Abstract
Poxviruses are important human and animal pathogens that have evolved elaborate strategies for antagonizing host innate and adaptive immunity. The E3 protein of vaccinia virus, the prototypic member of the orthopoxviruses, functions as an inhibitor of innate immune signaling and is essential for vaccinia virus replication in vivo and in many human cell culture systems. However, the function of orthologues of E3 expressed by poxviruses of other genera with different host specificity remains largely unknown. In the present study, we characterized the E3 orthologues from sheeppox virus, yaba monkey tumor virus, swinepox virus, and myxoma virus for their ability to modulate protein kinase R (PKR) function, cytokine responses and virus pathogenicity. We found that the E3 orthologues of myxoma virus and swinepox virus could suppress PKR activation and interferon (IFN)-induced antiviral activities and restore the host range function of E3 in HeLa cells. In contrast, the E3 orthologues from sheeppox virus and yaba monkey tumor virus were unable to inhibit PKR activation. While the sheeppox orthologue was unable to restore the host range function of E3, the yaba monkey tumor virus orthologue partially restored E3-deficient vaccinia virus replication in HeLa cells, correlated with its ability to suppress IFN-induced antiviral activities. Moreover, poxvirus E3 orthologues show varying ability to inhibit the induction of antiviral and proinflammatory cytokines. Despite these in vitro results, none of the E3 orthologues tested was capable of restoring pathogenicity to E3-deficient vaccinia virus in vivo.
INTRODUCTION
Poxviruses are a diverse family of double-stranded DNA viruses which replicate exclusively in the cytoplasm of infected cells (26). The Poxviridae include numerous important human and animal pathogens such as variola virus, the causative agent of smallpox, and monkeypox virus, an emerging zoonosis also capable of causing lethal disease in humans. Poxviruses infecting vertebrate animals encode a large array of immunomodulatory proteins that function to suppress cellular innate and adaptive immunity (27, 36). Many innate antiviral defense mechanisms are triggered by viral double-stranded RNA (dsRNA) generated during virus replication. The E3 protein of vaccinia virus, the prototypic member of the Orthopoxvirus genus, antagonizes several dsRNA-activated signaling pathways (13, 15, 29). The E3 protein is composed of a carboxy-terminal dsRNA binding domain and an amino-terminal Z-DNA binding domain. While wild-type vaccinia virus displays a broad cellular tropism and is highly resistant to the effects of interferon (IFN), deletion of E3 results in restricted tropism (5, 12) and sensitivity to IFNs (2, 4). Although the amino-terminal domain of E3 is dispensable in cell culture for virus replication, it is required for pathogenicity in vivo (7), where it contributes to neurovirulence of the virus (6).
The primary function of E3 identified to date is the inhibition of dsRNA-activated protein kinase R (PKR) function (13). This inhibitory activity requires the dsRNA binding domain of E3 (11). PKR regulates numerous antiviral responses (16), and the replication of E3L-deficient vaccinia virus can be partially restored when PKR expression is suppressed (44). Activated PKR phosphorylates the serine 51 residue of the alpha subunit of eukaryotic initiation factor 2 (eIF2α), resulting in a shutdown of general protein translation, an event believed to suppress viral replication. However, recent evidence suggests that PKR-dependent formation of cytoplasmic antiviral granules, an event downstream of eIF2α phosphorylation, suppresses E3L-deficient vaccinia virus replication (39). Thus, the overall contribution of downstream targets of PKR to the inhibition of vaccinia virus replication in cell culture remains to be clarified. In addition to the above described functions, PKR can also regulate vaccinia virus induced apoptosis and cytokine expression (29, 44).
Several viral and bacterial proteins have been reported to complement deletion of E3L from the vaccinia virus genome. For example, whereas vaccinia virus lacking E3L cannot replicate in HeLa cells, expression of the RNase III gene of Escherichia coli reverses this phenotype (38). RNase III mutants able to bind dsRNA but lacking RNase catalytic activity poorly rescue viral replication, suggesting that E3 dsRNA binding alone may not be sufficient for its host range function. Moreover, the IFN resistance phenotype can be restored in E3L-deficient viruses by expression of the reovirus S4 gene (4). While the E3L gene is relatively well conserved among orthopoxviruses, there is comparatively less sequence conservation among more distantly related members of the Poxviridae. To date, only the orf virus E3L orthologue has been studied and was shown to restore IFN resistance to E3L-deficient vaccinia virus (42).
Analysis of the interplay between poxvirus proteins and the host innate immune system is important for understanding the pathogenicity of poxviruses and may foster a greater understanding of the barriers mediating the strict species tropism of poxviruses. While there is concern that variola virus could be released as a bioterrorist agent, other poxviruses also remain a public health threat. For example, monkeypox virus represents an emerging zoonotic pathogen capable of causing severe disease in humans (10, 31). Also, infection of sheep and goats with sheeppox virus in developing countries can be a significant economic burden due to lost productivity (3). Besides the threat posed to human and animal health by poxviruses, there is also a considerable effort under way to develop recombinant poxvirus-based vaccine vectors. Among such vectors, vaccinia virus E3L mutants are being investigated as a vaccine platform due to their greater safety profile in comparison to wild-type vaccinia virus (18, 41). Moreover, the use of myxoma virus as an oncolytic agent for the treatment of cancer is being studied (23). Optimizing the efficacy of such vaccines and oncolytic agents will require a greater understanding of the interplay between poxvirus proteins and the immune system (30).
In the present study, we performed a comparative analysis of poxvirus orthologues of the vaccinia virus E3 protein. Specifically, we analyzed the role of the Nigeria goat and sheeppox virus (SPPV) (genus Capripoxvirus) SPPV34L (40), yaba monkey tumor virus (YMTV) (genus Yatapoxvirus) YMTV34L (8), swinepox virus (SPV) (genus Suipoxvirus) SPV032L (1), and myxoma virus (MYXV) (genus Leporipoxvirus) M029L (9) E3L orthologues to modulate PKR function, cytokine responses, and virus pathogenicity. To our knowledge, we provide the first characterization of the function of these four viral proteins. We found these proteins differ in their ability to complement important functions of E3, including suppression of PKR activation and cytokine expression and inhibition of the antiviral effects of IFN. However, in vivo, none of the E3 orthologues tested was able to restore pathogenicity to E3-deficient vaccinia virus.
MATERIALS AND METHODS
Cell culture and viruses.
HeLa, Huh7, BHK21, RK13, PK15, OA3.Ts, and CV-1 cells were maintained in Dulbecco modified Eagle medium (Gibco), supplemented with 10% fetal calf serum and 1% penicillin-streptomycin at 37°C in 5% CO2. SPV virus was a gift from Richard Moyer, University of Florida, and YMTV and MYXV were gifts from Grant McFadden, University of Florida. SPPV was from the Canadian Food Inspection Agency collection and originally obtained from the Institute for Animal Health, Pirbright, United Kingdom.
Construction of recombinant viruses.
All recombinant viruses were generated by homologous recombination and based on the Western Reserve strain of vaccinia virus. Generation of the E3L-deficient virus (ΔE3L) was previously described (25). For generation of the revertant virus, ΔE3L-Rev, the E3L gene, under the control of its authentic promoter, was reinserted back into the E3L locus, as per previously described protocols (2). Recombinant viruses expressing SPPV34L, YMTV34L, SPV032L, and M029L were generated in a manner similar to that for ΔE3L-Rev. Expression of all E3 orthologues is driven by a synthetic poxvirus early/late promoter. The SPPV34L and YMTV34L ORFs were synthesized by Genscript, while the ORFs of SPV032L and M029L were amplified from swinepox virus and myxoma virus genomic DNA, respectively. All viruses express enhanced green fluorescent protein (eGFP) under the control of a poxvirus early/late promoter. The eGFP coding sequence was cloned into the cassette used to replace E3L by homologous recombination (2). Thus, eGFP is expressed from the same genomic locus as E3 or the E3 orthologues in the respective viruses. A hemagglutinin (HA) tag was added at the N termini of SPPV34L, YMTV34L, SPV032L, and M029L to facilitate protein detection.
