Abstract
The two major antiviral effector mechanisms of CD8+ T cells are thought to be perforin (Prf)-mediated cell lysis and gamma interferon (IFN-γ)-mediated induction of an antiviral state. By affecting the expression of proteins involved in antigen presentation, IFN-γ is also thought to shape the magnitude and specificity of the CD8+ T cell response. Here we studied the roles of Prf and IFN-γ in shaping the effector and memory CD8+ T cell responses to vaccinia virus (VACV). IFN-γ deficiency resulted in increased numbers of anti-VACV effector and memory CD8+ T cells, which were partly dependent on increased virus loads. On the other hand, Prf-deficient mice showed an increase in the number of VACV-specific CD8+ T cells only in the memory phase. Treatment of the mice with the antiviral drug cidofovir reduced the numbers of effector and memory cells closer to wild-type levels in IFN-γ-deficient mice and reduced the numbers of memory CD8+ T cells to wild-type levels in Prf-deficient mice. These data suggest that virus loads are the main reason for the increased strength of the CD8 response in IFN-γ- and Prf-deficient mice. Neither Prf deficiency nor IFN-γ deficiency had an effect on the immunodominance hierarchy of five Kb-restricted CD8+ T cell determinants either during acute infection or after recovery. Thus, our work shows that CD8+ T cell immunodominance during VACV infection is not affected by the effects of IFN-γ on the antigen presentation machinery.
INTRODUCTION
Upon antigen recognition, antiviral CD8+ T cells expand and kill infected cells mostly by the exocytosis of granules containing perforin (Prf) and granzyme B (GzB). In addition, effector CD8+ T cells produce the antiviral cytokine gamma interferon (IFN-γ). Even though CD8+ T cells also produce other proinflammatory and cytolytic cytokines, such as tumor necrosis factor (TNF), Prf/GzB-mediated killing and IFN-γ production are thought to be the major effector mechanisms whereby CD8+ T cells clear viral infections (15). Once the antigen is cleared, many of the virus-specific CD8+ T cells die of apoptosis but leave behind a pool of resting memory CD8+ T cells with the capacity to respond rapidly to a secondary viral challenge (15, 27).
Vaccinia virus (VACV) is an orthopoxvirus (OPV) that served as the vaccine that eradicated smallpox, an often fatal human disease produced by the OPV variola virus (13). In addition, VACV is being developed as a vector for vaccines against various infectious agents and tumors (4, 12, 17, 24, 37). Because it is the only vaccine that has eradicated a disease, VACV serves as a unique model that can advance our understanding of the induction of protective immune responses. Thus, it is of interest to fully dissect the immune mechanisms that control VACV and shape the anti-VACV immune response.
To exert their effector functions, CD8+ T cells must recognize viral antigens as small peptides bound to major histocompatibility complex class I (MHC I) molecules. The vast majority of these peptides derive from the cytosolic degradation of viral proteins by the proteasome (34, 35). Despite the large number of potentially immunogenic peptides, virus-specific CD8+ T cell responses are often focused on a limited number of dominant and subdominant peptides (22, 32, 33). Previously, Tscharke et al. used a VACV genomic library to screen for VACV CD8+ T cell determinants in H-2b C57BL/6 (B6) mice (25). This resulted in the identification of the Kb-restricted immunodominant determinant TSYKFESV from the B8R protein (referred to below as B8R) and four additional subdominant determinants restricted to Kb or Db. B8R-specific CD8+ T cells accounted for 25%, and the five peptides together for 40%, of the total anti-VACV CD8+ T cell response (25). Later work using a VACV synthetic peptide library confirmed these determinants and identified 44 additional subdominant determinants covering 95% of the anti-VACV response in B6 mice. All these peptides displayed strong affinity for the restricting MHC I molecules (20).
