Abstract
Reactive oxygen species (ROS) are generated as by-products of many cellular processes and can modulate cellular signaling pathways. However, high ROS levels are toxic; thus, intracellular ROS need to be tightly controlled. Therefore, cells use a group of antioxidant molecules and detoxifying enzymes that remove or detoxify reactive species. We found that the level of the antioxidant glutathione is greatly increased in human cytomegalovirus (HCMV)-infected cells due to activation of glutathione synthetic enzymes. In addition, our data suggest that virus-specific mechanisms are used to induce the expression of target antioxidant and detoxifying enzymes critical for the success of the infection. As a result of this virus-induced anti-ROS environment, key signaling kinases, such as the mammalian target of rapamycin (mTOR) kinase in mTOR complex 1 (mTORC1), are protected from inhibition by exogenous hydrogen peroxide (H2O2). In this regard, we found that phosphorylation of mTOR kinase at serine 2448 (suggested to be activating) was maintained during infection even under ROS stress conditions that inhibited it in uninfected cells. We also show that AMP-dependent kinase (AMPK)-mediated phosphorylation of serine 792 of raptor, the specificity subunit of mTORC1, increases in infected cells after H2O2 treatment. This phosphorylation is normally inhibitory for mTORC1. However, in infected cells this did not result in inhibition of mTORC1 activity, suggesting that inhibitory effects of raptor phosphorylation are circumvented. Overall, our data suggest that HCMV utilizes virus-specific mechanisms to activate a variety of means to protect the cell and mTORC1 from the effects of ROS.
INTRODUCTION
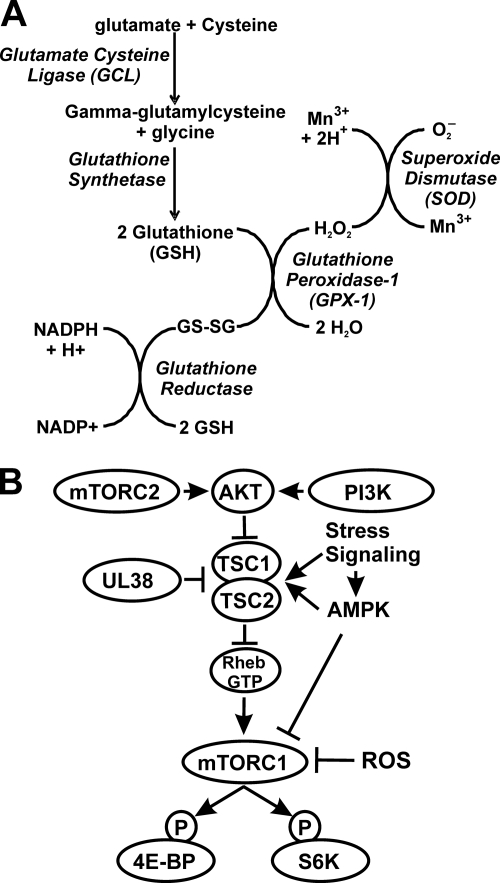
Free radicals and other reactive oxygen species (ROS) are generated as by-products of many cellular processes, primarily mitochondrial respiration. ROS can be an important modulator of cellular signaling pathways; however, if ROS levels increase too much they are capable of inflicting damage to biological macromolecules (18, 19). Given their potential toxicity, ROS need to be tightly controlled so that they do not accumulate in the cell. To do this, cells have evolved a group of antioxidant molecules and detoxifying enzymes that remove or detoxify reactive species. The thiol-containing tripeptide glutathione (GSH) is the most abundant small-molecule antioxidant. Antioxidant and detoxifying enzymes include superoxide dismutase (SOD), glutathione peroxidase 1 (GPX-1), catalase (CAT), glutathione reductase (GR), glutamate cysteine ligase (GCL), NAD(P)H:quinone oxidoreductase 1 (NQO1), heme oxygenase1 (HO-1), and other phase II xenobiotic-metabolizing enzymes, such as glutathione S-transferase (GST), UDP-glucuronyl transferase (UGT), and sulfotransferase (SULT) (11, 20, 21). These enzymes are distributed in various organelles and subcellular compartments and cooperate in interconnected reactions that eliminate ROS at the sites of origin. For example, Fig. 1A shows the integrated pathway for the synthesis, utilization, and regeneration of GSH used to rid the cells of superoxides and H2O2. Under nonstressed conditions these enzymes are expressed at levels capable of maintaining nontoxic levels of ROS within the cells so that redox-sensitive processes, such as protein folding in the endoplasmic reticulum, can operate (20, 21).
Fig. 1.
Synthesis, utilization, and regeneration of glutathione and regulation of mTORC1 activity. (A) Diagrammed is the pathway for the synthesis, utilization, and regeneration of reduced glutathione, which is needed to rid cells of superoxides (O2−) and H2O2. (B) mTORC1 activity is primarily mediated by the PI3K-Akt-TSC pathway. In this pathway, activation of PI3K leads to activation of Akt, which phosphorylates and inactivates the tuberous sclerosis complex (TSC1 and −2). This inhibits TSC's GTPase-activating protein (GAP) function, allowing the accumulation of Rheb-GTP, which activates mTORC1. In HCMV-infected cells, the activity of the TSC is inhibited by the viral protein UL38 (39). This allows Rheb-GTP levels to be maintained for mTORC1 activation. The UL38-mediated inhibition of the TSC blocks the effects of stress signaling and activated AMPK, which normally activate the TSC. However, ROS inactivation of mTORC1 is TSC independent; it has been suggested that this is mediated in part by AMPK activation and direct phosphorylation of the raptor subunit of mTORC1, which is thought to be inhibitory (22).
Human cytomegalovirus (HCMV), a betaherpesviruses, is the largest human herpesvirus, with a 230-kb double-stranded DNA genome and the potential to encode over 200 proteins (42, 43). HCMV shows slow growth in culture and, therefore, must maintain favorable cellular conditions for a long period. However, during this time, the increased transcription, translation, and metabolism that accompany infection cause cellular stress. This, in turn, results in the induction of a repertoire of cellular stress responses which provide means for the cell to survive and control the stress. Some consequences of stress response induction can be beneficial to the viral infection, while others are deleterious. We and others have shown that HCMV has multiple mechanisms to deal with the deleterious aspects of cellular stress responses while maintaining beneficial ones (2, 6, 7, 10, 23, 26, 27, 31–33, 38, 39, 52, 53). However, there has been little study on how HCMV deals with ROS and oxidative stress. HCMV infection induces the generation of ROS minutes after entry (51). This event is thought to be necessary for initiation of the virus life cycle, since high ROS levels activate NF-κB and lead to transactivation of the viral immediate-early promoter (51). However, HCMV has been shown to significantly activate and alter cellular metabolism (8, 40, 41) in ways that would significantly increase ROS throughout the infection; thus, a virally induced means to control ROS levels would be expected.
In the following work we show that during infection the various pathways and enzymes outlined in Fig. 1A are induced in HCMV-infected cells to produce glutathione and rid the cell of superoxides and ROS. The data suggest that multiple mechanisms are used by the virus to detoxify the cell. The effectiveness of this virally induced process was tested by examining the resistance of mammalian target of rapamycin (mTOR) kinase to inhibition by H2O2, a potent reactive oxygen species. Many stress responses target mTOR for inactivation to reduce cellular translation and save energy and resources during times of stress. Thus, the examination of mTOR kinase activity serves as an indicator of how well viral infection can combat the effects of ROS.
