Abstract
In cell culture experiments, phosphorylation appears to be a critical regulator of the herpes simplex virus 1 (HSV-1) immediate-early (IE) protein, ICP0, which is an E3 ubiquitin ligase that transactivates viral gene expression. Three major regions of phosphorylation in ICP0 (amino acids 224 to 232, 365 to 371, and 508 to 518) have been identified, and mutant viruses that block phosphorylation sites within each region (termed Phos 1, 2, and 3, respectively) have been constructed. Previous studies indicated that replication of Phos 1 is significantly reduced compared to that of wild-type virus in cell culture (C. Boutell, et al., J. Virol. 82:10647–10656, 2008). To determine the effects these phosphorylation site mutations have on the viral life cycle in vivo, mice were ocularly infected with wild-type HSV-1, the Phos mutants, or their marker rescue counterparts. Subsequently, viral replication, establishment of latency, and viral explant-induced reactivation of these viruses were examined. Relative to wild-type virus, Phos 1 eye titers were reduced as much as 7- and 18-fold on days 1 and 5 postinfection, respectively. Phos 2 eye titers showed a decrease of 6-fold on day 1 postinfection. Titers of Phos 1 and 2 trigeminal ganglia were reduced as much as 16- and 20-fold, respectively, on day 5 postinfection. Additionally, the reactivation efficiencies of Phos 1 and 2 were impaired relative to wild-type HSV-1, although both viruses established wild-type levels of latency in vivo. The acute replication, latency, and reactivation phenotypes of Phos 3 were similar to those of wild-type HSV-1. We conclude from these studies that phosphorylation is likely a key modulator of ICP0's biological activities in a mouse ocular model of HSV-1 infection.
INTRODUCTION
A hallmark of herpes simplex virus 1 (HSV-1) infection is its capacity to establish both lytic and latent infections in its host. HSV-1 lytic infections, either primary infections or those due to reactivation, can lead to the development of oral-facial and ocular sores (56). Latent infection differs from lytic infection in that viral transcription is largely repressed, with the exception of the latency-associated transcripts (63); consequently, no infectious virus is produced (63). The first viral genes to be expressed during lytic infection are the immediate-early (IE) genes (37), which regulate viral and host gene expression and impair the host immune response. HSV-1 synthesizes five IE proteins, known as infected cell proteins (ICPs) 0, 4, 22, 27, and 47. Of these IE proteins, ICP0 is known to be required for efficient viral gene expression, replication, and reactivation from latency (9, 18, 31, 32, 39, 57, 64).
ICP0's ability to promote viral replication is closely associated with its RING-finger motif, which possesses E3 ubiquitin ligase activity (8, 29, 67). As a typical E3 ubiquitin ligase, ICP0 interacts with components of the ubiquitin-proteasome pathway (8, 27), adding chains of ubiquitin to cellular substrates (5, 7), marking them for degradation by the 26S proteasome complex. ICP0's E3 ubiquitin ligase activity enhances viral transcription (10, 11, 19, 26, 55) and disrupts the subnuclear structure known as nuclear domain 10 (ND10) (46, 47). ND10 and several of its constituent proteins, the promyelocytic leukemia protein (PML), Sp100, hDaxx, and ATRX, have been shown to limit HSV replication (21, 22, 52). Specifically, ICP0's E3 ubiquitin ligase activity promotes the disruption of ND10 by mediating the proteolysis (directly or indirectly) of SUMO-conjugated isoforms of PML and Sp100 (12, 20, 50) or by dissociating the ND10 components hDaxx and ATRX (45), stimulating viral transcription. In addition to affecting viral transcription, ICP0 assists HSV-1 in counteracting other intrinsic and innate host defenses that include chromatinization or repression of the viral genome (13, 14, 23, 24, 28) and activation and establishment of the interferon response (35, 40, 48, 49, 54).
ICP0 is known to be phosphorylated, a posttranslational modification that acts to modulate the activities of many viral and cellular proteins (1, 68). Cellular cyclin-dependent kinases (cdks) and the viral kinase UL13 have been reported to phosphorylate ICP0 or to be required for maximal levels of ICP0 phosphorylation in the infected cells (3, 15, 53). The activities of cdks appear to modulate the biological properties of ICP0, including its transactivating activity (15, 17). To directly determine the role phosphorylation plays in ICP0 functions, we mapped three phosphorylated regions in ICP0 and mutated phospho-acceptor sites in each of these regions, creating the mutants Phos 1, 2, and 3 (16). In transient expression assays, these phosphorylation site mutations affected several properties of ICP0, including its subcellular localization, E3 ubiquitin ligase activity, ability to dissociate proteins from ND10, and transactivation activity (6, 16). When these mutations were introduced into the HSV-1 genome, only Phos 1 was impaired for HSV-1 replication in cell culture (6). The purpose of the present study is to understand the impact these mutations have on HSV-1 acute replication, latency, and explant-induced reactivation in vivo. Consequently, the Phos mutants were tested in the mouse ocular model of HSV-1 latency and reactivation. Results from this study show that mutations in regions I and II of ICP0 affect acute replication and explant-induced reactivation, suggesting that phosphorylation regulates ICP0's biological functions in vivo.
