Abstract
Major depression disorder is a significant health problem with 10-20% of all adults suffering from this disease. The underlying causes of depression are still unclear and 15% of depressed patients are resistant to all known therapies. Monoamine therapies have so far been the most successful approach for treating depression. Triple monoamine reuptake inhibitors have recently been implicated in generation of potent antidepressant activity while possibly exhibiting a low side-effect profile in addition to treating anhedonia. The additional, previously under-appreciated involvement of dopaminergic systems in depression prompted our efforts to develop novel asymmetric trisubstituted and disubstituted pyran derivatives as triple reuptake inhibitors. One of the lead compounds, D-142, exhibited uptake inhibition (Ki) values of 29.3 nM, 14.7 nM and 37.4 nM for norepinephrine, serotonin and dopamine transporters, respectively. Its affinity for serotonin transporter was comparable to fluoxetine , a well known SSRI. In the rat forced swimming test, compound D-142 exhibited potent antidepressant activity in the dose range tested (2.5, 5 and 10 mg/kg) and was far more efficacious than the reference compound imipramine. In the mouse tail suspension test, compound D-142 reduced immobility in a dose (2.5, 5 and 10 mg/kg) dependent manner, indicating a potent antidepressant effect. In locomotor activity tests, compound D-142 did not exhibit any stimulation in the same dose ranges. In the extended CNS receptors screening assay this molecule exhibited little or no non-specific interaction in the CNS, indicating high specificity for monoamine transporters. These results advance D-142 as a potential potent antidepressant.
Keywords: Antidepressant, dopamine, serotonin, norepinephrine, monoamine transporters, Triple uptake inhibitor
1. Introduction
Environmental, genetic and other related factors can trigger major depression disorder. This disorder is a significant health problem and 10-20% of all adults suffer from this disease1. Unipolar depression is ranked as number one before all other somatic and psychiatric illnesses. In spite of its prevalence, the underlying causes of depression are still unclear (Millan, 2009) Over the past decades different classes of antidepressants have been developed. Antidepressants are traditionally thought to elicit their therapeutic effects by increasing extraneuronal concentrations of serotonin and norepinephrine (Richelson, 2003). Tricyclic antidepressants were the first to be synthesized in the category of monoamine reuptake inhibitors, binding to both serotonin transporters (SERTs) and norepinephrine transporters (NETs); in addition, tricyclic antidepressants display considerable affinity for muscarinic, histamine and adrenergic receptors along with activity for cardiac Na+ and Ca2+ channels (Baldessarini, 1996). However, although tricyclic antidepressants were effective in the treatment of depression, undesirable side effects, resulting from non-specific interactions, restricted their use. The second generation of antidepressants, the selective serotonin reuptake inhibitors (SSRIs) and serotonin/ norepinephrine inhibitors (SNRIs) provided drugs with much more selective interaction at serotonin and norepinephrine systems (Spinks and Spinks, 2002; Wong and Bymaster, 1995). By virtue of their interaction with the SERT and NET only, these drugs had appreciable less unwanted side effects than tricyclic antidepressants (Barbey and Roose, 1998; Goldstein and Goodnick, 1998). The mechanism of action of SNRIs is believed to involve an increase of extraneuronal levels of serotonin and norepinephrine due to blockade of SERT and NET followed by alleviation of depression (Hiemke and Hartter, 2000; Owens and Nemeroff, 1998). However, in spite of their relative better pharmacological profile compared to tricyclic antidepressants, these compounds still exhibit a number of limitations and they do not offer much improved efficacy over tricyclic antidepressants. An SNRI such as Venlafaxine exhibits antidepressant activity in clinical trials and displays somewhat greater response and remission rates compared to SSRIs (Thase et al., 2001).
There still remains a significant unmet need for much more improved therapy, as large numbers of depressed people, an estimated 15%, are still refractory to the current existing therapies. In addition, a significant number of people suffer from relapse after treatment with current therapies (Rush et al., 2006). Consequently, a majority of these people is or becomes refractory to any treatment. In the current pharmacotherapy of depression, a dopaminergic component has not been included in spite of existence of evidences pointing to a strong dopaminergic component. There is a strong correlation of depression with dysregulation of dopaminergic systems (Dunlop and Nemeroff, 2007). In addition, application of imaging indicated down regulation of the presynaptic dopamine transporter (DAT) and increased binding of D2/D3 receptors in depression (Klimek et al., 2002). Clinical studies either with dopamine agonists or dopamine uptake blockers indicate a strong dopaminergic component in antidepressant action (Corrigan et al., 2000).
To further support role of dopamine in depression, it has been shown that an adjunct therapy approach by combining both bupropion and SSRI was more effective in patients refractory to SSRIs (Mischoulon et al., 2000). Since bupropion is a blocker of both DAT and NET, efficacy in such treatment strongly points towards the involvement of a dopamine component (Ascher et al., 1995). In another study, pramipexole, an antiparkinsonian drug with D3 dopamine receptor preferring activity, exhibited effectiveness in both unipolar and bipolar depression (Sporn et al., 2000). Furthermore, in a controlled clinical study pramipexole was found to be as effective as fluoxetine and more effective than placebo in producing an antidepressant effect. In spite of strong evidence of role of dopamine in depression, the current pharmacotherapy for depression does not target dopaminergic activity. Broad spectrum antidepressants enhancing activity of all three monoamies simultaneously, are predicted to be more efficacious in relieving depression.
