Abstract
Human cytomegalovirus (HCMV) remains a major cause of viral disease in immunosuppressed transplant patients. The ability of HCMV to establish lifelong infection in humans and reactivate with devastating clinical consequences underscores the importance of understanding the triggers of HCMV reactivation in mature myeloid cells. Dendritic cell (DC) differentiation is concomitant with the activation of cellular signaling pathways and inflammatory gene expression and also HCMV reactivation. Here, we show a major role for interleukin-6 (IL-6) through extracellular signal-regulated kinase–mitogen-activated protein kinase (ERK-MAPK) signaling upon DC differentiation to promote HCMV reactivation. IL-6 drives reactivation by transcriptional upregulation of the major immediate-early (IE) genes, resulting in efficient progression of the virus life cycle and, ultimately, higher titers of infectious virus. Furthermore, the interception of IL-6 signaling with biological inhibitors significantly abrogated HCMV reactivation from experimental latency. Crucially, using cells derived from healthy seropositive donors, we observed a key role for IL-6 during reactivation from natural latency ex vivo in interstitial DCs. Clinically, HCMV reactivation occurs in highly inflammatory environments (i.e., transplantation); thus, the implications of this study could potentially provide novel approaches for therapeutic intervention.
INTRODUCTION
The opportunistic pathogen human cytomegalovirus (HCMV) remains a major cause of viral disease in immunocompromised patient groups, including transplant recipients, intensive care and cancer patients, as well as late-stage AIDS sufferers (21, 23). A major contribution to HCMV pathogenesis occurs following the reactivation of an existing HCMV latent infection within the myeloid cell populations in the host (40). As such, the ability of HCMV to establish lifelong infection in humans and reactivate with devastating clinical consequences underscores the importance of understanding the triggers of HCMV reactivation in mature myeloid cells.
Studies from a number of laboratories support the key tenet that HCMV latency and reactivation are intrinsically linked with the differentiation state of the cell (1, 11, 38, 42). Remaining latent in myeloid bone marrow CD34+ progenitor cells (20, 26, 39), and monocytes (43), reactivation occurs upon macrophage or dendritic cell (DC) differentiation (31, 42, 44). A key criterion of latency is the ability to reenter the lytic phase of infection (reactivation). Since viral lytic gene expression is driven by the immediate-early (IE) proteins IE72 and IE86 (25), which are regulated by the major immediate-early promoter (MIEP), it is postulated that the pivotal molecular switch from latency to reactivation is triggered by activation of the MIEP. However, the precise nature of the molecular trigger responsible for reactivation remains unknown.
Dendritic cell maturation is concomitant with the activation of a number of pathways associated with inflammatory gene expression and signaling (24). Similarly, HCMV reactivation and disease occur in highly inflammatory environments (23, 32) (i.e., transplantation); thus, there is a correlative link between the two events. Furthermore, the MIEP contains a number of binding sites for NF-κB, AP-1, and CREB transcription factors (25) and thus resembles the architecture of an inflammatory gene promoter. Given that the induction of inflammatory gene expression is often concomitant with myeloid cell differentiation, as is HCMV reactivation, it is an attractive model.
The interleukin-6 cytokine family can elicit a diverse number of biological responses with an apparent degree of redundancy (14). However, in vivo rapid localized production along with tissue-specific receptor subunit distribution enhances the biological specificity of IL-6 signaling, including defined roles in hematopoiesis (13). The best-characterized pathway is JAK/STAT signaling, which promotes the activation of a number of STAT-responsive genes, including the acute-phase response proteins (14). Alternatively, interleukin-6 (IL-6) signaling can also occur via the SHP2/extracellular signal-regulated kinase (ERK) pathway. A major component of this signaling pathway is growth factor receptor binding protein 2 (Grb2), which is constitutively associated with the Ras-GTP-exchange factor Sos (6). The complex interplay between these two pathways has made it hard to dissect the precise mechanisms that govern different aspects of IL-6 signaling. Indeed, the simultaneous activation of both pathways can often generate opposing signals which has led to the development of the “orchestration model,” where the relative intensities of the opposing signals determine the biological outcome (16).
There are precedents for an interaction between IL-6 and HCMV during lytic infection. For example, the IE86 gene product has been reported to downregulate IL-6 production by active destabilization of the IL-6 mRNA (8). Despite these effects, an analysis of the composition of the secretome from lytically infected cells identified IL-6 as one of the most abundant cytokines released (5). It is likely that this IL-6 production is, at least in part, due to the actions of the US28 G protein-coupled receptor encoded by HCMV. Recent data have shown that US28 can promote IL-6 production and subsequent signaling; this may result in elevated STAT-3 phosphorylation, which has been associated with a poor prognosis for cancer patients (41).
Most pertinent to this study was a recent report from the Shenk laboratory that documented an experimental model of HCMV latency in monocytes involving the long-term culture of CD14+ cells in cytokine-conditioned medium which, upon the addition of exogenous IL-6, was capable of supporting reactivation (12). Indeed, aspects of this study echoed an earlier study that reported that the culture of granulocyte-macrophage progenitors (GM-Ps) in a highly similar cytokine cocktail promoted commitment to a dendritic cell lineage and concomitant reactivation following culture with primary fibroblasts (11). Thus, although these data argued for a role for IL-6 in reactivation, the precise mechanisms of the IL-6-mediated effect—and any complementary effects associated with prolonged culture of the monocytes in the previously described cytokine cocktail—were not definitively delineated. However, the inference from the data was that IL-6 played a key role in the efficient reactivation of HCMV from myeloid cells. Aspects of this observation correlated with our own ongoing studies of HCMV latency, and here we show that we also observe that IL-6 is a major component of HCMV reactivation from dendritic cells, a known site of natural latency (31). Furthermore, we observe that neutralization of IL-6 activity or the inhibition of downstream ERK–mitogen-activated protein kinase (ERK-MAPK), but not JAK/STAT, markedly abrogated HCMV reactivation. Importantly, we observe that the effects of IL-6 are only manifest on myeloid cells differentiated prior to the addition of IL-6. Taken together, these data provide a mechanism by which inflammation and myeloid cell differentiation act in concert to promote reactivation of HCMV and, importantly, the potential for therapeutic intervention.
