Abstract
Gammaretrovirus receptors have been suggested to contain the necessary determinants to mediate virus binding and entry. Here, we show that murine NIH 3T3 and baby hamster kidney (BHK) cells overexpressing receptors for subgroup A, B, and C feline leukemia viruses (FeLVs) are weakly susceptible (101 to 102 CFU/ml) to FeLV pseudotype viruses containing murine leukemia virus (MLV) core (Gag-Pol) proteins, whereas FeLV receptor-expressing murine Mus dunni tail fibroblast (MDTF) cells are highly susceptible (104 to 106 CFU/ml). However, NIH 3T3 cells expressing the FeLV subgroup B receptor PiT1 are highly susceptible to gibbon ape leukemia virus pseudotype virus, which differs from the FeLV pseudotype viruses only in the envelope protein. FeLV resistance is not caused by a defect in envelope binding, low receptor expression levels, or N-linked glycosylation. Resistance is not alleviated by substitution of the MLV core in the FeLV pseudotype virus with FeLV core proteins. Interestingly, FeLV resistance is alleviated by fusion of receptor-expressing NIH 3T3 and BHK cells with MDTF or human TE671 cells, suggesting the absence of an additional cellular component in NIH 3T3 and BHK cells that is required for FeLV infection. The putative FeLV-specific cellular component is not a secreted factor, as MDTF conditioned medium does not alleviate the block to FeLV infection. Together, our findings suggest that FeLV infection requires an additional envelope-dependent cellular component that is absent in NIH 3T3 and BHK cells but that is present in MDTF and TE671 cells.
INTRODUCTION
Cell infection by retroviruses is mediated by interaction of the viral envelope glycoprotein with host cell surface receptors. Retroviruses such as human, feline, and simian lentiviruses and the human T-cell leukemia virus type I employ more than one cell surface receptor for infection (11, 16, 22, 30, 35, 46), whereas other retroviruses have been shown to use a single receptor for infection (30, 38). Receptors for gammaretroviruses have been identified to be multiple membrane-spanning transporter proteins (30, 38). These receptors contain multiple extracellular domains that provide several contact sites necessary for virus binding and infection. Thus, expression of a functional gammaretrovirus receptor is sufficient to render normally resistant cells highly susceptible to gammaretrovirus infection. The previous studies (reviewed in references 30 and 38) have led to the conclusion that gammaretrovirus receptors contain all the determinants necessary to mediate virus binding and infection. The use of additional receptors or cellular components required for gammaretrovirus entry has been difficult to establish because of the unavailability of cell lines that allow efficient virus binding but are resistant to infection when a functional receptor is overexpressed. A secreted accessory factor termed FeLIX that shares sequence identity to the receptor binding domain of subgroup B feline leukemia virus (FeLV-B) can trigger cellular entry of the subgroup T FeLV (1) but only in the presence of the FeLV-B receptor PiT1 (28, 42). A limiting accessory factor has also been suggested for ecotropic murine leukemia virus infection (45). Recently, the requirement of an envelope-dependent ancillary factor was implicated for entry by gibbon ape leukemia virus (GALV) and the xenotropic murine retrovirus-related virus (XMRV) (47).
FeLVs are pathogenic gammaretroviruses found in domestic cats (reviewed in reference 12). The three major FeLV subgroups, A, B, and C, use distinct cell surface transporters as receptors for infection (30, 38). The receptor for FeLV-A has been identified to be the thiamine transporter THTR1 (24), whereas the inorganic phosphate transporters PiT1 and PiT2 (17) function as receptors for FeLV-B (3, 28, 42). FeLV-C has been shown to use the heme exporter FLVCR1 for entry (31, 32, 41), whereas the FeLV-C variant FY981 can use THTR1, FLVCR1, and the heme importer FLVCR2 as receptors (8, 34). Expression of FeLV receptors in murine Mus dunni tail fibroblast (MDTF) cells and Chinese hamster ovary cells is sufficient to confer high susceptibility of these cells to the respective FeLVs (5, 24, 34, 37), thus confirming previous conclusions that gammaretroviruses require a single receptor for infection. We now provide evidence suggesting that FeLVs require an additional envelope-dependent cellular component at a post-receptor binding stage of infection. Murine NIH 3T3 and baby hamster kidney (BHK) cells overexpressing THTR1, FLVCR1, or PiT1 are weakly sensitive to infection by the respective FeLV pseudotype viruses. Resistance is alleviated only by fusion of these FeLV receptor-expressing cells with murine MDTF and human TE671 cells, suggesting that the MDTF and TE671 cells provide another cellular component required for FeLV infection.
MATERIALS AND METHODS
Cells.
MDTFs (CRL-2017; ATCC), murine NIH 3T3 cells (CRL-1658; ATCC), feline FEA cells (kindly provided by Brian Willett, Faculty of Veterinary Medicine, University of Glasgow, Glasgow, United Kingdom), and TELCeB6 retroviral packaging cells (6) were maintained in Dulbecco's minimal essential medium (DMEM) with low glucose (1,000 mg/ml) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Invitrogen). TELCeB6 cells are retroviral packaging cells that do not express retroviral envelope genes and produce noninfectious virus. As previously described (5), TELCeB6 cells were transfected with pFBsalf (6) envelope expression constructs to produce β-galactosidase (LacZ)-encoding pseudotype viruses containing murine leukemia virus (MLV) Gag-Pol proteins and bearing the respective gammaretrovirus envelope protein. Human embryonic kidney (HEK) 293T (HEK293T) cells (CRL-1573; ATCC) and Phoenix ampho packaging cells (SD-3443; ATCC) were maintained in Dulbecco's minimal essential medium with high glucose (4,500 mg/ml) supplemented with 10% FBS. Phoenix ampho packaging cells produce replication-defective virus expressing the amphotropic MLV envelope. BHK cells (CCL-10; ATCC) were maintained in alpha minimal essential medium supplemented with 10% FBS.
Receptor-expressing cells.
Hemagglutinin epitope (YPYDVPDYA)-tagged feline THTR1 (feTHTR1; FeLV-A receptor) and human FLVCR1 (huFLVCR1; FeLV-C receptor) were introduced into NIH 3T3 (NIH 3T3/feTHTR1 and NIH 3T3/huFLVCR1, respectively), MDTF (MTDF/feTHTR1 and MTDF/huFLVCR1, respectively), TE671, and BHK (BHK/feTHTR1 and BHK/huFLVCR1, respectively) cells by retrovirus infection. Retroviral vectors carrying the respective FeLV receptor sequences were generated as previously described (5, 34). NIH 3T3 and MDTF cells expressing the FeLV-B receptor were generated by transfection of cells with a hemagglutinin (HA)-tagged human PiT1 expression construct (kindly provided by Maribeth Eiden, National Institute of Mental Health, Bethesda, MD). Receptor-expressing cells were selected with G418 (1.5 mg/ml), and resistant cells were pooled and tested for susceptibility to virus infection. The human ASCT2 sequence tagged with a Myc epitope (23) was cloned in the pLPCX retroviral vector (Clontech), and retroviruses were produced as described above. ASCT2-transduced NIH 3T3 cells were selected with puromycin (5 μg/ml).