Two multiprotein complexes contain mTOR kinase, mTOR complex 1 (mTORC1) and complex 2 (mTORC2). The major difference between the two is the substrate specificity factors: raptor in mTORC1 and rictor in mTORC2 (30, 48). Functionally, the two complexes have a very different set of substrates in uninfected cells. The functions of mTORC2 are less well characterized than mTORC1; however, data suggest that mTORC2 functions in (i) promoting the phosphorylation of Akt and protein kinase C isoforms (reviewed in reference 1), (ii) regulating cell growth and proliferation, (iii) the organization of the actin cytoskeleton (29, 48), and (iv) activation of the serum glucocorticoid-induced protein kinase (SGK). A primary function of mTORC1 is to control cap-dependent translation (37, 46, 49). When mTORC1 is active, it phosphorylates p70S6 kinase (S6K) and the eukaryotic initiation factor 4E (eIF4E) binding protein (4E-BP). Phosphorylation of S6K renders it active and promotes the phosphorylation of ribosomal protein S6 and the formation of translation initiation complexes (37). The phosphorylation of 4E-BP is a major point of control in cap-dependent translation and regulates the function of the eIF4F translation-initiation complex. Under positive growth conditions, mTORC1 is active and maintains 4E-BP in its hyperphosphorylated state where it is incapable of inhibiting cap-dependent translation. However, under negative growth conditions, for example, those produced by stress responses, mTORC1 is inactive, and 4E-BP becomes hypophosphorylated (reviewed in reference 7). Thus, it is advantageous for HCMV during infection to protect mTORC1 from all stress effects that would inhibit it. Indeed, numerous studies have shown that HCMV is adroit at thwarting stress inhibition (2, 12, 31, 33, 39, 52), and a picture is emerging of how this is accomplished.
The activation of mTORC1 is primarily mediated by the phosphotidylinositol-3′-kinase (PI3K)–Akt–tuberous sclerosis complex (TSC) pathway (Fig. 1B). Activation of PI3K leads to activation of Akt, which phosphorylates and inactivates the tuberous sclerosis complex (TSC1 and −2) (reviewed in reference 3). This inhibits TSC's GTPase-activating protein (GAP) function, allowing the accumulation of Rheb-GTP, which activates mTOR kinase activity in mTORC1 (3, 35, 36). We have shown that the PI3K-Akt-TSC-mTORC1 pathway is activated during HCMV infection (31–33, 54). A major means by which many cellular stress responses inhibit mTORC1 is through activation of the TSC, either directly or by activating the AMP-dependent kinase (AMPK). We previously showed that mTORC1 activity in HCMV-infected cells is resistant to inhibition by activated AMPK (32). It was further shown that this resistance was, at least in part, due to the HCMV-induced inactivation of the TSC through binding to the HCMV protein UL38 (Fig. 1B) (39). Thus, the virus uses one key inhibitory interaction to bypass the effects of any stress response (or other inhibitory event) that functions through the activation of the TSC.
This UL38 mechanism accounts for the protection of mTORC1 from inhibition by many stress responses, but not from oxidative stress. Numerous studies have established that oxidative stress, induced by the addition of H2O2 to the cells, inhibits mTOR kinase in a TSC-independent manner; specifically, oxidative stress-mediated inhibition of mTOR kinase still occurs in TSC-deficient cells (22, 34). Thus, ROS inhibition of mTOR kinase occurs by mechanisms that do not involve TSC activation; therefore, it would not be blocked by the UL38-mediated inactivation of the TSC in HCMV-infected cells. However, the activation of AMPK does appear to be involved in mTOR kinase inhibition by ROS. It has been shown that AMPK directly phosphorylates raptor on two conserved serine residues, and this phosphorylation induces 14-3-3 protein binding to raptor, leading to inhibition of mTORC1 (22). As mentioned above, we previously showed that the mTORC1 activity is maintained in HCMV-infected cells when AMPK is activated (32); thus, HCMV-infected cells appear to also be able to bypass this inhibitory effect of AMPK.
In the following studies we show that virally infected cells are able to maintain mTOR kinase activity even after treatment with H2O2, a potent reactive oxygen species. In order to avoid the effects of ROS, the virus induces enzymes to synthesize glutathione and to efficiently utilize glutathione to eliminate ROS and maintain redox homeostasis. The induction of these enzymes is critical for the success of the viral infection. Our data suggest that HCMV utilizes virus-specific gene activation mechanisms to induce selected antioxidant genes instead of activating the master transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2), which normally enhances the expression of a number of antioxidant and phase I and II detoxifying enzymes. In sum, our data show that HCMV utilizes multiple means to combat reactive oxygen species.
MATERIALS AND METHODS
Cell culture, reagents, and antibodies.
Life-extended human foreskin fibroblasts (HFs) (5) were cultured at 37°C in 5% CO2 in Dulbecco's minimal essential medium (DMEM) supplemented with 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM Gluta-Max (all from GIBCO-BRL, Gaithersburg, MD). Serum starvation experiments were done in DMEM lacking fetal calf serum. All cells used in experiments were within 10 passages from the time the cells were taken from liquid nitrogen storage. Cell morphology and viability (measured by trypan blue staining) were monitored in all depletion and drug treatments. H2O2 solution and l-buthionine [S,R]sulfoximine (BSO) were purchased from Sigma (St. Louis, MO). Antibodies used in this study included total 4E-BP1, total and phospho-S6K (T389), total and phospho-AMPK (T172), and total and phospho-mTOR (S2448) (Cell Signaling); total and phospho-Raptor (T792) (Millipore and Cell Signaling, respectively); SOD1, UL44, and pp28 (Santa Cruz Biotechnology); GCLC (Thermo Fisher Scientific); GPX-1 (Cell Signaling); HO-1 (Santa Cruz Biotechnology); NQO1 (Santa Cruz Biotechnology); Nrf2 (Abcam); lamin B (Santa Cruz Biotechnology), and β-actin (Sigma). The antibody that recognizes the common exon 2 and 3 sequences of the HCMV major immediate-early proteins (MIEPs) was prepared by this laboratory and has been previously described (24).
Nuclear-cytoplasmic fractionation for Nrf2 analysis was done as previously described (16).
Virus preparation, titration, and infection.
HCMV (Towne strain) stocks were prepared and purified as previously described (31). Titers were determined using the 50% tissue culture infective dose (TCID50) method. All experiments were performed using a multiplicity of infection (MOI) of 3. Plasmids encoding lentiviruses that express shRNAs against SOD were purchased from Open Biosystems. The lentivirus containing the luciferase shRNA, used as a control, was constructed in our laboratory (55). Lentiviruses were prepared in 293T cells as previously described (55). Where applicable, lentiviruses were incubated with cells for 7 h in the presence of 10 μg/ml Polybrene. Cells were then maintained in serum-containing medium for 72 h before serum starvation prior to mock or HCMV infection.