Recently, triple monoamine uptake inhibitors have been shown to produce potent antidepressant activity (Shaw et al., 2007; Skolnick et al., 2003a; b). These evidences suggest that triple uptake inhibitors interacting at all three monoamine transporters in a calibrated way might provide superior treatment regimen for depression (Liang and Richelson, 2008). Along these lines,the triple uptake inhibitors DOV 21,947 and PCR200-SS have been suggested to be potent antidepressants (Shaw et al., 2007; Skolnick et al., 2003a; b). In a recent study with rodents, we demonstrated potent antidepressant-like activity of the asymmetric pyran derivative D-161 which is a triple uptake inhibitor (Dutta et al., 2008).
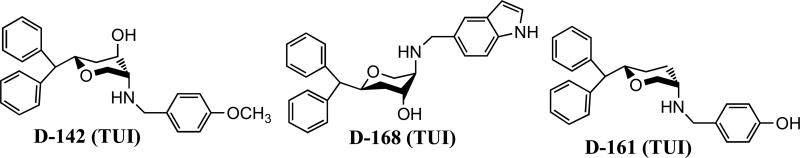
From a drug development point of view, development of triple uptake inhibitor is challenging as it involves a single molecule interacting with all three transporters. In our effort to develop a novel molecular template for monoamine transporter systems, we recently have developed novel asymmetric di- and tri-substituted pyran derivatives (Zhang et al., 2006; Zhang et al., 2005). The compound D-142 from this series exhibited high potency for NET, SERT and DAT (Table 1, Fig. 1), which qualifies this molecule as a triple uptake inhibitor. The compound is two-fold more potent at SERT compared to NET and DAT. Its potency at SERT is comparable to fluoxetine, a well-known SSRI (see Table 1). In our effort to evaluate antidepressant property of this molecule, we have carried out in vivo studies with this molecule in two well-known animal models, the forced swim test (FST) and tail suspension test (TST). Results from the known antidepressants evaluated in these two models seem to correlate with their clinical efficacies. These two tests measure immobility of animals under a state of despair (Porsolt et al., 1977; Steru et al., 1985). Imipramine was used as a reference drug in the study.
Table 1.
Affinity of Drugs at DAT, SERT, and NET in Rat Brain as Determined in Uptake Assays.
| Drugs | DAT uptake, Ki, nM, [3H]DAa | SERT uptake, Ki, nM, [3H]5-HTa | NET uptake, Ki, nM [3H]NEa |
|---|---|---|---|
| D-142 | 59.3 ± 13.7 | 14.7 ± 2.1 | 29.3 ± 7.9 |
| D-168 | 85.2 ± 8.2 | 25.0 ± 8.4 | 25.5 ± 9.6 |
| D-161 | 42.0 ± 3.3 | 29.1 ± 3.5 | 30.5 ± 7.8 |
| Fluoxetine | 1,092 ± 98 | 12.2 ± 2.4 | 713 ± 337 |
| Reboxetine | 2,908 ± 136 | 503 ± 61 | 0.79 ± 0.25 |
| Desipramine | NDb | 135 ± 62 | 1.45 ± 0.24 |
Uptake into rat brain synaptosomes was measured as described in Methods. Results are average ± S.E.M. for 3 to 6 independent determinations.
not determined in this set of experiments.
Fig. 1.
Molecular structures of lead pyran-molecule based triple uptake inhibitors.
2. Materials and methods
2.1 Reagents and drugs
Synthesis of D-142 is described in our earlier publication (Zhang et al., 2005). This compound was resynthesized by asymmetric synthetic pathway as developed by us. Similarly, compounds D-161 and D-168 were synthesized by us previously (Zhang et al., 2006). [Ring 2,5,6-3H]dopamine (38.7 Ci/mmol), [1,2-3H]serotonin (28.0 Ci/mmol), and levo-[ring-2,5,6-3H]norepinephrine (44.6 Ci/mmol) were obtained from Perkin-Elmer (Boston, MA, U.S.A). Imipramine, desipramine, fluoxetine, and reboxetine, and GBR 12909 dihydrochloride (1-[2-[bis(4-fluorophenyl)methoxy]ethyl]-4-[3-phenylpropyl]piperazine) were purchased from SIGMA-ALDRICH (St. Louis, MO).
2.2 Animals
Male Sprague-Dawley rats (200-225 g) and male C57bl/6 mice (25-30 g) were purchased from Harlan. Animals were housed in a temperature and humidity controlled room with 12 h light /dark cycle. Food and water were accessible to animals freely through out the duration of study. All testing occurred during the light component. All animal procedures were reviewed and approved by Wayne State University animal investigation committee consistent with AALAC guidelines.
