Abstract
The myxoma virus (MYXV)-encoded pyrin domain-containing protein M013 coregulates inflammatory responses mediated by both the inflammasome and the NF-κB pathways. Infection of human THP-1 monocytic cells with a MYXV construct deleted for the M013 gene (vMyxM013-KO), but not the parental MYXV, activates both the inflammasome and NF-κB pathways and induces a spectrum of proinflammatory cytokines and chemokines, like interleukin-1β (IL-1β), tumor necrosis factor (TNF), IL-6, and monocyte chemoattractant protein 1. Here, we report that vMyxM013-KO virus-mediated activation of inflammasomes and secretion of IL-1β are dependent on the adaptor protein ASC, caspase-1, and NLRP3 receptor. However, vMyxM013-KO virus-mediated activation of NF-κB signaling, which induces TNF secretion, was independent of ASC, caspase-1, and either the NLRP3 or AIM2 inflammasome receptors. We also report that early synthesis of pro-IL-1β in response to vMyxM013-KO infection is dependent upon the components of the inflammasome complex. Activation of the NLRP3 inflammasome and secretion of IL-1β was also dependent on the release of cathepsin B and production of reactive oxygen species (ROS). By using small interfering RNA screening, we further demonstrated that, among the RIG-I-like receptors (RLRs) and Toll-like receptors (TLRs), only TLR2, TLR6, TLR7, and TLR9 contribute to the NF-κB-dependent secretion of TNF and the inflammasome-dependent secretion of IL-1β in response to vMyxM013-KO virus infection. Additionally, we demonstrate that early triggering of the mitogen-activated protein kinase pathway by vMyxM013-KO virus infection of THP-1 cells plays a critical common upstream role in the coordinate induction of both NF-κB and inflammasome pathways. We conclude that an additional cellular sensor(s)/receptor(s) in addition to the known RLRs/TLRs plays a role in the M013 knockout virus-induced activation of NF-κB pathway signaling, but the activation of inflammasomes entirely depends on sensing by the NLRP3 receptor in response to vMyxM013-KO infection of human myeloid cells.
INTRODUCTION
Invading microbial pathogens, including viruses, are initially recognized by dedicated cellular receptors called pathogen recognition receptors (PRRs), which trigger the activation of the early proinflammatory responses responsible for the establishment of innate and adaptive immune responses. Based on recent findings, the PRRs can be grouped into four main classes: Toll-like receptors (TLRs), retinoic acid inducible gene I (RIG-I)-like receptors (RLRs), nucleotide binding oligomerization domain (NOD)-like receptors (NLRs), and the newly identified Absent in Melanoma-2 (AIM2)-like receptors (ALRs). These receptors sense pathogens or pathogen-associated molecular patterns (PAMPs), such as nonself entities, mislocalized nucleic acids, or overexpressed glycoproteins, either at the cell surface or within intracellular compartments. For example, exogenous cytosolic double-stranded DNA (dsDNA) can be sensed by endosomal TLR9 and/or intracellular ALRs (50), whereas viral envelope glycoproteins are sensed by extracellular TLR4 (36). Recent findings also suggest that the expression patterns and levels of PRRs significantly differ among various cell lineages and tissue types, and these differences govern the receptor specificity functions in a cell-, species- and pathogen-specific manner.
TLRs can sense viruses and virus-derived PAMPs at either the cell surface or within endosomal compartments. Among the TLRs, TLR3, TLR7, and TLR9, which are present in the endosomal compartment, sense both single- and double-stranded RNA and DNA viruses, whereas TLR2 and TLR4 recognize either DNA or RNA viruses from the cell surface. In some cases, TLRs collaborate with other receptors, like the RLRs and NLRs, and together they play an important coordinate role in triggering innate responses and facilitating adaptive immunity (23). The PAMP-activated TLRs recruit the adaptor proteins MyD88 and TRIF to activate downstream kinases, including IκB kinase complex (IKK) and IKK-related kinases (TBK1 and IKKε), which then activate the NF-κB transcription factors and interferon (IFN) regulatory factors (IRFs). Indeed, this NF-κB activation pathway is frequently subverted by a wide variety of pathogens (34). Members of the RLR family of RNA helicases, RIG-I, melanoma differentiation-associated gene 5 (MDA5), and laboratory of genetics and physiology-2 (LGP2), have been shown to be critical for detecting RNA viruses by sensing single-stranded RNAs and dsRNAs in the cytosol (52). RIG-I and MDA5 interact with the caspase activation and recruitment domain (CARD) of mitochondrial antiviral signaling protein (MAVS; also known as IPS-1, VISA, and CARDIF) using its N-terminal CARD. The main function of RLR activation is the production of type I IFN by activating the IRF3, IRF7, and NF-κB pathways. In addition, RIG-I was recently described as an upstream activator of the inflammasome complex that triggers IL-1β and/or IL-18 production through caspase-1 and caspase-3 activation (33). This activation of caspase-1 initiated by RIG-I, but not MDA5, was mediated by protein-protein interaction with the pyrin-containing cellular adaptor protein ASC. This suggests that RIG-I uniquely mediates cross talk between the type I IFN and inflammasome pathways.
Members of the NLR family of PAMP sensors are the major intracellular receptors involved in the activation of the inflammasome complex (21, 26, 40). To date, the NLR members NLRP1, NLRP3, and NLR family CARD-containing 4 (NLRC4) have been shown to sense viral and other pathogen infections, leading to the activation of the inflammasomes that mediate the cleavage of caspase 1 and thereby trigger release of the proinflammatory cytokines IL-1β and IL-18. Although both DNA and RNA viruses, such as the attenuated vaccinia virus (VACV) strain MVA and influenza A virus, have been shown to activate NLRP3, no direct interaction between NLRP3 and RNA or DNA has yet been established (8, 22). In addition to microbial pathogens, the NLRP3 inflammasome is also activated by cellular damage-associated molecular patterns, such as monosodium urate (MSU), calcium pyrophosphate dehydrate, and ATP, with the common inducer molecule being the reactive oxygen species (ROS) generated by NADP (NADPH) oxidase (48).
Members of AIM2-like intracellular receptors sense cytosolic DNA from either viruses or bacteria and induce the secretion of type I IFN and other proinflammatory mediators. AIM2 is a member of PYHIN protein family (pyrin and HIN200 domain-containing proteins; also known as p200 or HIN200 proteins) and interacts with dsDNA through its HIN domain, which then promotes the assembly of the inflammasome complex via PYD-PYD interactions with the adaptor protein ASC (6, 10, 14, 38). The AIM2 inflammasome plays a critical role in host defenses against some DNA viruses, like VACV and murine cytomegalovirus but not, for example, herpes simplex virus 1 (11, 37). This suggests that AIM2 is either unable to recognize certain DNA viruses or, more likely, many DNA viruses have evolved mechanisms to suppress and or bypass AIM2 signaling. Another member of the PYHIN protein family, IFI16 has been recently identified as a cytosolic DNA sensor that signals via STING, TBK1, and IRF3 in human cells (49). The murine homolog of IFI16, p204, also acts as a DNA sensor in mouse cells. IFI16 and p204 each interact with STING and trigger TBK1-mediated phosphorylation of IRF3 and activation of NF-κB, which then leads to the induction of IFN-α/β expression. However, IFI16 has no direct role in inflammasome activation or in IL-1β production (6, 10, 14). Apart from IFI16, cytosolic DNA is also reported to be sensed by RNA polymerase III and DNA-dependent activator of IRFs (DAI), which then trigger the transcription of genes involved in the innate immune response (7, 46).