Time course viral infections.
HFs were seeded onto 60-mm dishes and allowed to grow to 80% confluence. Cells were mock or HCMV infected at an MOI of 3. After a 2-h incubation, cells were washed two times with DMEM, and fresh medium was added to each well. At designated time points after infection, the plates were harvested by washing once with ice-cold phosphate-buffered saline (PBS), followed by a 5-min incubation at 4°C in lysis buffer (200 mM Tris-HCl [pH 7.5], 140 mM NaCl, 1% NP-40, 10 mM NaF, 1 mM EDTA, 200 μM NaVO4, 2 mM phenylmethylsulfonyl fluoride, 1.5 μg aprotinin/ml, 1 μg leupeptin/ml). Cells were scraped from the plates, and lysates were centrifuged at 13,000 rpm at 4°C. The supernatants were transferred to new tubes and stored at −80°C.
HCMV growth curves.
HFs were grown in six-well plates and infected at an MOI of 3. At designated times, 1 ml of medium was removed from one well of each plate and placed in a 15-ml conical tube. The cells in the well were scraped into the remaining 1 ml of medium, sonicated 10 times with 1-s pulses, and centrifuged for 10 min in a microcentrifuge at 4°C. The supernatants were combined with previously collected medium and stored at −80°C for titer determination using the TCID50 method. When BSO was used it was added to the medium (to 100 μM) at either 2 h postinfection (hpi) or 24 h prior to harvest. The use of shRNAs in HCMV growth curves is described above.
Hydrogen peroxide treatment.
For the H2O2 treatments, HFs were seeded onto 60-mm dishes. After reaching 80% confluence, the cells were serum starved for 48 h. Cells were then either mock infected or infected with purified HCMV at an MOI of 3 for 24, 48, or 72 h in serum-free DMEM. As a control, a separate set of mock-infected cells was serum stimulated for 1 h prior to H2O2 treatment and maintained in serum-containing medium during the H2O2 treatment. Indicated amounts of H2O2 were diluted in prewarmed medium and added to the cells every 15 min for 1 h. Cell extracts were then harvested at the various time points up to 24 h and stored at −80°C for subsequent Western analysis.
Western analyses.
Cellular lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. The membranes were blocked for 30 min in blocking buffer consisting of 5% nonfat dry milk in Tris-buffered saline (TBS) containing 0.1% Tween 20. Specific proteins were detected by incubating the membranes overnight at 4°C with the appropriate antibody diluted in 5% bovine serum albumin in TBS containing 0.1% Tween 20. Membranes were then incubated for 1 h with horseradish peroxidase-conjugated secondary antibodies in blocking buffer and visualized with enhanced chemiluminescence reagents (Amersham Biosciences).
Microscopy.
HFs were seeded in 6-well dishes containing glass coverslips. At 24 h after seeding, cells were either mock or HCMV infected at an MOI of 3. At the indicated time points after infection, cells were washed twice with warmed Dulbecco's phosphate-buffered saline (D-PBS) containing Ca2+ and Mg2+ and then incubated with 20 μM ThiolTracker violet (Invitrogen) for 15 min at 37°C. After incubation, cells were washed twice with D-PBS, fixed in 2% paraformaldehyde for 15 min, and then washed twice with D-PBS. The coverslips were immediately mounted on slides and examined with a Nikon Eclipse E600 microscope.
RT-PCR.
For reverse transcription-PCR (RT-PCR), a viral time course was set up as described above. Total mRNA was extracted from the infected cells using TRIzol reagent (Invitrogen) at the indicated times. Samples were DNase treated (Invitrogen), and cDNA was prepared using reagents from the Invitrogen SuperScript first-strand synthesis system for RT-PCR. Real-time PCR was performed using the TaqMan gene expression assay system (Applied Biosystems) according to the manufacturer's protocol. The gene expression levels were normalized against the endogenous control, phosphoglycerate kinase 1 (PGK1).
RESULTS
Glutathione levels are increased in HCMV-infected cells.
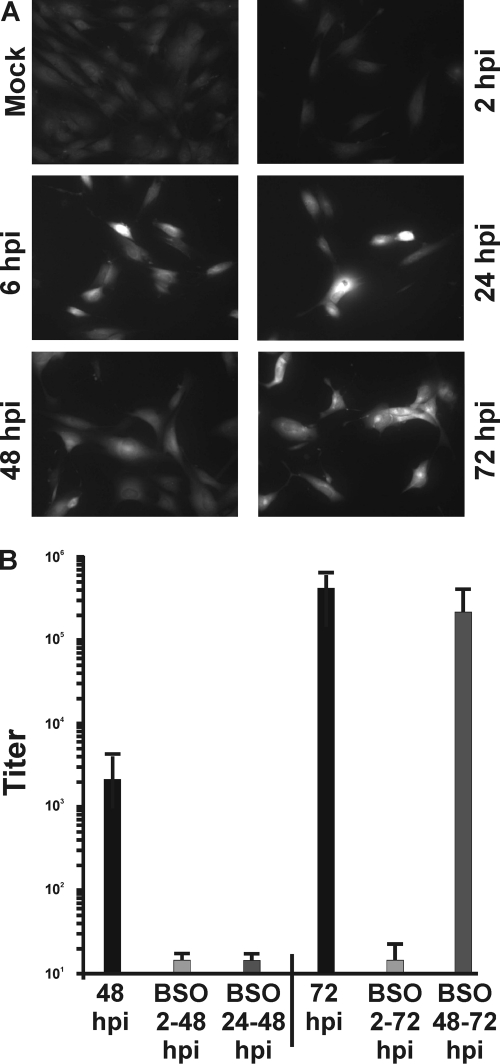
Since glutathione is a powerful intracellular antioxidant and plays a central role in protecting cells against damage incurred by free radicals and oxidants, we determined whether HCMV-infected cells exhibit increased levels of reduced glutathione. HFs were grown on coverslips and then mock infected or infected with HCMV at an MOI of 3. At various times after infection, the cells were incubated with ThiolTracker violet, a fluorescent reagent that reacts with reduced thiols in intact cells. Since reduced glutathione represents the majority of intracellular free thiols, the ThiolTracker signal provides a representation of the cellular level of reduced glutathione. Cells were then washed and fixed for fluorescence microscopic examination as described in Materials and Methods. The results demonstrated that HCMV-infected cells had markedly higher levels of GSH than mock-infected cells (Fig. 2A). This increase was observed as early as 6 hpi and continued throughout the course of infection. To show that increased glutathione synthesis is necessary for the success of the viral infection, BSO was added to infected cell cultures. BSO reversibly inhibits the activity of glutamate cysteine ligase (Fig. 1A), thereby inhibiting GSH synthesis and accumulation (4, 17). In this experiment, we added BSO from the beginning of the infection (at 2 hpi) or at 24 h before the harvest, e.g., 24 to 48 hpi and 48 to 72 hpi. As shown in Fig. 2B, the production of infectious progeny virions at 48 and 72 hpi was severely reduced when BSO was added at 2 hpi. In addition, BSO addition between 24 and 48 hpi was very deleterious to viral growth. However, addition of BSO between 48 and 72 hpi had significantly less effect on infectious progeny virion formation. These data suggest that the early accumulation of GSH is very important in order to proceed through the highly synthetic phase of the infection up to 48 hpi. After that point there is either sufficient GSH accumulated to sustain the infection or the demand for GSH declines during the virion assembly phase.