MATERIALS AND METHODS
Ethics statement.
All research describing studies on primary human material with HCMV were assessed and approved by the Novartis research ethics committee. The collection of venous blood samples from anonymous donors was performed in accordance with established guidelines for the handling and processing of said tissue by the Novartis Blood Donor Enrollment Program and Cambridge Local Research Ethics committee.
Virus, cell lines, culture, and reagents.
The clinical isolates TB40/e and VR1814 were purified from infected human retinal pigment epithelial cells using sorbitol gradients as previously described (3). Viruses for these studies were characterized by their ability to infect primary dendritic cells to assay myelotropism; routinely, virus preparations used functionally infected 30 to 40% of DCs when used at a multiplicity of infection (MOI) of 5 calculated with fibroblasts. Primary CD14+ monocytes were isolated from the venous blood of anonymous donors who had given informed consent under the appropriate local rules. Typically, 50 ml of venous blood was diluted in phosphate-buffered saline (PBS) 1:1 and, following separation on a Ficoll gradient (Lymphoprep, Nycomed, Melville, NY), peripheral blood mononuclear cells were then purified using either a CD14+ cell direct isolation kit (Miltenyi Biotec, Auburn, CA). Labeled CD14+ cells were rescued using magnet-activated cell sorting (MACS) and cultured in X-vivo 15 medium (Cambrex, Walkersville, MD) supplemented with 2 mM l-glutamine and cytokines to promote DC differentiation as appropriate.
Experimental infection of primary cells and differentiation.
The establishment of an in vitro experimental latent infection of primary CD14+ cells was performed by infecting CD14+ monocytes (5 × 105 cells/well) in 250 μl of X-vivo 15 medium plus VR1814 or TB40/e (MOI, 5) for 3 h and then rescuing the monocytes in 1 ml of fresh medium. After 3 days, experimentally infected CD14+ cells were differentiated to immature interstitial cell-like DCs (here referred to as “interstitial-like DCs”). To show differentiation, immature DCs were shown to be CD14neg, CD80+, CD83low, where “neg” and “low” refer to negative and low expression, respectively, and upon maturation with lipopolysaccharide (LPS), CD83+ as reported previously (10, 36). No overt differences were observed between mock-treated and experimentally latent populations of monocytes upon differentiation. Addition of IL-6 to immature DCs did not promote DC maturation (measured by CD83 induction and morphology) over the time frame of our analyses.
Cytokines, inhibitors, and antibodies.
Differentiation of primary cells to DCs was achieved as previously described (36), and all cytokines are from Peprotech, Rocky Hill, NJ, unless otherwise stated. Briefly, CD14+ monocytes were cultured with interleukin-4 (100 ng/ml) and granulocyte-macrophage colony-stimulating factor (GM-CSF; 100 ng/ml) to promote differentiation to an interstitial-like DC phenotype (10). Following culture, immature DCs was matured using lipopolysaccharide (500 ng/ml; Sigma, St. Louis, MO) or incubated with recombinant IL-6 (Novartis Pharma, Basel, Switzerland, or Peprotech, Rocky Hill, NJ), IL-8, IL-11, oncostatin, monocyte chemoattractant protein 1 (MCP-1), IL-1β, or prostaglandin E2 (Sigma, St. Louis, MO).
To test for inhibition of HCMV reactivation, a neutralizing IL-6, IL-8, or goat IgG isotype antibody (R&D systems, Minneapolis, MN) was added concomitantly with LPS (between 10 ng/ml and 10 μg/ml). Inhibition of the MAPK pathways was performed using a MAPK inhibitor set II. This kit contained MEK/ERK inhibitors U0126 and PD98059 (10 μM), p38 inhibitor SB203580 (25 μM), and the inactive analogue SB202474 (25 μM). To inhibit Jun N-terminal protein kinase (JNK) kinase activity, SP600125 stress-activated protein kinase Saran (SAPK) JNK inhibitor (10 μM) was used with N′-methyl-1,9-pyrazolanthrone as a negative control (all from Calbiochem, San Diego, CA). MAPK inhibitors were dissolved in dimethyl sulfoxide (DMSO) and added 2 h prior to the addition of LPS directly to the culture medium. JAK/STAT inhibitor AG490 (20 μM; Sigma, Poole, Dorset, United Kingdom) was dissolved in ethanol and added to the culture medium 2 h prior to LPS. Prior to use, all inhibitors were titrated to determine a dose that gave activity against their target without any carry-over effect on the viability of the cells, as measured by trypan blue staining. Activin A (Invitrogen, Carlsbad, CA) was added directly to the medium 1 h prior to addition of LPS or IL-6 and was used at concentrations previously shown to inhibit IL-6 activity (25 ng/ml).
For immunofluorescent detection of viral antigens, cells were fixed in 4% paraformaldehyde and then permeabilized with 0.1% Triton X/PBS. An anti-IE (1:1,000 in PBS; Millipore) or anti-pp28 (1:250 in PBS; Abcam) antibody was used, followed by an Alexa Fluor 594-nm conjugated goat anti-mouse antibody (1:1,000 in PBS; Millipore). All antibody incubations were performed for 1 h at room temperature.
Nucleic acid isolation, RT, and PCR.