The molecular and cellular bases that determine the hierarchy of immunodominance for peptides with strong affinity for MHC I remain a mystery (22, 32, 33). A possible contributing factor is that immunodominant peptides may be produced more efficiently by the antigen presentation machinery (18, 26, 32). In addition to its direct antiviral effects, IFN-γ affects the subunit composition and proteolytic specificity of the proteasome and induces the expression of other proteins involved in antigen processing and presentation (16, 35). Thus, it has been proposed that IFN-γ may affect peptide abundance, thereby playing a major role in shaping the hierarchy of immunodominance (14, 32). Indeed, IFN-γ has been shown to affect immunodominance during infection with an attenuated strain of Listeria monocytogenes (2) and after DNA immunization (21). In contrast, a deficiency in Prf, which does not modulate antigen presentation, resulted in overall increased CD8+ T cell responses to L. monocytogenes during the acute phase of the infection but did not affect immunodominance (2). The enhanced CD8+ T cell response to L. monocytogenes in the absence of Prf appeared to be independent of the ability of Prf to reduce bacterial loads, because Prf−/− mice also mounted enhanced responses to peptide-pulsed dendritic cells. Similarly, the absence of Prf resulted in increased magnitude of the response but did not affect immunodominance during influenza infection (5). Whether IFN-γ affected the magnitude or immunodominance of the influenza response was not addressed in that study. The roles of IFN-γ and Prf in the expansion and contraction of CD8+ T cells appear to differ for different infections. Studies by Andrews et al. with mouse cytomegalovirus showed that the absence of Prf and IFN-γ resulted in increased strength of the response but did alter the kinetics of expansion of CD8+ T cells (1). Conversely, Whitmire et al. showed that during lymphocytic choriomeningitis virus infection, IFN-γ enhanced T cell responses (28–30), while Christensen et al. demonstrated that the responses to vesicular stomatitis virus were not affected by either molecule (6). None of these studies addressed the role of IFN-γ or Prf in immunodominance.
Here we examined the roles of IFN-γ and Prf in shaping the anti-VACV CD8+ T cell response. We found that IFN-γ deficiency resulted in an increased number of anti-VACV effector and memory CD8+ T cells, which was partly dependent on increased virus loads during the acute phase of the infection. On the other hand, Prf deficiency resulted in an increased number of VACV-specific memory CD8+ T cells only during the memory phase. Remarkably, neither Prf deficiency nor IFN-γ deficiency had any effect on the immunodominance hierarchy of B8R and four of the most prominent Kb-restricted subdominant CD8+ T cell determinants either during acute infection or after recovery.
MATERIALS AND METHODS
Viruses.
Initial stocks of VACV Western Reserve were obtained from Bernard Moss (National Institute of Allergy and Infectious Diseases, Bethesda, MD) and were amplified in HeLa S3 cells as described previously (8). Briefly, HeLa S3 cells in T150 flasks were infected with 0.1 PFU/cell VACV. After 3 or 4 days, cells were collected, resuspended in phosphate-buffered saline (PBS), frozen and thawed three times, and stored in aliquots at −80°C as virus stocks. Virus titers in VACV stocks were determined by plaque assays on confluent BSC-1 cells using 10-fold serial dilutions of the stocks in 0.5 ml RPMI 2.5 medium in six-well plates (2 wells/dilution) for 1 h. A 2-ml volume of fresh RPMI 2.5 medium was added, and the cells were incubated at 37°C for 3 days (VACV). Next, the medium was aspirated, and the cells were fixed and stained for 10 min with 0.1% crystal violet in 20% ethanol. The fix/stain solution was subsequently aspirated, the cells air dried, the plaques counted, and the PFU/ml in stocks calculated accordingly.
For the determination of the virus titers in ovaries, both ovaries were homogenized in a medium using a TissueLyser (Qiagen). The virus titers were calculated as PFU/ovaries. To determine virus titers in spleens, the spleens were made into a single-cell suspension between two frosted slides and were resuspended in 10 ml complete RPMI medium. A 1-ml volume of the cell suspension was frozen and thawed three times, and titers were determined in 10-fold serial dilutions of the cell lysates as described above. Virus titers were calculated as PFU/spleen. To determine the virus titers in the liver, a portion of the liver was weighed and homogenized in the medium using a TissueLyser. The virus titers were calculated as PFU/g.
Mice and infections.
The Fox Chase Cancer Center Institutional Animal Care and Use Committee approved the experimental protocols involving animals. C57BL/6J and C57BL/6NTac (B6) mice were purchased from Taconic or the Jackson Laboratory, respectively, when they were 8 to 10 weeks of age. The mice were allowed to rest for at least 1 week before use in experiments. Experiments (not shown) demonstrated that the VACV-specific CD8+ T cell responses in B6 mice from Taconic and the Jackson Laboratory were not significantly different. All the figures in this report show the results of experiments carried out with Taconic B6 mice. B6.129S7-Ifngtm1Ts/J (IFN-γ−/−) and C57BL/6-Prf1tm1Sdz/J (Prf−/−) mice were initially purchased from the Jackson Laboratory and were bred in the Fox Chase Cancer Center Laboratory Animal Facility. IFN-γ−/− and Prf−/− mice were genotyped using the standard PCR protocols described by the Jackson Laboratory.
Unless otherwise indicated, VACV was inoculated via the intraperitoneal (i.p.) route with 500 μl PBS containing 106 PFU. Following infections, mice were observed daily for signs of disease (lethargy, ruffled hair, weight loss, skin rash, eye secretions) and imminent death (unresponsiveness to touch, lack of voluntary movements).
Antiviral treatment was administered systemically via the i.p. route with 500 μl PBS containing 400 μg of cidofovir (Vistide; Gilead Sciences).
