Abstract
Rotaviruses (group A rotaviruses) are the most important cause of severe gastroenteritis in infants and children worldwide. Currently, an antiviral drug is not available and information on therapeutic targets for antiviral development is limited for rotavirus infection. Previously, it was shown that lipid homeostasis is important in rotavirus replication. Since farnesoid X receptor (FXR) and its natural ligands bile acids (such as chenodeoxycholic acid [CDCA]) play major roles in cholesterol and lipid homeostasis, we examined the effects of bile acids and synthetic FXR agonists on rotavirus replication in association with cellular lipid levels. In a mouse model of rotavirus infection, effects of oral administration of CDCA on fecal rotavirus shedding were investigated. The results demonstrate the following. First, the intracellular contents of triglycerides were significantly increased by rotavirus infection. Second, CDCA, deoxycholic acid (DCA), and other synthetic FXR agonists, such as GW4064, significantly reduced rotavirus replication in cell culture in a dose-dependent manner. The reduction of virus replication correlated positively with activation of the FXR pathway and reduction of cellular triglyceride contents (r2 = 0.95). Third, oral administration of CDCA significantly reduced fecal virus shedding in mice (P < 0.05). We conclude that bile acids and FXR agonists play important roles in the suppression of rotavirus replication. The inhibition mechanism is proposed to be the downregulation of lipid synthesis induced by rotavirus infection.
INTRODUCTION
Rotaviruses are nonenveloped, icosahedral viruses with an 11-segment double-stranded-RNA genome. Rotavirus particles contain six structural proteins, which comprise a core (VP1 to -3), an inner capsid (VP6), and an outer capsid (VP4 and -7). Rotaviruses are divided into 7 morphologically indistinguishable but antigenically distinct serogroups (A to G) based on VP6 (16). Group A rotaviruses are the leading cause of severe gastroenteritis in infants and children worldwide, associated with approximately 111 million episodes of gastroenteritis that have required 25 million clinic visits and 2 million hospitalizations and have resulted in over 500,000 deaths in children younger than 5 years of age (16). Even though effective live-attenuated vaccines are available for human rotavirus infection (16), rotavirus still remains the most important cause of gastroenteritis in infants and children worldwide. Since there are no specific antiviral drugs available for rotavirus infection, treatment options for rotavirus-mediated gastroenteritis are limited to restoration and maintenance of hydration until the infection resolves (17). Therefore, development of a rotavirus-specific drug is important to reduce severity of disease and duration of rotavirus-related hospitalization. However, information on the therapeutic targets for rotavirus infection is limited.
Previously, it was shown that disruption of lipid rafts and/or lipid droplets decreased infectious rotaviruses by inhibition of rotavirus morphogenesis (7, 10). Lipid rafts are specialized membrane domains enriched in glycosphingolipids, cholesterol, and protein. Lipid droplets are the major lipid storage structure enriched in triglycerides and cholesterol ester and play a crucial role in regulating cellular lipid levels. The interaction of virus proteins with these subcellular lipid bodies is also important for infectious virus particle formation in some viruses, including human hepatitis C virus (30, 32) and dengue virus (38). These findings suggest that lipid homeostasis is important for the replication of certain viruses.
In the small intestines, where rotavirus replication occurs, de novo synthesis of lipids and absorption of dietary lipids affect cellular lipid contents in the epithelial cells (19). In the intestinal lumen, bile acids emulsify fats to form micelles to aid their absorption. Bile acids are synthesized from cholesterol in the liver, stored at the gallbladder, and released into the duodenum. The primary bile acids, cholic acid (CA) and chenodeoxycholic acid (CDCA), are synthesized in the liver from cholesterol by enzymes, including cholesterol 7α-hydroxylase, and subsequently conjugated with taurine or glycine to enhance affinity to both acids and bases. Primary bile acids are transformed by intestinal bacteria into secondary bile acids, deoxycholic acid (DCA), lithocholic acid (LCA), and ursodeoxycholic acid (UDCA) (11). While the secreted bile acids travel through the intestinal tracts, they are reabsorbed in the ileum and returned to the liver via the portal vein (25). This enterohepatic circulation is essential in maintaining an effective concentration of bile acids and cholesterol homeostasis. One of the bile acid receptors is farnesoid X receptor (FXR) (12, 27, 34). The activation of FXR by bile acids induces the expression of various proteins, including small heterodimer partner (SHP), which represses the expression of cholesterol 7α-hydroxylase, the rate-limiting enzyme in bile acid synthesis (27, 34). The FXR/SHP pathway is well developed in hepatic, intestinal, and renal cells and also participates in the regulation of fatty acid (including cholesterol) metabolism and glucose homeostasis (40, 41, 44). Previously, our group reported that bile acids play an important role in the replication of hepatitis C viruses and porcine enteric caliciviruses (5, 6). However, the role of bile acids and other FXR agonists in rotavirus replication has been unknown.
Here, we report that the intracellular contents of triglycerides increased significantly by rotavirus infection. Furthermore, we demonstrate that CDCA and DCA at physiologic concentrations and a synthetic FXR agonist, GW4064, at a low micromolar concentration significantly reduced rotavirus replication in cells in a dose-dependent manner. The reduction of virus replication correlated positively with activation of the FXR/SHP pathway and reduction of cellular lipid contents (triglycerides). Oral administration of CDCA significantly reduced the peak quantities of virus shedding in the mouse model of rotavirus infection compared to those for the no-treatment group (P < 0.05). We conclude that bile acids and FXR agonists play important roles in the suppression of rotavirus replication, and the inhibition mechanism is proposed to be the downregulation of lipid synthesis induced by rotavirus infection.
MATERIALS AND METHODS
Cells, antisera, and reagents.