RNA was isolated from 106 cells using RNAeasy spin columns as described by the manufacturer (Qiagen, Valencia, CA). Following isolation, 10 μg of total RNA was incubated with DNase I (Promega, Madison, WI) and then reverse transcribed using an ImpromII reverse transcription (RT) kit (Promega, Madison, WI). DNA was harvested from infected cells using the sodium perchlorate method. Cells (106) were resuspended in 100 mM NaCl–5 mM EDTA (pH 8.0) before lysis with 10% SDS. Protein was then aggregated with the addition of 5 M sodium perchlorate before phenol-chloroform extraction of DNA and isopropanol precipitation. Gene- and promoter-specific primers were then used to amplify target sequences by PCR using PCR 2× Master Mix (Promega, Madison, WI) with the following cycling conditions: 95°C (5 min); 0 to 30 cycles of 94°C (40 s), 55°C (40 s), and 72°C (60 s); and then a final extension at 72°C for 10 min. Primers were as follows: actin, 5′-GCT CCG GCA TGT GCA and 5′-AGG ATC TTC ATG AGG TAG T; GAPDH, 5′-GAG TCA ACG GAT TTG GTC GT and 5′-TTG ATT TTG GAG GGA TTC TCG; IE, 5′-CAT CCA CAT CTC CCG CTT AT and 5′-CAC GAC GTT CCT GCA GAC TAT G; MCP-1, 5′-TGG AAT CCT GAA CCC ACT TC and 5′-CCC AGT CAC CTG CTG TTA T; RL4, 5′-GTT TCG TTG TTG TCG GTA GT and 5′-CGT TAT CCG TTC CTC GTA GG; UL138, 5′-TGC GCA TGT TTC TGA GCT AC and 5′-ACG GGT TTC AAC AGA TCG AC; and UL81-82ast, 5′-ATG ACC TCT CCT CCA CAC C and 5′-GGA AAA ACA CGC GGG GGA. For detection of naturally latent HCMV gene expression, a nested PCR was performed with 5 μl of the primary PCR (30 cycles). The following cycling conditions were used: 95°C (5 min); 20 to 30 cycles of 94°C (40 s), 55°C (40 s), and 72°C (60 s); and then a final extension at 72°C for 10 min using IE (nest), 5′-GCG CCA GTG AAT TTC TCT TC and 5′-ACG AGA ACC CCG AGA AAG ATG.
For quantitative PCR, primers that amplified the IE region of HCMV were used (22). The following reaction conditions were used in a 96-well plate format with forward primer AGC GCC GCA TTG AGG A, reverse primer CAG ACT CTC AGA GGA TCG GCC, and probe ATC TGC ATG AAG GTC TTT GCC CAG TAC ATT (Fam probe with 6-carboxytetramethylrhodamine [TAMRA] quencher). PCRs were performed using TaqMan Master Mix (Applied Biosystems, Foster City, CA) in a 7500HT machine (Applied Biosystems, Foster City, CA). Actin was amplified using a VIC-actin commercial probe (Applied Biosystems, Foster City, CA), and statistical analysis and interpretation were performed as described previously (37).
RESULTS
CD14+ monocytes differentiated into interstitial-like DCs can be used to model experimental latency.
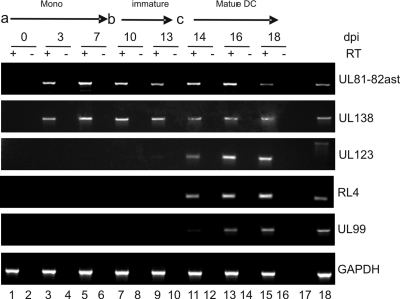
In order to investigate the role of inflammation in HCMV reactivation, we first used an experimental model of HCMV latency in the interstitial DC lineage. In principle, the model relies on the infection of the CD14+ peripheral blood monocyte population as a site of experimental latency. A latent infection of these cells was characterized by the detection of latency-associated transcripts UL138 and UL81-82ast in the absence of significant lytic IE, early (E), and late (L) gene expression upwards of 7 days postinfection (Fig. 1A, lanes 1 to 6). We note that we do not detect significant levels of viral lytic transcripts as was reported in an earlier study (12). Possibly, this is due to sensitivity and the endpoint PCR used, which gives a more comparative analysis of the levels of various transcripts during different phases of infection. However, we also routinely do not see IE protein expression in infected monocytes by immunofluorescence at 24 h postinfection (hpi) (data not shown), and we argue against the presence of a low-level infection, since we observe no effects of LPS or IL-6 on monocytes directly (Fig. 2D), consistent with the phenotype reported for natural latency (31, 42, 44). Subsequent differentiation of these experimentally latent cells into interstitial-like DCs does not trigger robust reactivation of lytic gene expression (Fig. 1A, lanes 7 to 10) until there is additional stimulation with LPS (Fig. 1A, lanes 11 to 16), upon which the efficient reactivation of IE, E, and L gene expression is observed. Thus, having observed that our monocyte model exhibited differentiation-dependent permissiveness for efficient reactivation, we chose to investigate this further.
Fig. 1.
Differentiation of CD14+ cells to interstitial DCs triggers lytic gene expression from latently infected cells. CD14+ monocytes infected with VR1814 (a) were cultured for 7 days in X-vivo 15 medium. The medium was then was exchanged and supplemented with IL-4/GM-CSF (b) to promote differentiation to an immature DC phenotype. At day 13 (c), and 6 days postdifferentiation, cells were stimulated with LPS to promote maturation and reactivation. At the times indicated, RT-PCR was performed with RNA samples to determine UL138, UL81-82ast, UL123, RL4, UL99, and GAPDH gene expression. dpi, days postinfection.
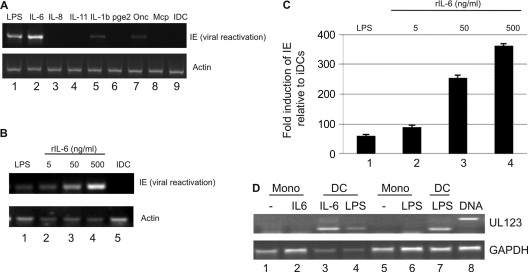
Fig. 2.
IL-6 promotes HCMV reactivation from immature interstitial-like DCs. (A) RT-PCR on experimentally latent CD14+-derived immature DCs for IE reactivation and actin gene expression treated with LPS (lane 1), IL-6 (lane 2), IL-8 (lane 3), IL-11 (lane 4), IL-1b (lane 5), prostaglandin E2 (pge2; lane 6), oncostatin (Onc; lane 7), or MCP-1 (Mcp; lane 8) or mock treated (lane 9). (B and C) CD14+-derived immature DCs incubated with LPS (lane 1), 5 to 500 ng/ml recombinant IL-6 (lanes 2 to 4), or mock treatment (lane 5) were analyzed for IE and actin gene expression by RT-PCR and real time qRT-PCR. Real time qRT-PCR analyses were expressed as the fold change in IE expression compared to that of immature DCs (n = 3). (D) Infected CD14+ monocytes (Mono) were cultured for 3 days in X-vivo 15 medium and then either left undifferentiated (lanes 1, 2, and 5) or stimulated to differentiate into DCs (lanes 3, 4, 6, and 7) and mock stimulated (−) or further stimulated with IL-6 or LPS for 24 h. Reactivation was measured by an intron-spanning IE RT-PCR. A DNA control (lane 8) and GAPDH RT-PCRs are also shown. rIL-6, recombinant IL-6.