MATERIALS AND METHODS
Cell lines and viruses.
Vero cells (African green monkey kidney cell line) and L7 cells (Vero cells that contain the ICP0 gene) were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with fetal bovine serum (FBS), 100 μg/ml penicillin, 100 U/ml streptomycin, and 2 mM l-glutamine as previously described (59, 60), except 5% fetal bovine serum (FBS) was used in our experiments. The wild-type HSV-1 strain KOS, 7134 (an ICP0-null mutant virus), Phos 1, 2, and 3, and their respective marker rescue viruses (Phos 1, 2, and 3 MR) were propagated and their titers determined as previously described (6, 11, 60).
Ocular infection of mice.
CD-1 outbred female mice (6 to 7 weeks old) were obtained from Charles Rivers Laboratories (Shrewsbury, MA) and cared for according to the Guide for the Care and Use of Laboratory Animals (51), and the protocol for their use was approved by University of Kansas Institutional Animal Care and Use Committee. Mice were infected as previously described (4). Briefly, mice were anesthetized by intraperitoneal injection with ketamine (100 mg/kg of body weight) and xylazine (6.6 mg/kg of body weight). Corneas were scarified with a 26-gauge needle and infected with one of 8 viruses (KOS, 7134, Phos 1, Phos 2, Phos 3, Phos 1 MR, Phos 2 MR, or Phos 3 MR) at 2 × 105 PFU of virus per eye in 3 μl medium. Mortality rates in our experiments (from day 0 to 30 days postinfection) for all viruses tested ranged from 0 to 19%.
Determination of viral titers in eyes and TG.
On indicated days 0 to 9 postinfection, eye swabs and trigeminal ganglion (TG) samples were collected. For eye swabs, a sterile cotton-tipped swab was moistened with Vero cell medium. Tear film was collected by swabbing each eye and then placing the swab in 500 μl growth medium. For TG samples, the mice were euthanized by CO2 asphyxiation, and the TG were removed and placed in microcentrifuge tubes with 500 μl 1% FBS growth medium and 100 μl of 1-mm-diameter glass beads. These samples were homogenized using a Mini-Beadbeater 8 (BioSpec, Bartlesville, OK). In all cases, the titers of the wild-type and MR viruses were determined on Vero cells, and those of mutant viruses were determined on L7 cells. Statistical analyses for acute viral titers were performed using Student's t test.
Latent viral genome loads in TG.
At 30 days postinfection, latently infected TG were collected, and DNA was isolated from each TG as reported by Halford et al. (32). PCR primers for the HSV-1 UL50 gene (61) and the mouse adipsin gene (65) were used to amplify viral DNA sequences and as a loading control for cellular DNA, respectively. Real-time PCR samples were performed in a total volume of 25 μl containing FastStart SYBR green master (Rox) (Roche, Indianapolis, IN) and primers (300 nM) in an ABI Prism 7500 real-time PCR system (Applied Biosystems, Foster City, CA). UL50 PCR samples contained 125 ng of DNA per reaction, adipsin PCR samples contained 10 ng of DNA per reaction, and all samples were analyzed in duplicate or triplicate. Standard curves for each PCR condition were carried out as described previously (65) to quantify the relative amount of viral DNA present in each sample relative to adipsin, using the 2−ΔΔCT method (44). One-way analysis of variance (ANOVA) was used to determine statistically significant differences in latent viral DNA loads.
Viral explant-induced reactivation studies.
Thirty days postinfection, latently infected mice were sacrificed, each trigeminal ganglion was collected and cut into 8 approximately equal pieces, and all 8 pieces were cultured in a well in 24-well plates (43), with each well containing Vero cells in 1.5 ml of Vero cell medium. The cultures were sampled daily (150 μl per sample) up to 16 days for the presence of infectious virus by cytopathic effect on Vero cell monolayers (for KOS and Phos 1, 2, and 3 MR) or L7 cell monolayers (for 7134 and Phos 1, 2, and 3). Vero cell medium (300 μl/well) was added to each explant culture every other day. After taking samples on day 10 postexplantation, cultures were heat shocked at 43°C for 3 h to provide an added stimulus for reactivation. Statistically significant differences for viral reactivation were determined using the Fisher's exact test.
RESULTS
Mutations in Phos 1 and 2 impair viral replication in the eyes.
