Abstract
HIV-1 viral protein R (Vpr) from laboratory-adapted virus strains activates the DNA damage/stress sensor ATR kinase and induces cell cycle arrest at the G2/M phase through a process that requires Vpr to engage the DDB1-CUL4A (VprBP/DCAF-1) E3 ligase complex. Activation of this DNA damage/stress checkpoint in G2 by Vpr was shown to modulate NKG2D-dependent NK cell effector functions via enhancing expression of NKG2D ligands, notably ULBP2. However, it is unknown whether Vpr from HIV-1 primary isolates (groups M, N, O, and P) could modulate NKG2D-mediated cytotoxic functions of NK cells. Here, we report that Vpr from most HIV-1 primary isolates can upregulate ULBP2 expression and induce NKG2D-dependent NK cell killing. Importantly, these activities were always accompanied by an active G2 cell cycle arrest function. Interestingly, Vpr variants from group P and a clade D isolate of group M were defective at enhancing NKG2D-mediated NK cell lysis owing to their inability to augment ULBP2 expression. However, distinct mechanisms were responsible for their failure to do so. While Vpr from group P was deficient in its ability to engage the DDB1-CUL4A (VprBP/DCAF-1) E3 ligase complex, the Vpr variant from group D was unable to properly localize to the nucleus, underlining the importance of these biological properties in Vpr function. In conclusion, the ability of Vpr from HIV-1 primary isolates to regulate NK cell effector function underscores the importance of this HIV-1 accessory protein in the modulation of the host's innate immune responses.
INTRODUCTION
Human immunodeficiency virus type 1 (HIV-1) is the causative agent of AIDS and responsible for nearly 33 million infections worldwide according to the latest Joint United Nations Programme on HIV/AIDS (UNAIDS) estimate (27). HIV-1 isolates are divided into four groups: M (main), N (non-M non-O), O (outlier), and P (pending), with the pandemic group M accounting for about 95% of the world's HIV/AIDS cases (reviewed in reference 26). Isolates of the nonpandemic groups N, O, and P account for the remaining HIV infections. Heterogeneity in the structural genes env and gag classifies group M viruses into at least nine clades (identified by letters A through K) and numerous subclades.
The HIV-1 RNA genome, aside from encoding the structural proteins, also codes for four accessory proteins, one of which is viral protein R (Vpr). The 96-amino-acid Vpr is highly conserved across primate lentiviruses and could be found in vivo as an intravirion, an intracellular, and a soluble molecule. The multifunctional Vpr is thought to assist in viral transcription, to promote infection of nondividing cells, and to be involved in cell apoptosis, although it is perhaps best known for its ability to induce a G2 cell cycle arrest (14). Vpr-mediated G2 block relies on the execution of a stepwise process thought to require engagement of Vpr to the host DDB1-CUL4A (VprBP; also designated DCAF-1) E3 ubiquitin ligase complex, degradation of an as yet unknown chromatin-bound cellular target(s), and activation of the DNA damage/stress ataxia telangiectasia-mutated and Rad3-mutated (ATR)-mediated pathway (3, 5, 18). ATR is a DNA damage sensor kinase that is involved in activating the G2 checkpoint in response to genotoxic stress conditions, preventing cell entry into mitosis (1). Vpr interaction with components of the E3 ligase, especially the substrate specificity receptor VprBP, is crucial to its arrest activity, as Vpr mutants, such as Vpr Q65R, which are unable to bind VprBP fail to induce a G2 block (3, 18). That being said, it is important to recognize in this context that, although Vpr and VprBP association is essential, binding to VprBP alone is insufficient for Vpr to activate ATR since some Vpr mutants, such as Vpr R80A, display a G2 arrest-defective phenotype despite their efficient engagement to VprBP (3, 18). Activation of the ATR signaling pathway generally leads to phosphorylation and/or recruitment of several checkpoint mediators, including H2AX (histone 2A, variant X) and 53BP-1 (p53 binding protein 1) as well as to the formation of DNA damage foci containing these molecules (15, 30). On this note, we previously demonstrated that Vpr forms distinct nuclear punctuate structures which partially overlap with γ-H2AX (phosphorylated H2AX) and 53BP-1 and that formation of these foci is critical to the induction of G2 arrest (5).
Vpr-mediated cell cycle arrest is widely believed to be preserved in all primary lentiviruses during viral evolution (22), implying that this Vpr function is potentially significant for the overall pathogenesis of HIV in vivo. Since the relation between Vpr-induced G2 block and increased viral production has been implied in many studies (9, 12), the abnormal accumulation of cells arrested at G2 in HIV-infected patients (31) may seem paradoxically beneficial to the virus. On the other hand, recent data from our group and others revealed that HIV-1 infection of primary CD4+ T lymphocytes with a laboratory-adapted virus enhanced expression of specific ligands of the NKG2D (natural killer [NK] group 2, member D) receptor on T cells in a Vpr-dependent manner (24, 28). These studies further showed that expression of Vpr alone was sufficient to induce an upregulation of NKG2D ligands. Indeed, ULBP-2, a member of the human cytomegalovirus UL16 binding protein (ULBP) family, was most predominantly upregulated on Vpr-expressing T cells, and such an increase promoted T cell killing by NKG2D receptor-expressing NK cells (24, 28). Intriguingly, the enhancement of ULBP2 expression requires Vpr to engage the DDB1-CUL4A (VprBP/DCAF-1) E3 ligase complex and activate the ATR pathway, the exact prerequisite for Vpr-induced G2 arrest. Indeed, the R80A and Q65R Vpr mutants, which are defective for G2 arrest, are also attenuated for ULBP2 upregulation (24). Taken together, the data highlight the essentiality of ATR activation for both activities mediated by Vpr.
