Abstract
Mitogen-activated protein kinase (MAPK) signaling pathways are dynamic and sensitive regulators of T cell function and differentiation. Altered MAPK signaling has been associated with the inflammatory and autoimmune diseases lupus and arthritis and with some pathogenic viral infections. HIV-1 infection is characterized by chronic immune inflammation, aberrantly heightened CD8+ T cell activation levels, and altered T cell function. The relationship between MAPK pathway function, HIV-1-induced activation (CD38 and HLA-DR), and exhaustion (Tim-3) markers in circulating CD8+ T cells remains unknown. Phosphorylation of the MAPK effector proteins ERK and p38 was examined by “phosflow” flow cytometry in 79 recently HIV-1-infected, antiretroviral-treatment-naïve adults and 21 risk-matched HIV-1-negative controls. We identified a subset of CD8+ T cells refractory to phorbol 12-myristate 13-acetate plus ionomycin-induced ERK1/2 phosphorylation (referred to as p-ERK1/2-refractory cells) that was greatly expanded in HIV-1-infected adults. The CD8+ p-ERK1/2-refractory cells were highly activated (CD38+ HLA-DR+) but not exhausted (Tim-3 negative), tended to have low CD8 expression, and were enriched in intermediate and late transitional memory states of differentiation (CD45RA− CD28− CD27+/−). Targeting MAPK pathways to restore ERK1/2 signaling may normalize immune inflammation levels and restore CD8+ T cell function during HIV-1 infection.
INTRODUCTION
Activation of ERK, and p38 MAPK signaling molecules modulates T cell function, exerting differential effects on T cell development, cell cycle progression, and apoptosis (8, 14, 26). ERK signaling is critical for positive selection, promotes cell cycle progression, and inhibits apoptosis (13, 19, 20), while p38 signaling is necessary for negative selection, promotes cell cycle arrest, and induces apoptosis (1, 12). Alterations in ERK signaling have been associated with chronic inflammatory autoimmune conditions such as lupus and rheumatoid arthritis (15, 25) and with pathogenic viral infections (30). Several viral proteins are known to interact with MAPK signaling pathways (29). Attenuated ERK1/2 phosphorylation responses to T cell receptor stimulation have been observed in unfractionated peripheral blood mononuclear cells (PBMCs) in HIV-1 infection (18).
HIV-1 disease is characterized by immune inflammation, with highly elevated CD8+ T cell-activation levels and lower levels of CD4+ T cell-activation, measured by joint surface expression of CD38 and HLA-DR markers. A set point CD8+ T cell-activation level is established in early untreated HIV-1 infection and predicts clinical outcome independently of plasma HIV-1 RNA levels (9). However, the functional significance of CD38 and HLA-DR coexpression on CD8+ T cells, a population that is not infected by HIV-1, has not been resolved. A detailed understanding of the functional changes to activated CD8+ T cells may aid in the development of therapeutic strategies to halt or reverse HIV immunopathogenesis.
HIV-1-associated CD8+ T cell activation has been linked to atypical T cell differentiation, (5) a process that involves MAPK signaling pathways (11). Previous studies of HIV-1-infected adults have reported altered CD8+ T cell differentiation profiles, specifically, a large expansion of transitional intermediate/late memory (CD45RA− CD28− CD27+/−) subsets and a reduction in the proportion of naïve (CD27+ CD28+ CD45RA+) subsets (2, 3, 22). An expansion of intermediate memory cells during HIV-1 infection may have negative functional consequences, such as increased CD8+ T cell replicative senescence or a failure to differentiate into functional effectors (28). In contrast, CD8+ T cells in the “terminally differentiated” CD45RA+ CD27− pool, referred to as the effector/memory RA (EMRA) pool, exhibit enhanced effector activities (27). An expanded TEMRA CD8+ T cell population has been associated with a lower viral load set point in early HIV-1 infection (21).
To evaluate MAPK signaling in activated CD8+ T cells during early untreated HIV-1 infection, we implemented a flow cytometry-based signaling assay termed “phosflow” (7, 24). Phosflow combines multiparameter phenotyping of surface antigen expression with simultaneous detection of phosphorylated forms of intracellular signaling protein intermediates. We examined ERK (ERK1/2) and p38 phosphorylation responses to phorbol 12-myristate 13-acetate and ionomycin (PMA+I) stimulation at the single-cell level in T cell subsets defined by expression of CD38, HLA-DR, and Tim-3. PMA is an analog of diacylglycerol, a key mediator of MAPK signaling through protein kinase C (PKC) (4). Ionomycin stimulates Ca2+ release from the endoplasmic reticulum, activating Ca2+-sensitive enzymes and synergizing with PMA (6). PMA+I is a potent stimulator of MAPK signaling cascades, resulting in the accumulation of phosphorylated, kinase-active ERK1/2 and p38 signaling intermediates (10). We hypothesized that activated CD38+ HLA-DR+ CD8+ T cells would display intact but attenuated MAPK signaling responses in HIV-1-infected adults compared to HIV-1-negative controls. Our findings did not confirm our hypothesis but instead revealed a novel, large population of highly activated CD8+ T cells not merely lacking robust MAPK pathway signaling responses but also displaying a complete abrogation of signaling through the ERK1/2 MAPK module.
