in human cells; †, SIVmac251 propagated in macaque cells.
Detection of viral variants by single genome analysis.
Plasma SIV RNA was quantified by nucleic acid sequence-based amplification (NASBA), as previously described (28). SIV DNA was quantified in the blood and tissue of macaques by quantitative PCR, as previously described (33). Transmitted or founder viruses and their progeny were identified by single-genome amplification of plasma SIV RNA, direct amplicon sequencing (ENV PRIMERS), and phylogenetic analysis, as previously described (13). SIV RNA was extracted from plasma and from rectal pinches, and limiting-dilution PCR of newly synthesized cDNA was performed. Transmitted or founder virus lineages were identified by low-diversity sequences and by single sequences with unique mutations. Phylogenetic trees were generated using CLUSTAL W.
A portion of the gag gene surrounding the CypA binding site was sequenced at peak viremia using a limiting-dilution PCR, such that only one amplifiable molecule is present in each reaction from each Trim5 restrictive and sensitive homozygous control animal, as previously described (12). Briefly, 20,000 viral RNA copies were isolated by using a QIAamp viral RNA minikit (Qiagen) and immediately subjected to cDNA synthesis with 1× reverse transcriptase buffer, 0.5 mM concentrations of each deoxynucleoside triphosphate, 5 mM dithiothreitol, 2 U of RNaseOUT (RNase inhibitor)/ml, 10 U of SuperScript III reverse transcriptase/ml, and a 0.25 mM concentration of the gene-specific antisense primer SIVrev10 (5′-CTA GTC CTG CAG GGT GTG GTA TTC C-3′). The cDNA mixture was incubated at 50°C for 60 min and 55°C for 60 min and then heat inactivated at 70°C for 15 min, followed by treatment with 2 U of RNase H at 37°C for 20 min. PCR amplification was performed with 1× PCR buffer, 2 mM MgSO4, 0.2 mM concentrations of each deoxynucleoside triphosphate, 0.2 μM concentrations of each primer, and 0.025 U Platinum Taq high-fidelity polymerase (Invitrogen)/μl in a 20-μl reaction with the sense primer SIVfor4 (5′-ACA GGG ACTT GAA GGA GAG TGA G-3′) and the antisense primer SIVrev10. Next, 1 μl from the first-round PCR product was added to a second-round PCR that included the sense primer SIVfor6 (5′-GGC AGA GGA GGA AAT TAC CCA G-3′) and antisense primer SIVrev7 (5′-AAT GTT GCC TAC TGG TAT GGG GT-3′) performed under the same conditions used for the first-round PCR, but with a total of 45 cycles. Correctly sized amplicons were identified by agarose gel electrophoresis and directly sequenced with the second-round PCR primer SIVfor6.
TRIM5α genotyping.
The TRIM5α genotypes were retrospectively determined for all of the macaques included in the study by isolation of genomic DNA from peripheral blood mononuclear cells (PBMC) or whole blood and direct sequencing of the 526-nucleotide PCR product of the B30.2/SPRY domain of TRIM5α. The sequences of the primers utilized both for PCR and for the sequencing reaction were CAGTGCTGACTCCTTTGCTTG for the forward primer and GCTTCCCTGATGTGATAC for the reverse primer. The sequences were then aligned with the genomic DNA sequence of the rhesus macaque (accession number DQ842021.1) to characterize the polymorphisms at nucleic acid positions 997, 1015 to 1020, and 1022 of TRIM5α. This analysis did not allow us to identify animals that expressed a splice variant of TRIM5α, the TRIM5-CypA chimera (TRIM5-CypA), since this single G-to-T mutation occurs 5′ to the region analyzed. However, TRIM5-CypA is at a relatively low frequency in rhesus macaque populations. Based upon sequence of the B30.2/SPRY domain, macaques expressing TRIM5-CypA would have been misclassified as having one or two permissive alleles but would not affect the classification of animals with restrictive alleles.
Enumeration of CD4+ T cell in blood and tissues.
The CD4+ T cell count in the blood was quantified by flow cytometry, as previously described (34). Immunohistochemistry for CD4+ T cells in rectal mucosa biopsy specimens was also previously described (9). Briefly, slides were stained using an Autostainer (Dako, Inc., Carpinteria, CA). Slides were visualized with epifluorescence illumination using a Zeiss Axioplan 2 microscope (Carl Zeiss) and appropriate filters. Digital images were captured and analyzed by using a Zeiss Axiocam system and OpenLab software (Inprovision, Inc., Waltham MA) (32). We used monoclonal anti-CD4 mouse serum (clone IF6; Vector, Burlingame, CA) as the primary antibody. Binding of CD4 was detected using Alexa Fluor 488-labeled polyclonal goat anti-rabbit IgG (Molecular Probes, Eugene, OR). Slides were visualized with epifluorescence illumination using a Zeiss Axioplan 2 microscope (Carl Zeiss, Inc., Thornwood, NY) and appropriate filters. Digital images were captured and analyzed by using a Zeiss Axiocam System and OpenLab software (Inprovision). Only clearly positive cells were considered positive. The number of positive cells is presented as the number of cells per square millimeter.
Statistical analysis.
Exact Jonckheere-Terpstra and Kruskal-Wallis tests were used to compare viral and proviral loads, CD4+ T cell outcomes, and numbers of transmitted variants between the macaques that carried TRIM5α restrictive alleles and those that carried nonrestrictive TRIM5α alleles. Tests were conducted separately in vaccinated animals and in controls. Comparisons were made, both within each subgroup defined by the dose of SIVmac251 challenge and in stratified tests across dose subgroups. The number of viral exposures needed for infection of the animals in the low-dose experiments was compared using the exact log-rank test.
RESULTS
Distribution of TRIM5α alleles in control and vaccinated macaques.