The MA104, Huh-7, and Caco-2 cell lines were maintained in minimum essential medium (MEM) or Dulbecco's MEM (DMEM) containing 5% fetal bovine serum and antibiotics (penicillin and streptomycin). Antibodies specific to rotavirus VP6 or SHP were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). A polyclonal goat antibody specific for rotavirus was also obtained from Fisher Scientific (Palatine, IL). Fluorescein isothiocyanate-conjugated anti-mouse IgG and peroxidase-conjugated goat anti-mouse IgG or rabbit anti-goat IgG were purchased from Sigma-Aldrich (St. Louis, MO). Rotavirus strains, including Wa (human G1 type) and SA11 (primate G3 type), were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Nitazoxanide, which was shown to reduce rotavirus replication in vitro and in vivo (37), was obtained from Fisher Scientific. The bile acids used in this study, including CA, glycocholic acid (GCA), CDCA, glycochenodeoxycholic acid (GCDCA), DCA, and UDCA, were obtained from Sigma-Aldrich. The synthetic FXR agonists 6-α-ethyl-chenodeoxycholic acid (6-ECDCA), GW4064, and fexaramine and an FXR and retinoid X receptor agonist, TTNPB {4-[(E)-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1-propenyl]benzoic acid}, were obtained from commercial sources, including Sigma-Aldrich and Enzo Life Sciences (Plymouth Meeting, PA). The conjugated bile acids were prepared in sterile water, and unconjugated bile acids and synthetic agonists were prepared in dimethyl sulfoxide (DMSO) as a 100 mM stock solution. Trypsin (Sigma-Aldrich) was used at 1 μg/ml in medium for rotavirus replication.
Rotavirus infection and treatment in cell culture.
Fully confluent monolayered MA104 cells grown in 6- or 12-well plates were inoculated with Wa or SA11 rotavirus for 1 h at a high (2) or low (0.01) multiplicity of infection (MOI) based on 50% tissue culture infective dose (TCID50) titers. Following a washing step with phosphate-buffered saline (PBS), MEM containing each compound or solvent (distilled water or DMSO) was added to each well. The concentration of each compound in the cells was less than 200 μM for bile acids, 6-ECDCA, and fexaramine or 10 μM for GW4064 and/or TTNPB. As a control, nitazoxanide was used at 0.1 to 10 μM. At these concentrations, all compounds showed little cytotoxicity to MA104 cells. Virus replication was monitored by immunofluorescence assay (IFA) or Western blot analysis with antibodies against VP6, and the TCID50/ml was also determined (36). We also examined the effects of CDCA and GW4064 on the replication of SA11 in Caco-2 cells (human colon carcinoma cells) to confirm the results obtained with MA104 cells. Because porcine reproductive respiratory syndrome virus (PRRSV) replicates well in MA104 cells, PRRSV strain P129 was used to examine the effects of each compound on viral replication as a negative control (35).
Nonspecific cytotoxic effect.
We determined the toxic concentration of each bile acid and compound in MA104 cells. Confluent MA104 cells grown in 24-well plates were treated for 24 h with various concentrations of bile acids, including CDCA, DCA, UDCA, and GCDCA, or synthetic FXR agonists, including 6-ECDCA, GW4064, fexaramine, and TTNPB. Cell cytotoxicity was measured by a CytoTox 96 nonradioactive cytotoxicity assay kit (Promega, Madison, WI).
IFA, Western blot analysis, TCID50 method, and real-time qRT-PCR.
Virus replication in cell culture was assessed by IFA or Western blot analysis at 12 or 24 h postinfection, before extensive cytopathic effects are induced by viral infection. Viral replication was also titrated with the TCID50 method at 12, 24, 48, and 72 h postinfection, after cells were lysed with repeated freezing and thawing (36). For IFA, cells were fixed with 100% methanol (MeOH) at room temperature for 30 min. Then, monoclonal antibody specific for rotavirus VP6, followed by fluorescein isothiocyanate-conjugated goat anti-mouse IgG, was applied to the cells. The stained cells were observed under fluorescence microscopy. For Western blot analysis, the expression levels of structural proteins, including VP6, in virus-infected cells with or without each compound were assessed by using the polyclonal antibody specific to rotavirus. The cells were lysed at 12 or 24 h postinfection, and the cell lysates were prepared in SDS-PAGE sample buffer containing 1% β-mercaptoethanol and sonicated for 20 s. The proteins were resolved in multiple 10% Novex Tris-Bis gels (Invitrogen, Carlsbad, CA) and transferred to nitrocellulose membranes. Each membrane was probed with antibody specific for rotavirus, β-actin, or SHP, and the binding of the antibodies was detected with peroxidase-conjugated goat anti-mouse IgG or rabbit anti-goat IgG. Following incubation with a chemiluminescent substrate (Pierce Biotechnology, Rockford, IL), signals were detected on X-ray film. For the TCID50 method, a standard method with 10-fold dilutions of each sample was used for virus titration in MA104 cells according to the Reed and Muench method (36). For real-time quantitative reverse transcriptase PCR (qRT-PCR), viral RNAs were extracted from viral suspension or fecal samples with an RNeasy kit according to the manufacturer's directions (Qiagen, Valencia, CA). Virus-specific primers and probe were synthesized based on the rotavirus VP6 gene. The primer sequences for the SA11 rotavirus VP6 gene were 5′-GGCTTTTAAACGAAGTCTTCAAC-3′ (forward) and 5′-CCATTCATAGTAATTATCATTTG-3′ (reverse). The probe sequence was FAM–5′-TTTGTCYCTAGCRTCTTTAAGAG-3′–Iowa Black (where FAM is 6-carboxyfluorescein). Using a one-step Platinum qRT-PCR kit (Invitrogen), the qRT-PCR amplification was performed with a Rotor-Gene Q (Qiagen, Valencia, CA) with the following parameters: 45°C for 30 min and 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 30 s, annealing at 50°C for 1 min, and elongation at 72°C for 30 s. The mRNA levels of SHP or FXR in MA104 or Huh-7 cells treated with each compound were also monitored using predesigned TaqMan probes and primer pairs obtained from Applied Biosystems (Foster City, CA). Constitutively expressed β-actin mRNA was measured as an internal standard for sample normalization (Applied Biosystems).