2.3.1 Uptake inhibition assays
The ability of test compounds to inhibit substrate uptake by rat monoamine transporters was monitored as described by us previously (Dutta et al., 2008; Kharkar et al., 2009; Zhen et al., 2004). Briefly, uptake of [3H]dopamine by DAT was measured in rat striatum, and uptake of [3H]serotonin by SERT and [3H]norepinephrine by NET was monitored in rat cerebral cortex. Dissected tissue was homogenized in ice-cold 0.32 M sucrose in a glass homogenizer with motor-driven Teflon pestle, and the synaptosomal P2 pellet was prepared by differential centrifugation as described by us previously (Zhen et al., 2004). The P2 pellet was resuspended in 0.32 M sucrose with the glass-teflon homogenizer, and aliquots of the suspension were incubated at 25°C in uptake buffer (for composition see “uptake buffer A” in Zhen et al., 2004) for 5 min with test compound followed by the additional presence of [3H]dopamine for 4 min, or [3H]serotonin for 8 min, or [3H]norepinephrine for 6 min (for DAT, SERT, or NET, respectively; all times within linear phase of ligand uptake). Assays were terminated by filtration with a Brandel 96-pin harvester (Brandel Inc., Gaithersburg, MD, USA) under reduced pressure. Nonspecific uptake at DAT, SERT, and NET was defined with 100 μM cocaine, 10 μM citalopram, and 10 μM desipramine, respectively. In the drug screening literature, it is customary to compare results from transporter assays done on different regions optimized for the particular monoamine transporter (Carroll et al., 1995), and there is no strong evidence to suggest that the pharmacological profile of DAT, SERT, or NET varies with brain region (Elsworth et al., 1993; Shank et al., 1987; Thomas et al., 1987). At least five triplicate concentrations of each test compound were studied, spaced evenly around the IC50 value. The latter was estimated by nonlinear computer curve-fitting procedures and converted to Ki with the Cheng-Prusoff equation as in our previous work (Dutta et al., 2008). Under the present conditions, IC50 values and Ki values were very close as [3H]substrate concentrations were much lower than the Km for uptake for each monoamine transporter. Slope factors of inhibition curves were routinely close to unity, suggesting competitive inhibition. We acknowledge that this is an underlying assumption in using Ki values as it is in many other studies aimed at screening uptake inhibitors (Liang et al., 2008).
2.3.2. CNS receptor screening
Compound D-142 was further characterized in several CNS receptor binding assays to assess selective and specific interactions of D-142 with monoamine transporters. The assays were carried out generously by the National Institute of Mental Health's Psychoactive Drug Screening Program, Contract # NO1MH32004 (NIMH PDSP). The NIMH PDSP isdirected by Bryan L. Roth MD, PhD at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscol at NIMH, Bethesda MD, USA (http://pdsp.med.unc.edu/).
Compound D-142 was first evaluated in primary binding assays targeting, among others, cloned human dopamine receptor subtypes, serotonin receptor subtypes, α-adrenergic receptors, and opioid receptors. The description of all receptors targeted and corresponding radioligand used, is provided in Table 2 in the Discussion section. The default concentration for primary binding experiments was 10 μM. Compound with inhibition > 50% at 10 μM in the primary assay was moved into the secondary assay with full concentration curves of the test compound in order to calculate the Ki value for inhibition. For experimental details please refer to the PDSP web site <http://pdsp.med.unc.edu/> and click on “Binding Assay” or “Functional Assay” on the menu bar. We have also added brief description of assay protocols in the supplementary material section.
Table 2.
Binding affinity of D-142 for CNS receptors. The default concentration of drugs for primary binding experiments was 10 μM, and % inhibition (mean of 4 determinations) is shown.
| Target Receptor | Radioligand | % Inhibition of Binding at 10 μM D-142 | Ki for D-161 (nM) |
|---|---|---|---|
| D1 | [3H]SCH 23390 | 2.5 | |
| D2 | [3H]N-methylspiperone | 3.2 | |
| D3 | [3H]N-methylspiperone | 15.3 | |
| D4 | [3H]N-methylspiperone | 3.1 | |
| D5 | [3H]SCH 23390 | 17.2 | |
| 5HT1a | [3H]8-OH-DPAT | 45.9 | |
| 5HT1b | [3H]GR-125743 | 9.1 | |
| 5HT1e | [3H]5-HT | 3.4 | |
| 5HT1d | [3H]GR-125743 | -6.2 | |
| 5HT2a | [3H]ketanserin | 12.7 | |
| 5HT2b | [3H]LSD | 46.3 | |
| 5HT2c | [3H]Mesulergine | 30.6 | |
| 5HT3 | [3H] LY 278,584 | 18 | |
| 5HT5a | [3H]LSD | 22.5 | |
| 5HT6 | [3H]LSD | 8.7 | |
| 5HT7 | [3H]LSD | 15.6 | |
| GABAA | [3H]Muscimol | 0.6 | |
| Alpha1A | [3H]Prazosin | 51.6 | >10,000 |
| Alpha1B | [3H]Prazosin | 24.8 | |
| Alpha1D | [3H]Prazosin | 30 | |
| Alpha2A | [3H]Clonidine | 45.8 | |
| Alpha2B | [3H]Clonidine | 28.5 | |
| Alpha2C | [3H]Clonidine | 32.9 | |
| Beta1 | [125I]Iodopindolol | -4.7 | |
| Beta2 | [125I]Iodopindolol | -3.8 | |
| Beta3 | [125I]Iodopindolol | 60.7 | >10,000 |
| BZP Rat Brain Site | 3H-Flunitrazepam | 20.5 | |
| Ca2+ Channel | [3H]Nitrendipine | 3.1 | |
| δ-opioid | [3H]DADLE | 3.3 | |
| κ-opioid | [3H]Bremazocine | 54.3 | 721 ± 70.28 |
| H1 | [3H]Pyrilamine | -16.9 | |
| H2 | [3H]Tiotodine | 100 | 90 ± 7 |
| H3 | [3H]Alpha-methyl Histamine | 3 | |
| H4 | [3H]Histamine | -13.7 | |
| μ-Opioid | [3H]Diprenorphine | 48.8 | |
| M1 | [3H]QNB | -1.7 | |
| M2 | [3H]QNB | 23 | |
| M3 | [3H]QNB | -3.1 | |
| M4 | [3H]QNB | -3.4 | |
| M5 | [3H]QNB | 16 | |
| NMDA PCP Site | MK801 | 15.4 |
2.4 In vivo studies
2.4.1 Effect of compound D-142 in the rat forced swimming test
The subjects were male Sprague Dawley rats (Harlam Sprague Dawley Inc., Indianapolis, Ind., USA) weighing 200-225 g housed in cages for at least 1 week prior to testing. Animals were maintained in a temperature-controlled environment under a 12 hr light-dark cycle. All subjects were naive and were used only once.