Given that NKG2D ligands have been shown to be expressed on infected cells of HIV-infected patients (10), the current study aimed to dissect whether Vpr from HIV-1 primary isolates could modulate NK cell cytotoxic function through the NKG2D pathway and whether there was an association between ULBP2 upregulation and G2 arrest in the context of primary Vpr. Herein, we document that Vpr from most HIV-1 groups can alter NKG2D-mediated NK cell cytotoxic functions through regulating ULBP2 expression on target cells. Coincidentally, we also find that the G2 arrest-inducing activity is preserved only in primary Vpr variants (interchangeably referred to in the text as primary Vprs) that can modulate the NKG2D pathway in NK cells, strengthening the link with the DNA damage sensor ATR-induced pathway. Overall, our data indicate that the ability of Vpr to modulate NK cell activation extends beyond laboratory-made HIV-1, lending further support to its role as an immunomodulator of the host's innate immune responses.
MATERIALS AND METHODS
Cell lines and isolation of primary cells.
CEM.NKR T, HEK293T, and HeLa cells were cultured as described previously (24). Natural killer cells and CD4+ T cells were purified from peripheral blood mononuclear cells (PBMC) by negative selection using appropriate immunomagnetic beads (Stem Cell Technologies) and cultured as detailed elsewhere (24). PBMC were obtained from individuals with no documented HIV or hepatitis C virus infections. The study was approved by the IRCM Research Ethic Review Board. Samples were collected after written informed consent had been obtained.
Plasmids and antibodies.
All primary vpr alleles were cloned into a green fluorescent protein (GFP)-marked pWPI lentiviral vector (generously provided by D. Trono, School of Life Sciences, Swiss Institute of Technology, Lausanne, Switzerland) as described previously (3). psvCMV-HA-tagged Vpr and pCMV-myc-tagged VprBP were generated as detailed previously (3, 29).
Mouse anti-human ULBP2 monoclonal antibody (MAb) was obtained from R&D Systems (Minneapolis, MN). Anti-Vpr MAb (clone 8D1) was a generous gift from Y. Ishizaka (National Center for Global Health and Medicine, Tokyo, Japan). Rabbit anti-Vpr polyclonal antibody (pAb) was prepared as described previously (17). The mouse anti-hemagglutinin (anti-HA) tag and anti-Myc tag MAbs (clones 12CA5 and 9E10, respectively) were produced from hybridomas (ATCC). Rabbit pAbs against DDB1 and actin were purchased from Santa Cruz Biotechnology. Mouse anti-phosphoS139-H2AX MAb (clone JBW301) was obtained from Upstate Biotechnology. All fluorochrome-conjugated secondary antibodies were from Molecular Probes (Invitrogen).
Viral protein R from HIV-1 primary isolates and laboratory-adapted virus.
Nine primary vpr alleles spanning four HIV-1 groups (M, N, O, and P) were selected. For pandemic group M, two alleles were from clade B (termed M/B-13C and M/B-18E alleles) (21), one from clade C (M/C allele), two from clade D (M/D-lo and M/D-sh alleles), and one from clade H (M/H allele). For nonpandemic groups N, O, and P, one vpr allele was selected for each group. Except for the M/D-sh, N, O, and P alleles, all alleles were amplified by PCR from the respective molecular clones. Three of these clones (94UG114 for the Vpr M/D-lo allele, 98IN022 for the Vpr M/C allele, and 90CF056 for the Vpr M/H allele) were obtained through the NIH AIDS Research and Reference Reagent Program from Beatrice Hahn and Feng Gao (11). The GenBank accession numbers for these three alleles are U88824, AF286232, and AF005496, respectively. For this study, a truncated version of Vpr M/D-lo, termed Vpr D-lo-12, was also generated by PCR from Vpr M/D-lo. The Vpr M/D-sh and N, O, and P alleles were synthetically made (GenScript) using published prototype sequences for the respective primary isolates (GenBank accession numbers AB485650, AJ006022, AB485667, and GQ328744, respectively). In all experiments, the activity of Vpr variants from primary isolates was compared to that of laboratory-adapted HxBRU (HxB) Vpr (7).
Production of Vpr-expressing lentiviral vectors, transduction of T cells, and analysis of ULBP2 and Vpr expression.
Production and titration of pWPI lentiviral vectors were performed as described previously (3).
CEM.NKR T cells and CD4+ T cells were transduced with GFP-marked pWPI.Vpr (HxB or primary Vpr) or pWPI (no Vpr) and analyzed 48 h later as described previously (24). ULBP2 cell surface expression and Vpr intracellular levels were analyzed on GFP+ cells by flow cytometry as previously reported (24).