Effects of CDCA, GCDCA, and GW4064 on the FXR and SHP mRNA expression levels in cells with or without rotavirus infection.
Confluent MA104 cells in 6-well plates were infected with mock medium or SA11 rotavirus at an MOI of 10. The mock medium, CDCA, GCDCA, or GW4064 was added to the medium with trypsin. At 30 min or 2 h postinfection, cells were collected to determine mRNA levels of FXR and SHP by qRT-PCR as described above. The effects of the mock medium, CDCA, GCDCA, or GW4064 on mRNA levels of FXR and SHP were also examined in the confluent Huh-7 or Caco-2 cells in 6-well plates.
Transfection of SHP expression plasmid pCI-SHP and rotavirus infection.
To confirm the effects of SHP on rotavirus replication, a plasmid expressing SHP (pCI-SHP) (4) was used in this study. Empty pCI vector was used as a control. One-day-old semiconfluent MA104 cells in 6-well plates were transfected with pCI-SHP (0.05 to 3 μg) or pCI (3 μg). At 24 h after transfection, the transfected cells were inoculated with SA11 rotavirus at an MOI of 0.5 and incubated with medium with trypsin. Virus replication was examined by Western blot analysis.
Pretreatment of viruses with CDCA or GW4064.
To investigate if CDCA or GW4064 has virucidal effects on rotavirus, SA11 rotavirus of high titer (>109 TCID50/ml) was preincubated with PBS (or DMSO [0.1%]), CDCA (200 μM), or GW4064 (10 μM) at 37°C for 2 h. Then, the mixture was diluted up to 100 times for cell inoculation. Virus-infected cells were incubated with medium containing trypsin for up to 24 or 48 h, and virus replication was monitored by the titration of progeny viruses using the TCID50 method.
Lipid staining of cells infected with rotavirus.
Confluent MA104 cells in 6-well plates were infected with mock medium or SA11 rotavirus at an MOI of 10. Cells were treated with DMSO (0.1%), CDCA (100 μM), or GW4064 (2 μM) and incubated with medium with 22-(N-7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-23,24-bisnor-5-cholen-3β-ol (NBD-cholesterol; 1 μg/ml) or Nile red (2.5 μg/ml). NBD-cholesterol binds specifically to lipid droplet-specific protein with high affinity and is a useful probe of sterol uptake and intracellular sterol targeting to lipid droplets (18). Nile red is a selective lipid droplet stain (21). The fluorescence signals of the cells were observed under fluorescence microscopy with corresponding filters for NBD-cholesterol (475 to 490 nm) or Nile red (>590 nm) at 4, 6, 8, or 10 h postinfection. The fluorescence signal by NBD-cholesterol was also monitored by a fluorometer (DinaTeck Laboratories, Chantilly, VA), with an excitation/emission wavelength of 485/535 nm.
Determination of triglyceride concentrations in neutral lipid extracts from cells.
To examine the effects of rotavirus infection on cellular triglyceride contents, confluent MA104 cells were infected with mock medium or SA11 rotavirus at an MOI of 10. Cells were incubated with mock medium (DMSO, 0.1%), CDCA (100 μM), or GW4064 (2 μM) for up to 10 h, and cells were then collected for triglyceride measurement by the method of Bligh and Dyer (3). Briefly, cells in 6-well plates were washed with cold PBS three times, and 3 ml of MeOH-H2O solution was added to the wells for cell scraping. Scraped cells were transferred into 15-ml centrifuge tubes each containing 1 ml of CHCl3. The tubes were vortexed for 30 s and centrifuged for 1 min at 400 × g. The top layer was transferred to a new tube containing 1 ml of CHCl3. Following vortexing and phase separation, two bottom layers were combined and washed again by adding 3 ml of MeOH-H2O, vortexing for 30 s, and centrifugation. The bottom solution was collected into a small glass tube and evaporated to dryness in a speed vacuum (∼1 h). The dried lipid phase was solubilized with the reaction buffer from a triglyceride assay kit (Cayman Chemical, Ann Arbor, MI), and triglyceride concentration was measured with the kit according to the manufacturer's protocol.
Determination of triglyceride concentrations in neutral lipid extracts from Huh-7 cells.
To confirm the effects of CDCA and GW4064 on the triglyceride concentrations, confluent Huh-7 cells in 12-well plates were supplemented with oleic acid at 100 μM and incubated with medium for 6 h. Then, cells were incubated with mock medium (DMSO, 0.1%), CDCA (100 μM), or GW4064 (2 μM) for up to 48 h. The same procedures were followed for collection of cells and determination of triglyceride concentration. In this study, Huh-7 cells were used because the triglyceride response to oleic acid was minimal in MA104 cells in our preliminary study.
Mouse study.
To examine the effects of oral administration of CDCA on rotavirus shedding, an adult mouse model of rotavirus infection was used. The animal study was performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at Kansas State University. The 4-week-old female BALB/c mice were purchased from Charles River Lab (Wilmington, MA).
Rotavirus shedding patterns in mice.
Mice were infected with SA11 rotavirus (2 × 108, 2 × 109, or 1 × 1010 TCID50) in a volume of 150 μl via oral gavage (5 animals per group). Because initial experiments with lower titers (<108 TCID50/mouse) of SA11 resulted in inconsistent virus shedding, the high titers of virus were used for the study. To get the high titers for the inocula, SA11 rotaviruses were concentrated up to 1,000-fold using Amicon Ultracel (3K [3,000-molecular-weight]) centrifugal filters (Fisher Scientific). A group of mice received the same volume of PBS. The fresh fecal samples were collected daily and resuspended in PBS (5%, wt/vol) for extraction of viral RNA. The virus titers in the fecal samples were determined by real-time qRT-PCR using primers for the VP6 gene. Ten 10-fold dilutions of cell-culture-grown SA11 virus (1 × 109 TCID50/ml) were used for real-time qRT-PCR to generate a standard curve. The log10 titer of virus in a specimen was plotted against the cycle threshold (CT) value, and a best-fit line was constructed. Rotavirus quantity in the fecal samples, expressed as viral RNA equivalent to log10 TCID50/ml, is derived by plotting the CT of a sample on the standard curve. Selected fecal samples (n = 10) were also examined with virus titration with the TCID50 method to confirm the viral RNA equivalent. In a separate experiment, concentrated SA11 rotaviruses were inactivated by EDTA as described previously (15). The inactivated viruses (equivalent to 1 × 1010.2 TCID50, 150 μl) were administered to mice by oral gavage.
