Abstract
Background
Several lines of evidence suggest that inflammatory mechanisms may be involved in the severity and progression of depression. One pathway implicated is the production of prostaglandins via the enzyme cyclooxygenase (COX). Although late life depression in particular has been associated with inflammation, we know of no published studies using COX inhibitors, such as nonsteroidal anti-inflammatory drugs (NSAIDs), in the treatment of depressive syndromes in this population.
Objective
To evaluate the effect of the NSAIDs celecoxib and naproxen on depressive symptoms in older adults.
Methods
The Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT) was a randomized, placebo-controlled, double-masked clinical trial conducted at six U.S. memory clinics. Cognitively normal volunteers aged 70 and over with a family history of Alzheimer-like dementia were randomly assigned to receive celecoxib 200mg BID, naproxen sodium 220mg BID, or placebo. The 30-item version of the Geriatric Depression Scale (GDS) was administered to all participants at enrollment and at yearly follow-up visits. Participants with a GDS score >5 at baseline were classified as depressed.
Results
Of 2,528 participants enrolled 2,312 returned for at least one follow-up visit. Approximately one-fifth had significant depressive symptoms at baseline. Mean GDS score, and the percentage with significant depressive symptoms, remained similar over time across all three treatment groups. Furthermore, there was no treatment effect on GDS scores over time in the subgroup of participants with significant depressive symptoms at baseline. In longitudinal analysis using Generalized Estimating Equations (GEE) regression, higher baseline GDS scores, a prior psychiatric history, older age, time in the study, and lower cognition interacting with time, but not treatment assignment, were associated with significantly higher GDS scores over time.
Conclusions
Treatment with celecoxib or naproxen did not improve depressive symptoms over time compared with placebo. While inflammation has been implicated in late life depression, these results do not support the hypothesis that inhibition of the cyclooxygenase pathway with these NSAIDs at these doses alleviates depressive symptoms in older adults.
Introduction
Several lines of evidence suggest an association between activation of the immune system and major depression. Proinflammatory cytokines - namely IL-6, IL-1beta, IFN-gamma, and TNF-alpha - are elevated in individuals with major depression (1-5). Levels of prostaglandins such as prostaglandin E2 (PGE2) are also increased in major depression (6-8) and may reflect depression severity (9, 10). A recent meta-analysis points to differences between the immune systems of depressed and non-depressed individuals (11). The high incidence of major depression in inflammatory medical illnesses (12, 13) and in patients undergoing cytokine therapies (5, 14, 15) also suggests a role for inflammation in the etiology and pathogenesis of depression. Furthermore, administration of cytokines and cytokine-inducers, such as endotoxin, to rodents can induce sickness behavior, a well-accepted animal model of depression (16-18). Important aspects of sickness behavior may be mediated by elevated brain prostaglandin levels (19).
Of particular interest to this study are the immunologic changes in late life depression. Depression in the elderly is a major public health concern. Major depression affects 1-4% of the general elderly population, and minor depression another 4-13% (20). Estimates of late-life depressive syndromes are even higher in medical and nursing home settings, and individuals with cognitive impairment are particularly vulnerable to the disorder (21). Although the exact mechanism remains unclear, inflammation has been cited as a biological risk factor for late-life depression (22, 23). In several recent studies, major depression in later life was associated with increased levels of proinflammatory cytokines, including IL-6 (24-27), IL-1beta (28, 29), IL-1 receptor antagonist (29, 30), TNF-alpha (26), and prostaglandins (27). Studies of aged mice have yielded similar findings (31-33).
One inflammatory pathway implicated in major depression is the conversion of arachidonic acid to prostaglandins via the enzyme cyclooxygenase (COX). COX exists in two isoforms: COX-1, which is constitutively expressed and is cytoprotective (34), and COX-2, which is inducible by cytokines and promotes further inflammation (35). Both isoforms are expressed in the central nervous system (36) and serve as potential inflammatory mediators. Naproxen is a non-steroidal anti-inflammatory agent that inhibits both COX-1 and COX-2, while celecoxib selectively inhibits COX-2. COX-2 inhibitors such as celecoxib inhibit PGE2 production and reduce the production of proinflammatory cytokines. Celecoxib treatment of aging (37) and depressed mice (38, 39) has been shown to reduce inflammation in the brain. Given that inflammation may be involved in late life depressive syndromes, one possible anti-inflammatory target is the cyclooxygenase enzyme and synthesis of prostaglandins.
Not surprisingly, cyclooxygenase inhibitors have been used in the treatment of major depression. Recent studies of celecoxib as an add-on treatment for major depression (40, 41) and for depressed or mixed episodes of bipolar disorder (42) have demonstrated greater response rates in the add-on groups. Augmentation of selective serotonin reuptake inhibitors (SSRIs) with acetylsalicylic acid (ASA), a strong inhibitor of COX-1, has also shown promising results (43, 44). We searched for anti-inflammatory treatment studies in late life depression and could not find published studies using cyclooxygenase inhibitors.
