Abstract
Noninvasive, ion-selective vibrating microelectrodes were used to measure the kinetics of H+, Ca2+, K+, and Cl− fluxes and the changes in their concentrations caused by illumination near the mesophyll and attached epidermis of bean (Vicia faba L.). These flux measurements were related to light-induced changes in the plasma membrane potential. The influx of Ca2+ was the main depolarizing agent in electrical responses to light in the mesophyll. Changes in the net fluxes of H+, K+, and Cl− occurred only after a significant delay of about 2 min, whereas light-stimulated influx of Ca2+ began within the time resolution of our measurements (5 s). In the absence of H+ flux, light caused an initial quick rise of external pH near the mesophyll and epidermal tissues. In the mesophyll this fast alkalinization was followed by slower, oscillatory pH changes (5–15 min); in the epidermis the external pH increased steadily and reached a plateau 3 min later. We explain the initial alkalinization of the medium as a result of CO2 uptake by photosynthesizing tissue, whereas activation of the plasma membrane H+ pump occurred 1.5 to 2 min later. The epidermal layer seems to be a substantial barrier for ion fluxes but not for CO2 diffusion into the leaf.
The onset of illumination triggers a cascade of electrical events in thylakoid and PMs of green plant tissues (Vredenberg and Tonk, 1975; Fujii et al., 1978; Hansen et al., 1987, 1989, 1993; Elzenga et al., 1995; Johannes et al., 1997). Enhanced H+ extrusion induced by light may be an important factor promoting leaf enlargement through an increase in wall extensibility (Linnemeyer et al., 1990; Elzenga et al., 1995). Activation of the H+ pump by photosynthesis might also be relevant to phloem loading and the removal of photosynthate from the mesophyll cells (Marrè et al., 1989). To understand metabolic control at the whole-plant level it is essential that such physiological implications of light-induced electrical signaling be taken into account.
Reports on the ionic basis of electrical responses to light in plants are as controversial as they are numerous. Conclusions reported from different species or under different experimental conditions are often diametrically opposite. The shape of the responses, their magnitude, and the number of phases depend strongly on the ionic composition (Elzenga et al., 1995; Johannes et al., 1997) and pH (Fujii et al., 1979; Kura-Hotta and Enami, 1981; Prins et al., 1982; Remis et al., 1994) of the medium. The typical scenario is a quick initial depolarization of the PM potential, followed in 1 to 2 min by a slower repolarization, which often (but not always) results in the hyperpolarization of the PM at the end of the transient response, 20 to 40 min after the onset of illumination (Fujii et al., 1979; Prins et al., 1980; Tazawa et al., 1986; Marrè et al., 1989; Spalding et al., 1992; Hansen et al., 1993; Blom-Zandstra et al., 1995, 1997; Johannes et al., 1997).
In spite of a large number of experimental studies, mechanisms of transient membrane potential changes and their ionic bases remain obscure. Involvement of numerous ion transporters in electrical events at the PM, including those for H+, K+, Cl−, and Ca2+, have been documented (Spalding et al., 1992; Blom-Zandstra et al., 1997; Johannes et al., 1997). However, there is no clear answer about which ion is acting as the depolarizing agent in the initial phases of PM depolarization. Most experiments have been carried out using ion-substitution protocols. So far no direct measurements of specific ion fluxes have been performed in relation to membrane depolarization by light. In addition, contributions of various ion transporters to the resulting electrical changes at the PM are very different for epidermal and mesophyll cells (Elzenga et al., 1995), which complicates the problem even more. Direct ion-specific measurements may provide a breakthrough in this old mystery.
Another question of specific interest is the involvement of the plasmalemma H+ pump in PM electrical responses. Proton pumps are central in maintaining the PM in its polarized state (Spanswick, 1981; Linnemeyer et al., 1990). There are numerous reports that the activity of the H+ pump is increased after the onset of illumination (Prins et al., 1982; Tazawa et al., 1986; Marrè et al., 1989, and refs. therein; Linnemeyer et al., 1990; Okazaki et al., 1994; Remis et al., 1994). However, it has also been observed in many cases that light causes a brief initial alkalinization of the medium (Atkins and Graham, 1971; Neuman and Levine, 1971; Hope et al., 1972; Brinckmann and Lüttge, 1975; Prins et al., 1982), not the acidification that would be produced by the activation of H+-extrusion pumping. For many years the apparent controversy between these two groups of observations has remained a submerged rock threatening the electrophysiological ship.
The reason for this controversy could be that there have been no direct measurements of net H+ fluxes from plant tissues caused by light changes. The conclusions presented in the literature were based on inferring H+ movement from measured pH changes, or on interpreting PM electrical activity suppressible by specific inhibitors of ion transport. Because pH changes may not always be accompanied by H+ transport, we needed a direct comparative measurement of pH changes and H+ fluxes caused by light.
In this study we addressed these two specific questions, the ionic basis of transient depolarization of the PM potential and the apparent inconsistency between initial alkalinization of the external medium caused by illumination and light-induced activation of the H+ pump in the PM. Using the noninvasive ion-specific microelectrode ion-flux measurement technique, we determined the kinetics of H+, Ca2+, K+, and Cl− fluxes and changes in their concentrations near bean (Vicia faba) mesophyll and attached epidermis due to illumination. It appears that the influx of Ca2+ is the main depolarizing agent in mesophyll electrical responses to light, whereas Cl− fluxes seem to be one of the major contributors to the subsequent repolarization. High temporal resolution (5 s) allowed us to find a significant delay between observed pH and H+-flux changes near the mesophyll tissue. We explain the initial alkalinization of the medium as a result of CO2 uptake by photosynthesizing tissue, whereas activation of the PM H+ pump occurs 1.5 to 2 min later. The epidermal layer seems to be a substantial barrier for ion fluxes but not for CO2 diffusion into the leaf.
MATERIALS AND METHODS
Plant Material
Plants of bean (Vicia faba L. cv Early Long Pod; Creswell's Seeds, New Norfolk, Australia) were grown from seeds in 0.5-L plastic pots containing a commercially available professional potting mixture (Debco, Tyabb, Australia). Growth conditions were 16 h/8 h light/dark (model M1500-A lighting unit, Thorne, Moonah, Australia; total irradiance = 150 W m−2 at the leaf level) with temperature ranging from 20°C (dark) to 28°C (light). Watering was four times per week with tap water. Plants were used for measurements after 20 d.