Virus yield assays.
Confluent BHK21 and HeLa cells in six-well plates were infected with the indicated viruses at a multiplicity of infection (MOI) of 0.5. After a 1-h incubation at 37°C, the virus inoculum was removed, fresh growth media were added, and cells were collected at 5 and 48 h postinfection (hpi). The virus was released by three cycles of freeze and thaw. Serial dilutions of each sample were then added to confluent BHK21 cells in 12-well plates. eGFP-positive plaques were counted at 24 hpi by fluorescence microscopy.
Antibodies and reagents.
The total PKR, cleaved caspase-7, and cleaved PARP antibodies were purchased from Cell Signaling Technologies. Phospho-specific PKR antibody (Thr446) was purchased from Epitomics. Eukaryotic translation initiation factor 2α (eIF2α) antibodies were from Invitrogen, and the actin and HA antibodies were from Sigma. The E3 and D12 polyclonal antibodies were generated by Genscript, and the H3 antibody was a generous gift from Wen Chang, Institute of Molecular Biology, Academia Sinica, Taiwan.
RT-PCR.
RNA was extracted by using an RNeasy minikit (Qiagen). Reverse transcription-PCR (RT-PCR) was performed on 1 μg of RNA using a QuantiTect RT kit (Qiagen). PCR was performed with GoTaq Green (Promega). The GAPDH (glyceraldehyde-3-phosphate dehydrogenase) forward primer sequence is 5′-AAGGTGAAGGTCGGAGTCAA-3′, and the reverse primer sequence is 5′-TTACTCCTTGGAGGCCATGT-3′. The RNase L forward primer sequence is 5′-TATGGCTTCACAGCCTTCATGGAA-3′, and the reverse primer sequence is 5′-ACAATCTGTACTGGCTCCACGTTT-3′.
Detection of E3L orthologue expression by RT-PCR.
Cells were pretreated with or without 50 μg of araC/ml for 1 h at 37°C. Reference cell lines were then infected with the corresponding poxvirus (SPPV, YMTV, SPV, or MYXV) in the presence or absence of araC, and cells were collected at 1, 3, and 8 hpi. RNA was extracted using an RNeasy minikit (Qiagen). RT-PCR was performed on 0.5 μg of RNA using the QuantiTect reverse transcription kit (Qiagen). PCR was performed with GoTaq Green (Promega). The SPPV34L forward primer sequence was 5′-TCACGAATTCATGTACCCATACGATGTTCCAGATTACGCTATGTATTCTTGTGATGAAGTAGATTCTT-3′, and the reverse primer sequence was 5′-TCATCCCGGGTTAAAATTTGATAATAGATGTATTGATT-3′. The YMTV34L forward primer sequence was 5′-TTAAGGATCCATGTACCCATACGATGTTCCAGATTACGCTATGGACTCTCCCGGTTGTGAGAACGA-3′, and the reverse primer sequence was 5′-ATTACCCGGGTTAAAATTTTACAATTGACGATTTTAATATCTCTTCCA-3′. The SPV032L forward primer sequence was 5′-ATTAGGATCCATGTACCCATACGATGTTCCAGATTACGCTATGTGTTCTGATATCTCAAATGAAGA-3′, and the reverse primer sequence was 5′-TGCACCCGGGTTAGAATTTTATAATAGTTTTATTTAATATGA-3′. The M029L forward primer sequence was 5′-TTAAGAATTCATGTACCCATACGATGTTCCAGATTACGCTATGGATCCCATTAACACGCTATGGCACA-3′, and the reverse primer sequence was 5′-TAATCCCGGGTTAAAACTTTATAACGACGTGTTTTAGT-3′.
Real-time PCR.
A total of 0.5 μg of RNA was reverse transcribed using a QuantiTect RT kit (Qiagen). Real-time PCR was performed using the TaqMan gene expression master mix (Applied Biosystems) on a StepOnePlus 96-well real-time PCR machine (Applied Biosystems). The actin forward primer sequence was 5′-CACACTGTGCCCATCTACGA-3′, the reverse primer sequence was 5′-GCCAGCCAGGTCCAGAC-3′, and the probe sequence was 5′-CCCATGCCATCCTGC-3′. The IFN-β forward primer sequence was 5′-TGGCTGGAATGAGACTATTGTTGAG-3′, the reverse primer sequence was 5′-CAGGACTGTCTTCAGATGGTTTATCT-3′, and the probe sequence was 5′-CCTCCTGGCTAATGTC-3′. The tumor necrosis factor alpha (TNF-α) forward primer sequence was 5′-GCCCCTCCACCCATGTG-3′, the reverse primer sequence was 5′-GGTTGACCTTGGTCTGGTAGGA-3′, and the probe sequence was 5′-ACCCACACCATCAGCC-3′. The interleukin-6 (IL-6) forward primer sequence was 5′-AGATGGATGCTTCCAATCTGGATTC-3′, the reverse primer sequence was 5′-TCAAACTCCAAAAGACCAGTGATGA-3′, and the probe sequence was 5′-ACCAGGCAAGTCTCCTCA-3′. Actin expression was used to normalize total RNA loading within each sample.
siRNA knockdown.
Approximately 1.5 × 105 HeLa cells were seeded in six-well plates. Cells were transfected with 100 nM control, PKR, or RNase L small interfering RNA (siRNA; Dharmacon) using HiPerFect (Qiagen). For the double-knockdown experiments, each siRNA was used at 50 nM. The PKR siRNA target sequence is 5′-GCGAGAAACUAGACAAAGU-3′. The RNase L siRNA target sequence is 5′-GUAAACGCCUGUGACAAUA-3′. The siRNA-transfected cells were incubated for 48 h, infected with the indicated viruses at an MOI of 1, and then collected at 24 hpi for virus yield assays.
IFN sensitivity assays.
Huh7 cell monolayers in 12-well plates were treated overnight at 37°C with or without 1,000 U of human IFN-β (PBL Biomedical Laboratories)/ml. Cell monolayers were infected at an MOI of 1 and incubated at 37°C for 1 h. Media containing the virus were then aspirated and replaced with fresh media with or without 1,000 U of IFN-β/ml. Cells were collected at 5 and 48 hpi, and virus yields were determined by plaque assays in BHK21 cells.
Western blotting.
Cells were washed in 1 ml of phosphate-buffered saline (PBS) and pelleted by centrifugation at 6,000 rpm for 2 min. Cell pellets were lysed in 200 μl of protein loading buffer. Protein samples were separated on Criterion XT precast gels (Bio-Rad) and transferred to Hybond-C nitrocellulose membranes (Amersham Bioscience). Membranes were incubated with the indicated antibodies and developed with Western Lightning Chemiluminescent Reagent Plus (Perkin-Elmer).
dsRNA binding assays.