Here we present the results of a treatment study using nonsteroidal anti-inflammatory drugs (NSAIDs) in adults aged 70 and over with and without significant depressive symptoms. In the Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT), 2,528 individuals were randomized to receive celecoxib, naproxen, or placebo. The 30-item version of the Geriatric Depression Scale (GDS) (45) was administered to all participants at enrollment and follow-up visits allowing us the opportunity to study treatment effects on depressive symptoms. We used GDS scores to classify participants at baseline as depressed (GDS>5) or not depressed (GDS≤5); we then followed GDS scores over time to see if monotherapy with either naproxen or celecoxib was associated with less severe depressive symptoms. We hypothesized that, if COX-2 related inflammatory mechanisms are involved in the severity and progression of depressive symptoms, inhibition of COX-2 would lead to fewer depressive symptoms over time.
Methods
The methods of the ADAPT study have been detailed in previous publications (46, 47). Here we summarize aspects of the methods relevant to these analyses.
Design overview
ADAPT was a randomized, placebo-controlled, double-masked, primary prevention trial for Alzheimer’s dementia sponsored by the National Institute on Aging (NIA; U01 AG15477). ADAPT was conducted at six U.S. memory clinics, and recruitment was accomplished primarily via mass mailings to Medicare beneficiaries in surrounding zip codes. Eligible participants were at least 70 years of age, cognitively normal, with a family history of Alzheimer-like dementia in at least one first-degree relative. Enrollment began in March 2001. All participants signed informed consent for participant under oversight of local Institutional Review Boards (IRBs).
Treatment
Participants were randomly assigned to receive monotherapy with celecoxib (Celebrex; Pfizer, New York, NY) 200 mg twice daily, naproxen sodium (Aleve; Bayer, Robinson, PA) 220 mg twice daily or matching placebos. On December 17, 2004, the ADAPT Steering Committee suspended treatment with celecoxib and naproxen secondary to an announcement by the National Cancer Institute of significantly increased cardiovascular risk with celecoxib in its Adenoma Prevention with Celecoxib (APC) trial. Treatment suspension was made permanent on March 31, 2005.
Procedures
Eligibility of prospective participants was determined using specified health criteria and cut-off scores on an Eligibility Battery of cognitive tests. At the enrollment visit, baseline cognitive and functional abilities were assessed using the Cognitive Assessment Battery (CAB), which included the 30-item GDS administered by self-report and the Modified Mini-Mental State Examination (3MS-E) (48). The CAB was administered every twelve months thereafter at follow-up visits. Median length of follow-up after treatment assignment was greater than 2 years.
Outcome assessment
The 30-item GDS was administered to all ADAPT participants at enrollment and follow-up. The GDS discriminates well between groups of normal, mildly depressed, and severely depressed subjects, including in physically ill and cognitively impaired individuals (49, 50). Participants with a baseline GDS score > 5 were classified as depressed and those with a GDS score ≤ 5 as not depressed. The cut-off of 5/6 (case/non-case) is indicative of probable depression (45), with sensitivities and specificities of 75.3% and 77.0%, respectively (51).
Data analysis
Outcome analyses followed the modified intention to treat principle (ITT) but included only participants with at least one GDS score in follow-up assessment. By design, active treatments were compared individually with placebo and not with each another. Mean scores in GDS (with 95% confidence intervals), as well as percent with significant depressive symptoms, at baseline and then 12, 24, 36, 48, or 60 months following randomization were calculated for each treatment. Longitudinal analyses comparing GDS scores over time by each treatment compared to placebo, controlling for baseline GDS scores, were conducted using generalized estimating regression equations (GEE) to account for correlations of within-person measures. This method provided estimates with confidence intervals of the difference between the active treatment groups and the placebo group averaged across all follow-up times. Analyses were done using SAS version 9.2. The GENMOD routine was used for GEE analysis.
Results
Study sample
Of the 2,528 participants enrolled in ADAPT, 2,312 returned for at least one follow-up visit. The flow of these participants from randomization forward is detailed elsewhere (47). Table 1 shows baseline characteristics by treatment group for all randomized participants and for the subgroup of participants with significant depressive symptoms at the time of enrollment. The median age at randomization was 74.5 years (range of 70-90). More men than women were enrolled (54% vs. 46%). Despite significant efforts to recruit minorities, participants were predominantly white (97%). Over three-quarters of the sample enrolled had some college education, with roughly half having earned a college degree. Nearly three-quarters of participants were married upon study entry. The median 3MS-E score was 94, and most participants had a Karnofsky functional rating of 100.
Table 1.