Fig. 2.
HCMV-infected cells express increased levels of reduced glutathione, which is required for the production of infectious viral progeny. (A) HFs grown on coverslips were mock or HCMV infected (MOI, 3). At 0, 2, 6, 24, 48, or 72 hpi, the cells were incubated with 20 μM ThiolTracker violet to estimate intercellular levels of reduced glutathione (GSH). Cells were then washed and fixed for fluorescence microscopy examination. (B) The production of infectious virions was determined at 48 and 72 hpi, either without or with the addition of BSO to infected cells at 2 hpi, 2 to 48 hpi, and 2 to 72 hpi, or 24 h before harvest, i.e., 24 to 28 hpi and 48 to 72 hpi.
HCMV-infected cells have increased levels of antioxidant and detoxifying enzymes.
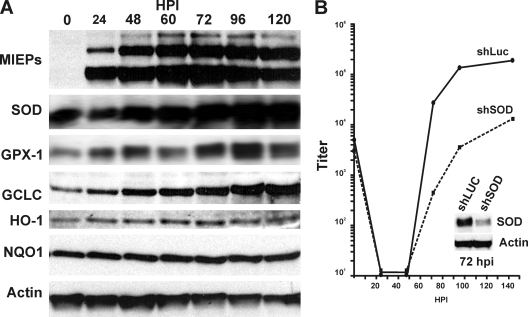
Given the necessity for increased GSH levels in infected cells, we asked whether GSH synthetic enzymes and other enzymes related to ROS detoxification (Fig. 1A) were increased during infection. Figure 3A shows that the levels of the catalytic subunit of GCL, the glutamate-cysteine ligase catalytic subunit (GCLC), SOD, and GPX-1 were all increased during an infection time course, suggesting that the infected cell not only increases GSH synthesis but also the levels of enzymes that will utilize GSH to reduce ROS. It should be noted that not all enzymes involved in redox homeostasis were upregulated; this is shown in Fig. 3A by the phase I and II detoxifying enzymes heme oxygenase 1 and NAD(P)H:quinone oxidoreductase 1, which will be discussed below.
Fig. 3.
Expression of enzymes involved in ROS detoxification during HCMV infection. (A) HF lysates collected at 0, 24, 48, 60, 72, 96, and 120 hpi were subjected to SDS-PAGE and Western blot analysis to determine the levels of the HCMV MIEPs, GCLC, SOD, GPX-1, HO-1, and NQO-1. (B) Serum-starved HFs were infected with control (shLuc) or SOD-specific (shSOD) shRNA for 72 h, followed by 48 h of serum starvation and then HCMV infection (MOI, 3) (see Materials and Methods for details). The viral titers were determined using the TCID50 method. The inset shows the effectiveness of the SOD knockdown at 72 hpi.
To demonstrate the significance of increased enzyme production for an HCMV infection, we examined the effect of depleting SOD, by using shRNA-expressing lentiviral vectors, on the production of infectious HCMV virions during an HCMV infection time course. It should be noted that of the three enzymes upregulated in Fig. 3A (SOD, GCLC, and GPX-1), only the depletion of SOD had minimal effects on the viability and morphology of uninfected cells (data not shown); presumably, depleting glutathione synthetic (GCLC) and regenerative (GPX-1) enzymes is deleterious to HFs. However, as shown in Fig. 3B, the depletion of SOD significantly decreased viral growth compared to a control lentiviral vector that expresses an shRNA to luciferase (Luc). These data indicate the importance of upregulation of ROS-controlling genes during HCMV infection.
The activity of mTOR kinase is maintained in HCMV-infected cells in the presence of ROS.
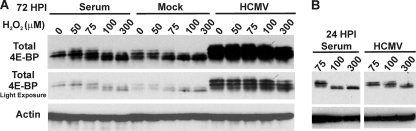
The data thus far suggest that HCMV infection coordinates conditions where ROS levels should be controlled and oxidative stress minimized. To determine this, we used mTOR kinase activity, based on 4E-BP and S6K phosphorylation, as a measure of oxidative stress in mock- and HCMV-infected cells. HFs were serum starved for 48 h and then either mock or HCMV infected (MOI, 3) using serum-free medium for 72 h and then treated with increasing amounts of hydrogen peroxide. A separate set of serum-starved, mock-infected cells were returned to serum-containing medium 1 h prior to H2O2 treatment. After the 72 h of infection, or the 1 h of serum stimulation, cells were treated with either 0, 50, 75, 100, or 300 μM H2O2 every 15 min for 1 h. The H2O2 was diluted in complete DMEM to treat serum-stimulated cells or in serum-free DMEM to treat HCMV-infected cells. After the 1-h H2O2 treatment, all cells were harvested and lysates were analyzed by Western analysis using antibodies that recognize total 4E-BP1 or actin.
Figure 4A shows the phosphorylation of 4E-BP1 under the above conditions. It is important to note that an antibody specific for total 4E-BP1 was used, and thus a number of phosphorylated forms were seen (31); progressively greater hyperphosphorylation causes slower-migrating forms to be detected. Two exposures of the 4E-BP1 data are shown for comparison. This was necessitated by the increased levels of total 4E-BP1 that accumulated in infected cells by 72 hpi. A comparison of the two exposures indicated that the most hyperphosphorylated forms of 4E-BP1 (the slower-migrating forms) persisted at 100 and even 300 μM H2O2 in the HCMV-infected cells, whereas they were depleted in the serum-stimulated and mock-infected cells. These data indicate that in infected cells, despite increased levels of 4E-BP1, there is sufficient mTOR kinase activity to maintain hyperphosphorylation, even in the presence of high H2O2 concentrations that inhibit mTOR activity in serum-stimulated, mock-infected cells. Figure 4B shows similar data for cells infected for only 24 h. Again, there was significantly more hyperphosphorylated 4E-BP1 in infected cells in the presence of 100 μM H2O2 than in comparably treated serum-stimulated cells. In the 24-hpi samples, 4E-BP levels had not yet increased in infected cells, and therefore the total levels were equivalent in the infected and serum-stimulated cells. These data show that in HCMV-infected cells the protection of mTOR kinase activity from ROS is effective by 24 hpi.
Fig. 4.
HCMV-infected cells maintain mTORC1 activity in the presence of hydrogen peroxide. (A) Serum-starved HFs were mock or HCMV infected (MOI, 3). At 72 hpi, cells were treated with various concentrations of H2O2 for 1 h. Where indicated, mock-infected cells were serum stimulated for 1 h prior to H2O2 treatment. Cells were harvested following H2O2 treatment, lysates were subjected to SDS-PAGE, and levels of total 4E-BP1 or actin were examined by Western blot analysis. Two exposures of total 4E-BP1 are included for clarity. (B) Cells were treated as for panel A, except H2O2 treatment and cell collection occurred at 24 hpi (instead of 72 hpi).