To prepare beads for pulldown assays, CNBr-activated Sepharose 4B beads (GE Healthcare) were conjugated with a 10-mg/ml solution of poly(I-C) (pIC; Sigma) in coupling buffer (0.1 M NaHCO3 [pH 8.3], 0.5 M NaCl). Next, unconjugated active sites were blocked by incubating the beads with 0.1 M Tris-HCl (pH 8.0) for 2 h at room temperature. The beads were subsequently washed in alternating cycles of low-pH buffer (0.1 M sodium acetate [pH 4.0], 0.5 M NaCl) and high-pH buffer (0.1 M Tris-HCl [pH 8.0], 0.5 M NaCl). Beads were stored at 4°C in 0.1 M Tris-HCl (pH 7.0). For pulldown assays, confluent BHK21 cells were infected with the indicated viruses at an MOI of 1. Cells were collected at 16 hpi and washed with PBS. Cells were then resuspended in nondenaturing lysis buffer (20 mM Tris-HCl [pH 8.0], 137 mM NaCl, 10% glycerol, 1% Triton X-100, 2 mM EDTA) in the presence of protease inhibitors (Roche) and incubated on ice for 30 min. Samples were then centrifuged at 5,000 × g for 10 min at 4°C. Clarified supernatant was then incubated with end-over-end mixing for 2 h at room temperature with pIC-coated Sepharose beads. Beads were then washed three times with nondenaturing lysis buffer and incubated for 10 min at 95°C in protein loading buffer to elute associated proteins. Samples were then analyzed by Western blotting.
Pathogenicity assay in mice.
All animal experiment protocols were reviewed and approved by the National Microbiology Laboratory Animal Care Committee of the Canadian Science Centre for Human and Animal Health. Then, 4- to 6-week-old female BALB/c mice were obtained from Charles River. Animals (four animals per group) were housed in separate cages for each experimental group. For infections, the mice were anesthetized with isoflurane and infected via intranasal inoculation with 105 or 106 PFU per mouse as indicated. Animals were monitored daily for clinical signs and weight loss. The animals were euthanized at an endpoint defined as a weight loss of >20% in combination with clinical signs including piloerection, hunched posture, and inactivity in the cage.
RESULTS
Amino acid sequence comparison of E3 orthologues.
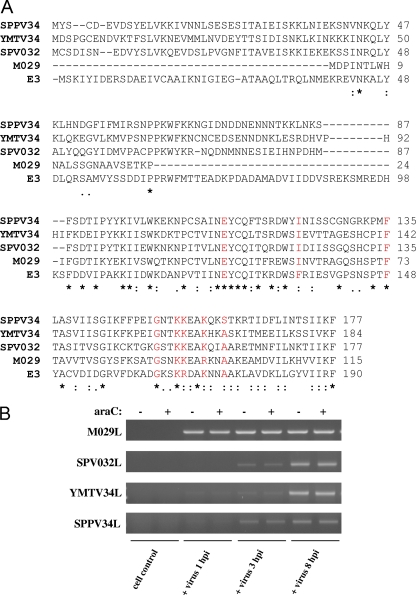
The amino acid sequences of E3, SPPV34, YMTV34, SPV032, and M029 proteins were aligned using CLUSTAL W2 (Fig. 1A). The SPPV34, YMTV34, SPV032, and M029 proteins share approximately 36, 32, 31, and 43% amino acid sequence identities with E3, respectively. Overall, the C-terminal dsRNA binding domains display comparatively higher amino acid conservation than do the N-terminal Z-DNA binding domains. It should also be noted that MYXV M029 has deletions in the N terminus in comparison to E3 and the other poxvirus orthologues. Previously, amino acid residues E124, F135, F148, G164, K167, R168, K171, and A174 have been shown to contribute to E3 dsRNA binding (11, 17) and are highlighted in red. E124, F148, G164, and K167 are conserved in each of the E3 orthologues in comparison to E3. F135I and R168K conserved substitutions are present in the sequence of each orthologue in comparison to E3. The K171 residue is also conserved in all of the orthologues except M029, where a K→R substitution has occurred, while A174 is conserved in each orthologue except SPPV34L, where an A→S substitution has occurred. Therefore, residues previously identified to be important for dsRNA binding by E3 are relatively well conserved among the proteins analyzed in the present study.
Fig. 1.
E3L orthologues are early genes whose protein products display significant amino acid divergence. (A) Protein sequence alignments were generated using the CLUSTAL W2 program. The “.” symbol denotes semiconserved substitutions, the “:” symbol denotes conserved substitutions, and the “*” symbol denotes complete conservation of the residue across all sequences. The amino acids highlighted in red indicate residues previously identified to contribute to E3 dsRNA binding. (B) OA3.Ts, CV-1, PK15, and RK13 cells were infected with SPPV, YMTV, SPV, and MYXV, respectively. Infections were performed in the presence or absence of araC, and cells were collected at 1, 3, and 8 hpi. The expression of SPPV34L, YMTV34L, SPV032L, and M029L was detected by RT-PCR in cells infected with the respective viruses.
The E3L orthologues SPPV34L, YMTV34L, SPV032L, and M029L are early genes.
Next, it was determined whether SPPV, YMTV, SPV, and MYXV express their respective E3L orthologue and whether these genes belong to the early class of poxvirus genes, as E3L does. To this end, reference cell lines OA3.Ts, CV-1, PK15, and RK13 were infected with SPPV, YMTV, SPV, and MYXV, respectively. Infections were performed in the presence or absence of araC to inhibit viral DNA replication and therefore intermediate and late poxvirus gene expression. It was found that each of the viruses expressed their respective E3L orthologue within infected cells (Fig. 1B). M029L expression was detected as early as 1 hpi, while the expression of SPV032L, YMTV34L, and SPPV34L was not detected until 3 hpi, with increased transcript levels present at 8 hpi. The expression of these E3L orthologues was not inhibited by araC treatment, indicating that SPPV34L, YMTV34L, SPV032L, and M029L are early genes.
SPV032 and M029, but not SPPV34 or YMTV34, restore the host range function of E3 in HeLa cells.
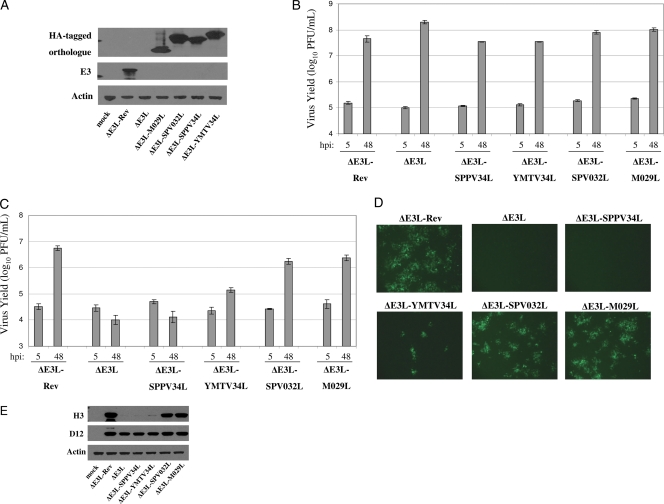
To investigate the potential of poxvirus orthologues of vaccinia virus E3 to complement the loss of E3, we constructed recombinant vaccinia viruses expressing the sheeppox virus SPPV34L, yaba monkey tumor virus YMTV34L, swinepox virus SPV032L, and myxoma virus M029L genes in place of the E3L locus. An HA tag was added to the amino terminus of each orthologue gene to facilitate detection of the proteins, a modification that has been shown to have no effect on the ability of E3 to bind dsRNA or inhibit PKR (34). We previously described the generation of a vaccinia virus mutant lacking the E3L gene (ΔE3L) (25). We also constructed a control revertant virus based on ΔE3L in which the E3L gene was reinserted into the original E3L locus (ΔE3L-Rev). BHK21 cells were infected with the recombinant viruses to detect expression of the gene products. As expected, E3 was only detected in ΔE3L-Rev-infected cells (Fig. 2A). Expression of SPPV34, YMTV34, SPV032, and M029 was detected in cells infected with the respective viruses.
Fig. 2.
