Abstract
BACKGROUND
Light transmission aggregometry (LTA) is considered the gold-standard for investigating platelet activity ex vivo. However, LTA protocols are not standardized and differences in LTA procedure are a potential source of variance in results. Centrifugation speed is an essential component of platelet preparation in LTA, has yet to be standardized, and may affect platelet aggregation results. We sought to investigate the effect of relative centrifugal force (RCF) intensity on LTA results.
METHODS
Ten healthy controls had venous blood drawn and centrifuged at 150g, 200g, 300g, and 500g for 10 minutes. Cell counts in whole blood and PRP were measured using a hematology analyzer. LTA was performed using 1.0uM ADP and 0.4uM epinephrine as an agonist. Aggregation (%) was compared at 60, 120, 180, and 300 seconds (s) and at maximum aggregation.
RESULTS
Centrifugation speed was associated with decreasing platelet count (P<0.001) and decreasing MPV (P<0.001) in platelet rich plasma. Maximum aggregation decreased with increasing speeds for ADP 1.0uM (150g-89%, 200g-93%, 300g-71%, 500g-17%; P<0.001). Similar findings were noted at 120s (150g-69%, 200g-50%, 300g-35%, 500g-12%; P<0.001), 180s (150g-82%, 200g-74%, 300g-44%, 500g-13%; P<0.001), and 300s (150g-85%, 200g-88%, 300g-55%, 500g-14%; P<0.001). Consistently, platelet aggregation in response to epinephrine 0.4uM decreased significantly with increasing centrifuge RCF at 60s, 120s, 180s, 300s, and at maximum aggregation (P<0.05 for each comparison).
CONCLUSIONS
Our data demonstrate the importance of centrifugation speed in the interpretation of LTA results, supporting the need for standardization of centrifugation RCF in LTA protocols.
Keywords: Light transmission aggregometry, Methodology, Centrifuge, Platelets
Introduction
Light transmission aggregometry (LTA) is the gold-standard for investigating platelet aggregation ex vivo. A recently conducted survey from the International Society of Thrombosis and Haemostasis (ISTH) reported a wide range in LTA protocols among research and clinical laboratories world-wide and suggested the need for LTA protocol standardization[Cattaneo et al 2009]. This survey reported discrepancies in LTA protocols regarding agonist concentrations, duration of assay, time-frame of performance, stirring parameters, and preparation of platelet rich plasma (PRP). Differences in procedures across laboratories make it difficult to compare LTA results across samples performed with different protocols.
Many parts of LTA have been studied and optimized. However, to our knowledge, no study has investigated how centrifugation may affect LTA results. Increasing centrifugation speed may remove larger, more active platelets. Furthermore, centrifugation can disturb and activate remaining platelets, thereby decreasing platelet aggregation in subsequent LTA[Sixma 1972]. Though platelets recover from centrifugation and show increased aggregation following a 30-minute rest after centrifugation[Cattaneo 2009], it is not known if platelets fully regain aggregability. The degree of centrifugation may affect LTA variability. In 1988, the British Committee for Standards in Haematology (BCSH) Haemostasis and Thrombosis Task Force provided guidelines for performing LTA[Guidelines on Platelet Function Testing 1988]. To obtain PRP, the BCSH recommended centrifuging at 170 × g for 10 minutes[Guidelines on Platelet Function Testing 1988]. In the recent ISTH survey, PRP was most frequently prepared by centrifuging blood at 150 × g for 10 minutes in 78% of all sites (thus almost a quarter of all sites were using another method) [Cattaneo et al 2009]. Furthermore, methodology for obtaining PRP in the literature is quite heterogeneous; various reports use centrifugation to generate PRP using relative centrifugal force (RCF) as low as 80 × g to as high as 500 × g for different intervals of time [Breddin 2005, Naylander et al 2006, Sbrana et al 2008, Yee et al 2005]. The goal of the current study is to investigate the effect of whole blood centrifuge speed, over a constant time interval, on platelet count, mean platelet volume, and LTA.
Methods
Healthy subjects that reported no use of aspirin or NSAID in the 10 days prior to participation, no bleeding disorders, nor any other significant medical history, were selected for participation in this study. Blood was drawn using a 21-gauge needle. The first 2ml was discarded and the remaining blood was collected into a vacutainer tube containing EDTA for complete blood count (CBC) and vacutainers containing 3.2% (0.105 moles/liter) sodium citrate for LTA (Becton Dickenson, Franklin Lakes, NJ). Complete blood count was performed within 30 minutes of phlebotomy. Blood drawn into citrated vacutainers were randomly selected to be spun at 150 × g, 200 × g, 300 × g, and 500 × g for 10 minutes, each separated by 30 minutes intervals. The order of centrifugation was random in each of the 10 samples. In earlier samples, tubes were also spun at 80 ×g and 120 × g, but samples did not yield sufficient PRP to perform an adequate number of LTA runs.