Viruses.
LacZ-encoding FeLVs, amphotropic MLVs, and RD114 pseudotype viruses were generated by transfection of TELCeB6 cells with the pFBsalf expression vector containing the respective gammaretrovirus envelope cDNA, as previously described (34, 39). Transfected cells were selected with phleomycin (50 μg/ml), resistant cells were pooled, and virus was harvested for infection studies (see below). Pseudotype viruses produced contain identical MLV Gag-Pol proteins and differ only in their envelope proteins. LacZ-encoding GALV pseudotype virus was generated by transfection of TELCeB6 cells with a pFBsalf GALV envelope expression construct. This construct was generated by PCR amplification of GALV envelope cDNA (provided by Maribeth Eiden, National Institute of Mental Health, Bethesda, MD) and the primers 5′-GGGGTCTAGACACCATGGTATTGCTGCCTGGGTCC-3′ and 5′-GGGGATCGATTCAAAGGTTACCTTCGTTCTCTAG-3′. Amplified cDNA was digested with XbaI and ClaI restriction enzymes and ligated into XbaI-ClaI-digested pFBsalf expression vector. LacZ-encoding GALV pseudotype virus was also provided by Maribeth Eiden and used in infection studies. This virus was generated by transfection of HEK293T cells with MLV Gag-Pol, LacZ, and GALV envelope expression constructs as described previously (47). LacZ-encoding FeLV-A containing FeLV core (Gag-Pol) proteins was produced by first transducing feline FEA cells with β-galactosidase-encoding FeLV-C to introduce the lacZ gene and then transfecting the transduced FEA cells with FeLV-A molecular clone (kindly provided by Brian Willett) using PolyFect transfection reagent (Qiagen, Toronto, ON, Canada). Transfected cells were incubated for 3 weeks to allow replication-competent FeLV-A to propagate and for the production of LacZ-encoding FeLV-cored FeLV-A. FeLV-cored FeLV-A was measured using a PetCheK enzyme-linked immunosorbent assay (ELISA) kit (IDEXX Laboratories, Westbrook, ME), which detects the FeLV p27 capsid protein in the viral supernatant. LacZ-encoding GALV with GALV core proteins was harvested from TElac2/GALV producer cells as previously described (40). All viruses were used in infection assays as described below.
Virus infection.
Target cells were seeded in 24-well plates (1.0 × 104 cells/well) and incubated overnight at 37°C. On the following day, cells were incubated with 1 ml of serially diluted LacZ-encoding retrovirus supernatant for 4 h in the presence of Polybrene (8 μg/ml). Virus supernatant was then replaced with fresh growth medium, and cells were allowed to incubate for a further 2 days before 5-bromo-4-chloro-3-indoyl-β-d-galactopyranoside (X-Gal; Sigma-Aldrich, Canada) staining. LacZ virus infection titers were determined by counting the number of blue CFU, and titers were expressed as the number of CFU obtained per milliliter of virus supernatant.
For N-linked glycosylation assay, NIH 3T3 and NIH 3T3/feTHTR1 cells were treated with tunicamycin (250 ng/ml) overnight at 37°C. The cells were infected on the following day with LacZ-encoding FeLV-A and RD114 pseudotype viruses (MLV cored).
For MDTF conditioned medium infection assay, overnight and 4-h-conditioned medium from MDTF cells was filtered using a 0.45-μm-pore-size filter to remove cell debris, and undiluted filtered conditioned medium was used in virus infection as described above.
Generation of soluble FeLV-A SU protein and SU binding assay.
A pCS-FSCHA expression construct containing FeLV-C/Sarma surface (SU) cDNA fused in frame with a double HA epitope was kindly provided by Julie Overbaugh (Fred Hutchinson Cancer Research Center, Seattle, WA). This construct was generated as described previously by Sugai and colleagues (36). FeLV-A SU expression constructs were generated by first amplifying the FeLV-A SU sequence using the upstream primer 5′-GGACGTCGGAGGAAGCTTGAT-3′ and downstream primer 5′-GGGGGAGCTCGTAAATATATTCGGGTTGATG-3′ and cloning the amplified SU cDNAs in a SacI-digested pCS-HA vector in frame with the double HA epitope. HEK293T cells, seeded at 1 × 106 cells in a 100-mm culture dish 1 day prior, were transfected with 8 μg of FeLV-A SU expression construct using PolyFect transfection reagent (Qiagen). At 2 days posttransfection, supernatant was harvested and filtered using a 0.45-μm-pore-size filter. The HA-tagged FeLV-A SU protein was subsequently stored at −80°C or used for envelope binding studies.
Binding of soluble SU to target cells was carried out as previously described (5). MDTF/feTHTR1 and NIH 3T3/feTHTR1 cells were treated with a cell dissociation buffer (Invitrogen) to dislodge cells. Approximately 1 × 106 cells were used for each binding assay. The cells were first incubated with 1 ml of HA-tagged FeLV-A SU in the presence of Polybrene (8 μg/ml) for 30 min at 37°C. Cells were then centrifuged at 4,000 rpm for 3 min. All subsequent spins were carried out at 4,000 rpm for 3 min. Cells were then washed two times with cold PBS containing 2% FBS (2% PFBS). Target cells were then incubated with 100 μl 2% PFBS containing 1:200-diluted monoclonal HA.11 antibody (Covance, Berkeley, CA) on ice for 30 min. Cells were washed again two times with 2% PFBS before incubation with 100 μl PFBS containing 1:25-diluted donkey antimouse antibody conjugated to fluorescein isothiocyanate (FITC; 1 mg/ml; Sigma) for 30 min on ice. Cells were then analyzed for envelope binding by flow cytometry (Beckman Coulter, Mississauga, ON, Canada).
Generation of hybrid cell lines.
Puromycin-resistant MDTF (MDTFpuro) cells were generated by transducing MDTF cells with Phoenix ampho virus expressing the retroviral expression vector pBabe-puro (27). MDTFpuro cells were selected using puromycin (5 μg/ml), and resistant colonies were pooled and used to generate hybrid cells.
Approximately 5 × 105 NIH 3T3/feTHTR1 or NIH 3T3/huFLVCR1 cells were cocultured with 2 × 105 MDTFpuro cells in a 60-mm cell culture dish. The cells were incubated at 37°C in 5% CO2 overnight. On the next day, the culture medium was removed and the cells were washed once with PBS and then overlaid with 1.5 ml of polyethylene glycol 1500 (PEG 1500) (Roche). Cells were then incubated at room temperature for 1 min, after which PEG 1500 was aspirated and cells were washed three times with minimal essential medium with low glucose (1,000 mg/ml) and without FBS. The cells were then maintained in 2% FBS minimal essential medium for 4 to 6 h and transferred to a 100-mm culture dish, and dual antibiotics (1.5 mg/ml G418 and 5 μg/ml puromycin) were added for selection. Clones resistant to both G418 and puromycin were pooled and used for infection studies.