Rats were transported to the testing room at least for one hour prior to testing for acclimatization and adaptation purposes. Experimental sessions were conducted between 9 AM to 2 PM daily. Animals were assigned randomly and were placed individually in a glass cylinder (24.5 cm × 35.5 cm) filled with water at room temperature to a depth of 22 cm. All the test sessions were recorded by a video camera. The water was changed in the beginning of each session and the temperature was maintained constant at 24-25 °C. Rats were judged to be immobile if they made only minimal movement, barely keeping afloat.
The test procedure consisted of a pretest and test session separated by 24 h (Porsolt et al., 1977). During the pretest period, rats were placed in the swim chamber for 15 min. Followed by the initial swim exposure; rats were patted dry and were transferred to the individual cages. Drugs or vehicle were then administered (i.p.) 15 min after the initial swim exposure and were then transported to their home cages. On the following day the rats were brought back to the testing room at least 1 h before the beginning of test session. Rats were administered either drugs or vehicle 1 h before the swim test. Each rat underwent a 5-min swim session, which was videotaped and scored later. In the case of imipramine, pretreatment time was 30 min.
All drugs were prepared freshly on the test days. Compound D-142 and imipramine were dissolved in deionized water. All drugs and vehicles were administered i.p. D-142 was administered at a dose of 2.5, 5 and 10 mg/kg and the volume of injections was maintained at 2 ml/kg. Imipramine was administered at 15 mg/kg. All drugs and vehicles were administered 1 h prior to testing for forced swimming. Imipramine was administered 30 min prior testing. An individual, blinded to the treatment, scored the videotapes for immobility. Immobility scores were analyzed by one way ANOVA test.
2.4.2 Effect of compound D-142 in the tail suspension test
The mouse tail suspension test was carried out as described previously (Steru et al., 1985). Briefly, C57Bl/6 mice weighing approximately 25 g was housed in cages with 12 h dark-light cycle in a temperature controlled room. Mice were brought to the testing room at least 1 h before the beginning of the test session. Mice were selected randomly and grouped in five different treatment groups consisting of seven to nine mice in each group. Each mouse was treated with either drug or vehicle 30 min before testing. Imipramine (15 mg/kg) was used as a positive control. Three different doses of D-142, 2.5, 5 and 10 mg/kg, were tested. Each mouse was suspended individually by its tail from a horizontal bar by using an adhesive tape placed 1 cm from the tip of the tail. Each test session lasted for six minutes and was videotaped. Duration of immobility was scored by an unbiased scorer. A mouse was considered immobile when it stayed motionless.
2.4.3 Locomotor Activity Test
Sprague Dawley Rats were tested at two doses of D-142 (5 and 10 mg/kg) to monitor changes of any locomotor activity in acrylic Versamax monitor chambers (AccuScan Instrument, Inc; Columbus, Ohio). The purpose of locomotor activity measurement was to evaluate locomotor activity of the doses of drug, which were used in forced swim and tail suspension tests. Rats were acclimated in the test chambers for 1 h prior to administration of drugs. Locomotor activity of the drugs were measured for half an hour in 1 h post administration of drug which corresponded to the time of measurement in the forced swimming experiment.
2.4.4 Data Analysis
Behavioral results were analyzed with Prism 3.0 (GraphPad Software, San Diego, CA). The immobility time for forced swimming test and tail suspension test was analyzed by one-way ANOVA (analysis of variance) followed by post Dunnett's multiple comparison test vs. vehicle. Data for vehicle and imipramine were used as negative and positive control, respectively. Statistical significance was assumed in analysis if p≤0.05. The data were used straight in the analysis without transformation, as the Bartlett test indicated that for these data the differences among the standard deviations of the groups were not significant (GraphPad Instat, GraphPad Software, San Diego, CA: corrected Bartlett statistic = 8.951 for forced swimming data, and 1.379 for tail suspension data, P>0.05). In addition, the untransformed data were found to be sampled from Gaussian distributions as indicated by the method of Kolmogorov and Smirnov (KS > 0.151, P > 0.10; for one group consisting of 4 subjects the KS test did not apply – all other groups had a size of ≥ 5).
3.Results
3.1.1 Inhibition of rat synaptosomal dopamine, serotonin, and norepinephrine uptake
In Table 1, the affinity constants (Ki) for D-142 and its analogues D-168 and D-161 in inhibiting monoamine uptake are compared with those for known antidepressants determined under identical conditions. As expected, fluoxetine, an SSRI, inhibited SERT with a potency more than one magnitude greater than that for inhibition of NET or DAT, whereas reboxetine, a known NET selective uptake inhibitor, inhibited NET with a potency greater (exceeding two orders of magnitude) than that for inhibition of SERT or DAT (Table 1). Likewise, desipramine, another NET selective uptake inhibitor, inhibited NET with a potency two orders of magnitude than that for inhibition of SERT (Table 1) and DAT (Dutta et al., 2008). Previously, we reported that under the same assay conditions imipramine was much stronger in inhibiting SERT than NET or DAT(Dutta et al., 2008); the metabolite desipramine formed in vivo contributes to imipramine's SNRI-like antidepressant profile (Baldessarini, 2006). In the monoamine uptake assays, the potency of D-142 for SERT was comparable to that of fluoxetine indicating potent SERT blockade by D-142. In addition, D-142 exhibited comparable potency for DAT and NET. Overall, a balanced activity for all three transporters was displayed by D-142. Two other lead compounds we developed in relation to D-142 (Fig. 1), D-168 and D-161, were, as D-142, triple uptake inhibitors in the present transporter assays (Table 1).