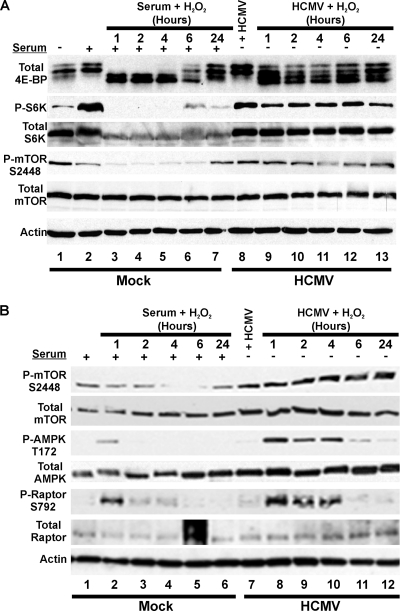
To further establish that mTOR signaling is maintained in HCMV-infected cells in the presence of high ROS levels, we infected serum-starved HFs with HCMV (MOI, 3). At 24 hpi, cells were treated with 100 μM H2O2. As the control, serum-starved, mock-infected cells were placed in serum-containing medium for 1 h prior to H2O2 treatment and maintained in serum-containing medium for the duration. Cell lysates were harvested 1, 2, 4, 6, and 24 h after H2O2 treatment and examined by Western analysis using antibodies that recognize total 4E-BP1, as shown in Fig. 4A. In addition, in this experiment (Fig. 5A) we examined phosphorylated or total S6K, another mTORC1 substrate. The data showed that in the absence of H2O2 both HCMV infection of serum-starved cells and serum stimulation of serum-starved, uninfected cells significantly activated mTOR, as indicated by the increased amount of hyperphosphorylated 4E-BP1 and phosphorylated S6K (P-S6K) (Fig. 5A, compare lane 1 with lanes 2 and 8). H2O2 treatment for 1 h significantly reduced the hyperphosphorylated forms of 4E-BP1 in serum-stimulated cells to below that seen in uninfected cells that had not been serum stimulated (Fig. 5A, compare lane 1 with lane 3). In addition, the effect of H2O2 treatment on serum-stimulated cells lasted for nearly 6 h; pre-serum stimulation levels of the hyperphosphorylated 4E-BP1 began to appear at 6 h and were returning to normal serum levels by 24 h. In contrast, a significant level of hyperphosphorylated 4E-BP1 was maintained in HCMV-infected cells, even 1 h after H2O2 treatment. Similarly, the analysis of P-S6K also showed a significant decrease in P-S6K in H2O2-treated, serum-stimulated cells but not in HCMV-infected cells. Overall, the results shown in Fig. 5A suggest that HCMV infection induces means to counteract the inhibitory effects of oxidative stress on the mTOR pathway; the data shown in Fig. 4B suggest that this protective effect is functioning by 24 hpi.
Fig. 5.
Effects of hydrogen peroxide treatment on the phosphorylation of 4E-BP, S6K, mTOR, AMPK, and raptor. Serum-starved HFs were mock or HCMV infected (MOI, 3). At 24 hpi (A) or 48 hpi (B), cells were treated with 100 μM H2O2 for 1 h. Cells were harvested at 1, 2, 4, 6, or 24 h post-H2O2 treatment. Mock-infected cells were serum stimulated for 1 h prior to H2O2 treatment. (A) Western blot analysis was conducted for total 4E-BP1, total or phosphorylated S6K (P-S6K), total or P-mTOR (S2448), and actin. (B) A similar experiment to that in panel A but with samples treated at 48 hpi with H2O2 and analyzed for total and P-mTOR (S2448) and total and P-AMPK (T172), as well as total and P-raptor (S792).
The phosphorylation of mTOR kinase at serine 2448 (S2448) was also tested and found to be inhibited by H2O2 in uninfected cells but not in infected cells (Fig. 5A). Although the effects of phosphorylation of sites in mTOR are not fully understood, it has been shown that mTORC1 contains mTOR phosphorylated predominantly on S2448 (14), suggesting that this phosphorylation is important for mTORC1 formation and/or activity. In this regard, it has also been reported that phosphorylation of mTOR at S2448 correlates with increased mTORC1 activity (47). Thus, the maintenance of S2448 phosphorylation would be beneficial to the viral infection and an additional way to combat oxidative stress.
The analysis of the phosphorylation of mTOR shown in Fig. 5A was repeated (Fig. 5B), with similar results (H2O2 was added at 48 hpi). In addition, Fig. 5B shows the analysis of the phosphorylation status of AMPK and raptor, the specificity factor for mTOR kinase in mTORC1. As diagrammed in Fig. 1B, the stress-induced phosphorylation (activation) of AMPK leads to the direct phosphorylation of raptor. It has been suggested that AMPK-mediated phosphorylation of raptor on serine 792 (S792) mediates H2O2-induced inhibition of mTORC1 (22). In serum-stimulated, mock-infected cells, AMPK became phosphorylated by 1 h after H2O2 treatment, but then this subsided by 2 h after treatment. Raptor phosphorylation on S792 coordinated with AMPK activation, appearing at 1 h post-H2O2 treatment and subsiding. Surprisingly, the activation of AMPK in infected cells continued at substantial levels until 4 h post-H2O2 treatment and could still be detected at 6 h. Coordinately, raptor phosphorylation increased between 1 and 4 h post-H2O2 treatment. That mTORC1 activity is maintained under these conditions, as measured by 4E-BP and S6K phosphorylation (Fig. 5A), suggests that raptor phosphorylation has no detectable effect in HCMV-infected cells, or that any discernible inhibitory effects of this phosphorylation are compensated by a virally induced mechanism.
HCMV's ability to maintain mTORC1 activity in the presence of ROS is partially maintained during SOD depletion.
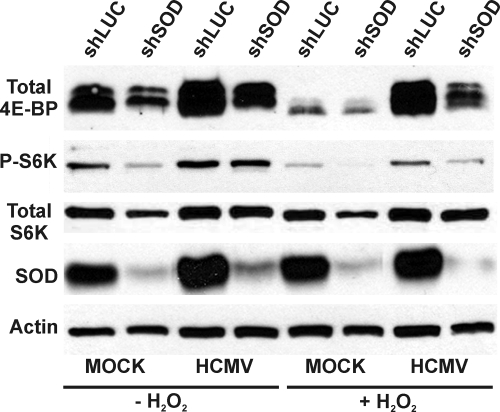
The data in Fig. 3B show that the depletion of SOD has deleterious effects on HCMV infection. The data in Fig. 4 and 5 show that mTOR kinase activity is maintained in the presence of H2O2. Thus, we asked whether mTOR activity, as measured by 4E-BP1 and S6K phosphorylation, could be maintained in the presence of H2O2 when SOD was depleted. Cells were infected with luciferase- or SOD1-specific shRNAs for 72 h followed by 24 h of serum starvation prior to mock or HCMV infection. At 48 hpi, mock-infected cells were serum stimulated for 30 min followed by a 1-h treatment of both mock-infected and HCMV-infected cells with 100 μM H2O2. Cells were harvested after the H2O2 treatment, and lysates were separated by SDS-PAGE. The results in Fig. 6 show that depletion of SOD caused a decrease in the total levels of 4E-BP1 in both mock- and HCMV-infected cells, but hyperphosphorylated forms of 4E-BP were substantially maintained in each case. As noted previously, the addition of H2O2 to mock-infected cells causes the loss of hyperphosphorylation of 4E-BP; Fig. 6 shows that this occurred regardless of SOD. In infected cells, 4E-BP hyperphosphorylation was maintained in SOD-depleted, H2O2-treated cells. Thus, although the depletion of SOD lowered the total level of 4E-BP, the hyperphosphorylation of the remaining 4E-BP was maintained.
