Abstract
Background
Helicobacter pylori causes gastritis, peptic ulcer and is a risk factor for adenocarcinoma and lymphoma of the stomach. Gastric mucins, carrying highly diverse carbohydrate structures, present functional binding sites for H. pylori and may play a role in pathogenesis. However, little information is available regarding gastric mucin in children with and without stomach diseases.
Material & Methods
Expression of mucins and glycosylation was studied by immunohistochemistry on gastric biopsies from 51 children with and without H. pylori infection and/or peptic ulcer disease.
Results
In all children, MUC5AC was present in the surface epithelium and MUC6 in the glands. No MUC6 in the surface epithelium or MUC2 was detected in any section. The Leb and Lea blood-group antigens were present in the surface epithelium of 80% and 29% of children respectively. H. pylori load was higher in Leb negative children than in Leb positive individuals (means ± SEM 17.8±3.5 vs 10.8±1.5; p < 0.05), but there was no correlation between Lea or Leb status and gastritis, nodularity, and gastric or duodenal ulcer. Expression of sialyl-Lex was associated with H. pylori infection, and DU.
Conclusions
Mucin expression and glycosylation is similar in children and adults. However, in contrast to adults, pediatric H. pylori infection is not accompanied by aberrant expression of MUC6 or MUC2. Furthermore, the lower H. pylori density in Leb positive children indicates that H. pylori is suppressed in the presence of gastric mucins decorated with Leb, the binding site of the H. pylori BabA adhesin.
Keywords: Gastric Mucosa, Mucins, Helicobacter pylori, Carbohydrate Antigens, Pediatric, gastritis, peptic ulcer
Introduction
H. pylori is the main causative agent of gastric and duodenal ulcers in adults and children (1, 2). However, only a fraction of H. pylori infected subjects develop ulcers, an observation attributed to concurrent differences in bacterial virulence as well as host and environmental factors (3). One virulence factor potentially involved in the pathogenesis of peptic ulcer disease (PUD) is the blood antigen binding adhesin BabA (4–7). The majority of H. pylori strains investigated carry the babA gene, (4, 6), and since the BabA adhesin adheres to gastric blood group antigens expressed on epithelial cells and mucus (8, 9), the expression of specific host receptors to bacterial adhesins are likely to play a role in the outcome of the infection (10).
Gastric mucosal surfaces are covered with a mucus layer primarily composed of secreted mucins. This mucus layer is continuously secreted and transports away trapped material. The mucus polymer is formed by oligomeric mucin glycoproteins that provide a matrix for a rich array of anti-microbial molecules. These mucins inhibit bacterial access to the mucosa by binding microbes (9) and, in the case of MUC6 oligosaccharides, by providing a direct antimicrobial activity (11). In adults, gastric MUC5AC and MUC6 are the major secreted mucins and are produced by the surface epithelium and by the glands, respectively (12). In the fetal stomach, in addition, MUC2 and MUC5B are also expressed (13) and mucin gene expression can be “fetal-like” in adults with gastric precancerous lesions and cancer (14, 15). Other disease-related changes in mucin expression are the presence of MUC2 and MUC6 in intestinal metaplasia and in the surface epithelium of H. pylori infected adults, respectively (16).
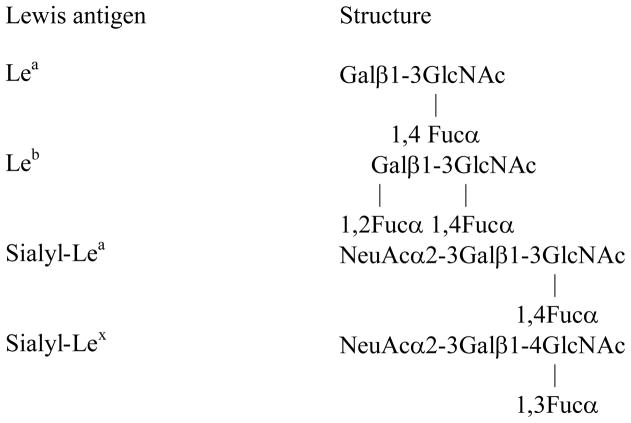
Each mucin carry a vast array of oligosaccharide structures, and the glycosyltransferases expressed by the individual determines the type of carbohydrate structures present on the secreted mucins. The H1 structure is made by the secretor gene and the majority of people carry this structure and are thus referred to as ‘secretors’ (17). Individuals may also express the Lewis gene (90% of the Caucasian population) and express the Leb histo-blood group antigen if they are secretors and Lea if they are non-secretors (Table 1)(17, 18). A third secretor phenotype, the weak-secretor phenotype Sew, is characterized by expression of both Lea and Leb antigens (19). In the healthy adult stomach, the surface epithelium expresses Lea and Leb structures and the Leb structure is expressed on the MUC5AC mucin (9, 12, 20).
Table 1.