ADAPT study sample: baseline characteristics of ADAPT
| All participants | Depressed at baseline | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Total | Celecoxib | Naproxen Sodium |
Placebo | Total | Celecoxib | Naproxen Sodium |
Placebo |
| Number of participants | 2528 | 726 | 719 | 1083 | 449 | 133 | 124 | 192 |
| Age, y | ||||||||
| Median | 74.5 | 74.5 | 74.5 | 74.4 | 75.1 | 75.9 | 74.4 | 75.0 |
| First/third quartile | 72.3/77.5 | 72.1/77.5 | 72.3/77.5 | 72.4/77.5 | 72.7/78.1 | 73.4/78.7 | 72.2/78.1 | 72.6/77.9 |
| Range | 70.0- 90.6 |
70.0-90.6 | 70.0-89.0 | 70.0-90.5 | 70.1-90.6 | 70.5-90.6 | 70.1-89.0 | 70.1-85.5 |
| Sex, % | ||||||||
| F | 45.9 | 47.1 | 45.9 | 45.1 | 46.5 | 51.9 | 46.0 | 43.2 |
| M | 54.1 | 52.9 | 54.1 | 54.9 | 53.5 | 48.1 | 54.0 | 56.8 |
| Ethnicity/race,% | ||||||||
| white | 97.0 | 96.1 | 97.1 | 97.4 | 94.2 | 93.2 | 93.5 | 95.3 |
| African-American | 1.5 | 1.8 | 1.8 | 1.0 | 2.4 | 2.3 | 4.0 | 1.6 |
| Hispanic | 0.7 | 1.4 | 0.3 | 0.6 | 1.6 | 3.8 | 0.8 | 0.5 |
| Other | 0.8 | 0.6 | 0.7 | 0.9 | 1.6 | 0.0 | 1.6 | 2.6 |
| Did not answer | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.8 | 0.0 | 0.0 |
| Marital status, % | ||||||||
| Married | 71.9 | 70.2 | 75.0 | 70.9 | 68.2 | 60.2 | 76.6 | 68.2 |
| Widowed | 18.2 | 19.7 | 16.1 | 18.7 | 21.6 | 28.6 | 16.9 | 19.8 |
| Divorced/separated | 7.3 | 7.3 | 6.1 | 8.0 | 7.1 | 6.8 | 4.0 | 9.4 |
| Single | 2.6 | 2.8 | 2.8 | 2.3 | 3.1 | 4.5 | 2.4 | 2.6 |
| Education, % | ||||||||
| <High school | 4.0 | 3.9 | 4.9 | 3.6 | 6.2 | 6.8 | 5.6 | 6.3 |
| High School degree | 19.9 | 20.8 | 17.5 | 20.9 | 24.3 | 24.8 | 22.6 | 25.0 |
| College, no degree | 27.5 | 27.7 | 28.4 | 26.8 | 26.9 | 29.3 | 29.0 | 24.0 |
| College degree | 19.1 | 19.1 | 17.0 | 20.6 | 17.6 | 13.5 | 15.3 | 21.9 |
| Post graduate | 29.4 | 28.5 | 32.3 | 28.2 | 24.9 | 25.6 | 27.4 | 22.9 |
| Karnofsky scoring, % | ||||||||
| 100 | 82.3 | 84.3 | 80.1 | 82.5 | 78.6 | 76.7 | 85.5 | 75.5 |
| 90 | 15.3 | 13.5 | 18.2 | 14.6 | 17.6 | 19.5 | 12.1 | 19.8 |
| 80 | 2.2 | 2.1 | 1.4 | 2.8 | 3.3 | 3.0 | 2.4 | 4.2 |
| 60-70 | 0.2 | 0.1 | 0.3 | 0.2 | 0.4 | 0.8 | 0.0 | 0.5 |
| GDS scores distribution, % | ||||||||
| GDS score 0-5 | 79.7 | 79.9 | 79.7 | 79.6 | 0.0 | 0.0 | 0.0 | 0.0 |
| GDS score 6-10 | 15.7 | 15.4 | 15.7 | 16.0 | 78.0 | 75.2 | 76.6 | 80.7 |
| GDS score 11-15 | 3.6 | 3.3 | 4.2 | 3.3 | 17.8 | 17.3 | 22.6 | 15.1 |
| GDS score >15 | 0.9 | 1.4 | 0.4 | 1.0 | 4.2 | 7.5 | 0.8 | 4.2 |
|
ADAPT measures GDS score |
||||||||
| Mean (sd) | 3.4 (3.4) | 3.4 (3.5) | 3.3 (3.4) | 3.4 (3.4) | 8.7 (3.1) | 9.1(3.6) | 8.5 (2.5) | 8.6 (3.0) |
| First/third quartile | 1.0/5.0 | 1.0/5.0 | 1.0/5.0 | 1.0/5.0 | 6.0/10.0 | 6.0/10.0 | 6.0/10.0 | 6.0/10.0 |
| Range | 0.0-24.0 | 0.0-23.0 | 0.0-24.0 | 0.0-22.0 | 6.0-23.0 | 6.0-23.0 | 6.0-18.0 | 6.0-22.0 |