MATERIALS AND METHODS
Study subjects.
Cryopreserved PBMCs were selected from the OPTIONS cohort study of early HIV-1 infection in San Francisco. The first clinical visit was always prior to administration of antiretroviral treatment. Early HIV-1 infection was identified as previously described (17). HIV-1-negative risk-matched controls were identified through OPTIONS project screening of adults with suspected HIV-1 sexual exposure who subsequently were found to be HIV-1 negative. All persons gave informed consent to participate, and the UCSF Committee on Human Research approved this study.
Cell culture, staining, stimulation, and flow-cytometric analysis.
Cryopreserved PBMCs stored by the UCSF/AIDS Research Institute (ARI) AIDS Specimen Bank were thawed, and 1 × 106 cells were immediately stained for T cell immunophenotypic markers. The remaining cells were allowed to rest for 18 h in 5% cRPMI (RPMI 1640 medium; UCSF Cell Culture Facility [CCF]) supplemented with 5% fetal bovine serum (FBS) (JR Scientific), 1% penicillin-streptomycin (CCF), 10 mM HEPES (CCF), 2 mM l-glutamine (Invitrogen), and 10 μg/ml DNase I (Sigma). Cells (1 × 106) were stained with aqua amine reactive dye (AARD; Invitrogen) and then anti-CD3 Alexa 700 (clone SP34-2), anti-CD4 PE-TR (clone S3.5), and anti-CD8 Pacific Blue (clone 3B5), along with anti-CD38 PE-Cy5 (clone HIT2), anti-HLA-DR APC-H7 (clone L243), and anti-Tim-3 PE (clone 344823) to identify activated and exhausted T cell subsets, respectively, or anti-CD28 PE-Cy5 (clone CD28.2), CD27 APC-eF780 (clone O323), and CD45RA PE (clone HI100) to identify differentiation subsets. Cells were stimulated with 100 ng/ml PMA and 1 μg/ml ionomycin (both from Sigma) for 15 min at 37°C, fixed in 4% paraformaldehyde (PFA; Electron Microscopy Sciences), permeabilized with custom phosflow buffer 643435 (courtesy of BD Bioscience), stained for intracellular phosphorylation-specific ERK1/2 Alexa 488 and p38 Alexa 647 (p-ERK1/2 and p-p38, clones 20A and 36/p38, respectively), suspended in 1% PFA in PBS, and analyzed by flow cytometry using a 4-laser LSR-II instrument (BD Bioscience). AARD, CD4, and CD8 were obtained from Invitrogen, CD3, CD38, HLA-DR, CD28, CD45RA, p-ERK1/2, and p-p38 from Becton Dickinson, CD27 from eBiosciences, and Tim-3 from R&D Systems. Fluorescence minus one (FMO) samples were prepared for each fluorochrome to facilitate gating. Anti-mouse or anti-rat IgG-coated beads (Becton Dickinson) were stained with each fluorochrome-conjugated antibody separately and used for software-based compensation. Data analysis was performed using FlowJo (Treestar).
Five CD8+ T cell maturation stages were defined by the use of three markers: (i) naïve, CD45RA+ CD28+ CD27+; (ii) early memory, CD45RA− CD28+ CD27+; (iii) intermediate memory, CD45RA− CD28− CD27+; (iv) late memory, CD45RA− CD28− CD27−; and (v) effector, CD45RA+ CD28− CD27− (27) (gating is depicted in Fig. S3 in the supplemental material).
Statistical analysis.
Statistical analysis was performed using GraphPad Prism statistical software (GraphPad Software, San Diego, CA). The nonparametric Mann-Whitney U was used for comparison tests, and the Spearman Rank test was used for correlation analyses. Measures of central tendency are expressed as medians and interquartile ranges (IQRs; given in the form 25th percentile, 75th percentile).
RESULTS
Clinical cohort description.
Frozen peripheral blood mononuclear cells (PBMCs) were obtained from the OPTIONS Project cohort study of early HIV-1 infection (San Francisco General Hospital, University of California, San Francisco). The study included 79 recently HIV-1-infected adults and 21 HIV-1-negative adult risk-matched controls (Table 1). PBMC samples were obtained during early infection prior to initiation of an antiretroviral treatment (HIV-1-infected patients) or during screening for HIV-1 infection that subsequently proved negative (controls). HIV-1-infected adults had lower CD4+ counts and higher CD8+ T cell activation than controls and had been infected an estimated median 98 days at the time of the study (Table 1).
Table 1.