Flux Measurements
Fluxes of specific ions were measured generally as described in our previous papers (Shabala et al., 1997; Shabala and Newman, 1997; Shabala et al., 1998) using a noninvasive microelectrode ion-flux measurement system (MIFE, Unitas Consulting, Hobart, Australia; additional information is available at http://www.phys.utas.edu.au/physics/biophys). Electrode blanks were pulled from 1.5-mm borosilicate glass capillaries (GC150-10, Clark Electromedical Instruments, Pangbourne, UK), dried in the oven at 220°C for 5 h, and silanized with tributylchlorsilane (catalog no. 90796, Fluka). Cooled microelectrodes were backfilled with 500 mol m−3 CaCl2 for Ca2+, 500 mol m−3 KCl for K+ and Cl−, and 15 mol m−3 NaCl plus 40 mol m−3 KH2PO4 (adjusted to pH 6.0 using NaOH) for H+. Electrode tips were then filled with commercially available ion-selective H+ (95297), Ca2+ (21048), K+ (60031), and Cl− (24902) cocktails (all from Fluka), and electrodes were calibrated in a known set of standards. The average slope was 53 to 54 mV/pIon for monovalent ions and 26 to 27 mV/pCa for Ca2+ electrodes.
The ion-selective electrodes were mounted on an electrode holder (MMT-5, Narishige, Tokyo, Japan) providing three-dimensional positioning. Plant tissue was placed into the measuring chamber, filled with solution, and electrodes were positioned in line 50 μm above the leaf surface with their tips spaced 3 to 4 μm apart. Three different ions were measured at the same time; in all measurements a H+ electrode was used as a reference point to make results comparable. The chamber was placed on a three-way hydraulic micromanipulator (WR-88, Narashige) driven by a computer-controlled stepper motor (MO61-CE08, Superior Electric, Bristol, CT). During the flux measurements, the MIFE computer gently moved the chamber up and down, providing virtual movement of electrode tips between two positions above the plant tissue. In this study the electrodes were moved in a 10-s square-wave cycle between 50 and 90 μm above the leaf surface. The concentration of each ion was calculated from its electrochemical potential for each position. The flux of each specific ion was calculated later from the measurements of the difference in the electrochemical potential between these positions (Shabala et al., 1997). During analysis the 1st s of each half-cycle was ignored (time required for both the movement and the electrochemical settling of the electrodes).
Experimental Procedure
We used expanding leaves in positions 3 to 6 on the stem (leaf age 7–10 d) in the experiments. The leaf was excised with a razor blade 4 to 5 h before measurements were taken. If fluxes were to be measured near the intact epidermal tissue, we cut out leaf segments of 5 × 8 mm from the apical part of the leaf, avoiding major veins. For mesophyll measurements we removed the epidermal tissue before cutting the segment. To do this, we first cut leaf strips 5 mm wide and peeled off the lower epidermal layer using fine forceps. Cut leaf or mesophyll segments floated peeled-side or abaxial-surface down on the experimental solution under light from a fiber-optic light source (40 W m−2; EK1, Euromex, Sydney, Australia). No wounding effects were noticeable when fluxes were measured 4 to 5 h after segments were cut (data not shown). After 3 to 3.5 h of floating, the cut segment was mounted and transferred into the measuring chamber. During the measurements, local pH values near the tissue varied slightly in the range of 5.3 to 5.5 depending on the magnitude and direction of H+ fluxes.
We used a Perspex holder that provided gentle bending of the plant tissue to mount the cut leaf or mesophyll segment. This arrangement allowed a clear view for electrode positioning compared with planar leaf arrangement. As the 5- to 6-mm radius of the leaf bending was close to that naturally occurring, it should not have affected the cell ion exchange. A few control experiments with plane-mounted segments showed the same steady fluxes in the dark (data not shown). The holder was installed in a measuring chamber with a volume of 10 mL. The chamber was filled with solution and fixed on the hydraulic micromanipulator under the microscope. Dim-green microscope light of about 12 W m−2 was used as the background illumination tangential to the leaf surface. Experiments started 1 h after plants adapted to the dim light.
Our preliminary studies showed that ion fluxes can vary significantly with position over a range of several millimeters, even for apparently uniform mesophyll tissue in steady conditions (S. Shabala and I. Newman, unpublished data). To minimize the variability of flux measurements, we chose to perform experiments in the regions where initial flux values (dark level) were close to the average (near zero for H+ flux in control). After a suitable spot on the mesophyll tissue was selected, we measured ion fluxes for about 5 min (microscope light only) before the projector light was turned on. We used a fiber-optic projector (Intralux 4000, Volpi AG, Urdorf, Switzerland) providing 60 W m−2 illumination. Although we used dark plastic tubes covering the chlorided wire region of the electrode capillaries in most experiments, there was no special need for electrode shielding from direct light. Flux measurements continued for 40 to 60 min after the onset of illumination. The light was then turned off, the measuring chamber removed, and a new leaf or mesophyll sample was installed.
All experiments were performed in unbuffered solution containing 0.5 mol m−3 CaCl2 plus 1 mol m−3 KCl, pH 5.4, at room temperature (22°C–24°C). Arif et al. (1995) have explained the reasons for not using buffers in the bath. Heat emission from the light source was negligible.
Membrane Potential Measurements
We measured the electrical potential difference across the PM in the standard way, by impaling the cell with a microelectrode filled with 500 mol m−3 KCl. Impalements were made using the same hydraulically driven, three-dimensional manipulator that was used for flux measurements. Because the membrane-potential-measuring electrode and the ion-selective electrodes were mounted in the same holder, we were not able to move the electrodes up and down to measure fluxes of ions while measuring membrane potential. Therefore, we measured concentrations only at a position about 60 μm above the leaf tissue.
For both flux and membrane-potential measurements, we used the same reference electrode. A chlorided silver wire was inserted into a thin plastic tube or glass microelectrode with a broken tip containing 1000 mol m−3 KCl in 2% agar. Because it was at least 6 cm from the measured leaf sample, the diffusion of K+ ions from it to the leaf was negligible. We based this conclusion on the absence of any measurable drift in K+ concentration near the tissue in steady conditions.
Statistics
We obtained most of the data shown in the figures from five to eight segments taken from five or six individual plants. Because a simple averaging could mask important features such as oscillations in plant transient responses, we have in some cases shown the records for several individual plants in each variant (see Fig. 4). Finally, we felt that the simple averaging under genetic or physiological variability could also mask the time delay between changes in PM potential and changes in ion flux or concentrations. For this reason, the data presented in Figures 1 and 2 are for experiments taken as typical of this set of experimental conditions. The qualitative character of these data was reproduced for leaf segments obtained from several (four or five) individual leaves. Statistical information on the magnitude and phase duration of such responses appears in the text.