Whole blood and PRP CBC were performed using a Sysmex (Mundelein, Illinois) XE-2100 analyzer. White blood cell count (WBC), hematocrit (HCT), platelet count (10^3 cells/µL), and mean platelet volume (MPV) were measurements of interest. LTA was performed using the Helena (Beaumont, Texas) AggRAM light transmission aggregometer, reagents, cuvettes, and stir bars. Agonists were purchased from a single lot at the onset of the study. LTA was performed according to manufacturer’s specification and tests ran for 10 minutes. Platelet aggregation (%) was recorded at 60 seconds (s), 90s, 120s, 180s, 300s, and at maximum aggregation. We tested aggregation in response to: adenosine diphosphate (ADP) at 1µM and 4µM, and epinephrine at 0.4 µM and 1.5µM.
Statistical Analysis
Data is presented as median (interquartile range [IQR] 25th, 75th percentile), and significance is developed at the 5% probability level. Statistical analysis was performed using non parametric related sample Friedman’s two-way analysis of variance by ranks and Wilcoxon signed ranks test. Calculations were performed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA)
All participants provided informed consent before participation in the study approved by the local institutional review board
Results
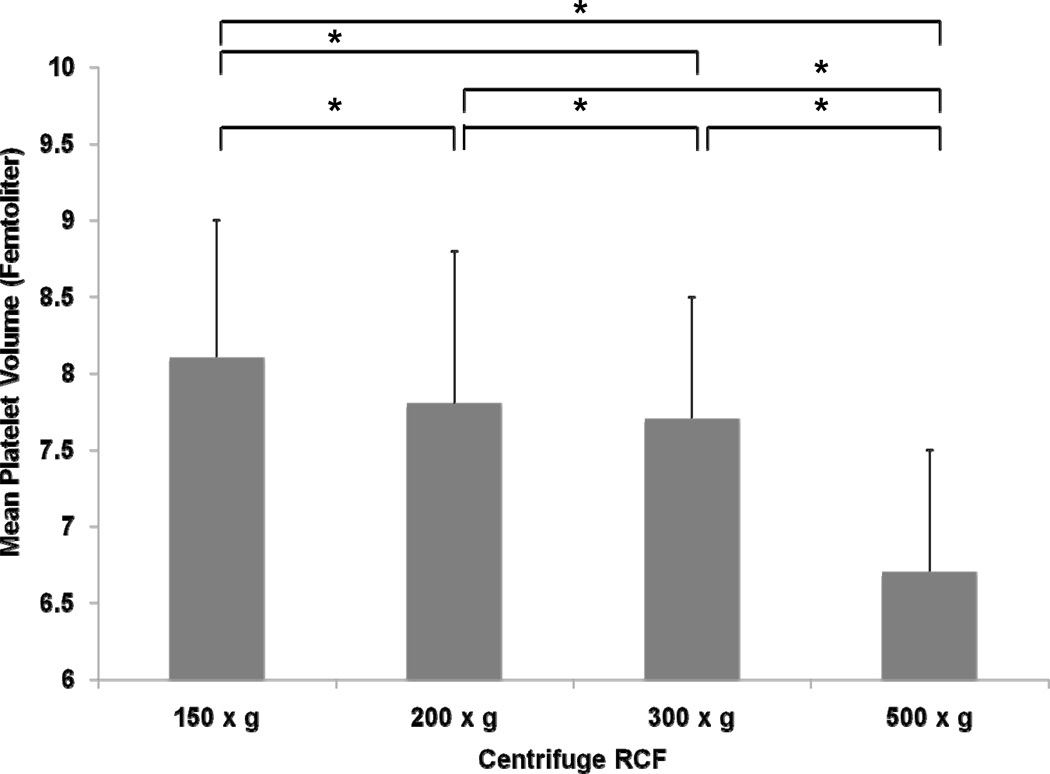
Median whole blood platelet count was 249 (180, 293.3) and MPV was 9.4 (8.1, 9.8) femtoliters (fl). Complete blood count demonstrated a significant association between centrifuge RCF and platelet count and MPV. Increasing centrifuge RCF decreased the platelet count (150 × g- 464.5 [399, 536.3], 200 × g- 441.5 [382, 540], 300 × g- 375.5 [338.8, 453.3], 500 × g- 257.5 [187.8, 345]; P<0.001) and MPV (150 × g- 8.1 fl [7.2, 9.0], 200 × g- 7.8 fl [7.1, 8.8], 300 × g- 7.7 fl [6.8, 8.5], 500 × g- 6.7 fl [6.1, 7.5]; P<0.001). When comparing platelet count between individual centrifugation RCFs, there were significant differences among the following: 500 × g vs 300 × g, 500 × g vs 200 × g, 500 × g vs 150 × g, 300 × g vs 200 × g, 300 × g vs 150 × g (P≤0.01 for each comparison, see figure 1a). Platelet count was not significantly different comparing 200 × g vs 150 × g (P=0.203). Significant differences in MPV were detected between each centrifuge RCF (P≤0.01 for each comparison, see Figure 1). Hematocrit and WBC were not associated with centrifuge RCF (data not shown).