SPV032 and M029 restore ΔE3L replication in HeLa cells. (A) BHK21 cells were infected with the indicated viruses and collected at 24 hpi. Western blotting was used to detect expression of actin, E3, and HA-tagged SPPV34, YMTV34, SPV032, and M029. (B) BHK21 cells were infected with the indicated viruses at an MOI of 0.5 and collected at 5 and 48 hpi. Virus yields were determined by plaque assays in BHK21 cells. (C) HeLa cells were infected with the indicated viruses at an MOI of 0.5 and collected at 5 and 48 hpi. Virus yields were determined by plaque assays in BHK21 cells. (D) HeLa cells were infected with the indicated viruses at an MOI of 0.01, and eGFP expression was analyzed by fluorescence microscopy at 24 hpi. Microscope settings were kept consistent for all images taken. (E) HeLa cells were infected with each of the indicated viruses at an MOI of 5 and collected at 6 hpi. Western blotting was used to analyze expression of vaccinia virus D12 and H3. The results are representative of three independent experiments. Error bars represent the standard errors of the mean.
The capacity of each virus to replicate in BHK21 cells (permissive for ΔE3L) and HeLa cells (nonpermissive for ΔE3L) (5) was compared. BHK21 and HeLa cells were infected with each of the recombinant viruses and collected at 5 and 48 hpi to determine the virus yield. All of the recombinant viruses produced comparable viral yields in BHK21 cells (Fig. 2B). In HeLa cells, ΔE3L-SPPV34L was unable to replicate (Fig. 2C). In contrast, ΔE3L-SPV032L and ΔE3L-M029L replicated to titers comparable to ΔE3L-Rev. An intermediate virus yield was obtained from cells infected with ΔE3L-YMTV34L. Each of the recombinant viruses expresses eGFP under the control of a poxvirus early/late promoter. eGFP expression was detected by fluorescence microscopy at 24 hpi (when eGFP expression is driven by late promoter activity) in infected cells (Fig. 2D). Robust expression of eGFP was detected in ΔE3L-Rev-, ΔE3L-SPV032L-, and ΔE3L-M029L-infected cells and a lower level of eGFP in ΔE3L-YMTV34L-infected cells. EGFP was not easily detectable in ΔE3L- and ΔE3L-SPPV34L-infected cells. Since the hallmark of the restricted replication phenotype of ΔE3L in HeLa cells is a block in intermediate and late protein translation (24), the expression of the vaccinia virus D12 (early) and H3 (late) proteins was analyzed in infected HeLa cells (Fig. 2E). D12 protein expression was comparable in all infected cells. In contrast, the H3 protein was almost undetectable in ΔE3L- and ΔE3L-SPPV34L-infected cells, while a faint band was detected in ΔE3L-YMTV34L-infected cells. Robust expression of H3 was observed in ΔE3L-Rev-, ΔE3L-SPV032L-, and ΔE3L-M029L-infected cells. Thus, late protein translation is consistent with the virus yield data obtained from HeLa cells.
SPV032 and M029, but not SPPV34 or YMTV34, inhibit PKR antiviral activity.
PKR is a major antiviral protein limiting the replication of E3L-deficient vaccinia virus in HeLa cells (44). Therefore, the activation of PKR, as measured by phosphorylation of threonine-446, and its downstream substrate, eIF2α, was examined by Western blotting. Robust phosphorylation of PKR and eIF2α was detected in ΔE3L-infected, but not ΔE3L-Rev-infected, HeLa cells (Fig. 3A). Significant PKR and eIF2α phosphorylation were also observed in ΔE3L-SPPV34L- and ΔE3L-YMTV34L-infected cells. In contrast, phosphorylation of PKR and eIF2α was significantly inhibited in ΔE3L-SPV032L-infected cells and completely blocked in ΔE3L-M029L-infected cells. In addition to mediating eIF2α phosphorylation, PKR also regulates apoptosis induced by ΔE3L (21, 44). The activation of apoptosis was examined in cells infected with each of the recombinant viruses by Western blotting to detect cleavage of caspase-7 and poly(ADP-ribose) polymerase (PARP) as apoptotic markers (28). Cleavage of both caspase-7 and PARP was detected in ΔE3L and ΔE3L-SPPV34L-infected cells and to a lesser degree in ΔE3L-YMTV34L-infected cells (Fig. 3B). In contrast, cleavage of these apoptotic markers was not detected in ΔE3L-Rev- and ΔE3L-M029L-infected cells, and minimal cleavage of caspase-7 was detected in ΔE3L-SPV032L-infected cells.
Fig. 3.
SPV032 and M029 suppress PKR activation. (A) HeLa cells were infected with the indicated viruses at an MOI of 5 and collected at 6 hpi. The phosphorylation of PKR and eIF2α was assessed by Western blotting. (B) HeLa cells were infected with the indicated viruses at an MOI of 5 and collected at 9 hpi. Cleavage of caspase-7 and PARP was assessed by Western blotting. The results are representative of four independent experiments.
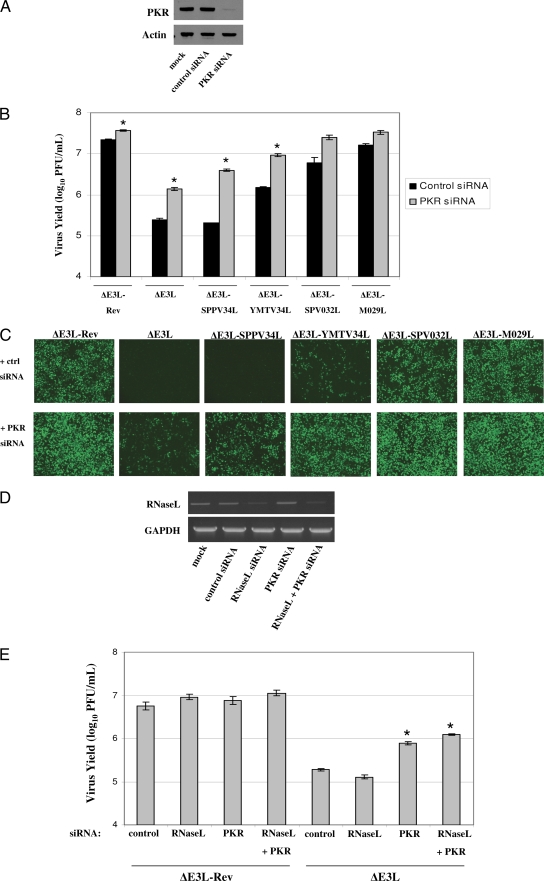
Given that PKR suppresses ΔE3L replication, we also sought to determine whether PKR inhibits the replication of the E3L orthologue-expressing viruses. To this end, HeLa cells were treated with control or PKR specific siRNAs (Fig. 4A), infected with each of the recombinant viruses, and virus yields were determined at 24 hpi (Fig. 4B). In comparison to control siRNA treated cells, treatment of cells with PKR siRNAs significantly increased virus yields in ΔE3L-, ΔE3L-SPPV34L-, and ΔE3L-YMTV34L-infected cells. Virus yields were also moderately increased in ΔE3L-SPV032L- and ΔE3L-M029L-infected cells treated with PKR siRNAs, although these results were not statistically significant (P > 0.05). Expression of virally expressed eGFP at 24 hpi in control and PKR siRNA-treated cells correlated well with the virus yield data (Fig. 4C).
Fig. 4.