Administration of CDCA in mice with rotavirus infection.
To study the effects of CDCA on viral shedding, mice were randomly divided into two groups (5 mice/group). Groups 1 and 2 received CDCA at 150 mg/kg body weight/day in three divided doses (in PBS) and PBS by oral gavage, respectively. The treatment started 2 days prior to virus inoculation and continued for 9 days postinoculation. On the day of virus inoculation, mice were infected with SA11 rotavirus (2 × 109 TCID50/mouse, 150 μl) diluted in PBS via oral gavage. The fecal samples were collected daily for 9 days following viral inoculation and processed for real-time qRT-PCR as described previously for virus shedding experiments.
Statistics.
The Student t test was used to compare the significance of the unpaired sample means. P values of <0.05 were considered significant.
RESULTS
Effects of various bile acids on rotavirus replication in cells.
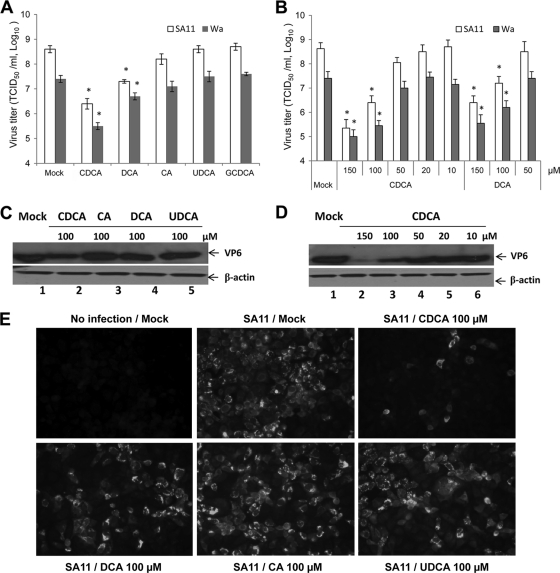
Among the bile acids tested, CDCA and DCA significantly reduced the replication of rotaviruses (Wa and SA11 strains) at 100 μM compared to that seen with mock treatment (P < 0.05) (Fig. 1A and B). The inhibitory effects of DCA and CDCA were also shown by Western blot analysis and IFA (Fig. 1C to E). CDCA was more effective in suppression of virus replication, with an effective dose causing 50% inhibition of virus replication (ED50) of 45 μM, than DCA, with an ED50 of 80 μM against both rotavirus strains. CA, UDCA, and GCDCA had little or no effect on rotavirus replication at the concentrations we tested (up to 200 μM) (Fig. 1A, C, and E). The cell toxicity by each bile acid was minimal (cell viability of >90%) at the concentrations used in the study. When various concentrations (0.1 to 10 μM) of nitazoxanide were used to treat MA104 cells infected with Wa and SA11 rotaviruses, they significantly reduced virus replication, as expected. The ED50 of nitazoxanide against both rotavirus strains was determined to be approximately 1.5 μM at 24 h postinfection in our study.
Fig. 1.
Effects of various bile acids on the replication of SA11 and Wa rotaviruses in MA104 cells. (A and B) The inhibition of virus replication in MA104 cells treated with DMSO (Mock) or various bile acids was evaluated by the TCID50 method. The final concentration of all compounds was 100 μM for results shown in panel A. Each bar represents the log10 TCID50/ml (mean ± standard error of the mean [SEM]) (*, P < 0.05 compared to mock treatment). (C and D) Expression of rotavirus VP6 protein in rotavirus-infected MA104 cells treated with DMSO or various bile acids was analyzed by Western blot analysis. β-Actin was loaded as an internal control. (E) Immunofluorescence of MA104 cells infected by SA11 rotavirus and treated with DMSO or various bile acids at 100 μM. Rotavirus VP6 protein was detected with a monoclonal antibody followed by a fluorescein isothiocyanate-labeled secondary antibody. Cells were fixed at 12 h after virus inoculation.
Effects of FXR agonists on rotavirus replication.
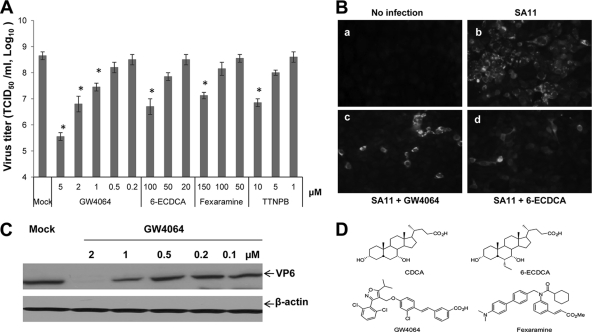
Because FXR is abundantly expressed in enterohepatic tissues and is activated by bile acids, we examined various synthetic FXR agonists, including 6-ECDCA, GW4064, fexaramine, and TTNPB (FXR and retinoid X receptor agonist), against rotavirus replication. All agonists tested significantly reduced rotavirus replication (both SA11 and Wa strains) in cells in a dose-dependent manner (Fig. 2A to D). Among the tested compounds, GW4064 was the most effective against rotavirus replication, with an ED50 of 1.2 μM against both rotavirus strains. When effects of CDCA or GW4064 on the replication of SA11 in Caco-2 cells were examined, the ED50 was determined to be 51 or 3.1 μM, respectively, which is similar to those seen with MA104 cells.
Fig. 2.