Figure 1.
Association between relative centrifugal force (150 × g, 200 × g, 300 × g, and 500 × g) and mean platelet volume.
* <0.01
† <0.05
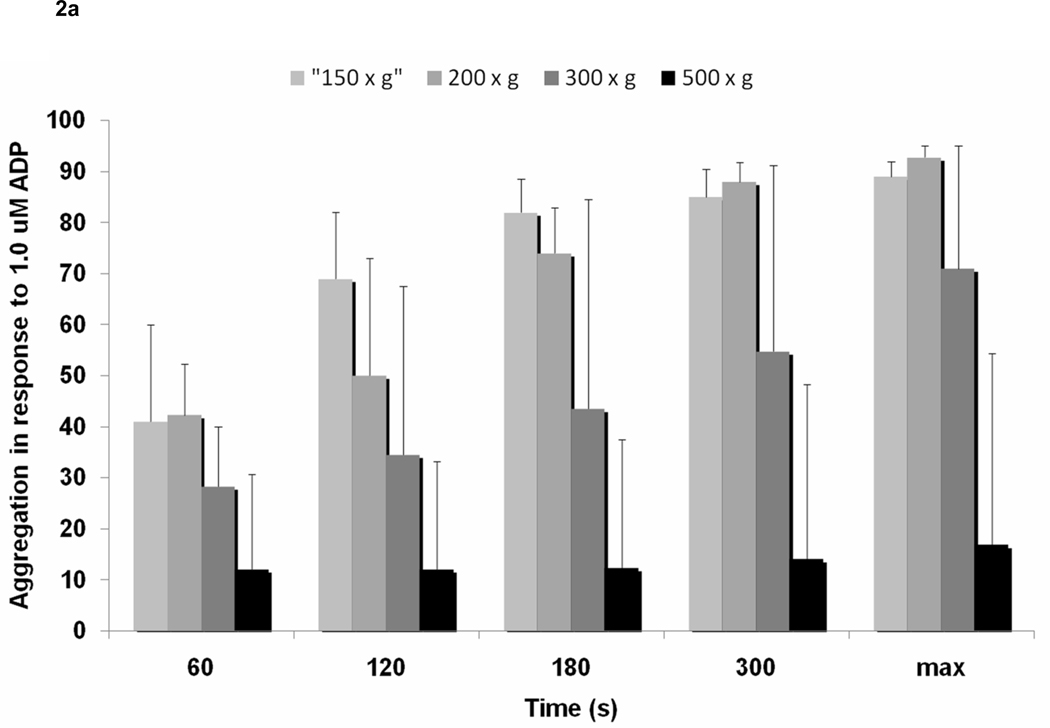
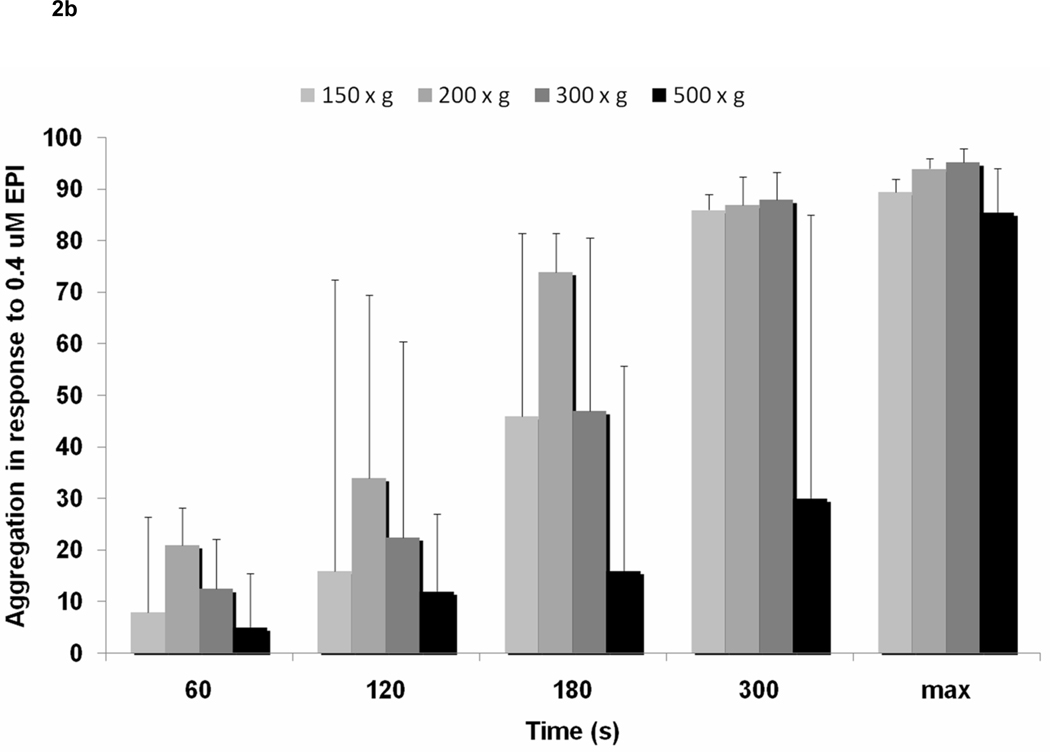
Increased centrifuge RCF resulted in lower platelet aggregation. In response to 1 µM ADP, maximum platelet aggregation decreased with increasing centrifuge RCF (150 × g – 89% [51.1, 92], 200 × g – 92.8% [44, 95.1], 300 × g 71% [35.9, 95], 500 × g – 16.8% [5.3, 54.4], P<0.001). This data was consistent at multiple time points, including 120s (150g-69% [13.5, 82], 200g-50% [24.4, 73], 300g-34.5% [10.5, 67.5], 500g-12% [0, 33.1]; P<0.001), 180s (150g-82% [13, 88.5], 200g-74% [34.5, 82.9], 300g-43.5% [19.5, 84.5], 500g-12.3% [0, 37.5]; P<0.001), and 300s (150g-85% [32.3, 90.5], 200g-88% [38.3, 28.8], 300g-54.8% [28.8, 91.3], 500g-14% [0, 48.3]; P<0.001) (Figure 2a). Consistently, platelet aggregation in response to epinephrine 0.4uM decreased with increasing centrifuge RCF at 60s, 120s, 180s, 300s, and maximum aggregation (Figure 2b). Differences in aggregation were most apparent between 500g-150g, 500g-200g, 500g-300g, 300g-150g, and 300g-200g. No significant difference was noted between 200g and 150g.
Figure 2.
Association between relative centrifugal force (150 × g, 200 × g, 300 × g, and 500 × g) and a) light transmission aggregometry in response to ADP 1uM, and b) light transmission aggregometry in response to epinephrine 0.4uM.
* <0.01
† <0.05
Centrifugation at 80 × g and 120 × g did not yield a sufficient volume of PRP to perform LTA. Therefore, we were unable to determine the effect of centrifuge RCF lower than 150 × g.