TE671 cells containing a puromycin resistance gene (TEpuro) were generated using the protocol described above for generation of MDTFpuro cells. Fused TEpuro and NIH 3T3/feTHTR1 cells were generated as described above and tested for susceptibility to LacZ-encoding FeLVs.
Thiamine uptake assay.
Thiamine uptake assay was performed as described previously (9). MDTF and NIH 3T3 cells expressing feTHTR1 were seeded in a 24-well plate at a density of 2 × 105 cells/well. On the following day, medium was aspirated and cells were washed once in transport buffer (25 mM Tris-HEPES, pH 8.0, supplemented with 140 mM N-methyl-d-glucamine chloride, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, and 5 mM glucose). Cells were then incubated with 2.5 μM [3H]thiamine (10 Ci/mmol) in transport buffer for 10 min at 37°C. Transport was terminated by aspiration of the [3H]thiamine uptake buffer, followed by two washes in ice-cold transport buffer. The cells were then lysed with 0.3 ml of 1% SDS in 0.2 M NaOH and transferred to scintillation vials, and [3H]thiamine uptake was measured using a scintillation counter. [3H]thiamine uptake was measured as pmol/min/mg of protein.
PiT1 surface expression.
Surface expression of HA-tagged human PiT1 on NIH 3T3/human PiT1 (huPiT1) and MDTF/huPiT1 cells was determined by flow cytometry. Cells were dislodged from culture plates using cell dissociation buffer and then incubated with monoclonal HA.11 and FITC-conjugated donkey antimouse antibody. Cells were then analyzed as described above.
RESULTS
Murine NIH 3T3 overexpressing FeLV receptors are weakly sensitive to FeLVs.
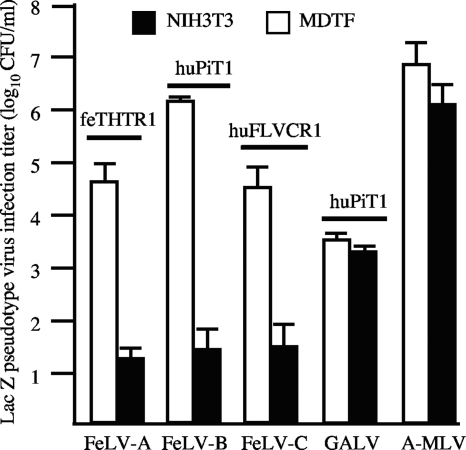
Murine NIH 3T3 cells are naturally resistant to all FeLV subgroups. We expressed receptors for FeLV subgroups A (feTHTR1 [24]), B (huPiT1 [28, 42]), and C (huFLVCR1 [31, 41]) in NIH 3T3 cells and tested the susceptibility of receptor-expressing cells to the respective LacZ-encoding FeLV pseudotype viruses containing MLV Gag-Pol proteins with FeLV envelope proteins. We observed weak infection of FeLV-A, -B, and -C pseudotype viruses on their respective receptor-expressing NIH 3T3 cells (Fig. 1). In contrast, and in agreement with previous reports (5, 24, 40), we observed high FeLV pseudotype virus infection titers on receptor-expressing murine MDTF cells (Fig. 1). To ascertain the resistance of receptor-expressing NIH 3T3 cells to the FeLV pseudotype viruses, we tested the susceptibility of huPiT1 expressing NIH 3T3 cells to a GALV pseudotype virus, which uses huPiT1 as a receptor. The GALV and FeLV pseudotype viruses contain MLV Gag-Pol and differ only in their envelope proteins. We used GALV generated by transient transfection of three components (MLV Gag-Pol, LacZ, and GALV envelope) in HEK293T cells and by transfection of the GALV envelope construct in TELCeB6 packaging cells. Both types of GALV pseudotype viruses showed comparable infection titers. We found that both MDTF/huPiT1 and NIH 3T3/huPiT1 cells were susceptible to GALV with comparable efficiency (Fig. 1). This is in contrast to the FeLV-B infection titer, which was approximately 50,000-fold lower on NIH 3T3/huPiT1 cells than MDTF/huPiT1 cells (Fig. 1). This finding suggests that FeLV resistance is envelope specific and implicates that resistance is not attributed to a postentry block. Consistent with this, we found that both NIH 3T3 and MDTF cells were highly susceptible to amphotropic murine leukemia virus (A-MLV) pseudotype virus with comparable infection titers (Fig. 1) and NIH 3T3 and MDTF cells overexpressing the neutral amino acid transporter ASCT2 were highly susceptible to feline RD114 pseudotype virus (data not shown). As described above, the A-MLV and RD114 pseudotype viruses differ from the FeLV and GALV pseudotype viruses only in their envelope proteins.
Fig. 1.
Murine NIH 3T3 cells expressing FeLV receptors are weakly sensitive to FeLV infection. Susceptibility of murine NIH 3T3 and MDTF cells expressing FeLV receptors to FeLV pseudotype virus. The FeLV receptor expressed in both MDTF and NIH 3T3 cells is shown above the bar graph. Murine cells expressing human PiT1 were also tested for susceptibility to GALV pseudotype virus, and control murine cells were tested for susceptibility to A-MLV pseudotype virus. The FeLV, GALV, and A-MLV pseudotype viruses differ only in their envelope proteins. Titers are averages of three infection studies. Standard deviation bars are shown.
The block to FeLV infectivity is not caused by a defect in envelope binding or by receptor expression levels.
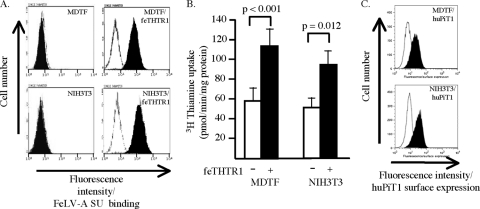
We used NIH 3T3/feTHTR1 cells as our representative model to further investigate the relative FeLV resistance of receptor-expressing NIH 3T3 cells. Using flow cytometry, we tested the ability of soluble surface envelope glycoprotein of FeLV-A to bind to NIH 3T3/feTHTR1 and MDTF/feTHTR1 cells. FeLV-A SU bound to NIH 3T3/feTHTR1 cells with comparable efficiency as SU binding on MDTF/feTHTR1 cells (Fig. 2A; see shift in fluorescence [black histogram]). FeLV-A SU did not bind to control MDTF and NIH 3T3 cells (Fig. 2A).
Fig. 2.