Expression of particular TRIM5α alleles has been reported to restrict SIVmac251 replication in vitro and in rhesus macaques in vivo following SIVmac251 exposure by the intravenous route (18, 19). To investigate whether this TRIM5α polymorphism impacts SIVmac251 infection following intrarectal inoculation, we studied a cohort of 82 Indian rhesus macaques. Thirty-nine of these macaques were mock vaccinated or naive, and 43 were vaccinated with either DNA-SIV-gag, pol, and env and then boosted with the recombinant avian poxvirus ALVAC-SIVgpe (24), expressing the SIV gag, pol, and envelope genes alone or in combination with the SIVmac251 gp120 envelope protein (DNA/ALVAC-SIVgpe/gp120) (20 macaques), ALVAC-SIVgpe plus gp120 (11 macaques), or gp120 alone (12 macaques) (Table 1). Of these animals, 16 macaques were exposed intrarectally to 6,100 TCID50 and 24 macaques were exposed intrarectally to 470 TCID50 of a SIVmac251 stock propagated in human cells. The remaining 42 macaques were exposed to a 120-TCID50 dose of SIVmac251 propagated in rhesus PBMC (Table 2). PBMC were purified from the blood of all the animals, and the DNA sequence of the TRIM5α B30.2 (SPRY) domain was obtained. The animals were grouped according to the allele-determined resistance to SIVmac251 infection (19), based on the polymorphisms at nucleotide positions 997, 1015 to 1020, and 1022 of TRIM5α.
Table 1.
Treatment of macaques in four cohorts
| Cohort | No. of animals | Treatment | No. of animals MamuA01+ or MamuB08+ or MamuB017+ |
|---|---|---|---|
| 1 | 39 | Mock-vaccinated/naive | 13 |
| 2 | 20 | DNA/ALVAC/gp120 | 6 |
| 3 | 11 | ALVAC/gp120 | 3 |
| 4 | 12 | gp120 | 3 |
As summarized in Tables 3 and 4, DNA sequencing of this region identified 32 animals homozygous for the restrictive TRIMTFP allele (R = 1-5/1-5 alleles (19) that we designated “R,” 25 macaques homozygous for the susceptible TRIMQ allele (S = 6-11/6-11 alleles) (14, 19) that were designated “S,” and 25 heterozygous with one copy each of 1-5 and 6-11, which would be predicted to have an intermediate ability to restrict SIV infection, that were designated “M.”
Table 3.
TRIM5α alleles in mock-vaccinated animals
| Treatment and animal no. | TRIM5α allelea | Nucleotide(s) at position(s): |
MHC-I | |||
|---|---|---|---|---|---|---|
| 979 | 997 | 1015–1020 | 1022 | |||
| 120 TCID50 | ||||||
| P064 | R | C | G | ACGTTT (TFP) | C | A01 |
| P146 | M | C | G/T | ACGTTT/ΔΔ (TFP/Q) | A/C | |
| P149 | M | C | G/T | ACGTTT/ΔΔ (TFP/Q) | A/C | |
| P158 | R | C | G | ACGTTT (TFP) | C | |
| P161 | R | C | G | ACGTTT (TFP) | C | |
| P258 | M | C | G/T | ACGTTT/ΔΔ (TFP/Q) | A/C | |
| P262 | S | C/A | T | ΔΔ (Q) | A | |
| M381 | S | C | T | ΔΔ (Q) | A | A01 |
| M385 | M | C/A | G/T | ACGTTT/ΔΔ (TFP/Q) | A/C | A01 |
| M895 | M | C/A | G/T | ACGTTT/ΔΔ (TFP/Q) | A/C | B17 |
| M903 | M | C/A | G/T | ACGTTT/ΔΔ (TFP/Q) | A/C | |
| G402 | R | C | G | ACGTTT (TFP) | C | A01 |
| G417 | R | C | G | ACGTTT (TFP) | C | |
| G419 | M | C | G/T | ACGTTT/ΔΔ (TFP/Q) | A/C | A01 |
| G420 | M | C | G/T | ACGTTT/ΔΔ (TFP/Q) | A/C | |
| G421 | S | C | T | ΔΔ (Q) | A | A01 |
| G422 | S | C | T | ΔΔ (Q) | A | |
| G423 | S | A | T | ΔΔ (Q) | A | A01 |
| G428 | M | C | G/T | ACGTTT/ΔΔ (TFP/Q) | A/C | |
| 470 TCID50 | ||||||
| M998 | S | C | T | ΔΔ (Q) | A | A01 |
| P059 | R | C | G | ACGTTT (TFP) | C | A01 |
| P017 | S | C | A | ΔΔ (Q) | A | A01 |
| P143 | R | C | G | ACGTTT (TFP) | C | |
| P144 | M | C | G/T | ACGTTT/ΔΔ (TFP/Q) | A/C | |
| P088 | R | C | G | ACGTTT (TFP) | C | |
| P130 | M | C/A | G/T | ACGTTT/ΔΔ (TFP/Q) | A/C | |
| P121 | S | C | T | ΔΔ (Q) | A | |
| P089 | S | C | T | ΔΔ (Q) | A | B08 |
| P095 | R | C | G | ACGTTT (TFP) | C | |
| P113 | R | C | G | ACGTTT (TFP) | C | |
| P071 | R | C | G | ACGTTT (TFP) | C | B17 |
| 6,100 TCID50 | ||||||
| M881 | R | C | G | ACGTTT (TFP) | C | A01 |
| P061 | S | A | T | ΔΔ (Q) | A | A01 |
| P015 | R | C | G | ACGTTT (TFP) | C | A01 |
| P066 | M | C/A | G/T | ACGTTT/ΔΔ (TFP/Q) | A/C | B08 |
| P136 | M | C | G/T | ACGTTT/ΔΔ (TFP/Q) | A/C | |
| P134 | M | C/A | G/T | ACGTTT/ΔΔ (TFP/Q) | A/C | |
| P084 | S | A | T | ΔΔ (Q) | A | B08 |
| P074 | R | C | G | ACGTTT (TFP) | C | |
R, homozygous restrictive (1-5/1-5); S, homozygous susceptible (6-11/6-11); M, heterozygous intermediate (1-5/6-11).