Discussion
Centrifuge speed had a significant impact on platelet count, MPV and LTA. The differences in platelet count, MPV and percent aggregation were most pronounced at the highest speed − 500 × g. However, subtle (and potentially important) differences existed at the lower speeds of 300 × g and 200 × g. Decreases in platelet count with increasing RCF confirm that higher centrifuge RCF remove a larger number of platelets out of plasma. Decreases in MPV with increasing RCF demonstrate that higher centrifuge RCF preferentially remove larger platelets from plasma. It is well known that larger platelets are metabolically more active and have greater prothrombotic potential[Bath & Butterworth 1996], which may explain the decrease in aggregation with higher centrifuge speed. While changes in platelet count and MPV account for at least some differences observed in LTA, platelet disturbance rendered during centrifugation also may contribute to these differences.
Since a number of studies have suggested that adjusting platelet count in plasma using autologous platelet-poor plasma may introduce artifacts and contribute to assay variability[Cattaneo et al 2007, Hayward et al 2008, Linnemann et al 2008], platelet count was not adjusted for. It is uncertain if adjustment of the platelet count would attenuate some of the present findings. Additionally, duration of centrifuge time may impact the results of LTA; however, it was our intention to look at a single variable - centrifugation speed - and compare different centrifugation RCFs on platelet population properties and platelet aggregation.