Myxoma virus (MYXV) is a prototype member of the Leporipoxvirus genus of the Poxviridae family and causes a lethal disease called myxomatosis, only in European rabbits (Oryctolagus cuniculus) (44). In most cases, myxomatosis is fatal and is accompanied by the total collapse of the rabbit immune system, but the virus is essentially harmless to all nonlagomorphs, such as mice and humans. The extreme virulence of MYXV in European rabbits is mediated by dozens of virus-encoded immune evasion molecules that are directed at numerous host innate and acquired immune pathways. Some of these MYXV-encoded immunomodulators are relatively rabbit specific, depending on the degree of conservation of the host target pathways across species, but many others are capable of recognizing and inhibiting their targets in nonrabbit cells as well. To date, a wide spectrum of MYXV-encoded immunomodulators have been identified that target diverse host cytokines, host cell signaling pathways, apoptosis, and other innate immune pathways (3, 24, 27).
The MYXV-encoded protein M013 is a functional ortholog of the cellular PYRIN domain (PYD)-containing protein and has been shown to be an intracellular inhibitor of both the inflammasome and the NF-κB pathway (18, 35). The cellular PYD proteins are also involved in the regulation of apoptosis, NF-κB activation, and proinflammatory cytokine production by mediating protein-protein interactions (45). An engineered MYXV construct deleted for the M013 gene (vMyxM013-KO) was significantly attenuated in European rabbits because of decreased virus dissemination and enhanced inflammatory responses at the tissue sites of virus infection (18). The regulation of at least two distinct immune pathways by M013 is mediated by protein-protein interactions with two independent cellular regulators of the inflammasome and NF-κB signaling pathways. First, M013 protein interacts with the apoptosis-associated Speck-like protein, which contains a caspase recruitment domain (ASC), a key conserved component of the cellular inflammasome complexes, and inhibits caspase-1 activation and the processing of proinflammatory cytokines IL-1β and IL-18 (18). Second, M013 protein also interacts with NF-κB1/p105, a member of the NF-κB protein family, and inhibits the activation of NF-κB by preventing the degradation of NF-κB1/p105 in response to upstream proinflammatory signals (35). Importantly, both of these binding/inhibitory properties of M013 are relatively species nonspecific, and thus M013 is an effective inhibitor of both pathways in human cells. Thus, infection of human myeloid cells (e.g., THP-1 cells) with vMyxM013-KO virus, but not wild-type MYXV, is rapidly sensed and then induces a variety of proinflammatory cytokine responses. However, it is not known which inflammasome sensor becomes activated by vMyxM013-KO infection, or how the NF-κB pathway is activated.
In this study, we report the mechanism(s) of inflammasome and NF-κB activation in human myeloid cells infected by vMyxM013-KO virus. We found that NLRP3, but not AIM2, together with ASC and caspase-1, is crucial for the activation of cellular inflammasomes and the release of IL-1β by vMyxM013-KO virus. The M013 knockout virus-induced activation of the NLRP3 inflammasome is dependent upon the release of the lysosomal protease cathepsin B into the cytoplasm. However, unexpectedly, the activation of the NF-κB pathway by M013 knockout virus infection is essentially independent of these inflammasome sensor components. Instead, the activation of the NF-κB pathway is dependent on the signaling from several TLRs, including TLR2, -6, -7, and -9. We also demonstrate that MEK–extracellular signal-regulated kinase 1/2 (MEK-ERK1/2) signaling is essential for the activation of both NF-κB and inflammasomes in response to vMyxM013-KO virus infection.
MATERIALS AND METHODS
Reagents and antibodies.
Rabbit polyclonal antibodies for RIG-I, MAVS, MDA5, MyD88, IκBα, IL-1β, and caspase-1 were purchased from Cell Signaling Technology. Mouse monoclonal antibody for NLRP3 was obtained from Alexis Biochemicals, rabbit polyclonal antibody for ASC was obtained from Santa Cruz Biotechnology, rabbit polyclonal antibody for AIM2 came from abcam, and mouse monoclonal antibody for β-actin was obtained from Ambion. Horseradish peroxidase (HRP)-conjugated goat anti-rabbit and anti-mouse IgG antibodies were purchased from Jackson Laboratory. Antibodies against human TLRs were purchased from Imgenex Corp. ATP, phorbol-12-myristate-13-acetate (PMA), and the caspase-1 inhibitor zVAD-fmk were purchased from Sigma. MEK1/2 inhibitor U0126 and the phosphatidylinositol 3-kinase (PI3 kinase) inhibitor LY294002 were purchased from Cell Signaling Technology. Cathepsin B-specific inhibitor, CA074Me, and NADPH oxidase inhibitor, diphenyleneiodonium (DPI) were purchased from Calbiochem. Ultrapure lipopolysaccharide from E. coli K-12 strain (LPS-EK), poly(dA:dT), Pam3CSK4, Pam2CSK4, CL075, E. coli DNA, alum crystals, and poly(I:C) were purchased from InvivoGen.
Cell lines and cell culture.
The human monocytic THP-1 cell line was cultured in RPMI 1640 medium (Lonza) supplemented with 10% heat-inactivated fetal bovine serum, 100 IU/ml of penicillin, 100 μg/ml of streptomycin. For differentiation into macrophages, THP-1 cells were stimulated for 12 to 18 h with 100 ng/ml of PMA. In all experiments, THP-1 cells were activated with PMA before infection or stimulation with ligands (32). All cultures were maintained at 37°C in a humidified 5% CO2 incubator. THP-1 cells stably expressing control shRNA, or NLRP3, caspase-1, and ASC shRNAs have been described previously (8).
Transfection of cells.
THP-1 cells were seeded at 5 × 104 cells per well in 24-well plates in growth medium without antibiotics. All the small interfering RNAs (siRNAs) used were ON-TARGETplus siRNA purchased from Thermo Scientific (Dharmacon). siRNA solution (50 to 100 nM final concentration/well) was prepared in 50 μl Opti-MEM I reduced serum medium (Invitrogen). Lipofectamine RNAiMAX (Invitrogen) solution was prepared in 50 μl Opti-MEM I reduced serum medium and incubated for 5 min at room temperature (RT). siRNA and Lipofectamine solutions were mixed and incubated for 20 min at RT, added to the cells to make a final volume of 500 μl, and incubated at 37°C in a CO2 incubator. The knockdown was verified after 48 to 72 h of transfection.
Viral preparation.
Construction of a wild-type MYXV that expressed green fluorescent protein (GFP) under the control of a synthetic VACV early-late promoter was described previously. Construction of the vMyxM013-KO virus was described previously (18). Viruses were purified by centrifugation through a sucrose cushion and two successive sucrose gradient sedimentations as described previously (20, 43).
Quantification of cytokine secretion by ELISA.
THP-1 cells were plated in multiwell plates in the presence of PMA (100 ng/ml) overnight. The following day, culture medium was replaced with fresh RPMI medium, and the cells were infected with wild-type MYXV (WT-MYXV) or vMyxM013-KO virus at a multiplicity of infection (MOI) of 3 or treated with inducers like lipopolysaccharide (LPS; 100 ng/ml) or ATP (5 mM), and the supernatants (media) were collected at different time points. Whenever mentioned, inhibitors were added 1 h before infection. The levels of TNF and IL-1β were determined using enzyme-linked immunosorbent assay (ELISA) kits (eBioscience) following the manufacturer's protocol.
Western blot analysis.
THP-1 cells were harvested at different time points after infection with viruses, washed with phosphate-buffered saline (PBS), and stored at −80°C or processed immediately with radioimmunoprecipitation assay lysis buffer (50 mM Tris, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% NP-40, 1 mM phenylmethylsulfonyl fluoride [PMSF], and protease inhibitor cocktail [Roche]). Amounts of total proteins were estimated by Bradford assay (Bio-Rad), and equal amounts of total proteins were used for Western blot analyses. Protein samples were separated on 10% SDS-PAGE gels and transferred to polyvinylidene difluoride membrane (GE Healthcare) using a wet transfer apparatus (Invitrogen). Membranes were blocked in TBST buffer (20 mM Tris, 150 mM NaCl, 0.1% Tween 20; pH 7.6) containing 5% nonfat dry milk for 1 h at room temperature and then incubated overnight with appropriate primary antibody at 4°C. The membranes were washed three times for 15 min each with TBST, incubated with HRP-conjugated goat anti-mouse (1:10,000) or goat anti-rabbit (1:10,000) secondary antibody in TBST containing 5% nonfat dry milk for 1 h at room temperature with gentle agitation, and then washed three times, 15 min each, with TBST. The proteins were detected using chemiluminescence substrate (Pierce) and exposure to X-ray film (Kodak).