Table 4.
TRIM5α alleles in vaccinated animals
| Treatment and animal no. | TRIM5α allelea | Nucleotide(s) at position(s): |
MHC-I | |||
|---|---|---|---|---|---|---|
| 979 | 997 | 1015-1020 | 1022 | |||
| gp120 (120 TCID50) | ||||||
| P065 | R | C | G | ACGTTT (TFP) | C | A01 |
| P122 | R | C | G | ACGTTT (TFP) | C | |
| P123 | S | C | T | ΔΔ (Q) | A | |
| P124 | R | C | G | ACGTTT (TFP) | C | |
| P126 | S | C/A | T | ΔΔ (Q) | A | |
| P127 | R | C | G | ACGTTT (TFP) | C | |
| P132 | S | C | T | ΔΔ (Q) | A | |
| P133 | R | C | G | ACGTTT (TFP) | C | |
| P135 | S | A | T | ΔΔ (Q) | A | |
| P137 | M | C/A | G/T | ACGTTT/ΔΔ (TFP/Q) | A/C | |
| P139 | R | C | G | ACGTTT (TFP) | C | A01 |
| M927 | R | C | G | ACGTTT (TFP) | C | A01 |
| ALVAC-SIV/gp120 (120 TCID50) | ||||||
| P062 | S | C | T | ΔΔ (Q) | A | A01 |
| P063 | R | C | G | ACGTTT(TFP) | C | |
| P067 | M | C/A | G/T | ACGTTT/ΔΔ (TFP/Q) | A/C | |
| P112 | R | C | G | ACGTTT (TFP) | C | A01 |
| P147 | S | C/A | T | ΔΔ (Q) | A | |
| P148 | M | C/A | G/T | ACGTTT/ΔΔ (TFP/Q) | A/C | |
| P150 | R | C | G | ACGTTT (TFP) | C | B08 |
| P172 | M | C | G/T | ACGTTT/ΔΔ (TFP/Q) | A/C | |
| P250 | M | C/A | G/T | ACGTTT/ΔΔ (TFP/Q) | A/C | |
| P254 | R | C | G | ACGTTT (TFP) | C | |
| M624 | S | A | T | ΔΔ (Q) | A | A01 |
| DNA/ALVAC-SIV/gp120 (470 TCID50) | ||||||
| P019 | M | C/A | G/T | ACGTTT/ΔΔ (TFP/Q) | A/C | A01 |
| P013 | R | C | G | ACGTTT (TFP) | C | A01 |
| P006 | M | C/A | G/T | ACGTTT/ΔΔ (TFP/Q) | A/C | A01 |
| P016 | M | C/A | G/T | ACGTTT/ΔΔ (TFP/Q) | A/C | |
| P014 | S | C | A | ΔΔ (Q) | A | |
| P009 | S | C | T | ΔΔ (Q) | A | |
| M890 | S | C | T | ΔΔ (Q) | A | |
| M898 | S | C | T | ΔΔ (Q) | A | |
| P008 | R | C | G | ACGTTT (TFP) | C | |
| P001 | M | C | G/T | ACGTTT/ΔΔ (TFP/Q) | A/C | B08 |
| P018 | S | C | T | ΔΔ (Q) | A | B17 |
| P010 | M | C | G/T | ACGTTT/ΔΔ (TFP/Q) | A/C | |
| DNA/ALVAC-SIV/gp120 (6,100 TCID50) | ||||||
| P002 | S | C | T | ΔΔ (Q) | A | A01 |
| P012 | R | C | G | ACGTTT (TFP) | C | A01 |
| M899 | R | C | G | ACGTTT (TFP) | C | A01 |
| P007 | R | C | G | ACGTTT (TFP) | C | |
| M891 | S | C | A | ΔΔ (Q) | A | |
| M896 | R | C | G | ACGTTT (TFP) | C | |
| M887 | M | C/A | G/T | ACGTTT/ΔΔ (TFP/Q) | A/C | |
| M904 | R | C | G | ACGTTT (TFP) | C | B08 |
R, homozygous restrictive (1-5/1-5); S, homozygous susceptible (6-11/6-11); M, heterozygous intermediate (1-5/6-11).
TRIM5α alleles are not associated with lower SIVmac251 levels or preservation of CD4+ T cells in unvaccinated Indian rhesus macaques.