Study participant characteristics
| Characteristic | Median value (IQR) for group |
|
|---|---|---|
| HIV negative (n = 21) | HIV positive (n = 79) | |
| Age | 38.1 (32.4, 40.9) | 34.9 (29.0, 39.9) |
| HIV-1 RNA level (log10 copies/ml) | NAa | 4.7 (4.2, 5.1) |
| CD4+ T cell count (cells/μl) | 1,038 (816, 1,133) | 593 (466, 750) |
| CD8+ (% CD38+ HLA− DR+) | 4.5 (3.5, 9.0) | 36.5 (27.5, 51.8) |
| % Male (no.) | 90.5 (19) | 96.2 (76) |
| % on ARTb at time of study | 0 | 0 |
| Time since infection (days) | NA | 98 (72, 130) |
NA, not applicable.
ART, antiretroviral treatment.
A subset of CD8+ T cells refractory to ERK1/2 phosphorylation is expanded in early untreated HIV-1 infection.
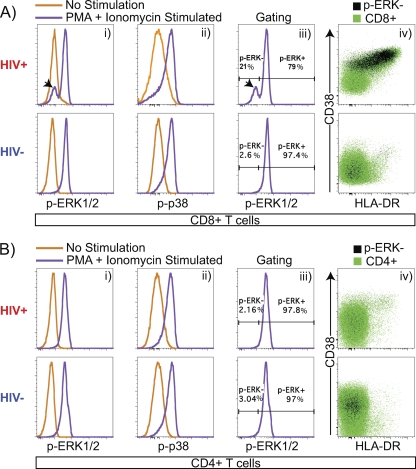
To examine MAPK pathway intracellular signaling events in T cell subsets, phosflow analysis was performed on PBMCs to detect phosphorylation of ERK1 and -2 (p-ERK1/2) and p38 (p-p38). Figure 1 illustrates a typical example of the CD8+ T cell p-ERK1/2 and p-p38 response to PMA+I stimulation and shows an increase in median fluorescence intensity (MFI) of p-ERK1/2 and p-p38 expression relative to unstimulated controls. Strikingly, in HIV-1-infected subjects, a large population of CD8+ T cells do not respond to PMA+I stimulation and have a p-ERK1/2 MFI similar to that of the unstimulated control (Fig. 1A, panel i). The resulting bimodal p-ERK1/2 histogram profile was gated into two distinct CD8+ subsets based on p-ERK1/2 responsiveness. The histogram peak containing cells shifted positively along the p-ERK1/2 axis was labeled p-ERK+, and this subset is referred to as p-ERK1/2 responsive. The histogram peak containing cells that failed to shift along the p-ERK1/2 axis was labeled p-ERK−, and this subset is referred to as p-ERK1/2 refractory. The term “refractory” was chosen to reflect the fact that while this CD8+ T cell subset exhibits an impaired p-ERK1/2 response after 15 min of PMA+I stimulation, the cells might still retain the capacity to respond through ERK1/2 with different signaling kinetics.
Fig. 1.
Identification of a CD8+ T cell subset refractory to PMA+I-induced ERK1/2 phosphorylation. (A) (Panel i) CD8+ T cells exhibit a shift in phosphorylation-specific ERK1/2 MFI (p-ERK1/2) in response to PMA+I stimulation. A subset of CD8+ cells that fails to phosphorylate ERK1/2 (black arrowhead) is expanded during HIV-1 infection. (Panel ii) CD8+ T cells exhibit a shift in phosphorylation-specific p38 MFI (p-p38) in response to PMA+I stimulation. (Panel iii) CD8+ T cells were gated for CD8+ p-ERK1/2-refractory (p-ERK−, black arrowhead) and p-ERK1/2-responsive (p-ERK+) subsets. (Panel iv) CD8+ T cells exhibit a p-ERK− subset. p-ERK1/2-refractory cells are predominantly activated (CD38+ HLA-DR+) and are increased in frequency during early untreated HIV-1 infection. (B) (Panel i) CD4+ T cells exhibit a shift in p-ERK1/2 MFI in response to PMA+I stimulation. (Panel ii) CD4+ T cells exhibit a shift in p-p38 MFI in response to PMA+I stimulation. (Panel iii) CD4+ T cells exhibit gating for CD4+ p-ERK1/2-refractory (p-ERK−) and -responsive (p-ERK+) populations. (Panel iv) CD4+ T cells exhibit a CD4+ p-ERK− population. Data are from one HIV− patient and one HIV+ patient.
As illustrated in Fig. 1A and in Table S1 in the supplemental material, the frequency of CD8+ p-ERK1/2-refractory T cells was significantly expanded in HIV-1-infected individuals relative to uninfected controls (median frequencies [IQRs], 19.0 [12.1, 28.7] for the infected group and 7.1 [3.4, 10.4] for the uninfected group; P < 0.0001). The fraction of CD4+ T cells falling within the p-ERK1/2-refractory gate was also significantly increased in HIV-1-infected individuals relative to uninfected controls (medians [IQRs], 3.1 [2.3, 5.1] for the infected group and 1.8 [1.4, 2.8] for the uninfected group; P = 0.0007) (Fig. 1B and data not shown), although the effect was more modest than for CD8+ T cells. The frequency of CD8+ p-ERK1/2-refractory T cells exhibited a significant correlation with viral load (Spearman r = 0.39, P = 0.0007) but not with CD4+ T cell count (Spearman r = −0.02, P = 0.8).