Interleukin-6 promotes efficient reactivation of HCMV from immature interstitial-like DCs.
Clinically, HCMV reactivation and disease is associated with highly inflammatory environments; thus, since dendritic cell maturation is a major trigger of inflammation, we first asked whether any inflammatory cytokines could promote HCMV reactivation from immature DCs directly. A number of cytokines were tested for their ability to promote reactivation of IE gene expression by RT-PCR. As expected, LPS maturation triggered the reactivation HCMV from interstitial-like DCs. Interestingly, similarly robust reactivation was induced only by IL-6 in the panel of cytokines we analyzed (Fig. 2A). Furthermore, semiquantitative PCR and quantitative RT-PCR (qRT-PCR) analysis revealed that the reactivation of HCMV IE gene expression by IL-6 occurred in a dose-dependent manner (Fig. 2B and C). Importantly, direct stimulation of CD14+ monocytes with either IL-6 or LPS (500 ng/ml) did not induce reactivation of HCMV without prior differentiation to an immature DC phenotype first (Fig. 2D).
The effects of IL-6 are observed only during HCMV reactivation from immature DCs and not upon lytic infection.
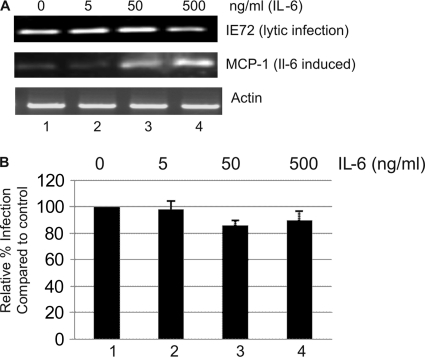
We next asked whether the effects of IL-6 were specific to reactivation or whether they had an impact on HCMV lytic infection also. Immature interstitial-like DCs were preincubated with a concentration of IL-6 shown to promote reactivation for 1 h and then infected, in the presence of IL-6, with HCMV. Intriguingly, we observed that the effects of IL-6 on the induction of IE gene expression were evident only during reactivation, since the incubation of interstitial-like DCs with IL-6 prior to de novo infection with HCMV had no impact on the level of IE gene expression in the infected cells (Fig. 3A) nor on the number of cells infected per se (Fig. 3B).
Fig. 3.
The effects of IL-6 on HCMV IE gene expression are specific to reactivation. (A) CD14+-derived immature DCs were incubated with IL-6 for 1 h and then infected with HCMV and RT-PCR for IE72. MCP-1 and actin gene expression was determined at 8 hpi. (B) CD14+-derived immature DCs were incubated with 0 to 500 ng/ml of IL-6 1 h prior to infection. At 24 h postinfection, cells were stained for IE gene expression to measure virus infection; infection was expressed as a percentage of DCs not incubated with IL-6. Results shown are representative of those of three experiments.
Interleukin-6 enhances IE gene reactivation, which correlates with elevated levels of virus production.
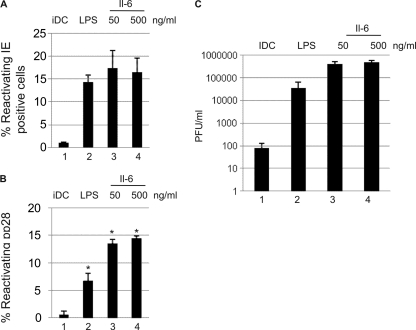
Potentially, the elevated IE gene expression seen by qRT-PCR in response to IL-6 could be due to one of two scenarios: that there is an increased frequency of reactivating cells in the presence of IL-6 or that IL-6 enhances IE gene expression in cells already undergoing reactivation. To address this, we performed IF analysis to quantify the number of reactivating cells in response to no stimulation, LPS, or IL-6 (Fig. 4). Minimal IE-positive cells were observed in immature interstitial-like DCs. Maturation with LPS promoted a significant increase in the number of IE-positive cells (14% ± 2%), which was mimicked by the addition of IL-6 (Fig. 4A). Thus, despite the elevated IE gene expression observed by qRT-PCR following the addition of IL-6, the absolute numbers of reactivating cells in the LPS- and IL-6-stimulated interstitial-like DC populations was not significantly different. However, an analysis of late gene expression in reactivating DCs at 7 days postreactivation showed that the addition of IL-6 significantly increased the number of pp28-positive DCs (Fig. 4B), suggesting that in the presence of IL-6, more cells go on to complete the life cycle. Consistent with this possibility, the production of infectious virus in the presence of recombinant IL-6 was at least 1 log higher than that seen with LPS alone (Fig. 4C).
Fig. 4.
Elevated IE expression induced by IL-6 promotes more efficient reactivation of infectious virus. (A and B) CD14+-derived immature DCs incubated with mock treatment (lane 1), LPS (lane 2), or 50 or 500 ng/ml IL-6 (lanes 3 and 4) were stained for IE (A) or pp28 (B) expression and counterstained with Hoechst, and the percent positive cells was enumerated (10 fields) from three separate wells per condition. Statistical significance was determined using the nonparametric Mann U Whitney test. (C) Shown is the 50% tissue culture infective dose (TCID50) for cell lysates and supernatants 10 days postcoculture to quantify the production of infectious virus. iDC, immature DCs.
ERK-MAPK signaling is important for LPS- and IL-6-induced reactivation.