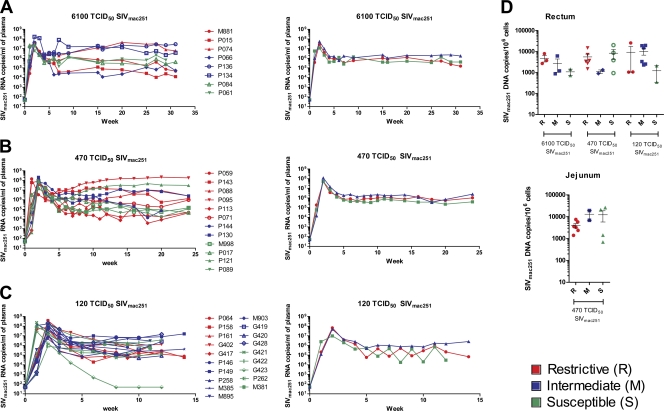
A previous study has shown that Indian rhesus macaques homozygous for the TRIM5α restrictive allele had a significantly lower peak and set-point plasma virus levels following intravenous challenge with SIVmac251 than those homozygous for the permissive allele (18, 19). We analyzed plasma virus levels at first in mock-vaccinated macaques following mucosal challenge exposure to two different doses of the same SIVmac251 stock used in this previous report (M. Vaccari et al., unpublished data). The data presented are based on the time of infection for each animal (week 0), regardless of the time of exposure. Of the 39 macaques, 8 were challenged with a single high dose of 6,100 TCID50, a dilution that corresponds to 100% infectivity and results in the transmission of multiple SIV variants. The plasma virus level measured during the duration of the study did not differ significantly between the three R, three M, and two S macaques at any time point (Fig. 1A). The absence of a restrictive effect of TRIM5α genotype on SIVmac251 did not appear to relate to the dose of challenge virus used since the same virus stock at 470 TCID50 that transmits an average of eight virus variants did not result in better control of SIV mac251 replication in the six R macaques, the remaining four S macaques, and the two M macaques (Fig. 1B). When we used a dose of 120 TCID50 of SIVmac251 propagated in macaque cells that transmits an average of one virus variant, we observed no significant differences in plasma virus level among the five R macaques versus the nine M and five S macaques (Fig. 1C).
Fig. 1.
SIVmac251 viral and proviral load in control rhesus macaques. (A) The left panel shows the viral load in the plasma of eight control animals challenged with 6,100 TCID50 of SIVmac251. Marked in red are the animals with a restrictive TRIM5α allele (R), whereas marked in green are the animals with nonrestrictive TRIM5α alleles (S), and in blue the TRIM5α heterozygous genotype (M). The right panel shows the geometric mean of the three R, three M, and two S animals. (B) Plasma SIV RNA is depicted on the left for 12 control animals exposed to 470 TCID50 of SIVmac251, and the geometric mean of the six R, two M, and four S animals is given on the right. (C) Plasma SIV RNA is depicted on the left for 19 control animals challenged with 120 TCID50 of SIVmac251 and the geometric mean of the five R, nine M, and five S animals on the right. (D) Proviral DNA copies in snap-frozen rectal (left) or jejunal (right) biopsy specimens collected at week 3 after infection from control animals exposed to 6,100, 470, or 120 TCID50 of SIVmac251 for rectum and 470 TCID50 of SIVmac251 only for jejunum.
Accordingly, the analysis of the viral DNA copy number in the rectal mucosa of these groups of animals, collected at 3 weeks after infection, demonstrated no significant differences, regardless of the dose or the type of virus used (Fig. 1D, left panel). Similar results were observed in the jejunum of animals challenged at the 470-TCID50 dose (Fig. 1D, right panel).
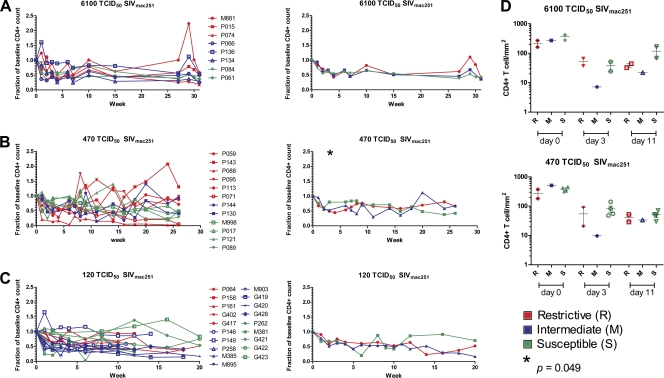
Since the presence of the TRIM5α restrictive alleles was also associated with better preservation of CD4+ T cells during either the acute or chronic phase of infection (18, 19), we evaluated the blood CD4+ T cell counts in all macaques. The CD4+ absolute T cell number at baseline was normalized for each macaque, and the changes in CD4+ T cell number over time were monitored. Consistent with the lack of an effect on SIV replication, the restrictive TRIM5α alleles had no apparent impact on the CD4+ T cell number following SIVmac251 infection in any of the groups studied here (Fig. 2A to C), except for the group of animals challenged with 470 TCID50 of SIVmac251 at the week 3, where the group that carries the nonrestrictive TRIM5α alleles appeared to have better CD4+ T cell preservation (P = 0.049) (Fig. 2B). Similarly, enumeration of CD4+ T cells at mucosal sites by immunohistochemistry, following SIVmac251 infection in animals exposed to the two higher doses of SIVmac251, did not reveal significant differences among the R, M, or S macaques regardless of the dose of virus used (Fig. 2D).
Fig. 2.
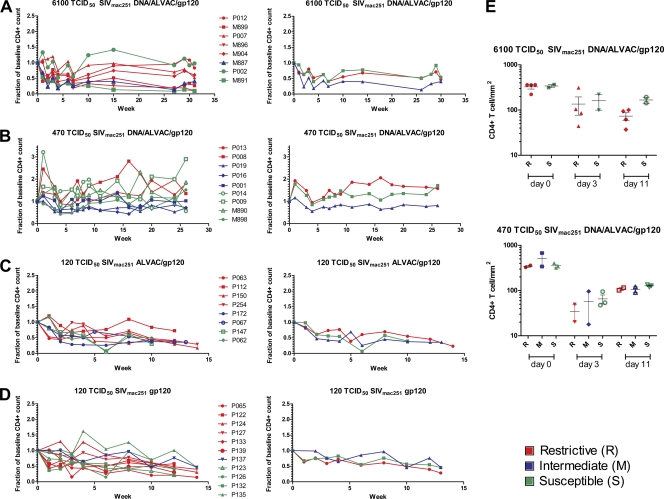
CD4+ T cell count in blood and rectal mucosa in control rhesus macaques. (A) Fraction of baseline of CD4+ T cell in the blood from eight animals challenged with a single 6,100 TCID50 of SIVmac251 dose in the left panel. The right panel shows the averages of the three R, three M, and two S animals. The relative CD4+ T cell count of animals challenged with two doses of 470 (B) or 120 TCID50 of SIVmac251 (C) are depicted in the left panels, whereas the averages of the R and S animals are shown in the right panels, respectively. (D) The count of CD4+ T cell was evaluated at 0, 3, and 11 weeks from the SIVmac251 challenge in the rectal mucosa from control animals exposed to 6,100 TCID50 (upper panel) and 470 TCID50 of SIVmac251 (lower panel). R animals are marked in red, M animals are marked in blue, and S animals are marked in green.