Detection of ROS.
The intracellular levels of ROS were measured using 2′,7′-dichlorofluroescein (DCF). Briefly, THP-1 cells were plated in multiwell plates in the presence of PMA overnight. Medium was replaced with fresh complete medium and infected with viruses for 6 h. Cells were then loaded with 10 μM 2′,7′dichlorofluroesceindiacetate (H2DCF-DA; Molecular Probes) for 30 min at 37°C. In some cases cells were treated with 100 μM NADPH oxidase inhibitor DPI for 1 h before virus infection. H2DCF-DA is a cell membrane-permeable nonfluorescent compound that is hydrolyzed to DCF and becomes fluorescent when it is oxidized by ROS. Cells were then washed and resuspended in PBS, and fluorescence was measured at 520 nm following excitation with a 488-nm light using an Appliskan plate reader (Thermo Scientific).
Statistics.
Data are expressed as means ± standard deviations (SD) and were analyzed by the paired t test. A significant difference was accepted at a P level of <0.05.
RESULTS
ASC and caspase-1 are required for vMyxM013-KO-mediated release of IL-1β, but not TNF, from infected THP-1 cells.
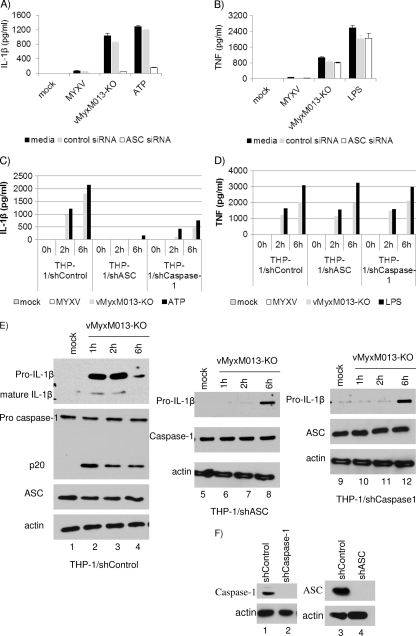
MYXV-encoded PYD-containing protein M013 interacts with the adaptor protein ASC of the inflammasome complex and inhibits the activation of inflammasomes and release of proinflammatory cytokines (18). In the absence of functional M013 protein, vMyxM013-KO virus infection of human macrophage-like differentiated THP-1 cells induces inflammasome activation and the cleavage of caspase-1, leading to the secretion of proinflammatory cytokines IL-1β and IL-18 (18). We have also demonstrated that vMyxM013-KO virus infection of THP-1 cells rapidly activates the NF-κB signaling pathway, leading to the secretion of NF-κB-regulated proinflammatory cytokines and chemokines, such as TNF, IL-6, and MCP-1 (35). Additionally, we have demonstrated that treatment of THP-1 cells with the caspase-1 inhibitor zVAD-fmk inhibits vMyxM013-KO virus infection-induced secretion of IL-1β (35). To investigate if these two cellular pathways (see Fig. 8) are coordinately triggered by the same cellular sensors in response to infection with M013-KO MYXV, we first examined the role of the common inflammasome adaptor component ASC in vMyxM013-KO virus-mediated activation of inflammasome and NF-κB pathways. We observed that silencing ASC in THP-1 cells, by using siRNAs, completely abolished IL-1β secretion following either vMyxM013-KO virus or ATP treatment (as a control), while no significant inhibition of IL-1β secretion was observed in control untreated cells or those transfected with nontargeting control siRNA (Fig. 1A). In contrast, silencing of ASC expression had no significant effect on NF-κB-mediated TNF secretion from the same vMyxM013-KO virus-infected or LPS-treated (as control) THP-1 cells (Fig. 1B). To further confirm these results using transient knockdown of ASC, we generated stable knockdown clones of THP-1 cells with lentiviruses expressing shASC or shCaspase-1. The reduced expression levels of ASC and caspase-1 in the stable knockdown THP-1 cells, compared to WT cells or cells treated with nontarget shRNA (ShControl), were confirmed by Western blot analysis (Fig. 1F). When these cells were infected with vMyxM013-KO virus or treated with ATP (as control), they secreted significantly less IL-1β than WT or shControl THP-1 cells, confirming that vMyxM013-KO virus-mediated IL-1β secretion relies on ASC and caspase-1 activation (Fig. 1C). However, the secretion of TNF from vMyxM013-KO virus-infected shASC or shCaspase-1 THP-1 cells was unaffected, suggesting that ASC and caspase-1 have no direct role in the activation of the NF-κB pathway (Fig. 1D). Infection of PMA-differentiated THP-1 cells with vMyxM013-KO virus upregulated the synthesis of pro-IL-1β protein and cleavage of pro-caspase-1 very rapidly, which resulted in the release of mature IL-1β (Fig. 1E, left, first and second panels). We examined the level of pro-IL-1β synthesis in both shASC and shCaspase-1 THP-1 cells and cleavage of caspase-1 in shASC THP-1 cells by Western blot analysis. The synthesis of pro-IL-1β was significantly delayed by the knockdown of either ASC or caspase-1, compared to the WT or shControl THP-1 cells, following infection with vMyxM013-KO virus (Fig. 1E). As expected, there was no cleavage of caspase-1 and formation of p20 in response to vMyxM013-KO virus infection in the ASC or caspase-1 knockdown cells compared to the shControl THP1 cells (Fig. 1E). However, the level of ASC (Fig. 1E) and NLRP3 (data not shown) in the control and knockdown THP-1cells remained unchanged. These results suggest that components of the inflammasome complex ASC and caspase-1 have direct and obligatory roles in the signaling that lead to the rapid induced synthesis of the pro-IL-1β protein precursor needed as substrate for caspase-1.
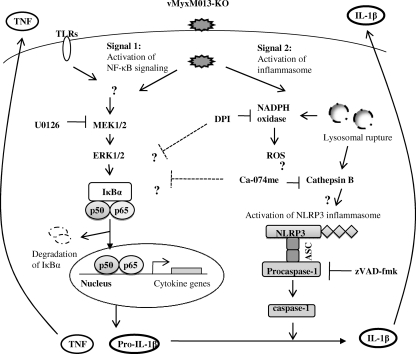
Fig. 8.
Schematic showing the mechanism(s) of activation of the NLRP3 inflammasome and NF-κB signaling pathways by vMyxM013-KO virus infection in human monocytic cell line THP-1. Infection of differentiated THP-1 cells by vMyxM013-KO virus activates the NLRP3 inflammasome by the release of ROS and cathepsin B from lysosomal destabilization. vMyxM013-KO virus activates the NF-κB pathway for the synthesis of cytokines via activation of the MEK-ERK1/2 pathway and sensing by the TLRs.
Fig. 1.