Cell cycle analysis.
Vpr-expressing GFP+ T cells were sorted directly into staining buffer (0.1% sodium citrate, 0.3% NP-40, 0.05 mg/ml propidium iodide, and 0.02 mg/ml RNase) using an Influx cell sorter (BD Biosciences) and analyzed for DNA content by flow cytometry. Alternatively, cell cycle analysis was performed on HEK293T cells transfected with psvCMV-HA-tagged Vpr (HxB, M/D-lo, M/D-sh, and M/D-lo-12) as described previously (3, 29). The ModFit mathematical model (Verity Software House) was used to enumerate proportions of cells in G1 and G2 phases.
Natural killer cell-mediated cell lysis.
Sorted GFP+ T cells were used as targets in a standard chromium release assay (6), while purified unactivated NK cells from healthy individuals were used as effector cells. Percent specific lysis was determined according to the formula [(experimental lysis − spontaneous lysis)/(total lysis − spontaneous lysis)] × 100. Data analysis was done using GraphPad (San Diego, CA) Prism 4.0 software.
Immunoprecipitation and immunoblotting.
HEK293T cells were cotransfected for 48 h using a standard calcium phosphate method with (i) pCMV plasmid expressing myc-tagged VprBP (or, as a control, pCMV-myc) and (ii) pWPI.Vpr lentiviral vector (or, as a control, pWPI). Immunoprecipitation was performed on total cell lysates using anti-myc antibody followed by a purification step on protein A-conjugated Sepharose beads. Western blotting was carried out as described previously (4).
Fluorescence microscopy.
HeLa cells (50,000) were seeded on coverslips in 24-well plates and transfected with psvCMV-HA-tagged Vpr (or empty vector psvCMV-HA) as detailed previously (5). Transfected cells were processed 48 h later for laser scanning confocal microscopy (8). Images were acquired using the Zeiss LSM10 system with ZEN 2009 software and processed using AxioVision version 4.7.
RESULTS
Heterogeneity of Vpr in different HIV-1 groups.
To determine whether Vpr from HIV-1 primary isolates can modulate NK cell functions as does that from laboratory-adapted virus (24, 28), we examined nine variants spanning all HIV-1 groups: M, N, O, and P (Fig. 1). Six came from 4 clades (B, C, D, and H) of group M, and these were specifically chosen to reflect different levels of amino acid sequence variations compared to laboratory-adapted HxB Vpr (belonging to clade B). The two variants from clade B, termed M/B-18E and M/B-13C, and isolated from patients with or without HIV-related dementia, respectively (21), showed 93 to 95% sequence similarity to HxB Vpr. Clade C (M/C) and H (M/H) Vprs displayed approximately 90% sequence identity with the reference strain. The two variants from clade D, M/D-sh (for short, 96 amino acids [aa]) and M/D-lo (for long, 108 aa), were chosen since they were most divergent from HxB Vpr (≤87% similarity), with one (M/D-lo) having a C-terminal 12-amino-acid extension not found in the other variants. Of note, Vpr M/D-lo was derived from a virus isolate carried by an asymptomatic African subject who was naive to therapy (11). A review of the Los Alamos HIV database revealed that this strain was unique, and the virus genome encoded a biologically competent virus with no inactivating mutations (11). The remaining three Vpr variants from groups N, O, and P shared at most 87% sequence identity with HxB Vpr, with those from groups O and P having a unique alignment at the C terminus. Of note, while there are many primary virus isolates reported for groups N and O, there have been only two isolates described for group P (Los Alamos HIV database). In any case, despite the noted single-amino-acid polymorphisms, Vpr remains highly conserved among the 4 groups, especially at positions known to be functionally important to its G2 arrest activity (e.g., Q65 and R80) (3, 4), implying likely conservation of this function across the different groups.
Fig. 1.
Genetic heterogeneity of HIV-1 Vpr from primary isolates. Vpr amino acid sequences from HIV-1 primary isolates of groups M (clades B, C, D, and H), N, O, and P were compared to that of the laboratory-adapted virus HxB (reference). There were two isolates each from clades B (B-13C and B-18E) and D (D-sh and D-lo). Residues identical to those of the reference sequence are indicated with dots, and deleted residues are indicated with dashes. The conservation of functionally important residues at positions 65 and 80 across Vprs from different HIV-1 groups was denoted.
Examining the G2 arrest-inducing function of primary Vpr.