3.1.2. CNS Receptor Screening
Table 2 shows the results for the various receptors. Selective radioligands were employed for each receptor to evaluate primary binding potency. It is clear from the results that at 10 μM D-142 does not potently bind to any of the receptor tested. In only three cases (alpha1A, beta3, kappa-opioid) somewhat greater than 50% inhibition was observed with 10μM D-142, but follow up assays indicated affinities at least an order of magnitude lower than those for monoamine transporters. In one case, H2 receptors, appreciable affinity was detected (Ki of 90 nM).
3.2 Effect of D-142 in the forced swim test in rats
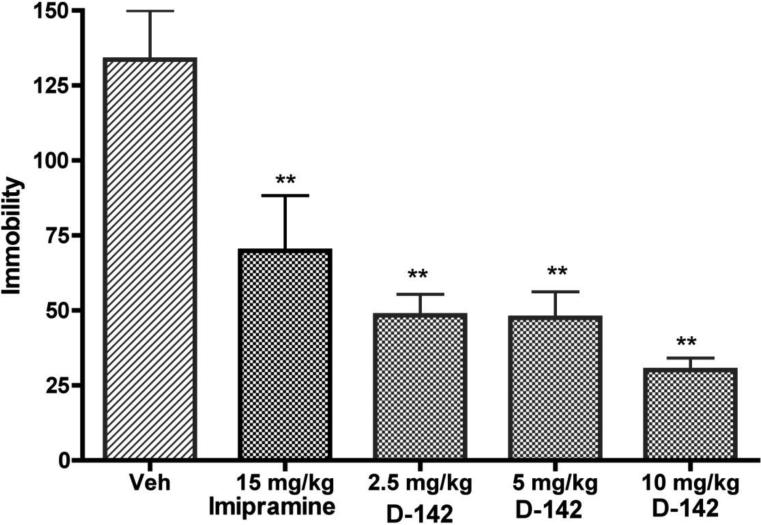
The results from the forced swim test indicate (Fig. 2) that at the three doses of 2.5, 5 and 10 mg/kg, D-142 significantly (P<0.01) reduced the duration of immobility compared to vehicle control. The highest dose of 10 mg/kg exhibited maximal efficacy. The effect from the two lower doses (2.5 and 5 mg/kg) was indistinguishable as they produced comparable reduction of immobility in swimming. The reference antidepressant compound imipramine at 15 mg/kg produced a reduction in immobility and the effect was significantly different from control (P<0.01).
Fig. 2.
Effect of sub-chronic administration of vehicle D-142 and imipramine on the duration of immobility in the forced swimming test in rats. One way ANOVA analysis demonstrates significant effect among treatments: F (4,95) = 11.03 (P< 0.0001). Dunnett's analysis showed that the effect of D-142 at three doses (2.5, 5 and 10 mg/kg n immobility was statistically significant different compared to vehicle (P< 0.01). The effect of reference imipramine (15 mg/kg) on immobility was also statistically significantly different (P< 0.01) from vehicle. Asterisks indicate a statistically significant difference toward control group that received saline i.p. **P < 0.01. Each treatment group contained four to seven rats.
3.3 Effect of D-142 in the mouse tail suspension test
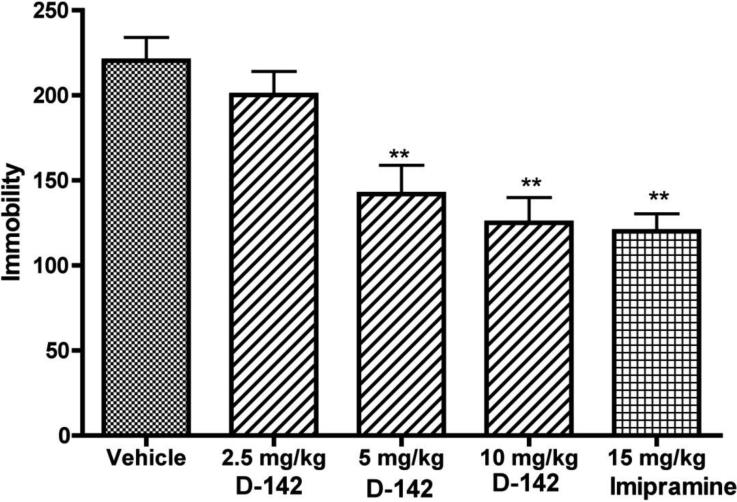
A dose dependent result was observed which is shown in Fig. 3. The results indicate that D-142 could reduce immobility dose-dependently with the highest dose (10 mg/kg) producing maximal reduction of immobility (P<0.01). The reference compound imipramine (15 mg/kg) reduced immobility (P<0.01), with the effect of imipramine at 15 mg/kg being comparable to that of D-142 at 10 mg/kg.
Fig. 3.
Effects of test drug D-142, imipramine and vehicle on immobility of mice in the tail suspension test. One way ANOVA analysis demonstrates significant effect among treatments: F (4,95) = 10.80 (P< 0.0001). Dunnett's analysis showed that the effect of D-142 at two doses (5 and 10 mg/kg) and the reference imipramine (15 mg/ kg) on immobility was significantly different compared to vehicle (P< 0.01). Asterisks indicate a statistically significant difference toward control group that received saline i.p. **P < 0.01. Each treatment group contains seven to eight mice.