PKR suppresses replication of ΔE3L-SPPV34L and ΔE3L-YMTV34L. (A) HeLa cells were mock transfected or transfected with control or PKR siRNA at 100 nM for 48 h. Western blotting was used to determine the expression of PKR and actin. (B) HeLa cells were treated as in panel A and then infected at an MOI of 1 with the indicated virus. Cells were collected at 24 hpi, and virus yields were determined by plaque assays in BHK21 cells. (C) eGFP expression was analyzed by fluorescence microscopy at 24 hpi in the siRNA-treated, virus-infected cells evaluated in panel B. Microscope settings were kept consistent for all images taken. (D) HeLa cells were mock transfected or transfected with control, RNase L, or PKR siRNA at 100 nM for 48 h. Cells were also transfected with both RNase L (50 nM) and PKR siRNA (50 nM) for 48 h. The expression of GAPDH and RNase L was analyzed by RT-PCR. (E) HeLa cells were transfected as in panel D and then infected at an MOI of 1 with the indicated virus. The cells were collected at 24 hpi, and virus yields were determined by plaque assays in BHK21 cells. The results are representative of three independent experiments. Error bars represent the standard errors of the mean. The “*” symbol signifies statistically significant differences (P < 0.05) in virus yield in comparison to control siRNA-treated cells infected with the same virus.
RNase L represents another host protein with antiviral function that can be activated in ΔE3L-infected cells (33). In an attempt to identify additional host antiviral factors which may limit replication of ΔE3L in cell culture, the role of RNase L was also addressed using an siRNA approach. HeLa cells were transfected with siRNAs targeting RNase L, PKR, or a combination of both RNase L and PKR siRNAs to assess the effects of a double knockdown. The knockdown efficiency of RNase L was analyzed by RT-PCR since it is difficult to detect RNase L protein by Western blotting in HeLa cells (Fig. 4D). There was no increase in ΔE3L virus yields in RNase L siRNA treated cells, in contrast to PKR siRNA-treated cells (Fig. 4E). Furthermore, the combined suppression of RNase L and PKR expression did not significantly increase virus yields in comparison to PKR siRNA-treated cells.
SPPV34, YMTV34, SPV032, and M029 bind dsRNA.
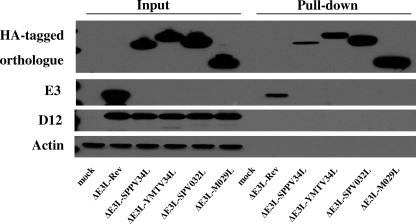
Binding to dsRNA is believed to be a critical function of E3 for suppression of PKR activation. The dsRNA binding capacity of the E3 orthologues was tested using pIC-conjugated Sepharose beads. BHK21 cells were infected overnight with each of the recombinant viruses, and clarified supernatants from these cell lysates were used in pulldown assays. As controls, we analyzed the presence of actin and vaccinia virus D12, which are not known to bind dsRNA. Although these proteins were detected in pre-pull-down samples, neither was detected following pull-down with pIC beads (Fig. 5). All E3 orthologues were pulled-down using pIC beads. However, significantly less SPPV34 was found associated with the pIC beads in comparison to the other E3 orthologues. We were also able to pull down E3 and observed less E3 associated with pIC beads in comparison to YMTV34, SPV032, and M029. We did not observe binding of any of these proteins to beads coated with the ssRNA analogue, poly(C) (data not shown).
Fig. 5.
dsRNA binding capacity of orthologues of the E3 protein. BHK21 cells were infected with the indicated viruses at an MOI of 1 for 16 h. Cells were then collected and lysed in nondenaturing lysis buffer. Lysates were incubated with pIC-conjugated beads, and proteins associated with the beads were isolated using pulldown assays. The presence of actin, D12, E3, HA-tagged SPPV34, YMTV34, SPV032, and M029 were analyzed by Western blotting. The results are representative of four independent experiments.
Differential suppression of cytokine expression by E3 orthologues.
Previously, we demonstrated that E3 suppresses cytokine induction through inhibition of PKR-, IRF3-, and NF-κB-dependent pathways (29). We next assessed the ability of the poxvirus orthologues to complement the function of E3 in regard to suppressing cytokine induction. HeLa cells were infected with the recombinant viruses and collected during the late phase of infection. The expression of IFN-β, TNF-α, and IL-6 was quantified by real-time PCR (Fig. 6). Consistent with previous reports (20, 29), infection of HeLa cells with ΔE3L resulted in increased IFN-β, TNF-α, and IL-6 gene transcription. In comparison to ΔE3L-Rev-infected cells, expression of IFN-β was higher in cells infected with each of the E3 orthologue expressing viruses with the exception of ΔE3L-M029L. In comparison to ΔE3L-infected cells, IFN-β expression was moderately reduced in ΔE3L-SPPV34L-infected cells and more significantly reduced in ΔE3L-YMTV34L- and ΔE3L-SPV032L-infected cells. TNF-α gene transcription was moderately reduced in ΔE3L-SPPV34L- and ΔE3L-YMTV34L-infected cells in comparison to cells infected with ΔE3L and more significantly reduced in ΔE3L-SPV032L- and ΔE3L-M029L-infected cells. Infection of cells with all of the recombinant viruses except ΔE3L-M029L also induced greater expression of IL-6 in comparison to ΔE3L-Rev-infected cells. IL-6 expression was slightly reduced in ΔE3L-SPPV34L- and ΔE3L-SPV032L-infected cells compared to ΔE3L-infected cells. The expression of IL-6 was comparable in ΔE3L- and ΔE3L-YMTV34L-infected cells.
Fig. 6.
Inhibition of cytokine expression by orthologues of the E3 protein. HeLa cells were infected with the indicated viruses at an MOI of 5 and collected at 9 hpi. The expression of IFN-β (A), TNF-α (B), and IL-6 (C) was quantified by real-time PCR. Shown is the gene expression fold change in infected cells in comparison to mock-infected cells. Actin expression was used as an internal normalization control for total RNA loading. Error bars represent one standard deviation. The results are representative of four independent experiments.
YMTV34, SPV032, and M029, but not SPPV34, inhibit IFN-β-induced antiviral activity.
Wild-type vaccinia virus is highly resistant to IFN treatment, while deletion of the E3L gene results in an IFN-sensitive phenotype (2, 4). We sought to determine whether SPPV34, YMTV34, SPV032, or M029 could compensate for the function of E3 in inhibiting the antiviral effects induced by IFN-β. To this end, Huh7 cells were treated with or without IFN-β overnight and then infected with each of the recombinant viruses. Virus yields were determined at 5 and 48 hpi by plaque assays. IFN-β treatment completely blocked the replication of ΔE3L but did not affect ΔE3L-Rev replication (Fig. 7A). Replication of ΔE3L-SPPV34L was also completely abolished in IFN-β-treated cells. In contrast, IFN-β treatment had no effect on ΔE3L-SPV032L and ΔE3L-M029L replication, while replication of ΔE3L-YMTV34L was slightly inhibited in the presence of IFN-β. The phosphorylation of PKR and eIF2α was examined in IFN-β-treated cells infected with each of the recombinant viruses (Fig. 7B). IFN-β treatment upregulated the expression of PKR in all samples. The phosphorylation of PKR was enhanced in IFN-β-treated cells infected with ΔE3L, ΔE3L-SPPV34L, and ΔE3L-YMTV34L. In ΔE3L-Rev-, ΔE3L-SPV032L-, and ΔE3L-M029L-infected cells, PKR is not phosphorylated in the absence of IFN-β. However, following IFN-β treatment, a low level of PKR phosphorylation is detected during infection with these viruses. In the absence of IFN-β treatment, phosphorylation of eIF2α generally correlated with the activation of PKR. However, IFN-β-induced enhancement of PKR expression and activation did not increase the level of phosphorylated eIF2α. Phosphorylation of eIF2α was also slightly lower in IFN-β-treated cells infected with ΔE3L-SPPV34L compared to untreated cells. Although this result was consistently observed, we currently do not understand the basis for this observation.