Flow cytometry.
T cell responses were detected as described previously (9–11, 31). Briefly, for T cell responses, lymphocytes were obtained from mice and were made into single-cell suspensions. Following osmotic lysis of red blood cells with 0.84% NH4-Cl, cells were washed; 2 × 106 cells were added to the wells of 96-well plates; and supernatants of hybridoma 2.4G2 (anti-Fcγ II/III receptor [ATCC]) were added to block nonspecific binding of the labeled antibody (Ab) to Fc receptors. The cells were stained for cell surface molecules and were then fixed with 0.5% paraformaldehyde in PBS. The following Abs and staining reagents were used: anti-CD4 (clone GK1.5), anti-CD14 (clone Sa14-2), anti-CD16 (clone 93), and anti-CD19 (clone 6D5) conjugated with fluorescein isothiocyanate (FITC) for a dump gate, phycoerythrin (PE)-conjugated anti-CD8 (clone 2.43) (all from BioLegend), and an H-2Kb–Ig recombinant fusion protein (DimerX; BD), which had been incubated with the synthetic peptide TSYKFESV (B8R), ITYRFYLI (A8R), STLNFNNL (A3L), KSYNYMLL (J3R), or SIFRFLNI (E7R) (GenScript) and used as recommended by the manufacturer. At least 100,000 cells were analyzed by flow cytometry using an LSR II system (BD Biosciences).
Data analysis and statistics.
Unless otherwise indicated, all data presented correspond to one experiment representative of at least two similar experiments with groups of four to five mice. Spleens were analyzed individually except for the experiment for which results are shown in Fig. 1, where data correspond to pooled spleens and four independent experiments. Statistical analysis was performed using GraphPad Prism software. All statistical analyses were performed using an unpaired two-tailed t test. When applicable, data are displayed as means ± standard errors of the means (SEM) with P values (represented by asterisks as explained in the figure legends).
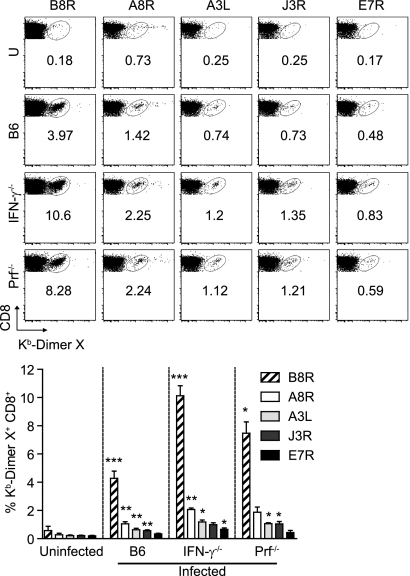
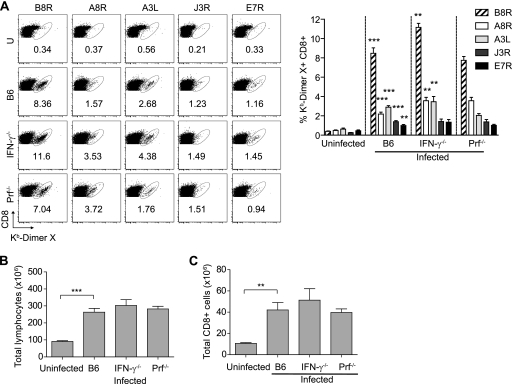
Fig. 1.
IFN-γ−/− and Prf−/− mice mount adaptive immune responses with increased frequencies of anti-VACV memory CD8+ T cells. B6, IFN-γ−/−, and Prf−/− mice were infected with 106 PFU VACV i.p.; ≥30 dpi, sera and spleens were collected, processed, and analyzed. (Top) Representative flow cytometry plots show the percentages of DimerX staining. U, uninfected. (Bottom) Bar graphs show summary data for the percentages of DimerX staining. Data are results of four independent experiments (except for Prf−/− mice, with two independent experiments) with pooled spleens from five mice. Means ± SEM are shown. Asterisks for infected B6 mice represent P values for comparisons to uninfected B6 mice. Asterisks for IFN-γ−/− and Prf−/− mice represent P values for comparisons to infected B6 mice. *, P = 0.05; **, P = 0.01; ***, P = 0.001.
RESULTS
IFN-γ−/− and Prf−/− mice mount adaptive immune responses with increased frequencies of anti-VACV memory CD8+ T cells.