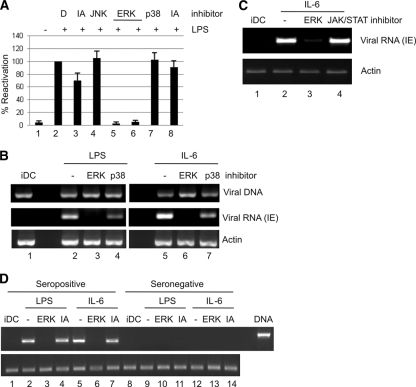
HCMV infection triggers multiple pathways upon virus binding and entry (18). A number of these pathways are modulated by HCMV throughout infection to promote efficient lytic infection (7). In contrast, reactivation of HCMV must, by definition, initiate in the absence of these virally induced effects. We, and others, have reasoned that the trigger to reactivate HCMV is a combination of cellular differentiation to a permissive environment and the concomitant activation of pathways that augment viral gene expression. The MIEP of HCMV bears the hallmarks of a cellular inflammatory promoter and, given the role of DC maturation in HCMV reactivation, we hypothesized that the MAPK pathways, which represent important mediators of the inflammatory response in DCs, could also play a role in reactivation. Consistent with this hypothesis, we observed that inhibition of the ERK, but not that of p38 or JNK/SAPK, MAPK pathway had the most pronounced effect on the reactivation of IE gene expression following stimulation of interstitial DCs with LPS (Fig. 5A). Activation of the ERK-MAPK pathway by LPS can occur directly or via intermediate signaling, which could include IL-6. We therefore asked whether ERK-MAPK signaling was important for the inhibition of IL-6-induced reactivation also. Interstitial DCs, preincubated with inhibitors of ERK-MAPK or the p38 MAPK signaling pathway and then analyzed for HCMV reactivation of IE gene expression, showed that IL-6-induced reactivation was predominantly ERK-MAPK dependent, although p38 inhibition also appeared to have some minor impact (Fig. 5B). Furthermore, an inhibitor of JAK/STAT signaling had no impact on IL-6-induced reactivation (Fig. 5C), suggesting that IL-6/ERK signaling, but not that of IL-6/JAK-STAT, was a major component of reactivation. Finally, the inhibition of ERK-MAPK signaling blocked both LPS- and IL-6-induced reactivation from immature DCs derived from the cells of seropositive donors (Fig. 5D).
Fig. 5.
ERK MAPK inhibitors block LPS- and IL-6-induced HCMV reactivation. (A) CD14+-derived immature DCs were mock cultured (lane 1) or cultured with LPS (lanes 2 to 8) after 2 h pretreatment with 0.1% DMSO (lanes 1 and 2), JNK-negative control (lane 3), SPB610025 (lane 4), PD98059 (lane 5), U0126 (lane 6), SB230580 (lane 7), or S202474 (lane 8) and analyzed for IE and actin gene expression by RT-PCR. IE expression levels were expressed as a function of LPS alone (n = 3). (B) CD14+-derived immature DCs (iDC; lane 1) stimulated with LPS (lanes 2 to 4) or 50 ng/ml IL-6 (lanes 5 to 7) after 2 h pretreatment with 0.1% DMSO (lanes 1, 2, and 5), U0126 (lanes 3 and 6), or SB230580 (lanes 4 and 7) were analyzed for IE and actin gene expression by RT-PCR or by PCR to detect viral DNA. (C) Experimentally infected CD14+ immature DCs pretreated with DMSO (lane 2), ERK (lane 3), or JAK/STAT (lane 4) inhibitors were incubated with IL-6 and analyzed 8 h postmaturation by RT-PCR for IE gene expression. (D) CD14+ cells isolated from CMV-seropositive (lanes 1 to 4) and -seronegative (lanes 5 to 8) donors were differentiated to immature DCs and then, prior to LPS (lanes 2 and 3) or IL-6 (lanes 4 and 5) stimulation, were incubated with an ERK or inactive analogue (IA) control for 1 h and analyzed for IE and actin gene expression by RT-PCR 24 h later. For experimental latency (B and C), results of one of three representative experiments are shown, and for natural latency (D), results of one of two experiments are shown.
Antagonists of IL-6 signaling block reactivation in experimental and natural latency.
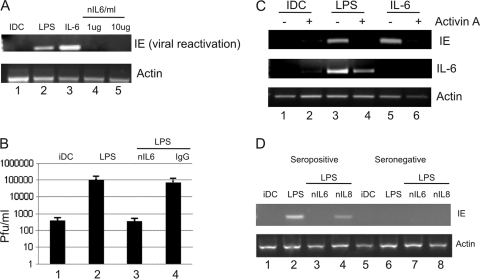
The data derived from our studies with interstitial-like DCs suggested that an important component of HCMV reactivation was maturation, which could be functionally substituted for by using IL-6 alone. Since LPS-induced maturation promotes inflammatory gene expression from DCs, including IL-6, we hypothesized that the induction of IL-6 production was an important mechanism for LPS-induced reactivation of HCMV. To test this, we incubated experimentally latent immature interstitial-like DCs with LPS in the presence of a neutralizing IL-6 antibody. Consistent with a role for IL-6, the neutralization of IL-6 blocked LPS-induced reactivation of IE gene expression from both interstitial-like DCs (Fig. 6A), which resulted in a significant reduction of the reactivation of infectious virus (Fig. 6B). We next asked whether activin A, a naturally occurring inhibitor of IL-6 signaling in certain settings (34), could also inhibit reactivation. A member of the transforming growth factor beta (TGF-β) superfamily, activin A is thought to act to regulate excessive inflammatory responses to prevent tissue damage (45), and in our interstitial-like DC model, activin A also had a profound impact on HCMV reactivation (Fig. 6C), again linking inflammation, IL-6, and HCMV reactivation.
Fig. 6.
Inhibition of IL-6 signaling abrogates LPS-induced HCMV reactivation from DCs. (A) CD14+-derived immature DCs were incubated with mock treatment (lane 1), LPS (lane 2), 50 ng/ml IL-6 (lane 3), or LPS plus 1 to 10 μg/ml nIL6ab (4 and 5) and analyzed for IE gene expression by RT-PCR. (B) CD14+-derived immature DCs were incubated with LPS alone or LPS plus nIL6 or isotype IgG and then cocultured with fibroblasts for 14 days, and virus production was measured by TCID50. (C) CD14+-derived immature DCs were incubated with 25 μg/ml activin A (lanes 2, 4, and 6) and then cultured with mock treatment (lanes 1 and 2), LPS (lanes 3 and 4), or 50 ng/ml IL-6 (lanes 5 and 6). Twenty-four h later, an RT-PCR for IE, IL-6, and actin gene expression was performed. (D) CD14+ cells isolated from CMV-seropositive (lanes 1 to 4) and -seronegative (lanes 5 to 8) donors were differentiated to immature DCs and then incubated with mock treatment (lanes 1 and 5), LPS (lanes 2 and 6), LPS plus 10 μg/ml nIL6ab (lanes 3 and 7), or LPS plus 10 μg/ml nIL8ab (lanes 4 and 8) and analyzed for IE and actin gene expression by RT-PCR. Panels are representative of three independent analyses.
