Abstract
VCP/p97 is a multifunctional AAA+-ATPase involved in vesicle fusion, proteasomal degradation, and autophagy. Reported dysfunctions of these processes in Alzheimer disease (AD), along with the linkage of VCP/p97 to inclusion body myopathy with Paget’s disease and frontotemporal dementia (IBMPFD) led us to examine the possible linkage of VCP to the AD-relevant protein, tau. VCP levels were reduced in AD brains, but not in the cerebral cortex of an AD mouse model, suggesting that VCP reduction occurs upstream of tau pathology. Genetic reduction of VCP in a primary neuronal model led to increases in the levels of tau phosphorylated at Ser262/356, indicating that VCP may be involved in regulating post-translational processing of tau in AD, demonstrating a possible functional linkage between tau and VCP.
Keywords: tau, valosin-containing protein, p97, autophagy
1. Introduction
Valosin-containing protein (VCP) (also known as p97) is a multifunctional AAA+ (ATPase associated with various cellular activities) protein involved in a wide variety of cellular functions including cytosolic proteasomal degradation [1] and autophagy [2]. VCP has been shown to interact with various proteins implicated in neurodegenerative diseases including ataxin-3 [3] and huntingtin [4]. In 2004 it was discovered that the autosomal-dominant degenerative disease Inclusion Body Myositis with Paget’s disease of the bone and Frontotemporal Dementia (IBMPFD) was linked to the VCP gene [5]. Affected tissue in this disorder contains both cytoplasmic and nuclear insoluble protein aggregates that are both ubiquitin- and VCP-positive [6].
Though the mechanistic role of VCP in endoplasmic reticulum associated degradation (ERAD) is well described [7], a full understanding of its role in cytosolic protein degradation has been more elusive. Genetic reduction of VCP results in large increases of ubiquitylated proteins and ubiquitin-positive aggregates [8]. VCP has been implicated in proteasomal degradation of ataxin-3 [3] and huntingtin [4], as well as the formation of proteinaceous aggregates [9]. In addition, it has been demonstrated that these aggregates contain the autophagosome markers LC3-II and p62 [2]. This dual role of VCP in both proteasomal degradation and autophagy suggests a central role of VCP in determining the degradative fate of aggregative proteins.
The main pathological hallmarks of Alzheimer disease (AD) are beta-amyloid (Aβ) plaques and neurofibrillary tangles composed of the microtubule-associated protein tau. Dysfunction in both the ubiquitin-proteasome system [10] and autophagic clearance pathways [11] have been proposed as potential contributors to the accumulation of both Aβ and tau in AD. In particular, tau has been described as a substrate of both the proteasome [12] and autophagy pathway [13] and in a previous study our lab showed that a C-terminal cleavage event was an important factor in directing tau towards autophagic rather than proteasomal degradation [14]. Because of VCP’s dualistic nature in modulating protein degradation, as well as case studies indicating accumulations of phospho-tau in IBMPFD cortex [15], we examined VCP protein content in AD brain and the effect of VCP reduction on total and phospho-tau levels in a neuronal model.
2. Materials and Methods
2.1. Antibodies and Plasmids
Tau antibodies used were: Tau5 (provided by Dr. L. Binder), PHF1 (provided by Dr. P Davies), and 12E8 (provided by Dr. P. Seubert). Other antibodies used were anti-VCP (Affinity Bioreagents), anti-ubiquitin (Biomol), and anti-actin (Chemicon). The shRNA lentiviral shuttle vectors were constructed by subcloning the cassette containing the H1 promoter and shRNA sequence from pSUPER.retro.p97 and pSUPER.retro.ran1 [16] followed by ligation into the vector FG12 (Addgene). The vectors pVSV-G (encoding the viral envelope) and psPAX2 (encoding gag-pol) were from Addgene.org. The identities of the constructs were confirmed by DNA sequencing.
2.2. Tissue fractionation
Human brain samples were prepared as described previously [17]. The protocol was designated as exempt by the Institutional Review Boards of University of Alabama at Birmingham and the University of Rochester. Information on the cases used in this study is provided in Table 1. 3xTg-AD [18] and background-matched nontransgenic (NTg) mice were housed in microisolators, and all experiments were in compliance with University of Rochester Committee on Animal Resources guidelines. Aged animals (NTg: 26 months, 3xTg-AD: 13–26 months) were anesthetized by intraperitoneal injection of nembutol (100 mg/kg), and transcardiac perfusion was performed using PBS. Brains were removed, and cortices dissected and immediately snap-frozen until use. Homogenization was performed exactly as for human brain tissue.
Table 1.