Effects of various FXR agonists on the replication of SA11 rotavirus in MA104 cells. (A) The inhibition of virus replication in MA104 cells treated with DMSO or various agonists was evaluated by the TCID50 method. Each bar represents the log10 TCID50/ml (mean ± SEM) (*, P < 0.05). (B) Immunofluorescence of MA104 cells infected by SA11 rotaviruses. Panel a shows cells without virus infection, and panel b shows cells infected with SA11 rotavirus without treatment. Panels c and d show virus-infected cells treated with GW4064 (2 μM) and 6-ECDCA (100 μM), respectively. Cells were fixed at 12 h after virus inoculation. (C) Western blot analysis of SA11 virus-infected cell lysates treated with DMSO (0.1%) or various concentrations of GW4064 for 24 h. β-Actin was loaded as an internal control. (D) Chemical structures of FXR agonists CDCA, 6-ECDCA, GW4064, and fexaramine.
Pretreatment of viruses demonstrated that antiviral effects of CDCA or GW4064 were not associated with a direct viral neutralizing or virucidal effect on rotavirus (data not shown). We also tested CDCA and GW4064 against PRRSV, which replicates in the same cell line (MA104 cells), as an unrelated virus control. When CDCA (100 μM) or GW4064 (5 μM) was added to virus-infected cells (at an MOI of 5), no inhibitory effect on the replication of PRRSV was observed. However, pretreatment of PRRSV with CDCA, but not GW4064, inactivated PRRSV. The inactivation of PRRSV, an enveloped RNA virus, may be due to detergent effects of bile acids.
Effects of FXR agonists on SHP mRNA in cells.
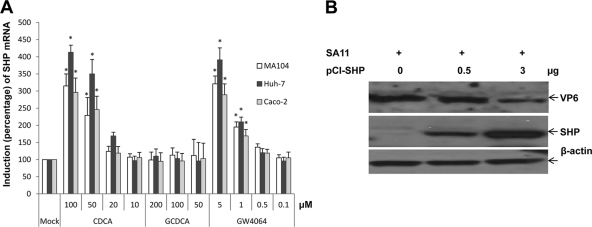
CDCA or GW4064 treatment significantly increased the expression of SHP mRNA, but not FXR mRNA, in MA104, Huh-7, or Caco-2 cells in a dose-dependent manner (Fig. 3A), in comparison to no treatment (P < 0.05), regardless of virus infection. The inhibition of virus replication positively correlated with increasing SHP mRNA expression (r2 = 0.95) in MA104 cells. GW4064 or CDCA significantly increased SHP mRNA expression at concentrations similar to those that showed antiviral effects on MA104 and Caco-2 cells. However, GCDCA treatment and viral infection itself did not increase the expression of SHP mRNA in MA104 cells. The overexpression of SHP by transfection of pCI-SHP reduced virus replication in a dose-dependent manner (Fig. 3B).
Fig. 3.
Effects of CDCA, GCDCA, and GW4064 on the expression of SHP mRNA in SA11-infected MA104, Huh-7, or Caco-2 cells treated for 2 h. The bar graphs show fold changes in SHP mRNA levels compared to levels seen with no treatment (mock) (means ± SEMs) (*, P < 0.05). (B) Western blot analysis of SHP and rotavirus VP6 expression. MA104 cells were transfected with different concentrations of SHP-expressing plasmid (pCI-SHP) (0, 0.5, or 3 μg) for 24 h prior to virus infection. Cell lysates were prepared 8 h after virus inoculation. β-Actin was loaded as an internal control.
CDCA and GW4096 reduced the increase of lipid contents in cells induced by rotavirus infection.
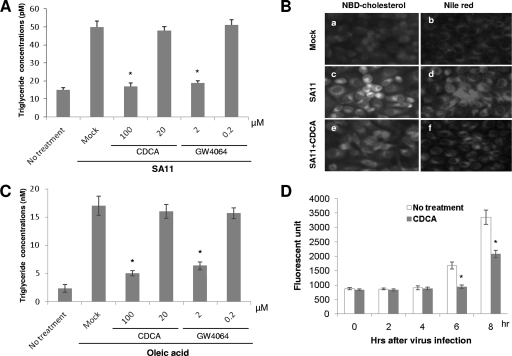
Rotavirus infection markedly increased cellular triglyceride contents, and CDCA or GW4064 significantly suppressed the increase of triglyceride contents in virus-infected cells compared to the result seen with no treatment (Fig. 4A). A similar effect was observed in Huh-7 cells supplemented with oleic acid, which is commonly used to induce lipid droplet formation (Fig. 4C). Importantly, CDCA and GW4064 significantly suppressed the increase of cellular triglyceride contents induced by virus infection or lipid supplementation at concentrations that significantly inhibited virus replication (Fig. 1B, 2A, and 4A and C). Rotavirus infection also increased the fluorescence intensity of NBD-cholesterol as well as Nile red staining in cells (Fig. 4B and D). In the virus-infected cells, the fluorescence signals by NBD-cholesterol were observed at 6 and 8 h postinfection (Fig. 4D). However, treatment of CDCA significantly suppressed the virus-induced increase in NBD-cholesterol fluorescence as well as Nile red staining, compared to results seen with no treatment (Fig. 4B and D). These findings indicate that bile acids are involved not only in regulation of triglycerides but also in cholesterol accumulation in cells.
Fig. 4.
(A) Effects of CDCA and GW4064 on triglyceride concentration in MA104 cells infected with rotavirus. The bar graph values shown are the means ± SEMs of triglyceride concentrations (*, P < 0.05 compared to mock treatment). (B) Nile red and NBD-cholesterol fluorescence of cells with or without virus infection and CDCA (100 μM) treatment. (C) Effects of CDCA and GW4064 on triglyceride concentration in Huh-7 cells (means ± SEMs) (*, P < 0.05 compared to mock treatment). (D) The fluorescence signals by NBD-cholesterol in rotavirus-infected MA104 cells at 0, 2, 4, 6, and 8 h postinfection were monitored by a fluorometer. Virus-infected cells were incubated in the presence of NBD-cholesterol (1 μg/ml) and trypsin with or without CDCA (100 μM), and the fluorescence signals were detected at the indicated times (*, P < 0.05 compared to no treatment).