Expression of the MHC-I MamuA01+, MamuB08+, and MamuB017+ alleles has been associated previously with better control of SIVmac251 replication (24, 36). Among the mock-vaccinated MamuA01+ macaques, five of them also carried restrictive TRIM5α alleles (Table 3). However, these five MamuA01+ R macaques did not control significantly better virus replication regardless of the dose of challenge exposure of SIVmac251 and, accordingly, these animals did not preserve CD4+ T cells better than the eight other MamuA01+ macaques (two M and six S) (data not shown).
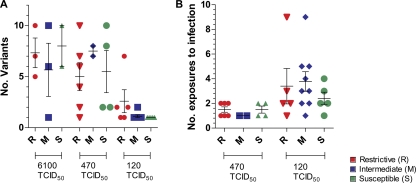
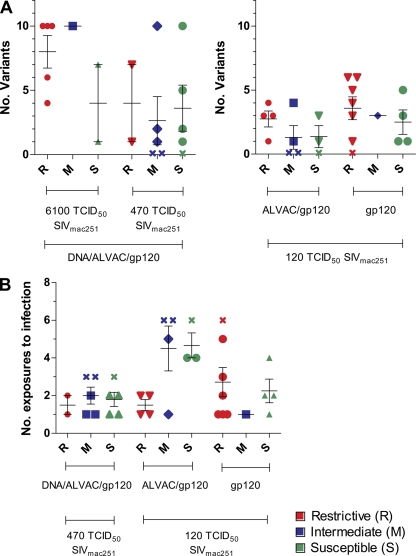
Recent studies have demonstrated that the number of SIVmac251 variants transmitted is dependent on the dose of the inoculum (13, 20). SIVmac251 exposure to low repeated doses typically results in transmission of few virus variants. The underlying reason for this bottleneck is unknown and does not appear to relate directly to the genetic composition of the virus (21). Therefore, we assessed whether restrictive TRIM5α alleles affect the number of the transmitted SIVmac251 variants by performing single-genome amplification and sequence analysis of the SIV envelope gene. The number of transmitted virus variants in the plasma of each animal was characterized within the first week of infection. We also investigated whether the presence of restrictive TRIM5α alleles was associated with the high level of SIVmac251 exposure necessary to infect the animals. We observed no significant difference in the number of variants transmitted regardless of the TRIM5α genotype (Fig. 3A). Likewise, the number of exposures required to infect the animals did not differ significantly in the S, M, or R macaques (Fig. 3B).
Fig. 3.
(A) Number of transmitted/founder variants after 2 weeks postinfection in R, M, and S control animals challenged with 6,100, 470, or 120 TCID50 of SIVmac251. The bars indicate the means ± the standard errors of the mean. (B) Number of SIVmac251 exposures to infection in R, M, and S animals challenged with 120 or 470 TCID50 of SIVmac251. The first group received weekly intrarectal challenges of 120 TCID50 of SIVmac251 until all of the animals became infected up to 10 exposures, whereas the other animals received up to two challenges of 470 TCID50 of SIVmac251.
TRIM5α polymorphism does not affect relative vaccine efficacy following mucosal challenge with SIVmac251.
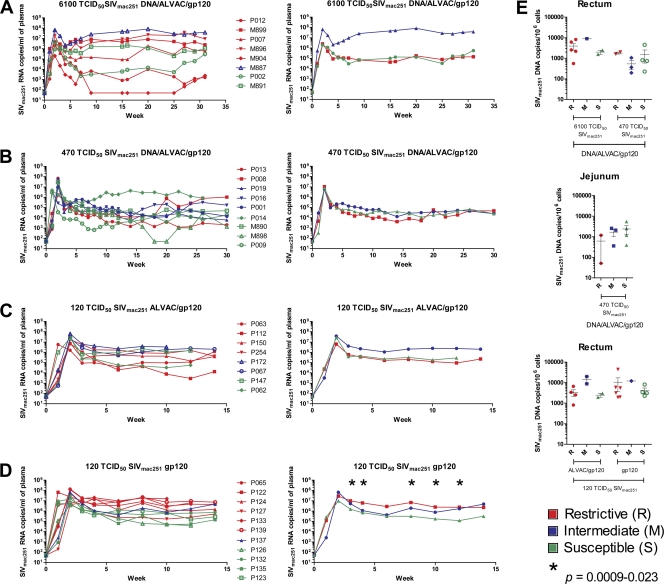
Next, we analyzed whether the TRIM5α allele, together with a vaccine-induced immune response to SIVmac251, resulted in better protection from infection and/or disease. Of the macaques vaccinated with DNA/ALVAC/gp120, eight macaques were then intrarectally challenged with 6,100 TCID50 of SIVmac251 and 12 macaques were challenged with 470 TCID50 of the same stock of SIVmac251. Comparisons of the plasma virus levels in the groups of vaccinated macaques that received either 6,100 TCID50 or 470 TCID50 of SIVmac251, respectively, demonstrated no significant differences (Fig. 4A and B). Next, we analyzed animals vaccinated with ALVAC-SIVgpe/gp120 and animals vaccinated with gp120 alone and exposed to 120 TCID50 of SIV mac251. We found no statistical significant differences in plasma virus levels, in blood, in the group of macaques given ALVAC/gp120 (Fig. 4C). Surprisingly, a significantly higher SIV RNA plasma level was found at weeks 3, 4, 8, 10, and 12 (P = 0.0009 to 0.023) in animals vaccinated with gp120 alone that carried the R allele (Fig. 4D). However, no significant difference in the level of SIV DNA was observed in the rectal mucosa of vaccinated animals in the groups that carried the TRIM5α R allele and those that did not (Fig. 4E). Importantly, the MamuA01+, MamuB08+, and MamuB017+ alleles, in conjunction with vaccination and the TRIM5α allele, were not associated with lower viremia or protection from CD4+ T cell loss (data not shown).