Structure of Lea, Leb, sialyl-Lea and sialyl-Lex
|
H. pylori is mainly found within the mucus layer and it is also attached to, or within, gastric epithelial cells.(21, 22) Mucins have a high and specific binding capacity for H. pylori (9, 23, 24). As a result, mucins function as decoys for bacterial binding (23) and they prevent most H. pylori from approaching epithelial cell surface. In the human-like rhesus monkey model (25), animals secreting mucins with higher H. pylori binding capacity developed lower H. pylori density infection and less gastritis (10, 25). Similarly, humans with primary Sjögren’s syndrome produce smaller quantities of mucins and have more H. pylori-associated pathology (26), further suggesting that the ability of secreted mucins to bind H. pylori protects the gastric epithelium. Three different modes of adhesion have been implicated in H. pylori binding to gastric mucins: the Leb and sialyl-Lex/sialyl-Lea binding adhesins and a charge/low pH mode of adhesin (9, 23, 24, 27, 28). In the healthy stomach, H. pylori binding to the gastric MUC5AC via the Leb mucin structure is the dominating mode of adhesion (9, 23, 24, 27, 28). Mucin glycosylation is predominantly neutral in the healthy stomach, whereas both sulfated and sialylated structures appear in infection, inflammation and cancer (20, 28). These disease-related changes in mucin expression and glycosylation modify the H. pylori adhesion targets,(10) and enhance H. pylori binding ability of secreted mucins (24).
Gastric colonization with H. pylori usually begins in childhood (29), although, in contrast to adults, gastro-duodenal ulcers and intestinal metaplasia are rare in children (30). Furthermore, children with H. pylori infection predominantly have pangastritis instead of the diffuse antral or multifocal atrophic gastritis described in adults (31). In addition, the numbers of inflammatory cells present in H. pylori-infected biopsy specimens is higher in children than adults (30) although IFN-γ secretion in the stomach of H. pylori-infected patients is lower in children than in adults (32). Finally, H. pylori contact with microvilli is higher in children compared to adults whereas the more intimate adhesion with abutting or adhesive pedestals dominate in adults (33).
Given these differences and the fact that the effect of H. pylori infection on mucin expression and glycosylation was extensively studied in adults (9, 15, 34–44) but not in children, it is important to investigate mucin expression and glycosylation in pediatric H. pylori infection. Here, we determined the expression and morphological location of the MUC5AC, MUC6, MUC2, MUC5B mucins and the Lea, Leb, sialyl-Lea, and sialyl-Lex carbohydrate structures (Table 1) in children and determined whether these parameters were different in the presence of gastritis, peptic ulcer disease and/or H. pylori infection.
Materials and Methods
Materials
Monoclonal antibody against the MUC5AC mucin (45M1) and Iodoacetamide were obtained from Sigma, BSA from Serva and 1,4-dithiothreitol (DTT) from Merck. Polyclonal antibodies against MUC6 (LUM6-3),(45) MUC5B (LUM5B-2 (46)) and MUC2 (LUM2-3 (47)) were kind gifts from Professor Ingemar Carlstedt, Lund University, Lund, Sweden. Monoclonal anti-Leb antibody (clone 2–25 LE) and anti Lea (7LE) were kind gifts from Dr. J. Bara, INSERM, France. Antibodies against Lea (BG-5), Leb (BG-6), were from Signet pathology systems, antibodies against sialyl-Lex (clone KM93) and sialyl-Lea (clone 1H4) from Seikagaku America, Biotinylated goat anti mouse and goat anti rabbit antisera, Strept AB complex/HRP from Vector and biotin blocking system from Dako. Thiopropyl Sepharose 6B was from Amersham Bioscience. DAB (3,3-diaminobenzidine tetrahydrochloride) was from (Sigma).
Study Population
Specimens used herein were harvested in a study approved by the Institutional Review Boards of Walter Reed Army Medical Center and the Uniformed Services University of the Health Sciences. The study included 51 children with either normal endoscopy, nodularity, gastric ulcer or duodenal ulcer as recently reported (48). Mean age was 12.2 years (range: 3 to 18; Since the youngest patients are 3 years old, mucin intake via mothers milk is not likely to confound the results from this study), and the ethnic distribution was 53% Caucasians, 27% African-Americans, 14% Hispanics, and 6% Asians. The indications for endoscopy included abdominal pain (n=38, 74%), nausea and vomiting (n=33, 65%), upper gastrointestinal bleeding (n=8, 16%), weight loss (n=4, 8%) and heartburn (n=8, 16%). The age, gender, and H. pylori positivity are listed in table 2. The ethnicity and subjective symptoms of the patients were not significantly different among the endoscopic groups, as reported (48).
Table 2.
Demographics and H. pylori prevalence in all patients.
| Normal (n=14) | Nodularity (n=18) | GU (n=8) | DU (n=11) | |
|---|---|---|---|---|
| Mean Age [years (range)] | 13.1 (10–17) | 12 (4–17) | 10.4 (3–17) | 12.7 (8.5–17) |
| Males [N (%)] | 7 (50) | 10 (53) | 5 (63) | 9 (82) |
| H. pylori positive [N (%)] | 7 (50) | 18 (100) | 6(75) | 10(91) |
Endoscopy
After obtaining informed consent, all patients underwent upper gastrointestinal endoscopy with the XP-20, P140, or GIF-100 endoscope (Olympus). The endoscopy reports were graded as follows: normal (n = 14), nodularity (n = 18), gastric ulceration (GU, n = 8) and duodenal ulceration (DU, n = 11).