Patient information of cortical specimens examined in Fig. 1.
| Specimen | Age | Sex | PMI (hr) | Diagnosis |
|---|---|---|---|---|
| C1 | 83 | M | NA | Hemorrhagic Gastritis |
| C2 | 70 | F | NA | Coronary Artery Disease |
| C3 | 65 | M | 6 | Lymphoma |
| C4 | 79 | F | 22 | Heart Failure |
| C5 | 63 | M | 12 | Hepatitis/Cirrhosis |
| AD1 | 85 | M | 2.5 | AD |
| AD2 | 74 | M | 11 | AD |
| AD3 | NA | F | NA | AD |
| AD4 | 64 | M | 6 | AD |
| AD5 | 78 | F | 4 | AD |
| AD6 | 79 | F | 5 | AD |
2.3. Lentiviral vector production
HEK 293TN cells were transfected with pLenti/psPAX2/pVSV-G using Lipofectamine 2000 (Invitrogen). Twelve hours post-transfection, media was replaced with DMEM supplemented with 1% Fetalclone II and 2 mM L-glutamine, and the cells were incubated at 33°C. Forty-eight hours later the media was collected, spun at 500×g for 10 min at room temperature and the resulting supernatant was then spun at 60,000×g for 2h at 4°C. The visible pellet was resuspended in 500 μl sterile PBS containing 0.1% BSA per 100 mm dish, and the lentiviral solution was either used immediately or snap-frozen for later use.
2.4. Primary neuronal culture and transduction
Primary cortical neuronal cultures from rat embryonic forebrain were prepared and maintained as described previously [19]. On DIV 1, 25 μl of virus was added directly to the media on the neurons, and medium replaced after 4 h. Experiments were performed on DIV 7–8. For treatment with autophagy inhibitor, neurons were treated with either vehicle or 10 mM 3-methyladenine (3-MA; Sigma-Aldrich) for 12 h and collected on DIV 8. Neuronal viability was determined using resazurin, as previously described [14].
2.5. Sarkosyl Fractionation, Immunoblotting, and qRT-PCR
Neurons were washed once with cold PBS and sarkosyl lysis buffer (50 mM Tris pH 7.4, 150 mM NaCl, 1% sodium N-lauryl sarkosamine) was added directly to cells. Plates were incubated at 4°C for 30 min, and samples were briefly sonicated and incubated 30 min at 25°C with occasional vortexing. Samples were centrifuged at 3000×g for 20 min at 4°C, and supernatants were centrifuged further at 150,000×g for 2h at room temperature using a benchtop airfuge (Beckman) to separate sarkosyl-soluble and –insoluble fractions. Immunoblotting was carried out as previously described [14]. Quantitative RT-PCR was carried out as described previously [20].
2.6. Statistics
Analyses of differences in protein levels (Figs. 1–2, 4–6) were determined using Student’s t-test. Differences in cDNA levels by qRT-PCR (Fig. 3) were analyzed using one-way ANOVA and post-hoc t-tests. A threshold of p<0.05 was used for determining significance. All data are expressed as means ±SEM.
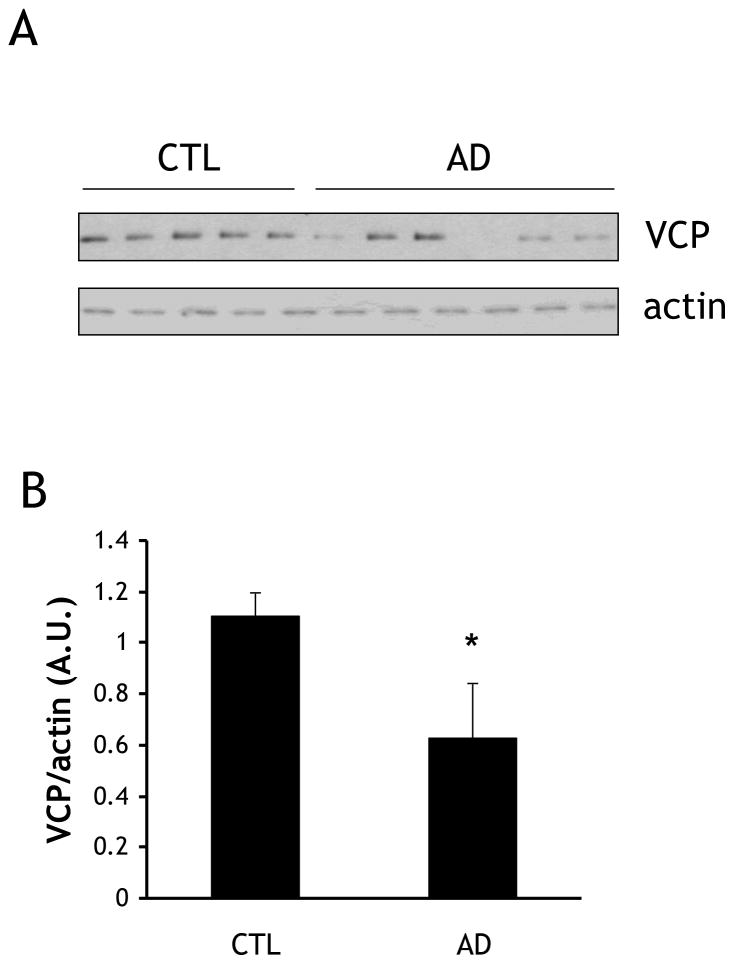
Fig. 1. VCP levels are lower in AD brain cortex than in age-matched controls.
