Summary
Objective
To determine the likelihood that recommended doses of acetaminophen (APAP) are associated with acute liver failure (ALF) in patients with myopathies
Design
retrospective analysis
Setting
level III pediatric intensive care unit
Patients
two pediatric patients with myopathies and acute liver failure
Clinical Investigations
We determined acetaminophen protein adduct levels, in combination with a literature review and systematic evaluation of the cases, using the Roussel Uclaf Causality Assessment Method (RUCAM) for drug-induced liver injury (DILI) to assess causality between recommended acetaminophen dosing and acute liver failure in two children with myopathies.
Main Results
The serum adduct levels were consistent with the values previously reported in children with acute liver injury following APAP overdose. We found four similar cases of ALF in pediatric and adult patients with myopathies following recommended APAP doses in the literature (n=3) and personal communication (n=1).The RUCAM suggested a probable relationship between APAP use at recommended doses and ALF in our myopathy patients.
Conclusions
Our data suggest that some patients with myopathies receiving recommended doses of APAP may be at increased risk for the development of toxicity resulting in ALF. More studies are needed to corroborate these findings. In the meantime, we would advise physicians to be alert in these patients while taking APAP, especially when critically ill or postoperative.
Keywords: Acetaminophen, pediatrics, acute liver failure, myopathy, acetaminophen adduct, adverse event
Introduction
Acetaminophen (N-acetyl-paraaminophenol (APAP)) is a commonly used analgesic and antipyretic agent, which is generally safe at recommended therapeutic doses. Overdosing may result in acute liver failure (ALF) however, and thus constitutes a serious public health concern, notably in children (1, 2). Data from the US suggest that up to 50% of all pediatric patients experiencing ALF die or require liver transplantation(3).
Most of a single dose (>90%) of APAP is metabolized to non-toxic metabolites by glucuronidation or sulphation. Approximately 5% of a therapeutic dose is metabolized to N-acetyl-p-benzoquinone (NAPQI) by cytochrome P450 2E1, CYP2E1 (to a lesser extent by CYP1A2 or CYP3A4) (4, 5). NAPQI is rapidly detoxified by interaction with glutathione to form cysteine and mercapturic acid conjugates. When glutathione is depleted, NAPQI binds to cysteine groups on protein, forming APAP-CYS adducts. Adduct formation correlates with toxicity in experimental models of APAP toxicity and in APAP overdose patients (6, 7). Recently, quantitative assessment of APAP adduct concentrations in patient sera has been shown to be a highly sensitive and specific biomarker of suspected APAP toxicity, even in the absence of toxic APAP blood concentrations(8) (9) (10).
We describe two children with underlying myopathies who developed ALF after having received therapeutic doses of APAP. Furthermore, we report the use of adduct measurements in these patients, so as to raise clinicians’ awareness of therapeutic doses of APAP as a possible cause of ALF in children with myopathies.
Patients and methods
Patients
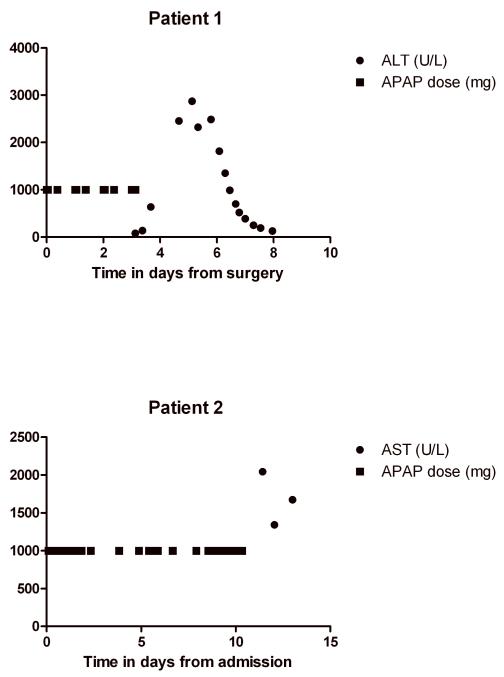
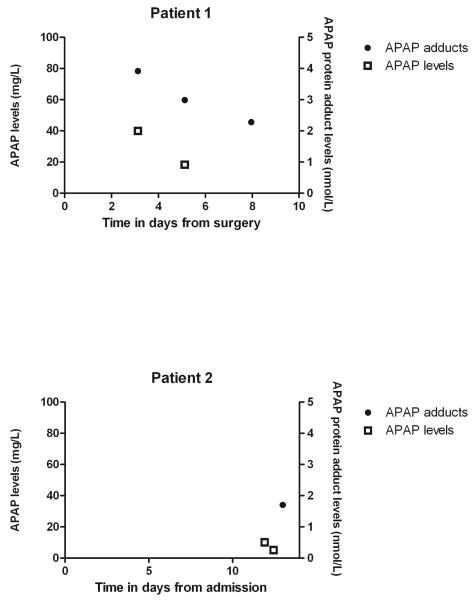
Two patients admitted to the Erasmus MC-Sophia Children’s Hospital in Rotterdam, the Netherlands developed acute liver failure, as defined by the Pediatric Acute Liver Failure Study Group (3). The Institutional Review Board of the Erasmus MC Sophia Children’s Hospital approved of this study and waived the need for informed consent. Plasma AST and APAP levels in relation to APAP dosing are shown in Figures 1 and 2, respectively. All prescribed drugs and their doses in relation to clinical events, acute liver failure and other diagnostic tests can be found in the supplementary files.