Histology
Serial paraffin sections from archived biopsies (4 μm) were stained with either H&E, according to Genta (49), or for in situ hybridization (48) and imunohistochemistry (see below). Chronic and acute inflammation, intestinal metaplasia, atrophy, and H. pylori density were scored according to the Updated Sydney System (50). Inflammation and atrophy scores represent the degree of either acute or chronic inflammation (0=normal, 1=mild, 2=moderate, and 3=severe). A pediatric pathologist blinded to the results of the endoscopy, Genta stain, in situ hybridization and immunohistochemistry performed the histological assessment (48).
H. pylori quantification
Fluorescence in situ hybridization (FISH) was used to determine concurrently the expression of H. pylori 16S rRNA and cagA using specific probes as described (48). Briefly, the 16S rRNA and cagA probes were labeled with biotin and digoxigenin, respectively, and binding was detected with Avidin-Texas red and anti-digoxigenin-FITC, respectively. Specific controls of method were also performed to verify specificity of the technique. Specimens were considered H. pylori positive when Genta-stained organisms of typical curved-shape morphology were present and if there was a positive FISH reaction for H. pylori 16S rRNA. An Eclipse E-800 Nikon microscope was used to examine the sections. Morphometric quantification of H. pylori by Genta and expression of 16S rRNA and cagA was performed using a previously published point-counting stereological technique (22). This method utilizes an intraocular reticule (No. Kr409, Klarman Rulings, Inc. Litchfield, NH) that has a 27 mm diameter grid covering 3,577 μm2, thus 14,314 μm3 for 4 μm-thick sections. Bacterial clusters that were Genta-stained (and displayed the typical curved shape) or expressed H. pylori 16S rRNA were quantified at 400X.
Immunohistochemistry
MUC2, MUC5AC, MUC5B, MUC6, Lea, Leb, sialyl-Lea and sialyl-Lex immunohistochemistry was performed on formalin fixed, paraffin-embedded tissue sections according to standard procedures as previously described (25). The investigator scoring sections stained via immunohistochemistry (SL) was unaware of the clinical diagnosis or H. pylori infection density when performing the analysis. Human adult gastric and intestinal biopsies were used as positive controls.
Purification of anti-MUC2 antibodies by affinity chromatography of LUM2-3 antiserum
The synthetic peptide (NGLQPVRVEDPDGC) used for production of the LUM2-3 antiserum (47) was covalently conjugated to Thiopropyl Sepharose 6B (2 mg peptide/g dry Thiopropyl Sepharose 6B) according to the protocol supplied by the manufacturer. The LUM2-3 antiserum was diluted 1:10 in 10 mM Tris buffer, pH 7.5 and applied to the column at a flow rate of 0.05 ml/min. The column was washed with 10 mM Tris buffer, pH 7.5 and 0.5 M NaCl in 10 mM Tris buffer, pH 7.5 before bound antibodies were eluted with 0.1 M Triethylamine pH 11.5.
Statistics
Data are reported as frequency (percent) or means ± standard error. Student’s t test for independent samples was used to compare means between groups, and the chi square test was used to compare proportions. A p <0.05 was considered statistically significant.
Results
H. pylori infection
H. pylori were observed either free in the lumen or adherent to the foveolar and epithelial surface (Figure 1). As previously reported (48), Genta staining and FISH demonstrated their presence in half of the children with normal endoscopy, and in all or almost all patients with nodularity, DU or GU. In addition, FISH showed that H. pylori and cagA expression was significantly higher in ulcer patients compared to children with normal endoscopy (48).
Figure 1. H. pylori in antral gastric gland.