(A) Frozen tissue from five control and six AD patients were homogenized and fractionated, and equal amounts of homogenate were immunoblotted for VCP and actin. (B) Quantification of VCP immunoblotting described in (A). Protein levels were quantified by scanning densitometry, normalized to actin, and expressed as arbitrary units (A.U.). *=p<0.05.
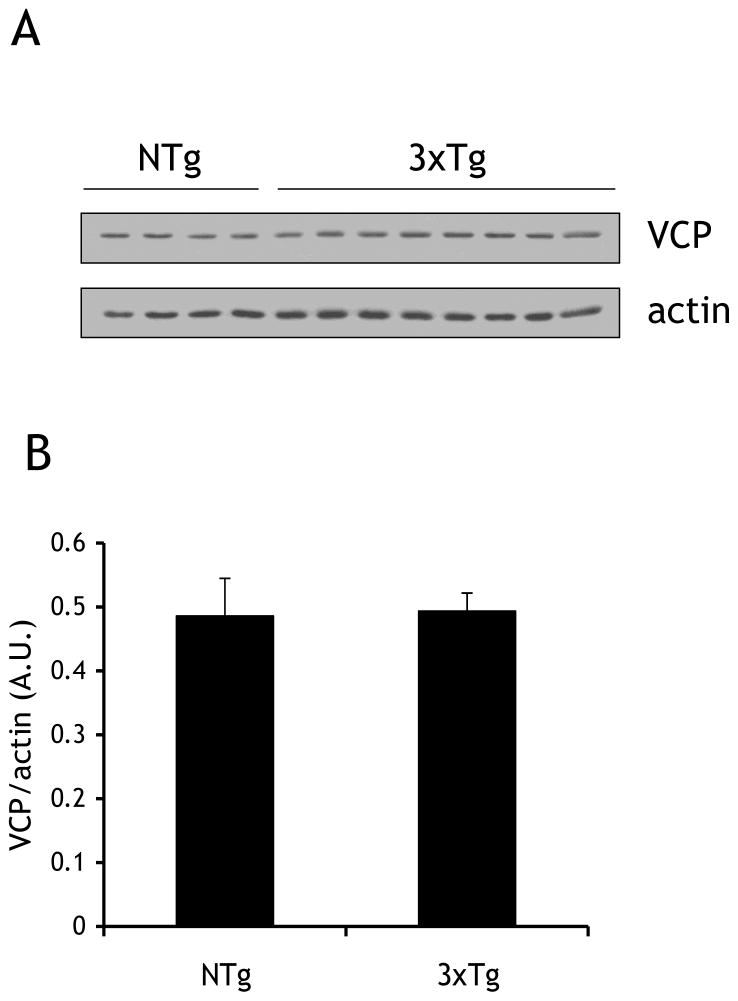
Fig 2. VCP levels in the cortex of 3xTg-AD and NTg mice are similar.
(A) Cortical tissue samples from 4 non-transgenic (NTg) (26 months) and 8 triple transgenic (3xTg) (13–22 months) were processed identically to human tissue in Fig. 1. Lysates were immunoblotted for VCP and actin. (B) Quantification of immunoblotting described in (A), protein levels were quantified by scanning densitometry, normalized to actin, and expressed as arbitrary units (A.U.). There was no significant difference between the two groups.
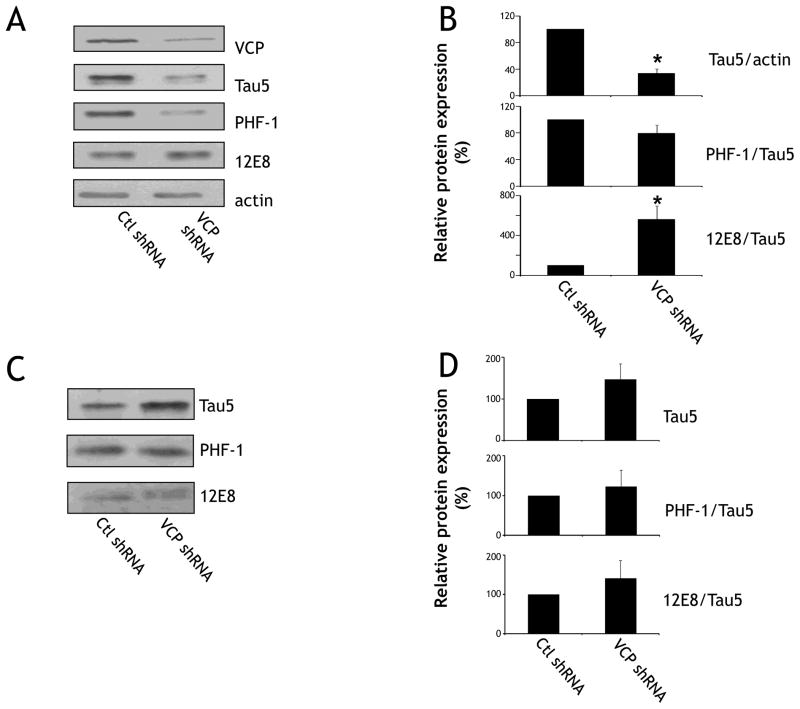
Fig. 4. Knockdown of VCP results in decreases in total soluble tau protein, but increases in soluble phosphorylated Ser262/356-tau protein.