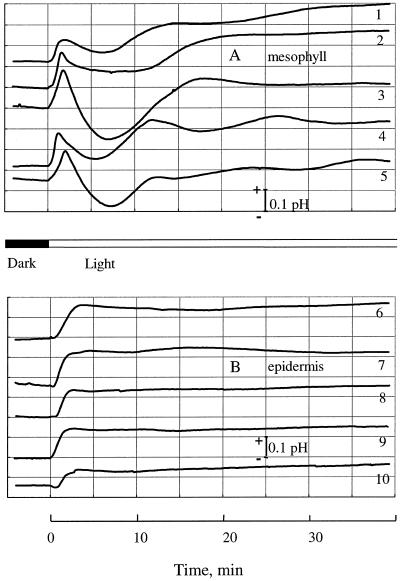
Figure 4.
Light-induced changes in pHo near the mesophyll (A) and the attached epidermis (B). Five individual traces for each variant are shown. Traces 1 to 5 were used for calculating the means shown in Figure 3A.
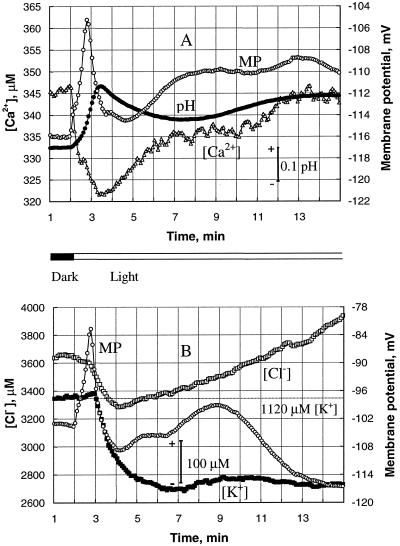
Figure 1.
Transient changes in mesophyll cell membrane potential (○) and pHo (•), and [Ca2+]o (▵), [K+]o (▪), and [Cl−]o (□) caused by transition from dark to light (at 2 min). The data shown are from two typical individual plants (A and B). A, Changes in pHo and [Ca2+]o measured with the membrane potential. B, Changes in [K+]o and [Cl−]o measured with the membrane potential. Each point is the average of measurements over a 5-s interval. Both [K+]o and [Cl−]o started to decrease only after the PM was significantly depolarized, with a delay of about 50 s after illumination.
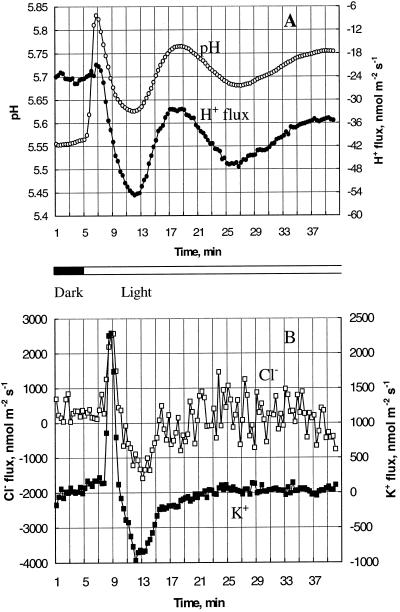
Figure 2.
Light-induced changes in net fluxes (inward positive) of H+ (•), K+ (▪), and Cl− (□), and pHo (○) near the mesophyll tissue measured simultaneously for a typical bean plant. Each point is the average of measurements over a 20-s interval. A, pHo and H+ flux; B, K+ and Cl− flux. A significant delay of about 2 min can be observed for fluxes of all three ions, although the pH change occurred immediately after the light was turned on at 5 min.
RESULTS
Light-Induced Transients in Mesophyll Membrane Potential and Solution Ion Concentration
Transition from dark to light triggered a multiphase, transient change of the membrane potential in the mesophyll cells of bean leaves (Fig. 1). The resting potential of the PM was slightly more negative than −100 mV in the dark. The onset of illumination caused a rapid (45–50 s) depolarization of 15 to 20 mV, which was followed by a slower repolarization lasting 2 to 2.5 min. Afterward, the membrane potential fluctuated in a complex way (individually for each plant), showing several oscillatory cycles of 5 to 15 min each. Often (but not always) there was a significant hyperpolarization of up to −20 mV compared with the dark level 20 to 30 min after the light was turned on.
Changes in membrane potential were always accompanied by changes in [H+]o, [Ca2+]o, [K+]o, and [Cl−]o near the mesophyll tissue. Figure 1 shows examples of individual records from two typical plants. For one of them (Fig. 1A), changes in pHo and [Ca2+]o were measured together with membrane potential; for the second plant, transient changes in [K+]o and [Cl−]o were recorded (Fig. 1B).
Although the time course of membrane-potential transients was qualitatively similar for all plants, concentration changes for the different ions were different. pHo and [Ca2+]o changes started immediately (within the 5-s time resolution) after the light was turned on (Fig. 1A). The onset of illumination caused an initial quick rise of the pHo near the mesophyll tissue; this fast alkalinization was followed by slower, transient pHo changes. Oscillations of 5 to 15 min were also evident (see Fig. 4A) for most plants. As in the case of membrane potential, the number of oscillatory cycles and their durations varied between individual plants. However, for each plant the final pHo value in light was more alkaline by 0.16 ± 0.02 (n = 7) than it was before the onset of illumination.
Changes in [Ca2+]o generally were of similar form as changes in [H+]o. There was an immediate drop in the [Ca2+]o of about 25 to 30 μm, followed by slow recovery in 1.5 to 2 min (Fig. 1). Minimum [Ca2+]o always occurred at the same time as maximum alkalinization of the adjacent medium. Another regular feature of light-induced transients was a small but significant delay between the first peak in membrane potential and the pHo changes. For each plant, the first extreme in pHo and [Ca2+]o transients occurred 25 to 30 s after the membrane-potential maximum.
However, unlike the pHo and [Ca2+]o changes for the similar membrane potential transient, noticeable changes in [K+]o and [Cl−]o occurred only after a significant delay (Fig. 1B). Both [K+]o and [Cl−]o started to decrease only when membrane potential reached its peak of depolarization. Although between-plant variability in transient [K+]o and [Cl−]o responses was much larger than that for pHo and [Ca2+]o changes, it is clear that K+ and Cl− are not required as depolarizing agents for the PM in bean mesophyll cells.