Fig. 7.
Inhibition of the antiviral effects of IFN-β by orthologues of the E3 protein. (A) Huh7 cells were treated overnight with 1,000 U of recombinant human IFN-β/ml. Cells were then infected with the indicated viruses in the presence or absence of 1,000 U of IFN-β/ml and collected at 5 and 48 hpi. Virus yields were determined by plaque assays in BHK21 cells. (B) Huh7 cells were treated overnight with 1,000 U of recombinant human IFN-β/ml. Cells were then infected with the indicated viruses at an MOI of 5 and collected at 6 hpi. The phosphorylation of PKR and eIF2α was analyzed by Western blotting. The results are representative of three independent experiments.
SPPV34, YMTV34, SPV032, and M029 do not restore pathogenicity to E3-deficient vaccinia virus in mice.
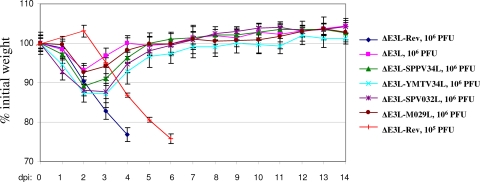
The pathogenesis of the recombinant viruses was tested in a BALB/c mouse model of vaccinia virus infection via intranasal inoculation. First, the pathogenicity of wild-type vaccinia virus was compared to that of the control ΔE3L-Rev virus. No significant differences were found in either weight loss or survival following infection of mice with 106 PFU/mouse of either virus (data not shown). Based on experiments infecting mice with different doses of ΔE3L-Rev, it was found that 105 PFU/mouse caused 100% mortality by 6 days postinfection (dpi) (Fig. 8). Next, mice were infected (four mice per experimental group) with 106 PFU of the indicated recombinant virus/mouse. It was reasoned that 106 PFU/mouse would be sufficient to observe differences in the pathogenicity of the recombinant viruses since this dose is at least 10 times greater than the dose of ΔE3L-Rev required to cause 100% lethality in this model. Animals were monitored daily for weight loss and clinical signs. Mice infected with 106 PFU of ΔE3L-Rev began to lose weight at 1 dpi and reached >20% weight loss by 4 dpi (Fig. 8). All animals in the ΔE3L-Rev group were euthanized at 4 dpi (106 PFU/mouse) or 6 dpi (105 PFU/mouse) on the basis of weight loss and severe clinical signs, including piloerection, inactivity, and hunched posture. In contrast, the ΔE3L virus was not pathogenic, and mice infected with this virus displayed only minor clinical signs. These results for ΔE3L-Rev and ΔE3L are similar to previous results with wild-type and E3L-deficient vaccinia virus (7, 32, 43). Similar to ΔE3L, all of the recombinant viruses expressing orthologues of E3L did not cause serious disease at a dose of 106 PFU/mouse. Mice infected with ΔE3L-SPPV34L, ΔE3L-YMTV34L, ΔE3L-SPV032L, or ΔE3L-M029L reached a maximum weight loss at 2 to 3 dpi, with ΔE3L-YMTV34L- and ΔE3L-SPV032L-infected mice experiencing the greatest weight loss (ca. 13%). The clinical signs in mice infected with the E3 orthologue-expressing recombinant viruses were mild and transient.
Fig. 8.
SPPV34, YMTV34, SPV032, and M029 cannot complement the deletion of E3 in vivo. BALB/c mice were infected with 105 or 106 PFU per mouse of the indicated viruses via intranasal inoculation. Animals were weighed daily and monitored for clinical signs. Mice were euthanized when they reached >20% weight loss in combination with clinical signs including inactivity, hunched posture, and piloerection.
DISCUSSION
All vertebrate poxviruses encode orthologues of E3, with the exception of the avipoxviruses and molluscum contagiosum virus. Currently, little is known about the function of orthologues of E3 encoded by other poxviruses. We chose to study orthologues of E3 encoded by poxviruses outside the orthopoxvirus genus, since the sequence similarity is high for orthologues of E3 within the Orthopoxviridae. The E3, SPPV34, YMTV34, SPV032, and M029 proteins display considerable divergence, particularly in the Z-DNA binding domain (Fig. 1A). Since the N terminus of E3 is required for in vivo pathogenesis (7), it is possible that the divergence of the N-terminal domains plays a role in the species specificity of the viruses naturally expressing these E3 orthologues. The majority of highly conserved residues are located in the dsRNA binding domain and amino acids previously identified to be important for E3 dsRNA binding (11, 17) are relatively well conserved among the E3 orthologues in the present study.
We found that SPV032 and M029 restored replication of ΔE3L in HeLa cells (Fig. 2C, D, and E). In contrast, YMTV34 only moderately restored ΔE3L replication, while SPPV34 was unable to rescue replication of ΔE3L. These results suggest that YMTV34, SPV032, and M029 are able to at least partially complement the deletion of E3, whereas SPPV34 cannot. To identify the mechanisms restricting replication of ΔE3L-YMTV34L and ΔE3L-SPPV34L, we analyzed the activation of PKR, which is known to inhibit the replication of E3-deficient vaccinia virus in HeLa cells (44). M029 could completely suppress PKR activation, while SPV032 also significantly inhibited PKR activation (Fig. 3A). In contrast, neither SPPV34 nor YMTV34 was capable of inhibiting PKR phosphorylation. In support of this result, suppression of PKR expression significantly enhanced the replication of ΔE3L, ΔE3L-SPPV34L, and ΔE3L-YMTV34L, demonstrating that SPPV34 and YMTV34 do not inhibit PKR antiviral activity. Thus, the inability of SPPV34 to block PKR function explains the nonpermissive infection of HeLa cells by ΔE3L-SPPV34L.
Although robust PKR activation occurs during ΔE3L-YMTV34L infection, this virus is still capable of replicating to some degree in HeLa cells, as evidenced by virus yield assays and expression of the late protein, H3. The activation of apoptosis is slightly reduced in ΔE3L-YMTV34L-infected cells in comparison to ΔE3L (Fig. 3B), but this effect is unlikely to be related to virus replication in cell culture since it was previously shown that inhibition of apoptosis does not rescue ΔE3L (44). Although YMTV34 does not suppress PKR phosphorylation, it is possible that it inhibits PKR through a mechanism such as protein-protein interactions. However, the finding that suppression of PKR expression significantly enhances ΔE3L-YMTV34L replication argues against YMTV34 having significant PKR inhibitory function in HeLa cells. Therefore, YMTV34 may block an alternate, PKR-independent antiviral mechanism active against ΔE3L. Such a mechanism is likely to exist, given that suppression of PKR expression does not fully restore ΔE3L replication in HeLa cells (44) (Fig. 4B). ΔE3L also activates the 2′-5′ oligoadenylate synthetase/RNase L system (33). However, in HeLa cells suppression of RNase L expression does not restore ΔE3L replication (Fig. 4E). Thus, this antiviral mechanism does not inhibit ΔE3L replication in HeLa cells, and therefore it is unlikely that YMTV34 contributes to ΔE3L replication by antagonizing RNase L activity. Analysis of the function of the YMTV34 protein should prove useful in future studies to identify the mechanism(s) by which it promotes viral replication.