ASC and caspase-1 are important for activation of inflammasome and release of IL-1β after vMyxM013-KO virus infection of THP-1 cells. (A and B) THP-1 cells were transfected with control siRNA or ASC siRNA, differentiated for 12 to 18 h with PMA, and then infected with MYXV or vMyxM013-KO viruses at an MOI of 3 or treated with ATP (5 mM) or LPS (500 ng/ml). Cell supernatants were collected after 6 h, and the secretion of IL-1β (A) and TNF (B) was quantified by ELISA. (C and D) THP-1 cells were stably transduced with control shRNA or ASC or caspase-1 shRNA. The cells were differentiated with PMA and were infected with WT-MYXV or vMyxM013-KO viruses or treated with ATP or LPS. Cell supernatants were collected at the indicated time points to quantify secretion of IL-1β (C) and TNF (D) by ELISA. Data are means ± SD of triplicate samples from one experiment and are representative of three independent experiments. (E and F) Western blots of intracellular IL-1β, caspase-1, ASC, and actin (loading control).
NLRP3 is required for activation of inflammasomes in THP-1 cells infected with vMyxvM013-KO.
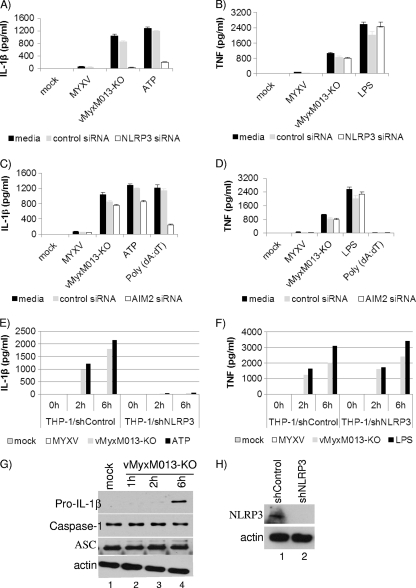
Among the intracellular PRRs identified so far, the NLRP3 and AIM2 receptors have been reported to sense infection by DNA viruses and activate the inflammasome complexes. We examined whether NLRP3 or AIM2 is involved in sensing MYXV lacking M013 and then signals the activation of the inflammasome complex in THP-1 cells. We transiently silenced the expression of NLRP3 or AIM2 by using corresponding siRNAs in THP-1 cells and tested the release of various indicator proinflammatory cytokines after virus infection. We found that silencing the expression of NLRP3 completely abolished the induced IL-1β secretion following either vMyxM013-KO virus or ATP (as control), while no significant inhibition of IL-1β secretion was observed in cells transfected with AIM2 siRNA or a nontargeting control siRNA (Fig. 2A and C). The secretion of TNF from the NLRP3 or AIM2 knockdown THP-1 cells after vMyxM013-KO virus infection remained unaffected (Fig. 2B and D). This observation suggests that the NLRP3 receptor is involved in the M013 knockout virus-induced activation of inflammasomes and the release of IL-1β. To further study the role of NLRP3, we next generated stable knockdown of THP-1 cells by using lentivirus-expressed shNLRP3 and confirmed the reduced expression of NLRP3 by Western blot analysis (Fig. 2H). In these NLRP3 stable knockdown THP-1 cells, secretion of IL-1β was significantly reduced, while the secretion of NF-κB-dependent TNF was unaffected (Fig. 2E and F). We also examined the level of pro-IL-1β synthesis, the cleavage of caspase-1, and the expression of ASC in these shNLRP3 THP-1 cells at various time points after infection. The synthesis of pro-IL-1β was significantly delayed at early time points after infection by vMyxM013-KO virus infection, when NLRP3 is knocked down, compared with the WT virus or shControl THP-1 cells (Fig. 2G, top panel). As expected, in the NLRP3 knockdown cells, there was no cleavage of caspase-1 in response to vMyxM013-KO virus, and the level of ASC remained unchanged during the tested period of infection (Fig. 2G, second and third panels). These results again support the conclusion that the inflammasome complex, together with the receptor NLRP3, plays a key role in the signaling events that lead to the very early synthesis of pro-IL-1β protein in response to vMyxM013-KO virus infection of human myeloid cells.
Fig. 2.
NLRP3, but not AIM2, is required for THP-1 inflammasome activation by vMyxM013-KO virus. (A to D) THP-1 cells were transfected with control, NLRP3 (A and B), or AIM2 (C and D) siRNA, differentiated for 12 to 18 h with PMA, and then infected with MYXV or vMyxM013-KO viruses (MOI, 3) or treated with ATP (5 mM), poly(dA:dT) (5 μg/ml), or LPS (500 ng/ml). Cell supernatants were collected after 6 h to measure the secretion of IL-1β (A and C) or TNF (B and D) by ELISA. THP-1 cells were stably transduced with control and NLRP3 shRNAs, differentiated with PMA, and infected with MYXV or vMyxM013-KO viruses or treated with ATP or LPS. (E to G) Cell supernatants were collected at the indicated time points to measure secretion of IL-1β (E) and TNF (F) by ELISA. Data are means ± SD of triplicate samples from one experiment and are representative of three independent experiments. (G and H) Western blots of intracellular IL-1β, caspase-1, ASC,NLRP3, and actin (loading control).
Cathepsin B and ROS activate the NLRP3 inflammasome in response to vMyxM013-KO infection.
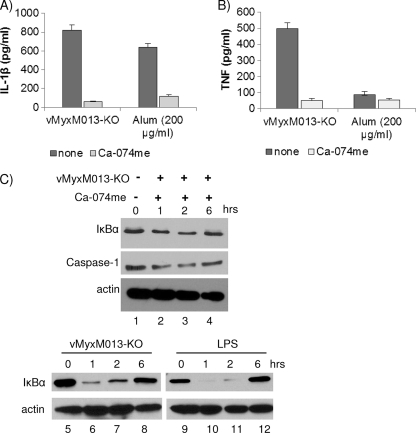
Recent studies have suggested that the NLRP3 inflammasome is activated by multiple mechanisms, including lysosomal damage and release of cathepsin B from lysosomal compartments (2, 13, 15). To determine whether vMyxM013-KO virus infection-mediated release of lysosomal cathepsin B into the cytoplasm might be required for NLRP3 inflammasome activation, PMA-differentiated THP-1 cells were treated with increasing concentrations (25 to 200 μM) cathepsin B inhibitor Ca-074me for 1 h before infection with the test virus or treatment with alum (as control). Release of IL-1β at 6 h postinfection was inhibited in THP-1 cells pretreated with all the different concentrations of Ca-074me, suggesting that the catalytic activity of cathepsin B is required for NLRP3 inflammasome activation by vMyxM013-KO virus (Fig. 3A [only the 100 μM Ca-074me treatment data are shown). Treatment with Ca-074me also inhibited the release of TNF from vMyxM013KO-infected THP-1 cells (Fig. 3B). This could be due to the role of cathepsin B in the trafficking of TNF-containing vesicles to the plasma membrane, which is inhibited by the treatment of Ca-074me, as reported recently (12). This was supported by the observation that treatment of THP-1 cells with Ca-074me inhibited activation of the NF-κB pathway, as observed by monitoring the degradation of IκBα and cleavage of caspase-1 by Western blot analysis (Fig. 3C). Infection of PMA-activated THP-1 cells with vMyxM013-KO virus alone or treatment with LPS induced the degradation of IκBα, as reported before (and included as a control) (Fig. 3C).
Fig. 3.
Cathepsin B is involved in the activation of NLRP3 inflammasome activation by vMyxM013-KO virus. (A and B) THP-1 cells were differentiated with PMA, treated with the cathepsin B inhibitor Ca-074me (100 μM) for 1 h, and then infected with vMyxM013-KO virus at an MOI of 3 or treated with alum (as a positive control). Cell supernatants were collected after 6 h to measure the secretion of IL-1β (A) and TNF (B) by ELISA. Data are means ± SD of triplicate samples from one experiment and are representative of two independent experiments. (C) THP-1 cells were differentiated with PMA and treated with LPS (500 ng/ml) as control or infected with vMyxM013-KO virus in the presence or absence of cathepsin B inhibitor Ca-074me, and Western blot analysis was done for the detection of intracellular IκBα, caspase-1, and actin (loading control).