Figure 1.
AST levels and APAP doses after surgery in patient 1 and after admission in patient 2
Figure 2.
APAP serum levels and APAP protein adduct levels in days after surgery in patient 1 and after admission in patient 2.
Patient 1
A 12-year-old, 40-kilogram girl with spinal muscular atrophy (SMA) type II underwent spinal fusion surgery for the correction of scoliosis under general anesthesia with sevoflurane and remifentanil. Liver function tests at pre-operative screening were normal. Postoperative analgesics consisted of hydromorphine, diclofenac and APAP rectally (69 mg/kg/d for 4 days).
Three days postoperatively, the patient developed a hematothorax. Pre-procedure screening by anaesthesiology showed increased hepatic transaminases that were greater than 2 times above normal values. A chest drain and central venous catheter were placed under general anesthesia after a difficult intubation. Ventilatory support was continued post-procedure under propofol sedation (2.5 mg/kg/h for 17 hours) (Table 1). Propofol and APAP were discontinued after laboratory findings showed liver injury (Figure 1). The patient ultimately developed ALF with encephalopathy and required prolonged ventilatory support. N-acetylcysteine (150 mg/kg bolus IV in 15 minutes, followed by 50 mg/kg/dose IV every 4 hrs for 17 doses) was started on day 4. The ALF resolved with supportive measures; on day 6 she was extubated and on day 8 she was transferred to the referring hospital.
Table 1.
| Patient 1 | Days | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical course | Units/results | ||||||||||
| scoliosis surgery | |||||||||||
| medium care ward | |||||||||||
| hematothorax: thoraxdrain + central line placement | |||||||||||
| intubation and ventilation | |||||||||||
| fulminant liver failure | |||||||||||
| ICU stay | |||||||||||
| Diagnostics | |||||||||||
| AST | U/L | 91 | 639 | 2456 | 2485 | 521 | 130 | ||||
| ALT | U/L | 81 | 626 | 2875 | 4173 | 3369 | 1799 | ||||
| AF | U/L | 147 | 127 | 152 | ND | 206 | 207 | ||||
| gamma GT | U/L | 48 | 52 | 104 | 210 | 231 | 408 | ||||
| LDH | U/L | 237 | 810 | 2372 | 6369 | 849 | 542 | ||||
| bilirubin total | umol/L | 40 | 45 | 89 | 90 | 77 | 92 | ||||
| bilirubin unconjugated | umo/L | 29 | 33 | 73 | 60 | ND | 58 | ||||
| Ammonia | umol/L | 42 | 42 | 31 | 36 | ||||||
| PT | sec | 35,6 | 25,7 | 45,1 | 24,2 | 16,3 | |||||
| APTT | sec | 33 | 28,9 | 33 | 32 | 29 | |||||
| INR | INR | 2,9 | 2,6 | ||||||||
| ultrasound liver | Normal liver aspect, size for age normal, no biliary obstruction |
||||||||||
| hep A IgM | negative | ||||||||||
| hep B IgM | negative | ||||||||||
| ep nonAnonB | negative | ||||||||||
| respiratory viruses | negative | ||||||||||
| CMV, EBV, B19 IgM | negative | ||||||||||
| Concomitant drugs | Dose | ||||||||||
| omeprazol | 1 dd 20 mg IV | ||||||||||
| ondansetron | 3 dd 2 mg IV | ||||||||||
| morfine | 10 mg PRN | ||||||||||
| diclofenac | 3 dd mg PR | ||||||||||
| fraxiparine | 1 dd 1900 IE | ||||||||||
| amoxicillin-clavulanic acid | 4 dd 1000 mg IV | ||||||||||
| fentanyl | 40 mcg IV | ||||||||||
| propofol | 80 mg IV | ||||||||||
| propofol | 10 mg/h | ||||||||||
| sevoflurane | inhalation during surgery /procedure |
||||||||||
| remifentanyl | 100 ucg/h | ||||||||||
| ceftriaxone | 1 dd 2 g | ||||||||||