3.4 Effect of D-142 on locomotor activity
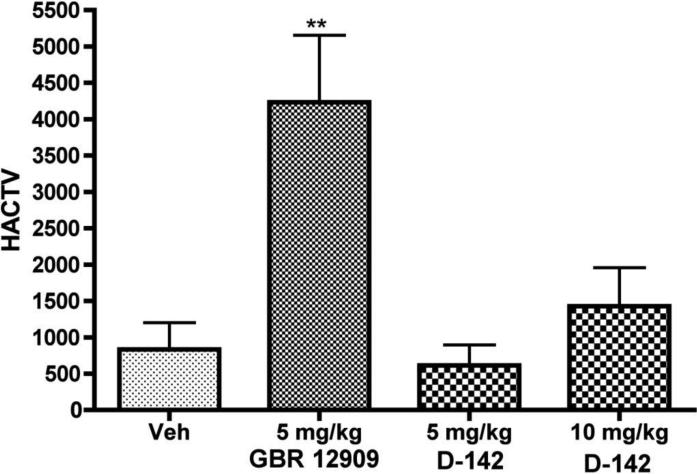
Two doses (5 and 10 mg/kg) of D-142 were tested for their effect on locomotor activity in rats and the result is shown in Fig. 4. The activity from the lower dose of 5 mg/kg measured for 0.5 h following 1 h post injection was indistinguishable from vehicle. The higher dose of 10 mg/kg under similar conditions did not produce a statistically significant effect compared to vehicle (P>0.05). In contrast, both doses of D-142 were able to produce a significant effect in reducing immobility in the rat forced swim and mouse tail suspension tests (P<0.01, Fig. 2 and Fig. 3). On the other hand, the well known DAT blocker GBR 12909 at a dose of 5 mg/kg produced a significant rise (P<0.01) in locomotor activity and thus exhibited a much more stimulant effect.
Fig. 4.
Effects of drugs, D-142 and GBR 12909, on locomotor activity (horizontal activity, HACTV). Rats were injected (i.p.) with either vehicle, D-142 and GBR 12909 followed by measurement of locomotor activity for ½ h after post 1 h of administration of drugs. This time frame mimicked the condition of rat FST test. One way ANOVA analysis demonstrates non significant effect between control and the two doses of D-142 but significant effect between control and GBR 12909 : F (4,95) = 5.84 (P< 0.01). Asterisks indicate a statistically significant difference toward control group that received saline i.p. **P < 0.01. Each treatment group contains six to eight rats.
4. Discussion
A role for dopamine in depression has been firmly established. Considerable evidence links the mesocorticolimbic dopamine system with depression and anhedonia, which is associated with a lack of motivation (D'Aquila et al., 2000). Changes in this system produce loss of interest and pleasure through neuronal circuitries mediating reward, marking a depressed state of mind. More specifically, diminished dopaminergic activity has been implicated in major depression disorder. A number of evidences indicate that a low level of dopamine along with a low level of dopamine metabolites such as homovanillic acid (HVA) is associated with a depressed state (Hamner and Diamond, 1996; Roy et al., 1985). In addition, imaging studies indicate down-regulation of presynaptic DAT and an increase in binding affinity of dopamine D2/D3 receptors in depression (Klimek et al., 2002). Dopaminergic drugs which either enhance or decrease dopamine activity, can produce either antidepressant-like or depression-like states (Dunlop and Nemeroff, 2007; Jimerson, 1987).
In this report, we have demonstrated that the triple uptake inhibitor D-142 exhibits a balanced activity for all three monoamine transporters with two fold higher potency for SERT compared to DAT and NET. As eluded to earlier, the SERT uptake inhibitory potency of D-142 is similar to that of the well-known SSRI fluoxetine (Table 1). Thus, high potency for SERT coupled with comparable high potencies at NET and DAT makes this molecule an ideal TUI. Furthermore, in an extended receptor screening study, D-142 did not exhibit appreciable action at other important CNS receptors at a concentration of 10 μM. This should translate into D-142 producing minimal side effects. D-142 was evaluated in FST in rats at three different doses with the lowest dose being 2.5 mg/kg. The compound exhibited significant reduction of immobility at the lowest dose indicating high efficacy. In this regard, the effect from 2.5 mg/kg and 5 mg/kg was essentially similar. Highest reduction of immobility was achieved at the 10 mg/kg dose. Overall, FST results indicate the ability of D-142 to reverse the immobility in rats in an efficient way. In this regard, D-142 was more efficacious than the reference compound imipramine. In the mouse TST, D-142 exhibited dose dependent activity with the highest dose of 10 mg/kg exhibiting the highest reduction of immobility. However, the effect from the lowest dose (2.5 mg/kg) was not significant compared to the control.
In order to determine whether the efficacy of D-142 originated, in part or in full, from locomotor activation, we carried out locomotor activity studies with the same doses of D-142 as applied in the FST. The results indicate that D-142 was ineffective in producing locomotor activity at the same dose range as tested under same experimental protocol applied in rat FST. Thus, the reduction of immobility by D-142 in these animal models was not due to increase in locomotor activity.