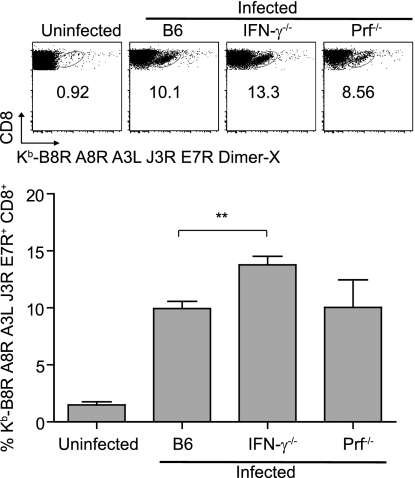
To examine the roles of IFN-γ and Prf in anti-VACV CD8+ T cell responses, we infected B6, IFN-γ−/−, and Prf−/− mice with 106 PFU VACV. All the B6 and Prf−/− mice and most IFN-γ−/− mice survived the infection without overt symptoms of disease. In four experiments where we infected a total of 40 IFN-γ−/− mice (10 in each experiment), 6 animals became very sick 7 to 10 days postinfection and had to be euthanized. None of the other 34 animals showed signs of disease. This is consistent with a previous report showing that IFN-γ−/− mice control VACV following infection by tail scarification (19). Sixty days postinfection (dpi), the anti-VACV CD8+ T cell responses were analyzed. Compared with those in B6 mice, both Prf−/− and IFN-γ−/− mice had significantly higher frequencies of Kb-restricted memory CD8+ T cells specific for the dominant Kb-restricted B8R peptide and four other subdominant Kb-restricted peptides (20). However, the hierarchy of immunodominance for the five determinants (B8R, A8R, A3L, J3R, and E7R, listed in decreasing order of immunodominance) remained unchanged (Fig. 1). The increased frequencies of memory CD8+ T cells in IFN-γ−/− and Prf−/− mice were not due to virus persistence, because (i) no virus was detectable in the spleen, liver, and ovaries 30 dpi; (ii) the memory cells were GzB negative, indicating that they were resting (11, 36); and (iii) anti-VACV memory CD8+ T cells from VACV-immune B6 mice did not expand when transferred to VACV-immune IFN-γ−/− or Prf−/− mice (not shown). Thus, IFN-γ- and Prf-deficient mice cleared VACV by the i.p. route of infection, and compared to that in wild-type (WT) mice, mice deficient in IFN-γ or Prf displayed increased frequencies of memory CD8+ T cells with an unchanged immunodominance hierarchy.
Increased number of anti-VACV CD8+ T cell responses to acute VACV in IFN-γ−/− but not Prf−/− mice.
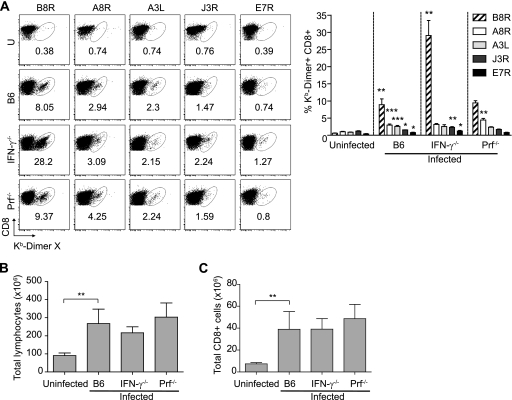
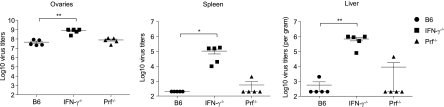
It has been reported that mice deficient in IFN-γ had normal frequencies but altered immunodominance of L. monocytogenes-specific CD8+ T cells during the acute phase of L. monocytogenes infection and increased frequencies of memory CD8+ T cells after recovery. This indicated that during L. monocytogenes infection, the increased frequency of memory CD8+ T cells was due to decreased contraction and not to increased expansion of responding CD8+ T cells. On the other hand, Prf−/− mice showed increased expansion of specific CD8+ T cells in the acute phase of L. monocytogenes infection, which was independent of the effector function of Prf (2). In contrast, we found that during the acute phase of VACV infection (7 dpi), the frequencies of CD8+ T cells specific for B8R and most subdominant determinants were significantly increased in IFN-γ−/− mice, but only the frequency of CD8+ T cells specific for the subdominant determinant A8R was increased in Prf−/− mice. The hierarchy of immunodominance was unaltered in both IFN-γ−/− and Prf−/− mice (in descending order, B8R, A8R, A3L, J3R, and E7R) (Fig. 2A). The differences in frequencies resulted in differences in absolute numbers, because B6, IFN-γ−/−, and Prf−/− mice had similar increases in the cellularity of their spleens (Fig. 2B) and similar calculated absolute numbers of CD8+ cells (Fig. 2C). At 7 dpi, IFN-γ−/− mice had significantly higher virus titers in the ovaries, spleen, and liver than B6 mice (Fig. 3), suggesting that the increased number of virus-specific effector CD8+ T cells in IFN-γ−/− mice could have been driven by the increase in the virus load. Virus was barely detectable in the livers and spleens of some Prf−/− mice, but the difference from levels in B6 mice was not significant, possibly because the titers were close to the limit of detection of the plaque assay.