To examine the relationship between activation and the p-ERK1/2-refractory population, CD8+ T cells were divided into subsets with the activation markers CD38 and HLA-DR. We found that p-ERK1/2-refractory cells were primarily contained within the highly activated CD38+ HLA-DR+ population (Fig. 1A, panel iv). In HIV-1-infected patients, the frequency of CD8+ p-ERK1/2-refractory cells strongly correlated with CD38+ HLA-DR+ frequency (Spearman r = 0.71, P < 0.0001).
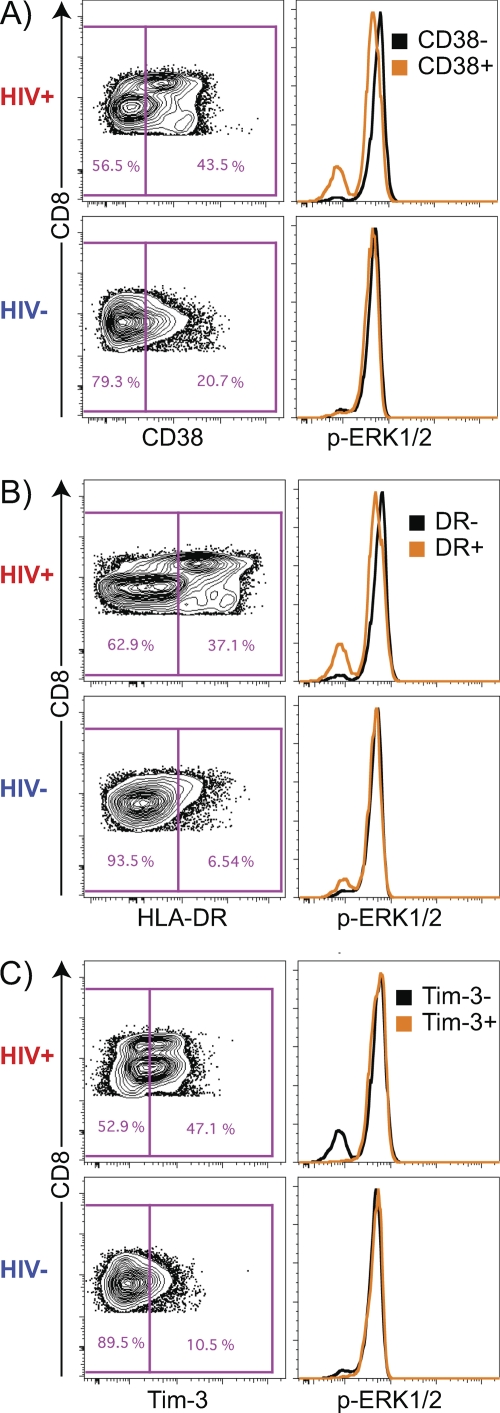
To define the p-ERK1/2-refractory phenotype, CD8+ T cells were gated for the presence or absence of CD38, HLA-DR, and Tim-3 markers individually, and histogram profiles of p-ERK1/2 MFI in the single-marker subsets were examined (Fig. 2). Of the p-ERK1/2-refractory CD8+ T cells in HIV-1-infected subjects, 69.9% (IQR, 53.1, 84.1) were CD38+ and 50.7% (IQR, 39.0, 70.4) were HLA-DR+, but only 11.7% (IQR, 8.6, 16.6) were Tim-3+. We then examined the MFIs of CD38, HLA-DR, and Tim-3 in p-ERK1/2-refractory and -responsive populations. The CD38 MFI of p-ERK1/2-refractory cells was significantly higher than that of the p-ERK1/2-responsive population (medians [IQRs], 705 [397, 1,221] for p-ERK1/2-refractory cells and 262 [224, 341] for p-ERK1/2-responsive cells; P < 0.0001). We observed a similar difference for HLA-DR (medians [IQRs] 835 [545, 1,548] for p-ERK1/2-refractory cells and 289 [225, 415] for p-ERK1/2-responsive cells; P < 0.0001), while Tim-3 expression showed the opposite pattern (medians [IQRs] 74.6 [67.0, 88.3] for p-ERK1/2-refractory cells and 129 [110, 149] for p-ERK1/2-responsive cells; P < 0.0001). Together, these data indicate that CD8+ T cells exhibiting a p-ERK1/2-refractory phenotype are predominately activated, as they express high levels of CD38+ and/or HLA-DR+, but are not exhausted, as they express low levels of Tim-3.
Fig. 2.
Gating strategy to identify the location of p-ERK1/2-refractory cells in CD38, HLA-DR, and Tim-3+ CD8+ T cell subsets. (A) CD38+ and CD38− gates in the CD8+ T cell compartment. p-ERK1/2-refractory cells are located predominantly within the CD38+ subset. (B) HLA-DR+ and HLA-DR− gates in the CD8+ T cell compartment. p-ERK1/2-refractory cells are located predominantly within the HLA-DR+ subset. (C) TIM3+ and TIM3− gates in the CD8+ T cell compartment. p-ERK1/2-refractory cells are located predominantly in the TIM3− subset. Data are from one HIV− patient and one HIV+ patient.