Fig. 4.
SIVmac251 viral and proviral load in vaccinated rhesus macaques. (A) The left panel shows the viral load in the plasma of eight animals challenged with 6,100 TCID50 of SIVmac251 and vaccinated with DNA/ALVAC/gp120. Red, R animals; blue, M animals; green, S animals. The right panel shows the geometric mean of the five R, one M, and two S animals. (B) Plasma SIV RNA is depicted on the left for nine mock-vaccinated animals challenged with 470 TCID50 of SIVmac251 and vaccinated with DNA/ALVAC/gp120. The geometric means of two R, three M, and four S animals are shown in the right panel. (C) Plasma SIV RNA is depicted on the left for eight mock-vaccinated animals challenged with 120 TCID50 of SIVmac251 and vaccinated with ALVAC/gp120. The geometric means of four R, two M, and two S animals are shown in the right panel. (D) The left panel shows the viral load in plasma of 11 animals challenged with 120 TCID50 of SIVmac251 and vaccinated with gp120 only. The right panel shows the geometric mean viral load of six R, one M, and four S animals. (E) In the upper panels, proviral DNA copies in snap-frozen rectal (left) or jejunal (right) biopsy specimens collected after week 3 postinfection from animals vaccinated with DNA/ALVAC/gp120 and exposed to 6,100 or 470 TCID50 of SIVmac251 for rectum and 470 TCID50 of SIVmac251 only for jejunum. The lower panel shows proviral DNA copies in rectal biopsy specimens of R, M, and S animals challenged with 120 TCID50 of SIVmac251 and vaccinated with ALVAC/gp120 or gp120 only.
Analysis CD4+ T cell numbers in the blood and at mucosal sites in vaccinated animals did not reveal any differences in the preservation or reconstitution of CD4+ T cell in macaques that carried the R, M, or S genotypes (Fig. 5A to E). TRIM5α alleles did not affect the number of transmitted SIVmac251 variants at any of the doses used in the challenge exposure (Fig. 6A). Similarly, the number of SIVmac251 exposures necessary to infect the vaccinated macaques did not differ significantly in animals that carried the R, M, or S TRIM5α alleles. Overall, our data demonstrate that the polymorphisms of TRIM5α alleles are not associated with better control of viral replication or protection of CD4+ T cell following mucosal exposure to SIVmac251 in Indian rhesus macaques, regardless of vaccination. In addition, no synergy was observed between the TRIM5α alleles and protective MHC-I alleles.
Fig. 5.
CD4+ T cell count in blood and rectal mucosa in vaccinated rhesus macaques. (A) Fraction of baseline of CD4+ T cell in the blood from eight animals challenged with 6,100 TCID50 of SIVmac251 and vaccinated with DNA/ALVAC/gp120 in the left panel. The right panel shows the averages of five R, one M, and two S animals. (B) The relative CD4+ T cell counts of nine animals challenged with 470 are depicted in the left panels, whereas the averages of the two R, three M, and four S animals are shown in the right panels. Nineteen animals were infected with 120 TCID50 of SIVmac251; eight of them were vaccinated with ALVAC/gp120 (C), and eleven were vaccinated with gp120 only (D). The averages of the R, M, and S animals from these two groups are shown in the right panels. (E) The CD4+ T cell count was evaluated in the rectal mucosa at 0, 3, and 11 weeks postchallenge in animals vaccinated with DNA/ALVAC/gp120 and exposed to 6,100 (upper panel) and 470 TCID50 of SIVmac251 (lower panel).
Fig. 6.
(A) Number of transmitted or founder variants at 2 weeks postinfection in R, M, and S animals vaccinated with DNA/ALVAC/gp120 challenged with 6,100 or 470 TCID50 of SIVmac251 (left panel). In the right panel is depicted the number of transmitted or founder variants in R, M, and S animals challenged with 120 TCID50 of SIVmac251 and vaccinated with ALVAC/gp120 or gp120 only. The bars indicate the means ± the standard errors of the mean. (B) Number of SIVmac251 exposures to infection in R, M, and S animals challenged with 120 or 470 TCID50 of SIVmac251. The first group was vaccinated with DNA/ALVAC/gp120 and received up to two challenges of 470 TCID50 of SIVmac251. One M and two S animals were not infected after the two exposures, and they are marked with the symbol “x” and assigned to 3. The other animals were either vaccinated with ALVAC/gp120 or gp120 only and received weekly intrarectal challenges of 120 TCID50 of SIVmac251 until all of the animals became infected up to five exposures. Two M animals and one S animal from the group vaccinated with ALVAC/gp120 and one R animal from the group vaccinated with gp120 only were not infected after five exposures, and they are marked with the symbol “x” and assigned to 6.
The viral genotypes within the SIVmac251 virus stocks are resistant to the TRIM5α polymorphic forms.