Fig. 2.
Increased number of anti-VACV CD8+ T cell responses to acute VACV in IFN-γ−/− but not in Prf−/− mice. B6, IFN-γ−/−, and Prf−/− mice were infected with 106 PFU VACV i.p., and at 7 dpi, individual spleens were collected and processed, and total lymphocytes were counted and analyzed. (A) (Left) Representative flow cytometry plots show the percentages of DimerX staining. U, uninfected. (Right) Bar graphs show summary data for the percentages of DimerX staining for five individual mice. (B) Total lymphocyte numbers. (C) The number of total CD8+ cells increases in infected mice. Data are means for five mice per group ± SEM (four IFN-γ−/− mice, because one died 7 dpi) and are representative of three independent experiments. Asterisks for infected B6 mice represent P values for comparisons to uninfected B6 mice. Asterisks for IFN-γ−/− and Prf−/− mice represent P values for comparisons to infected B6 mice. *, P = 0.05; **, P = 0.01; ***, P = 0.001.
Fig. 3.
VACV-infected IFN-γ−/− mice have higher virus titers in the spleen and liver than WT mice. B6, IFN-γ−/−, and Prf−/− mice were infected with 106 PFU VACV i.p., and virus titers in their ovaries, spleens, and livers were determined 7 dpi. Data correspond to individual mice. Horizontal lines and error bars indicate the mean ± SEM for each group and are representative of three independent experiments. *, P = 0.05; **, P = 0.01.
Antiviral treatment partially reduces the increased frequency of anti-VACV effector and memory CD8+ T cells.
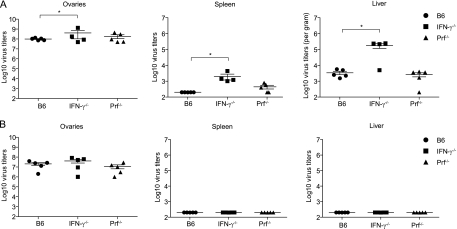
Cidofovir [(S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine] (Vistide; Gilead Sciences), inhibits the VACV polymerase and dramatically decreases VACV replication in vitro and in vivo (7, 23). Cidofovir is highly effective at protecting mice from a lethal respiratory infection with either VACV or cowpox virus after a single systemic (i.p.) or aerosolized (intranasal) dose (3). Additionally, cidofovir is highly effective for treating VACV-infected severe combined immune-deficient (SCID) mice (7). To test whether the increased expansion of effector cells in IFN-γ−/− mice was due to an increased antigenic load, mice infected with VACV i.p. were treated 2 dpi (to permit some viral replication and CD8+ T cell priming) with a single dose of 400 μg of cidofovir given i.p. At 7 dpi, VACV was undetectable in the spleens and livers of B6, IFN-γ−/−, and Prf−/− mice. Cidofovir moderately reduced virus titers in the ovaries to similar loads in all mice (Fig. 4). Interestingly, treatment with cidofovir did not affect the frequency of the anti-VACV CD8+ T cell response in B6 and Prf−/− mice but reduced the frequency of anti-B8R cells ∼3-fold in IFN-γ−/− mice (Fig. 5A; compare with Fig. 2A). Still, the frequencies of anti-B8R, anti-A8R, and anti-A3L CD8+ T cells were significantly higher in cidofovir-treated IFN-γ−/− mice than in cidofovir-treated B6 mice. The numbers of total lymphocytes and CD8+ T cells in the spleen were similar in all infected mice and were significantly higher than those in uninfected mice (Fig. 5B and C), indicating that the differences in frequencies reflected differences in absolute numbers. Therefore, the increased expansion of virus-specific CD8+ T cells in IFN-γ−/− mice was attributable mostly to the viral load, though other factors could also be involved. Importantly, the immunodominance hierarchy was maintained in cidofovir-treated mice (Fig. 5A) (in descending order, B8R, A8R, A3L, J3R, and E7R).
Fig. 4.
Cidofovir treatment equalizes virus titers. B6, IFN-γ−/−, and Prf−/− mice were infected with 106 PFU VACV i.p. and were either left untreated (A) or treated 2 dpi with 400 μg of cidofovir i.p. (B). At 7 dpi, virus titers in the ovaries, spleen, and liver were determined. The quantitation of virus titers in the treated and untreated groups are from a different experiment than that for which data are shown in Fig. 3. Data are results for individual mice. Horizontal lines and error bars indicate the mean ± SEM for each group and are representative of two independent experiments. *, P = 0.05.
Fig. 5.