Fig. 6.
Maintenance of mTORC1 activity in HCMV-infected cells depleted of SOD. Control (shLuc) or SOD1-specific (shSOD) shRNA was added to HFs via lentiviral vectors. At 72 h, the cells were serum starved for 48 h, followed by mock or HCMV infection (MOI, 3). At 48 hpi, cells were treated with 100 μM H2O2 for 1 h, where applicable. Mock-infected cells were serum stimulated for 1 h prior to H2O2 treatment. Western blot analysis was conducted for total 4E-BP1, total and P-S6K, SOD, and actin.
Examination of S6K showed that the depletion of SOD caused a moderate decrease in total S6K in mock-infected cells but not in infected cells. In agreement with the data in Fig. 5A, the phosphorylation of S6K is lowered substantially by H2O2 addition to mock-infected cells expressing the control shRNA and is dramatically lowered in SOD-depleted cells. In HCMV-infected HFs expressing the control shRNA, S6K phosphorylation was substantially maintained after H2O2 treatment. SOD-depleted, HCMV-infected cells treated with H2O2 maintained a detectable level of S6K phosphorylation, but lower than in HCMV-infected controls. Thus, SOD depletion appears to deter the ability of HCMV to maintain S6K phosphorylation. These data suggest a differential effect of SOD depletion on the mTOR substrates: 4E-BP levels appear to be reduced but hyperphosphorylation is maintained, whereas S6K levels and phosphorylation are reduced. Differential effects of HCMV infection on mTOR kinase activity toward 4E-BP and S6K have been previously noted (27).
Nrf2 target genes are independently activated by HCMV.
One means by which cells augment their antioxidant capacity quickly to counteract ROS is through the activation of the master regulating transcription factor Nrf2 (15, 16, 28, 45, 56). This Nrf2-centered network is adaptively activated, enhancing the expression of a group of antioxidant and phase I and II detoxifying enzymes to restore redox homeostasis. Nrf2's cytoplasmic partner Keap1 suppresses Nrf2 by maintaining it in the cytoplasm. In order to be activated, Nrf2 must be phosphorylated and released from Keap1; one way of accomplishing this is through the action of PKR-like ER kinase (PERK), which is a kinase activated by the unfolded protein response (15, 16). Phosphorylation releases Nrf2 from Keap1 and allows it to enter the nucleus, where it activates promoters containing antioxidant responsive elements (AREs). We previously showed that PERK is activated during HCMV infection (27). Thus, it is reasonable to propose that ROS resistance in HCMV-infected cells may be mediated, at least in part, by Nrf2 activation. However, previous studies have suggested that HCMV infection antagonizes Nrf2 function through an interaction with the IE2 major immediate-early protein (25).
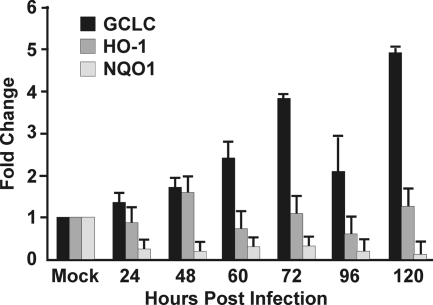
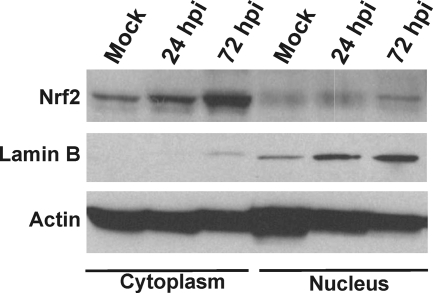
In the experiment shown in Fig. 3A, we examined the expression of three enzymes that are activated by Nrf2: GCLC, HO-1, and NQO1. We have already discussed the increase in GCLC levels during HCMV infection and the importance of GCLC in glutathione production. However, the other two Nrf2 target genes are not coordinately upregulated with GCLC. This was confirmed by RT-PCR analysis of the RNA levels of these gene products in HCMV-infected cells compared to mock-infected cells (Fig. 7). GCLC mRNA clearly increased during the course of the infection, whereas HO-1 and NQO1 RNAs did not. In fact, NQO1 RNA appeared to decrease during infection. Thus, our data suggest that HCMV utilizes virus-specific mechanisms, rather than Nrf2, to activate those Nrf2 target genes (e.g., GCLC) that are specifically needed during infection. This is further supported by the data in Fig. 8, which show the results of a Western analysis of Nrf2 in the cytoplasmic fraction and the nuclear fraction from mock-infected and HCMV-infected (24 and 72 hpi) HFs. The data show that although there was an increase in Nrf2 in the cytoplasm over the course of the infection, there was no increase in the level of nuclear Nrf2; it remained at levels comparable to those in unstressed, mock-infected cells. Lamin B, a nuclear marker, was also analyzed to show the effectiveness of the fractionation. These results suggested that Nrf2 is maintained in the cytoplasm in infected cells, in agreement with a model whereby HCMV utilizes virus-specific means to activate select genes that are normally activated by Nrf2.
Fig. 7.
HCMV infection increases RNA levels of specific Nrf2 target genes. Real-time PCR analysis of GCLC, HO-1, and NQO1 RNA levels in HCMV-infected cells allowed comparisons to mock-infected cells. Total mRNA was extracted from HFs at 0, 24, 48, 60, 72, 96, or 120hpi. Quantitative gene expression was normalized against phosphoglycerate kinase 1.
Fig. 8.
Nrf2 is maintained in the cytoplasm during HCMV infection. HFs were mock infected or HCMV infected for 24 and 72 h. Infected cells were fractionated into nuclear and cytoplasmic fractions and subjected to SDS-PAGE and Western analysis for Nrf2, lamin B, and actin.
DISCUSSION
HCMV has evolved mechanisms to modulate many cellular stress responses to favor efficient replication. It is widely accepted that ROS are involved in the regulation of HCMV gene transcription early in infection (51). However, overproduction of ROS leads to oxidative stress, which can inhibit vital cellular processes and signaling. In this study we found that virally infected cells increase enzymes that synthesize and utilize glutathione, essentially providing a milieu that can quickly eliminate ROS and maintain redox homeostasis. This provides the ability to maintain critical cell signaling, such as mTOR kinase activity, even after treatment with hydrogen peroxide, a potent ROS and an inhibitor of mTOR kinase. We showed that the induction of these enzymes and glutathione synthesis is critical for the success of the viral infection.