We found significant variability in the dsRNA binding capacity of the E3 orthologues. SPPV34 had the lowest dsRNA binding capacity, while SPV032 and M029 displayed the highest dsRNA binding capacity, which correlated with their ability to suppress PKR activation (Fig. 5). YMTV34 binds dsRNA much more efficiently than SPPV34 but is still unable to inhibit the activation of PKR. Although different antibodies must be used to detect E3 and the E3 orthologues in our study, it seems E3 binds dsRNA with less affinity than YMTV34, SPV032, and M029. Nonetheless, E3 can potently inhibit PKR activation. These results support the concept that dsRNA binding is not the sole factor in PKR inhibition, which may additionally require protein-protein interactions between E3 and PKR (34, 37). We have found that the A175 residue in E3 is also required for dsRNA binding and inhibition of PKR (Jingxin Cao, unpublished data). The corresponding alanine residue is conserved in SPV032 and M029. In YMTV34, an A→S substitution has occurred, while in SPPV34 an A→T substitution has occurred. Thus, the presence of alanine at this residue correlates with the ability of the protein to suppress PKR activation and to promote viral replication. It will be interesting to investigate further the functional consequence of mutation of this residue in E3, SPPV34, and YMTV34.
E3 suppresses the expression of cytokines during vaccinia virus infection (14, 20, 29) and also antagonizes the antiviral effects of IFN-β (2, 4). We found that all of the E3 orthologues were able to inhibit the induction of IFN-β expression, although to various degrees (Fig. 6A). M029 was the most effective inhibitor of IFN-β expression in this system, and SPPV34, the least effective. However, the partial inhibition of cytokine gene expression was the only function identified for SPVV34 in our study. We recently reported that IFN-β induction is regulated by both RIG-I and MDA5 during ΔE3L infection of HeLa cells (28). Thus, each of the E3 orthologues can inhibit RIG-I/MDA5 signaling to some extent, but whether this is the consequence of sequestering viral dsRNA or occurs through an alternate mechanism remains unknown. YMTV34, SPV032, and M029, but not SPPV34, were also able to suppress the antiviral effects of IFN-β (Fig. 7A). Induction of TNF-α and IL-6 during ΔE3L infection is partially mediated by PKR (29). SPPV34 and YMTV34 only slightly reduced TNF-α expression during virus infection, while SPV032 and M029 more significantly inhibited TNF-α expression, correlating with the ability of each viral protein to suppress PKR function. M029 was also the only E3 orthologue capable of significantly suppressing IL-6 expression in this system. IFN-β is induced by late gene products in ΔE3L-infected cells, while TNF-α and IL-6 can be induced by early gene products (15, 29). Since the E3 orthologues inhibit IFN-β expression to a greater extent than TNF-α and IL-6, these viral proteins are more effective at suppressing cytokines induced by late gene products as opposed to early gene products.
Despite results obtained in cell culture, none of the E3 orthologues tested could restore pathogenicity to ΔE3L in vivo at a dose of 106 PFU/mouse (Fig. 8). Likewise, expression of the orf virus E3 orthologue in place of E3L is sufficient to rescue virus replication in vitro but not in vivo (42). Although the dose of 106 PFU/mouse is at least 10 times higher than the dose of ΔE3L-Rev required to cause 100% lethality in our model, we cannot rule out differences in pathogenicity at higher doses of virus. Furthermore, although the HA tag added to each orthologue does not appear to affect protein function in cell culture, we cannot rule out effects of the HA tag on protein function in vivo. Currently, the role of host proteins such as PKR in the in vivo response to ΔE3L infection remains to be clarified. In one study, intranasal inoculation of PKR/RNase L double-deficient mice with ΔE3L failed to result in severe disease (43). However, in an alternate, intratracheal mouse model, inoculation with higher doses of ΔE3L resulted in severe disease in ∼80% of PKR/RNase L double-deficient mice (32). Thus, although SPV032 and M029 can suppress PKR activation and the antiviral effect of IFN-β in vitro, these functions may not be sufficient to promote viral replication following intranasal infection of mice. It has been shown that the N-terminal domain of E3 is required for pathogenesis in mice (7). The N-terminal divergence of the E3 orthologues may reflect species specific evolution and could explain the inability SPPV34, YMTV34, SPV032, and M029 to complement loss of E3 in vivo. It is also possible that inhibition of cytokine production by E3 contributes to vaccinia virus replication in vivo. For example, TNF-α treatment has no effect on vaccinia virus replication in cell culture but potently suppresses vaccinia virus replication in mice (22, 35), whereas IL-6-deficient mice are more susceptible to vaccinia virus infection than wild-type mice (19). We have found that none of the E3 orthologues tested can fully compensate for the ability of E3 to inhibit TNF-α and/or IL-6 expression, and induction of these cytokines during infection may result in activation of mechanisms to clear virus-infected cells.
In summary, we have provided the first characterization of the SPPV34, YMTV34, SPV032, and M029 proteins (Table 1). SPPV34 and YMTV34 cannot suppress PKR activity in HeLa cells. However, YMTV34 promotes vaccinia virus replication through a PKR-independent mechanism. We cannot rule out the possibility that SPPV34 and YMTV34 can inhibit PKR in the natural host during SPPV and YMTV infection, respectively. While SPV032 and M029 can complement the deletion of E3 in vitro through the inhibition of PKR activity, they cannot complement E3 function in vivo. Therefore, further work is required to understand the mechanism by which E3 supports vaccinia virus replication in vivo. It will also be interesting to study the role of the E3 orthologues in the biology of the viruses that naturally express them. Overall, our results contribute to an understanding of how poxviral proteins subvert host innate immune signaling to support virus replication.
Table 1.
Characterization of SPPV34, YMTV34, SPV032, and M029 protein function
| Characterization | Protein functiona |
|||||
|---|---|---|---|---|---|---|
| ΔE3L-Rev | ΔE3L | ΔE3L-SPPV34L | ΔE3L-YMTV34L | ΔE3L-SPV032L | ΔE3L-M029L | |
| Replication in HeLa cells | +++ | − | − | + | ++ | ++ |
| Inhibition of PKR | +++ | − | − | − | ++ | +++ |
| Inhibition of cytokine expression | +++ | − | + | + | ++ | +++ |
| Inhibition of antiviral effects of IFN-β | +++ | − | − | ++ | +++ | +++ |
| Pathogenicity in mice | +++ | − | − | − | − | − |
For comparative purposes, ΔE3L-Rev was denoted “+++”, while ΔE3L was denoted “−” for all of the functions analyzed, and each of the orthologues was compared with respect to ΔE3L-Rev and ΔE3L.
ACKNOWLEDGMENTS
This study was supported by funding from the Public Health Agency of Canada, the Natural Sciences and Engineering Council of Canada, and the Manitoba Health Research Council.
Footnotes
Published ahead of print on 14 September 2011.