FeLV SU binds to receptor-expressing cells, and receptors are efficiently expressed on the cell surface. (A) MDTF, NIH 3T3, MDTF/feTHTR1, and NIH 3T3/feTHTR1 cells were incubated with (black histograms) or without (white histograms) soluble HA-tagged FeLV-A SU protein. Bound SU was detected by flow cytometry using HA.11 anti-HA monoclonal antibody, followed by an FITC-conjugated donkey antimouse antibody. An increase in fluorescence denotes FeLV-A SU binding. (B) FeLV-A receptor-expressing murine cells show enhanced uptake of [3H]thiamine. Murine MDTF and NIH 3T3 cells expressing (+) or not expressing (−) the FeLV-A receptor feTHTR1 were incubated with [3H]thiamine, and the amount of uptake was measured using a scintillation counter. Values shown are averages of at least three uptake assays. Standard deviations are shown. (C) Cell surface expression of FeLV-B receptor huPiT1 on NIH 3T3/huPiT1 and MDTF/huPiT1 cells. Targets cells were incubated with HA.11 anti-HA monoclonal antibody, followed by incubation with FITC-conjugated donkey antimouse antibody. Cells were analyzed by flow cytometry. An increase in fluorescence denotes an increase in huPiT1 surface expression.
To test whether low surface receptor expression levels were responsible for FeLV resistance, we determined [3H]thiamine uptake by NIH 3T3/feTHTR1 and MDTF/feTHTR1 cells. Because the FeLV-A receptor is a cell surface thiamine transporter (24), overexpression of feTHTR1 should lead to enhanced uptake of [3H]thiamine, which can be used as a measure of feTHTR1 surface expression. As shown in Fig. 2B, NIH 3T3 cells showed an approximately 2-fold increase in [3H]thiamine uptake. This uptake was comparable to the approximately 2-fold [3H]thiamine uptake observed with MDTF/feTHTR1 cells. Using flow cytometry, we also determined surface expression of huPiT1 on MDTF/huPiT1 and NIH 3T3/huPiT1 cells (see Materials and Methods). We observed comparable levels of huPiT1 surface expression on NIH 3T3/huPiT1 and MDTF/huPiT1 cells (Fig. 2C). Together, these findings suggest that low cell surface receptor expression is not responsible for FeLV resistance of receptor-expressing NIH 3T3 cells.
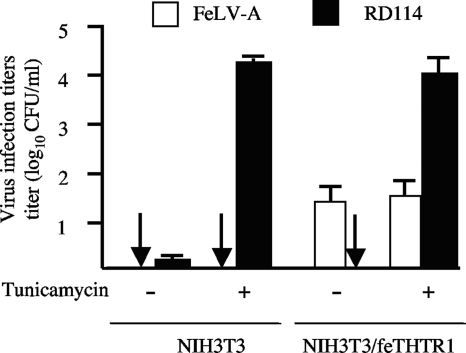
Treatment of NIH 3T3/feTHTR1 cells with the N-linked glycosylation inhibitor tunicamycin does not enhance FeLV-A infection.
Previous studies (25, 26) have shown that functional cell surface receptor proteins can be masked by N-linked glycosylation, which prevents virus infection. The block to infection can be alleviated by treatment with the N-linked glycosylation inhibitor tunicamycin. Treatment of NIH 3T3 cells with tunicamycin alleviates the block to feline RD114 endogenous virus infection (23), suggesting that N-linked glycosylation in NIH 3T3 cells could be responsible for the block to FeLV infection. However, we found that treatment of NIH 3T3/feTHTR1 cells with tunicamycin did not enhance FeLV-A pseudotype virus infection titers compared to those for untreated cells (Fig. 3). As a control, infection titers of RD114 pseudotype virus were enhanced 1,000-fold compared to untreated cells (Fig. 3).
Fig. 3.
N-linked glycosylation is not responsible for resistance of NIH 3T3/feTHTR1 to FeLV-A. NIH 3T3/feTHTR1 cells treated with (+) or without (−) tunicamycin, an inhibitor of N-linked glycosylation, were challenged with FeLV-A (white bars) or with RD114 virus (black bars). Arrows, infection titers of zero. Titers are averages of six infection experiments. Standard deviation bars are shown.
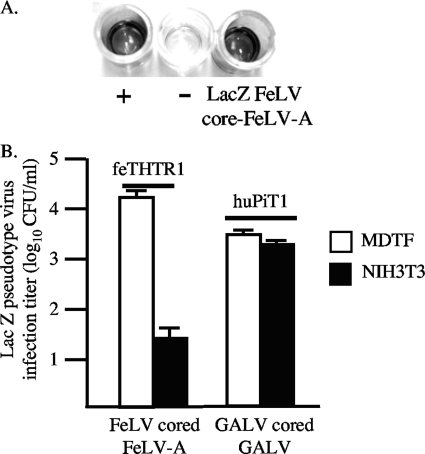
Resistance is not alleviated using FeLV with FeLV core (Gag-Pol) proteins.
A previous study (29) has shown that gammaretrovirus core proteins, specifically, the capsid protein, can influence virus infection. To ascertain the influence of Gag proteins, we generated LacZ-encoding FeLV-A containing FeLV core (Gag-Pol) proteins (see Materials and Methods) and tested its infectivity on MDTF/feTHTR1 and NIH 3T3/feTHTR1 cells. The presence of FeLV-cored FeLV-A was confirmed using the PetChek ELISA (see Materials and Methods), which detects the p27 FeLV capsid protein in virus supernatant (Fig. 4A; dark coloration depicts the presence of FeLV-cored virus). We observed a similar reduced (500-fold) infection titer of FeLV-cored FeLV-A on NIH 3T3/feTHTR1 cells relative to MDTF/feTHTR1 cells, as observed with MLV-cored FeLV-A. We also tested wild-type GALV (GALV-cored GALV) infection on MDTF/huPiT1 and NIH 3T3/huPiT1 cells and found that infection titers were similar to those observed with MLV-cored GALV (Fig. 1).
Fig. 4.
Substitution of MLV core protein with FeLV core protein does not alleviate FeLV resistance. (A) PetChek ELISA (IDEXX Laboratories) to test production of LacZ-encoding FeLV-cored FeLV-A. ELISA is based on detection of p27 FeLV capsid protein in viral supernatant. A dark color indicates detection of p27 and, hence, the presence of FeLV-cored virus. The positive (+) and negative (−) controls from the detection kit are shown. The LacZ FeLV-cored FeLV-A was generated by transfection of feline FEA cells with FeLV-A molecular clone and then transduction with LacZ-encoding FeLV-C to introduce the lacZ gene. (B) LacZ FeLV-cored FeLV-A and LacZ GALV-cored GALV infection on receptor-expressing MDTF and NIH 3T3 cells. The LacZ GALV-cored GALV was harvested from TElac2/GALV cells (40). Titers are averages of three infection experiments. Standard deviation bars are shown.