Crucially, the effects of IL-6 neutralizing antibodies (nIL6abs) were also evident in natural latency. CD14+ cells isolated from seropositive donors (results from 1 of 3 are shown in Fig. 6) were differentiated to immature interstitial-like DCs and then stimulated with LPS to induce maturation and reactivation (Fig. 6D). Preincubation of immature DCs with an nIL6ab, but not an isotype-matched control antibody with neutralizing capability (anti-IL-8), had a profound effect on the reactivation of IE gene expression from latency from cells carrying naturally latent HCMV (Fig. 6D).
Conclusions.
The mechanisms by which HCMV maintains latency and, arguably more importantly, reactivates remain an enigmatic problem. Evidence from a number of studies of both experimental and natural latency has corroborated the idea that the myeloid lineage is a key site of latency and reactivation (1, 9, 11, 31, 42, 44). Critically, a number of these studies have linked the differentiation of myeloid progenitors into terminally differentiated macrophages or dendritic cells as a key event for the induction of reactivation (11, 31, 42). Thus, at a phenotypic level, changes in cellular identity and the implications for reactivation are becoming increasingly understood. However, much less is known about the intracellular events which accompany these changes. Of note is the apparent ease and rapidity with which HCMV reactivates in vivo compared with that seen in experimental and natural latency analyses in vitro, suggesting that further undefined stimuli which are clearly absent in experimental models must play a role in efficient reactivation. Thus, we chose to further investigate the role of inflammation.
We utilized a tractable model of HCMV latency that relied on the experimental infection of nonpermissive CD14+ monocytes and subsequent differentiation to promote reactivation. Critically, the biology of this experimental model is recapitulated on studies of natural latency (31), suggesting that the latent infections we establish in these cells are biologically relevant regarding HCMV using either the TB40/e or the VR1814 strain. Reactivation is commonly observed to occur when the latently infected cell differentiates to a permissive phenotype and has been a hallmark of a number of studies that have employed different protocols. However, we were intrigued that immature interstitial-like DCs, which are known to be permissive for lytic infection (33), did not support robust HCMV reactivation compared with mature interstitial-like DCs. Consequently, we hypothesized that elements associated with inflammation and DC maturation were key aspects of HCMV reactivation in our in vitro system. What became evident from these studies was that IL-6 was a key component of HCMV reactivation from potentially permissive cells. Furthermore, the reactivation associated with LPS-induced reactivation upon maturation was also shown to rely substantially on IL-6 signaling, as evidenced by the substantial effect neutralizing IL-6 antibodies had on reactivation from CD14+-derived interstitial-like DCs. Key to these observations is that the effects of IL-6 were observed only during reactivation and were evident only for cell types with the capability to support active HCMV infection (15, 33), suggesting that multiple criteria have to be met to support reactivation, of which the activity of IL-6 is just one important component.
The reactivation-specific effects of IL-6 were an intriguing aspect of this study. The addition of IL-6 to lytically infected cells had no impact on IE gene expression nor, unlike with studies using the histone deacetylase inhibitor trichostatin A, did it enhance the overall level of infection at low MOIs. Unsurprisingly, preincubation with an nIL6ab had little impact on the level of lytic infection (data not shown), although we did observe that addition of activin A prior to infection imparted a minor inhibitory effect on lytic infection at higher doses (data not shown). These data would suggest that either IL-6 activates a pathway that is already activated by virus binding and thus becomes redundant during lytic infection or that the activation of other pathways upon virus binding are sufficient for maximal activation of the MIEP. Alternatively, it may point toward a latency-specific region of the MIEP that is predominantly important for reactivation (19). It is of importance that we observed that the effects of IL-6 are manifest via the ERK-MAPK pathway. During lytic infection, HCMV binding to the surface of cells promotes ERK-MAPK signaling, which is then continued throughout infection via the activity of IE72 (35). In this context, it is clear that the effects of IL-6 would be redundant during lytic infection but may play a major role in the initial stages of reactivation prior to the induction of substantial IE gene expression as the virus reenters the lytic life cycle.
During the course of our own study, a report from another laboratory (12) also suggested that IL-6 could have a role in reactivation from experimentally latent CD14+ cells precultured in a cytokine cocktail with similarities to one that has previously been shown to promote HCMV reactivation from bone marrow progenitor cells (11). The specific mechanism of the effect of IL-6 in this particular system remained not wholly defined. However, in a study by Hahn et al. (11), the culture of granulocyte/macrophage progenitors in a similar cytokine cocktail had profound effects on the cellular differentiation of the myeloid progenitors to dendritic precursors, which was an important mediator of reactivation itself upon explant and coculture with fibroblasts and is similar to the observations in the later study. Given that, in our hands, the rapid effects of IL-6 on HCMV reactivation are manifest only for cells we observe to be capable of supporting a lytic infection of HCMV (i.e., differentiated DCs), the results reported by Hahn et al. may suggest that there is some level of activation and differentiation occurring with the culture of the monocytes in that system. Indeed, the long-term culture of monocytes with serum has been proposed to promote a heterogeneous myeloid differentiation phenotype, even in IL-4/GM-CSF-stimulated populations, due to the presence of contaminating IL-6 in the serum (27). Since we perform all our experiments under serum-free conditions, this was not an issue in our studies, and we argue that the effects of IL-6 in our study are due to direct reactivation of viral gene expression rather than the induction of change from a nonpermissive to permissive phenotype. Of considerable interest will be the addition of IL-6 to naturally latent monocytes cultured under the same conditions to see if the effects Hargett and Shenk observed in their latent system were reflective of natural latency. However, the observation that IL-6 may play a role was consistent with our data and, herein, we also provide evidence for a potential mechanism of IL-6-induced reactivation from dendritic cells. However, we do not rule out, with regard to the previous observations (12), the explanation that IL-6 plays a role in reactivation that is similarly pleiotropic as that in normal cell biology, which would underpin its importance in both studies.