| 3MS-E score | ||||||||
| Mean (sd) | 94.1(3.6) | 94.3 (3.4) | 94.1(3.6) | 94.0 (3.6) | 94.0 (3.7) | 94.0 (3.3) | 94.1 (3.4) | 93.9 (4.0) |
| First/third quartile | 92.0/96.0 | 92.0/97.0 | 92.0/96.0 | 92.0/96.0 | 92.0/96.0 | 92.0/96.0 | 92.5/96.0 | 92.0/96.0 |
| Range | 74.0- 100.0 |
80.0- 100.0 |
76.0- 100.0 |
74.0- 100.0 |
78.0- 100.0 |
84.0-100.0 | 82.0- 100.0 |
78.0- 100.0 |
GDS score by treatment assignment
Table 2 displays mean GDS scores (and 95% CIs) by visit for all three treatment groups along with the percent with significant depressive symptoms, i.e. GDS > 5. At enrollment the celecoxib-, naproxen-, and placebo-assigned groups had comparable mean GDS scores (3.4, 3.2, and 3.3, respectively) and percentages with significant depressive symptoms (19.9%, 18.9%, and 19.5%, respectively). While the proportion of participants with significant depressive symptoms remained similar after baseline, overall the mean GDS scores if anything increased slightly, but not significantly, over time in all treatment groups. Over time, mean GDS scores for participants in the active treatment groups did not differ significantly from those in the placebo group.
Table 2.
Visit-wise mean GDS scores for all participants (N) with number (%) significant depressive symptoms by group
| All participants | Celecoxib | Naproxen | Placebo | |||||
|---|---|---|---|---|---|---|---|---|
| Visit | N (%) | Mean GDS (sd) |
N (%) | Mean GDS (sd) |
N (%) | Mean GDS (sd) |
N (%) | Mean GDS (sd) |
| EN | 2311 (19.43) | 3.29 (3.33) | 669 (19.88) | 3.38 (3.59) | 656 (18.90) | 3.19 (3.17) | 986 (19.47) | 3.30 (3.25) |
| F12 | 2233 (22.84) | 3.57 (3.61) | 650 (23.69) | 3.81 (3.88) | 633 (23.38) | 3.53 (3.38) | 950 (21.89) | 3.43 (3.56) |
| F24 | 2044 (23.34) | 3.67 (3.69) | 600 (22.67) | 3.72 (3.75) | 577 (24.09) | 3.71 (3.87) | 867 (23.3) | 3.60 (3.52) |
| F36 | 1659 (25.80) | 3.84 (3.82) | 467 (26.77) | 4.00 (3.93) | 477 (26.42) | 3.81 (3.79) | 715 (24.76) | 3.75 (3.77) |
| F48 | 1080 (27.22) | 4.09 (3.97) | 310 (30.65) | 4.20 (4.11) | 310 (24.84) | 4.08 (4.03) | 460 (26.52) | 4.02 (3.85) |
| F60 | 323 (25.39) | 3.90 (3.95) | 94 (26.60) | 3.73 (3.91) | 88 (27.27) | 4.15 (3.96) | 141 (23.40) | 3.85 (4.01) |
Significant depressive symptoms and GDS score
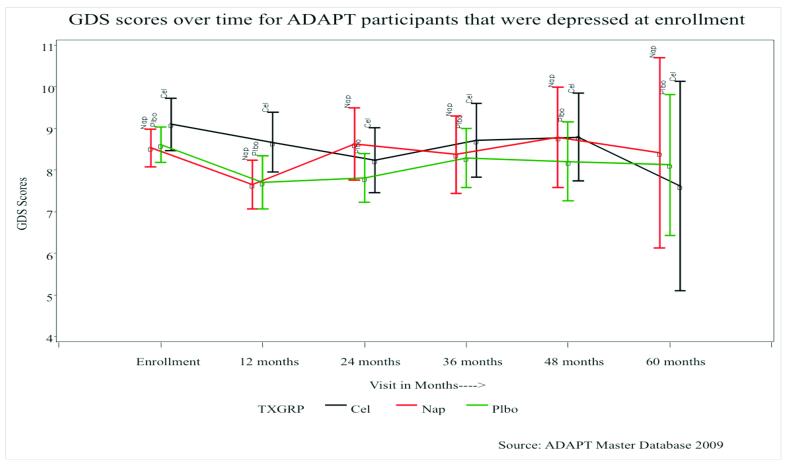
Figures 1a and 1b illustrate the follow-up mean GDS scores (and 95% CIs) for all participants and for those with significant depressive symptoms at baseline, respectively. At enrollment participants with significant depressive symptoms in the celecoxib-, naproxen-, and placebo-assigned groups had mean GDS scores of 9.1, 8.5, and 8.6, respectively. After an initial decrease at the 12-month follow-up, the mean GDS scores stayed fairly constant over time.