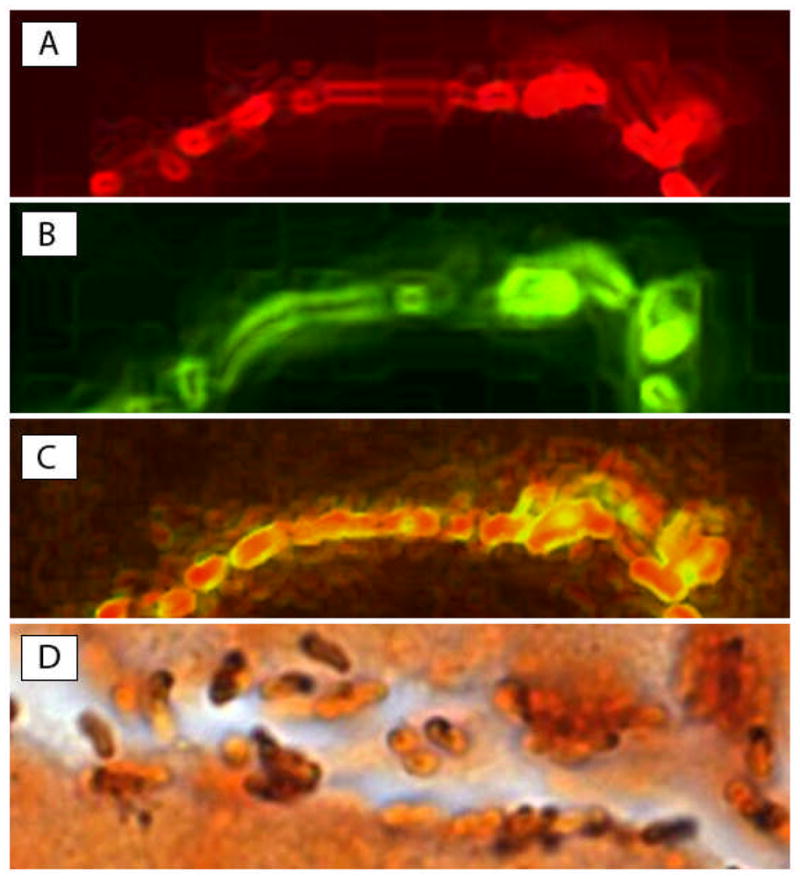
H. pylori colonization in superficial gland of a child with duodenal ulcer in two 5-μm-thick serial sections (Original magnification X1000). (A) 16S rRNA in red (FISH-Texas red); (B) cagA in green (FISH-FITC); (C) merge of A and B, demonstrating colocalization of 16S rRNA and cagA (in yellow) in the same bacteria. Note that only cagA (green) is expressed in the outer part of bacteria while both 16S rRNA and cagA are co-localized in the center (yellow); (D) Genta stain of a gastric gland, showing many curved H. pylori-shaped bacteria colonizing the lumen mucus or adherent to the apical part of mucus-secreting cells. Note that some of the bacteria appear intensely stained black by silver, while others are weakly stained (in part or completely). These variations may represent different capture of silver stain and may be due to the presence of mucus on part of the bacterial membrane.
Mucin expression and tissue localization
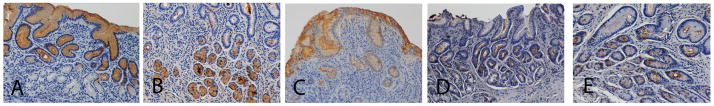
MUC5AC was detected in the surface/foveolar epithelial cells in all sections. The cytoplasm of the epithelial cells was mainly comprised of MUC5AC positive mucus granules, and in sections where the mucus layer had been retained, the mucus layer was also positive for MUC5AC (Figure 2A). MUC6 was located in the antral glands and in the larger oval neck cells of the corpus (Figure 2B). Secreted MUC6 was detected in the lumen of the foveolae, but no MUC6 was detected in the surface/foveolar epithelium in any of the biopsies.
Figure 2. Tissue localization of mucins and carbohydrate antigens.
Section from a H. pylori negative patient was stained brown using immunohistochemistry for MUC5AC (A). Sections from H. pylori positive patients were stained brown for MUC6 (B), Leb (C), Sialyl-Lex (D) and Sialyl-Lea (E).
Sialomucins were mainly detected in the neck region. Neither MUC2 nor MUC5B were detected in any of the biopsies. There were fewer mucin producing cells in the epithelia with intense inflammation and thicker lamina propria. As a result, the tall normal epithelial cells loaded with mucus were replaced by short or flat cells containing little mucus in the mucin thecae. However, no difference in tissue localization of mucins was detected among children with and without gastritis, PUD and/or H. pylori infection.
Lewis antigens
In the surface/foveolar epithelium, Leb (Figure 2C) and Lea were detected in 80% and 29% of children (Table 3), respectively, but not in the glands of any of the children. In biopsies where Leb and/or Lea was present, the mucous cells of the entire surface and foveolar epithelium tended to be positive, although there were slight variations in stain intensity between individuals. Leb and Lea thus have tissue localizations similar to the MUC5AC mucin (Compare Figure 2A with 2C). The Leb+/Lea- phenotype was inversely associated with presence of sialomucin (13/29 [45%] children with this phenotype had sialomucin present, compared with 15/20 [75%] of children with other phenotypes, p =0.038).
Table 3.
Individuals with clinical diagnosis of the different Lewis phenotypes.
| Lewis phenotype | All individuals n=49* | Controls n=6 | H. pylori infection n=39 | Gastritis n=39 | Nodularity N=18 | Gastric Ulcer n=8 | Duodenal ulcer n=10 |
|---|---|---|---|---|---|---|---|
| Leb+ | 39 (80%) | 5 (83%) | 31 (79%) | 31 (79%) | 13 (72%) | 6 (75%) | 9 (90%) |
| Lea+ | 14 (31%) | 2 (33%) | 11 (29%) | 11 (29%) | 3 (17%) | 3 (38%) | 4 (40%) |
| Leb+,Lea+ | 11 (22%) | 2 (33%) | 9 (23%) | 9 (23%) | 2 (11%) | 1 (13%) | 4 (40%) |
| Leb+,Lea- | 28 (59%) | 3 (50%) | 21 (54%) | 20 (51%) | 11 (61%) | 5 (63%) | 5 (50%) |
| Lea+, Leb- | 3 (6%) | 0 | 3 (8%) | 3 (8%) | 1 (6%) | 2 (25%) | 0 |
| Lea-, Leb- | 7 (14%) | 1 (17%) | 6 (15%) | 7 (18%) | 4 (22%) | 0 | 1 (10%) |
Insufficient material was available to analyze Lewis type in 2 of the 51 patients. Some patients had several clinical diagnoses, which is why the numbers of gastritis, nodularity, GU and DU add up to more than the number of patients in the study.