Since we did not observe an effect of the TRIM5α alleles on SIVmac251 replication and the SIVmac251 challenge stocks used are constituted by multiple virus variants, we hypothesized that the lack of restriction may be due to viral selection based on host TRIM5α restriction. To address this possibility, we amplified and sequenced a portion of SIV gag from plasma collected during acute infection from each mock-vaccinated animal that was identified as homozygous restrictive (n = 14) or homozygous susceptible (n = 11) (Table 3). Viral sequences from all 25 animals (n = 235 sequences) were identical within the CypA binding site in capsid of Gag (PQPAPQQGQLREPS) to the reported SIVmac239 clone (16). Importantly, the DNA sequence of both viral stocks demonstrated that mutations, within the Gag gene previously associated with resistance in SIVmac251, were present at high frequency, which might explain the lack of difference of SIVmac251 replication in animals with TRIM5α alleles.
DISCUSSION
Animal models are important tools to evaluate the relative efficacy of HIV vaccine candidates. It is becoming evident that different species of nonhuman primates have evolved strategies to restrict retroviral infection that may differ from those evolved in humans (8, 14). Thus, uncovering restrictive genes for SIV infection and replication in the rhesus macaque, a widely used model in the evaluation of vaccine efficacy, is of paramount importance. TRIM5α is a natural host factor that is known to act as a species-specific restriction factor against HIV/SIV. Recently, polymorphisms in the B30.2 (SPRY) domain of this protein have been related to different levels of restriction of viral replication in vivo and protection from CD4+ cells loss in rhesus macaques intravenously infected with SIVmac251 (18).
In our cohort of 82 macaques, the polymorphisms in B30.2 (SPRY) domain of TRIM5α did not appear to have a significant effect on SIVmac251 replication in vivo or on CD4+ loss regardless of vaccination, dose of challenge, or the presence of protective MHC-I alleles. These results are not surprising since we also demonstrated that the SIVmac251 variants, transmitted by the same virus stock to macaques, which have the restrictive and nonrestrictive TRIM5α alleles, had mutations in the CypA binding site in the p27Gag protein that have been associated with resistance to TRIM5α. This result is consistent with the barely detectable effect in single cycle replication assay of the virus stock used in our study as reported by Lim et al. (19). Indeed, the magnitude of effect previously reported after intravenous exposure to the same stock of SIVmac251, although significant, was small compared to that observed in a similar study using the highly resistant SIVsmE543-3 (14, 18). The effect of TRIM5α, an interferon-inducible gene, may be better detected following intravenous exposure to a single high dose of virus, as done by Lim and coworkers, rather than following a single or multiple mucosal exposures to lower doses of SIVmac251, as was done in the present study. Studies by Abel et al. (1, 2) demonstrate a higher production of interferon-inducible genes at mucosal sites than systemic in rhesus macaques. In addition, because TRIM5α also promotes innate immune signaling by increasing NF-κB activation, it is possible that this antiviral effect varies at different sites of infection (26, 31). The mucosa is a bottleneck for SIV and HIV, as demonstrated by the studies in acute infection, whereby typically, a few virus variants are transmitted by the mucosal route, whereas multiple variants are transmitted by the intravenous route. This hypothesis could be tested by studying the level of induction and expression of TRIM5α at mucosal and systemic sites following a different route of infection. There is still the possibility that the virus stocks, used by us and others, contained a mixture of variants, constituted by less-frequent sensitive and more-frequent resistant capsid binding sites to TRIM5α within the p27Gag proteins. We demonstrated by DNA sequence that both virus stocks used in our study were constituted entirely of genotype expressing Gag proteins resistant to TRIM5α restriction. However, we cannot rule out that TRIM5α-sensitive strains, present at very low frequency in the challenge stock, may be selected differently following the mucosal or the intravenous route. In conclusion, while the use of low-dose mucosal challenge exposure to SIVmac251 did not affect the evaluation of vaccine efficacy, the use of the same virus by the intravenous route at higher doses may pose more challenges in the interpretation of vaccine efficacy.
ACKNOWLEDGMENTS
We thank Shari Gordon and Namal Liyanage for critical reading of the manuscript and Teresa Habina for editorial assistance. We also thank Sharon Orndorff, Jim Treece, Deborah Weiss, and Phil Markham for execution of the animal studies.
Footnotes
Published ahead of print on 14 September 2011.