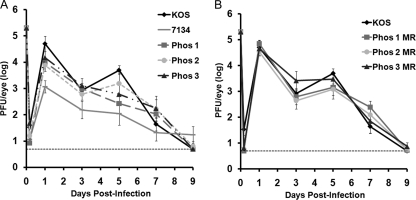
We have previously shown that cluster mutations in one of three phosphorylated regions of ICP0 (Fig. 1) altered several properties of the protein, and at least one ICP0 phosphorylation mutant (Phos 1) was impaired for viral replication in cell culture (6, 16). To determine whether the relative replication efficiencies of the Phos mutant viruses were altered in vivo, we used the mouse ocular model of HSV latency and reactivation. Initially, to examine acute replication at the periphery, CD-1 mice were infected with 2 × 105 PFU of virus per eye, and eye swabs were taken up to 9 days postinfection. Viral titers were determined by standard plaque assay. For wild-type HSV-1 (KOS), viral replication in the eyes was biphasic (Fig. 2), peaking on days 1 and 5 postinfection, which has been observed in other studies (30, 33, 58); this increase in ocular replication on day 5 is likely attributed to viral spread from the TG back to the eye (66). The replication of Phos 1 was reduced relative to that of KOS, on days 1 and 5 postinfection, with a 7-fold reduction (P = 0.047) on day 1 and an 18-fold reduction (P = 0.01) on day 5 (Fig. 2A). Interestingly, Phos 2 replication was only diminished 6-fold (P = 0.039) on day 1 postinfection. In contrast to the first two mutants, the replication of Phos 3 was comparable to that of KOS on all days tested (Fig. 2A). In the case of Phos 1 and 2, their replication was not as reduced as that of the ICP0 null mutant, 7134, which reached a 64-fold decrease on day 5 postinfection. As expected, the marker rescue (MR) viruses (Phos 1 MR, Phos 2 MR, and Phos 3 MR) replicated at levels similar to KOS (Fig. 2B). Collectively, these data indicate that the mutations in Phos 1 and 2 impair viral replication in the eyes.
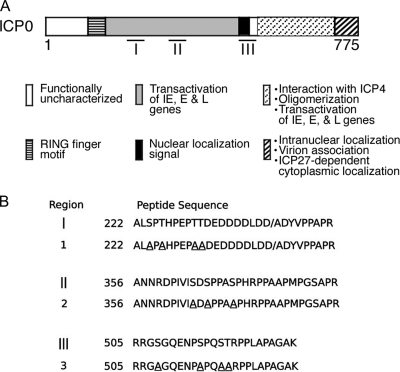
Fig. 1.
Location of ICP0 functional domains, phosphorylation sites, and phosphorylation site mutants. (A) The 775 amino acids of ICP0 and the locations of its major functional domains. The regions of ICP0 phosphorylation are indicated by bars below its structure. IE, E, and L are immediate-early, early and late, respectively. (B) Location of the mutated phosphorylation sites for each Phos mutant. The serines (S) or threonines (T) identified within regions I, II, and III replaced with alanines (A) in Phos 1, Phos 2, and Phos 3 are underlined. Phos 1 is mutated at S224, T226, T231, and T232. Phos 2 is mutated at S365, S367, and S371. Phos 3 is mutated at S508, S514, S517, and T518. This figure was modified from Davido et al. (16) and Boutell et al. (6).
Fig. 2.
Viral titers of eye swabs taken on days 1, 3, 5, 7, and 9 postinfection. Mice were infected with 2 × 105 PFU per eye, and tear film was collected from each eye on the aforementioned days. The amount of infectious virus collected in each sample was determined by plaque assay. The results shown are logarithmic means (n = 10 eyes per group), with the error bars indicating the standard error of the mean (SEM). The horizontal dotted lines represent the lower limit of detection. The experiments were performed simultaneously for all viral groups, but the results have been separated for ease of interpretation. (A) KOS, 7134, Phos 1, Phos 2, and Phos 3 titers. (B) KOS and Phos 1 MR, Phos 2 MR, and Phos 3 MR titers.
Phos 1 and 2 are diminished for acute replication in the TG.
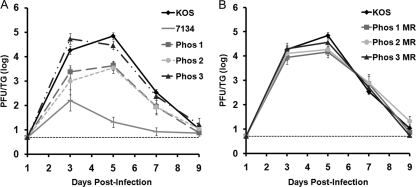
To determine if the Phos mutations affected productive infection in the sensory neurons, viral replication of the Phos mutant viruses was monitored in the TG of ocularly infected mice. For TG titers on days 3 and 5 postinfection, Phos 1 replication was diminished 7-fold (P = 0.01) and 16-fold (P = 0.00009), respectively, compared to KOS replication (Fig. 3A). In the case of Phos 2, viral titers were reduced 18-fold day 3 postinfection (P = 0.04) and 20-fold day 5 postinfection (P = 0.0002) (Fig. 3A). Decreases in the TG viral titers of Phos 1 and Phos 2, however, did not approach those of 7134, which were reduced as much as ∼2,000-fold on days 3 to 7 postinfection. The acute replication of Phos 3, however, showed no significant difference compared with the wild-type virus (Fig. 3A). Titers of the Phos MR viruses were comparable to those of KOS (Fig. 3B). Our results show that the Phos 1 and 2 mutations impair acute viral replication in the TG, whereas the Phos 3 mutation does not influence the viral growth in the TG.
Fig. 3.
Viral titers of TG collected on days 1, 3, 5, 7, and 9 postinfection. Mice were infected with 2 × 105 PFU per eye, and TG were collected on the indicated days. TG were then homogenized, and the amount of infectious virus present was determined by plaque assay. The results shown are logarithmic means (n = 10 TG per group), with the error bars indicating the SEM. The horizontal dotted lines represent the lower limit of detection. The experiments were performed simultaneously for all groups, but the results have been separated for ease of interpretation. (A) KOS, 7134, Phos 1, Phos 2, and Phos 3 titers. (B) KOS, Phos 1 MR, Phos 2 MR, and Phos 3 MR titers.
The Phos mutants are not impaired in establishing a latent infection.