| metronidazole | 3 dd 400 mg | ||||||||||
| vit K | 5 mg IV | ||||||||||
| fentanyl | 150 mcg IV | ||||||||||
| dopamine | 8 ucg/kg/min | ||||||||||
| midazolam | 0,04 mg/kg/h | ||||||||||
| lactulose | 1 dd 15 ml | ||||||||||
| lactulose | 3 dd 20 ml | ||||||||||
| pantoprazole | 1 dd 20 mg IV | ||||||||||
| N-acetylcysteine | 6 dd 2 g IV (17 doses) | ||||||||||
| neomycin | 4 dd 1125 mg IV | ||||||||||
| furosemide | 60 mg IV (total in 24 hrs) |
Patient 2
A 17-year-old, 55-kilogram girl with congenital muscular dystrophy, carnitine deficiency and home ventilator dependency was admitted to the intensive care unit due to respiratory insufficiency and pneumonia. Liver function tests obtained at the last regular outpatient clinic visit were normal. Initially, pneumonia was treated with amoxicillin-clavulanic acid. When the clinical signs did not improve, antibiotics were switched to cefuroxime, in addition to clarithromycin. She received APAP rectal pro re nata (on average 40 mg/kg/d, max 90 mg/kg/d). On day 10, tracheotomy, under propofol, fentanyl and rocuronium, was performed and invasive ventilation was initiated. On day 11 she developed icterus, abdominal pain, nausea and vomiting. Liver tests showed ALF (Table 2 and Figure 1). Acetaminophen was discontinued immediately and N-acetylcysteine was started (150 mg/kg bolus IV in 15 minutes, followed by 50 mg/kg/dose IV every 4 hrs for 17 doses). Despite supportive therapy, she developed multiple organ failure with severe hypotension and died of refractory shock on day 14 (Table 2).
Table 2.
| Patient 2 | Days | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical course | Units/results | |||||||||||||||
| pneumonia: noninvasive ventilation | ||||||||||||||||
| metabolic acidosis | ||||||||||||||||
| hypokalemia | ||||||||||||||||
| hyperglycemia | ||||||||||||||||
| gastric retention | ||||||||||||||||
| increased CO2 en high ventilatory pressures: trachea canula and start invasive ventilation |
||||||||||||||||
| icterus | ||||||||||||||||
| shock | ||||||||||||||||
| ARDS | ||||||||||||||||
| Acute liver failure | ||||||||||||||||
| Deceased | ||||||||||||||||
| Diagnostics | ||||||||||||||||
| AST | U/L | 2043 | 1340 | 1671 | ||||||||||||
| ALT | U/L | 872 | 636 | 566 | ||||||||||||
| AF | U/L | 239 | 150 | 161 | ||||||||||||
| gamma GT | U/L | 352 | 221 | 220 | ||||||||||||
| LDH | U/L | 1858 | 3584 | |||||||||||||
| bilirubin total | umol/L | 28 | 77 | 92 | 94 | |||||||||||
| bilirubin unconjugated | umo/L | 52 | 83 | |||||||||||||
| Ammonia | umol/L | 132 | ||||||||||||||
| PT | sec | 32,4 | 37,9 | |||||||||||||
| APTT | sec | 41 | 64 | |||||||||||||
| ultrasound liver | normal size for age, no biliary obstruction, normal vascularization |
|||||||||||||||
| hep A IgM | - | |||||||||||||||
| hep B IgM | - | |||||||||||||||
| hep nonAnonB | - | |||||||||||||||
| respiratory viruses | - | |||||||||||||||
| mycoplasma serology | - | |||||||||||||||
| liver biopsy (postmortem) | diffuse micro vesiculair steatosis with bile pigment extended cellnecrosis |
|||||||||||||||
| Concomitant drugs | Dose | |||||||||||||||
| omeprazol | 1dd 40mg PO | |||||||||||||||
| lactulose | 1 dd 20 ml PO | |||||||||||||||
| lactulose | 3 dd 26 ml PO | |||||||||||||||
| broomhexine | 1 dd 16mg PO | |||||||||||||||