(A) Neurons were harvested on DIV 8 and fractionated using sarkosyl buffer. Equal amounts of soluble protein were immunoblotted for VCP, Tau5 (total tau) PHF1 (Ser396/404), 12E8 (Ser262/356) and actin. (B) Quantification of data described in (A). Tau5 immunoreactivity is normalized to actin, and PHF1 and 12E8 are both normalized to the Tau5/actin ratio. There was a significant difference in total tau and 12E8 levels (*p<0.05) between control and VCP shRNA-infected groups (n=9 from 3 different animals). Note that even though PHF1 levels are lower for shRNA-VCP, there is no difference when this is normalized to Tau5/actin. (C) Sarkosyl-insoluble pellets were resolubilized, and equal volumes of resulting sample were immunoblotted for Tau5, PHF1, and 12E8. (D) Quantification of data described in (C). For sarkosyl-insoluble pellets, there was no significant difference in any of the epitopes as a result of shRNA-mediated knockdown of VCP.
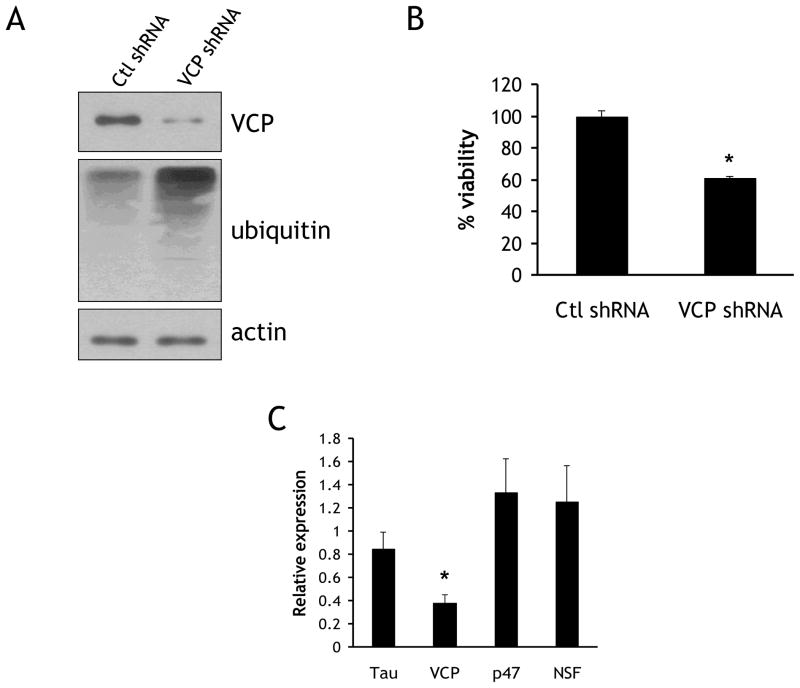
Fig. 3. VCP knockdown results in increased ubiqitylated proteins and decreased viability.
(A) Rat primary cortical neurons infected with shRNA-expressing lentivirus targeted to either VCP or a scrambled non-targeting sequence. Lysates were immunoblotted for VCP, ubiquitylated conjugates (FK2), and actin. (B) Neurons were infected as in (A), and subjected to a resazurin viability assay on DIV 8. (C) Neurons were infected as in (A), and RNA was collected on DIV8 for qRT-PCR. Data are normalized to the housekeeping gene actin and expressed as a ratio of cDNA levels in VCP knockdown cultures compared to control cultures. *=p<0.05.
3. Results
3.1. VCP protein levels are reduced in AD brain
Soluble protein lysates obtained from 5 control and 6 AD cortex tissue samples (Table 1) were immunoblotted for VCP and actin (Fig. 1A). Donors were similar in age (Control: 72 ± 3.9 y; AD: 76 ± 3.5 y). Band densitometry was performed and VCP levels were normalized to actin. Despite some variance in VCP levels in AD samples, the amount of VCP protein was significantly reduced in AD compared to the control samples (42.7±19.1%) (Fig. 1B). In contrast, this reduction in VCP levels was not observed in aged 3xTg-AD mice (Fig. 2).