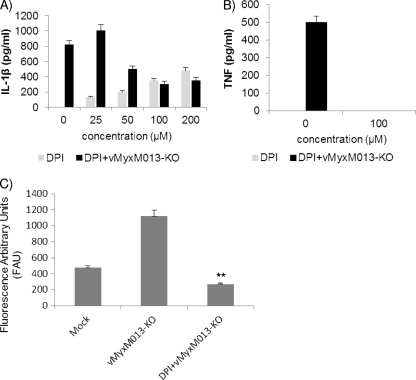
The activation of the NLRP3 inflammasome by numerous stimuli depends on the production of ROS released by NADPH oxidase. The requirement for ROS in vMyxM013-KO virus infection-mediated NLRP3 activation was tested by using the NADPH oxidase inhibitor DPI. Release of IL-1β was inhibited in virus-infected THP-1 cells pretreated with DPI in a dose-dependent manner, suggesting that ROS is required for activation of NLRP3 inflammasome activation by vMyxM013-KO virus infection (Fig. 4A). Treatment with DPI also inhibited the release of TNF from the infected THP-1 cells (Fig. 4B), which was reported to be an inhibitor of NF-κB pathway activation (25). The release of ROS by vMyxM013-KO infection was also confirmed in a DCF fluorescence assay, and treatment with DPI significantly reduced the level of fluorescence (Fig. 4C). Neither Ca-074me nor DPI significantly affected the cell viability during the time periods tested (data not shown).
Fig. 4.
ROS are involved in NLRP3 inflammasome activation by vMyxM013-KO virus. (A and B) THP-1 cells were differentiated with PMA, treated with DPI using the indicated concentrations for 1 h, and then infected with vMyxM013-KO virus at an MOI of 3. Cell supernatants were collected after 6 h to measure the secretion of IL-1β (A) and TNF (B). (C) PMA-differentiated THP-1 cells were mock treated or pretreated with ROS inhibitor DPI (100 μM) for 1 h and then infected with vMyxM013-KO virus. After 6 h cells were loaded with the ROS-sensitive fluorophore H2DCF-DA (10 μM), and fluorescence intensity was measured as described in Materials and Methods. Data are means ± SD of triplicate samples from one experiment and are representative of two or three independent experiments. **, P < 0.01 compared with vMyxM013-KO infection.
Release of TNF and IL-1β by vMyxM013-KO virus infection of THP-1 cells is independent of RLRs but dependent on MyD88.
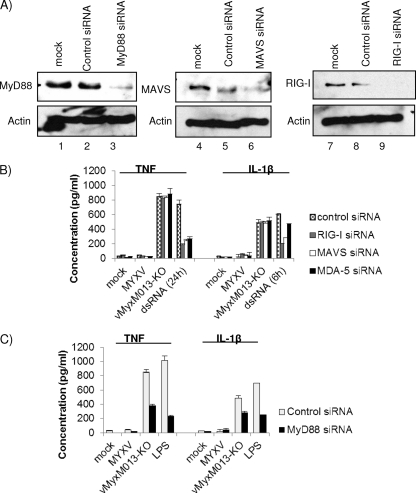
We have tested the potential roles of other known pathogen-sensing signaling pathways in the rapid activation of NF-κB and inflammasomes in THP-1 cells by vMyxM013KO virus infection. WT-MYXV is sensed by RIG-I in human primary macrophages, and this sensing leads to the coinduction of type I interferon and TNF (51), but at the present time it is not clear why the deletion of M013 is apparently needed in order for MYXV to induce TNF in THP-1 cells. MyD88 is an obligatory adaptor molecule for the majority of TLRs. In order to assess which signaling pathways might contribute to the activation of NF-κB in response to vMyxM013KO virus infection of THP-1 cells, we used siRNAs for RIG-I, MDA-5, MAVS, and MyD88 to knock down the expression of these proteins and tested the level of TNF and IL-1β production. The knockdown of these proteins was confirmed by checking the level of protein expression by Western blotting (Fig. 5A). The siRNA-transfected THP-1 cells were differentiated with PMA and infected with WT-MYXV or vMyxM013-KO viruses or treated with known inducers. Supernatants were collected at 6 h or 24 h postinfection/treatment. Our results demonstrated that knockdown of RIG-I, MDA-5, or MAVS had no effect on secretion of TNF or IL-1β (Fig. 5B), suggesting that RIG-I-like receptors are not required for TNF secretion from THP-1 cells in response to vMyxM013-KO virus infection. At present, the reason that RIG-I is apparently linked to MYXV-induced TNF secretion in primary human macrophages, but not THP-1 cells, is unknown. We previously reported that in THP-1 cells, vMyxM013-KO virus infection did not induce the secretion of type I IFN (35), which has been further supported by this observation that RIG-I does not apparently trigger TNF secretion following vMyxM013-KO virus (or WT-MYXV) infection of THP-1 cells. Knockdown of a key adaptor protein for TLR signaling pathways (MyD88) significantly reduced the level of TNF and IL-1β secretion from the vMyxM013-KO virus-infected THP-1 cells (Fig. 5C). This suggests that one or more TLRs have a role in the activation of the NF-κB signaling pathway in THP-1 cells infected with vMyxM013-KO.
Fig. 5.
Roles of RLRs and MyD88 in the activation of inflammasomes and NF-κB signaling by vMyxM013-KO. THP-1 cells were transfected with siRNAs for nontargeting control, RIG-I, MDA5, MAVS, or MyD88, differentiated for 12 to 18 h with PMA, and then infected with vMyxM013-KO virus (MOI, 3), transfected with dsRNA poly(I:C) (1 μg/ml) for the indicated time periods, or treated with LPS (500 ng/ml). (A) Western blots showing the levels of protein knockdown using MyD88, MAVS, and RIG-I siRNA. (B and C) Cell supernatants were collected after 6 h or 24 h of infection or treatment with the inducers to quantify the secretion of TNF and IL-1β by ELISA. Data are means ± SD of triplicate samples from one experiment and are representative of two or three independent experiments. P was <0.05 under all conditions in panel C.
Role of TLRs in activation of NF-κB and inflammasomes by vMyxM013-KO in THP-1 cells.
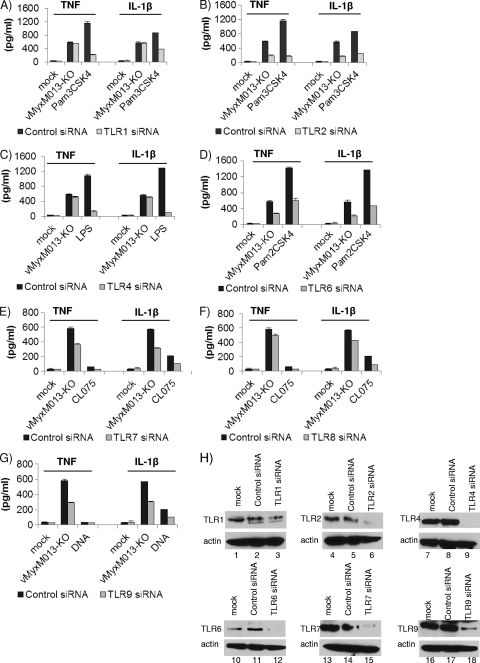
TLRs play an important role in early detection of viruses and in the initiation of the antiviral host defense responses (5). TLR-mediated sensing of viruses is mainly linked to the rapid activation of the NF-κB signaling pathway. TLR-mediated activation of the NF-κB pathway also regulates the synthesis of pro-IL-1β precursor protein, which is then utilized as substrate for cleavage into mature IL-1β by the inflammasome complex. Several TLRs have been shown to be important for sensing poxviruses, including vaccinia virus and MVA, the attenuated smallpox vaccine candidate (8, 29, 39, 54). In order to test which TLRs might be involved in sensing vMyxM013-KO virus and in activation of the NF-κB pathway, we used siRNAs against all the known human TLRs. Among the TLRs, TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are present on the cell surface, while TLR3, TLR7, TLR8, and TLR9 are present in the endosomes. To test which of these TLRs might contribute to the activation of the NF-κB pathway, we used siRNAs for all of them to transiently knock down the expression of these TLRs in THP-1 cells and tested the levels of TNF and IL-1β production in response to vMyxM013-KO virus infection. The siRNA-transfected THP-1 cells were differentiated with PMA and infected with WT-MYXV (data not shown, as there was no change compared with the mock treatment) or vMyxM013-KO viruses or treated with the known ligand inducers for TLRs. Supernatants were collected at 6 h or 24 h postinfection/treatment for ELISA analysis of secreted cytokines. Our results demonstrated that knockdown of TLR2 and TLR6 has significant effects on secretion of both TNF and IL-1β (Fig. 6B and D), suggesting that these extracellular TLRs play a role in sensing vMyxM013-KO infection and activation of NF-κB signaling. Among the endosomal TLRs, TLR7 and TLR9 knockdown also had significant effects on the secretion of TNF and IL-1β by vMyxM013-KO virus infection in THP-1 cells (Fig. 6E and G). The knockdown of representative TLR proteins was confirmed by checking the level of protein expression by Western blotting (Fig. 6H).