| levocarnitine | 1 dd 1000 mg PO | |||||||||||||||
| amoxicillin-clavulanic acid | 4 dd 1650 mg IV | |||||||||||||||
| clarithromycin | 2 dd 500 mg PO | |||||||||||||||
| furosemide | 20 - 40 mg PRN iv | |||||||||||||||
| cefuroxim | 3 dd 2 gram IV | |||||||||||||||
| actrapid | 10-100 IU/kg/h | |||||||||||||||
| alimemazine | 25 mg once daily PO | |||||||||||||||
| klysma | 120 ml RECTAL | |||||||||||||||
| nadroparine | 1 dd 2850 IE sc | |||||||||||||||
| propofol | PRN total 60 mg | |||||||||||||||
| fentanyl | PRN total 150 mcg | |||||||||||||||
| rocuronium | 10mg once IV | |||||||||||||||
| domperidone | 3 dd 20 mg PO | |||||||||||||||
| cardiotonics (dobutamine, dopamine, noradrenalin, vasopressin) |
||||||||||||||||
| hydrocortisone | 3 dd 100 mg IV | |||||||||||||||
| midazolam | 0,1-0,3 mg/kg/h | |||||||||||||||
| ketamine | 1-2 mg/kg/h | |||||||||||||||
| metoclopramide | 4 dd 10 mg IV |
Laboratory analysis
Serum samples (0.5 mL) were obtained and stored at −80C until analysis. APAP protein adducts in serum were quantified by means of a previously reported assay for determination of APAP protein adducts (APAP-CYS) in serum (8, 10).
Literature search
We performed a literature search using PubMed and Embase databases from inception - July 2010. We used the following search terms: [(acetaminophen OR paracetamol) AND (myopathy OR muscle dystrophy)]. Papers were evaluated by two authors (IC, SNW) for relevance. Literature references of identified papers were checked for additional references.
To determine ‘recommended’ dosage of APAP, we used the Dutch national formulary (www.fk.cvz.nl, accessed July 6, 2010) and the Dutch national pediatric formulary (www.kinderformularium.nl; accessed July 6, 2010). For adults, the recommended oral dose is 4 g/d (2.5 g/d for chronic use). For children, the oral and rectal recommended dose is 60-90 mg/kg/d, with a maximum of 4 g/d.
Causality assessment
The causality assessment of our cases was based on the standard liver-specific Council for International Organizations of Medical Sciences / Roussel Uclaf Causality Assessment Method scale for DILI (11). The RUCAM consensus was developed in 1993, based on international consensus meeting with hepatology and pharmacovigilance experts. The method has a high reproducibility and validity for drug-induced liver injuries (11, 12). The method was validated using reported cases with positive re-challenge. Despite the shortcomings of all existing causality assessment scales, we chose this scale as it appears the best validated scale to date (13). The RUCAM is based on seven components including time to onset and clinical course of the reactions, risk factors, concomitant drugs, screening for other causes, previous information of hepatotoxicity of the drugs and response to re-administration, toxic concentration or validated laboratory test. To determine if concomitantly prescribed drugs were potential hepatotoxins, we searched the Dutch National Formulary for serious hepatotoxicity listed as reported adverse event. Theoretically the scale has a range from −9 to +15, but in reality only scores between −5 and +13 are found. The classification of the degrees of DILI diagnosis was as follows: score <0: ‘relationship excluded’; 1-2: ‘unlikely’; 3-5: ‘possible’; 6-8: ‘probable’; and above 8: ‘highly probable’.