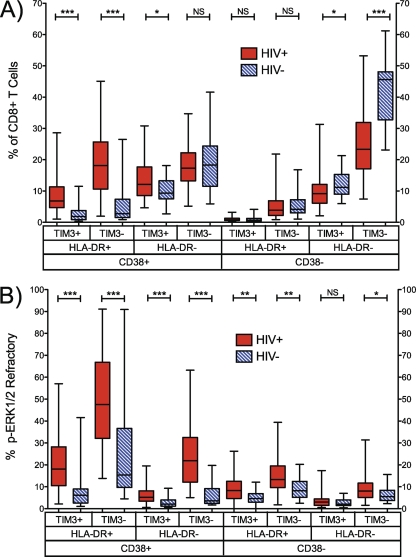
To explore the p-ERK1/2-refractory phenotype, individually gated CD38, HLA-DR, and Tim-3 subsets (Fig. 2) were used to generate eight Boolean gate populations in both HIV-1-infected and uninfected populations (Fig. 3). As expected, we observed significant expansions of activated (CD38+ HLA-DR+ Tim-3+/−) subsets, and a reduction in the nonactivated (CD38− HLA-DR− Tim-3−) subset, in recently HIV-1-infected patients versus controls (Fig. 3A). The frequency of p-ERK1/2-refractory cells was significantly increased in 7 of 8 Boolean subsets in HIV-1-infected patients versus uninfected controls (Fig. 3B). Notably, the highly activated CD38+ HLA-DR+ Tim-3− subset contained the largest proportion of p-ERK1/2-refractory cells in both HIV-1-infected and control subjects (medians [IQRs], 47.6% [32.1, 66.8] for the infected group and 15.4% [9.7, 36.7] for the uninfected group) (Fig. 3B, columns 3 and 4), and the level was significantly higher in HIV-1-positive patients (P > 0.0001).
Fig. 3.
Activation and p-ERK1/2-refractory phenotypes in CD8+ T cells. (A) Frequency of CD8+ CD38, HLA-DR, and Tim-3 Boolean-gated subsets in HIV-1-infected and HIV-1-negative patients. (B) Frequency of each subset exhibiting the p-ERK1/2-refractory phenotype. n = 21 HIV− and 79 HIV+ patients. NS, not significant (P > 0.1); *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
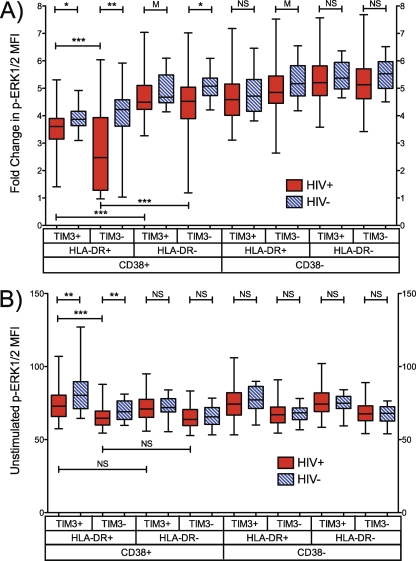
Next, we examined the magnitude of the PMA+I-induced p-ERK1/2 response in CD38, HLA-DR, and Tim-3 Boolean gated subsets, recorded as fold change (FC) in p-ERK1/2 MFI upon stimulation. The p-ERK1/2 FC response tended to be lower for the HIV-1-infected group than for uninfected controls in all subsets. Significant differences between infected and uninfected groups were observed in highly activated CD38+ HLA-DR+ Tim-3+/− and CD38+ HLA-DR− Tim-3− populations (Fig. 4A). There was no significant difference in p-ERK1/2 FC between groups in any of the CD8+ T cell populations that did not express CD38. In HIV-1-infected patients, the activated CD38+ HLA-DR+ Tim-3− subset exhibited the lowest p-ERK1/2 response (FC = 2.47 [IQR, 1.28, 3.93]). The reduced magnitude of p-ERK1/2 FC response in highly activated subsets was not attributable to differences in unstimulated (basal) p-ERK1/2 levels, as there were no significant differences in p-ERK1/2 MFIs between CD38+ HLA-DR+ double-positive subsets and subsets not expressing HLA-DR (Fig. 4B, compare column 1 with column 5 and column 3 with column 7). Taken together with the finding that almost 50% of CD8+ T cells in the CD38+ HLA-DR+ Tim-3− compartment were p-ERK1/2 refractory (Fig. 3B), these results indicate that the reduced p-ERK1/2 responsiveness (p-ERK1/2 FC) observed in the highly activated CD38+ HLA-DR+ Tim-3− subset in HIV-1-infected individuals is primarily due to the large percentage of p-ERK1/2-refractory cells present in this compartment, not simply an attenuated but intact per-cell responsiveness.
Fig. 4.