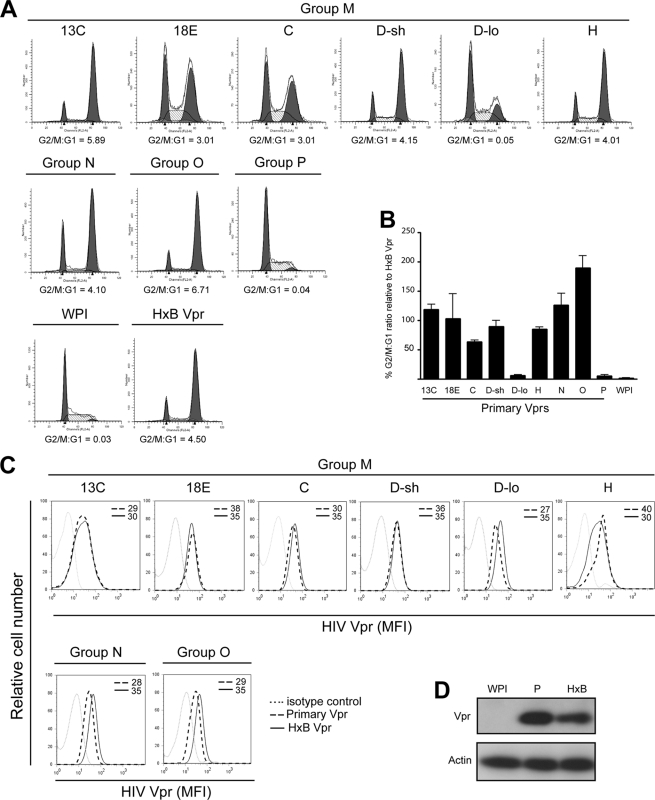
In the first instance, we assessed whether all primary Vprs are indeed able to mediate G2 arrest. CEM.NKR T cells were transduced with GFP-marked lentiviral vectors expressing laboratory-adapted (HxB) or primary Vpr. Forty-eight hours later, GFP+ cells were sorted and analyzed for cell cycle profiles. As illustrated (Fig. 2A and B), most primary Vprs were efficient at blocking cell division in G2 (G2/G1: 3.01 to 6.71 compared to 4.50 for HxB Vpr in a representative experiment or overall, 63% to 189% relative to HxB Vpr). In contrast, Vpr M/D-lo and Vpr P were both defective for G2 arrest (G2/G1: 0.04 and 0.05 versus 0.03 for no Vpr, WPI in a representative experiment or overall, about 5% relative to HxB Vpr). It is unlikely that the lack of G2 arrest induced by Vpr M/D-lo or Vpr P was due to inefficient protein expression since the data from flow cytometry (Fig. 2C) and/or Western blotting (Fig. 2D) clearly indicated that both variants were expressed at levels similar to or even higher than their G2 arrest-competent counterparts. Further, a comparative analysis of Vpr expression in lentiviral-vector-transduced T cells and HIV-infected T cells showed that Vpr was not overexpressed in our experimental system (data not shown). Of note, although Vpr P was not detectable by flow cytometry with anti-Vpr MAb (data not shown), the protein was readily identifiable by Western blotting using anti-Vpr pAb (Fig. 2D).
Fig. 2.
Most HIV-1 primary Vpr variants can efficiently induce a G2 cell cycle arrest in T cells. CEM.NKR T cells were transduced with GFP-marked lentiviral vectors expressing Vpr (primary laboratory-adapted [HxB]) or not expressing Vpr (WPI). (A and B) GFP+ cells were sorted and analyzed for cell cycle profiles by flow cytometry. Proportions of cells in G1 and G2 phases were enumerated using the ModFit mathematical model. (A) Representative results from one experiment. (B) Compilation of 3 experiments in which mean G2/G1 ratios ± standard deviations (SD) for primary Vprs were expressed as percentages of that obtained for HxB Vpr. (C) CEM cells were also stained with anti-Vpr MAb (8D1) and analyzed by flow cytometry for Vpr expression in GFP+ cells. MFI values were obtained after subtraction of signals given by cells stained with a relevant isotype control antibody. Shown are data representative of 3 experiments. (D) HEK293T cells were transfected with lentiviral vectors expressing no Vpr (WPI), Vpr P, or HxB Vpr and assessed for Vpr expression by Western blotting using anti-Vpr pAb.
Investigating possible modulation of NKG2D-mediated NK cell function by primary Vprs.
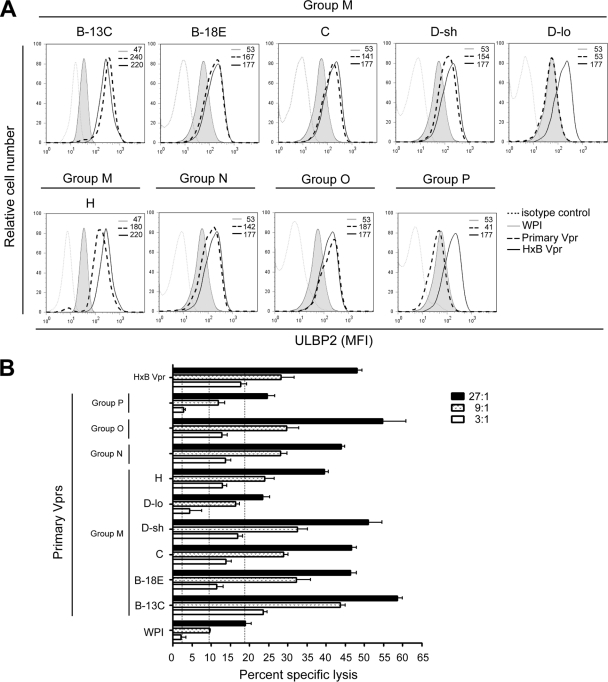
To ascertain whether HIV-1 primary Vpr variants could enhance ULBP2 expression as does laboratory-made Vpr, CEM.NKR T cells were transduced with Vpr-expressing lentiviral vectors as above and evaluated for cell surface ULBP2 expression (Fig. 3A). To this end, we observed that most primary Vprs (i.e., M/B-13C, M/B-18E, M/C, M/D-sh, M/H, and the group N and O Vprs) enhanced ULBP2 expression at levels comparable to that observed for HxB Vpr (average mean fluorescence intensity [MFI] for the primary variants: 173 ± 34 versus 199 ± 30 for HxB Vpr on average), with the exception of Vpr M/D-lo and Vpr P, where ULBP2 staining was essentially at a background level (MFI: 47 ± 8 versus 50 ± 4 for cells expressing no Vpr, WPI). The fact that Vpr M/D-lo and Vpr P were also defective for G2 arrest (Fig. 2) clearly demonstrates a universal correlation between increased ULBP2 expression and the induction of DNA damage/stress checkpoint in G2.