3.2. Knockdown of VCP levels in rat primary cortical neurons
Primary rat cortical neurons transduced with shRNA targeted to VCP were used to model the lowered levels of VCP found in AD brain. Previous studies using VCP-targeted knockdown methods revealed a resulting accumulation of ubiquitylated conjugates, as well as a decrease in cell viability [8]. Neurons were transduced on DIV 1, and lysates were collected on DIV 7–8. Using this paradigm, VCP levels were consistently lowered 70–80% in comparison to neurons equivalently transduced with control shRNA. In addition, ubiquitylated conjugates accumulated upon VCP knockdown, in accordance with previous studies (Fig. 3A). The viability of neurons transduced either with control or VCP shRNA was determined using the resazurin metabolic assay, and the viability of VCP shRNA-infected neurons was found to be significantly reduced by 26.5±3.5% (Fig 3B). To ensure the specificity of shRNA targeting, the message levels of four different proteins were examined: (1) VCP, its functional homologue N-ethylmaleimide Sensitive Factor (NSF), (3) the VCP-associated protein p47, and (4) tau. RNA was extracted from neurons transduced with control or VCP shRNA and assayed using qRT-PCR. Transduction with VCP shRNA resulted in the specific reduction of VCP message (38.1±7.1%) compared to control (Fig. 3C). NSF and p47 were both increased, but not significantly (126±31% and 133±29%, respectively). Tau cDNA levels were lowered slightly but not significantly (85±16%).
3.3. Soluble tau phosphorylated at Ser262/356 phosphorylation is increased as a result of VCP reduction
Because of the central role of VCP in both proteasomal and autophagic degradation, we examined steady state tau levels in neurons with reduced VCP expression. In addition, it has been demonstrated that the sensitivity of tau to either the proteasome or autophagy systems can be determined by post-translational processing, such as phosphorylation [21]. Therefore we examined whether there were changes in the levels of phosphorylated tau as a result of VCP knockdown. Primary rat cortical neurons were transduced with control and VCP shRNA lentivirus on DIV1, subjected to sarkosyl fractionation on DIV 8, and immunoblotted (Fig. 4A). Reduction of VCP levels resulted in a significant reduction in the levels of soluble total tau levels to 33.5±6.8% of control levels (Fig. 4B). While the phosphorylation of the PHF1 epitope (Ser396/404) remained unchanged, 12E8 phosphorylation (Ser262/356) immunoreactivity was significantly increased to 561±134% as a result of the reduction of VCP levels. No changes in total or Ser262/356-phosphorylated tau were observed in the sarkosyl-insoluble fractions (Fig. 4C).
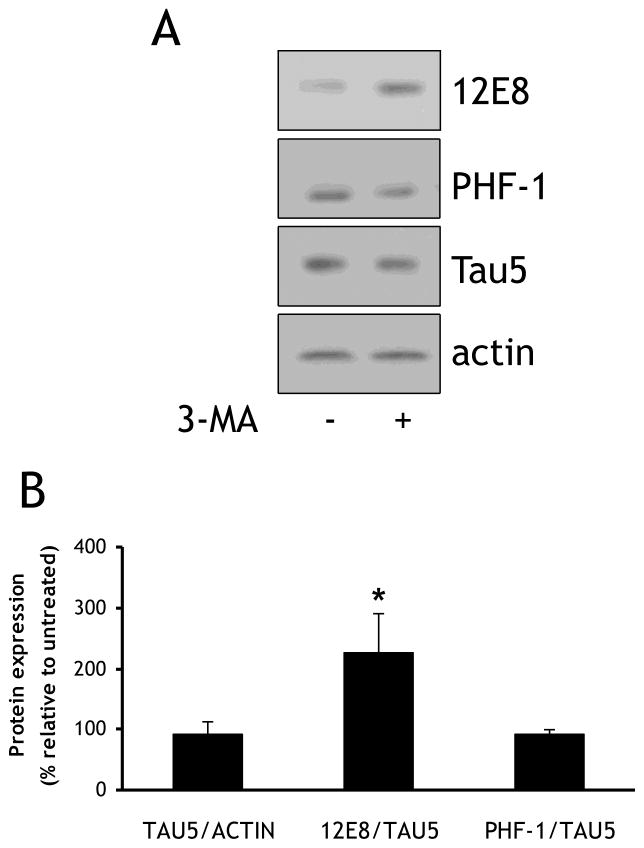
3.4. Treatment with the macroautophagy inhibitor 3-methyladenine results in increases in tau phosphorylated at Ser262/356
Because of its multivariate involvement in autophagy, proteasomal degradation, and aggresome formation, VCP may play an important modulatory role in determining the stability and degradation of tau. The expression of dysfunctional mutants of VCP related to IBMPFD has been found to result in increased accumulation of puncta containing processed LC3-II, a hallmark of dysfunction in the autophagosome maturation pathway [2]. In order to determine if autophagic inhibition resulted in similar increases in the levels of soluble Ser262/356-phosphorylated tau that we noted in neurons with reduced VCP levels, neurons were treated with either vehicle or 10 mM 3-MA for 16 h prior to collection of lysates, and changes in 12E8, PHF1, and Tau5 immunoreactivity were measured in the soluble fraction (Fig. 5A). There was a significant increase in the amount of 12E8 immunoreactivity upon treatment with 3-MA (227±65.9%) (Fig. 5B). This is in concordance with previous findings in a different cell model which also reported increases in 12E8 immunoreactivity upon 3-MA treatment [22].