Results
APAP protein adduct levels
The serum APAP protein adduct levels for our two cases were consistent with the values previously reported in children with acute liver injury following APAP overdose (Figure 2)(9). Previous receiver operator curve analysis determined that APAP protein adduct levels in serum of > 1.1 nmol/mL had a sensitivity of 96.8% and a specificity of 95% for patients with ALT values > 1000 IU/L(10).
Literature search & personal communication
Of the 98 papers retrieved using the predefined search terms, only one was of interest. This report described two adult patients with muscular dystrophies who developed acute liver failure after receiving APAP (3g and 4 g, daily respectively) for treatment of pulmonary infections associated with end stage neuromuscular respiratory failure(14). A second report was identified, from reference list search, in which a 12-year-old patient with Duchenne’s muscular dystrophy developed hepatotoxicity in association with doses of APAP ranging from 70 to 108 mg/kg/day that were administered after posterior spinal fusion surgery (15). These three patients had full recovery after APAP was discontinued and supportive care and N-acetylcysteine treatment were initiated.
In addition to the three previously reported cases of APAP toxicity in patients with existing muscular disorders described in the literature, we identified another child with a myopathy who developed ALF requiring liver transplantation after the use recommended doses of APAP (personal communication, Dr S. Ito). This 12-year, 60-kilogram boy, with SMA type II underwent spinal fusion surgery for scoliosis repair and received on average APAP 23 mg/kg/day for 5 days. The highest measured APAP level was 248 mg/l for this patient but APAP protein adduct analysis was not performed.
Causality assessment
Using the RUCAM scale, we found a probable relationship between APAP and acute liver failure for both patients (RUCAM +6 and +8 for patient 1 and 2, respectively). Based on the criteria of the RUCAM, both patients developed acute liver injury (ALT >2N = ALT 872 U/l and 4173 U/L, respectively). The time to onset was compatible for patient 1 (<5 days from onset of drug and <15 days after cessation) and highly suggestive (5-90 days from onset of drug and <15 days after cessation) for patient 2. For patient 1, the course of reaction was highly suggestive as ALT levels decreased >50% within 8 days. The course of the reaction was inconclusive for patient 2, as the patient died within two days of liver failure and ALT levels did not decrease <50% in that time window. There were no known risk factors (age>55yr, ethanol use or pregnancy) for either patient. Other drugs with compatible time of onset and known hepatotoxins were sevoflurane for patient 1 and clarithromycin and amoxicillin-clavulanic acid for patient 2. We ruled out hepatitis, biliary obstruction, alcoholism, acute hypotension episode as non-drug causes. Abdominal ultrasound excluded biliary obstruction. Inspection of surgery and ICU charts did not reveal hypovolemic episodes. We could not rule out ALF as complication of myopathy for either patient. The specific drug reaction in this context (therapeutic dose in myopathy patients) has been published in this context, but is unlabelled. A validated test (APAP protein adducts) was positive for both patients. When we also applied RUCAM for the other potential hepatotoxic drugs, we found a possible relationship (RUCAM + 4) between each drug and ALF.
Discussion
Overall, the available data involving six patients (two clinical cases, three in the literature and one personal communication) with underlying myopathies and the development of ALF following the administration of manufacturer recommended doses of APAP suggest that some of these individuals may have increased susceptibility to APAP. This in an interesting finding in the light of a recent meeting held by the US Food and Drug Administration to address the public health problem of liver injury related to the use of acetaminophen in both over-the-counter (OTC) and prescription (Rx) products. The risk to the individual patient of developing liver injury after use of APAP at doses recommended by the manufacturer is very low. However, the agency recognizes that acetaminophen containing products are used extensively making the absolute number of liver injury cases a public health concern (16).The cases reported herein may represent a specific patient population at risk for acute liver failure with the use of doses of APAP recommended by the manufacturer. Our findings suggest that inter-individual variations in the metabolism of APAP may predispose certain subpopulations of patients to a higher risk for developing this serious adverse event(1).