Sialylated Lewis antigens
Sialyl-Lea was present in mucus producing cells, secreted mucin and lamina propria in 60% of the children and sialyl-Lex was positive in 29% of the children (Figure 2D and E). In the 14 sialyl-Lex positive individuals, secreted mucins and/or mucin producing cells were sialyl-Lex positive in 11, whereas only the lamina propria was positive in the other three. Only a minority of the cells stained for sialyl-Lex, and the staining occurred most often in the antral foveolar epithelium (Figure 2D). Expression of sialyl-Lex was associated with the Lea+ phenotype (9/13 (69%) vs. 7/34 (21%), p = 0.002).
Lewis expression, level of infection, and endoscopic diagnosis
H. pylori density was higher in Leb-negative children than in Leb-positive individuals (means ± SEM: 17.8±3.5, n=10 versus 10.8±1.5, n=39; p =0.045). In addition, presence of sialyl-Lex was associated with H. pylori infection and DU; 42% (16/38) of H. pylori infected patients were sialyl-Lex positive and 67% (6/9, p= 0.023) of DU patients were sialyl-Lex positive compared to 0% (0/9 p = 0.018) without H. pylori infection and DU. However, no significant relation was found between Leb status and endoscopical diagnosis (normal, nodularity, GU or DU).
Discussion
Here, we observed that the histological location of the MUC5AC and MUC6 mucins as well as Lewis fucosylation and sialylation are similar in normal pediatric and adult gastric tissue but that, in contrast to adults (16), pediatric H. pylori infection was not accompanied by aberrant expression of MUC6 or MUC2. In another study, MUC2 was detected in a few foveolar and surface cells in H. pylori–infected children, but the observation was made in only 2/18 children.(51). No aberrant expression of MUC6 in the surface or foveolar epithelium was found in the present study, although MUC6 has been shown to be aberrantly expressed in the surface epithelium in 72% of H. pylori-infected adults (16). These differences may be due to the fact that abnormal expression of MUC2 and MUC6 require a longer H. pylori exposure than that experienced by our group of children. This hypothesis is supported by the observation that Rhesus monkeys followed for 10 months after H. pylori inoculation did not show changes in MUC5AC, MUC6 or MUC2 tissue localization (10).
In children, as in adults, the cytotoxin-associated A (cagA) gene is the most frequently implicated virulence factor associated with increased risk of PUD (1). We recently demonstrated that the fraction of H. pylori bacteria expressing cagA in situ was higher in children with peptic ulcer (48). However, no association between host Lewis antigen expression and H. pylori cagA expression was detected in this study.
In the present group of children, prevalence of Lea and Leb histo-blood group antigens expression in the foveolar epithelium was similar to that found in adults (52). No significant correlation was found between Lea or Leb status and endoscopic findings of gastritis, nodularity, gastric ulcer, duodenal ulcer, or H. pylori infection. This is in agreement with previously published data from adults showing that the Leb host phenotype is not associated with H. pylori infection or PUD (38). However, we found that H. pylori density was significantly higher in Leb negative individuals than in Leb positive individuals, which is similar to results obtained in Rhesus monkeys (10). Secreted mucins function as decoys for bacterial binding to the epithelial surface, and they are produced in large amounts and constantly wash the mucosal surfaces (23). Mucins efficiently bind H. pylori via Leb, reducing the number of H. pylori available to bind to the mucosal cell surfaces (9, 10, 24). In the human-like rhesus monkey model, we previously observed that the Leb positive mucins act as functional glyco-decoys and clearance factors and therefore reduce infection density. Taken together, these results demonstrate that the secretor and Lewis status play an intrinsic role in resistance to H. pylori infection and suggest that the fucosylated secretor antigens constitute interactive members of the young human mucosal innate immune system. In contrast to the results from the young humans and rhesus monkeys, Taiwanese adult patients expressing Leb have a higher H. pylori density than those who do not, and H. pylori density increases with Leb expression (42). This difference may be due to the fact that the protective function of Leb may only be functional in young individuals because the adherent gastric antral and duodenal mucus gel layer thins with advancing age and duration of H. pylori infection (53).
In conclusion, the expression and tissue localization of mucins and Lea, Leb, Lex, sialyl-Lex and sialyl-Lea were similar in uninfected children and adults. However, in contrast to reports in adults, H. pylori infection was not commonly accompanied by aberrant expression of MUC6 or MUC2 in children, which may relate to duration of exposure to the bacterium. Furthermore, in contrast to reports in adults, Leb negative children had a greater H. pylori density than Leb positive children. These results strongly indicate that the secretor and Lewis status plays an intrinsic role in resistance to H. pylori infection and suggest that the fucosylated secretor antigens constitute interactive members of the young human mucosal innate immune system.
Acknowledgments
We thank Cara Olsen (Uniformed Services of the Health Sciences, Bethesda, Maryland, USA) for critical review of statistical analysis. The work was supported in part by grants from The Swedish Research Council (SL) and the National Institutes of Health (AD) (CA82312).