Fig. 5. Inhibition of macroautophagy with 3-methyladenine results in increased levels of 12E8 immunoreactivity.
(A) Rat primary cortical neurons were infected on DIV 1 with lentivirus expressing a scrambled non-targeting sequence, and treated on DIV8 for 16 h with either vehicle or 10 mM 3-methyladenine (3-MA). Equal amounts of lysate were blotted for Tau5, PHF1, 12E8, and actin. (B) Quantification of data described in (A). Normalization was performed as described in Figure 4.*=p<0.05.
4. Discussion
Tau is degraded through multiple clearance pathways, and it is likely that a significant factor dictating the pathway by which tau is degraded is its posttranslational state [21]. Further, it is important to examine the proteins that may be directing the processes that regulate the degradation of tau by these different pathways, as their study may provide insights into the mechanisms that regulate the fate of tau in different physiological and pathological states. These mechanisms may occur either through direct binding and tethering of tau to degradative processes, or through indirect regulation of tau stability through an intermediary. It has already been shown that tau interacts with p62 [23] and histone deacetylase 6 (HDAC6) [17], which both contain ubiquitin- and LC3-binding capacities. Another such protein is VCP, which is involved in both delivery of substrates to the proteasome [24] and autophagosome formation [25]. In this study we show that total VCP levels are reduced in the cortex of sporadic AD cases. When VCP levels were reduced by shRNA in primary cortical neurons there was a significant increase in soluble tau phosphorylated at Ser262/356 as indicated by an increase in 12E8 immunoreactivity. We also observed that total soluble tau levels as measured by Tau5 immunoreactivity were decreased when VCP levels were reduced. No changes in tau levels were observed in the insoluble fraction upon VCP knockdown.
A recent study determined that in AD brain VCP is cleaved by caspase 6 resulting into two N-terminal fragments of 28 and 20 kDa. These fragments inhibit the ubiquitin-proteasome system by effectively acting as competitive inhibitors of both ubiquitylated substrates and N-terminal binding co-factors, and also act as destabilizers of steady-state VCP levels [26]. In this present study these 28 and 20 kDa VCP fragments were not observed in AD brain samples, but it should be noted that the VCP antibody we used is specific for the C-terminus of VCP. We did note lower levels of VCP in AD brain, which could corroborate this finding of the N-terminal cleavage of VCP. It is difficult to positively determine a correlation between these observations, as the previous study did not examine total levels of VCP in human brain, but rather examined the appearance of the N-terminal fragments using a specific antibody raised against the cleavage site. The lack of similar results in 3xTg-AD mice indicates that VCP reduction occurs upstream of Aβ and tau pathology, and not directly as a result of pathology incurred by either of these proteins.
Knockdown of VCP resulted in a significant increase in soluble tau phosphorylated at the Ser262/356 sites as evidence by an almost six-fold increase in 12E8 immunoreactivity. Tau’s Ser262/356 sites lie within the microtubule binding domains, and are primary determinants of tau affinity for microtubules. The phosphorylation status of tau may impact its particular degradative fate as it has been reported that tau phosphorylated at Ser199/202 and Thr205 has an increased propensity for ubiquitylation [12]. In contrast, tau phosphorylated at the KXGS motifs is not degraded by the proteasome [21], but rather by autophagy [22]. VCP is required for the completion of macroautophagy, so one possible mechanism for the increase of 12E8-tau when VCP levels are reduced is that autophagy is inhibited, and this is very selective in preventing the degradation of tau phosphorylated at Ser262/356, while other forms of tau in the neuron are degraded. Indeed, inhibition of autophagy with 3-MA resulted in a selective increase in tau phosphorylated at Ser262/356.