Although APAP levels in our two patients were below the reported toxic range (Figure 2), the probability that ALF was APAP-induced is supported by the high levels of APAP protein adduct levels in both patients. Earlier studies reported a range of levels of APAP protein adducts in serum between 1 to 40 nmol/mL in patients with acute APAP overdose (9, 10). In addition, strong correlations were noted between peak adduct measurements and peak AST and ALT values (9, 10), While adducts have been detected in healthy adult volunteers receiving a seven day course of APAP at 4 grams/day, the mean Cmax for adducts in serum was 0.3 nmol/mL (17), Importantly, no adducts were observed in control patients who received placebo. Thus, the high levels of adducts in the two patients reported herein suggest a causal relationship between APAP consumption and the development of ALF. To the best of our knowledge, this is the first time that APAP protein adduct measurements were used in clinical care to assess the role of recommended APAP doses in the development of ALF.
Although we found a probable association between APAP exposure and acute liver failure in our two patients, we cannot exclude that other drugs also contributed to the liver failure. Patient 1 received sevoflurane twice, first during scoliosis surgery, next during chest tube and central venous line placement. Although sevoflurane is generally considered safe in comparison with other halogenated anesthetics, cases in the literature suggest that sevoflurane can lead to severe life-threatening hepatic necrosis in at-risk individuals (18). For patient 2, we identified amoxicillin-clavulanic acid and clarithromycin as possible other serious hepatotoxins.
For both these drugs serious hepatic failure has been reported rarely, most often in patients with serious underlying disease or in combination with other drugs. In addition, both patients received propofol, which has been associated with propofol transfusion syndrome. The limited doses and durations of treatment with propofol (2.5 mg/kg/h for 17 hours and 6 mg/kg/h for 2 hours, respectively) were less than that previously associated with the development of propofol infusion syndrome (19). In addition, the most striking symptoms of propofol transfusion syndrome, i.e. cardiac failure combined with lipemic plasma, fatty liver enlargement, metabolic acidosis with negative base excess >10 mmol/l, rhabdomyolysis or myoglobinuria were not present in our patients (19).
Despite the fact that we cannot exclude a possible role of other drugs in the development of acute liver failure, the causality between APAP and ALF appears more probable in our patients, as supported by the high APAP adduct levels.
The underlying mechanism in the development of APAP toxicity in patients with myopathies is unknown. Glutathione depletion and increased CYP 2E1 activity in relation to relative malnutrition may contribute to increased APAP toxicity (20, 21). Although both our patients had age-adequate weights, an undernourished status may have been present (22, 23). Second, as critically ill patients often receive multiple drugs concurrently, drug interactions at the level of APAP metabolism, e.g. induction of CYP2E1 or inhibition of alternative pathways, may contribute to increased APAP toxicity in this setting(24-26). Patient 2 also received clarithromycin, which is a cytochrome P450 3A substrate and inhibitor (27).Theoretically, it may change metabolic disposition of acetaminophen by blocking its CYP3A metabolic pathway, resulting in higher compensatory CYP2E1 metabolism, which in turn may increase the risk of APAP induced liver injury. However, as the main metabolic pathways of acetaminophen are sulphation and glucuronidation, we do not expect a significant effect of CYP3A inhibition on the formation of APAP adducts (4, 5). In addition, we could not find reports of clarithromycin and acetaminophen interaction in the literature. To our knowledge our patients did not receive any drugs, in addition to clarithromycin, that are known to interact with the metabolism of APAP. Third, inflammation has been shown to play a role in the mediation of APAP toxicity in experimental models but its relevance to the underlying muscular disorders in these children is unclear (28).
In addition, the adduction of mitochondrial proteins appears to trigger mitochondrial dysfunction,(29) and may contribute to the development of liver cell death after APAP exposure (30). The 12 year old patient referenced above (personal communication, Dr Ito) showed clinical signs and symptoms of severe mitochondrial derangement, including ALF. This patient ultimately required emergency liver transplantation. Also, animal studies suggest a protective role of L-carnitine in APAP-induced liver failure, which may hint to the underlying reason why the patient with carnitine deficiency developed ALF (31). In addition, a recent report found that patients with myopathies have evidence of increased oxidative stress in cells isolated from peripheral blood (32). Oxidative stress is a known mechanism in the pathogenesis of APAP toxicity in laboratory mice (33). Thus, further study is needed to understand the relative role and contribution of mitochondrial derangement and anti-oxidant status in patients with myopathies as these mechanisms may have relevance to understanding the potential for increased sensitivity to APAP.