References
- 1.Atherton JC. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu Rev Pathol. 2006;1:63–96. doi: 10.1146/annurev.pathol.1.110304.100125. [DOI] [PubMed] [Google Scholar]
- 2.Sherman P, Czinn S, Drumm B, Gottrand F, Kawakami E, Madrazo A, et al. Helicobacter pylori infection in children and adolescents: Working Group Report of the First World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2002;35 (Suppl 2):S128–33. doi: 10.1097/00005176-200208002-00010. [DOI] [PubMed] [Google Scholar]
- 3.Levenstein S, Ackerman S, Kiecolt-Glaser JK, Dubois A. Stress and peptic ulcer disease. JAMA. 1999 Jan 6;281(1):10–1. doi: 10.1001/jama.281.1.10. [DOI] [PubMed] [Google Scholar]
- 4.Gerhard M, Lehn N, Neumayer N, Boren T, Rad R, Schepp W, et al. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc Natl Acad Sci U S A. 1999;96(22):12778–83. doi: 10.1073/pnas.96.22.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prinz C, Schoniger M, Rad R, Becker I, Keiditsch E, Wagenpfeil S, et al. Key importance of the Helicobacter pylori adherence factor blood group antigen binding adhesin during chronic gastric inflammation. Cancer Res. 2001;61(5):1903–9. [PubMed] [Google Scholar]
- 6.Ilver D, Arnqvist A, Ogren J, Frick IM, Kersulyte D, Incecik ET, et al. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279(5349):373–7. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- 7.Yamaoka Y, Ojo O, Fujimoto S, Odenbreit S, Haas R, Gutierrez O, et al. Helicobacter pylori outer membrane proteins and gastroduodenal disease. Gut. 2006 Jun;55(6):775–81. doi: 10.1136/gut.2005.083014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borén T, Falk P, Roth KA, Larson G, Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993 Dec 17;262(5141):1892–5. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- 9.Lindén S, Nordman H, Hedenbro J, Hurtig M, Borén T, Carlstedt I. Strain- and blood group-dependent binding of Helicobacter pylori to human gastric MUC5AC glycoforms. Gastroenterology. 2002;123(6):1923–30. doi: 10.1053/gast.2002.37076. [DOI] [PubMed] [Google Scholar]
- 10.Linden S, Mahdavi J, Semino-Mora C, Olsen C, Carlstedt I, Boren T, et al. Role of ABO secretor status in mucosal innate immunity and H. pylori infection. PLoS Pathog. 2008 Jan 4;4(1):e2. doi: 10.1371/journal.ppat.0040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawakubo M, Ito Y, Okimura Y, Kobayashi M, Sakura K, Kasama S, et al. Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science. 2004 Aug 13;305(5686):1003–6. doi: 10.1126/science.1099250. [DOI] [PubMed] [Google Scholar]
- 12.De Bolos C, Garrido M, Real FX. MUC6 apomucin shows a distinct normal tissue distribution that correlates with Lewis antigen expression in the human stomach. Gastroenterology. 1995;109(3):723–34. doi: 10.1016/0016-5085(95)90379-8. [DOI] [PubMed] [Google Scholar]
- 13.Buisine MP, Devisme L, Savidge TC, Gespach C, Gosselin B, Porchet N, et al. Mucin gene expression in human embryonic and fetal intestine. Gut. 1998 Oct;43(4):519–24. doi: 10.1136/gut.43.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buisine MP, Devisme L, Maunoury V, Deschodt E, Gosselin B, Copin MC, et al. Developmental mucin gene expression in the gastroduodenal tract and accessory digestive glands. I. Stomach. A relationship to gastric carcinoma. J Histochem Cytochem. 2000;48(12):1657–66. doi: 10.1177/002215540004801209. [DOI] [PubMed] [Google Scholar]
- 15.Wang RQ, Fang DC. Effects of Helicobacter pylori infection on mucin expression in gastric carcinoma and pericancerous tissues. J Gastroenterol Hepatol. 2006 Feb;21(2):425–31. doi: 10.1111/j.1440-1746.2005.04006.x. [DOI] [PubMed] [Google Scholar]
- 16.Byrd JC, Yan P, Sternberg L, Yunker CK, Scheiman JM, Bresalier RS. Aberrant expression of gland-type gastric mucin in the surface epithelium of Helicobacter pylori-infected patients. Gastroenterology. 1997;113(2):455–64. doi: 10.1053/gast.1997.v113.pm9247464. [DOI] [PubMed] [Google Scholar]
- 17.Oriol R. ABO, Hh, Lewis, and secretion Serology, Genetics, and tissue distribution. In: Cartron JP, Rouger P, editors. Blood Cell Biochemistry. Vol. 6. New York: Plenum press; 1995. Chapter 2. [Google Scholar]
- 18.Oriol R, Mollicone R, Coullin P, Dalix AM, Candelier JJ. Genetic regulation of the expression of ABH and Lewis antigens in tissues. APMIS Suppl. 1992;27:28–38. [PubMed] [Google Scholar]
- 19.Henry S, Oriol R, Samuelsson B. Lewis histo-blood group system and associated secretory phenotypes. Vox Sang. 1995;69(3):166–82. doi: 10.1111/j.1423-0410.1995.tb02591.x. [DOI] [PubMed] [Google Scholar]