Conclusion
In this report, we demonstrated the development of a unique asymmetric tri-substituted pyran derivative as a promising lead triple uptake inhibitor (Zhang et al., 2006; Zhang et al., 2005). We have demonstrated that stereoselective orientation of the hydroxyl group in compound D-142 is important, as the (-)-isomers exhibited higher potency compared to the corresponding (+)-isomers (Zhang et al., 2005). D-142 was highly efficacious in reducing immobility in both the rat forced swimming test and mouse tail suspension test. These tests have been used widely for preclinical evaluation of test compounds for antidepressant activity including other triple uptake inhibitors. Interestingly, D-142 was more efficacious than the reference compound imipramine in both the rat forced swimming test and mice tail suspension test, with lower doses of D-142 than imipramine required for a given response. The additional dopaminergic activity along with possible favorable PK or other properties of D-142 may be responsible for its higher efficacy. In the screen for CNS multiple receptor binding, D-142 exhibited little or no non-specific interaction. Thus it is highly likely that the pharmacological response of D-142 derives from interactions at all three monoamine transporters. Taken together, the in vitro and in vivo results strongly suggest that compound D-142 possesses potent antidepressant activity.
Supplementary Material
Acknowledgement
This work is supported by National Institute of Mental Health/ National Institute of Health MH084888 (AKD). The CNS broad screening assays were carried out generously by the National Institute of Mental Health's Psychoactive Drug Screening Program, Contract # NO1MH32004 (NIMH PDSP). The NIMH PDSP is directed by Bryan L. Roth MD, PhD at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscol at NIMH, Bethesda MD, USA (http://pdsp.med.unc.edu/).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, Golden RN, Martin P, Potter WZ, Richelson E, et al. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry. 1995;56:395–401. [PubMed] [Google Scholar]
- Baldessarini RH. Goodman and Gilman's. The pharmacological basis of the therapeutica. McGrawe Hills; New York: 1996. [Google Scholar]
- Baldessarini RJ. In: Goodman & Gilman's The Pharmnacological Basis of Therapeutics. 11th ed. Brunton LL, Lazo JS, Parker KL, editors. McGraw-Hill; New York: 2006. p. 443. [Google Scholar]
- Barbey JT, Roose SP. SSRI safety in overdose. J Clin Psychiatry. 1998;59(Suppl 15):42–48. [PubMed] [Google Scholar]
- Carroll FI, Kotian P, Dehghani A, Gray JL, Kuzemko MA, Parham KA, Abraham P, Lewin AH, Boja JW, Kuhar MJ. Cocaine and 3 beta-(4'-substituted phenyl)tropane-2 beta-carboxylic acid ester and amide analogues. New high-affinity and selective compounds for the dopamine transporter. J Med Chem. 1995;38:379–388. doi: 10.1021/jm00002a020. [DOI] [PubMed] [Google Scholar]
- Corrigan MH, Denahan AQ, Wright CE, Ragual RJ, Evans DL. Comparison of pramipexole, fluoxetine, and placebo in patients with major depression. Depress Anxiety. 2000;11:58–65. doi: 10.1002/(sici)1520-6394(2000)11:2<58::aid-da2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- D'Aquila PS, Collu M, Gessa GL, Serra G. The role of dopamine in the mechanism of action of antidepressant drugs. Eur J Pharmacol. 2000;405:365–373. doi: 10.1016/s0014-2999(00)00566-5. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Dutta AK, Ghosh B, Biswas S, Reith ME. D-161, a novel pyran-based triple monoamine transporter blocker: behavioral pharmacological evidence for antidepressant-like action. Eur J Pharmacol. 2008;589:73–79. doi: 10.1016/j.ejphar.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Taylor JR, Berger P, Roth RH. Cocaine-sensitive and -insensitive dopamine uptake in prefrontal cortex, nucleus accumbens and striatum. Neurochem Int. 1993;23:61–69. doi: 10.1016/0197-0186(93)90144-t. [DOI] [PubMed] [Google Scholar]
- Goldstein BJ, Goodnick PJ. Selective serotonin reuptake inhibitors in the treatment of affective disorders--III. Tolerability, safety and pharmacoeconomics. Journal of psychopharmacology (Oxford, England) 1998;12:S55–87. doi: 10.1177/0269881198012003041. [DOI] [PubMed] [Google Scholar]
- Hamner MB, Diamond BI. Plasma dopamine and norepinephrine correlations with psychomotor retardation, anxiety, and depression in non-psychotic depressed patients: a pilot study. Psychiatry research. 1996;64:209–211. doi: 10.1016/s0165-1781(96)02879-x. [DOI] [PubMed] [Google Scholar]
- Hiemke C, Hartter S. Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol Ther. 2000;85:11–28. doi: 10.1016/s0163-7258(99)00048-0. [DOI] [PubMed] [Google Scholar]
- Jimerson DC. Psychopharmacology The third generation of progress. Raven Press; New York: 1987. Role of dopamine mechanisms in affective disorder. pp. 505–511. [Google Scholar]
- Kharkar PS, Batman AM, Zhen J, Beardsley PM, Reith ME, Dutta AK. Synthesis and biological characterization of (3R,4R)-4-(2-(benzhydryloxy)ethyl)-1-((R)-2-hydroxy-2-phenylethyl)-piperid in-3-ol and its stereoisomers for activity toward monoamine transporters. ChemMedChem. 2009;4:1075–1085. doi: 10.1002/cmdc.200900085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimek V, Schenck JE, Han H, Stockmeier CA, Ordway GA. Dopaminergic abnormalities in amygdaloid nuclei in major depression: a postmortem study. Biological psychiatry. 2002;52:740–748. doi: 10.1016/s0006-3223(02)01383-5. [DOI] [PubMed] [Google Scholar]