Taken together, our results suggest that the FeLV resistance of receptor-expressing NIH 3T3 cells is envelope dependent (Fig. 1) and is not caused by a disruption in envelope binding (Fig. 2A), by low receptor expression levels (Fig. 2B and C), by N-linked glycosylation (Fig. 3), or by the presence of MLV core proteins (Fig. 4B).
Fusion of NIH 3T3/FeLV receptor cells with MDTF cells rescues FeLV infection.
The specific block to FeLV infection could be attributed to expression of an NIH 3T3 inhibitor. Alternatively, NIH 3T3 cells may lack an additional cellular component required for FeLV infection. To ascertain the presence or absence of a cellular component responsible for FeLV resistance, we fused FeLV-resistant MDTF cells containing a puromycin resistance gene (MDTFpuro) with NIH 3T3/feTHTR1 cells containing a neomycin resistance gene to generate the MDTF-3T3/feTHTR1 fused cell line that was resistant to both puromycin and G418 selection. The fused cells were then tested for susceptibility to MLV-cored FeLV-A. Because MDTF/feTHTR1 cells are highly susceptible to FeLV-A infection, we hypothesized that if NIH 3T3 cells lack an additional cellular component required for infection, then fused MDTF-3T3/feTHTR1 cells should be highly susceptible to FeLV-A, as the cellular component is provided by MDTF cells. However, if NIH 3T3 cells express an FeLV inhibitor, then fused MDTF-3T3/feTHTR1 cells should be weakly susceptible to FeLV-A with infection titers similar to those observed on NIH 3T3/feTHTR1 cells. As shown in Fig. 5A, MDTF-3T3/feTHTR1 fused cells were highly susceptible to FeLV-A. Infection titers were approximately 3,000-fold greater than the titers on NIH 3T3/feTHTR1 cells. Fusion of MDTFpuro with NIH 3T3/huFLVCR1 cells did not alleviate the block to FeLV-A infection, suggesting that the fusion process was not responsible for the enhanced virus infection. Interestingly, MDTF-3T3/huFLVCR1 fused cells were highly susceptible to FeLV-C infection, with titers being approximately 2,000-fold greater than the titers on nonfused NIH 3T3/huFLVCR1 cells (Fig. 5A). Together, these findings suggest that NIH 3T3 cells lack an additional cellular component required for FeLV infection. Moreover, this cellular component is present in MDTF cells.
Fig. 5.
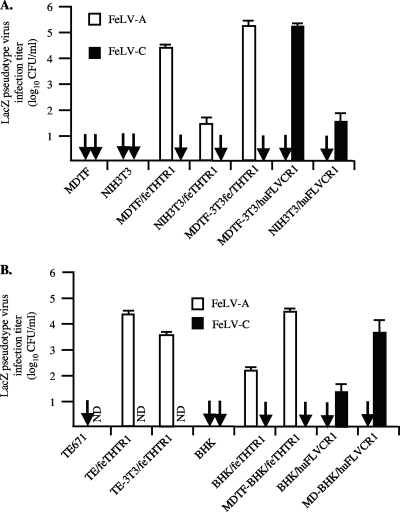
FeLV infection of NIH 3T3 and BHK cells expressing FeLV receptors can be rescued by fusion of cells with murine MDTF cells or with human TE671 cells. (A) Fused MDTF-3T3/feTHTR1 cells and MDTF-3T3/huFLVCR1 cells were tested for susceptibility to LacZ-encoding FeLV-A and FeLV-C. MDTF-3T3/huFLVCR1 fused cells were also used as controls to show that the fusion process was not responsible for enhancement in FeLV-A infection. Titers are averages of three infection experiments. Arrows, infection titers of zero; ND, not determined. (B) FeLV susceptibility of human TE671 cells fused with NIH 3T3/feTHTR1 cells and susceptibility of MDTF cells fused with BHK/feTHTR1 cells. Titers are averages of three infection experiments. Standard deviation bars are shown. Arrows, infection titers of zero; ND, not determined.
The FeLV-specific MDTF cellular component is not a secreted factor.
A secreted accessory factor termed FeLIX has been demonstrated to trigger FeLV-T infection of target cells in the presence of PiT1 (1). Using procedures described previously (1), we tested whether the MDTF cellular component required for FeLV infection was a soluble secreted factor. Incubation of NIH 3T3/feTHTR1 with overnight MDTF conditioned medium (see Materials and Methods) did not enhance FeLV-A infection titers, and titers were 100-fold lower than infection titers on MDTF/feTHTR1 cells treated with MDTF conditioned medium (MDTF/feTHTR1 cells, 1.6 × 103 ± 0.4 × 103 CFU/ml; NIH 3T3/feTHTR1 cells, 2.0 × 101 ± 1.0 × 101 CFU/ml). Similar titers were obtained on MDTF/feTHTR1 and NIH 3T3/feTHTR1 cells using a 4-h MDTF conditioned medium (data not shown). This finding suggests that the MDTF cellular component is not a secreted factor.
Human TE671 cells but not baby hamster kidney cells also express the FeLV envelope-specific cellular component.
To ascertain the specificity of the MDTF cellular component, we fused NIH 3T3/feTHTR1 cells with human TE671 cells and tested the susceptibility of fused cells to FeLV-A infection. Because human TE671 cells are naturally highly susceptible to FeLV-B and FeLV-C (41) and become highly susceptible to FeLV-A pseudotype virus when transduced with feTHTR1 (Fig. 5B; see TE/feTHTR1), we hypothesized that TE671 cells must also express a cellular component(s) required for FeLV infection. We observed a consistent 100-fold enhancement in FeLV-A infection titers on TE-3T3/feTHTR1 fused cells (Fig. 5B) compared to the titers on nonfused NIH 3T3/feTHTR1 cells (Fig. 5A). Thus, fusion of human TE671 cells with NIH 3T3/feTHTR1 cells also alleviates resistance to FeLV-A.
A recent report (47) has suggested that BHK cells lack an ancillary factor required for infection by GALV and by XMRV. To test whether BHK cells also lack a cellular component/ancillary factor required for FeLV infection, we tested the FeLV susceptibility of receptor-expressing BHK cells. We observed weak infection of BHK/feTHTR1 and BHK/huFLVCR1 cells by the respective FeLV pseudotype viruses (Fig. 5B). However, fusion of MDTFpuro cells with BHK/feTHTR1 or with BHK/huFLVCR1 cells enhanced the infection titer by a consistent 100-fold (Fig. 5B; see MDTF-BHK/feTHTR1 and MDTF-BHK/huFLVCR1). These results suggest that BHK cells also lack an additional cellular component for FeLV infection.