Figure 1a.
Mean GDS scores, and 95% confidence intervals, over time for all participants.
Figure 1b.
Mean GDS scores, and 95% confidence intervals, over time for participants that had significant depressive symptoms at enrollment.
Table 3 shows the longitudinal effect of each of the two treatments separately relative to placebo on depressive symptoms over time adjusted for covariates. In multivariable modeling, there was no effect of either treatment on follow-up GDS scores. Of the other variables examined, higher baseline GDS scores, a prior psychiatric history, older age, time in the study, and lower cognition interacting with time were all associated with significantly higher follow-up GDS scores (p < 0.01).
Table 3.
Longitudinal analysis of depressive symptoms by treatment and other covariates*
| Measure | Coefficient | df | Wald 95% CI | p-value | Measure | Coefficient* | df | Wald 95% CI | p-value |
|---|---|---|---|---|---|---|---|---|---|
| Celecoxib vs Placebo** | 0.11 | 1 | −0.11 to 0.33 | 0.34 | Naproxen vs Placebo** | 0.09 | 1 | −0.12 to 0.30 | 0.42 |
| Baseline GDS | 0.73 | 1 | 0.68 to 0.78 | <0.01 | Baseline GDS | 0.76 | 1 | 0.72 to 0.80 | <0.01 |
| 3MS-E | −0.03 | 1 | −0.09 to 0.03 | 0.37 | 3MS-E | 0.03 | 1 | −0.03 to 0.09 | 0.28 |
| Gender (Female vs Male) | −0.05 | 1 | −0.28 to 0.18 | 0.66 | Gender (Female vs Male) | −0.02 | 1 | −0.23 to 0.20 | 0.89 |
| Time | 1.51 | 1 | 0.03 to 2.99 | 0.04 | Time | 3.09 | 1 | 1.65 to 4.53 | <0.01 |
| Education | −0.04 | 1 | −0.08 to 0.01 | 0.12 | Education | −0.01 | 1 | −0.06 to 0.03 | 0.56 |
| 3MS-E*Time | −0.01 | 1 | −0.03 to 0.01 | 0.07 | 3MS-E*Time | −0.03 | 1 | −0.05 to −0.02 | <0.01 |
| Age (70-74 vs 80+) | −0.76 | 1 | −1.16 to −0.36 | <0.01 | Age (70-74 vs 80+) | −0.47 | 1 | −0.79 to −0.15 | <0.01 |
| Age (75-79 vs 80+) | −0.59 | 1 | −1.00 to −0.17 | 0.01 | Age (75-79 vs 80+) | −0.23 | 1 | −0.58 to 0.12 | 0.19 |
|
Depression or Psychiatric History |
1.01 | 1 | 0.49 to 1.53 | <0.01 |
Depression or Psychiatric History |
0.77 | 1 | 0.29 to 1.25 | <0.01 |
Estimates obtained by generalized estimating equations using a Gaussian link. Estimates are adjusted for all covariates listed as well as the stratification variable, field site.
Discussion
In this randomized controlled trial of NSAID treatment in over 2500 older adults, neither celecoxib nor naproxen improved depressive symptoms over time compared with placebo. In contrast with prior reports suggesting benefit of cyclooxygenase inhibitors as an adjunct in the treatment of younger and middle-aged adults with depression (40-42), these results do not support the hypothesis suggesting a role for inflammation in the etiology and pathogenesis of depression in older adults. Nevertheless, due to study limitations articulated below, these results alone do refute this inflammatory hypothesis.
On the whole, with a mean GDS score of only 3.29±3.33 at baseline, ADAPT participants were not a depressed sample. Such is the nature of recruitment for prevention trials, with healthy volunteers comprising the majority of participants. However, approximately one-fifth had significant depressive symptoms at enrollment, mostly with mild depression. This proportion did not change much over time. Unexpectedly and for unknown reasons, mean GDS scores increased slightly, but not significantly with time in all treatment groups.
Of interest here are the 449 participants with significant depressive symptoms at the time of enrollment. We interpret their brief initial decrease in GDS scores as a placebo effect. Thereafter, in contrast to trends for the entire sample, their mean GDS scores stayed fairly constant regardless of assigned treatment. The latter finding suggests that depressive symptoms are not alleviated by NSAID treatment.
Of the variables examined, the factors associated with higher GDS scores over time were as expected: higher baseline GDS scores, a prior psychiatric history (depression or other psychiatric illness), older age, time in the study, and worse baseline cognitive performance interacting with time. Importantly, neither treatment assignment nor worse cognitive performance was associated with higher GDS scores over time.
This study has several strengths. It is the first large-scale randomized trial to examine the effects of NSAIDs on depressive symptoms in later life. Its large sample size provides significant power to detect even small treatment effects on depressive symptoms. In addition, the median length of follow-up of two years is longer than previously published studies, the drop-out rate was low and participants generally reported good adherence to treatment (47).