Crucially, because we used primary cells for these studies, it was possible to perform parallel studies in natural latency ex vivo which, in the absence of an animal model for HCMV infection, is becoming essential to sensibly address specific biochemical questions regarding HCMV reactivation. Although these data do not preclude a role for other inflammatory molecules in efficient HCMV reactivation in vivo—the MIEP is also NF-κB and TNF-α responsive (29, 32), and IL-8 (28) has been shown to enhance replication during lytic infection—they do suggest a major role for IL-6 for efficient initiation and progression of reactivation to infectious virus production. However, epidemiological studies that have attempted to correlate inflammation and reactivation are not definitive. It has been suggested that elevated IL-6 levels in the sera of seropositive patients correlates with HCMV reactivation and disease (17). Furthermore, HCMV seropositivity and elevated IL-6 levels have been suggested to be a predictor of cardiac disease (2) and are associated with increased risk for inflammatory bowel diseases (30) as well as with disease following bone marrow transplantation (17). Of course, a caveat of such studies is that HCMV infection induces substantial inflammatory gene expression (including that of the IL-6 gene), and thus it is unclear which event is precedent. However, in this study, we have observed that there is a dose-dependent effect of IL-6 on the reactivation of IE gene expression, which manifests as elevated virus production. We did not see an overt difference in the numbers of reactivating cells with different doses of IL-6 (by IE immunofluorescence), which suggests that the threshold to trigger reactivation requires a lower than maximal occupancy of the IL-6 receptor. However, as the dose increases, an enhancement of IE gene expression is seen, probably due to increased receptor occupancy, which likely drives a more efficient reactivation event and which would be consistent with increased reactivation in highly inflammatory environments.
Overall, this report represents the characterization of a specific cytokine that promotes the reactivation of naturally latent HCMV ex vivo and, more importantly, illustrates a potential mechanism to limit reactivation from these cells. Of paramount importance are the juxtapositions of inhibiting reactivation and the potential triggering of viral diseases using anti-inflammatory treatments, e.g., TNF antagonists (4); long-term nIL6ab therapies are under further evaluation. However, such an approach could be readily viable in already-immunosuppressed patients, a patient population highly susceptible to HCMV reactivation disease; thus, the modulation of IL-6 could have important implications for future therapeutic strategies.
ACKNOWLEDGMENTS
This work was funded by the Novartis Institutes for Biomedical Research (NIBR) and, subsequently, by a UK MRC CDA Fellowship to M.B.R. (G:0900466).
We are grateful to Carol Lachance for coordinating the Novartis Blood Donor Enrollment Program at NIBR that made these studies possible.
Footnotes
Published ahead of print on 21 September 2011.
REFERENCES
- 1. Bego M. G., St Jeor S. 2006. Human cytomegalovirus infection of cells of hematopoietic origin: HCMV-induced immunosuppression, immune evasion, and latency. Exp. Hematol. 34:555–570 [DOI] [PubMed] [Google Scholar]
- 2. Blankenberg S., et al. 2001. Cytomegalovirus infection with interleukin-6 response predicts cardiac mortality in patients with coronary artery disease. Circulation 103:2915–2921 [DOI] [PubMed] [Google Scholar]
- 3. Compton T. 2000. Analysis of cytomegalovirus ligands, receptors, and the entry pathway, p. 53–65 In Sinclair J. (ed.), Methods in molecular medicine: cytomegalovirus protocols. Humana Press, New York, NY: [DOI] [PubMed] [Google Scholar]
- 4. Domm S., Cinatl J., Mrowietz U. 2008. The impact of treatment with tumour necrosis factor-alpha antagonists on the course of chronic viral infections: a review of the literature. Br. J. Dermatol. 159:1217–1228 [DOI] [PubMed] [Google Scholar]
- 5. Dumortier J., et al. 2008. Human cytomegalovirus secretome contains factors that induce angiogenesis and wound healing. J. Virol. 82:6524–6535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ernst M., Jenkins B. J. 2004. Acquiring signalling specificity from the cytokine receptor gp130. Trends Genet. 20:23–32 [DOI] [PubMed] [Google Scholar]
- 7. Fortunato E. A., McElroy A. K., Sanchez I., Spector D. H. 2000. Exploitation of cellular signaling and regulatory pathways by human cytomegalovirus. Trends Microbiol. 8:111–119 [DOI] [PubMed] [Google Scholar]
- 8. Gealy C., et al. 2005. Posttranscriptional suppression of interleukin-6 production by human cytomegalovirus. J. Virol. 79:472–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goodrum F., Reeves M., Sinclair J., High K., Shenk T. 2007. Human cytomegalovirus sequences expressed in latently infected individuals promote a latent infection in vitro. Blood 110:937–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grassi F., et al. 1998. Monocyte-derived dendritic cells have a phenotype comparable to that of dermal dendritic cells and display ultrastructural granules distinct from Birbeck granules. J. Leukoc. Biol. 64:484–493 [DOI] [PubMed] [Google Scholar]
- 11. Hahn G., Jores R., Mocarski E. S. 1998. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc. Natl. Acad. Sci. U. S. A. 95:3937–3942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hargett D., Shenk T. E. 2010. Experimental human cytomegalovirus latency in CD14+ monocytes. Proc. Natl. Acad. Sci. U. S. A. 107:20039–20044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heike T., Nakahata T. 2002. Ex vivo expansion of hematopoietic stem cells by cytokines. Biochim. Biophys. Acta 1592:313–321 [DOI] [PubMed] [Google Scholar]