DISCUSSION
Previous characterizations of FeLV receptors have implicated that FeLVs use a single cell receptor or cellular component for infection, with the exception of FeLV-T, which requires the secreted cellular protein FeLIX and PiT1 inorganic phosphate transporter for virus entry (1). We now provide substantial evidence suggesting that the major FeLV subgroups A, B, and C require an additional cellular component at a post-receptor binding stage of infection. This cellular component is not a secreted factor, is envelope dependent, and is expressed by murine MDTF and human TE671 cells but not by murine NIH 3T3 or BHK cells.
The requirement of an additional, FeLV envelope-specific cellular component was based on our initial observation that murine NIH 3T3 cells overexpressing FeLV receptor feTHTR1, huFLVCR1, or huPiT1 are relatively resistant to the respective FeLV pseudotype viruses, compared to the high susceptibility of FeLV receptor-expressing murine MDTF cells (Fig. 1). FeLV resistance is not attributed to a disruption in envelope binding, as soluble FeLV-A SU bound efficiently to both NIH 3T3/feTHTR1 and MDTF/feTHTR1 cells (Fig. 2A). Resistance is also not attributed to low surface receptor expression levels, as shown by the comparable surface expression of huPiT1 on NIH 3T3/huPiT1 and MDTF/huPiT1 cells (Fig. 2C) and by the similar fold increase in [3H]thiamine uptake by NIH 3T3/feTHTR1 and MDTF/feTHTR1 cells compared to the uptake by control NIH 3T3 and MDTF cells, respectively (Fig. 2B). Interestingly, NIH 3T3/huPiT1 and MDTF/huPiT1 cells showed comparable susceptibility to GALV pseudotype virus (Fig. 1), in contrast to the low susceptibility of NIH 3T3/huPiT1 cells to FeLV-B. Because the GALV and FeLV pseudotype viruses used in this study contain MLV core (Gag-Pol) proteins, our results suggest that resistance is envelope specific. Our results would also implicate that resistance is not attributed to a postentry block. However, additional experiments quantifying reverse transcriptase products, as described by Oliveira and colleagues (29), are needed to fully confirm this conclusion.
Several mechanisms of gammaretrovirus resistance have been previously described. Oliveira and colleagues (29) recently described an envelope (Env)- and capsid-dependent resistance of gammaretroviruses. The authors showed that pseudotype viruses containing an RD114 envelope with core proteins derived from MLV-B showed an approximately 16-fold reduced infectivity on human HeLa cells than on human NP2 glioma cells. In contrast, pseudotype virus containing the GALV envelope with the MLV-B core showed comparable infectivities on both human cell lines, thus implicating an envelope-dependent restriction of RD114 on HeLa cells. However, RD114 infection was rescued by substitution of the MLV-B core with an MLV-NB core, which implicates a core-dependent restriction for RD114 as an additional mechanism. In our study, we replaced the MLV core with the FeLV core to determine whether this would rescue FeLV infection on receptor-expressing NIH 3T3 cells. We observed the similar weak infection of FeLV-cored FeLV-A on NIH 3T3/feTHTR1 cells as observed with MLV-cored FeLV-A. Virus infection titers were approximately 500-fold lower than titers on MDTF/feTHTR1 cells (Fig. 4). Similarly, we did not observe a difference in relative infectivity between MLV-cored GALV and GALV-cored GALV on MDTF/huPiT1 and NIH 3T3/huPiT1 cells (Fig. 1 and Fig. 4). Thus, our findings suggest that resistance to FeLVs on receptor-expressing NIH 3T3 cells is not attributed to Gag-Pol proteins and is envelope dependent. However, the use of pseudotype viruses with MLV-B and MLV-NB cores may be required to conclusively confirm that FeLV resistance is envelope dependent. Another study (33) has reported the presence of a novel restriction factor (Lv2) for human immunodeficiency virus type 2 (HIV-2) that is also envelope and capsid dependent. The authors did not ascertain whether the restricted cells expressed an inhibitor or lacked a factor for HIV-2 infection. However, restriction was alleviated (2- to 9-fold) by treatment of cells with drugs or small interfering RNA against actin and clathrin, thus suggesting that cellular entry through an actin- and clathrin-dependent route is necessary for Lv2 restriction of HIV-2 (13). In this study, the dramatic difference (1,000- to 50,000-fold) in FeLV infection observed between receptor-expressing MDTF and NIH 3T3 cells, and the 3,000- to 5,000-fold enhancement in infection observed when receptor-expressing NIH 3T3 cells are fused with MDTF cells (Fig. 5A), would strongly suggest that NIH 3T3 cells lack an additional cellular component required for FeLV entry rather than favor a role for actin and clathrin in the FeLV resistance. Our findings show strong similarity to previous reports of an ancillary factor for GALV and XMRV (47) and to the requirement of a second cell component for HIV-1 infection (4). However, we cannot exclude the possibility that MDTF and TE671 cells express a cellular factor that counteracts an inhibitor expressed by NIH 3T3 cells or that actin and clathrin are involved in resistance.
N-linked glycosylation of cell surface receptors has also been reported to restrict gammaretrovirus infection (23, 25, 26). N-linked glycosylation can mask receptor sites critical for virus binding and infection and/or influence cellular factors that inhibit gammaretrovirus infection. The block to infection can often be alleviated by treatment of cells with the N-linked glycosylation inhibitor tunicamycin. Indeed, the block to RD114 infection of NIH 3T3 cells can be alleviated by treatment of cells with tunicamycin (23), thus implicating N-linked glycosylation as a potential mechanism for the FeLV resistance of receptor-expressing NIH 3T3 cells. However, findings in this study clearly suggest that resistance is not attributed to N-linked glycosylation. Tunicamycin treatment of NIH 3T3/feTHTR1 cells did not enhance the FeLV-A infection titer (Fig. 3), whereas RD114 infection was enhanced 1,000-fold compared to untreated cells.