Among the study’s weaknesses, the sample was almost entirely Caucasian, limiting generalizability to other demographic groups. Broad screening for depressive disorders using the GDS-30, although a widely used method, may not be as accurate as a clinical interview. However, sensitivity analyses on this point using cut-offs of GDS>6 or GDS>11 post hoc showed similar findings. Furthermore, because the GDS was administered yearly, short-term responses to treatment would have been missed.
Although these findings provide no support for the hypothesis that anti-inflammatory treatments can help late life depression, they certainly do not refute this hypothesis. Our results assess only the effects of celecoxib and naproxen, each in a single dosage regimen, and they may not apply to other doses or other NSAIDs. As well, the failure here of NSAIDs to ameliorate late-life depressive symptoms does not address their possible utility as adjuncts to other forms of treatment. Finally, this study investigated just one pathway of inflammation, the arachidonic acid cascade and its inhibition at one particular point, the oxidation of arachidonic acid to prostaglandin H2. Non-COX-dependent mechanisms and cytokines may also be important to the pathophysiology of inflammation. Thus, inflammatory mechanisms may yet play a role in late life depression via some other pathway that remains to be elucidated.
Acknowledgments
Support: Grants U01-AG15477 (ADAPT study), and P50-AG005146 (Johns Hopkins ADRC).
Footnotes
Disclosures: Dr. Lyketsos has received Grant support (research or CME) from NIMH, NIA, Associated Jewish Federation of Baltimore, Weinberg Foundation, Forest, Glaxo-Smith-Kline, Eisai, Pfizer, Astra-Zeneca, Lilly, Ortho-McNeil, Bristol-Myers, Novartis, National Football League, Elan. He has been Consultant/Advisor for Astra-Zeneca, Glaxo-Smith Kline, Eisai, Novartis, Forest, Supernus, Adlyfe, Takeda, Wyeth, Lundbeck, Merz, Lilly, Genentech, Pfizer, NFL Players Association, NFL Benefits Office. He has received honorarium or travel support from Pfizer, Forest, Glaxo-Smith Kline, Health Monitor, Lundbeck.
The other authors have no disclosures.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Smith RS. The macrophage theory of depression. Med Hypotheses. 1991 Aug;35(4):298–306. doi: 10.1016/0306-9877(91)90272-z. [DOI] [PubMed] [Google Scholar]
- 2.Maes M, Scharpe S, Meltzer HY, Bosmans E, Suy E, Calabrese J, et al. Relationships between interleukin-6 activity, acute phase proteins, and function of the hypothalamic-pituitary-adrenal axis in severe depression. Psychiatry Res. 1993 Oct;49(1):11–27. doi: 10.1016/0165-1781(93)90027-e. [DOI] [PubMed] [Google Scholar]
- 3.Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997 Nov;9(11):853–8. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- 4.van West D, Maes M. Activation of the inflammatory response system: A new look at the etiopathogenesis of major depression. Neuro Endocrinol Lett. 1999;20(1-2):11–7. [PubMed] [Google Scholar]
- 5.Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005 Feb;29(2):201–17. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Linnoila M, Whorton AR, Rubinow DR, Cowdry RW, Ninan PT, Waters RN. CSF prostaglandin levels in depressed and schizophrenic patients. Arch Gen Psychiatry. 1983 Apr;40(4):405–6. doi: 10.1001/archpsyc.1983.01790040059008. [DOI] [PubMed] [Google Scholar]
- 7.Lieb J, Karmali R, Horrobin D. Elevated levels of prostaglandin E2 and thromboxane B2 in depression. Prostaglandins Leukot Med. 1983 Apr;10(4):361–7. doi: 10.1016/0262-1746(83)90048-3. [DOI] [PubMed] [Google Scholar]
- 8.Calabrese JR, Skwerer RG, Barna B, Gulledge AD, Valenzuela R, Butkus A, et al. Depression, immunocompetence, and prostaglandins of the E series. Psychiatry Res. 1986 Jan;17(1):41–7. doi: 10.1016/0165-1781(86)90040-5. [DOI] [PubMed] [Google Scholar]
- 9.Ohishi K, Ueno R, Nishino S, Sakai T, Hayaishi O. Increased level of salivary prostaglandins in patients with major depression. Biol Psychiatry. 1988 Feb 15;23(4):326–34. doi: 10.1016/0006-3223(88)90283-1. [DOI] [PubMed] [Google Scholar]
- 10.Nishino S, Ueno R, Ohishi K, Sakai T, Hayaishi O. Salivary prostaglandin concentrations: Possible state indicators for major depression. Am J Psychiatry. 1989 Mar;146(3):365–8. doi: 10.1176/ajp.146.3.365. [DOI] [PubMed] [Google Scholar]
- 11.Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, et al. The relationship of depression and stressors to immunological assays: A meta-analytic review. Brain Behav Immun. 2001 Sep;15(3):199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]