Highly activated CD8+ T cells exhibit attenuated PMA+I-induced p-ERK1/2 responses without increased basal p-ERK1/2 levels. (A) Magnitude of PMA+I-induced p-ERK1/2 response in CD8+ T cells shown as fold change (stimulated MFI/unstimulated MFI) of CD38, HLA-DR, and TIM3 Boolean-gated subsets in HIV-1-infected and HIV-1-negative patients. (B) Unstimulated p-ERK1/2 MFI in each subset. n = 21 HIV− and 79 HIV+ patients. NS, not significant (P > 0.1); M, marginally significant (P = 0.1 to 0.05); *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
Similar to the p-ERK1/2 response, the PMA+I-induced p38 phosphorylation response (fold change in p-p38 MFI) was significantly reduced in highly activated CD38+ HLA-DR+ subsets compared to populations not coexpressing these two markers (left four columns of Fig. S2A in the supplemental material). However, unlike with p-ERK1/2, significantly higher unstimulated basal p-p38 levels were observed in CD38+ HLA− DR+ subsets (Fig. S2B). These data indicate that, in contrast to p-ERK1/2, reduced p-p38 fold changes in activated CD8+ T cell compartments during early untreated HIV-1 infection are at least partially driven by higher basal levels of p38 phosphorylation.
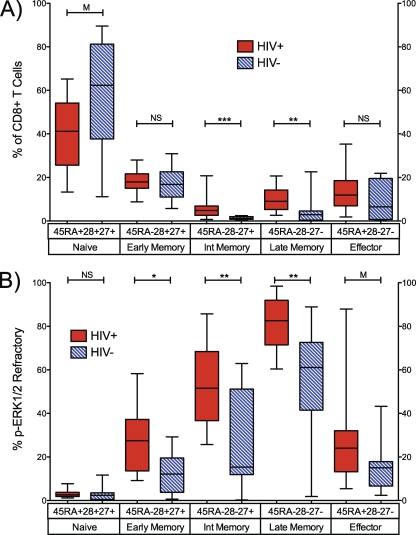
Impairment of p-ERK1/2 responses, which participate in cell cycle and cell differentiation processes in CD8+ T cells (13), might associate with the shifts in T cell maturation profiles observed in HIV-1 infection. We therefore examined the coexpression of CD45RA, CD28, and CD27 in PMA+I-stimulated samples stained for p-ERK1/2 expression. Five maturation stages were defined and are described in Materials and Methods. As previously described, recently HIV-1-infected, treatment-naïve patients exhibited significantly elevated frequencies of intermediate (CD45RA− CD28− CD27+) and late (CD45RA− CD28− CD27−) memory maturation stages relative to HIV-1-negative controls (Fig. 5A; see also Table S1 in the supplemental material).
Fig. 5.
p-ERK1/2-refractory CD8+ T cells are significantly enriched in early, intermediate, and late memory maturation stages in HIV-1-infected adults. (A) Frequency of CD45RA, CD27, and CD28 maturation marker subsets in CD8+ T cells from HIV-1-infected and HIV-1-negative patients. (B) Frequency of p-ERK1/2-refractory cells within each maturation subset. p-ERK1/2-refractory cells localize predominantly to intermediate and late memory compartments. n = 8 HIV− and 22 HIV+ patients. NS, not significant (P > 0.1); M, marginally significant (P = 0.1 to 0.05); *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
Figure 5B illustrates the proportion of p-ERK1/2-refractory cells that were identified within each of the five maturation stages (see Materials and Methods for descriptions of the stages). We observed p-ERK1/2-refractory cells in all stages in both HIV-1-infected and uninfected subjects, but they differed substantially in distribution by HIV infection status and differentiation state. The frequency of p-ERK1/2-refractory cells was lowest in the naïve (CD45RA+ CD28+ CD27+) compartment and increased in proportion through to the late memory stage (CD45RA− CD28− CD27−) in both HIV-1-infected and HIV-1-negative individuals (Fig. 5B). With the exception of naïve-phenotype CD8+ T cells, the p-ERK1/2-refractory population was significantly expanded or trended toward significant expansion in every maturation stage in recently HIV-1-infected, treatment-naïve adults relative to HIV-1-negative adults. These expansions of the p-ERK1/2-refractory population were most pronounced in the intermediate (CD45RA− CD28− CD27+) and late (CD45RA− CD28− CD27−) memory populations. These are the same two memory differentiation stages that are themselves expanded in frequency in HIV-1 infection (2, 3, 22).
DISCUSSION
We observed a highly expanded p-ERK1/2-refractory CD8+ T cell population residing within the activated (CD38+ HLA-DR+) CD8+ T cell compartment in HIV-1-infected adults. In contrast, we did not observe a large expansion of a p-ERK1/2-refractory population in CD4+ T cells in HIV-1 infection. In HIV-1-negative risk-matched controls, we observed a smaller, lower-frequency population of p-ERK1/2-refractory CD8+ T cells, suggesting that a functional blockade in the ERK1/2 MAPK pathway is a normal, if far less common, process in CD8+ T cells in healthy adults. CD8+ T cells refractory to phosphorylation of ERK1/2 not only were highly activated but also were enriched in intermediate (CD45RA− CD28− CD27+) and late memory (CD45RA− CD28− CD27−) CD8+ T cell populations, which are greatly expanded in HIV-1-infected persons (3, 22). However, we found the ERK1/2-refractory phenotype to be present in both CD28+ and CD28− CD8+ T cells, suggesting that the loss of PMA+I-induced ERK1/2 responsiveness was not due exclusively to the loss of CD28 expression, which signals through ERK1/2.