Centrifugation is just one of many variables involved in PRP preparation that may affect LTA results. Yet, unlike other variables, centrifugation in LTA has not been standardized. As noted in the current study, using different centrifuge RCF results in changes in PRP properties and LTA. This difference in methodology gives pause to comparing LTA from studies with different protocols and may help explain some of the inconsistencies observed in the field. Consequently, consideration should be given to standardize centrifugation parameters in performing LTA.
Table.
A brief, quick, and focused summary of this study
| What is known on this topic? |
|
|
|
| What this paper adds? |
|
|
|
|
Acknowledgements
Supported in part by grant 1UL1RR029893 from the National Center for Research Resources, National Institutes of Health. Dr Berger was partially funded by an American Heart Association Fellow to Faculty Award (0775074N) and a Doris Duke Clinical Scientist Award. Michael Merolla, Michael A. Nardi, and Jeffrey S. Berger designed the research study. Michael Merolla performed the research. Jeffrey S. Berger analyzed the data. Michael Merolla, Michael A. Nardi, and Jeffrey S. Berger wrote the manuscript.
Footnotes
Conflicts of Interest
There are no potential conflicts of interest to disclose.
CITATIONS
- 1.Bath PM, Butterworth RJ. Platelet size: measurement, physiology and vascular disease. Blood Coagulation and Fibrinolysis. 1996;7:157–161. [PubMed] [Google Scholar]
- 2.Breddin HK. Can platelet aggregometry be standardized? Platelets. 2005;16:151–158. doi: 10.1080/09537100400020161. [DOI] [PubMed] [Google Scholar]
- 3.Cattaneo M, Lecchi A, Zighetti ML, Lussana F. Platelet aggregation studies: autologous platelet-poor plasma inhibits platelet aggregation when added to platelet-rich plasma to normalize platelet count. Haematologica. 2007;92 doi: 10.3324/haematol.10999. 694-7.2. [DOI] [PubMed] [Google Scholar]
- 4.Cattaneo M, Hayward CP, Moffat KA, Pugliano MT, Liu Y, Michelson AD. Results of a worldwide survey on the assessment of platelet function by light transmission aggregometry: a report from the platelet physiology subcommittee of the SSC of the ISTH. Journal of Thrombosis and Haemostasis. 2009;7:1029.3. doi: 10.1111/j.1538-7836.2009.03458.x. [DOI] [PubMed] [Google Scholar]
- 5.Cattaneo M. Light transmission aggregometry and ATP release for the diagnostic assessment of platelet function. Seminars in Thrombosis and Hemostasis. 2009;35:158–167. doi: 10.1055/s-0029-1220324. [DOI] [PubMed] [Google Scholar]
- 6.Guidelines on platelet function testing. The British Society for Haematology BCSH Haemostasis and Thrombosis Task Force. Journal of Clinical Pathology. 1988;41:1322–1330. doi: 10.1136/jcp.41.12.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayward CP, Moffat KA, Pai M, Liu Y, Seecharan J, McKay H, Webert KE, Cook RJ, Heddle NM. An evaluation of methods for determining reference intervals for light transmission platelet aggregation tests on samples with normal or reduced platelet counts. Journal of Thrombosis and Haemostasis. 2008;100:134–145. [PubMed] [Google Scholar]
- 8.Linnemann B, Schwonberg J, Mani H, Prochnow S, Lindhoff-Last E. Standardization of light transmittance aggregometry for monitoring antiplatelet therapy: an adjustment for platelet count is not necessary. Journal of Thrombosis and Haemostasis. 2008;6:677–683. doi: 10.1111/j.1538-7836.2008.02891.x. [DOI] [PubMed] [Google Scholar]
- 9.Nylander S, Johansson K, Van Giezen JJ, Lindahl TL. Evaluation of platelet function, a method comparison. Platelets. 2006;17:49–55. doi: 10.1080/09537100500197448. [DOI] [PubMed] [Google Scholar]
- 10.Sbrana S, Della Pina F, Rizza A, Buffa M, De Filippis R, Gianetti J, Clerico A. Relationships between optical aggregometry (type born) and flow cytometry in evaluating ADP-induced platelet activation. Cytometry Part B Clincal Cytometry. 2008;74:30–39. doi: 10.1002/cyto.b.20360. [DOI] [PubMed] [Google Scholar]
- 11.Sixma JJ. Methods for platelet aggregation. Advances in Experimental Medicine and Biology. 1972;34:79–95. doi: 10.1007/978-1-4684-3231-2_5. [DOI] [PubMed] [Google Scholar]
- 12.Yee DL, Sun CW, Bergeron AL, Dong JF, Bray PF. Aggregometry detects platelet hyperreactivity in healthy individuals. Blood. 2005;106:2723–2729. doi: 10.1182/blood-2005-03-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]