- 12.Bruce TO. Comorbid depression in rheumatoid arthritis: Pathophysiology and clinical implications. Curr Psychiatry Rep. 2008 Jun;10(3):258–64. doi: 10.1007/s11920-008-0042-1. [DOI] [PubMed] [Google Scholar]
- 13.Gold SM, Irwin MR. Depression and immunity: Inflammation and depressive symptoms in multiple sclerosis. Neurol Clin. 2006 Aug;24(3):507–19. doi: 10.1016/j.ncl.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001 Mar 29;344(13):961–6. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 15.Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, et al. Neurobehavioral effects of interferon-alpha in cancer patients: Phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002 May;26(5):643–52. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 16.Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996 Mar 4;711(1-2):163–74. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- 17.Yirmiya R, Pollak Y, Barak O, Avitsur R, Ovadia H, Bette M, et al. Effects of antidepressant drugs on the behavioral and physiological responses to lipopolysaccharide (LPS) in rodents. Neuropsychopharmacology. 2001 May;24(5):531–44. doi: 10.1016/S0893-133X(00)00226-8. [DOI] [PubMed] [Google Scholar]
- 18.Dantzer R, Bluthe RM, Laye S, Bret-Dibat JL, Parnet P, Kelley KW. Cytokines and sickness behavior. Ann N Y Acad Sci. 1998 May 1;840:586–90. doi: 10.1111/j.1749-6632.1998.tb09597.x. [DOI] [PubMed] [Google Scholar]
- 19.Yirmiya R, Barak O, Avitsur R, Gallily R, Weidenfeld J. Intracerebral administration of mycoplasma fermentans produces sickness behavior: Role of prostaglandins. Brain Res. 1997 Feb 21;749(1):71–81. doi: 10.1016/s0006-8993(96)01295-4. [DOI] [PubMed] [Google Scholar]
- 20.Blazer DG. Depression in late life: Review and commentary. J Gerontol A Biol Sci Med Sci. 2003 Mar;58(3):249–65. doi: 10.1093/gerona/58.3.m249. [DOI] [PubMed] [Google Scholar]
- 21.Alexopoulos GS. Depression in the elderly. Lancet. 2005 Jun 4-10;365(9475):1961–70. doi: 10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- 22.Tiemeier H. Biological risk factors for late life depression. Eur J Epidemiol. 2003;18(8):745–50. doi: 10.1023/a:1025388203548. [DOI] [PubMed] [Google Scholar]
- 23.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008 Jan;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bremmer MA, Beekman AT, Deeg DJ, Penninx BW, Dik MG, Hack CE, et al. Inflammatory markers in late-life depression: Results from a population-based study. J Affect Disord. 2008 Mar;106(3):249–55. doi: 10.1016/j.jad.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Tiemeier H, Hofman A, van Tuijl HR, Kiliaan AJ, Meijer J, Breteler MM. Inflammatory proteins and depression in the elderly. Epidemiology. 2003 Jan;14(1):103–7. doi: 10.1097/00001648-200301000-00025. [DOI] [PubMed] [Google Scholar]
- 26.Penninx BW, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, et al. Inflammatory markers and depressed mood in older persons: Results from the health, aging and body composition study. Biol Psychiatry. 2003 Sep 1;54(5):566–72. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- 27.Dimopoulos N, Piperi C, Psarra V, Lea RW, Kalofoutis A. Increased plasma levels of 8-iso-PGF2alpha and IL-6 in an elderly population with depression. Psychiatry Res. 2008 Oct 30;161(1):59–66. doi: 10.1016/j.psychres.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 28.Thomas AJ, Davis S, Morris C, Jackson E, Harrison R, O’Brien JT. Increase in interleukin-1beta in late-life depression. Am J Psychiatry. 2005 Jan;162(1):175–7. doi: 10.1176/appi.ajp.162.1.175. [DOI] [PubMed] [Google Scholar]
- 29.van den Biggelaar AH, Gussekloo J, de Craen AJ, Frolich M, Stek ML, van der Mast RC, et al. Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Exp Gerontol. 2007 Jul;42(7):693–701. doi: 10.1016/j.exger.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Milaneschi Y, Corsi AM, Penninx BW, Bandinelli S, Guralnik JM, Ferrucci L. Interleukin-1 receptor antagonist and incident depressive symptoms over 6 years in older persons: The InCHIANTI study. Biol Psychiatry. 2009 Jun 1;65(11):973–8. doi: 10.1016/j.biopsych.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, O’Connor J, et al. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008 Sep;33(10):2341–51. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol. 1999 Jan 1;93(1-2):139–48. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- 33.Ye SM, Johnson RW. An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation. 2001;9(4):183–92. doi: 10.1159/000049025. [DOI] [PubMed] [Google Scholar]