Fig. 3.
G2 arrest-competent primary Vpr variants can modulate NK cell functions through the NKG2D pathway. (A) CEM.NKR T cells were transduced with GFP-marked Vpr-expressing (or non-Vpr-expressing) lentiviral vectors as indicated in the Fig. 2 legend and stained for ULBP2. Expression levels were gated on GFP+ cells. MFI values were obtained after subtraction of signals given by cells stained with a relevant isotype control antibody. (B) GFP+ cells were sorted and used as targets in a standard 51Cr release assay. Purified resting NK cells from healthy donors were used as effector cells at the indicated effector/target ratios. Shown is mean percent specific cell lysis ± SD (triplicate samples) representative of three experiments with different donors. Vertical dotted lines indicated background killing.
Functionally, T cells with elevated ULBP2 levels were highly susceptible to NK cell-mediated lysis. Conversely, in the case of T cells expressing Vpr M/D-lo or Vpr P, where there was no detectable upregulation of ULBP2, the level of NK cell killing was comparable to that of cells expressing the WPI control (Fig. 3B). Nonetheless, the extent of ULBP2 enhancement per se did not necessarily predict the degree of NK cell killing. It is important to mention here that, although CEM.NKR T cells were the model cells for this study owing to their easy access and the lack of donor variability, the ability of primary Vprs to modulate ULBP2 expression has also been observed in primary CD4+ T cells (see Fig. S1 in the supplemental material).
Distinct mechanisms responsible for the lack of ULBP2 modulation by Vpr M/D-lo and Vpr P.
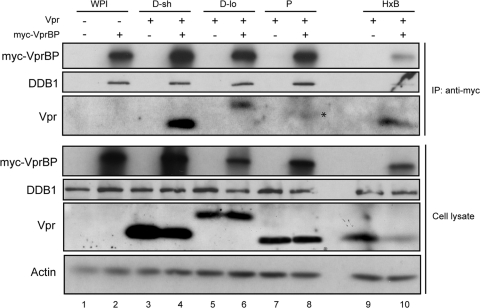
At this point, our data revealed that two of the nine primary Vprs, namely, Vpr P and Vpr M/D-lo, were unable to upregulate ULBP2 expression (or induce a G2 block). Since Vpr binding to the VprBP adaptor of the DDB1-CUL4A (VprBP/DCAF-1) E3 ubiquitin ligase is instrumental to both activities (24), we asked whether the dual defect could be attributed to their failed association with VprBP. For this purpose, cells expressing Vpr M/D-lo or Vpr P were evaluated for their ability to interact with VprBP by coimmunoprecipitation. As with the positive controls Vpr M/D-sh and HxB Vpr (Fig. 4, lanes 4 and 10, respectively), Vpr M/D-lo was present in the immunocomplexes containing VprBP and DDB1 (lane 6, IP). Nevertheless, putative Vpr P was not detected under the same experimental conditions (lane 8). This indicated that the inability of Vpr P to modulate ULBP2 expression was likely due to failed engagement to the said E3 ligase. As for Vpr M/D-lo, binding to the ligase was clearly insufficient to promote ATR activation and G2 arrest.
Fig. 4.
Analysis of engagement of Vpr M/D-lo and Vpr P to the DDB1-CUL4A (VprBP/DCAF-1) E3 ubiquitin ligase. HEK293 T cells were transfected with plasmids expressing Vpr (D-sh, D-lo, P, or HxB) or no Vpr (WPI). Cells were transcomplemented with plasmids expressing myc-VprBP (lanes 2, 4, 6, 8, and 10) or a myc tag alone (lanes 1, 3, 5, 7, and 9). Immunoprecipitation (IP) of total cell lysates was performed using anti-myc antibody. Resulting immunocomplexes (and total lysates, as a control) were analyzed for myc-VprBP, DDB1, and Vpr by Western blotting using the respective antibodies. The asterisk indicates an unidentified species having a higher molecular size than the expected size (∼14 kDa) for Vpr P. Shown are data representative of 3 experiments.