- Liang Y, Richelson E. Triple reuptake inhibitors: Next-generation antidepressants. Primary Psychiatry. 2008:50–56. [Google Scholar]
- Liang Y, Shaw AM, Boules M, Briody S, Robinson J, Oliveros A, Blazar E, Williams K, Zhang Y, Carlier PR, Richelson E. Antidepressant-like pharmacological profile of a novel triple reuptake inhibitor, (1S,2S)-3-(methylamino)-2-(naphthalen-2-yl)-1-phenylpropan-1-ol (PRC200-SS). J Pharmacol Exp Ther. 2008;327:573–583. doi: 10.1124/jpet.108.143610. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Dual- and triple-acting agents for treating core and co-morbid symptoms of major depression: novel concepts, new drugs. Neurotherapeutics. 2009;6:53–77. doi: 10.1016/j.nurt.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischoulon D, Nierenberg AA, Kizilbash L, Rosenbaum JF, Fava M. Strategies for managing depression refractory to selective serotonin reuptake inhibitor treatment: a survey of clinicians. Canadian journal of psychiatry. 2000;45:476–481. doi: 10.1177/070674370004500509. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. The serotonin transporter and depression. Depress Anxiety 8 Suppl. 1998;1:5–12. [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Richelson E. Interactions of antidepressants with neurotransmitter transporters and receptors and their clinical relevance. The Journal of clinical psychiatry. 2003;64(Suppl 13):5–12. [PubMed] [Google Scholar]
- Roy A, Pickar D, Linnoila M, Potter WZ. Plasma norepinephrine level in affective disorders. Relationship to melancholia. Archives of general psychiatry. 1985;42:1181–1185. doi: 10.1001/archpsyc.1985.01790350055010. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Shank RP, Gardocki JF, Schneider CR, Vaught JL, Setler PE, Maryanoff BE, McComsey DF. Preclinical evaluation of McN-5707 as a potential antidepressant. J Pharmacol Exp Ther. 1987;242:74–84. [PubMed] [Google Scholar]
- Shaw AM, Boules M, Zhang Y, Williams K, Robinson J, Carlier PR, Richelson E. Antidepressant-like effects of novel triple reuptake inhibitors, PRC025 and PRC050. Eur J Pharmacol. 2007;555:30–36. doi: 10.1016/j.ejphar.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Skolnick P, Popik P, Janowsky A, Beer B, Lippa AS. Antidepressant-like actions of DOV 21,947: a “triple” reuptake inhibitor. Eur J Pharmacol. 2003a;461:99–104. doi: 10.1016/s0014-2999(03)01310-4. [DOI] [PubMed] [Google Scholar]
- Skolnick P, Popik P, Janowsky A, Beer B, Lippa AS. “Broad spectrum” antidepressants: is more better for the treatment of depression? Life sciences. 2003b;73:3175–3179. doi: 10.1016/j.lfs.2003.06.007. [DOI] [PubMed] [Google Scholar]
- Spinks D, Spinks G. Serotonin reuptake inhibition: an update on current research strategies. Curr Med Chem. 2002;9:799–810. doi: 10.2174/0929867024606795. [DOI] [PubMed] [Google Scholar]
- Sporn J, Ghaemi SN, Sambur MR, Rankin MA, Recht J, Sachs GS, Rosenbaum JF, Fava M. Pramipexole augmentation in the treatment of unipolar and bipolar depression: a retrospective chart review. Ann Clin Psychiatry. 2000;12:137–140. doi: 10.1023/a:1009060800999. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Thase ME, Entsuah AR, Rudolph RL. Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. Br J Psychiatry. 2001;178:234–241. doi: 10.1192/bjp.178.3.234. [DOI] [PubMed] [Google Scholar]
- Thomas DR, Nelson DR, Johnson AM. Biochemical effects of the antidepressant paroxetine, a specific 5-hydroxytryptamine uptake inhibitor. Psychopharmacology. 1987;93:193–200. doi: 10.1007/BF00179933. [DOI] [PubMed] [Google Scholar]
- Wong DT, Bymaster FP. Development of antidepressant drugs. Fluoxetine (Prozac) and other selective serotonin uptake inhibitors. Adv Exp Med Biol. 1995;363:77–95. [PubMed] [Google Scholar]
- Zhang S, Fernandez F, Hazeldine S, Deschamps J, Zhen J, Reith ME, Dutta AK. Further structural exploration of trisubstituted asymmetric pyran derivatives (2S,4R,5R)-2-benzhydryl-5-benzylamino-tetrahydropyran-4-ol and their corresponding disubstituted (3S,6S) pyran derivatives: a proposed pharmacophore model for high-affinity interaction with the dopamine, serotonin, and norepinephrine transporters. J Med Chem. 2006;49:4239–4247. doi: 10.1021/jm0601699. [DOI] [PubMed] [Google Scholar]
- Zhang S, Zhen J, Reith ME, Dutta AK. Discovery of novel trisubstituted asymmetric derivatives of (2S,4R,5R)-2-benzhydryl-5-benzylaminotetrahydropyran-4-ol, exhibiting high affinity for serotonin and norepinephrine transporters in a stereospecific manner. J Med Chem. 2005;48:4962–4971. doi: 10.1021/jm049021k. [DOI] [PubMed] [Google Scholar]
- Zhen J, Maiti S, Chen N, Dutta AK, Reith ME. Interaction between a hydroxypiperidine analogue of 4-(2-benzhydryloxy-ethyl)-1-(4-fluorobenzyl)piperidine and Aspartate 68 in the human dopamine transporter. Eur J Pharmacol. 2004;506:17–26. doi: 10.1016/j.ejphar.2004.10.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.