REFERENCES
- 1. Afonso C. L., et al. 2002. The genome of swinepox virus. J. Virol. 76:783–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arsenio J., Deschambault Y., Cao J. 2008. Antagonizing activity of vaccinia virus E3L against human interferons in Huh7 cells. Virology 377:124–132 [DOI] [PubMed] [Google Scholar]
- 3. Babiuk S., Bowden T. R., Boyle D. B., Wallace D. B., Kitching R. P. 2008. Capripoxviruses: an emerging worldwide threat to sheep, goats and cattle. Transbound. Emerg. Dis. 55:263–272 [DOI] [PubMed] [Google Scholar]
- 4. Beattie E., et al. 1995. Reversal of the interferon-sensitive phenotype of a vaccinia virus lacking E3L by expression of the reovirus S4 gene. J. Virol. 69:499–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beattie E., et al. 1996. Host-range restriction of vaccinia virus E3L-specific deletion mutants. Virus Genes 12:89–94 [DOI] [PubMed] [Google Scholar]
- 6. Brandt T., et al. 2005. The N-terminal domain of the vaccinia virus E3L-protein is required for neurovirulence, but not induction of a protective immune response. Virology 333:263–270 [DOI] [PubMed] [Google Scholar]
- 7. Brandt T. A., Jacobs B. L. 2001. Both carboxy- and amino-terminal domains of the vaccinia virus interferon resistance gene, E3L, are required for pathogenesis in a mouse model. J. Virol. 75:850–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brunetti C. R., et al. 2003. Complete genomic sequence and comparative analysis of the tumorigenic poxvirus yaba monkey tumor virus. J. Virol. 77:13335–13347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cameron C., et al. 1999. The complete DNA sequence of myxoma virus. Virology 264:298–318 [DOI] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention 2003. Multistate outbreak of monkeypox–Illinois, Indiana, and Wisconsin, 2003. MMWR Morb. Mortal. Wkly. Rep. 52:537–540 [PubMed] [Google Scholar]
- 11. Chang H. W., Jacobs B. L. 1993. Identification of a conserved motif that is necessary for binding of the vaccinia virus E3L gene products to double-stranded RNA. Virology 194:537–547 [DOI] [PubMed] [Google Scholar]
- 12. Chang H. W., Uribe L. H., Jacobs B. L. 1995. Rescue of vaccinia virus lacking the E3L gene by mutants of E3L. J. Virol. 69:6605–6608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang H. W., Watson J. C., Jacobs B. L. 1992. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc. Natl. Acad. Sci. U. S. A. 89:4825–4829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deng L., Dai P., Ding W., Granstein R. D., Shuman S. 2006. Vaccinia virus infection attenuates innate immune responses and antigen presentation by epidermal dendritic cells. J. Virol. 80:9977–9987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deng L., et al. 2008. Vaccinia virus subverts a MAVS-dependent innate immune response in keratinocytes through its dsRNA binding protein E3. J. Virol. 82:10735–10746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia M. A., et al. 2006. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol. Mol. Biol. Rev. 70:1032–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ho C. K., Shuman S. 1996. Mutational analysis of the vaccinia virus E3 protein defines amino acid residues involved in E3 binding to double-stranded RNA. J. Virol. 70:2611–2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jentarra G. M., et al. 2008. Vaccinia viruses with mutations in the E3L gene as potential replication-competent, attenuated vaccines: scarification vaccination. Vaccine 26:2860–2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kopf M., et al. 1994. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368:339–342 [DOI] [PubMed] [Google Scholar]
- 20. Langland J. O., et al. 2006. Suppression of proinflammatory signal transduction and gene expression by the dual nucleic acid binding domains of the vaccinia virus E3L proteins. J. Virol. 80:10083–10095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee S. B., Esteban M. 1994. The interferon-induced double-stranded RNA-activated protein kinase induces apoptosis. Virology 199:491–496 [DOI] [PubMed] [Google Scholar]
- 22. Lidbury B. A., Ramshaw I. A., Sambhi S. K. 1995. The role for host-immune factors in the in vivo antiviral effects of tumour necrosis factor. Cytokine 7:157–164 [DOI] [PubMed] [Google Scholar]
- 23. Liu J., Wennier S., McFadden G. 2010. The immunoregulatory properties of oncolytic myxoma virus and their implications in therapeutics. Microbes Infect. 12:1144–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ludwig H., et al. 2006. Double-stranded RNA-binding protein E3 controls translation of viral intermediate RNA, marking an essential step in the life cycle of modified vaccinia virus Ankara. J. Gen. Virol. 87:1145–1155 [DOI] [PubMed] [Google Scholar]
- 25. Meng X., et al. 2009. Vaccinia virus K1L and C7L inhibit antiviral activities induced by type I interferons. J. Virol. 83:10627–10636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moss B. 2000. Poxviridae: the viruses and their replication, p. 2906–2945 In Knipe D. M., Howley P. M. (ed.), Fields virology, 4th ed Lippincott-Raven Publishers, Philadelphia, PA [Google Scholar]
- 27. Moss B., Shisler J. L. 2001. Immunology 101 at poxvirus U: immune evasion genes. Semin. Immunol. 13:59–66 [DOI] [PubMed] [Google Scholar]
- 28. Myskiw C., et al. 2011. RNA species generated in vaccinia virus-infected cells activate cell type-specific MDA5 or RIG-I-dependent interferon gene transcription and PKR-dependent apoptosis. Virology 413:183–193 [DOI] [PubMed] [Google Scholar]
- 29. Myskiw C., Arsenio J., van Bruggen R., Deschambault Y., Cao J. 2009. Vaccinia virus E3 suppresses expression of diverse cytokines through inhibition of the PKR, NF-κB, and IRF3 pathways. J. Virol. 83:6757–6768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pickup D. J. 2007. Understanding orthopoxvirus interference with host immune responses to inform novel vaccine design. Expert Rev. Vaccines 6:87–95 [DOI] [PubMed] [Google Scholar]
- 31. Regnery R. L. 2007. Poxviruses and the passive quest for novel hosts. Curr. Top. Microbiol. Immunol. 315:345–361 [DOI] [PubMed] [Google Scholar]
- 32. Rice A. D., et al. 2011. Roles of vaccinia virus genes E3L and K3L and host genes PKR and RNase L during intratracheal infection of C57BL/6 mice. J. Virol. 85:550–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rivas C., Gil J., Melkova Z., Esteban M., Diaz-Guerra M. 1998. Vaccinia virus E3L protein is an inhibitor of the interferon (IFN)-induced 2-5A synthetase enzyme. Virology 243:406–414 [DOI] [PubMed] [Google Scholar]
- 34. Romano P. R., et al. 1998. Inhibition of double-stranded RNA-dependent protein kinase PKR by vaccinia virus E3: role of complex formation and the E3 N-terminal domain. Mol. Cell. Biol. 18:7304–7316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sambhi S. K., Kohonen-Corish M. R., Ramshaw I. A. 1991. Local production of tumor necrosis factor encoded by recombinant vaccinia virus is effective in controlling viral replication in vivo. Proc. Natl. Acad. Sci. U. S. A. 88:4025–4029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seet B. T., et al. 2003. Poxviruses and immune evasion. Annu. Rev. Immunol. 21:377–423 [DOI] [PubMed] [Google Scholar]
- 37. Sharp T. V., et al. 1998. The vaccinia virus E3L gene product interacts with both the regulatory and the substrate binding regions of PKR: implications for PKR autoregulation. Virology 250:302–315 [DOI] [PubMed] [Google Scholar]
- 38. Shors T., Jacobs B. L. 1997. Complementation of deletion of the vaccinia virus E3L gene by the Escherichia coli RNase III gene. Virology 227:77–87 [DOI] [PubMed] [Google Scholar]
- 39. Simpson-Holley M., et al. 2010. Formation of antiviral cytoplasmic granules during Orthopoxvirus infection. J. Virol. 85:1581–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tulman E. R., et al. 2002. The genomes of sheeppox and goatpox viruses. J. Virol. 76:6054–6061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vijaysri S., et al. 2008. Vaccinia viruses with mutations in the E3L gene as potential replication-competent, attenuated vaccines: intra-nasal vaccination. Vaccine 26:664–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vijaysri S., et al. 2003. The Orf virus E3L homologue is able to complement deletion of the vaccinia virus E3L gene in vitro but not in vivo. Virology 314:305–314 [DOI] [PubMed] [Google Scholar]
- 43. Xiang Y., et al. 2002. Blockade of interferon induction and action by the E3L double-stranded RNA binding proteins of vaccinia virus. J. Virol. 76:5251–5259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang P., Jacobs B. L., Samuel C. E. 2008. Loss of protein kinase PKR expression in human HeLa cells complements the vaccinia virus E3L deletion mutant phenotype by restoration of viral protein synthesis. J. Virol. 82:840–848 [DOI] [PMC free article] [PubMed] [Google Scholar]