Effects of CDCA on the replication of rotavirus in a mouse model.
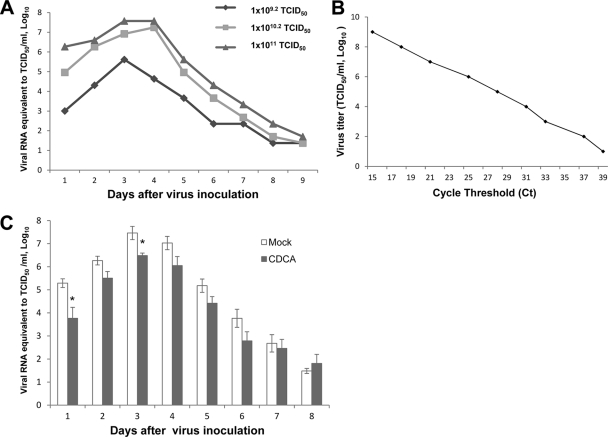
The virus shedding patterns with various inoculum doses of SA11 rotaviruses are shown in Fig. 5A. No animals showed clinical symptoms, including diarrhea or behavioral changes, during the course of experiments. The viral shedding patterns showed that viral shedding peaked on 3 to 4 days postinoculation and then rapidly decreased, which is similar to previous reports where the detection of virus in fecal samples peaked 2 to 5 days postinoculation and rapidly decreased after inoculation of a homologous mouse strain (EDIM) (8, 29, 33). However, administration of inactivated rotaviruses of high titer did not result in fecal virus shedding. The CT values from samples were converted to TCID50/ml using a standard curve generated from 10 10-fold dilutions of titrated virus stocks (Fig. 5B). The log10 titer of virus in a specimen was plotted against the CT value, and a best-fit line was constructed, with a correlation coefficient of 0.9914. Rotavirus quantity in the samples, expressed as viral RNA equivalent to log10 TCID50/ml, is derived by plotting the CT of a sample on the standard curve. We used a viral inoculum dose of 1 × 1010.2 TCID50/ml in further animal experiments. In mice orally infected with 1 × 1010.2 TCID50/ml SA11 rotavirus, the viral shedding significantly decreased in mice given CDCA on days 1 and 3 postinfection compared to that for the no-treatment group. When selected fecal samples (n = 10) were examined for virus titration with the TCID50 method to confirm the viral RNA equivalent, comparable levels of TCID50 titers (approximately 5-fold lower) corresponding to their viral RNA equivalent values were identified from the samples.
Fig. 5.
(A) Fecal viral RNA equivalent to log10 TCID50/ml in mice orally inoculated with SA11 rotavirus of various doses. (B) Standard curve generated by plotting the CT value versus log10 titer of virus (TCID50/ml). (C) Quantitation of virus shedding in feces in mice inoculated with 1 × 1010.2 TCID50 (in 150 μl) SA11 rotavirus and treated with PBS (mock) or CDCA. Animals received 150 mg/kg/day of CDCA in three divided doses by oral gavage. Each bar represents the log10 TCID50/ml (mean ± SEM) (*, P < 0.05 compared to mock treatment).
DISCUSSION
The association of rotavirus replication and lipid homeostasis was suggested by various reports. For example, cholesterol depletion by lovastatin, an inhibitor of cholesterol biosynthesis, was shown to reduce rotavirus replication in MA104 cells (31). Likewise, drugs that disperse lipid droplets by promoting lipolysis were shown to interfere with rotavirus particle or viroplasm formation (7). Therefore, lipid metabolism was suggested as a novel therapeutic target for rotavirus infections. Since FXR is reported to play major roles in lipid homeostasis, we examined effects of bile acids and synthetic FXR agonists on rotavirus replication in association with cellular lipid levels. In this study, we report that rotavirus replication induces cellular lipid accumulation and that disruption of this process by bile acids or FXR agonists significantly inhibits rotavirus replication.
Earlier studies during the 1970s and 1980s showed that bile acids (CDCA) lowered triglyceride levels in blood through a mechanism not fully understood. Recent studies suggested that triglyceride-lowering effects of bile acids and some synthetic FXR agonists were mediated by FXR/SHP (13, 43). The expression of SHP by activation of FXR led to the repression of sterol regulatory element-binding protein 1c, a transcription factor that controls genes involved in fatty acid and triglyceride synthesis (43). It is also reported that FXR regulates several genes in fatty acid and triglyceride synthesis, as well as lipoprotein metabolism (2, 14, 39). It was previously reported that bile acids differ markedly in binding affinity to FXR (27, 34). The activation of FXR is specific and limited to CDCA, the most potent activator, and DCA to a much lesser degree (27, 34). The ED50s of CDCA for activating FXR were reported to be 50 μM and 10 μM on murine and human FXR, respectively (27), which are within the physiological intracellular range (1). GW4064 is known as one of the most potent synthetic FXR agonists and has no activity on other nuclear receptors, including retinoic A receptor, at concentrations up to 1 mM (28, 45). In our study, GW4064 significantly inhibited virus replication at concentrations as low as 1 μM, and the ED50 of CDCA (45 μM) for inhibiting rotavirus infection in cell culture was similar to the reported ED50 of CDCA for activating FXR.
It is notable that cell lines used in this study express no or few bile acid transporters, such as the apical sodium-dependent bile acid transporter (ASBT) and the sodium-independent organic-anion-transporting peptide. While unconjugated bile acids may passively diffuse across the small intestinal and colonic epithelia, conjugated bile acids are actively absorbed in the distal ileum via ASBT (9, 20). With the presence of bile acid transporter in cells, conjugates of CDCA or DCA were previously shown to activate FXR (24). Therefore, the absence of bile acid transporter could probably explain the lack of activity of GCDCA on viral replication in our study.