Conclusion
Our data suggest that some children with myopathies receiving recommended doses of APAP may be at increased risk for the development of toxicity resulting in ALF. . Despite a possible relationship between therapeutic APAP use and ALF in myopathic patients, we do not recommend dose adjustment of APAP at this time. Further research is needed to validate our findings and to reveal the underlying mechanisms, underlying the interactions between myopathy and potential sensitivity to APAP toxicity’.
In the meantime, we would advise physicians to be alert in these patients while taking APAP, especially when critically ill or postoperative
Acknowledgments
Financial disclosure: Dr. James has grant support from the National Institutes of Health (79387 and 81406) for the study of acetaminophen toxicity and acetaminophen protein adducts in human samples and has a pending patent with the National Institutes of Diabetes and Digestive Diseases. All other authors have no potential conflicts of interest to disclose.
Footnotes
No reprints will be ordered
References
- 1.Kuehn BM. FDA focuses on drugs and liver damage: labeling and other changes for acetaminophen. Jama. 2009;302(4):369–371. doi: 10.1001/jama.2009.1019. [DOI] [PubMed] [Google Scholar]
- 2.Watkins PB, Kaplowitz N, Slattery JT, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial. Jama. 2006;296(1):87–93. doi: 10.1001/jama.296.1.87. [DOI] [PubMed] [Google Scholar]
- 3.Squires RH, Jr., Shneider BL, Bucuvalas J, et al. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006;148(5):652–658. doi: 10.1016/j.jpeds.2005.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patten CJ, Thomas PE, Guy RL, et al. Cytochrome P450 enzymes involved in acetaminophen activation by rat and human liver microsomes and their kinetics. Chem Res Toxicol. 1993;6(4):511–518. doi: 10.1021/tx00034a019. [DOI] [PubMed] [Google Scholar]
- 5.Dahlin DC, Miwa GT, Lu AY, et al. N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci U S A. 1984;81(5):1327–1331. doi: 10.1073/pnas.81.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 2003;31(12):1499–1506. doi: 10.1124/dmd.31.12.1499. [DOI] [PubMed] [Google Scholar]
- 7.Hinson JA, Pohl LR, Monks TJ, et al. Acetaminophen-induced hepatotoxicity. Life Sci. 1981;29(2):107–116. doi: 10.1016/0024-3205(81)90278-2. [DOI] [PubMed] [Google Scholar]
- 8.Muldrew KL, James LP, Coop L, et al. Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high-performance liquid chromatography with electrochemical detection. Drug Metab Dispos. 2002;30(4):446–451. doi: 10.1124/dmd.30.4.446. [DOI] [PubMed] [Google Scholar]
- 9.James LP, Capparelli EV, Simpson PM, et al. Acetaminophen-associated hepatic injury: evaluation of acetaminophen protein adducts in children and adolescents with acetaminophen overdose. Clin Pharmacol Ther. 2008;84(6):684–690. doi: 10.1038/clpt.2008.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James LP, Letzig L, Simpson PM, et al. Pharmacokinetics of acetaminophen-protein adducts in adults with acetaminophen overdose and acute liver failure. Drug Metab Dispos. 2009;37(8):1779–1784. doi: 10.1124/dmd.108.026195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danan G, Benichou C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46(11):1323–1330. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 12.Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs--II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol. 1993;46(11):1331–1336. doi: 10.1016/0895-4356(93)90102-7. [DOI] [PubMed] [Google Scholar]
- 13.Liss G, Lewis JH. Drug-induced liver injury: what was new in 2008? Expert Opin Drug Metab Toxicol. 2009;5(8):843–860. doi: 10.1517/17425250903018904. [DOI] [PubMed] [Google Scholar]
- 14.Pearce B, Grant IS. Acute liver failure following therapeutic paracetamol administration in patients with muscular dystrophies. Anaesthesia. 2008;63(1):89–91. doi: 10.1111/j.1365-2044.2007.05340.x. [DOI] [PubMed] [Google Scholar]
- 15.Hynson JL, South M. Childhood hepatotoxicity with paracetamol doses less than 150 mg/kg per day. Med J Aust. 1999;171(9):497. doi: 10.5694/j.1326-5377.1999.tb123758.x. [DOI] [PubMed] [Google Scholar]