Fig. 6.
Roles of TLRs in the activation of inflammasomes and NF-κB signaling by vMyxM013-KO. THP-1 cells were transfected with siRNAs for nontargeting control or human TLRs (1 to 10), differentiated for 12 to 18 h with PMA, and then infected with vMyxM013-KO virus (MOI, 3) or treated with known ligands for TLRs: LPS (100 ng/ml), Pam3CSK4 (10 ng/ml), Pam2CSK4 (0.1 ng/ml), CL075(5 μg/ml), or E. coli DNA (6 μg/ml; control). Cell supernatants were collected after 6 h of infection or treatment with LPS, Pam3CSK4, or Pam2CSK4 and 24 h after treatment with CL075 and E. coli DNA to measure the secretion of TNF and IL-1β by ELISA. Results shown here are from A) TLR1 (A), TLR2 (B), TLR4 (C), TLR6 (D), TLR7 (E), TLR8 (F), or TLR9 (G) siRNA-treated THP-1 cells. Data are means ± SD of triplicate samples from one experiment and are representative of two or three independent experiments. P was <0.05 under all conditions. (H) Western blots showing the levels of protein knockdown when using TLR1, TLR2, TLR4, TLR6, TLR7, and TLR9 siRNAs.
The requirement of MEK-ERK1/2 for the activation of NF-κB signaling in THP-1 cells.
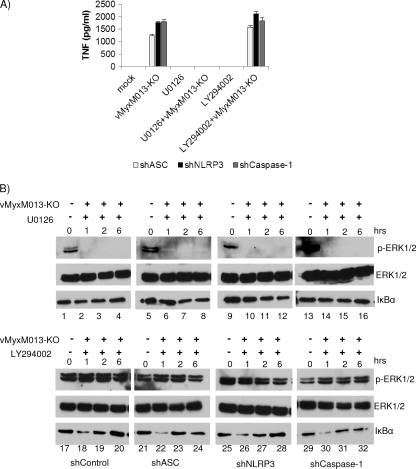
We previously demonstrated that vMyxM013-KO virus-mediated activation of NF-κB is dependent on mitogen-activated protein kinase pathway, but not the PI3 kinase pathway (35). Inhibition of ERK1/2 activation by U0126 inhibited TNF secretion and NF-κB-dependent synthesis of pro-IL-1β protein. Inhibition of PI3 kinase by LY294002 had no effect on activation of the NF-κB pathway. We have further extended this observation and studied the role of ERK1/2 in the absence of ASC, NLRP3, and caspase-1. As we have shown, constitutive knockdown of the expression of ASC, NLRP3, or caspase-1 with shRNA in THP-1 cells had no effect on secretion of TNF induced by M013-KO virus infection. We then tested whether inhibition of several key kinases would inhibit this TNF secretion response. The cells were pretreated with U0126 or LY294002 for 1 h and infected with vMyxM013-KO virus. The supernatants were collected 6 h after infection and tested for secretion of TNF by ELISA. Treatment of shASC, shNLRP3, and shCaspase-1 THP-1 cells with U0126 totally inhibited the vMyxM013-KO virus-induced secretion of TNF (Fig. 7A). The synthesis of pro-IL-1β protein was also inhibited, extending out to late time points of infection in these THP-1 cells (data not shown). This is because treatment with U0126 blocked the phosphorylation of ERK1/2 and activation of the NF-κB pathway, as observed in the absence of IκBα degradation in response to vMyxM013-KO virus infection (Fig. 7B, lanes 1 to 16 in the top and bottom panels). The PI3 kinase inhibitor LY294002 had no effect on secretion of TNF from these cells, as observed with THP-1 cells (Fig. 7A). This was further supported by the observation that the NF-κB pathway was still activated at early time points by vMyxM013-KO virus infection in the presence of a PI3 kinase inhibitor (Fig. 7B, lanes 17 to 32 in the bottom panels). This suggests that ERK1/2 signaling plays a common central early role in sensing vMyxM013-KO virus infection via multiple TLRs and the NLRP3 inflammasome complex.
Fig. 7.
The MEK-ERK1/2 pathway is involved in activation of both NF-κB and inflammasomes by vMyxM013-KO. (A) THP-1 cells stably transduced with control, ASC, caspase-1, or NLRP3 shRNAs. The cells were differentiated with PMA, treated with U0126 (20 μm) or LY294002 (20 μm) for 1 h, and infected with vMyxM013-KO virus. Cell supernatants were collected after 6 h of infection to quantify the secretion of TNF by ELISA. Data are means ± SD of triplicate samples from one experiment and are representative of three independent experiments (B) Western blots of intracellular phospho-EFK1/2 (pERK1/2), total ERK1/2, and IκBα after treatment with U0126 or LY294002 and infection with vMyxM013-KO.
DISCUSSION
Poxviruses represent a large family of DNA viruses that replicate exclusively in the host cytoplasm. Members of the poxvirus family harbor unique sets of immunomodulatory proteins, which are in most cases essential for virus virulence, evasion of host immune responses, and host range functions but which are not required for virus replication in cultured cells (42). The MYXV-encoded PYD-containing protein M013 is required for viral pathogenicity in rabbits, and infection with the M013 knockout virus induces an exacerbated proinflammatory response that clears the virus infection (18). At the molecular level, M013 inhibits two crucial innate immune pathways regulated by NF-κB and the inflammasome complexes in a species pan-specific fashion by virtue of binding independently to NF-κB1/p105 and the ASC adaptor protein (18, 19, 35). The present study provides insights into the mechanisms by which MYXV infection is sensed and activates these two immune pathways in human myeloid cells (Fig. 8). Since MYXV replicates as well in many human cancer cells as it does in rabbit cells, but is essentially nonpathogenic to any nonlagomorph host such as mice or humans, it is being developed for cancer virotherapy, and thus the specific innate interactions of MYXV with human cells of various lineages are of particular interest.
Here we have shown that the cellular NLRP3 receptor, in conjunction with ASC and caspase-1, plays a critical role in the sensing by activated human monocytic THP-1 cells of infection with vMyxM013-KO virus and the activation of the inflammasome-dependent release of IL-1β. In contrast, AIM2, a documented cytosolic DNA sensor that is also linked to inflammasome activation, has no apparent role in the IL-1β release in response to vMyxM013-KO virus infection in THP-1 cells (Fig. 2). Furthermore, we have demonstrated that at least 4 TLR receptors, coupled with rapid triggering of the MEK-ERK1/2 pathway, are required for the NF-κB activation that is needed to induce the secretion of proinflammatory cytokines such as TNF (Fig. 6 and 7).