Transient Ion-Flux Changes in the Mesophyll
In other experiments the net fluxes of H+, Ca2+, K+, and Cl− were measured in response to illumination. Figure 2 shows a typical example from one individual plant, where net fluxes of H+, K+, and Cl− were measured simultaneously in the same experiment. As membrane-potential measurements were impossible with the moving electrode probe, transient pHo changes have been used as a reference point instead of membrane potential. These typical pHo changes appear in Figure 2A and make these results comparable with those reported in Figure 1.
The data presented in Figure 2 confirm our previous findings. Light caused a significant influx of both K+ and Cl− (which is in good agreement with the decrease in [K+]o and [Cl−]o shown in Fig. 1B), but only after a significant delay of 2 to 3 min (Fig. 2B). The most surprising result was that, in spite of the significant initial increase in pHo near the mesophyll tissue, no significant change in net H+ flux was observed during the first 2 min after light application (Fig. 2A). The H+ flux started to change only after the pHo value had reached its first peak at 1.5 min and had started to decrease.
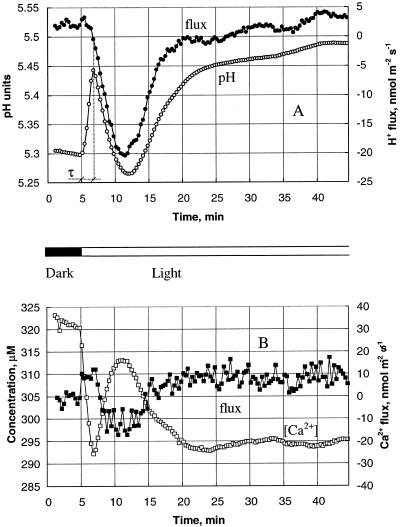
We studied this last observation in more detail in the next experiments. Figure 3A shows average pHo and H+ flux changes measured near the mesophyll tissues of eight individual plants after the onset of illumination. As a result of the light-induced transient, the average H+ flux decreased from a slightly positive value of 1.7 ± 5.9 nmol m−2 s−1 (net influx) down to −20 nmol m−2 s−1. The H+ flux reached its minimum value 7 min after the light was turned on, and slowly returned to its dark level (3.6 ± 4.3 nmol m−2 s−1) in the next 15 to 20 min. The delay (τ) between the start of the pHo rise and the beginning of H+-flux change (see Fig. 3A) was 100 ± 13 s (n = 8). Afterward, pHo changes were qualitatively consistent with those expected from the measured H+ flux.
Figure 3.
Average changes in ion fluxes (solid symbols) and concentrations (open symbols) for five plants. A, pHo and H+ flux; B, [Ca2+]o and Ca2+ flux. Means are over 20-s intervals and the se for fluxes is shown in Figure 7. The se for concentration = 5.7 μm; the se for pH changes = 0.03.
There was no such delay between changes in Ca2+ flux and its concentration (Fig. 3B). Light application immediately initiated Ca2+ uptake by the mesophyll. This resulted in decreased [Ca2+]o close to the tissue (Fig. 3B). The Ca2+ influx lasted only 2 to 2.5 min, and was followed by transient efflux over the next 7 to 8 min. Ca+ fluxes stabilized at a slightly positive level (9.7 ± 5.6 nmol m−2 s−1) 15 to 20 min after the onset of illumination. Changes in the Ca2+ flux were always qualitatively consistent with changes in [Ca2+]o.
Effect of Epidermis on pHo and Ion Fluxes
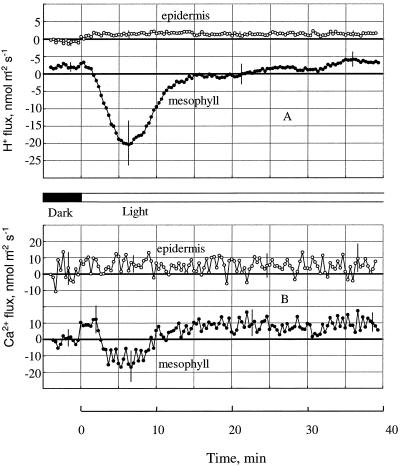
When we measured pHo changes near the intact leaf segment (with epidermis present), the qualitative course of transient responses was very different from that measured near the isolated mesophyll (Fig. 4). After the onset of illumination, the pHo for the epidermis rose steadily for a few minutes before reaching a plateau (Fig. 4B). Afterward, the pHo remained constant, without the significant drop and subsequent fluctuations for the pHo measured near the mesophyll. The magnitude of the transient alkalinization was similar for both tissues (ΔpH 0.15 ± 0.02). The pHo saturation near the epidermis occurred later than the first peak in mesophyll pH changes (2.8 ± 0.25 and 1.7 ± 0.15 min, respectively). There was also a slight delay of 30 ± 5 s (n = 6) between the onset of illumination and the beginning of pHo changes from the epidermis. We observed no such delay for mesophyll tissue.
Even more pronounced was the difference between ion fluxes from mesophyll and epidermis (Fig. 5). Both H+ and Ca2+ fluxes from epidermis were negligible, and, when the light was turned on, there was a barely noticeable increase in H+ influx measured near the epidermis. Changes in Ca2+ fluxes were less than the level of noise in the system.
Figure 5.
Fluxes of H+ (A) and Ca2+ (B) induced by light near the mesophyll (solid symbols) and attached epidermis (open symbols). Average data from five plants in each variant are shown. Bars = ±se.
DISCUSSION
The Ionic Basis of Electrical Events at the PM: Is Ca2+ a Depolarizing Agent?
In general, changes in membrane potential reflect underlying changes in the conductance of ion channels and the activity of pumps. For this reason, the ionic basis of the transient depolarization of the PM seems to be a foundation for our understanding of light-induced bioelectrogenesis in plants. The involvement of all major ions, in particular H+, K+, Ca2+, and Cl−, has been suggested previously by numerous researchers; however, the reported data are controversial.