- 20.Murata K, Egami H, Shibata Y, Sakamoto K, Misumi A, Ogawa M. Expression of blood group-related antigens, ABH, Lewis(a), Lewis(b), Lewis(x), Lewis(y), CA19-9, and CSLEX1 in early cancer, intestinal metaplasia, and uninvolved mucosa of the stomach. Am J Clin Pathol. 1992;98(1):67–75. doi: 10.1093/ajcp/98.1.67. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu T, Akamatsu T, Sugiyama A, Ota H, Katsuyama T. Helicobacter pylori and the surface mucous gel layer of the human stomach. Helicobacter. 1996;1(4):207–18. doi: 10.1111/j.1523-5378.1996.tb00041.x. [DOI] [PubMed] [Google Scholar]
- 22.Semino-Mora C, Doi SQ, Marty A, Simko V, Carlstedt I, Dubois A. Intracellular and interstitial expression of Helicobacter pylori virulence genes in gastric precancerous intestinal metaplasia and adenocarcinoma. J Infect Dis. 2003;187(8):1165–77. doi: 10.1086/368133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linden S, Mahdavi J, Hedenbro J, Boren T, Carlstedt I. Effects of pH on Helicobacter pylori binding to human gastric mucins: identification of binding to non-MUC5AC mucins. Biochem J. 2004 Dec 1;384(Pt 2):263–70. doi: 10.1042/BJ20040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linden SK, Wickstrom C, Lindell G, Gilshenan K, Carlstedt I. Four modes of adhesion are used during Helicobacter pylori binding to human mucins in the oral and gastric niches. Helicobacter. 2008 Apr;13(2):81–93. doi: 10.1111/j.1523-5378.2008.00587.x. [DOI] [PubMed] [Google Scholar]
- 25.Lindén S, Borén T, Dubois A, Carlstedt I. Rhesus monkey gastric mucins and their Helicobacter pylori binding properties. Biochem J. 2004;379:1–11. doi: 10.1042/BJ20031557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El Miedany YM, Baddour M, Ahmed I, Fahmy H. Sjogren’s syndrome: concomitant H. pylori infection and possible correlation with clinical parameters. Joint Bone Spine. 2005 Mar;72(2):135–41. doi: 10.1016/j.jbspin.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Ilver D, Arnqvist A, Ogren J, Frick IM, Kersulyte D, Incecik ET, et al. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279(5349):373–7. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- 28.Mahdavi J, Sonden B, Hurtig M, Olfat FO, Forsberg L, Roche N, et al. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science. 2002;297(5581):573–8. doi: 10.1126/science.1069076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kivi M, Tindberg Y, Sorberg M, Casswall TH, Befrits R, Hellstrom PM, et al. Concordance of Helicobacter pylori strains within families. J Clin Microbiol. 2003 Dec;41(12):5604–8. doi: 10.1128/JCM.41.12.5604-5608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitney AE, Guarner J, Hutwagner L, Gold BD. Helicobacter pylori gastritis in children and adults: comparative histopathologic study. Ann Diagn Pathol. 2000 Oct;4(5):279–85. doi: 10.1053/adpa.2000.17871. [DOI] [PubMed] [Google Scholar]
- 31.Cohen MC, Rua EC, Balcarce N, Drut R. Sulfomucins in Helicobacter pylori-associated chronic gastritis in children: is this incipient intestinal metaplasia? J Pediatr Gastroenterol Nutr. 2000 Jul;31(1):63–7. doi: 10.1097/00005176-200007000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Bontems P, Robert F, Van Gossum A, Cadranel S, Mascart F. Helicobacter pylori modulation of gastric and duodenal mucosal T cell cytokine secretions in children compared with adults. Helicobacter. 2003 Jun;8(3):216–26. doi: 10.1046/j.1523-5378.2003.00147.x. [DOI] [PubMed] [Google Scholar]
- 33.Blom J, Gernow A, Holck S, Wewer V, Norgaard A, Graff LB, et al. Different patterns of Helicobacter pylori adherence to gastric mucosa cells in children and adults. An ultrastructural study. Scand J Gastroenterol. 2000 Oct;35(10):1033–40. doi: 10.1080/003655200451144. [DOI] [PubMed] [Google Scholar]
- 34.Byrd JC, Yan P, Sternberg L, Yunker CK, Scheiman JM, Bresalier RS. Aberrant expression of gland-type gastric mucin in the surface epithelium of Helicobacter pylori-infected patients. Gastroenterology. 1997 Aug;113(2):455–64. doi: 10.1053/gast.1997.v113.pm9247464. [DOI] [PubMed] [Google Scholar]