Among the cytosolic inflammasome receptors identified so far, AIM2 senses certain DNA viruses, including VACV, whereas the NLRP3 inflammasome has been reported to sense both RNA and DNA viruses. The viruses sensed by the NLRP3 inflammasome include Sendai virus (22), influenza A virus (1, 16, 47), adenovirus (2, 31), and the attenuated vaccinia virus strain MVA (8). In vivo studies have demonstrated that sensing of influenza A virus is mediated by NLRP3 (1, 47). Thus, NLRP3-deficient mice produce reduced levels of IL-1β in response to intranasal challenge by influenza A virus. In addition to NLRP3-deficient mice, ASC- and caspase-1-deficient mice also displayed significantly reduced survival compared to wild-type mice following influenza virus infection (1, 47). This suggests that NLRP3, ASC, and caspase-1 all play important roles in the immune responses against at least some pathogenic virus infections. Recently, it has been reported that the influenza virus-encoded M2 protein, which acts as a proton ion channel essential for viral entry and replication, is required and sufficient for NLRP3 activation by influenza A virus (17). In contrast, the available data on the innate inflammasome-linked sensors that trigger early host responses to poxvirus infections are not yet definitive. For example, MVA infection is sensed by the NLRP3 inflammasome in THP-1 cells, but the in vivo role of NLRP3 and ASC for any poxvirus infection remains to be studied. It was recently reported that AIM2 is required for caspase-1 activation and release of IL-1β in response to VACV-WR (strain Western Reserve) infection of murine bone marrow-derived macrophages and dendritic cells (DCs) (37). However, in activated THP-1 cells, VACV-WR did not induce IL-1β secretion, suggesting that AIM2 sensing of poxvirus infection might only operate in a cell- or species-specific manner (unpublished observations).
Recent studies suggest that activation of the NLRP3 inflammasome and release of IL-1β require at least two upstream signals. The NLRP3 inflammasome becomes primed when NF-κB signaling from activated TLRs upregulates the levels of the NLRP3 and pro-IL-1β proteins. An additional signal is then required to activate NLRP3, leading to the assembly of the active inflammasome complex. This is supported by the observation that either TLR signaling or ROS generation alone is insufficient for NLRP3 inflammasome activation (28, 53). Here, we have observed that infection of activated THP-1 cells with vMyxM013-KO virus increased the level of pro-IL-1β protein very rapidly (Fig. 1E). However, the protein levels of the other key components of the inflammasome complex, specifically NLRP3 and ASC, remained unchanged. We also observed that in the absence of NLRP3, ASC, or caspase-1, the induced synthesis of pro-IL-1β in response to vMyxM013-KO virus infection was delayed, suggesting that activation of the inflammasome complex is required to initiate the rapid synthesis of pro-IL-1β protein (Fig. 1E and 2G). It also suggests that the NLRP3 inflammasome complex might have direct or indirect links with the NF-κB activation pathway. It has been recently demonstrated that synthesis of NLRP3 protein is dependent on NF-κB, which is induced by many other PRRs, particularly the TLRs (4).
The NLRP3 inflammasome is activated by diverse pathogens, toxins, and particulates (30, 41). However, unlike the inflammasome-linked DNA sensor AIM2, direct association of NLRP3 with inflammasome activators has not been reported. Previous studies have demonstrated that release of lysosomal cathepsins into the cytoplasm can function as an additional trigger for the activation of the NLRP3 inflammasome (9, 15). In addition to phagocytosed particulates, such as silica particles or asbestos, intracellular bacteria and RNA or DNA viruses can also activate the NLRP3 inflammasome via the release of cathepsin B into the cytoplasm (1, 2, 9). Pharmacological inhibition or gene silencing of cathepsin B leads to reduced NLRP3 inflammasome activation. The requirement of the NLRP3 receptor in sensing MYXV lacking M013 has been further supported by the observation that inhibition of cathepsin B results in the inhibition of release of IL-1β (Fig. 3). It has been proposed that release of cathepsin B induces ROS production, which might activate the NLRP3 inflammasome. The release of ROS has been reported for many stimuli (including viruses) that activate NLRP3. NADPH oxidase has been shown to be a source of ROS, as inhibition of NADPH with DPI affects the release of IL-1β. Here, we observed that DPI also inhibited vMyxM013-KO virus-mediated release of both IL-1β and TNF from THP-1 cells (Fig. 4).
The activation of NF-κB pathway signaling in response to virus infection, which is linked to the induction of many other proinflammatory cytokines, such as TNF and IL-6, can also be mediated by TLRs or RLRs. TLRs in particular are capable of sensing both DNA and RNA viruses and rapidly inducing the activation of NF-κB and the IRFs needed to initiate effective antiviral immune responses. Our results suggest that at least in THP-1 cells, RLRs are not required for the release of TNF in response to vMyxM013-KO virus infection (Fig. 5B). Interestingly, primary human macrophages sense WT-MYXV (i.e., virus that expresses M013) via RIG-I, which then induces release of both TNF and type I IFN (51), whereas WT MYXV infection of differentiated THP-1 cells does not release the secretion of TNF (this study and reference 35). This suggests that either MYXV is sensed differently in primary human macrophages versus differentiated THP-1 cells or else RIG-I integration with the TNF secretion pathway is functionally different in primary versus transformed human myeloid cells.
In this study we showed that sequential transient siRNA knockdown of all known human TLRs suggests that TLR2, TLR6, TLR7, and TLR9 all have roles in sensing of vMyxM013-KO virus and inducing the release of TNF and IL-1β (Fig. 6). Other members of the poxvirus family appear to be sensed by different TLRs, depending on the species or cell types. For example, MVA is a particularly immunogenic variant of VACV that is sensed by TLR2 and/or TLR6 and then drives the activation of NF-κB and synthesis of pro-IL-1β (8). VACV-WR is sensed by the TLR2-MyD88 pathway on conventional DCs (cDCs) for the production of proinflammatory cytokines IL-6, IL-1, and IL-12 (54); however, in murine pDCs VACV-WR is sensed by TLR8 for the activation of NF-κB and type I IFN production (29). Ectromelia virus, the causative agent of mousepox, is sensed by TLR9 in mouse DCs (39). This all suggests that the functionality of TLRs against specific poxviruses is very dependent on the host species, the cell lineage, and possibly even the transformed status of the cells in question. It is also possible that this variation among cell types is a result of differences in the expression levels of PRRs or adaptor molecules.
In conclusion, the present study demonstrates that MYXV is sensed by multiple pattern recognition receptors in human myeloid cells and that M013 has evolved to inhibit both the inflammasome and NF-κB signaling pathways. In human monocytic THP-1 cells, the NLRP3, ASC, and caspase-1 inflammasome is required for the early synthesis and release of IL-1β precursor protein in response to vMyxM013-KO virus infection. This virus-induced activation of NLRP3 involves cathepsin B leakage and the release of ROS into the cytoplasm. We also observed that multiple TLRs, including TLR2, TLR6, TLR7, and TLR9, contribute to the activation of NF-κB, which is required for the release of other proinflammatory cytokines, like TNF and IL-6, and for the upregulated synthesis of the pro-IL-1β precursor. MYXV is currently being developed as an oncolytic virus candidate for the treatment of various human cancers, and it is critical to better understand how this rabbit-specific virus is sensed by human innate sentinel cells and how this sensing triggers the ensuing immune responses to the virus infection.
ACKNOWLEDGMENTS
Our laboratory is supported by a start-up grant from the University of Florida, College of Medicine, NIH grants AI080607 and CA13854, and the Bankhead Coley Foundation.
We thank Thierry Roger for sharing the shRNA plasmids and D. Smith for preparing reagents.
Footnotes
Published ahead of print on 28 September 2011.