We also observed that knocking down VCP resulted in a decrease in soluble total tau levels as indicated by a decrease in Tau5 immunoreactivity. This is in apparent conflict with the functions of VCP that have been previously described—namely substrate delivery to the proteasome and autophagic vacuole maturation. A possible explanation for the reduction in tau levels noted here is that VCP plays a role in maintaining the stability of particular post-translationally modified forms of tau protein, while leaving others unperturbed. A role for VCP in maintaining proper folding and stability of substrate proteins is not unprecedented. Rumpf and Jentsch [27] outline a model by which the ubiquitylation and proteasome-targeting activity of VCP, when bound to the E4 ligase Ufd2, can be counteracted by either simultaneous binding of either the deubiquitylase Otu1 or the competing cofactor Ufd3. This could result in the release of a substrate, possibly after modification of its ubiquitylation status to maintain proper folding and stability. Alternatively, it is possible that VCP binds tau through an alternate substrate binding region in the D2 loop of VCP that has the capacity to bind hydrophobic patches of substrate proteins [28]. One other distinct possibility that cannot be ruled out is that changes in tau stability are an indirect result of the reduction of VCP levels. VCP reduction results in the loss of cell viability (Fig. 3; [8]), and the loss of neuronal integrity may be affecting total and phosphorylated tau levels in the neuron. A recent study [29] demonstrated that after antimycin A treatment, the presence of Ser262/356-tau increases, while total tau and tau phosphorylated at Ser396/404 decreases. This was shown to be a result of the specific recruitment of Ser262/356-tau to actin-cofilin rods. While knockdown of VCP resulted in an increase in Ser262/356-tau, a concomitant decrease in tau phosphorlyated at the Ser396/404 epitope was not observed. Nevertheless, the similarities between the two models are intriguing and may underlie a common mechanism present during the early pathogenesis of AD. However it is clear that further investigation is needed to determine the mechanisms by which VCP regulates the stability/turnover of tau and how the site specific phosphorylation state of tau determines its fate.
VCP levels are decreased in Alzheimer disease brain
VCP knockdown in neurons decreases total soluble tau levels
VCP knockdown increases the levels of tau phosphorylated at Ser262/356
Inhibition of autophagy increases soluble tau phosphorylated at Ser262/356
Acknowledgments
This work was supported by NIH grants NS051279 and NS041744 and a grant from the Alzheimer’s Association. The authors would like to thank Drs. Oddo and LaFerla for providing the 3xTg-AD mouse, Dr. Wojcikiewicz for providing pSUPER.retro.p97 and pSUPER.retro.ran1 constructs, and Drs. Binder, Davies, and Seubert for providing the Tau5, PHF1, and 12E8 antibodies, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dai RM, Chen E, Longo DL, Gorbea CM, Li CC. Involvement of valosin-containing protein, an ATPase Co-purified with IkappaBalpha and 26 S proteasome, in ubiquitin-proteasome-mediated degradation of IkappaBalpha. J Biol Chem. 1998;273:3562–73. doi: 10.1074/jbc.273.6.3562. [DOI] [PubMed] [Google Scholar]
- 2.Ju JS, Fuentealba RA, Miller SE, Jackson E, Piwnica-Worms D, Baloh RH, Weihl CC. Valosin-containing protein (VCP) is required for autophagy and is disrupted in VCP disease. J Cell Biol. 2009;187:875–88. doi: 10.1083/jcb.200908115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doss-Pepe EW, Stenroos ES, Johnson WG, Madura K. Ataxin-3 interactions with rad23 and valosin-containing protein and its associations with ubiquitin chains and the proteasome are consistent with a role in ubiquitin-mediated proteolysis. Mol Cell Biol. 2003;23:6469–83. doi: 10.1128/MCB.23.18.6469-6483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang H, Zhong X, Ballar P, Luo S, Shen Y, Rubinsztein DC, Monteiro MJ, Fang S. Ubiquitin ligase Hrd1 enhances the degradation and suppresses the toxicity of polyglutamine-expanded huntingtin. Exp Cell Res. 2007;313:538–50. doi: 10.1016/j.yexcr.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 5.Watts GD, et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36:377–81. doi: 10.1038/ng1332. [DOI] [PubMed] [Google Scholar]
- 6.Guyant-Marechal L, et al. Valosin-containing protein gene mutations: clinical and neuropathologic features. Neurology. 2006;67:644–51. doi: 10.1212/01.wnl.0000225184.14578.d3. [DOI] [PubMed] [Google Scholar]
- 7.Rabinovich E, Kerem A, Frohlich KU, Diamant N, Bar-Nun S. AAA-ATPase p97/Cdc48p, a cytosolic chaperone required for endoplasmic reticulum -associated protein degradation. Mol Cell Biol. 2002;22:626–34. doi: 10.1128/MCB.22.2.626-634.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wojcik C, Yano M, DeMartino GN. RNA interference of valosin-containing protein (VCP/p97) reveals multiple cellular roles linked to ubiquitin/proteasome-dependent proteolysis. J Cell Sci. 2004;117:281–92. doi: 10.1242/jcs.00841. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi T, Manno A, Kakizuka A. Involvement of valosin-containing protein (VCP)/p97 in the formation and clearance of abnormal protein aggregates. Genes Cells. 2007;12:889–901. doi: 10.1111/j.1365-2443.2007.01099.x. [DOI] [PubMed] [Google Scholar]