Antiviral treatment partially reduces the increased frequency of anti-VACV effector CD8+ T cells. B6, IFN-γ−/−, and Prf−/− mice were infected with 106 PFU VACV i.p. and treated with 400 μg cidofovir i.p. 2 dpi. At 7 dpi, individual spleens were collected, processed, and analyzed. (A) (Left) Representative flow cytometry plots show the percentages of DimerX staining. (Right) Bar graphs show cumulative data for the percentages of DimerX staining. (B) Total lymphocyte numbers. (C) The number of total CD8+ cells increases in infected mice. Data correspond to the same mice as those for which results are shown in Fig. 4 and are representative of two independent experiments. **, P = 0.01; ***, P = 0.001.
We next determined the long-term effects of treating the mice with cidofovir at 2 dpi. All B6, IFN-γ−/−, and Prf−/− mice (5/group) survived the infection without noticeable symptoms of disease (not shown). At 30 dpi, the spleens were harvested and stained with pooled Kb-B8R, -A8R, -A3L, -J3R, and -E7R dimers. As in the acute phase of the infection, IFN-γ−/− mice showed significant increases in the overall frequencies of VACV-specific CD8+ T cells specific for the five determinants combined (∼1.3-fold higher [Fig. 6]), but these were much lower than the increases we had observed in untreated mice. The frequencies of VACV-specific memory CD8+ T cells in cidofovir-treated B6 and Prf−/− mice were similar. Thus, virus loads were responsible for the increase in memory CD8+ T cells in untreated Prf−/− mice and for most, if not all, of the increase in untreated IFN-γ−/− mice.
Fig. 6.
Antiviral treatment partially reduces the increased frequency of anti-VACV memory CD8+ T cells. B6, IFN-γ−/−, and Prf−/− mice were infected with 106 PFU VACV i.p., and 400 μg of cidofovir was administered i.p. at 2 dpi. At ≥30 dpi, individual spleens were collected, processed, and analyzed. (Top) Representative flow cytometry plots show the percentages of DimerX staining. (Bottom) Bar graph shows summary data for the percentages of DimerX staining for all the mice in the experiment. Data are means for five mice per group ± SEM and are representative of two independent experiments. **, P = 0.01.
DISCUSSION
Here we infected IFN-γ−/− and Prf−/− mice with VACV i.p. and analyzed their CD8+ T cell responses to the dominant determinant B8R and the subdominant determinants A8R, A3L, J3R, and E7R.
In results reminiscent of previous work with L. monocytogenes (2), Prf−/− mice and, more notably, IFN-γ−/− mice showed higher frequencies of VACV-specific memory CD8+ T cells than B6 mice, despite virus clearance. However, the hierarchy of dominance for B8R and the four subdominant determinants was not affected in either deficient strain. Moreover, analysis of the CD8+ T cell response at the acute phase of the infection showed normal CD8+ T cell responses in Prf−/− mice but increased responses in IFN-γ−/− mice, which also had increased virus titers in the ovaries, spleen, and liver. As in the memory phase, the hierarchy of immunodominance was unaffected. This was surprising, because IFN-γ was hypothesized to be important in shaping CD8+ T cell immunodominance (32, 33), and a role for IFN-γ in immunodominance was later found in mice infected with attenuated L. monocytogenes or immunized with DNA (2, 21). VACV is a large virus with more than 200 proteins and a large number of MHC class I determinants (20); therefore, it would be expected that changes associated with IFN-γ signaling would be important in shaping the hierarchy of dominance in the anti VACV response. However, this did not occur.
Treatment of mice with the antiviral drug cidofovir at 2 dpi reduced virus loads in Prf−/− and IFN-γ−/− mice to undetectable levels in the liver and spleen and to levels similar to those for B6 mice in the ovaries. Cidofovir treatment partially decreased the difference in the number of antiviral CD8+ T cells between IFN-γ−/− and B6 mice during acute infection and after recovery. This demonstrates that virus loads, and not immunomodulation, play the most important role in regulating the strength of the CD8+ T cell response to VACV in the absence of IFN-γ. Our experiments could not distinguish whether the remaining difference was due to disparities in virus loads between B6 and IFN-γ−/− mice during the first 2 dpi or to other reasons. Cidofovir treatment reduced the memory CD8+ T cells in Prf−/− mice to numbers similar to those in B6 mice, suggesting that differences in virus loads (though not significant by the plaque assay at 7 dpi) may also affect the formation of memory CD8+ T cells in the absence of Prf. Interestingly, cidofovir treatment did not have a detectable effect on the strength of the CD8+ T cell response in B6 mice. This suggests that cidofovir treatment could be used to reduce the complications of VACV vaccination, in particular in immunocompromised individuals, without affecting the effectiveness of the vaccine at inducing CD8+ T cell responses.