REFERENCES
- 1. Abel K., Alegria-Hartman M. J., Rothaeusler K., Marthas M., Miller C. J. 2002. The relationship between simian immunodeficiency virus RNA levels and the mRNA levels of alpha/beta interferons (IFN-α/β) and IFN-α/β-inducible Mx in lymphoid tissues of rhesus macaques during acute and chronic infection. J. Virol. 76:8433–8445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abel K., Rocke D. M., Chohan B., Fritts L., Miller C. J. 2005. Temporal and anatomic relationship between virus replication and cytokine gene expression after vaginal simian immunodeficiency virus infection. J. Virol. 79:12164–12172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson J. L., et al. 2006. Proteasome inhibition reveals that a functional preintegration complex intermediate can be generated during restriction by diverse TRIM5 proteins. J. Virol. 80:9754–9760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Asaoka K., et al. 2005. A retrovirus restriction factor TRIM5α is transcriptionally regulated by interferons. Biochem. Biophys. Res. Commun. 338:1950–1956 [DOI] [PubMed] [Google Scholar]
- 5. Carthagena L., et al. 2008. Implication of TRIM alpha and TRIMCyp in interferon-induced anti-retroviral restriction activities. Retrovirology 5:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chackerian B., Long E. M., Luciw P. A., Overbaugh J. 1997. Human immunodeficiency virus type 1 coreceptors participate in postentry stages in the virus replication cycle and function in simian immunodeficiency virus infection. J. Virol. 71:3932–3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diaz-Griffero F., et al. 2006. Rapid turnover and polyubiquitylation of the retroviral restriction factor TRIM5. Virology 349:300–315 [DOI] [PubMed] [Google Scholar]
- 8. Dietrich E. A., Jones-Engel L., Hu S. L. 2010. Evolution of the antiretroviral restriction factor TRIMCyp in Old World primates. PLoS One 5:e14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gordon S. N., et al. 2010. Preexisting infection with human T-cell lymphotropic virus type 2 neither exacerbates nor attenuates simian immunodeficiency virus SIVmac251 infection in macaques. J. Virol. 84:3043–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hatziioannou T., et al. 2006. Generation of simian-tropic HIV-1 by restriction factor evasion. Science 314:95. [DOI] [PubMed] [Google Scholar]
- 11. Javanbakht H., Diaz-Griffero F., Stremlau M., Si Z., Sodroski J. 2005. The contribution of RING and B-box 2 domains to retroviral restriction mediated by monkey TRIM5α. J. Biol. Chem. 280:26933–26940 [DOI] [PubMed] [Google Scholar]
- 12. Keele B. F., et al. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 105:7552–7557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keele B. F., et al. 2009. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J. Exp. Med. 206:1117–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kirmaier A., et al. 2010. TRIM5 suppresses cross-species transmission of a primate immunodeficiency virus and selects for emergence of resistant variants in the new species. PLoS Biol. 8:e1000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knapp L. A., Lehmann E., Piekarczyk M. S., Urvater J. A., Watkins D. I. 1997. A high frequency of Mamu-A*01 in the rhesus macaque detected by PCR-SSP and direct sequencing. Tissue Antigens 50:657–661 [DOI] [PubMed] [Google Scholar]
- 16. Kono K., et al. 2010. Multiple sites in the N-terminal half of simian immunodeficiency virus capsid protein contribute to evasion from rhesus monkey TRIM5α-mediated restriction. Retrovirology 7:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Letvin N. L., et al. 2006. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 312:1530–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lim S. Y., et al. 2010. Contributions of Mamu-A*01 status and TRIM5 allele expression, but not CCL3L copy number variation, to the control of SIVmac251 replication in Indian-origin rhesus monkeys. PLoS Genet. 6:e1000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lim S. Y., et al. 2010. TRIM5α modulates immunodeficiency virus control in rhesus monkeys. PLoS Pathog. 6:e1000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu J., et al. 2010. Low-dose mucosal simian immunodeficiency virus infection restricts early replication kinetics and transmitted virus variants in rhesus monkeys. J. Virol. 84:10406–10412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Masek-Hammerman K., et al. 2010. Mucosal trafficking of vector-specific CD4+ T lymphocytes following vaccination of rhesus monkeys with adenovirus serotype 5. J. Virol. 84:9810–9816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakayama E. E., Shioda T. 2010. Anti-retroviral activity of TRIM5α. Rev. Med. Virol. 20:77–92 [DOI] [PubMed] [Google Scholar]
- 23. Owens C. M., Yang P. C., Gottlinger H., Sodroski J. 2003. Human and simian immunodeficiency virus capsid proteins are major viral determinants of early, postentry replication blocks in simian cells. J. Virol. 77:726–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pal R., et al. 2002. ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J. Virol. 76:292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perez-Caballero D., Hatziioannou T., Yang A., Cowan S., Bieniasz P. D. 2005. Human tripartite motif 5α domains responsible for retrovirus restriction activity and specificity. J. Virol. 79:8969–8978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pertel T., et al. 2011. TRIM5 is an innate immune sensor for the retrovirus capsid lattice. Nature 472:361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rold C. J., Aiken C. 2008. Proteasomal degradation of TRIM5α during retrovirus restriction. PLoS Pathog. 4:e1000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Romano J. W., Williams K. G., Shurtliff R. N., Ginocchio C., Kaplan M. 1997. NASBA technology: isothermal RNA amplification in qualitative and quantitative diagnostics. Immunol. Invest. 26:15–28 [DOI] [PubMed] [Google Scholar]
- 29. Shibata R., Sakai H., Kawamura M., Tokunaga K., Adachi A. 1995. Early replication block of human immunodeficiency virus type 1 in monkey cells. J. Gen. Virol. 76(Pt. 11):2723–2730 [DOI] [PubMed] [Google Scholar]
- 30. Stremlau M., Perron M., Welikala S., Sodroski J. 2005. Species-specific variation in the B30.2(SPRY) domain of TRIM5α determines the potency of human immunodeficiency virus restriction. J. Virol. 79:3139–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tareen S. U., Emerman M. 2011. Human Trim5α has additional activities that are uncoupled from retroviral capsid recognition. Virology 409:113–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vaccari M., et al. 2008. CD4+ T-cell loss and delayed expression of modulators of immune responses at mucosal sites of vaccinated macaques following SIVmac251 infection. Mucosal Immunol. 1:497–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vaccari M., et al. 2008. Reduced protection from simian immunodeficiency virus SIVmac251 infection afforded by memory CD8+ T cells induced by vaccination during CD4+ T-cell deficiency. J. Virol. 82:9629–9638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vaccari M., Trindade C. J., Venzon D., Zanetti M., Franchini G. 2005. Vaccine-induced CD8+ central memory T cells in protection from simian AIDS. J. Immunol. 175:3502–3507 [DOI] [PubMed] [Google Scholar]
- 35. Wu X., Anderson J. L., Campbell E. M., Joseph A. M., Hope T. J. 2006. Proteasome inhibitors uncouple rhesus TRIM5α restriction of HIV-1 reverse transcription and infection. Proc. Natl. Acad. Sci. U. S. A. 103:7465–7470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang Z. Q., et al. 2002. Mamu-A*01 allele-mediated attenuation of disease progression in simian-human immunodeficiency virus infection. J. Virol. 76:12845–12854 [DOI] [PMC free article] [PubMed] [Google Scholar]