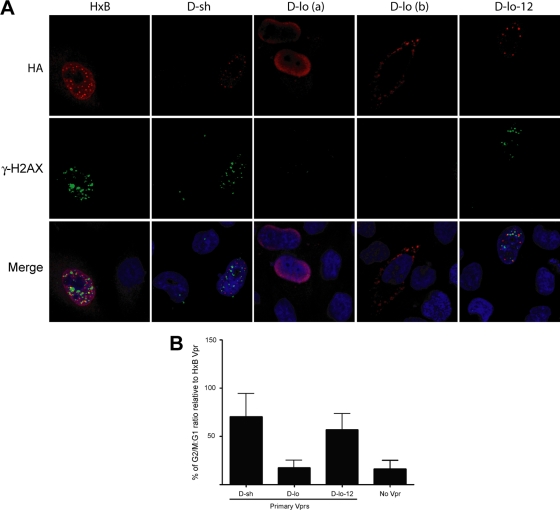
To gain a further insight as to why Vpr M/D-lo was unable to activate ATR, we took advantage of our earlier finding demonstrating the importance of formation of Vpr's nuclear foci for G2 arrest activity (5) and assessed the capability of Vpr M/D-lo to make similar structures. By confocal microscopy, we found that, unlike HxB Vpr or Vpr M/D-sh, M/D-lo could not make these nuclear foci (Fig. 5A). Instead, this Vpr variant appeared highly diffused (Vpr shown in red) and mislocalized to either the perinuclear membrane [Fig. 5A, D-lo (a)] or even external to the nucleus [Fig. 5A, D-lo (b)]. A multiple-field analysis revealed that Vpr M/D-lo was outside the nucleus approximately 70% of the time. Further, while the structures made by HxB Vpr and Vpr M/D-sh appeared to partially overlap with γ-H2AX (shown in green), a surrogate marker of ATR activation, this feature was clearly not observed for Vpr M/D-lo.
Fig. 5.
Formation of DNA damage nuclear foci by primary Vprs competent for G2 arrest. (A) HeLa cells were transfected with plasmids expressing HA-tagged Vpr, immunostained for Vpr (red) and γ-H2AX (green), and analyzed by confocal microscopy. Nuclei are shown in blue (DAPI [4′,6-diamidino-2-phenylindole] staining). Merge images reveal partial colocalization between nuclear Vpr foci and γ-H2AX. Images were acquired by confocal microscopy with a 63× objective. Shown are representative images of multiple fields. (B) HEK293T cells were cotransfected with plasmids expressing GFP- and HA-tagged Vpr. GFP+ cells were analyzed for cell cycle profiles as described in the Fig. 2 legend. Shown are mean G2/G1 ratios ± SD (n = 2 experiments) expressed as percentages of that obtained for HxB Vpr.
From the sequence standpoint, Vpr M/D-lo is unique in that it has a 12-amino-acid extension at the C terminus (Fig. 1). To explore whether the tail would somehow hinder Vpr M/D-lo's ability to activate the ATR—a prerequisite for both ULBP2 upregulation and G2 cell cycle arrest—a truncated version of the allele (without the extra 12 amino acids), termed D-lo-12, was examined for its ability to induce a G2 block. Intriguingly, unlike Vpr M/D-lo, D-lo-12 could promote cell cycle arrest essentially as efficiently as does Vpr M/D-sh (Fig. 5B). In addition, Vpr D-lo-12 (Fig. 5A) could form nuclear foci, similar to those observed for G2-competent HxB Vpr or Vpr M/D-sh. These structures were found, unsurprisingly, in the proximity of phosphorylated γ-H2AX, a marker of ATR activation. Taking these results together, we submit that the C-terminal extension of Vpr M/D-lo is likely responsible for its failure to trigger ATR and G2 arrest. These findings thus explain why this particular Vpr variant is unable to upregulate ULBP2.
DISCUSSION
In this study, we show that Vprs from primary isolates from three of the four HIV-1 groups are capable of inducing a G2 arrest and upregulating ULBP2 expression on T cells. The enhanced presence of this NKG2D ligand promotes T cell killing by NK cells through the NKG2D receptor. Further, in all variants investigated, the observation of Vpr-induced augmentation of ULBP2 is invariably accompanied by evidence of activation of DNA damage sensor ATR.
Nine primary Vprs from HIV-1 groups M, N, O, and P were initially evaluated for their G2 arrest activity. Vpr P and Vpr M/D-lo are clearly defective in this regard but differ greatly in how this effect is brought about. For Vpr P, the inactivity appears to be due to its inability to engage the substrate specificity receptor VprBP (Fig. 4) of the DDB1-CUL4A (VprBP/DCAF-1) ubiquitin E3 ligase complex; it is thus unable to activate ATR and induce a G2 arrest. The same, however, cannot be said for Vpr M/D-lo, as it is clearly able to directly associate with VprBP (Fig. 4). Since triggering ATR requires engagement of Vpr to the E3 ligase and the recruitment of an as-yet-unknown cellular target(s) for polyubiquitination and proteasomal degradation, the unique C-terminal extension of Vpr M/D-lo (Fig. 1) may hinder such a process. In that regard, the fact that this extension causes a mislocalization of the protein from the nucleus most probably underlies the defect since recent indirect evidence from our laboratory suggests that the cellular target(s) of Vpr is likely to be a chromatin-associated protein(s) (5). Indeed, we demonstrate herein that removal of the extra end renders Vpr M/D-lo able to activate ATR, as evidenced by its formation of DNA damage nuclear foci and mediation of a G2 arrest (Fig. 5).