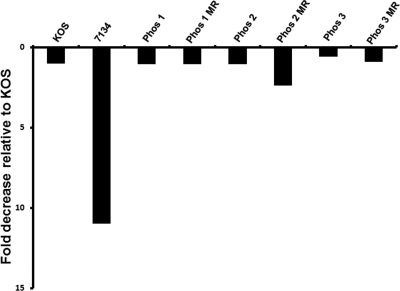
To quantify the amount of viral DNA present in latently infected neurons, TG were collected 30 days postinfection and assayed for the presence of HSV-1 DNA by real-time PCR. As shown in Fig. 4, the relative amounts of latent viral DNA present in TG were comparable to those of KOS for all of the Phos mutants. The ICP0 null mutant, 7134, was the only virus that showed a significant reduction (11-fold; P < 0.05) in viral DNA levels relative to KOS, and this reduction corresponded with previously published studies (31, 32). As expected, there were no appreciable differences in the latent viral genome loads among Phos 1 MR, Phos 2 MR, Phos 3 MR, and KOS.
Fig. 4.
Viral genome loads in latent TG. Mice were infected with 2 × 105 PFU per eye, and TG were collected 30 days postinfection, with the exception of one set of KOS samples taken 21 days postinfection. DNA was extracted from latent TG, and the amount of HSV-1 DNA present was determined by real-time PCR (n = 4 to 10 TG per group). The graph shown is the relative fold reduction in viral DNA levels for each virus compared to KOS by the 2−ΔΔCT method, and the viral DNA level for KOS was given the value of 1. Mock-infected samples were below the limit of detection (data not shown).
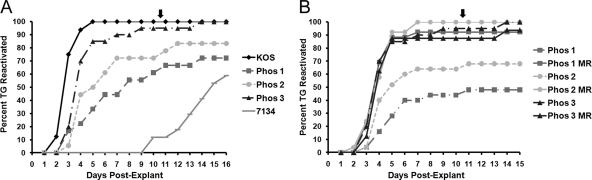
Phos 1 and 2 show reduced efficiencies of explant-induced reactivation from latency.
The efficiencies and kinetics of explant-induced reactivation from latency were established for all viral groups by collecting latently infected TG 30 days postinfection and culturing them ex vivo. Samples were examined daily for cytopathic effect, which indicates reactivation of virus from latent TG, and the total percentage of reactivating samples per virus was determined. In our initial set of studies, we examined the rates of reactivation for KOS, 7134, and the Phos mutants. KOS began to reactivate on day 3 postexplant (p.e.) at 66% and reached 100% reactivation by day 5 p.e. (Fig. 5A). Compared with KOS, Phos 1 and 2 reactivated at reduced rates of 33% (P = 0.0003) and 50% (P = 0.0009), respectively, on day 5 p.e. (Fig. 5A). Phos 3 reactivated efficiently, achieving 85% (P = 0.15) reactivation efficiency on day 5 p.e., which was comparable to that of KOS (Fig. 5A). The ICP0 null mutant, 7134, reactivated on day 10 p.e. and after heat shock reached its highest levels (58.8%) on day 16 p.e. (Fig. 5A). Notably, the reactivation phenotypes of Phos 1 and 2 were intermediate to those of KOS and 7134. In a second set of studies, we examined the reactivation efficiencies of the Phos mutants relative to their MR viruses. The MR viruses behaved as expected, rapidly reactivating from days 2 to 5 p.e., eventually reaching efficiencies of 87 to 92% by day 5 p.e. (Fig. 5B). In contrast, Phos 1 reached a peak efficiency of 48% by day 11 p.e. (P = 0.0002), and Phos 2 peaked at 68% by day 11 p.e. (P = 0.01) (Fig. 5B). The Phos 3 reactivation rate resembled its MR virus with a rate of 85% on day 5 p.e. and reached 100% at the end of the study (Fig. 5B). These explant-induced reactivation studies show that the frequencies and kinetics of reactivation are altered for two of the Phos mutants, with Phos 1 showing the greatest deficiency followed by Phos 2.
Fig. 5.
Virus explant-induced reactivation from latent TG. Mice were infected with 2 × 105 PFU per eye. On day 30 postinfection, TG were collected and explanted onto Vero cells. The time required for each virus to reactivate from the latent TG was determined by assaying the culture medium daily for the presence of infectious virus. Each time point represents the cumulative percentage of samples that reactivated (n = 19 or 20 TG per group). Two groups of experiments are shown. (A) KOS, 7134, and phosphorylation site mutants. (B) Phosphorylation site mutants and MR viruses. The arrows at the top of both graphs indicate that after day 10, samples were heat shocked at 43°C for 3 h.