- 16.FDA Joint Meeting of the Drug Safety and Risk Management Advisory Committee with the Anesthetic and Life Support Drugs Advisory Committee and the Nonprescription Drugs Advisory Committee; 2008. cited Available from:http://www.fda.gov/advisorycommittees/calendar/ucm143083.htm. [Google Scholar]
- 17.James LP, Simpson P, Russo M, et al. Detection of acetaminophen protein adducts in serum during therapeutic exposure to acetaminophen in healthy volunteers; The Liver Meeting 2007; 2007. [Google Scholar]
- 18.Singhal S, Gray T, Guzman G, et al. Sevoflurane hepatotoxicity: a case report of sevoflurane hepatic necrosis and review of the literature. Am J Ther. 17(2):219–222. doi: 10.1097/MJT.0b013e318197eacb. [DOI] [PubMed] [Google Scholar]
- 19.Fudickar A, Bein B. Propofol infusion syndrome: update of clinical manifestation and pathophysiology. Minerva Anestesiol. 2009;75(5):339–344. [PubMed] [Google Scholar]
- 20.Hwang J. Diets with corn oil and/or low protein increase acute acetaminophen hepatotoxicity compared to diets with beef tallow in a rat model. Nutr Res Pract. 2009;3(2):95–101. doi: 10.4162/nrp.2009.3.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoo JS, Park HS, Ning SM, et al. Effects of thiamine deficiency on hepatic cytochromes P450 and drug-metabolizing enzyme activities. Biochem Pharmacol. 1990;39(3):519–525. doi: 10.1016/0006-2952(90)90059-t. [DOI] [PubMed] [Google Scholar]
- 22.Zemel BS, Riley EM, Stallings VA. Evaluation of methodology for nutritional assessment in children: anthropometry, body composition, and energy expenditure. Annu Rev Nutr. 1997;17:211–235. doi: 10.1146/annurev.nutr.17.1.211. [DOI] [PubMed] [Google Scholar]
- 23.Khoshoo V. Nutritional assessment in children and adolescents. Curr Opin Pediatr. 1997;9(5):502–507. doi: 10.1097/00008480-199710000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Kaneko T, Wang Y, et al. Troglitazone enhances the hepatotoxicity of acetaminophen by inducing CYP3A in rats. Toxicology. 2002;176(1-2):91–100. doi: 10.1016/s0300-483x(02)00143-9. [DOI] [PubMed] [Google Scholar]
- 25.Kostrubsky SE, Sinclair JF, Strom SC, et al. Phenobarbital and phenytoin increased acetaminophen hepatotoxicity due to inhibition of UDP-glucuronosyltransferases in cultured human hepatocytes. Toxicol Sci. 2005;87(1):146–155. doi: 10.1093/toxsci/kfi211. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki A, Yuen N, Walsh J, et al. Co-medications that modulate liver injury and repair influence clinical outcome of acetaminophen-associated liver injury. Clin Gastroenterol Hepatol. 2009;7(8):882–888. doi: 10.1016/j.cgh.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 27.Tinel M, Descatoire V, Larrey D, et al. Effects of clarithromycin on cytochrome P-450. Comparison with other macrolides. J Pharmacol Exp Ther. 1989;250(2):746–751. [PubMed] [Google Scholar]
- 28.Jaeschke H. Role of inflammation in the mechanism of acetaminophen-induced hepatotoxicity. Expert Opin Drug Metab Toxicol. 2005;1(3):389–397. doi: 10.1517/17425255.1.3.389. [DOI] [PubMed] [Google Scholar]
- 29.Neustadt J, Pieczenik SR. Medication-induced mitochondrial damage and disease. Mol Nutr Food Res. 2008;52(7):780–788. doi: 10.1002/mnfr.200700075. [DOI] [PubMed] [Google Scholar]
- 30.Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci. 2006;89(1):31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- 31.Yapar K, Kart A, Karapehlivan M, et al. Hepatoprotective effect of L-carnitine against acute acetaminophen toxicity in mice. Exp Toxicol Pathol. 2007;59(2):121–128. doi: 10.1016/j.etp.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Mancuso M, Orsucci D, Logerfo A, et al. Oxidative stress biomarkers in mitochondrial myopathies, basally and after cysteine donor supplementation. J Neurol. 257(5):774–781. doi: 10.1007/s00415-009-5409-7. [DOI] [PubMed] [Google Scholar]
- 33.Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol. (196):369–405. doi: 10.1007/978-3-642-00663-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]