REFERENCES
- 1. Allen I. C., et al. 2009. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 30:556–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barlan A. U., Griffin T. M., McGuire K. A., Wiethoff C. M. 2011. Adenovirus membrane penetration activates the NLRP3 inflammasome. J. Virol. 85:146–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barrett J. W., Cao J. X., Hota-Mitchell S., McFadden G. 2001. Immunomodulatory proteins of myxoma virus. Semin. Immunol. 13:73–84 [DOI] [PubMed] [Google Scholar]
- 4. Bauernfeind F. G., et al. 2009. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183:787–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boehme K. W., Compton T. 2004. Innate sensing of viruses by toll-like receptors. J. Virol. 78:7867–7873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burckstummer T., et al. 2009. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 10:266–272 [DOI] [PubMed] [Google Scholar]
- 7. Chiu Y. H., Macmillan J. B., Chen Z. J. 2009. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138:576–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Delaloye J., et al. 2009. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog. 5:e1000480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9. Duncan J. A., et al. 2009. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J. Immunol. 182:6460–6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fernandes-Alnemri T., Yu J. W., Datta P., Wu J., Alnemri E. S. 2009. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458:509–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fernandes-Alnemri T., et al. 2010. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat. Immunol. 11:385–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ha S. D., et al. 2008. Cathepsin B is involved in the trafficking of TNF-alpha-containing vesicles to the plasma membrane in macrophages. J. Immunol. 181:690–697 [DOI] [PubMed] [Google Scholar]
- 13. Halle A., et al. 2008. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 9:857–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hornung V., et al. 2009. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458:514–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hornung V., et al. 2008. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9:847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ichinohe T., Lee H. K., Ogura Y., Flavell R., Iwasaki A. 2009. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J. Exp. Med. 206:79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ichinohe T., Pang I. K., Iwasaki A. 2010. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat. Immunol. 11:404–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnston J. B., et al. 2005. A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity 23:587–598 [DOI] [PubMed] [Google Scholar]
- 19. Johnston J. B., Rahman M. M., McFadden G. 2007. Strategies that modulate inflammasomes: insights from host-pathogen interactions. Semin. Immunopathol. 29:261–274 [DOI] [PubMed] [Google Scholar]
- 20. Joklik W. K. 1962. The purification of four strains of poxvirus. Virology 18:9–18 [DOI] [PubMed] [Google Scholar]
- 21. Kanneganti T. D. 2010. Central roles of NLRs and inflammasomes in viral infection. Nat. Rev. Immunol. 10:688–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kanneganti T. D., et al. 2006. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J. Biol. Chem. 281:36560–36568 [DOI] [PubMed] [Google Scholar]
- 23. Kawai T., Akira S. 2008. Toll-like receptor and RIG-I-like receptor signaling. Ann. N. Y. Acad. Sci. 1143:1–20 [DOI] [PubMed] [Google Scholar]
- 24. Kerr P., McFadden G. 2002. Immune responses to myxoma virus. Viral Immunol. 15:229–246 [DOI] [PubMed] [Google Scholar]
- 25. Kono H., et al. 2001. Diphenyleneiodonium sulfate, an NADPH oxidase inhibitor, prevents early alcohol-induced liver injury in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G1005–G1012 [DOI] [PubMed] [Google Scholar]
- 26. Lamkanfi M. 2011. Emerging inflammasome effector mechanisms. Nat. Rev. Immunol. 11:213–220 [DOI] [PubMed] [Google Scholar]
- 27. Liu J., Wennier S., McFadden G. 2010. The immunoregulatory properties of oncolytic myxoma virus and their implications in therapeutics. Microbes Infect. 12:1144–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maitra R., et al. 2009. Endosomal damage and TLR2 mediated inflammasome activation by alkane particles in the generation of aseptic osteolysis. Mol. Immunol. 47:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martinez J., Huang X., Yang Y. 2010. Toll-like receptor 8-mediated activation of murine plasmacytoid dendritic cells by vaccinia viral DNA. Proc. Natl. Acad. Sci. U. S. A. 107:6442–6447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martinon F., Mayor A., Tschopp J. 2009. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27:229–265 [DOI] [PubMed] [Google Scholar]
- 31. Muruve D. A., et al. 2008. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452:103–107 [DOI] [PubMed] [Google Scholar]
- 32. Netea M. G., et al. 2009. Differential requirement for the activation of the inflammasome for processing and release of IL-1β in monocytes and macrophages. Blood 113:2324–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Poeck H., et al. 2010. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nat. Immunol. 11:63–69 [DOI] [PubMed] [Google Scholar]
- 34. Rahman M. M., McFadden G. 2011. Modulation of NF-κB signaling by microbial pathogens. Nat. Rev. Microbiol. 9:291–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rahman M. M., Mohamed M. R., Kim M., Smallwood S., McFadden G. 2009. Co-regulation of NF-κB and inflammasome-mediated inflammatory responses by myxoma virus pyrin domain-containing protein M013. PLoS Pathog. 5:e1000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rassa J. C., Meyers J. L., Zhang Y., Kudaravalli R., Ross S. R. 2002. Murine retroviruses activate B cells via interaction with toll-like receptor 4. Proc. Natl. Acad. Sci. U. S. A. 99:2281–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rathinam V. A., et al. 2010. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11:395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roberts T. L., et al. 2009. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323:1057–1060 [DOI] [PubMed] [Google Scholar]
- 39. Samuelsson C., et al. 2008. Survival of lethal poxvirus infection in mice depends on TLR9, and therapeutic vaccination provides protection. J. Clin. Invest. 118:1776–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schroder K., Tschopp J. 2010. The inflammasomes. Cell 140:821–832 [DOI] [PubMed] [Google Scholar]
- 41. Schroder K., Zhou R., Tschopp J. 2010. The NLRP3 inflammasome: a sensor for metabolic danger? Science 327:296–300 [DOI] [PubMed] [Google Scholar]
- 42. Seet B. T., et al. 2003. Poxviruses and immune evasion. Annu. Rev. Immunol. 21:377–423 [DOI] [PubMed] [Google Scholar]
- 43. Smallwood S. E., Rahman M. M., Smith D. W., McFadden G. 2010. Myxoma virus: propagation, purification, quantification, and storage. Curr. Protoc. Microbiol. Chapter 14:Unit 14A1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stanford M. M., Werden S. J., McFadden G. 2007. Myxoma virus in the European rabbit: interactions between the virus and its susceptible host. Vet. Res. 38:299–318 [DOI] [PubMed] [Google Scholar]
- 45. Stehlik C. 2007. The PYRIN domain in signal transduction. Curr. Protein Pept. Sci. 8:293–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Takaoka A., et al. 2007. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448:501–505 [DOI] [PubMed] [Google Scholar]
- 47. Thomas P. G., et al. 2009. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 30:566–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tschopp J., Schroder K. 2010. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 10:210–215 [DOI] [PubMed] [Google Scholar]
- 49. Unterholzner L., et al. 2010. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 11:997–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vilaysane A., Muruve D. A. 2009. The innate immune response to DNA. Semin. Immunol. 21:208–214 [DOI] [PubMed] [Google Scholar]
- 51. Wang F., et al. 2008. RIG-I mediates the co-induction of tumor necrosis factor and type I interferon elicited by myxoma virus in primary human macrophages. PLoS Pathog. 4:e1000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wilkins C., Gale M., Jr 2010. Recognition of viruses by cytoplasmic sensors. Curr. Opin. Immunol. 22:41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhou R., Tardivel A., Thorens B., Choi I., Tschopp J. 2010. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 11:136–140 [DOI] [PubMed] [Google Scholar]
- 54. Zhu J., Martinez J., Huang X., Yang Y. 2007. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-beta. Blood 109:619–625 [DOI] [PMC free article] [PubMed] [Google Scholar]