Prins et al. (1982) reported a pause of approximately 5 min between the reduction of [K+]o and the pHo rise near the lower side of Potamogeton lucens. According to Fujii et al. (1978), K+ and Cl− ions in solution were not essential for light-induced membrane-potential changes, whereas such membrane responses were completely inhibited by the absence of Ca2+. Johannes et al. (1997) supported this point of view, showing that K+ influx was not crucial for membrane-potential depolarization, because K+ could be removed from the bathing medium without affecting the electrical response of the PM. According to these and other findings, the crucial ion for PM depolarization is Ca2+; when Ca2+ was omitted from the solution, membrane-potential transients were abolished (Ermolayeva et al., 1996; Johannes et al., 1997).
Remis et al. (1994) challenged this point of view by showing that transient changes in the PM depend strongly on the presence of K+ in the bathing medium. When K+ was present in the medium, light induced the extrusion of H+ and the uptake of K+ by Elodea densa, which caused membrane hyperpolarization, not depolarization. However, in the absence of K+, the PM was initially depolarized by the onset of illumination (Marrè et al., 1989).
Similar controversy exists for other ions. Conductance changes for Cl− have been suggested by Spalding et al. (1992), and were supported later by Blom-Zandstra et al. (1997) as one of the possibilities for PM depolarization. In their experiments in pea, Elzenga et al. (1995) found that in mesophyll cells the transient depolarization depended on the [Cl−]o and was unaffected by changes in the [Ca2+]o or [K+]o. In contrast, when isolated epidermal tissue was measured, the membrane depolarization was much smaller and was enhanced by increasing the [Ca2+]o. They concluded that the ionic basis of this depolarization differs qualitatively between the epidermis and the mesophyll, and suggested that light-induced depolarization of the PM in pea mesophyll seems to be mediated by an increased efflux of Cl−, whereas membrane-potential changes in the epidermis reflect changes in the fluxes of Ca2+ and the activity of an ATPase-dependent H+-pump in the PM (Elzenga et al., 1995).
The major reason for such controversy is that no direct flux measurements have been performed so far (to our knowledge) to elucidate the ionic basis of cell electrical responses to light. Because of the numerous feedback loops and interaction between ion transporters, reliable selective inhibition or enhancement of one of them is not feasible. Furthermore, even when the experimental solution is initially lacking one particular ion, it does not necessarily mean that there will be no flux of that ion from the measured tissue. We have previously observed that in a short time many ions (K+ in particular) can be released from the cell into the bath in concentrations large enough to produce flux able to change the membrane potential by 20 to 30 mV in 1 min (S. Shabala and I. Newman, unpublished data).
To our knowledge, the only reported data on light-induced flux measurements were given by Johannes et al. (1997), who linked patch-clamp measurements on caulomal filaments of the moss Physcomitrella patens with measurements of ion fluxes induced by red light. According to their findings, Ca2+, K+, and anion-permeable channels were open at the peak of light-induced membrane depolarization. Ca2+ influx and anion efflux coincided with the depolarizing phase, whereas K+ influx occurred only for the first 30 s. Dramatic transient K+ efflux associated with PM repolarization took place later (unfortunately, the flux data are only mentioned but not shown in that paper). In addition, Johannes et al. (1997) used absorption spectrometry applied to samples taken discretely at 30-s or 1-min intervals for their flux measurements, and, because the peak of membrane depolarization occurred within 2 to 15 s, this rate of sampling was clearly inadequate and made their conclusions on flux kinetics questionable.
Our study is the first to our knowledge to report direct measurements of light-induced ion fluxes near green plant tissue. The peak of the depolarization occurred at about 50 s, and the depolarization process was clearly biphasic, with a typical shoulder at about 15 s (Fig. 1). All of this is in good agreement with membrane-potential data reported previously (Spalding et al., 1992; Elzenga et al., 1995).
There were immediate changes in pHo and [Ca2+]o after the light was turned on (Fig. 1A). However, the immediate pHo changes were not accompanied by any significant change in net H+ flux near the tissue (Fig. 2A). The statistically significant delay of 100 ± 13 s (Fig. 3A) before H+ flux changed suggests that activation of the PM proton pump began only after the PM was depolarized.
Activities of K+ and Cl− transporters were also affected much later, when membrane potential reached its peak of depolarization (Fig. 1B). These data are supported by direct measurements of K+ and Cl− fluxes near the mesophyll tissue (Fig. 2B). We observed a delay of up to 2 min between the onset of illumination and the beginning of changes in K+ and Cl− fluxes. Although in some plants this delay was not very pronounced (largely due to extremely high variability in initial K+ fluxes), there is no doubt that neither K+ nor Cl− flux is required as a depolarizing agent in the PM of bean mesophyll cells.
Among the four different ions that we measured, the most likely candidate for membrane depolarization is Ca2+. There was an increase in the net Ca2+ uptake immediately after the light was turned on (within the 5-s time resolution) (Figs. 1A and 3B). If we assume a membrane capacitance of 2 μF cm−2, a Ca2+ influx as small as 0.05 nmol m−2 s−1 would be enough to depolarize the membrane by 25 mV in 50 s (the typical rate of depolarization shown in Fig. 1). In our experiments we observed a Ca2+ influx of approximately 10 nmol m−2 s−1 (Fig. 5B) near the mesophyll tissue within the first 2 min after the onset of illumination, which is 2 orders of magnitude greater than that needed to produce the observed depolarization of the PM. Thus, our data support the work of others in considering Ca2+ to be a potent depolarizing agent in the light-induced electrical responses at the PM (Weisenseel and Ruppert, 1977; Takagi and Nagai, 1988; Spalding and Cosgrove, 1992; Elzenga et al., 1995).
To explain our data, we suggest a scenario similar to the one proposed by Johannes et al. (1997) for phytochrome-mediated, red-light-induced membrane-potential transients in P. patens. A light-induced Ca2+ influx of about 10 nmol m−2 s−1 would be expected to cause a significant increase in [Ca2+]cyt. Increased [Ca2+]cyt can also stimulate H+-ATPase activity, resulting in an increased H+ efflux (Elzenga et al., 1995). It may take a while before [Ca2+]cyt is elevated high enough to make this activation possible, and this could be the reason for the approximately 2-min delay between light application and the beginning of the H+ efflux observed in our experiments (Fig. 3A).