To understand potential mechanisms, we studied the role of CDCA and GW4064 in lipid (triglyceride) synthesis with or without rotavirus infection. Interestingly, the intracellular concentrations of triglycerides were significantly increased by rotavirus infection, which is in line with a previous report that the size and number of lipid droplets whose major component is triglycerides increased in cells infected by rotavirus (7). Although the cholesterol contents were not measured in rotavirus-infected cells, increased fluorescence intensity of NBD-cholesterol as well as Nile red staining in cells also indicates that virus infection increases cellular neutral lipids, including triglycerides and cholesterol esters. CDCA or GW4064 treatment suppressed the increase in triglyceride content in MA104 cells infected with SA11 and in Huh-7 cells supplemented with oleic acid at concentrations that significantly inhibited virus replication. The reduction of virus replication correlated positively with reduction of cellular triglyceride contents (r2 = 0.95). These results suggest that the antiviral effects of CDCA and GW4064 are associated with the downregulation of cellular lipid accumulation induced by virus replication. CDCA and GW4064, but not GCDCA, treatment increased SHP mRNA in correlation with inhibition of cellular lipid accumulation and virus replication, and overexpression of SHP in cells reduced virus replication. These results suggest that rotavirus replication depends on lipid accumulation in the cells and that bile acids disrupt the process, resulting in inhibition of virus replication by pathways involving FXR/SHP. However, it is not clear to what extent SHP contributes to the effects of FXR on virus replication. Thus, we are investigating the roles of SHP on the FXR-mediated disruption of cellular lipid accumulation and virus replication using small interfering RNA (siRNA) for SHP. Next, we investigated the effects of bile acids on rotavirus shedding in a mouse model of rotavirus infection. Rotavirus infection in mice older than 15 days results in virus shedding without diarrhea (26, 42), since these mice lack fluid secretion in the small intestines despite extensive viral replication (26). In our study, in the infected mice orally treated with CDCA at 150 mg/kg/day, the peak viral shedding significantly decreased compared to that for the no-treatment group, even though the lengths of time of virus shedding did not differ.
We previously reported that bile acids were essential for porcine enteric calicivirus replication in cell culture (6) and promoted the expression of hepatitis C virus proteins and genome in replicon-harboring cells (5), suggesting significant roles of bile acids in virus replication. The results of this study show for the first time that bile acids suppress rotavirus replication in vitro and in vivo. The inhibition mechanism of bile acids is proposed to be downregulation of cellular lipid accumulation in association with activation of FXR. In humans, rotavirus diarrhea occurs most frequently in infants and children younger than 5 years of age (16). However, the age-related loss of susceptibility of rotavirus diarrhea is not yet well explained. Since the size of bile acid pools increases with body size and age in infants and young children (22, 23), we speculate that bile acids contribute to age-dependent diarrhea by rotavirus infection: in older children and adults, larger amounts of bile acids may limit virus replication, leading to decreased susceptibility of rotavirus-induced diarrhea. Nonetheless, our results suggest that FXR agonists, including bile acids, may be a valuable treatment option and that the FXR pathway may represent a potential therapeutic target for rotavirus infection.
ACKNOWLEDGMENTS
This work was supported by NIH U01AI081891.
We thank David George for technical assistance.
Footnotes
Published ahead of print on 28 September 2011.
REFERENCES
- 1. Akashi Y., Miyazaki H., Nakayama F. 1983. Correlation of bile acid composition between liver tissue and bile. Clin. Chim. Acta 133:125–132 [DOI] [PubMed] [Google Scholar]
- 2. Anisfeld A. M., et al. 2003. Syndecan-1 expression is regulated in an isoform-specific manner by the farnesoid-X receptor. J. Biol. Chem. 278:20420–20428 [DOI] [PubMed] [Google Scholar]
- 3. Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911–917 [DOI] [PubMed] [Google Scholar]
- 4. Chang K. O. 2009. Role of cholesterol pathways in norovirus replication. J. Virol. 83:8587–8595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang K. O., George D. W. 2007. Bile acids promote the expression of hepatitis C virus in replicon-harboring cells. J. Virol. 81:9633–9640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chang K. O., et al. 2004. Bile acids are essential for porcine enteric calicivirus replication in association with down-regulation of signal transducer and activator of transcription 1. Proc. Natl. Acad. Sci. U. S. A. 101:8733–8738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheung W., et al. 2010. Rotaviruses associate with cellular lipid droplet components to replicate in viroplasms, and compounds disrupting or blocking lipid droplets inhibit viroplasm formation and viral replication. J. Virol. 84:6782–6798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choi A. H., Basu M., McNeal M. M., Clements J. D., Ward R. L. 1999. Antibody-independent protection against rotavirus infection of mice stimulated by intranasal immunization with chimeric VP4 or VP6 protein. J. Virol. 73:7574–7581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Craddock A. L., et al. 1998. Expression and transport properties of the human ileal and renal sodium-dependent bile acid transporter. Am. J. Physiol. 274:G157–G169 [DOI] [PubMed] [Google Scholar]
- 10. Cuadras M. A., Greenberg H. B. 2003. Rotavirus infectious particles use lipid rafts during replication for transport to the cell surface in vitro and in vivo. Virology 313:308–321 [DOI] [PubMed] [Google Scholar]
- 11. Danielsson H., Sjövall J. (ed.). 1985. Sterols and bile acids. Elsevier Science Publishers B.V., Amsterdam, Netherlands [Google Scholar]