In summary, we show that following VACV i.p. infection, IFN-γ deficiency results in slightly increased virus titers that are at least partly responsible for increased frequencies of VACV-specific effector and memory CD8+ T cells in acutely infected and recovered mice, respectively. A deficit in Prf results only in an enhanced frequency of VACV-specific memory CD8+ T cells. The hierarchy of immunodominance, however, was not affected by either Prf deficiency or (unexpectedly) IFN-γ deficiency. Together, our results contribute to an understanding of the mechanisms that control the strength and shape of the CD8+ T cell response. Of particular importance, our results show that IFN-γ does not play a major role in regulating the hierarchy of dominance during a viral infection.
ACKNOWLEDGMENTS
We thank the Fox Chase Cancer Center Laboratory Animal, Flow Cytometry, and Tissue Culture facilities for their services. We are grateful to Gilead Sciences for the generous gift of cidofovir. We also thank Holly Gillin for assistance in the preparation of the manuscript and Glenn Rall for critical reading of the manuscript.
This work was supported by grants R01AI048849 and 5U19AI083008 to L.J.S. and by grant P30CA006927 to the FCCC.
Footnotes
Published ahead of print on 14 September 2011.
REFERENCES
- 1. Andrews D. M., Andoniou C. E., Fleming P., Smyth M. J., Degli-Esposti M. A. 2008. The early kinetics of cytomegalovirus-specific CD8+ T-cell responses are not affected by antigen load or the absence of perforin or gamma interferon. J. Virol. 82:4931–4937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Badovinac V. P., Tvinnereim A. R., Harty J. T. 2000. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science 290:1354–1358 [DOI] [PubMed] [Google Scholar]
- 3. Bray M., et al. 2000. Cidofovir protects mice against lethal aerosol or intranasal cowpox virus challenge. J. Infect. Dis. 181:10–19 [DOI] [PubMed] [Google Scholar]
- 4. Chavan R., Marfatia K. A., An I. C., Garber D. A., Feinberg M. B. 2006. Expression of CCL20 and granulocyte-macrophage colony-stimulating factor, but not Flt3-L, from modified vaccinia virus Ankara enhances antiviral cellular and humoral immune responses. J. Virol. 80:7676–7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen W., Bennink J. R., Morton P. A., Yewdell J. W. 2002. Mice deficient in perforin, CD4+ T cells, or CD28-mediated signaling maintain the typical immunodominance hierarchies of CD8+ T-cell responses to influenza virus. J. Virol. 76:10332–10337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Christensen J. E., Wodarz D., Christensen J. P., Thomsen A. R. 2004. Perforin and IFN-γ do not significantly regulate the virus-specific CD8+ T cell response in the absence of antiviral effector activity. Eur. J. Immunol. 34:1389–1394 [DOI] [PubMed] [Google Scholar]
- 7. De Clercq E. 2002. Cidofovir in the treatment of poxvirus infections. Antiviral Res. 55:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Earl P. L., Moss B., Wyatt L. S., Carroll M. W. 2001. Generation of recombinant vaccinia viruses. Curr. Protoc. Mol. Biol. 2001:Chapter 16, Unit 16.17 [DOI] [PubMed] [Google Scholar]
- 9. Fang M., Sigal L. 2010. Studying NK cell responses to ectromelia virus infections in mice. Methods Mol. Biol. 612:411–428 [DOI] [PubMed] [Google Scholar]
- 10. Fang M., Sigal L. J. 2005. Antibodies and CD8+ T cells are complementary and essential for natural resistance to a highly lethal cytopathic virus. J. Immunol. 175:6829–6836 [DOI] [PubMed] [Google Scholar]
- 11. Fang M., Sigal L. J. 2006. Direct CD28 costimulation is required for CD8+ T cell-mediated resistance to an acute viral disease in a natural host. J. Immunol. 177:8027–8036 [DOI] [PubMed] [Google Scholar]
- 12. Feder-Mengus C., et al. 2005. Nonreplicating recombinant vaccinia virus expressing CD40 ligand enhances APC capacity to stimulate specific CD4+ and CD8+ T cell responses. Hum. Gene Ther. 16:348–360 [DOI] [PubMed] [Google Scholar]
- 13. Fenner F., et al. 1988. Smallpox and its eradication. World Health Organization, Geneva, Switzerland [Google Scholar]
- 14. Groettrup M., Kirk C. J., Basler M. 2010. Proteasomes in immune cells: more than peptide producers? Nat. Rev. Immunol. 10:73–78 [DOI] [PubMed] [Google Scholar]
- 15. Harty J. T., Tvinnereim A. R., White D. W. 2000. CD8+ T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 18:275–308 [DOI] [PubMed] [Google Scholar]