From the sequence standpoint (Fig. 1), seven of the nine primary Vpr variants that we studied coincidentally had a substitution at position 77, with six carrying the R77Q mutation. This substitution, found in a higher proportion of HIV-infected long-term nonprogressive patients (19, 20), has been shown to correlate with impaired apoptosis in some studies (19, 23) but not in others (2, 13). In the context of our current study, it is evident that R77Q is unlikely to have a negative effect on G2 arrest activity as 6 of the 7 Vprs with this mutation were still able to induce a cell division block. On this note, our latest data are in agreement with those previously reported (2, 16, 19).
Consistent with earlier findings reported for laboratory-adapted HIV-1 Vpr (24, 28), we show here that ULBP2 upregulation on T cells by primary Vprs is universally correlated with an active G2 arrest function. Given that it was only with Vpr M/D-lo and Vpr P that we observed no such modulation in ULBP2 expression (Fig. 3), the data point toward ATR activation as the common thread between the two events. Since T cells with elevated ULBP2 levels were more susceptible to NK cell-mediated lysis (Fig. 4), augmentation of this NKG2D ligand, in the context of HIV infection, may initially serve as a host's attempt to contain the viral presence. Nonetheless, prolonged engagement of the NKG2D receptor to its ligand(s) could conceivably lead to gradual deregulation of NK cell activity over time. In this context, it would be interesting to determine whether the lack of ULBP2 upregulation on T cells expressing Vpr P or the unique Vpr M/D-lo would inadvertently be beneficial to the integrity of NK cells in the long haul. Interestingly, the notion of derailed NKG2D-mediated NK cell effector functions in the face of NK cell activation was recently described to be a possible approach exploited by another HIV-1 accessory protein, namely, Vpu, to evade the host's immune responses. In this setting, Vpu appears to avoid NK cell activation but selectively downregulates NTBA—an NKG2D costimulatory receptor for degranulation—to ultimately impair this particular function in NK cells (25).
With our latest findings, it is now well established that, even in the absence of a bona fide infection, Vpr can modulate NK cell effector functions through the NKG2D, implying a potential effect of Vpr on bystander healthy cells (24). Since depletion of CD4+ T cells during HIV-1 infection is largely ascribed to the death of uninfected cells, it is conceivable that, through enhancing the expression of ULBP2 on target cells and subsequently promoting their lysis by NK cells, Vpr could contribute to the overall loss of healthy bystander CD4+ T cells during HIV infection. Further, the fact that Vpr-mediated cell surface ULBP2 upregulation can now be extended to most HIV-1 groups sheds light on the possibility of ULBP2 as a potential marker in the identification of low-level and chronically infected cells, a population that currently hampers the swift eradication of HIV.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) (MOP 12381 to E.A.C.) and Canadian Foundation for AIDS Research (to E.A.C. and T.N.Q.P). J.R. is a recipient of a Frederick Banting and Charles Best scholarship from the CIHR. F.C.A.G. is supported by a CIHR postdoctoral fellowship. C.P. and E.A.C. are Canada Research Chairs in Neurological Infection and Immunity and Human Retrovirology, respectively.
We thank Didier Trono and Yukihito Ishizaka for kindly providing reagents, Nicole Rougeau, Eric Massicotte, Martine Dupuis, and Julie Lord-Grignon for expert technical assistance, Mathieu Dube for critical reading of the manuscript, and Pierre Larochelle, the IRCM clinic staff, and all donors for providing blood samples. HIV molecular clones 94UG114, 98IN022, and 90CF056 were obtained through the NIH AIDS Research and Reference Reagent Program from Beatrice Hahn and Feng Gao.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 28 September 2011.