DISCUSSION
In this study, we have shown that two ICP0 phosphorylation site mutants are impaired for acute replication and explant-induced reactivation using the mouse ocular model of HSV infection. Specifically, Phos 1 and 2 were impaired for acute ocular and neuronal replication and explant-induced reactivation from latency. Interestingly, all Phos mutants established latency at wild-type levels, even though the levels of Phos 1 and 2 acute replication in the TG were intermediate compared to those of wild-type HSV-1 and the ICP0 null mutant, 7134. Given that the degree of acute viral replication in the TG correlates with the establishment of latency (38, 39), the decreases in Phos 1 and 2 acute neuronal replication were, nonetheless, sufficient to allow these viruses to establish efficient latent infections. The life cycle of Phos 3 in mice mirrored wild-type HSV-1 in our experiments, indicating that phosphorylation of residues in region III of ICP0 (Fig. 1A) is not required for productive infection and explant-induced reactivation in mice. Of the Phos mutants examined, Phos 1 was the only mutant to show replication deficiencies in cell culture (6, 16) and in vivo, as demonstrated in this report. Notably, Phos 2 had no obvious viral growth defects in cell culture (6, 16); however, decreased levels of viral replication and explant-induced reactivation observed with Phos 2 only became apparent when tested in the mouse ocular model, highlighting the importance of characterizing our mutants in vivo. While we tested only one viral dose in this study, it is also possible that the replication, latency, and/or reactivation phenotypes of Phos 1, 2, and 3 may be greater or become evident when infecting mice with lower input viral doses (<2 × 105 PFU/eye). Overall, our results suggest that phosphorylation plays an important role in the biology of ICP0 to enhance HSV-1 acute replication in vivo and reactivation ex vivo. Studies are being conducted to identify the mechanisms responsible for the in vivo phenotypes of Phos 1 and Phos 2.
For Phos 1, its mutation lies in the proline-rich transactivation domain and is adjacent to the RING-finger domain of ICP0 (Fig. 1). We have previously shown that Phos 1 is impaired for ICP0's E3 ubiquitin ligase activity, dispersal of ND10-associated proteins, and transactivator function, in the absence of other viral factors, and has enhanced protein stability during viral infection (6). Thus, defects in Phos 1 autoubiquitination and its ubiquitination of other cellular target proteins are likely contributors to its reduced levels of acute replication and reactivation. The delay and reduced efficiency of explant-induced reactivation observed with Phos 1 could also be linked to alterations in viral chromatinization and apoptosis. ICP0 is known to enhance viral gene expression during reactivation, which is associated with a decrease in the chromatinization of the viral genome (14, 24). Indeed, unlike wild-type strains of HSV-1, a RING-finger mutant of ICP0, which is impaired for ICP0's E3 ubiquitin ligase activity, was unable to induce viral gene expression in quiescently infected cultures (25). Because Phos 1 is defective or diminished for ubiquitin conjugation, Phos 1 may not be as proficient as wild-type ICP0 in altering the structure of viral chromatin to promote viral transcription and replication. Lastly, ICP0 has been shown to ubiquitinate the tumor suppressor protein, p53, and limit apoptosis in virally infected cells upon DNA damage (7). Thus, Phos 1 may not efficiently mediate the degradation of p53 or other antiapoptotic targets during reactivation, potentially increasing apoptotic signaling, thereby reducing the levels and kinetics of reactivation. Clearly, the impairment of these activities does not impede Phos 1's ability to establish an efficient latent infection, which is similar to that of KOS.
The Phos 2 mutation, which is also located in a proline-rich transactivation domain of ICP0 (Fig. 1), has a minor defect in ocular shedding and is significantly defective in neuronal replication and explant-induced reactivation from latency. These results indicate that phosphorylated residues in region II of ICP0 are important for efficient viral replication, especially in sensory neurons. One potential explanation for Phos 2's attenuation in vivo is its ability to differentially affect the dissociation of cellular proteins from ND10. The Phos 2 mutant form of ICP0 is unable to dissociate PML from ND10 but is able to dissociate Sp100 from ND10 in a cell-type-dependent manner (6). Thus, the inability of Phos 2 to alter the subcellular localization of PML, which is a part of the host's antiviral response, could diminish its viral replication. Related to its ND10-disrupting activity, ICP0 has been described to contain a SUMO-interacting motif (SIM) in region II (5a). A SIM facilitate interactions with SUMO isoforms or SUMO-conjugated proteins (reviewed in references 34 and 36). A recent publication showed that phosphorylation of SIM domains in at least three cellular proteins, including PML, significantly enhances their interaction with SUMO isoforms (62). Thus, in the case of Phos 2, blocking phosphorylation in region II may impede this putative SUMO-binding activity, affecting its interaction with SUMO-conjugated proteins (such as PML) or specific SUMO isoforms. Lastly, the Phos 2 mutation lies within a region that contains Src homology 3 (SH3) binding motifs, which allow for interaction with a subset of Src kinase family members and other SH3-containing proteins (41, 42). These kinases and factors play important roles in cell signaling. It has been suggested that the interaction between these factors and ICP0 perturbs normal cell signaling. Thus, Phos 2 might interrupt these interactions in neurons, impairing acute replication and explant-induced reactivation.
Of the three Phos mutants, only mutations in Phos 3 did not influence the replication of HSV-1 in vivo, similar to our previously published results with cell culture (6, 16). While mutations in Phos 3 appear to affect its subcellular localization and transactivating activity when expressed in transient-transfection assays (16), our results indicate that the phenotypes observed with Phos 3 in cell-based assays do not impact acute infection and latency in vivo and explant-induced reactivation. How phosphorylation of region III in ICP0 contributes to HSV replication remains to be determined.