In contrast, CD8+ T cells bearing the immunomodulatory receptor Tim-3 did not display this refractory signaling phenotype, suggesting that a proportion of Tim-3-bearing cells retain the ability to respond to certain stimuli during HIV-1 infection (16) and may be readily converted to effectors through blockade of Tim-3 ligands. Tim-3, commonly referred to as an exhaustion marker, may mark a population of CD8+ T cells that, while strongly repressed in exhibiting effector activities, retain functional signaling pathways and perhaps full effector activities, given the appropriate stimuli. Additionally, we observed the appearance of a bilobed CD8+ population among HIV-1-infected individuals not apparent in HIV-1-negative risk-matched controls (Fig. 2; also Fig. S1 in the supplemental material and data not shown) (23). We observed that the majority of activated (CD38+ HLA-DR+) CD8+ “low” cells exhibited the p-ERK1/2-refractory phenotype (data not shown).
In this study, we found evidence for and against the previously reported higher basal MAPK pathway phosphorylation in HIV-1-infected persons (24). Specifically, we found that p38 basal phosphorylation levels were higher in activated (CD38+ HLA-DR+) CD8+ T cell subsets, which likely contributed to the lower fold changes in p-p38 MFI observed in activated compartments (see Fig. S2 in the supplemental material). In contrast, reduced fold changes in p-ERK1/2 MFI observed in activated CD8+ T cells was not due to higher basal phosphorylation levels (Fig. 4B) but instead was driven primarily by the presence of cells refractory to potent PMA+I stimuli (Fig. 3B). These data suggest that HIV-1 infection, and associated activation levels, may differentially impact ERK1/2 and p38 MAPK modules.
An abrogated ERK1/2 signaling response indicates the presence of a block in the MAPK pathway. PMA induces ERK1/2 phosphorylation through activation of PKC, which in turn positively modulates activity of the Raf scaffolding protein upstream of ERK1/2 (4). This suggests a functional impediment either at the level of PKC or in one of the downstream components in the pathway, such as Raf, MEK, or ERK1/2 itself. Alternatively, low ERK1/2 expression levels or the induction of phosphatases with specificity for ERK1/2 might confer the observed phenotype. Abrogation of ERK1/2 signaling in a large fraction of CD8+ T cells could have multiple deleterious functional consequences. These include reduced T cell proliferation, altered differentiation profiles, changes to apoptotic programs, and altered effector functions, all of which are observed in CD8+ T cells in HIV-1 infection. Targeting MAPK pathways to restore ERK1/2 signaling may normalize immune inflammation levels and restore CD8+ T cell function during HIV-1 infection.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants K01 AI 066917 (NIAID, to J. D. Barbour) and R21 AI 076014 (NIAID, to J. D. Barbour), R56AI083112 (NIAID, to L. C. Ndhlovu), P01 AI071713 (NIAID, to F. M. Hecht), UL1 RR024131 (NCRR UCSF CTSA), and P30AI027763 (NIAID GIVI/UCSF CFAR Volberding/Greene) and by the UCSF REAC Award (to J. D. Barbour).
We thank J. M. McCune and M. Schweneker for assistance with initial implementation of the phosflow assay. We thank the OPTIONS study participants for their time and effort.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 21 September 2011.