REFERENCES
- 1. Abraham R. T. 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15:2177–2196 [DOI] [PubMed] [Google Scholar]
- 2. Andersen J. L., et al. 2006. HIV-1 Vpr-induced apoptosis is cell cycle dependent and requires Bax but not ANT. PLoS Pathog. 2:e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Belzile J. P., et al. 2007. HIV-1 Vpr-mediated G2 arrest involves the DDB1-CUL4AVPRBP E3 ubiquitin ligase. PLoS Pathog. 3:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belzile J. P., Richard J., Rougeau N., Xiao Y., Cohen E. A. 2010. HIV-1 Vpr induces the K48-linked polyubiquitination and proteasomal degradation of target cellular proteins to activate ATR and promote G2 arrest. J. Virol. 84:3320–3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belzile J. P., Abrahamyan L. G., Gerard F. C. A., Rougeau N., Cohen E. A. 2010. Formation of mobile chromatin-associated nuclear foci containing HIV-1 Vpr and VPRBP is critical for the induction of G2 cell cycle arrest. PLoS Pathog. 6:e1001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bonaparte M. I., Barker E. 2003. Inability of natural killer cells to destroy autologous HIV-infected T lymphocytes. AIDS. 17:487–494 [DOI] [PubMed] [Google Scholar]
- 7. Cohen E. A., et al. 1990. Identification of HIV-1 vpr product and function. J. Acquir. Immune Defic. Syndr. 3:11–18 [PubMed] [Google Scholar]
- 8. Dube M., et al. 2009. Suppression of tetherin-restricting activity upon human immunodeficiency virus type 1 particle release correlates with localization of Vpu in the trans-Golgi network. J. Virol. 83:4574–4590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Felzien L. K., et al. 1998. HIV transcriptional activation by the accessory protein, VPR, is mediated by the p300 co-activator. Proc. Natl. Acad. Sci. U. S. A. 95:5281–5286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fogli M., et al. 2008. Lysis of endogenously infected CD4+ T cell blasts by rIL-2 activated autologous natural killer cells from HIV-infected viremic individuals. PLoS Pathog. 4:e1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gao F., et al. 1998. A comprehensive panel of near full-length clones and reference sequences for non-subtype B isolates of human immunodeficiency virus type 1. J. Virol. 72:5680–5698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goh W. C., et al. 1998. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat. Med. 4:65–71 [DOI] [PubMed] [Google Scholar]
- 13. Jacquot G., et al. 2009. Characterization of the molecular determinants of primary HIV-1 proteins: impact of the Q65R and R77Q substitutions on Vpr functions. PLoS One. 4:e7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kogan M., Rappaport J. 2011. HIV-1 accessory protein Vpr: relevance in the pathogenesis of HIV and potential for therapeutic intervention. Retrovirology 8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lai M., Zimmerman E. S., Planelles V., Chen J. 2005. Activation of the ATR pathway by human immunodeficiency virus type 1 Vpr involves its direct binding to chromatin in vivo. J. Virol. 79:15443–15451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lai M., Chen J. 2006. The role of Vpr in HIV-1 disease progression is independent of its G2 arrest induction function. Cell Cycle 5:2275–2280 [DOI] [PubMed] [Google Scholar]
- 17. Lavallee C., et al. 1994. Requirement of the Pr55gag precursor for incorporation of the Vpr product into human immunodeficiency virus type 1 viral particles. J. Virol. 68:1926–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Le Rouzic E., et al. 2007. HIV1 Vpr arrests the cell cycle by recruiting DCAF1/VprBP, a receptor of the Cul4-DDB1 ubiquitin ligase. Cell Cycle 6:182–188 [DOI] [PubMed] [Google Scholar]
- 19. Lum J. J., et al. 2003. Vpr R77Q is associated with long-term nonprogressive HIV infection and impaired induction of apoptosis. J. Clin. Invest. 111:1547–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mologni D., et al. 2006. Vpr and HIV-1 disease progression: R77Q mutation is associated with long term control of HIV-1 infection in different groups of patients. AIDS 20:567–574 [DOI] [PubMed] [Google Scholar]
- 21. Na H., et al. 2011. Interactions between human immunodeficiency virus (HIV)-1 Vpr expression and innate immunity influence neurovirulence. Retrovirology 8:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Planelles V., et al. 1996. Vpr-induced cell cycle arrest is conserved among primate lentiviruses. J. Virol. 70:2516–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rajan D., Wildum S., Rücker E., Schindler M., Kirchhoff F. 2006. Effect of R77Q, R77A and R80A changes in Vpr on HIV-1 replication and CD4 T cell depletion in human lymphoid tissue ex vivo. AIDS 20:831–836 [DOI] [PubMed] [Google Scholar]
- 24. Richard J., Sindhu S., Pham T. N., Belzile J. P., Cohen E. A. 2010. HIV-1 Vpr up-regulates expression of ligands for the activating NKG2D receptor and promotes NK cell-mediated killing. Blood 115:1354–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shah A. H., et al. 2010. Degranulation of natural killer cells following interaction with HIV-1-infected cells is hindered by downmodulation of NTB-A by Vpu. Cell Host Microbe 8:397–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tebit D. M., Arts E. J. 2011. Tracking a century of global expansion and evolution of HIV to drive understanding and to combat disease. Lancet Infect. Dis. 11:45–56 [DOI] [PubMed] [Google Scholar]
- 27. UNAIDS 2010. UNAIDS report on the global AIDS epidemic. UNAIDS, Geneva, Switzerland: http://www.unaids.org (accessed 3 July 2011). [Google Scholar]
- 28. Ward J., et al. 2009. HIV-1 Vpr triggers natural killer cell-mediated lysis of infected cells through activation of the ATR-mediated DNA damage response. PLoS Pathog. 5:e1000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yao X. J., et al. 1998. Vpr stimulates viral expression and induces cell killing in human immunodeficiency virus type 1-infected dividing Jurkat T cells. J. Virol. 72:4686–4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zimmerman E. S., et al. 2004. Human immunodeficiency virus type 1 Vpr-mediated G2 arrest requires Rad17 and Hus1 and induces nuclear BRCA1 and γ-H2AX focus formation. Mol. Cell. Biol. 24:9286–9294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zimmerman E. S., et al. 2006. Human immunodeficiency virus type 1 Vpr induces DNA replication stress in vitro and in vivo. J. Virol. 80:10407–10418 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.