Another important observation is the initial increase in the net Cl− influx (Fig. 2B) (not an efflux, as was postulated by Elzenga et al. [1995]). Because inward Cl− movement in higher-plant cells is an active process mediated by a Cl− pump (Felle, 1994), we suggest that elevated [Ca2+]cyt activates the Cl− pump in a manner similar to that suggested for the H+ pump. This also provides a reason for the 2-min delay observed for Cl−-flux activation by light. Together with the H+ efflux, this increased Cl− influx causes PM repolarization. Therefore, our data rule out Cl− participation in the PM depolarization and indicate its involvement in the repolarization process. K+ seems to function as the equilibrium ion, moving passively to compensate for light-induced charge movement of Cl− or H+, which is consistent with other reports (Prins et al., 1982; Staal et al., 1994). It is known that Ca2+ can directly activate a Ca2+-dependent K+ channel in some species (Elzenga and Van Volkenburgh, 1993; Johannes et al., 1997). The subsequent membrane repolarization may trigger Ca2+-permeable channels to close, leading to a decrease in the [Ca2+]cyt and to a change in the net Ca2+ flux from influx to efflux (Fig. 3B).
Apparent Inconsistency between Light-Induced Changes in pHo and H+ Flux
If the initial alkalinization of the bath solution near the mesophyll induced by light that we (Figs. 1, 2A, and 4A) and others (Atkins and Graham, 1971; Neuman and Levine, 1971; Hope et al., 1972; Prins et al., 1982) have observed is due to modified activity of H+ transporters, it could be achieved by either a decrease in the active H+ extrusion or an increase in the activity of passive H+ inward transporters. In each case, we would expect an increased H+ influx. But in our experiments, switching on the light induced significant H+ efflux, which occurred after a distinct time delay (nearly 2 min after pHo changes started; see Fig. 3A). Alkalinization of similar magnitude (Fig. 4B) was also evident near the attached epidermal tissue, where H+ fluxes were nearly zero (Fig. 5A).
This apparent inconsistency cannot be explained by a methodological fault in the flux measurements obtained by using the MIFE technique. When measured at the same time as H+, changes in Ca2+ flux and [Ca2+]o were in good agreement with each other and showed no delay after light onset (Fig. 3B). We also ruled out the effect of light on the measuring electrodes, because there was no pHo change when the electrodes were far away from the tissue (data not shown). Therefore, this apparent inconsistency has a biological origin and means that, in spite of the alkalinization of the medium, there is no net H+ electrochemical gradient near the leaf surface in the first 100 s after the dark-to-light transition.
We believe that the initial alkalinization of the medium near the leaf tissue in the absence of net H+ fluxes observed in bean is a result of quick CO2 uptake by photosynthesizing tissue after the onset of illumination.
CO2 dissolved from the atmosphere is normally present in solution (Lucas and Berry, 1985; Arif et al., 1995; Raven, 1997). Following 1 h of dark adaptation, the amount of CO2 was expected to be significant. In solution, dissolved CO2 reacts with water to form HCO3−:
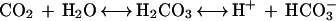 |
1 |
The combined pK of these reactions is 6.3 (Neumann and Levine, 1971), which is above the pH of our experimental solution (5.3–5.5), shifting the equilibrium toward CO2 formation. When the light is turned on, the uptake of CO2 by photosynthesizing cells would cause a decrease in the concentration of H2CO3, which would cause an association of H+ with HCO3−, resulting in H+ leaving the medium. If the CO2 flux is much faster than the H+ flux through the medium, there will be an increase in medium pH with a net H+ flux near zero. This is what we observed.
This explanation is in good agreement with reports that the direction of light-induced pH changes is strongly dependent on the pH of the external medium. Illumination of a Cyanidium caldarium cell suspension caused a rapid alkalinization of the medium at pH 7.0, whereas a slower acidification occurred at pH 4.0 in the light (Kura-Hotta and Enami, 1981). Light-induced transient acidification of the medium measured at pH 1.0 to 3.0 turned into a light-induced alkalinization in the range of pH 5.0 to 7.0 for the green alga Dunaliella acidophila (Remis et al., 1994).
We also found evidence supporting this explanation when we pretreated plants for 3 h at both pH 4.0 and pH 7.0 (data not shown). Changing the bathing solution to be more alkaline is known to shift the equilibrium between CO2 and HCO3− (see Eq. 1) toward HCO3− formation (Yin et al., 1996). Therefore, one would expect HCO3−-induced pHo changes of the bath solution to be more significant. In our experiments, pHo measured at 60 μm from the tissue increased by up to 0.63 ± 0.03 units (compared with 0.15 ± 0.02 for the control at pH 5.4) in less than 2 min after the light was turned on. On the other hand, a shift into the more acidic region (pH 4.0) was expected to reduce HCO3− formation and inhibit the rapid, light-induced pH rise observed in our experiments. In such experiments we found not only that the initial alkalinization was completely suppressed, but that even the barely noticeable alkalinization of the external solution took place 5 min after the onset of illumination, when activity of the H+ pump was expected to start decreasing (data not shown).
Both CO2 and HCO3− can be used by plants during photosynthesis (Prins et al., 1982; Lucas and Berry, 1985; Raven, 1997). It seems reasonable to assume, however, that an efficient mechanism of HCO3− transport through the PM would be more appropriate for aquatic than for terrestrial plant tissue. Most experiments with light-induced pH changes have used aquatic plant species. However, even for some cyanobacteria and microalgae, CO2 diffusion was preferred to HCO3− uptake (Lucas and Berry, 1985, and refs. therein). Some aquatic organisms can utilize only CO2 and not HCO3− (Prins et al., 1982, and refs. therein). We argue that CO2 transport is appropriate for the mesophyll tissues of terrestrial plants, in which atmospheric CO2 is the normal source of inorganic carbon.
Prins et al. (1980) reported an immediate decrease in the CO2 concentration after the onset of illumination for some aquatic angiosperms that use CO2 as their source of inorganic carbon for photosynthesis. The present study provides evidence that a similar mechanism exists in the mesophyll tissues of terrestrial plants. According to Hansen et al. (1993), CO2 reaches the photosynthetic apparatus quite rapidly, within a few seconds. This can explain the absence of a detectable delay in pHo rise close to the tissue as a result of the dark-to-light transition in our experiments.
Earlier we suggested that the nearly 2-min delay between light application and the beginning of H+ efflux observed in our experiments could be explained as the time required to elevate [Ca2+]cyt to a level that can stimulate H+-ATPase activity. This delay may also be mediated by some other metabolic process involved in the light signal transduction to the PM ATPase. Linnemeyer et al. (1990) discussed at least four different mechanisms for this process, and there is clearly a need for more experiments in this direction. It has been argued that light activation of H+ uptake from the stroma to the thylakoid lumen should result in alkalinization of the cytosol (Hansen et al., 1987; Linnemeyer et al., 1990; Heber et al., 1994; Okazaki et al., 1994; Yin et al., 1996). According to the findings of Linnemeyer et al. (1990), this shift in cytosolic pH from neutral to more alkaline should result in a significant decrease in PM ATPase activity (the optimal pH for which is close to 6.5 for bean mesophyll cells). Therefore, we can rule out the direct control of PM ATPase activity by cytosolic pH as the sole factor regulating the activity of the H+ pump. More experiments are required to clarify this issue.