It has been demonstrated that the phosphorylation state of ICP0 is altered during the course of the viral replication cycle (1, 2, 15, 53, 68), suggesting that differential phosphorylation of ICP0 might activate or inhibit one or more specific functions of this key viral regulatory protein. Consequently, the identity of specific phosphorylation sites in regions I and II of ICP0, the potential kinases that phosphorylate these sites, and their temporal regulation and roles in ICP0's functions will likely elucidate new mechanisms in the switch between the lytic and latent infections of HSV-1.
ACKNOWLEDGMENTS
This work was supported in part by Public Health Service grant R01AI72357 from the National Institute of Allergy and Infectious Diseases (D.J.D.) and Public Health Service grant R01CA20260 from the National Cancer Institute (for P.A.S.).
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank members of the Davido lab, former members of the Schaffer lab, and Steve Benedict for helpful comments and suggestions.
This article was written in honor of Priscilla Schaffer, who passed away late in 2009. Priscilla was an excellent virologist and a caring, dedicated mentor. Her enthusiasm for biology and scientific contributions to the field of herpesvirology will be greatly missed.
Footnotes
Published ahead of print on 21 September 2011.
REFERENCES
- 1. Ackermann M., Braun D. K., Pereira L., Roizman B. 1984. Characterization of herpes simplex virus 1 alpha proteins 0, 4, and 27 with monoclonal antibodies. J. Virol. 52:108–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Advani S. J., Hagglund R., Weichselbaum R. R., Roizman B. 2001. Posttranslational processing of infected cell proteins 0 and 4 of herpes simplex virus 1 is sequential and reflects the subcellular compartment in which the proteins localize. J. Virol. 75:7904–7912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Advani S. J., Weichselbaum R. R., Roizman B. 2000. The role of cdc2 in the expression of herpes simplex virus genes. Proc. Natl. Acad. Sci. U. S. A. 97:10996–11001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Balliet J. W., Schaffer P. A. 2006. Point mutations in herpes simplex virus type 1 oriL, but not in oriS, reduce pathogenesis during acute infection of mice and impair reactivation from latency. J. Virol. 80:440–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boutell C., Canning M., Orr A., Everett R. D. 2005. Reciprocal activities between herpes simplex virus type 1 regulatory protein ICP0, a ubiquitin E3 ligase, and ubiquitin-specific protease USP7. J. Virol. 79:12342–12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a. Boutell C., Cuchet-Lourenço D., Vanni E., Orr A., Glass M., McFarlane S., Everett R. D. 15 September 2011. A viral ubiquitin ligase has substrate preferential SUMO targeted ubiquitin ligase activity that counteracts intrinsic antiviral defence. PLoS Pathog. 7:e1002245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boutell C., et al. 2008. Herpes simplex virus type 1 ICP0 phosphorylation mutants impair the E3 ubiquitin ligase activity of ICP0 in a cell type-dependent manner. J. Virol. 82:10647–10656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boutell C., Everett R. D. 2003. The herpes simplex virus type 1 (HSV-1) regulatory protein ICP0 interacts with and ubiquitinates p53. J. Biol. Chem. 278:36596–36602 [DOI] [PubMed] [Google Scholar]
- 8. Boutell C., Sadis S., Everett R. D. 2002. Herpes simplex virus type 1 immediate-early protein ICP0 and is isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 76:841–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cai W., et al. 1993. The herpes simplex virus type 1 regulatory protein ICP0 enhances virus replication during acute infection and reactivation from latency. J. Virol. 67:7501–7512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cai W., Schaffer P. A. 1992. Herpes simplex virus type 1 ICP0 regulates expression of immediate-early, early, and late genes in productively infected cells. J. Virol. 66:2904–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cai W. Z., Schaffer P. A. 1989. Herpes simplex virus type 1 ICP0 plays a critical role in the de novo synthesis of infectious virus following transfection of viral DNA. J. Virol. 63:4579–4589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chelbi-Alix M. K., de The H. 1999. Herpes virus induced proteasome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene 18:935–941 [DOI] [PubMed] [Google Scholar]
- 13. Cliffe A. R., Knipe D. M. 2008. Herpes simplex virus ICP0 promotes both histone removal and acetylation on viral DNA during lytic infection. J. Virol. 82:12030–12038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coleman H. M., et al. 2008. Histone modifications associated with herpes simplex virus type 1 genomes during quiescence and following ICP0-mediated de-repression. J. Gen. Virol. 89:68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davido D. J., Leib D. A., Schaffer P. A. 2002. The cyclin-dependent kinase inhibitor roscovitine inhibits the transactivating activity and alters the posttranslational modification of herpes simplex virus type 1 ICP0. J. Virol. 76:1077–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davido D. J., von Zagorski W. F., Lane W. S., Schaffer P. A. 2005. Phosphorylation site mutations affect herpes simplex virus type 1 ICP0 function. J. Virol. 79:1232–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davido D. J., Von Zagorski W. F., Maul G. G., Schaffer P. A. 2003. The differential requirement for cyclin-dependent kinase activities distinguishes two functions of herpes simplex virus type 1 ICP0. J. Virol. 77:12603–12616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Everett R. D. 1989. Construction and characterization of herpes simplex virus type 1 mutants with defined lesions in immediate early gene 1. J. Gen. Virol. 70:1185–1202 [DOI] [PubMed] [Google Scholar]