- 12. De Fabiani E., et al. 2004. Bile acid signaling to the nucleus: finding new connections in the transcriptional regulation of metabolic pathways. Biochimie 86:771–778 [DOI] [PubMed] [Google Scholar]
- 13. Duran-Sandoval D., et al. 2004. Glucose regulates the expression of the farnesoid X receptor in liver. Diabetes 53:890–898 [DOI] [PubMed] [Google Scholar]
- 14. Edwards P. A., Kast H. R., Anisfeld A. M. 2002. BAREing it all: the adoption of LXR and FXR and their roles in lipid homeostasis. J. Lipid Res. 43:2–12 [PubMed] [Google Scholar]
- 15. Estes M. K., Graham D. Y., Smith E. M., Gerba C. P. 1979. Rotavirus stability and inactivation. J. Gen. Virol. 43:403–409 [DOI] [PubMed] [Google Scholar]
- 16. Estes M. K., Kapikian A. Z. 2007. Rotaviruses, p. 1917–1974 In Knipe D. M., et al. (ed.), Fields virology, 5th ed., vol. 2 Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 17. Farthing M. J. 2001. Treatment of gastrointestinal viruses. Novartis Found. Symp. 238:289–300; discussion, 300-305 [DOI] [PubMed] [Google Scholar]
- 18. Frolov A., et al. 2000. High density lipoprotein-mediated cholesterol uptake and targeting to lipid droplets in intact L-cell fibroblasts. A single- and multiphoton fluorescence approach. J. Biol. Chem. 275:12769–12780 [DOI] [PubMed] [Google Scholar]
- 19. Gangl A. 1975. The lipid metabolism of the small intestine and its correlation to the lipid and lipoprotein metabolism of the total organism. Acta Med. Austriaca Suppl. 2:1–49 [PubMed] [Google Scholar]
- 20. Geyer J., Wilke T., Petzinger E. 2006. The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships. Naunyn Schmiedebergs Arch. Pharmacol. 372:413–431 [DOI] [PubMed] [Google Scholar]
- 21. Greenspan P., Mayer E. P., Fowler S. D. 1985. Nile red: a selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 100:965–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heubi J. E., Balistreri W. F. 1980. Bile salt metabolism in infants and children after protracted infantile diarrhea. Pediatr. Res. 14:943–946 [DOI] [PubMed] [Google Scholar]
- 23. Heubi J. E., Balistreri W. F., Suchy F. J. 1982. Bile salt metabolism in the first year of life. J. Lab. Clin. Med. 100:127–136 [PubMed] [Google Scholar]
- 24. Karpen S. J. 1999. Bile acids go nuclear! Hepatology 30:1107–1109 [DOI] [PubMed] [Google Scholar]
- 25. Kelly D. A. (ed.). 2008. Diseases of the liver and biliary system in children. Blackwell, Oxford, United Kingdom [Google Scholar]
- 26. Kordasti S., et al. 2006. Rotavirus infection is not associated with small intestinal fluid secretion in the adult mouse. J. Virol. 80:11355–11361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Makishima M., et al. 1999. Identification of a nuclear receptor for bile acids. Science 284:1362–1365 [DOI] [PubMed] [Google Scholar]
- 28. Maloney P. R., et al. 2000. Identification of a chemical tool for the orphan nuclear receptor FXR. J. Med. Chem. 43:2971–2974 [DOI] [PubMed] [Google Scholar]
- 29. McNeal M. M., et al. 2006. Protection against rotavirus shedding after intranasal immunization of mice with a chimeric VP6 protein does not require intestinal IgA. Virology 346:338–347 [DOI] [PubMed] [Google Scholar]
- 30. Miyanari Y., et al. 2007. The lipid droplet is an important organelle for hepatitis C virus production. Nat. Cell Biol. 9:1089–1097 [DOI] [PubMed] [Google Scholar]
- 31. Mohan K. V., Muller J., Atreya C. D. 2008. Defective rotavirus particle assembly in lovastatin-treated MA104 cells. Arch. Virol. 153:2283–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ogawa K., et al. 2009. Hepatitis C virus utilizes lipid droplet for production of infectious virus. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 85:217–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O'Neal C. M., Harriman G. R., Conner M. E. 2000. Protection of the villus epithelial cells of the small intestine from rotavirus infection does not require immunoglobulin A. J. Virol. 74:4102–4109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parks D. J., et al. 1999. Bile acids: natural ligands for an orphan nuclear receptor. Science 284:1365–1368 [DOI] [PubMed] [Google Scholar]
- 35. Patton J. B., Rowland R. R., Yoo D., Chang K. O. 2009. Modulation of CD163 receptor expression and replication of porcine reproductive and respiratory syndrome virus in porcine macrophages. Virus Res. 140:161–171 [DOI] [PubMed] [Google Scholar]
- 36. Reed L. J., Muench H. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493–497 [Google Scholar]
- 37. Rossignol J. F., Abu-Zekry M., Hussein A., Santoro M. G. 2006. Effect of nitazoxanide for treatment of severe rotavirus diarrhoea: randomised double-blind placebo-controlled trial. Lancet 368:124–129 [DOI] [PubMed] [Google Scholar]
- 38. Samsa M. M., et al. 2009. Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS Pathog. 5:e1000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sirvent A., et al. 2004. The farnesoid X receptor induces very low density lipoprotein receptor gene expression. FEBS Lett. 566:173–177 [DOI] [PubMed] [Google Scholar]
- 40. Trauner M. 2003. When bile ducts say NO: the good, the bad, and the ugly. Gastroenterology 124:847–851 [DOI] [PubMed] [Google Scholar]
- 41. Trauner M., Boyer J. L. 2003. Bile salt transporters: molecular characterization, function, and regulation. Physiol. Rev. 83:633–671 [DOI] [PubMed] [Google Scholar]
- 42. Ward R. L., McNeal M. M., Sheridan J. F. 1990. Development of an adult mouse model for studies on protection against rotavirus. J. Virol. 64:5070–5075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Watanabe M., et al. 2004. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Invest. 113:1408–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yamagata K., et al. 2004. Bile acids regulate gluconeogenic gene expression via small heterodimer partner-mediated repression of hepatocyte nuclear factor 4 and Foxo1. J. Biol. Chem. 279:23158–23165 [DOI] [PubMed] [Google Scholar]
- 45. Yu J., et al. 2002. Lithocholic acid decreases expression of bile salt export pump through farnesoid X receptor antagonist activity. J. Biol. Chem. 277:31441–31447 [DOI] [PubMed] [Google Scholar]