We have previously shown that HCMV strives to maintain mTOR kinase activity in mTORC1 during infection regardless of the stress responses induced (reviewed in references 2 and 7). The present data add ROS stress to the list of stress responses that are successfully circumvented by HCMV to maintain mTOR activity. The present data cast new light on the interactions between viral infection and the components of mTORC1. We found that the phosphorylation of mTOR kinase at serine 2448 is maintained during infection even under ROS stress conditions that inhibit it in uninfected cells. Although the effects of phosphorylation of mTOR are not fully understood, data suggest that mTOR phosphorylated predominantly on S2448 is a component of active mTORC1 (14), indicating that this phosphorylation is important for mTORC1 formation and/or activity. Indeed, it has also been reported that phosphorylation of mTOR at S2448 correlates with mTORC1 activity (47). It has been suggested that Akt is the kinase involved in phosphorylation of mTOR kinase at S2448 (44, 50). However, more recent data suggest that S6K, the downstream target of mTORC1, is the S2448 kinase (9). We found that S6K is maintained in its phosphorylated, active state during ROS stress in infected cells. Thus, HCMV's ability to maintain S2448 phosphorylation may result from a feedback loop involving S6K. Whatever the case may be, it appears that maintenance of S2448 phosphorylation is another means by which the viral infection maintains mTOR activity and combats oxidative stress.
We also analyzed AMPK activation and phosphorylation of raptor, the specificity subunit of mTORC1, during ROS stress. It has been suggested that AMPK-mediated phosphorylation of raptor on serine 792 (S792) mediates H2O2-induced inhibition of mTORC1 (22). Interestingly, we found that the ROS-induced activation of AMPK, and the subsequent phosphorylation of raptor, continued substantially longer in infected cells than in uninfected cells. However, under these putative mTORC1 inhibitory conditions, infected cells maintained phosphorylation of 4E-BP and S6K. This suggests that in infected cells the inhibitory effects of raptor phosphorylation on S792 are also circumvented. Recent data showed that, during infection, mTORC1 and its activator, Rheb-GTP (Fig. 1), are maintained together in the HCMV cytoplasmic assembly compartment (13). In this sequestered position, mTOR can be maintained in an active state and possibly be protected from many inhibitory events.
Our data also suggest that HCMV utilizes virus-specific mechanisms to activate certain ROS-protective and detoxifying enzymes over others. This is especially indicated by the activation of only one of three Nrf2 target genes that we examined. These data suggest that the virus does not utilize Nrf2-mediated activation. As mentioned above, a previous study suggested that HCMV infection antagonizes Nrf2 function via interaction with the IE2 major immediate-early protein (25). From our data we conclude that HCMV uses a virus-specific mechanism to selectively activate specific genes within the family normally activated by Nrf2. Such selective activation of gene family members by HCMV has been previously reported; we have shown that HCMV activates specific ATF6 target genes (26). By selectively activating genes that are normally coordinately activated, the virus can restrict its effects to only those genes that are most beneficial to the viral infection. Our data suggest that this is the case with Nrf2 target genes. Overall, our data suggest that HCMV utilizes virus-specific mechanisms to coordinate conditions that readily and efficiently reduce ROS and maintain a redox environment that is conducive to viral replication.
ACKNOWLEDGMENTS
We thank Liping Liu and Celeste Simon for assistance with conditions for hydrogen peroxide treatments as well as all the members of the Alwine lab for support and critical evaluation of the experiments and data.
This work was supported by NIH grant R01 CA157679-01 awarded to J.C.A. A.J.C. and C.A.T. were supported by Training in Tumor Virology grant T32 CA115299 awarded to Erle Robertson. C.A.T. was also supported by NIH fellowship grant 1F32AI082893.
Footnotes
Published ahead of print on 21 September 2011.
REFERENCES
- 1. Alessi D. R., Pearce L. R., García-Martínez J. M. 2009. New Insights into mTOR signaling: mTORC2 and beyond. Sci. Signal. 2:pe27. [DOI] [PubMed] [Google Scholar]
- 2. Alwine J. C. 2008. Modulation of host cell stress responses by human cytomegalovirus. Curr. Top. Microbiol. Immunol. 325:263–279 [DOI] [PubMed] [Google Scholar]
- 3. Astrinidis A., Henske E. P. 2005. Tuberous sclerosis complex: linking growth and energy signaling pathways with human disease. Oncogene 24:7475–7481 [DOI] [PubMed] [Google Scholar]
- 4. Bailey H. H. 1998. l-S,R-Buthionine sulfoximine: historical development and clinical issues. Chem. Biol. Interact 111–112:239–254 [DOI] [PubMed] [Google Scholar]
- 5. Bresnahan W. A., Hultman G. E., Shenk T. 2000. Replication of wild-type and mutant human cytomegalovirus in life-extended human diploid fibroblasts. J. Virol. 74:10816–10818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buchkovich N. J., Maguire T. G., Paton A. W., Paton J. C., Alwine J. C. 2008. Human cytomegalovirus specifically controls the levels of the endoplasmic reticulum chaperone BiP/GRP78, which is required for virion assembly. J. Virol. 82:31–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buchkovich N. J., Yu Y., Zampieri C. A., Alwine J. C. 2008. The TORrid affairs of viruses: effects of mammalian DNA viruses on the PI3K-Akt-mTOR signaling pathway. Nat. Rev. Microbiol. 6:265–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chambers J. W., Maguire T. G., Alwine J. C. 2010. Glutamine metabolism is essential for human cytomegalovirus infection. J. Virol. 84:1867–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chiang G. G., Abraham R. T. 2005. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J. Biol. Chem. 280:25485–25490 [DOI] [PubMed] [Google Scholar]
- 10. Child S. J., Hakki M., De Niro K. L., Geballe A. P. 2004. Evasion of cellular antiviral responses by human cytomegalovirus TRS1 and IRS1. J. Virol. 78:197–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Circu M. L., Aw T. 2010. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 48:749–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clippinger A. J., Maguire T. G., Alwine J. C. 2011. The changing role of mTOR kinase in the maintenance of protein synthesis during human cytomegalovirus infection. J. Virol. 85:3930–3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clippinger A. J., Maguire T. G., Alwine J. C. 2011. Human cytomegalovirus infection maintains mTOR activity and its perinuclear localization during amino acid deprivation. J. Virol. 85:9369–9376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Copp J., Manning G., Hunter T. 2009. TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser 2481 is a marker for intact mTOR signaling complex 2. Cancer Res. 69:1821–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cullinan S. B., Diehl J. A. 2006. Coordination of ER and oxidative stress signaling: the PERK/Nrf2 signaling pathway. Int. J. Biochem. Cell Biol. 38:317–332 [DOI] [PubMed] [Google Scholar]
- 16. Cullinan S. B., et al. 2003. Identification of Nrf2 as a novel PERK substrate and critical effector of PERK-dependent cell survival. Mol. Cell. Biol. 23:7198–7209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Estrela J. M., Ortega A., Obrador E. 2006. Glutathione in cancer biology and therapy. Crit. Rev. Clin. Lab. Sci. 43:143–181 [DOI] [PubMed] [Google Scholar]