- 14. Heinrich P. C., et al. 2003. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 374:1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hertel L., Lacaille V. G., Strobl H., Mellins E. D., Mocarski E. S. 2003. Susceptibility of immature and mature Langerhans cell-type dendritic cells to infection and immunomodulation by human cytomegalovirus. J. Virol. 77:7563–7574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hirano T., Matsuda T., Nakajima K. 1994. Signal transduction through gp130 that is shared among the receptors for the interleukin 6 related cytokine subfamily. Stem Cells 12:262–277 [DOI] [PubMed] [Google Scholar]
- 17. Humar A., et al. 1999. Elevated serum cytokines are associated with cytomegalovirus infection and disease in bone marrow transplant recipients. J. Infect. Dis. 179:484–488 [DOI] [PubMed] [Google Scholar]
- 18. Isaacson M. K., Juckem L. K., Compton T. 2008. Virus entry and innate immune activation. Curr. Top. Microbiol. Immunol. 325:85–100 [DOI] [PubMed] [Google Scholar]
- 19. Keller M. J., et al. 2007. Reversal of human cytomegalovirus major immediate-early enhancer/promoter silencing in quiescently infected cells via the cyclic AMP signaling pathway. J. Virol. 81:6669–6681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kondo K., Kaneshima H., Mocarski E. S. 1994. Human cytomegalovirus latent infection of granulocyte-macrophage progenitors. Proc. Natl. Acad. Sci. U. S. A. 91:11879–11883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Legendre C., Pascual M. 2008. Improving outcomes for solid-organ transplant recipients at risk from cytomegalovirus infection: late-onset disease and indirect consequences. Clin. Infect. Dis. 46:732–740 [DOI] [PubMed] [Google Scholar]
- 22. Leruez-Ville M., et al. 2003. Monitoring cytomegalovirus infection in adult and pediatric bone marrow transplant recipients by a real-time PCR assay performed with blood plasma. J. Clin. Microbiol. 41:2040–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Limaye A. P., et al. 2008. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA 300:413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Macagno A., Napolitani G., Lanzavecchia A., Sallusto F. 2007. Duration, combination and timing: the signal integration model of dendritic cell activation. Trends Immunol. 28:227–233 [DOI] [PubMed] [Google Scholar]
- 25. Meier J. L., Stinski M. F. 1996. Regulation of human cytomegalovirus immediate-early gene expression. Intervirology 39:331–342 [DOI] [PubMed] [Google Scholar]
- 26. Mendelson M., Monard S., Sissons P., Sinclair J. 1996. Detection of endogenous human cytomegalovirus in CD34+ bone marrow progenitors. J. Gen. Virol. 77:3099–3102 [DOI] [PubMed] [Google Scholar]
- 27. Mitani H., et al. 2000. Activity of interleukin 6 in the differentiation of monocytes to macrophages and dendritic cells. Br. J. Haematol. 109:288–295 [DOI] [PubMed] [Google Scholar]
- 28. Murayama T., Mukaida N., Khabar K. S., Matsushima K. 1998. Potential involvement of IL-8 in the pathogenesis of human cytomegalovirus infection. J. Leukoc. Biol. 64:62–67 [DOI] [PubMed] [Google Scholar]
- 29. Prosch S., Wuttke R., Kruger D. H., Volk H. D. 2002. NF-kappaB: a potential therapeutic target for inhibition of human cytomegalovirus (re)activation? Biol. Chem. 383:1601–1609 [DOI] [PubMed] [Google Scholar]
- 30. Rahbar A. R., Sundqvist V. A., Wirgart B. Z., Grillner L., Soderberg-Naucler C. 2004. Recognition of cytomegalovirus clinical isolate antigens by sera from cytomegalovirus-negative blood donors. Transfusion 44:1059–1066 [DOI] [PubMed] [Google Scholar]
- 31. Reeves M. B., MacAry P. A., Lehner P. J., Sissons J. G., Sinclair J. H. 2005. Latency, chromatin remodeling, and reactivation of human cytomegalovirus in the dendritic cells of healthy carriers. Proc. Natl. Acad. Sci. U. S. A. 102:4140–4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reinke P., Prosch S., Kern F., Volk H. D. 1999. Mechanisms of human cytomegalovirus (HCMV) (re)activation and its impact on organ transplant patients. Transpl. Infect. Dis. 1:157–164 [DOI] [PubMed] [Google Scholar]
- 33. Riegler S., et al. 2000. Monocyte-derived dendritic cells are permissive to the complete replicative cycle of human cytomegalovirus. J. Gen. Virol. 81:393–399 [DOI] [PubMed] [Google Scholar]
- 34. Robson N. C., et al. 2008. Activin-A: a novel dendritic cell-derived cytokine that potently attenuates CD40 ligand-specific cytokine and chemokine production. Blood 111:2733–2743 [DOI] [PubMed] [Google Scholar]
- 35. Rodems S. M., Spector D. H. 1998. Extracellular signal-regulated kinase activity is sustained early during human cytomegalovirus infection. J. Virol. 72:9173–9180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sallusto F., Lanzavecchia A. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schmittgen T. D., Livak K. J. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3:1101–1108 [DOI] [PubMed] [Google Scholar]
- 38. Sinclair J., Sissons P. 2006. Latency and reactivation of human cytomegalovirus. J. Gen. Virol. 87:1763–1779 [DOI] [PubMed] [Google Scholar]
- 39. Sindre H., et al. 1996. Human cytomegalovirus suppression of and latency in early hematopoietic progenitor cells. Blood 88:4526–4533 [PubMed] [Google Scholar]
- 40. Sissons J. G., Bain M., Wills M. R. 2002. Latency and reactivation of human cytomegalovirus. J. Infect. 44:73–77 [DOI] [PubMed] [Google Scholar]
- 41. Slinger E., et al. 2010. HCMV-encoded chemokine receptor US28 mediates proliferative signaling through the IL-6-STAT3 axis. Sci. Signal. 3:ra58. [DOI] [PubMed] [Google Scholar]
- 42. Soderberg-Naucler C., Fish K. N., Nelson J. A. 1997. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell 91:119–126 [DOI] [PubMed] [Google Scholar]
- 43. Taylor-Wiedeman J., Sissons J. G., Borysiewicz L. K., Sinclair J. H. 1991. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J. Gen. Virol. 72:2059–2064 [DOI] [PubMed] [Google Scholar]
- 44. Taylor-Wiedeman J., Sissons P., Sinclair J. 1994. Induction of endogenous human cytomegalovirus gene expression after differentiation of monocytes from healthy carriers. J. Virol. 68:1597–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yu J., Dolter K. E. 1997. Production of activin A and its roles in inflammation and hematopoiesis. Cytokines Cell Mol. Ther. 3:169–177 [PubMed] [Google Scholar]