- 19. Everett R. D. 1984. Trans activation of transcription by herpes virus products: requirement for two HSV-1 immediate-early polypeptides for maximum activity. EMBO J. 3:3135–3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Everett R. D., et al. 1998. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J. Virol. 72:6581–6591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Everett R. D., Parada C., Gripon P., Sirma H., Orr A. 2008. Replication of ICP0-null mutant herpes simplex virus type 1 is restricted by both PML and Sp100. J. Virol. 82:2661–2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Everett R. D., et al. 2006. PML contributes to a cellular mechanism of repression of herpes simplex virus type 1 infection that is inactivated by ICP0. J. Virol. 80:7995–8005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferenczy M. W., DeLuca N. A. 2009. Epigenetic modulation of gene expression from quiescent herpes simplex virus genomes. J. Virol. 83:8514–8524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferenczy M. W., DeLuca N. A. 2011. Reversal of heterochromatic silencing of quiescent herpes simplex virus type 1 by ICP0. J. Virol. 85:3424–3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferenczy M. W., Ranayhossaini D. J., Deluca N. A. 2011. Activities of ICP0 involved in the reversal of silencing of quiescent herpes simplex virus 1. J. Virol. 85:4993–5002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gelman I. H., Silverstein S. 1985. Identification of immediate early genes from herpes simplex virus that transactivate the virus thymidine kinase gene. Proc. Natl. Acad. Sci. U. S. A. 82:5265–5269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gu H., Roizman B. 2003. The degradation of promyelocytic leukemia and Sp100 proteins by herpes simplex virus 1 is mediated by the ubiquitin-conjugating enzyme UbcH5a. Proc. Natl. Acad. Sci. U. S. A. 100:8963–8968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gu H., Roizman B. 2007. Herpes simplex virus-infected cell protein 0 blocks the silencing of viral DNA by dissociating histone deacetylases from the CoREST-REST complex. Proc. Natl. Acad. Sci. U. S. A. 104:17134–17139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hagglund R., Van Sant C., Lopez P., Roizman B. 2002. Herpes simplex virus 1-infected cell protein 0 contains two E3 ubiquitin ligase sites specific for different E2 ubiquitin-conjugating enzymes. Proc. Natl. Acad. Sci. U. S. A. 99:631–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Halford W. P., Balliet J. W., Gebhardt B. M. 2004. Re-evaluating natural resistance to herpes simplex virus type 1. J. Virol. 78:10086–10095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Halford W. P., Schaffer P. A. 2001. ICP0 is required for efficient reactivation of herpes simplex virus type 1 from neuronal latency. J. Virol. 75:3240–3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Halford W. P., Schaffer P. A. 2000. Optimized viral dose and transient immunosuppression enable herpes simplex virus ICP0-null mutants to establish wild-type levels of latency in vivo. J. Virol. 74:5957–5967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Halford W. P., et al. 2006. ICP0 antagonizes Stat 1-dependent repression of herpes simplex virus: implications for the regulation of viral latency. Virol. J. 3:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hannich J. T., et al. 2005. Defining the SUMO-modified proteome by multiple approaches in Saccharomyces cerevisiae. J. Biol. Chem. 280:4102–4110 [DOI] [PubMed] [Google Scholar]
- 35. Harle P., Sainz B., Jr., Carr D. J., Halford W. P. 2002. The immediate-early protein, ICP0, is essential for the resistance of herpes simplex virus to interferon-alpha/beta. Virology 293:295–304 [DOI] [PubMed] [Google Scholar]
- 36. Hecker C. M., Rabiller M., Haglund K., Bayer P., Dikic I. 2006. Specification of SUMO1- and SUMO2-interacting motifs. J. Biol. Chem. 281:16117–16127 [DOI] [PubMed] [Google Scholar]
- 37. Honess R. W., Roizman B. 1974. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol. 14:8–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Katz J. P., Bodin E. T., Coen D. M. 1990. Quantitative polymerase chain reaction analysis of herpes simplex virus DNA in ganglia of mice infected with replication-incompetent mutants. J. Virol. 64:4288–4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leib D. A., et al. 1989. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J. Virol. 63:759–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leib D. A., et al. 1999. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 189:663–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liang Y., Kurakin A., Roizman B. 2005. Herpes simplex virus 1 infected cell protein 0 forms a complex with CIN85 and Cbl and mediates the degradation of EGF receptor from cell surfaces. Proc. Natl. Acad. Sci. U. S. A. 102:5838–5843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liang Y., Roizman B. 2006. State and role of SRC family kinases in replication of herpes simplex virus 1. J. Virol. 80:3349–3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Link M. A., Schaffer P. A. 2007. Herpes simplex virus type 1 C-terminal variants of the origin binding protein (OBP), OBPC-1 and OBPC-2, cooperatively regulate viral DNA levels in vitro, and OBPC-2 affects mortality in mice. J. Virol. 81:10699–10711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 45. Lukashchuk V., Everett R. D. 2010. Regulation of ICP0-null mutant herpes simplex virus type 1 infection by ND10 components ATRX and hDaxx. J. Virol. 84:4026–4040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maul G. G., Guldner H. H., Spivack J. G. 1993. Modification of discrete nuclear domains induced by herpes simplex virus type 1 immediate early gene 1 product (ICP0). J. Gen. Virol. 74:2679–2690 [DOI] [PubMed] [Google Scholar]