- 18. Finkel T. 2003. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 15:247–254 [DOI] [PubMed] [Google Scholar]
- 19. Finkel T., Holbrook N. J. 2000. Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247 [DOI] [PubMed] [Google Scholar]
- 20. Go Y. M., Jones D. P. 2008. Redox compartmentalization in eukaryotic cells. Biochim. Biophys. Acta 1780:1273–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Go Y. M., Jones D. P. 2010. Redox control systems in the nucleus: mechanisms and functions. Antioxid. Redox Signal. 13:489–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gwinn D. M., et al. 2008. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 30:214–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hakki M., Marshall E. E., De Niro K. L., Geballe A. P. 2006. Binding and nuclear relocalization of protein kinase R by human cytomegalovirus TRS1. J. Virol. 80:11817–11826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harel N. Y., Alwine J. C. 1998. Phosphorylation of the human cytomegalovirus 86-kilodalton immediate-early protein IE2. J. Virol. 72:5481–5492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang C.-F., et al. 2000. Antagonism between members of the CNC-bZIP family and the immediate-early protein IE2 of human cytomegalovirus. J. Biol. Chem. 275:12313–12320 [DOI] [PubMed] [Google Scholar]
- 26. Isler J. A., Maguire T. G., Alwine J. C. 2005. Production of infectious HCMV virions is inhibited by drugs that disrupt calcium homeostasis in the endoplasmic reticulum. J. Virol. 79:15338–15397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Isler J. A., Skalet A. H., Alwine J. C. 2005. Human cytomegalovirus infection activates and regulates the unfolded protein response. J. Virol. 79:6890–6899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Itoh K., Ishii T., Wakabayashi N., Yamamoto M. 1999. Regulatory mechanisms of cellular response to oxidative stress. Free Radic. Res. 31:319–324 [DOI] [PubMed] [Google Scholar]
- 29. Jacinto E., et al. 2004. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 6:1122–1128 [DOI] [PubMed] [Google Scholar]
- 30. Kim D. H., et al. 2002. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110:163–175 [DOI] [PubMed] [Google Scholar]
- 31. Kudchodkar S., Yu Y., Maguire T., Alwine J. C. 2004. Human cytomegalovirus infection induces rapamycin insensitive phosphorylation of downstream effectors of mTOR kinase. J. Virol. 78:11030–11039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kudchodkar S. B., Del Prete G. Q., Maguire T. G., Alwine J. C. 2007. AMPK-mediated inhibition of mTOR kinase is circumvented during immediate-early times of human cytomegalovirus infection. J. Virol. 81:3649–3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kudchodkar S. B., Yu Y., Maguire T. G., Alwine J. C. 2006. Human cytomegalovirus infection alters the substrate specificities and rapamycin sensitivities of raptor- and rictor-containing complexes. Proc. Natl. Acad. Sci. U. S. A. 103:14182–14187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu L., Wise D. R., Diehl J. A., Simon M. C. 2008. Hypoxic reactive oxygen species regulate the integrated stress response and cell survival. J. Biol. Chem. 283:31153–31162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Long X., Lin Y., Ortiz-Vega S., Yonezawa K., Avruch J. 2005. Rheb binds and regulates the mTOR kinase. Curr. Biol. 15:702–713 [DOI] [PubMed] [Google Scholar]
- 36. Long X., Ortiz-Vega S., Lin Y., Avruch J. 2005. Rheb binding to mammalian target of rapamycin (mTOR) is regulated by amino acid sufficiency. J. Biol. Chem. 280:23433–23436 [DOI] [PubMed] [Google Scholar]
- 37. Mamane Y., Petroulakis E., LeBacquer O., Sonenberg N. 2006. mTOR, translation initiation and cancer. Oncogene 25:6416–6422 [DOI] [PubMed] [Google Scholar]
- 38. Mohr I. 2006. Phosphorylation and dephosphorylation events that regulate viral mRNA translation. Virus Res. 119:89–99 [DOI] [PubMed] [Google Scholar]
- 39. Moorman N. J., et al. 2008. Human cytomegalovirus protein UL38 inhibits host cell stress responses by antagonizing the tuberous sclerosis protein complex. Cell Host Microbe 3:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Munger J., Bajad S. U., Coller H. A., Shenk T., Rabinowitz J. D. 2006. Dynamics of the cellular metabolome during human cytomegalovirus infection. PLoS Pathog. 2:1165–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Munger J., et al. 2008. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat. Biotechnol. 26:1179–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Murphy E., Rigoutsos I., Shibuya T., Shenk T. E. 2003. Reevaluation of human cytomegalovirus coding potential. Proc. Natl. Acad. Sci. U. S. A. 100:13585–13590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Murphy E., et al. 2003. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc. Natl. Acad. Sci. U. S. A. 25:14976–14981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nave B. T., Ouwens D. M., Withers D. J., Alessi D. R., Shepherd P. R. 1999. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem. J. 344:427–431 [PMC free article] [PubMed] [Google Scholar]
- 45. Nguyen T., Sherratt P., Pickett C. 2003. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol. 43:233–260 [DOI] [PubMed] [Google Scholar]
- 46. Reiling J. H., Sabatini D. M. 2006. Stress and mTORture signaling. Oncogene 25:6373–6383 [DOI] [PubMed] [Google Scholar]
- 47. Rosner M., Siegel N., Valli A., Fuchs C., Hengstschlager M. 2010. mTOR phosphorylated at S2448 binds to raptor and rictor. Amino Acids 38:223–228 [DOI] [PubMed] [Google Scholar]
- 48. Sarbassov D. D., et al. 2004. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14:1296–1302 [DOI] [PubMed] [Google Scholar]
- 49. Sarbassov D. D., Ali S. M., Sabatini D. M. 2005. Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 17:596–603 [DOI] [PubMed] [Google Scholar]
- 50. Sekulic A., et al. 2000. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 60:3504–3513 [PubMed] [Google Scholar]
- 51. Speir E., Shibutani T., Yu Z. X., Ferrans V., Epstein S. E. 1996. Role of reactive oxygen intermediates in cytomegalovirus gene expression and in the response of human smooth muscle cells to viral infection. Circ. Res. 79:1143–1152 [DOI] [PubMed] [Google Scholar]
- 52. Walsh D., Perez C., Notary J., Mohr I. 2005. Regulation of the translation initiation factor eIF4F by multiple mechanisms in human cytomegalovirus-infected cells. J. Virol. 79:8057–8064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xuan B., Qian Z., Torigoi E., Yu D. 2009. Human cytomegalovirus protein pUL38 induces ATF4 expression, inhibits persistent JNK phosphorylation, and suppresses endoplasmic reticulum stress-induced cell death. J. Virol. 83:3463–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yu Y., Alwine J. C. 2002. Human cytomegalovirus major immediate-early proteins and simian virus 40 large T antigen can inhibit apoptosis through activation of the phosphatidylinositide 3′-OH kinase pathway and cellular kinase Akt. J. Virol. 76:3731–3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yu Y., Alwine J. C. 2008. Interaction between simian virus 40 large T antigen and insulin receptor substrate 1 is disrupted by the K1 mutation, resulting in the loss of large T antigen-mediated phosphorylation of Akt. J. Virol. 82:4521–4526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang Q., Pi J., Woods C. G., Andersen M. E. 2010. A systems biology perspective on Nrf2-mediated antioxidant response. Toxicol. Appl. Pharmacol. 244:84–97 [DOI] [PMC free article] [PubMed] [Google Scholar]