Retrovirus restriction can also be attributed to expression of a dominant inhibitory factor. Secreted protein factors expressed by Chinese hamster ovary cells have been shown to inhibit GALV and A-MLV infections (26). Similarly, the Fv-4 (Friend virus susceptibility factor 4) gene in mice encodes an envelope protein that inhibits ecotropic MLV but not A-MLV infection (14, 15). Thus, these expressed cellular factors inhibit specific retroviruses. The presence of an inhibitory factor in restricted cells can often be established by generating hybrid cells between the restricted and nonrestricted cells (20, 43). If hybrid cells show retrovirus restriction, this would implicate the presence of a cellular inhibitor. Interestingly, our cell-cell fusion experiments between murine MDTF cells and NIH 3T3- or BHK-expressing FeLV receptor cells showed a significant enhancement (100- to 1,000-fold) in FeLV infection titers compared to the titers observed on nonfused FeLV receptor-expressing cells (Fig. 5A). FeLV infectious titers were also enhanced when human TE671 cells were fused with NIH 3T3/FeLV receptor cells (Fig. 5B). These findings would suggest that NIH 3T3 and BHK cells do not express an inhibitor but they lack an additional cellular component that is required for FeLV infection. Moreover, this cellular component is expressed in MDTF and TE671 cells and is not a secreted factor. This is in contrast to the characteristics of the FeLIX factor that triggers FeLV-T infection (1). Our findings share surprising similarities to those of a previous study (4) reporting the requirement of an additional cellular component (later identified to be the chemokine receptors CCR5 and CXCR4 [2]) used for entry by HIV-1. Expression of the primary HIV-1 receptor CD4 (7, 18) in murine cells, including NIH 3T3 cells, is sufficient for HIV-1 gp120 envelope binding but not sufficient to mediate HIV-1 infection (21). However, fusion of human cells with CD4-expressing murine cells alleviates the block to HIV-1 infection (4), suggesting a requirement of an additional human component for HIV-1 entry. On the basis of this similarity, it would be interesting to speculate that the MDTF and TE671 cellular component is a second cell surface receptor or coreceptor specific for FeLV infection. Xu and Eiden (47) recently reported a requirement of an envelope-dependent ancillary factor for GALV infection at a post-receptor binding stage of infection. BHK/huPiT1 cells are resistant to MLV-cored GALV infection, despite binding of the GALV envelope to cells and efficient PiT1 surface expression. GALV infection is rescued by fusion of BHK/huPiT1 cells with GALV-resistant MDTF cells, thus suggesting that MDTF cells express a GALV ancillary factor. Our observation that GALV, but not FeLV-B, infects MDTF/huPiT1 and NIH 3T3/huPiT1 cells with comparable efficiency would suggest that the putative GALV ancillary factor implicated by Xu and Eiden (47) could be distinct from the putative FeLV-specific cellular component implicated in this study. Thus, these gammaretroviruses may use distinct additional cellular components/ancillary factors, despite using PiT1 as a receptor. There is precedence for use of distinct cellular components/receptors in retrovirus infection. For example, HIV-1 (X4 strain) uses the receptors CD4 and CXCR4 for entry (7, 10, 18), whereas feline immunodeficiency virus (FIV) has been shown to use CD134 and CXCR4 for entry (35, 46).
It is interesting that NIH 3T3 and BHK cells expressing FeLV receptors are weakly susceptible and not completely resistant to FeLVs. It is possible that the additional cellular component required for FeLV infection is expressed at low levels in NIH 3T3 and BHK cells that allow weak infection. Alternatively, the FeLV envelope may interact with a related NIH 3T3 cellular component that can mediate weak FeLV infection. It is also interesting that the two murine cell lines, NIH 3T3 and MDTFs, show different FeLV infection properties when the cognate FeLV receptors are expressed (Fig. 1) and that our results implicate MDTF but not NIH 3T3 cells as expressing the FeLV-specific cellular component. NIH 3T3 cells are derived from the NIH Swiss laboratory mouse, the Mus musculus house mouse (44), whereas MDTF cells are derived from the Asian wild mouse species, the Mus dunni wild mouse (19). House mice express an XPR1 protein that is nonfunctional as a receptor for xenotropic MLV, whereas wild mice express multiple XPR1 variants that are functional xenotropic MLV receptors (48). Similarly, it is possible that house mice may have evolved to lack the additional cellular component for FeLV entry as a means to further restrict the FeLV that can be transmitted from infected domestic cats. Further investigations are necessary to establish whether the absence and presence of an additional FeLV-specific cellular component are common phenomena for house and wild mice, respectively.
In conclusion, results in this study suggest that in addition to their cognate receptors, FeLV entry requires a nonsecreted envelope-dependent cellular component at a post-receptor binding stage of infection. Identification of the additional cellular components/ancillary factors will have major implications in understanding entry mechanisms of FeLVs and other related gammaretroviruses.
ACKNOWLEDGMENTS
We are grateful to Jaehun Ma (Hospital for Sick Children, Toronto, ON, Canada) for assistance in the envelope binding assay and to Christine Kozak (National Institute of Allergy and Infectious Diseases, Bethesda, MD) for helpful suggestions with the manuscript. We are also grateful to Maribeth Eiden (National Institute of Mental Health, Bethesda, MD) for providing the GALV pseudotype virus and GALV envelope expression construct.
This work was supported by Canadian Institutes of Health research grant MOP-79541.
Footnotes
Published ahead of print on 14 September 2011.
REFERENCES
- 1. Anderson M. M., Lauring A. S., Burns C. C., Overbaugh J. 2000. Identification of a cellular cofactor required for infection by feline leukemia virus. Science 287:1828–1830 [DOI] [PubMed] [Google Scholar]
- 2. Berger E. A., Murphy P. M., Farber J. M. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657–700 [DOI] [PubMed] [Google Scholar]
- 3. Boomer S., Eiden M., Burns C. C., Overbaugh J. 1997. Three distinct envelope domains, variably present in subgroup B feline leukemia virus recombinants, mediate Pit1 and Pit2 receptor recognition. J. Virol. 71:8116–8123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Broder C. C., Dimitrov D. S., Blumenthal R., Berger E. A. 1993. The block to HIV-1 envelope glycoprotein-mediated membrane fusion in animal cells expressing human CD4 can be overcome by a human cell component(s). Virology 193:483–491 [DOI] [PubMed] [Google Scholar]
- 5. Brown J. K., Fung C., Tailor C. S. 2006. Comprehensive mapping of receptor-functioning domains in feline leukemia virus subgroup C receptor FLVCR1. J. Virol. 80:1742–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cosset F. L., Takeuchi Y., Battini J. L., Weiss R. A., Collins M. K. L. 1995. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J. Virol. 69:7430–7436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dalgleish A. G., et al. 1984. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 312:763–767 [DOI] [PubMed] [Google Scholar]
- 8. Duffy S. P., et al. 2010. The Fowler syndrome-associated protein FLVCR2 is an importer of heme. Mol. Cell. Biol. 30:5318–5324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dutta B., et al. 1999. Cloning of the human thiamine transporter, a member of the folate transporter family. J. Biol. Chem. 274:31925-31929 [DOI] [PubMed] [Google Scholar]
- 10. Feng Y., Broder C. C., Kennedy P. E., Berger E. A. 1996. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 272:872–877 [DOI] [PubMed] [Google Scholar]