- 34.Suleyman H, Demircan B, Karagoz Y. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacol Rep. 2007 May-Jun;59(3):247–58. [PubMed] [Google Scholar]
- 35.Botting RM. Inhibitors of cyclooxygenases: Mechanisms, selectivity and uses. J Physiol Pharmacol. 2006 Nov;57(Suppl 5):113–24. [PubMed] [Google Scholar]
- 36.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 37.Casolini P, Catalani A, Zuena AR, Angelucci L. Inhibition of COX-2 reduces the age-dependent increase of hippocampal inflammatory markers, corticosterone secretion, and behavioral impairments in the rat. J Neurosci Res. 2002 May 1;68(3):337–43. doi: 10.1002/jnr.10192. [DOI] [PubMed] [Google Scholar]
- 38.Myint AM, Steinbusch HW, Goeghegan L, Luchtman D, Kim YK, Leonard BE. Effect of the COX-2 inhibitor celecoxib on behavioural and immune changes in an olfactory bulbectomised rat model of depression. Neuroimmunomodulation. 2007;14(2):65–71. doi: 10.1159/000107420. [DOI] [PubMed] [Google Scholar]
- 39.Guo JY, Li CY, Ruan YP, Sun M, Qi XL, Zhao BS, et al. Chronic treatment with celecoxib reverses chronic unpredictable stress-induced depressive-like behavior via reducing cyclooxygenase-2 expression in rat brain. Eur J Pharmacol. 2009 Jun 10;612(1-3):54–60. doi: 10.1016/j.ejphar.2009.03.076. [DOI] [PubMed] [Google Scholar]
- 40.Muller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Muller B, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: Results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006 Jul;11(7):680–4. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- 41.Akhondzadeh S, Jafari S, Raisi F, Nasehi AA, Ghoreishi A, Salehi B, et al. Clinical trial of adjunctive celecoxib treatment in patients with major depression: A double blind and placebo controlled trial. Depress Anxiety. 2009;26(7):607–11. doi: 10.1002/da.20589. [DOI] [PubMed] [Google Scholar]
- 42.Nery FG, Monkul ES, Hatch JP, Fonseca M, Zunta-Soares GB, Frey BN, et al. Celecoxib as an adjunct in the treatment of depressive or mixed episodes of bipolar disorder: A double-blind, randomized, placebo-controlled study. Hum Psychopharmacol. 2008 Mar;23(2):87–94. doi: 10.1002/hup.912. [DOI] [PubMed] [Google Scholar]
- 43.Brunello N, Alboni S, Capone G, Benatti C, Blom JM, Tascedda F, et al. Acetylsalicylic acid accelerates the antidepressant effect of fluoxetine in the chronic escape deficit model of depression. Int Clin Psychopharmacol. 2006 Jul;21(4):219–25. doi: 10.1097/00004850-200607000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Mendlewicz J, Kriwin P, Oswald P, Souery D, Alboni S, Brunello N. Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: A pilot open-label study. Int Clin Psychopharmacol. 2006 Jul;21(4):227–31. doi: 10.1097/00004850-200607000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Yesavage JA. Geriatric depression scale. Psychopharmacol Bull. 1988;24(4):709–11. [PubMed] [Google Scholar]
- 46.ADAPT Research Group. Martin BK, Szekely C, Brandt J, Piantadosi S, Breitner JC, et al. Cognitive function over time in the alzheimer’s disease anti-inflammatory prevention trial (ADAPT): Results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol. 2008 Jul;65(7):896–905. doi: 10.1001/archneur.2008.65.7.nct70006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.ADAPT Research G. Lyketsos CG, Breitner JC, Green RC, Martin BK, Meinert C, et al. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology. 2007 May 22;68(21):1800–8. doi: 10.1212/01.wnl.0000260269.93245.d2. [DOI] [PubMed] [Google Scholar]
- 48.Teng EL, Chui HC. The modified mini-mental state (3MS) examination. J Clin Psychiatry. 1987 Aug;48(8):314–8. [PubMed] [Google Scholar]
- 49.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1982 -1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 50.Debruyne H, Van Buggenhout M, Le Bastard N, Aries M, Audenaert K, De Deyn PP, et al. Is the geriatric depression scale a reliable screening tool for depressive symptoms in elderly patients with cognitive impairment? Int J Geriatr Psychiatry. 2009 Jan 8; doi: 10.1002/gps.2154. [DOI] [PubMed] [Google Scholar]
- 51.Wancata J, Alexandrowicz R, Marquart B, Weiss M, Friedrich F. The criterion validity of the geriatric depression scale: A systematic review. Acta Psychiatr Scand. 2006 Dec;114(6):398–410. doi: 10.1111/j.1600-0447.2006.00888.x. [DOI] [PubMed] [Google Scholar]