REFERENCES
- 1. Adler H. S., Steinbrink K. 2008. MAP kinase p38 and its relation to T cell anergy and suppressor function of regulatory T cells. Cell Cycle 7:169–170 [DOI] [PubMed] [Google Scholar]
- 2. Appay V., et al. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379–385 [DOI] [PubMed] [Google Scholar]
- 3. Barbour J. D., et al. 2009. High CD8+ T cell activation marks a less differentiated HIV-1 specific CD8+ T cell response that is not altered by suppression of viral replication. PLoS One 4:e4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carroll M. P., May W. S. 1994. Protein kinase C-mediated serine phosphorylation directly activates Raf-1 in murine hematopoietic cells. J. Biol. Chem. 269:1249–1256 [PubMed] [Google Scholar]
- 5. Champagne P., et al. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 410:106–111 [DOI] [PubMed] [Google Scholar]
- 6. Chatila T., Silverman L., Miller R., Geha R. 1989. Mechanisms of T cell activation by the calcium ionophore ionomycin. J. Immunol. 143:1283–1289 [PubMed] [Google Scholar]
- 7. Chow S., Patel H., Hedley D. W. 2001. Measurement of MAP kinase activation by flow cytometry using phospho-specific antibodies to MEK and ERK: potential for pharmacodynamic monitoring of signal transduction inhibitors. Cytometry 46:72–78 [DOI] [PubMed] [Google Scholar]
- 8. Cuadrado A., Nebreda A. R. 2010. Mechanisms and functions of p38 MAPK signalling. Biochem. J. 429:403–417 [DOI] [PubMed] [Google Scholar]
- 9. Deeks S. G., et al. 2004. Immune activation set point during early HIV infection predicts subsequent CD4+ T cell changes independent of viral load. Blood 104:942–947 [DOI] [PubMed] [Google Scholar]
- 10. DeSilva D. R., Jones E. A., Feeser W. S., Manos E. J., Scherle P. A. 1997. The p38 mitogen-activated protein kinase pathway in activated and anergic Th1 cells. Cell Immunol. 180:116–123 [DOI] [PubMed] [Google Scholar]
- 11. D'Souza W. N., Chang C. F., Fischer A. M., Li M., Hedrick S. M. 2008. The Erk2 MAPK regulates CD8 T cell proliferation and survival. J. Immunol. 181:7617–7629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farley N., et al. 2006. p38 mitogen-activated protein kinase mediates the Fas-induced mitochondrial death pathway in CD8+ T cells. Mol. Cell. Biol. 26:2118–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fischer A. M., Katayama C. D., Pages G., Pouyssegur J., Hedrick S. M. 2005. The role of erk1 and erk2 in multiple stages of T cell development. Immunity 23:431–443 [DOI] [PubMed] [Google Scholar]
- 14. Furler R. L., Uittenbogaart C. H. 2010. Signaling through the P38 and ERK pathways: a common link between HIV replication and the immune response. Immunol. Res. 48:99–109 [DOI] [PubMed] [Google Scholar]
- 15. Gorelik G., Richardson B. 2010. Key role of ERK pathway signaling in lupus. Autoimmunity 43:17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jones R. B., et al. 2008. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J. Exp. Med. 205:2763–2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kothe D., et al. 2003. Performance characteristics of a new less sensitive HIV-1 enzyme immunoassay for use in estimating HIV seroincidence. J. Acquir. Immune Defic. Syndr. 33:625–634 [DOI] [PubMed] [Google Scholar]
- 18. Leng Q., Borkow G., Bentwich Z. 2002. Attenuated signaling associated with immune activation in HIV-1-infected individuals. Biochem. Biophys. Res. Commun. 298:464–467 [DOI] [PubMed] [Google Scholar]
- 19. McNeil L. K., Starr T. K., Hogquist K. A. 2005. A requirement for sustained ERK signaling during thymocyte positive selection in vivo. Proc. Natl. Acad. Sci. U. S. A. 102:13574–13579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meloche S., Pouyssegur J. 2007. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene 26:3227–3239 [DOI] [PubMed] [Google Scholar]
- 21. Northfield J. W., et al. 2007. Human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T(EMRA) cells in early infection are linked to control of HIV-1 viremia and predict the subsequent viral load set point. J. Virol. 81:5759–5765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Papagno L., et al. 2004. Immune activation and CD8+ T cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2:E20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmitz J. E., et al. 1998. Expression of the CD8αβ-heterodimer on CD8+ T lymphocytes in peripheral blood lymphocytes of human immunodeficiency virus− and human immunodeficiency virus+ individuals. Blood 92:198–206 [PubMed] [Google Scholar]
- 24. Schweneker M., Favre D., Martin J. N., Deeks S. G., McCune J. M. 2008. HIV-induced changes in T cell signaling pathways. J. Immunol. 180:6490–6500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Singh K., et al. 2009. ERK-dependent T cell receptor threshold calibration in rheumatoid arthritis. J. Immunol. 183:8258–8267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Teixeiro E., Daniels M. A. 2010. ERK and cell death: ERK location and T cell selection. FEBS J. 277:30–38 [DOI] [PubMed] [Google Scholar]
- 27. Tomiyama H., Takata H., Matsuda T., Takiguchi M. 2004. Phenotypic classification of human CD8+ T cells reflecting their function: inverse correlation between quantitative expression of CD27 and cytotoxic effector function. Eur. J. Immunol. 34:999–1010 [DOI] [PubMed] [Google Scholar]
- 28. van Baarle D., Kostense S., van Oers M. H., Hamann D., Miedema F. 2002. Failing immune control as a result of impaired CD8+ T cell maturation: CD27 might provide a clue. Trends Immunol. 23:586–591 [DOI] [PubMed] [Google Scholar]
- 29. Witte V., et al. 2008. Induction of HIV transcription by Nef involves Lck activation and protein kinase Cθ raft recruitment leading to activation of ERK1/2 but not NFκB. J. Immunol. 181:8425–8432 [DOI] [PubMed] [Google Scholar]
- 30. Zampieri C. A., Fortin J. F., Nolan G. P., Nabel G. J. 2007. The ERK mitogen-activated protein kinase pathway contributes to Ebola virus glycoprotein-induced cytotoxicity. J. Virol. 81:1230–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.