- 11. Ghez D., et al. 2006. Neuropilin-1 is involved in human T-cell lymphotropic virus type 1 entry. J. Virol. 80:6844–6854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hardy W. D. 1993. Feline oncoretroviruses, p. 109–180 In Levy J. A. (ed.), The retroviridae, vol. 2 Plenum Press, New York, NY [Google Scholar]
- 13. Harrison I. P., McKnight A. 2011. Cellular entry via an actin and clathrin-dependent route is required for Lv2 restriction of HIV-2. Virology 415:47–55 [DOI] [PubMed] [Google Scholar]
- 14. Ikeda H., Laigret F., Martin M. A., Repaske R. 1985. Characterization of a molecularly cloned retroviral sequence associated with Fv-4 resistance. J. Virol. 55:768–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ikeda H., Sugimura H. 1989. Fv-4 resistance gene: a truncated endogenous murine leukemia virus with ecotropic interference properties. J. Virol. 63:5405–5412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jones K. S., Petrow-Sadowski C., Bertolette D. C., Huang Y., Ruscetti F. W. 2005. Heparan sulfate proteoglycans mediate attachment and entry of human T-cell leukemia virus type 1 virions into CD4+ T cells. J. Virol. 79:12692–12702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kavanaugh M. P., et al. 1994. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc. Natl. Acad. Sci. U. S. A. 91:7071–7075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klatzmann D., et al. 1984. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature 312:767–768 [DOI] [PubMed] [Google Scholar]
- 19. Lander M. R., Chattopadhyay S. K. 1984. A Mus dunni cell line that lacks sequences closely related to endogenous murine leukemia viruses and can be infected by ectropic, amphotropic, xenotropic, and mink cell focus-forming viruses. J. Virol. 52:695–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Madani N., Kabat D. 1998. An endogenous inhibitor of human immunodeficiency virus in human lymphocytes is overcome by the viral Vif protein. J. Virol. 72:10251–10255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maddon P. J., et al. 1986. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell 47:333–348 [DOI] [PubMed] [Google Scholar]
- 22. Manel N., et al. 2003. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell 115:449–459 [DOI] [PubMed] [Google Scholar]
- 23. Marin M., Lavillette D., Kelly S. M., Kabat D. 2003. N-linked glycosylation and sequence changes in a critical negative control region of the ASCT1 and ASCT2 neutral amino acid transporters determine their retroviral receptor functions. J. Virol. 77:2936–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mendoza R., Anderson M. M., Overbaugh J. 2006. A putative thiamine transport protein is a receptor for feline leukemia virus subgroup A. J. Virol. 80:3378–3385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miller D. G., Miller A. D. 1993. Inhibitors of retrovirus infection are secreted by several hamster cell lines and are also present in hamster sera. J. Virol. 67:5346–5352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miller D. G., Miller A. D. 1992. Tunicamycin treatment of CHO cells abrogates multiple blocks to retrovirus infection, one of which is due to a secreted inhibitor. J. Virol. 66:78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morgenstern J. P., Land H. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. O'Hara B., et al. 1990. Characterization of the human gene conferring sensitivity to infection by gibbon ape leukemia virus. Cell Growth Differ. 1:119–127 [PubMed] [Google Scholar]
- 29. Oliveira N. M., Trikha R., McKnight A. 2010. A novel envelope mediated post entry restriction of murine leukaemia virus in human cells is Ref1/TRIM5alpha independent. Retrovirology 7:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Overbaugh J., Miller A. D., Eiden M. V. 2001. Receptors and entry cofactors for retroviruses include single and multiple transmembrane-spanning proteins as well as newly described glycophosphatidylinositol-anchored and secreted proteins. Microbiol. Mol. Biol. Rev. 65:371–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Quigley J. G., et al. 2000. Cloning of the cellular receptor for feline leukemia virus subgroup C (FeLV-C), a retrovirus that induces red cell aplasia. Blood 95:1093–1099 [PubMed] [Google Scholar]
- 32. Quigley J. G., et al. 2004. Identification of a human heme exporter that is essential for erythropoiesis. Cell 118:757–766 [DOI] [PubMed] [Google Scholar]
- 33. Schmitz C., et al. 2004. Lv2, a novel postentry restriction, is mediated by both capsid and envelope. J. Virol. 78:2006–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shalev Z., et al. 2009. Identification of a feline leukemia virus variant that can use THTR1, FLVCR1, and FLVCR2 for infection. J. Virol. 83:6706–6716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shimojima M., et al. 2004. Use of CD134 as a primary receptor by the feline immunodeficiency virus. Science 303:1192–1195 [DOI] [PubMed] [Google Scholar]
- 36. Sugai J., et al. 2001. Identification of envelope determinants of feline leukemia virus subgroup B that permit infection and gene transfer to cells expressing human Pit1 or Pit2. J. Virol. 75:6841–6849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tailor C. S., Kabat D. 1997. Variable regions A and B in the envelope glycoproteins of feline leukemia virus subgroup B and amphotropic murine leukemia virus interact with discrete receptor domains. J. Virol. 71:9383–9391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tailor C. S., Lavillette D., Marin M., Kabat D. 2003. Cell surface receptors for gammaretroviruses. Curr. Top. Microbiol. Immunol. 281:29–106 [DOI] [PubMed] [Google Scholar]
- 39. Tailor C. S., Nouri A., Zhao Y., Takeuchi Y., Kabat D. 1999. A sodium-dependent neutral amino acid transporter mediates infections of feline and baboon endogenous retroviruses and simian type D retroviruses. J. Virol. 73:4470–4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tailor C. S., et al. 1993. Mutation of amino acids within the gibbon ape leukemia virus (GALV) receptor differentially affects feline leukemia virus subgroup B, simian sarcoma-associated virus, and GALV infections. J. Virol. 67:6737–6741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tailor C. S., Willet B. J., Kabat D. 1999. A putative cell surface receptor for anemia-inducing subgroup C feline leukemia virus is a member of a transporter superfamily. J. Virol. 73:6500–6505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Takeuchi Y., et al. 1992. Feline leukemia virus subgroup B uses the same cell surface receptor as gibbon ape leukemia virus. J. Virol. 66:1219–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tennant R. W., Myer F. E., McGrath L. 1974. Effect of the Fv-1 gene on leukemia virus in mouse cell heterokaryons. Int. J. Cancer 14:504–513 [DOI] [PubMed] [Google Scholar]
- 44. Todaro G. J., Green H. 1963. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell Biol. 17:299–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang H., Paul R., Burgeson R. E., Keene D. R., Kabat D. 1991. Plasma membrane receptors for ecotropic murine retroviruses require a limiting accessory factor. J. Virol. 65:6468–6477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Willett B. J., et al. 1997. Shared usage of the chemokine receptor CXCR4 by the feline and human immunodeficiency viruses. J. Virol. 71:6407–6415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xu W., Eiden M. V. 2011. Primate gammaretroviruses require an ancillary factor not required for murine gammaretroviruses to infect BHK cells. J. Virol. 85:3498–3506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yan Y., et al. 2010. Evolution of functional and sequence variants of the mammalian XPR1 receptor for mouse xenotropic gammaretroviruses and the human-derived retrovirus XMRV. J. Virol. 84:11970–11980 [DOI] [PMC free article] [PubMed] [Google Scholar]