- 47. Maul G. G., Ishov A. M., Everett R. D. 1996. Nuclear domain 10 as preexisting potential replication start sites of herpes simplex virus type-1. Virology 217:67–75 [DOI] [PubMed] [Google Scholar]
- 48. Melroe G. T., DeLuca N. A., Knipe D. M. 2004. Herpes simplex virus 1 has multiple mechanisms for blocking virus-induced interferon production. J. Virol. 78:8411–8420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mossman K. L., Saffran H. A., Smiley J. R. 2000. Herpes simplex virus ICP0 mutants are hypersensitive to interferon. J. Virol. 74:2052–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Muller S., Dejean A. 1999. Viral immediate-early proteins abrogate the modification by SUMO-1 of PML and Sp100 proteins, correlating with nuclear body disruption. J. Virol. 73:5137–5143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. National Research Council 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC [Google Scholar]
- 52. Negorev D. G., Vladimirova O. V., Ivanov A., Rauscher F., III, Maul G. G. 2006. Differential role of Sp100 isoforms in interferon-mediated repression of herpes simplex virus type 1 immediate-early protein expression. J. Virol. 80:8019–8029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ogle W. O., Ng T. I., Carter K. L., Roizman B. 1997. The UL13 protein kinase and the infected cell type are determinants of posttranslational modification of ICP0. Virology 235:406–413 [DOI] [PubMed] [Google Scholar]
- 54. Paladino P., Collins S. E., Mossman K. L. 2010. Cellular localization of the herpes simplex virus ICP0 protein dictates its ability to block IRF3-mediated innate immune responses. PLoS One 5:e10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Perry L. J., Rixon F. J., Everett R. D., Frame M. C., McGeoch D. J. 1986. Characterization of the IE110 gene of herpes simplex virus type 1. J. Gen. Virol. 67:2365–2380 [DOI] [PubMed] [Google Scholar]
- 56. Roizman R., Knipe D. M., Whitley R. J. 2007. Herpes simplex viruses, p. 2501–2601 In Knipe D. M., Howley P. M. (ed.), Fields virology, vol. 2 Lippincott Williams & Wilkins, New York, NY [Google Scholar]
- 57. Sacks W. R., Schaffer P. A. 1987. Deletion mutants in the gene encoding the herpes simplex virus type 1 immediate-early protein ICP0 exhibit impaired growth in cell culture. J. Virol. 61:829–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sainz B., Jr., Halford W. P. 2002. Alpha/beta interferon and gamma interferon synergize to inhibit the replication of herpes simplex virus type 1. J. Virol. 76:11541–11550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Samaniego L. A., Wu N., DeLuca N. A. 1997. The herpes simplex virus immediate-early protein ICP0 affects transcription from the viral genome and infected-cell survival in the absence of ICP4 and ICP27. J. Virol. 71:4614–4625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schaffer P. A., Aron G. M., Biswal N., Benyesh-Melnick M. 1973. Temperature-sensitive mutants of herpes simplex virus type 1: isolation, complementation and partial characterization. Virology 52:57–71 [DOI] [PubMed] [Google Scholar]
- 61. Schrimpf J. E., Tu E. M., Wang H., Wong Y. M., Morrison L. A. 2011. B7 costimulation molecules encoded by replication-defective, vhs-deficient HSV-1 improve vaccine-induced protection against corneal disease PLoS One 6:e22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stehmeier P., Muller S. 2009. Phospho-regulated SUMO interaction modules connect the SUMO system to CK2 signaling. Mol. Cell 33:400–409 [DOI] [PubMed] [Google Scholar]
- 63. Stevens J. G., Wagner E. K., Devi-Rao G. B., Cook M. L., Feldman L. T. 1987. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science 235:1056–1059 [DOI] [PubMed] [Google Scholar]
- 64. Stow N. D., Stow E. C. 1986. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J. Gen. Virol. 67:2571–2585 [DOI] [PubMed] [Google Scholar]
- 65. Strand S. S., Leib D. A. 2004. Role of the VP16-binding domain of vhs in viral growth, host shutoff activity, and pathogenesis. J. Virol. 78:13562–13572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Summers B. M., Margolis T. P., Leib D. A. 2001. Herpes simplex virus type 1 corneal infection results in periocular disease by zosteriform spread. J. Virol. 75:5069–5075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Van Sant C., Hagglund R., Lopez P., Roizman B. 2001. The infected cell protein 0 of herpes simplex virus 1 dynamically interacts with proteasomes, binds and activates the cdc34 E2 ubiquitin-conjugating enzyme, and possesses in vitro E3 ubiquitin ligase activity. Proc. Natl. Acad. Sci. U. S. A. 98:8815–8820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wilcox K. W., Kohn A., Sklyanskaya E., Roizman B. 1980. Herpes simplex virus phosphoproteins. I. Phosphate cycles on and off some viral polypeptides and can alter their affinity for DNA. J. Virol. 33:167–182 [DOI] [PMC free article] [PubMed] [Google Scholar]