- 16. Khan S., et al. 2001. Immunoproteasomes largely replace constitutive proteasomes during an antiviral and antibacterial immune response in the liver. J. Immunol. 167:6859–6868 [DOI] [PubMed] [Google Scholar]
- 17. Kim J. H., et al. 2006. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol. Ther. 14:361–370 [DOI] [PubMed] [Google Scholar]
- 18. Levitsky V., Zhang Q. J., Levitskaya J., Masucci M. G. 1996. The life span of major histocompatibility complex-peptide complexes influences the efficiency of presentation and immunogenicity of two class I-restricted cytotoxic T lymphocyte epitopes in the Epstein-Barr virus nuclear antigen 4. J. Exp. Med. 183:915–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mota B. E., et al. 2011. Adverse events post smallpox-vaccination: insights from tail scarification infection in mice with vaccinia virus. PLoS One 6:e18924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moutaftsi M., et al. 2006. A consensus epitope prediction approach identifies the breadth of murine TCD8+-cell responses to vaccinia virus. Nat. Biotechnol. 24:817–819 [DOI] [PubMed] [Google Scholar]
- 21. Rodriguez F., Harkins S., Slifka M. K., Whitton J. L. 2002. Immunodominance in virus-induced CD8+ T-cell responses is dramatically modified by DNA immunization and is regulated by gamma interferon. J. Virol. 76:4251–4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sercarz E. E., et al. 1993. Dominance and crypticity of T cell antigenic determinants. Annu. Rev. Immunol. 11:729–766 [DOI] [PubMed] [Google Scholar]
- 23. Smee D. F., Bailey K. W., Wong M. H., Sidwell R. W. 2001. Effects of cidofovir on the pathogenesis of a lethal vaccinia virus respiratory infection in mice. Antiviral Res. 52:55–62 [DOI] [PubMed] [Google Scholar]
- 24. Sutter G., Moss B. 1992. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc. Natl. Acad. Sci. U. S. A. 89:10847–10851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tscharke D. C., et al. 2005. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J. Exp. Med. 201:95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vijh S., Pilip I. M., Pamer E. G. 1998. Effect of antigen-processing efficiency on in vivo T cell response magnitudes. J. Immunol. 160:3971–3977 [PubMed] [Google Scholar]
- 27. Welsh R. M., Selin L. K., Szomolanyi-Tsuda E. 2004. Immunological memory to viral infections. Annu. Rev. Immunol. 22:711–743 [DOI] [PubMed] [Google Scholar]
- 28. Whitmire J. K., Benning N., Whitton J. L. 2005. Early IFN-γ signaling directly enhances primary antiviral CD4+ T cell responses. J. Immunol. 175:5624–5628 [DOI] [PubMed] [Google Scholar]
- 29. Whitmire J. K., Eam B., Benning N., Whitton J. L. 2007. Direct interferon-gamma signaling dramatically enhances CD4+ and CD8+ T cell memory. J. Immunol. 179:1190–1197 [DOI] [PubMed] [Google Scholar]
- 30. Whitmire J. K., Tan J. T., Whitton J. L. 2005. Interferon-gamma acts directly on CD8+ T cells to increase their abundance during virus infection. J. Exp. Med. 201:1053–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu R. H., Fang M., Klein-Szanto A., Sigal L. J. 2007. Memory CD8+ T cells are gatekeepers of the lymph node draining the site of viral infection. Proc. Natl. Acad. Sci. U. S. A. 104:10992–10997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yewdell J. W., Bennink J. R. 1999. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu. Rev. Immunol. 17:51–88 [DOI] [PubMed] [Google Scholar]
- 33. Yewdell J. W., Del Val M. 2004. Immunodominance in TCD8+ responses to viruses: cell biology, cellular immunology, and mathematical models. Immunity 21:149–153 [DOI] [PubMed] [Google Scholar]
- 34. York I. A., Goldberg A. L., Mo X. Y., Rock K. L. 1999. Proteolysis and class I major histocompatibility complex antigen presentation. Immunol. Rev. 172:49–66 [DOI] [PubMed] [Google Scholar]
- 35. York I. A., Rock K. L. 1996. Antigen processing and presentation by the class I major histocompatibility complex. Annu. Rev. Immunol. 14:369–396 [DOI] [PubMed] [Google Scholar]
- 36. Yuen T. J., et al. 2010. Analysis of A47, an immunoprevalent protein of vaccinia virus, leads to a reevaluation of the total antiviral CD8+ T cell response. J. Virol. 84:10220–10229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zurkova K., et al. 2009. The expression of the soluble isoform of hFlt3 ligand by recombinant vaccinia virus enhances immunogenicity of the vector. Oncol. Rep. 21:1335–1343 [DOI] [PubMed] [Google Scholar]