- 10.Hegde AN, Upadhya SC. The ubiquitin-proteasome pathway in health and disease of the nervous system. Trends Neurosci. 2007;30:587–95. doi: 10.1016/j.tins.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64:113–22. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- 12.Shimura H, Miura-Shimura Y, Kosik KS. Binding of tau to heat shock protein 27 leads to decreased concentration of hyperphosphorylated tau and enhanced cell survival. J Biol Chem. 2004;279:17957–62. doi: 10.1074/jbc.M400351200. [DOI] [PubMed] [Google Scholar]
- 13.Hamano T, Gendron TF, Causevic E, Yen SH, Lin WL, Isidoro C, Deture M, Ko LW. Autophagic-lysosomal perturbation enhances tau aggregation in transfectants with induced wild-type tau expression. Eur J Neurosci. 2008;27:1119–30. doi: 10.1111/j.1460-9568.2008.06084.x. [DOI] [PubMed] [Google Scholar]
- 14.Dolan PJ, Johnson GV. A caspase cleaved form of tau is preferentially degraded through the autophagy pathway. J Biol Chem. 2010;285:21978–87. doi: 10.1074/jbc.M110.110940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroder R, et al. Mutant valosin-containing protein causes a novel type of frontotemporal dementia. Ann Neurol. 2005;57:457–61. doi: 10.1002/ana.20407. [DOI] [PubMed] [Google Scholar]
- 16.Alzayady KJ, Panning MM, Kelley GG, Wojcikiewicz RJ. Involvement of the p97-Ufd1-Npl4 complex in the regulated endoplasmic reticulum-associated degradation of inositol 1,4,5-trisphosphate receptors. J Biol Chem. 2005;280:34530–7. doi: 10.1074/jbc.M508890200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding H, Dolan PJ, Johnson GV. Histone deacetylase 6 interacts with the microtubule-associated protein tau. J Neurochem. 2008;106:2119–30. doi: 10.1111/j.1471-4159.2008.05564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oddo S, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–21. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 19.Kress GJ, Dineley KE, Reynolds IJ. The relationship between intracellular free iron and cell injury in cultured neurons, astrocytes, and oligodendrocytes. J Neurosci. 2002;22:5848–55. doi: 10.1523/JNEUROSCI.22-14-05848.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filiano AJ, Tucholski J, Dolan PJ, Colak G, Johnson GV. Transglutaminase 2 protects against ischemic stroke. Neurobiol Dis. 2010;39:334–43. doi: 10.1016/j.nbd.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dickey CA, et al. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest. 2007;117:648–58. doi: 10.1172/JCI29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Martinez-Vicente M, Kruger U, Kaushik S, Wong E, Mandelkow EM, Cuervo AM, Mandelkow E. Synergy and antagonism of macroautophagy and chaperone-mediated autophagy in a cell model of pathological tau aggregation. Autophagy. 2009;6:182–3. doi: 10.4161/auto.6.1.10815. [DOI] [PubMed] [Google Scholar]
- 23.Babu JR, Geetha T, Wooten MW. Sequestosome 1/p62 shuttles polyubiquitinated tau for proteasomal degradation. J Neurochem. 2005;94:192–203. doi: 10.1111/j.1471-4159.2005.03181.x. [DOI] [PubMed] [Google Scholar]
- 24.Richly H, Rape M, Braun S, Rumpf S, Hoege C, Jentsch S. A series of ubiquitin binding factors connects CDC48/p97 to substrate multiubiquitylation and proteasomal targeting. Cell. 2005;120:73–84. doi: 10.1016/j.cell.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Krick R, Bremer S, Welter E, Schlotterhose P, Muehe Y, Eskelinen EL, Thumm M. Cdc48/p97 and Shp1/p47 regulate autophagosome biogenesis in concert with ubiquitin-like Atg8. J Cell Biol. 2010;190:965–73. doi: 10.1083/jcb.201002075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halawani D, Tessier S, Anzellotti D, Bennett DA, Latterich M, LeBlanc AC. Identification of Caspase-6-mediated processing of the valosin containing protein (p97) in Alzheimer’s disease: a novel link to dysfunction in ubiquitin proteasome system -mediated protein degradation. J Neurosci. 2010;30:6132–42. doi: 10.1523/JNEUROSCI.5874-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rumpf S, Jentsch S. Functional division of substrate processing cofactors of the ubiquitin-selective Cdc48 chaperone. Mol Cell. 2006;21:261–9. doi: 10.1016/j.molcel.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 28.DeLaBarre B, Christianson JC, Kopito RR, Brunger AT. Central pore residues mediate the p97/VCP activity required for ERAD. Mol Cell. 2006;22:451–62. doi: 10.1016/j.molcel.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 29.Whiteman ITMLS, Goh DL, Bamburg JR, Goldsbury C. Rapid changes in phospho-MAP/tau epitopes during neuronal stress: Cofilin-actin rods primarily recruit microtubule binding domain epitopes. PLoS One. 2011;6:e20878. doi: 10.1371/journal.pone.0020878. [DOI] [PMC free article] [PubMed] [Google Scholar]