The subsequent decrease in H+ efflux 7 min after the onset of illumination (Fig. 3A) could reflect the feedback mechanism of cytoplasmic pH homeostasis of the living cell. Its details are unknown but may include slowing down the activity of the H+ pump via a substrate-depletion effect (Hansen et al., 1987). Participation of other ion-transport systems should also be considered (Marrè et al., 1989; Blom-Zandstra et al., 1997).
Effect of the Epidermis
The epidermal layer seemed to be an effective barrier for ion fluxes in our experiments (Fig. 5). Only a slight, light-induced H+ influx was discernible outside the epidermis (Fig. 5A), and no changes in Ca2+ flux could be seen (Fig. 5 B). At the same time there was a significant initial alkalinization of the medium near the epidermis (Fig. 4B), of about the same magnitude as that observed near the isolated mesophyll tissue (ΔpH 0.15 ± 0.02). Unlike the classical multiphase transients from the mesophyll, however, the pHo near the attached epidermis rose steadily before reaching its saturation level about 3 min after the light application.
In spite of earlier reports on the lack of light-induced changes of the membrane potential in epidermal tissues (Lüttge and Pallaghy, 1969), it seems to be accepted now that chlorophyll-deficient tissues also exhibit electrical changes in response to light. Fujii et al. (1978) and Johannes et al. (1997) have also reported phytochrome-mediated, light-induced membrane-potential changes. Mechanisms of the light-induced depolarization in mesophyll and epidermis seem to be very different (Elzenga et al., 1995).
Although we also observed membrane-potential changes from epidermal cells in our experiments (data not shown), we measured the fluxes of H+ and Ca2+ at nearly zero levels outside the epidermis (Fig. 5). The epidermal layer (with its cuticule present) was an effective barrier for ion fluxes but not for CO2 diffusion into the leaf. Further experiments should take this point into account. Working on the isolated epidermis, an alternative method could measure fluxes from the inside of the strip, where the cutinized layer is absent.
conclusion
The first mention of plant bioelectric responses to light appeared in a study over 100 years ago (Haake, 1892), and hundreds of papers have been published on this subject since that time. Having been obtained from different plant materials and using different experimental conditions and techniques, these results are often quite contradictory. The interaction and interdependence of the numerous ion transporters are far from well understood. Recent publications on whole-cell, patch-clamp measurements from plant protoplasts have revealed the advantages of that technique in studying the mechanisms of ion-channel responses to light variations (Blom-Zandstra et al., 1995, 1997; Johannes et al., 1997). In this paper we have shown the value of noninvasive, specific ion-flux measurements in addressing the same problem. A combination of the two techniques may be ideal in casting more light on this old and mysterious problem of the mechanisms of light-induced plant bioelectrogenesis.
ACKNOWLEDGMENTS
We are grateful to Dr. Bruce Scott for his helpful discussion of this work. We also thank Mrs. Svetlana Shabala for her technical assistance in the preparation of the manuscript.
Abbreviations:
- [Ca2+]cyt
cytosolic free Ca2+ concentration
- [XX]o
external concentration
- pHo
external pH
- PM
plasma membrane
Footnotes
This work was supported by an Australian Research Council grant to I.N.
LITERATURE CITED
- Arif I, Newman IA, Keenlyside N. Proton flux measurements from tissues in buffered solution. Plant Cell Environ. 1995;18:1319–1324. [Google Scholar]
- Atkins CA, Graham D. Light-induced pH changes by cells of Chlamydomonas reinhardii: dependence on CO2 uptake. Biochim Biophys Acta. 1971;226:481–485. doi: 10.1016/0005-2728(71)90117-4. [DOI] [PubMed] [Google Scholar]
- Blom-Zandstra M, Koot HTM, van Hattum J, Vogelzang SA. Isolation of protoplasts for patch-clamp experiments: an improved method requiring minimal amounts of adult leaf or root tissue from monocotyledonous or dicotyledonous plants. Protoplasma. 1995;185:1–6. [Google Scholar]
- Blom-Zandstra M, Koot HTM, van Hattum J, Vogelzang SA. Transient light-induced changes in ion channel and proton pump activities in the plasma membrane of tobacco mesophyll protoplasts. J Exp Bot. 1997;48:1623–1630. [Google Scholar]
- Elzenga JTM, Prins HBA, Van Volkenburgh E. Light-induced membrane potential changes of epidermal and mesophyll cells in growing leaves of Pisum sativum. Planta. 1995;197:127–134. [Google Scholar]
- Elzenga JTM, Van Volkenburgh E. Ion channels in the plasma membrane of epidermal and mesophyll cells of growing pea leaves. Plant Physiol. 1993;102:S106. doi: 10.1104/pp.113.4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermolayeva E, Hohmeyer H, Johannes E, Sanders D. Calcium-dependent membrane depolarization activated by phytochrome in the moss Physcomitrella patens. Planta. 1996;199:352–358. [Google Scholar]
- Felle HH. The H+/Cl− symporter in root-hair cells of Sinapis alba. Plant Physiol. 1994;106:1131–1136. doi: 10.1104/pp.106.3.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S, Shimmen T, Tazawa M. Light-induced changes in membrane potential in Spirogyra. Plant Cell Physiol. 1978;19:573–590. [Google Scholar]
- Fujii S, Shimmen T, Tazawa M. Effect of intracellular pH on the light-induced potential change and electrogenic activity in tonoplast-free cells of Chara australis. Plant Cell Physiol. 1979;20:1315–1328. [Google Scholar]
- Haake O. Über die ursachen electrischer ströme in pflanzen. Flora. 1892;75:455–487. [Google Scholar]