- 35.Boren T, Falk P, Roth KA, Larson G, Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993;262(5141):1892–5. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- 36.Bravo JC, Correa P. Sulphomucins favour adhesion of Helicobacter pylori to metaplastic gastric mucosa. J Clin Pathol. 1999;52(2):137–40. doi: 10.1136/jcp.52.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Byrd JC, Bresalier RS. Alterations in gastric mucin synthesis by Helicobacter pylori. World J Gastroenterol. 2000 Aug;6(4):475–82. doi: 10.3748/wjg.v6.i4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Mattos LC, Rodrigues Cintra J, Sanches FE, Alves da Silva Rde C, Ruiz MA, Moreira HW. ABO, Lewis, secretor and non-secretor phenotypes in patients infected or uninfected by the Helicobacter pylori bacillus. Sao Paulo Med J. 2002 Mar 7;120(2):55–8. doi: 10.1590/S1516-31802002000200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heneghan MA, Moran AP, Feeley KM, Egan EL, Goulding J, Connolly CE, et al. Effect of host Lewis and ABO blood group antigen expression on Helicobacter pylori colonisation density and the consequent inflammatory response. FEMS Immunol Med Microbiol. 1998 Apr;20(4):257–66. doi: 10.1111/j.1574-695X.1998.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 40.Morgenstern S, Koren R, Fraser G, Okon E, Niv Y. Gastric corpus mucin expression after partial gastrectomy, in relation to colonization with Helicobacter pylori. J Clin Gastroenterol. 2001 Mar;32(3):218–21. doi: 10.1097/00004836-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Ota H, Nakayama J, Momose M, Hayama M, Akamatsu T, Katsuyama T, et al. Helicobacter pylori infection produces reversible glycosylation changes to gastric mucins. Virchows Arch. 1998;433(5):419–26. doi: 10.1007/s004280050269. [DOI] [PubMed] [Google Scholar]
- 42.Sheu BS, Sheu SM, Yang HB, Huang AH, Wu JJ. Host gastric Lewis expression determines the bacterial density of Helicobacter pylori in babA2 genopositive infection. Gut. 2003 Jul;52(7):927–32. doi: 10.1136/gut.52.7.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor DE, Rasko DA, Sherburne R, Ho C, Jewell LD. Lack of correlation between Lewis antigen expression by Helicobacter pylori and gastric epithelial cells in infected patients. Gastroenterology. 1998;115(5):1113–22. doi: 10.1016/s0016-5085(98)70082-4. [DOI] [PubMed] [Google Scholar]
- 44.Xia HH, Yang Y, Lam SK, Wong WM, Leung SY, Yuen ST, et al. Aberrant epithelial expression of trefoil family factor 2 and mucin 6 in Helicobacter pylori infected gastric antrum, incisura, and body and its association with antralisation. J Clin Pathol. 2004 Aug;57(8):861–6. doi: 10.1136/jcp.2003.015487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nordman H, Davies JR, Lindell G, de Bolos C, Real F, Carlstedt I. Gastric MUC5AC and MUC6 are large oligomeric mucins that differ in size, glycosylation and tissue distribution. Biochem J. 2002;364(Pt 1):191–200. doi: 10.1042/bj3640191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wickstrom C, Davies JR, Eriksen GV, Veerman EC, Carlstedt I. MUC5B is a major gel-forming, oligomeric mucin from human salivary gland, respiratory tract and endocervix: identification of glycoforms and C-terminal cleavage. Biochem J. 1998;334(Pt 3):685–93. doi: 10.1042/bj3340685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herrmann A, Davies JR, Lindell G, Martensson S, Packer NH, Swallow DM, et al. Studies on the “insoluble” glycoprotein complex from human colon. Identification of reduction-insensitive MUC2 oligomers and C-terminal cleavage. J Biol Chem. 1999;274(22):15828–36. doi: 10.1074/jbc.274.22.15828. [DOI] [PubMed] [Google Scholar]
- 48.Rick JR, Goldman M, Semino-Mora C, Liu H, Olsen C, Rueda-Pedraza E, et al. In situ expression of cagA and risk of gastroduodenal disease in Helicobacter pylori-infected children. J Pediatr Gastroenterol Nutr. 2010 Feb;50(2):167–72. doi: 10.1097/MPG.0b013e3181bab326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Genta RM, Robason GO, Graham DY. Simultaneous visualization of Helicobacter pylori and gastric morphology: a new stain. Hum Pathol. 1994;25(3):221–6. doi: 10.1016/0046-8177(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 50.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20(10):1161–81. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Cohen M, Drut R, Cueto Rua E. SIALYL-Tn antigen distribution in Helicobacter pylori chronic gastritis in children: an immunohistochemical study. Pediatr Pathol Mol Med. 2003 Mar-Apr;22(2):117–29. [PubMed] [Google Scholar]
- 52.Mollicone R, Bara J, Le Pendu J, Oriol R. Immunohistologic pattern of type 1 (Lea, Leb) and type 2 (X, Y, H) blood group-related antigens in the human pyloric and duodenal mucosae. Lab Invest. 1985;53(2):219–27. [PubMed] [Google Scholar]
- 53.Newton JL, Jordan N, Pearson J, Williams GV, Allen A, James OF. The adherent gastric antral and duodenal mucus gel layer thins with advancing age in subjects infected with Helicobacter pylori. Gerontology. 2000 May–Jun;46(3):153–7. doi: 10.1159/000022151. [DOI] [PubMed] [Google Scholar]