- Hansen U-P, Dau H, Vanselow KH, Fisahn J, Stein S, Kolbowski J. Thylakoid and plasma fluxes. In: Dainty J, DeMichelis MI, Marrè E, Rasa-Caldogno F, editors. Plant Membrane Transport: The Current Position. Amsterdam: Elsevier; 1989. pp. 345–351. [Google Scholar]
- Hansen U-P, Kolbowski J, Dau H. Relationship between photosynthesis and plasmalemma transport. J Exp Bot. 1987;38:1965–1981. [Google Scholar]
- Hansen U-P, Moldaenke C, Tabrizi H, Ramm D. The effect of transthylakoid proton uptake on cytosolic pH and the imbalance of ATP and NADPH/H+ production as measured by CO2- and light-induced depolarization of the plasmalemma. Plant Cell Physiol. 1993;34:681–695. [Google Scholar]
- Heber U, Wagner U, Kaiser W, Neimanis S, Bailey K, Walker D. Fast cytoplasmic pH regulation in acid-stressed leaves. Plant Cell Physiol. 1994;35:479–488. [Google Scholar]
- Hope AB, Lüttge U, Ball E. Photosynthesis and apparent proton fluxes in Elodea canadensis. Z Pflanzenphysiol. 1972;68:63–72. [Google Scholar]
- Johannes E, Ermolayeva E, Sanders D. Red light-induced membrane potential transients in the moss Physcomitrella patens: ion channel interaction in phytochrome signalling. J Exp Bot. 1997;48:599–608. doi: 10.1093/jxb/48.Special_Issue.599. [DOI] [PubMed] [Google Scholar]
- Kura-Hotta M, Enami I. Light-induced H+ efflux from intact cells of Cyanidium caldarium. Plant Cell Physiol. 1981;22:1175–1183. [Google Scholar]
- Linnemeyer PA, Van Volkenburgh E, Cleland RE. Characterization and effect of light on the plasma membrane H+-ATPase of bean leaves. Plant Physiol. 1990;94:1671–1676. doi: 10.1104/pp.94.4.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas WJ, Berry JA. Inorganic carbon transport in aquatic photosynthetic organisms. Physiol Plant. 1985;65:539–543. [Google Scholar]
- Lüttge U, Pallaghy CK. Light triggered transient changes of membrane potentials in green cells in relation to photosynthetic electron transport. Z Pflanzenphysiol. 1969;61:58–67. [Google Scholar]
- Marre MT, Albergoni FG, Moroni A, Marre E. Light-induced activation of electrogenic H+ extrusion and K+ uptake in Elodea densa depends on photosynthesis and is mediated by the plasma membrane H+-ATPase. J Exp Bot. 1989;40:343–352. [Google Scholar]
- Neumann J, Levine RP. Reversible pH changes in cells of Chlamydomonas reinhardtii resulting from CO2 fixation in the light and its evolution in the dark. Plant Physiol. 1971;47:700–704. doi: 10.1104/pp.47.5.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki Y, Tazawa M, Iwasaki N. Light-induced changes in cytosolic pH in leaf cells of Egeria densa: measurements with pH-sensitive microelectrodes. Plant Cell Physiol. 1994;35:943–950. [Google Scholar]
- Prins HBA, Harper JR, Higinbotham N. Membrane potentials of Vallisneria leaf cells and their relation to photosynthesis. Plant Physiol. 1980;65:1–5. doi: 10.1104/pp.65.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins HBA, Snel JFH, Zanstra PE, Helder RJ. The mechanism of bicarbonate assimilation by the polar leaves of Potamogeton and Elodea: CO2 concentrations at the leaf surface. Plant Cell Environ. 1982;5:207–214. [Google Scholar]
- Raven JA. CO2-concentrating mechanisms: a direct role for the thylakoid lumen acidification? Plant Cell Environ. 1997;20:147–154. [Google Scholar]
- Remis D, Treffny B, Gimmler H. Light-induced H+ transport across the plasma membrane of the acid-resistant green alga Dunaliella acidophila. Plant Physiol Biochem. 1994;32:75–84. [Google Scholar]
- Shabala SN, Newman IA. Proton and calcium flux oscillations in the elongation region correlate with root mutation. Physiol Plant. 1997;100:917–926. [PubMed] [Google Scholar]
- Shabala SN, Newman IA, Morris J. Oscillations in H+ and Ca2+ ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiol. 1997;113:111–118. doi: 10.1104/pp.113.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala SN, Newman IA, Whittington J, Juswono U. Protoplast ion fluxes: their measurement and variation with time, position and osmoticum. Planta. 1998;204:146–152. [Google Scholar]
- Spalding EP, Cosgrove DJ. Mechanism of blue-light-induced plasma-membrane depolarization in etiolated cucumber hypocotyls. Planta. 1992;188:199–205. doi: 10.1007/BF00216814. [DOI] [PubMed] [Google Scholar]
- Spalding EP, Slayman CL, Goldsmith MHM, Gradmann D, Bertl A. Ion channels in Arabidopsis plasma membrane. Transport characteristics and involvement in light-induced voltage changes. Plant Physiol. 1992;99:96–102. doi: 10.1104/pp.99.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanswick RM. Electrogenic ion pumps. Annu Rev Plant Physiol. 1981;32:267–289. [Google Scholar]
- Staal M, Elzenga JTM, Van Elk AG, Prins HBA, Van Volkenburgh E. Red and blue light-stimulated proton efflux by epidermal leaf cells of the argenteum mutant of Pisum sativum. J Exp Bot. 1994;45:1213–1218. [Google Scholar]
- Takagi S, Nagai R. Light-affected Ca2+ fluxes in protoplasts from Vallisneria mesophyll cells. Plant Physiol. 1988;88:228–232. doi: 10.1104/pp.88.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazawa T, Shimmen T, Mimura T. Spectrum of light-induced membrane hyperpolarization in Egeria densa. Plant Cell Physiol. 1986;27:163–168. [Google Scholar]
- Vredenberg WJ, Tonk WJM. On the steady-state electrical potential difference across the thylakoid membranes of chloroplasts in illuminated plant cells. Biochim Biophys Acta. 1975;387:580–587. doi: 10.1016/0005-2728(75)90095-x. [DOI] [PubMed] [Google Scholar]
- Weisenseel MH, Ruppert HK. Phytochrome and calcium ions are involved in light-induced membrane depolarization in Nitella. Planta. 1977;137:225–229. doi: 10.1007/BF00388154. [DOI] [PubMed] [Google Scholar]
- Yin ZH, Huve K, Heber U. Light-dependent proton transport into mesophyll vacuoles of leaves of C3 plants as revealed by pH-indicating fluorescent dyes: a reappraisal. Planta. 1996;199:9–17. [Google Scholar]