Abstract
N-terminal-pro-B-type natriuretic peptide (NT-proBNP) is a commonly measured cardiovascular biomarker in both ambulatory and hospital settings. Nonetheless, there are limited data regarding “normal” ranges for NT-proBNP in healthy individuals, despite the importance of such information for interpreting natriuretic peptide measurements. We examined a healthy reference sample free of cardiovascular disease from the Framingham Heart Study Generation 3 cohort; there were 2,285 subjects (mean age 38 years, 56% women). Plasma NT-proBNP levels were measured using the Roche Diagnostics Elecsys 2010 assay, and reference values (2.5, 50, 97.5 quantiles) were determined using empiric and quantile regression methods. Gender, age, and body mass index accounted for approximately 33% of the inter-individual variability in NT-proBNP in the reference sample. NT-proBNP values were substantially higher in women compared with men at every age, and levels increased with increasing age for both sexes. Using quantile regression, the upper reference values (97.5 quantile) for NT-proBNP were 42.5 pg/ml to 106.4 pg/ml in men (depending on age), and 111.0 pg/ml to 215.9 pg/ml in women. Intra-individual variability was assessed in an additional 12 healthy individuals, who had serial NT-proBNP measurements over a month. Intra-class correlation was 0.85, indicating that most of the variability in NT-proBNP concentrations was among-persons rather than within-persons. However, the reference change value was 100%, suggesting that small proportional differences in NT-proBNP could be attributable to analytic variability. In conclusion, the reference limits obtained from this large, healthy community-based sample may aid in the evaluation of NT-proBNP concentrations measured for both clinical and research purposes.
Keywords: Natriuretic peptides, Cardiac Biomarkers, Heart Failure
INTRODUCTION
The objectives of the current study were three-fold. First, we sought to establish reference limits for NT-proBNP, using a widely available commercial assay and a large, well-characterized community-based sample. Second, we examined the clinical correlates of NT-proBNP in this reference sample, and assessed the influence of age and gender on the normal ranges. Lastly, we set out to determine the intra-individual variability of NT-proBNP concentrations over serial measures.
METHODS
The design and selection criteria of the Framingham Heart Study Generation 3 cohort have been previously described.1 The Generation 3 cohort began in 2002 with the recruitment of 4,095 men and women who were the grandchildren of the original Framingham Heart Study participants. Participants who attended the first examination cycle (from 2002 to 2005) were eligible for this investigation. Individuals were excluded for the following reasons, in hierarchical manner: prevalent cardiovascular disease, including history of myocardial infarction, angina pectoris, coronary insufficiency, heart failure, stroke, transient ischemic attack, or intermittent claudication (n = 40); history of atrial fibrillation (n = 16); diabetes (n = 112); hypertension (systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥90 mm Hg, or use of antihypertensive medications; n = 595); obesity (body mass index ≥30 kg/m2; n = 591); valvular heart disease (grade 3/6 systolic murmur or any diastolic murmur on examination; n = 24); renal insufficiency (eGFR ≤60 ml/min using the modified diet in renal disease [MDRD] formula; n = 8); history of pulmonary disease (n = 304); history of thyroid disease (n = 99); age less than 20 or older than 59 years (n = 12); and missing NT-proBNP values (n = 4). The reference sample comprised a healthy subgroup of 2,285 individuals (56% of participants; 1,013 men and 1,272 women) after these exclusions.
A separate group of men and women between the ages of 18 and 75 (not part of the Framingham Health Study) was enrolled at Massachusetts General Hospital (MGH) for the intra-individual variability analyses. Exclusion criteria included history of congestive heart failure or myocardial infarction, prior coronary angioplasty/stent, history of coronary artery bypass graft surgery, history of stable or unstable angina, history of peripheral vascular surgery or procedure, hypertension or use of anti-hypertensive medications, use of nitrates/phosphodiesterase inhibitors, body mass index (BMI) ≥ 27 kg/m2 (calculated on the basis of reported height and weight), diabetes, renal failure, and pregnancy. After applying these exclusions, a sample of 12 healthy individuals (age range 26–53) were included in the analysis. Institutional Review Boards of Boston University and Massachusetts General Hospital approved these studies. All participants provided informed consent prior to their involvement.
At the Framingham examination, a detailed medical history was obtained to determine cardiovascular disease risk factors. Participants underwent a routine physical examination, venipuncture with collection of fasting blood samples, anthropometry and 12-lead electrocardiography. Systolic and diastolic blood pressures were measured in the left arm of participants seated for 5 minutes using a standard mercury column sphygmomanometer. The examining physician recorded values to the nearest even number, and the mean of 2 separate readings was used for study analysis.
In the intra-individual variability study at MGH, participants were examined up to 4 times over a one month period. Absence of intercurrent illness or use of new medications was confirmed by a nurse-administered questionnaire. Blood pressure was checked at each visit, following the protocol described above.
Blood samples were obtained from fasting participants in the morning, typically between 8:00 am and 9:00 am. Subjects were in the supine position and blood was obtained from the antecubital vein. Samples were immediately transferred to pre-chilled tubes containing EDTA and then stored at −70 °C for future analysis. Plasma NT-proBNP was measured with the standard electrochemiluminesence assay (Elecsys 2010, Roche Diagnostics, Indianapolis, Indiana) using established methodology.2 The lower limit of detection was 4 pg/ml. The mean coefficient of variation for these samples was 2.7%.
In the healthy Framingham sample, we estimated reference limits for NT-proBNP using two statistical approaches: empirical and quantile regression. To develop empirical reference limits, the sample was divided into subgroups based on sex and 5-year age bins; estimates of the 2.5th, 50th, and 97.5th quantiles were made within each subgroup. With moderate subgroup sizes, empirical estimates tend to vary substantially. Therefore, we also employed linear quantile regression (PROC QUANTREG),3 to estimate conditional quantile functions for log NT-proBNP.4 Sex-specific regressions were used with age as the predictor. The regression coefficients were estimated at the 2.5th, 50th, and 97.5th quantiles; reference limits were estimated at 5-year age increments for men and women separately. In addition, multivariable linear regression analyses were performed to assess the clinical correlates of log NT-proBNP, regressing log NT-proBNP on sex, age, body mass index, systolic blood pressure and diastolic blood pressure. Continuous predictor variables were standardized (standard deviation = 1) to facilitate comparison of effect sizes. We tested for a sex interaction, and repeated the analyses separately in men and women.
To assess intra-individual variability from the MGH sample, we calculated the mean intra-individual variance and the intraclass correlation (ICC) for the group. The intraclass correlation (ICC) varies from 0 to 1 and corresponds to the proportion of total variability in the analyte attributable to among-person rather than within-person variability. Additionally, we determined the reference change value (RCV) for the group using a Z value of 1.96 as described previously.5–7 All analyses were done using SAS version 9.1.3 (SAS Institute, Cary, N.C.).
RESULTS
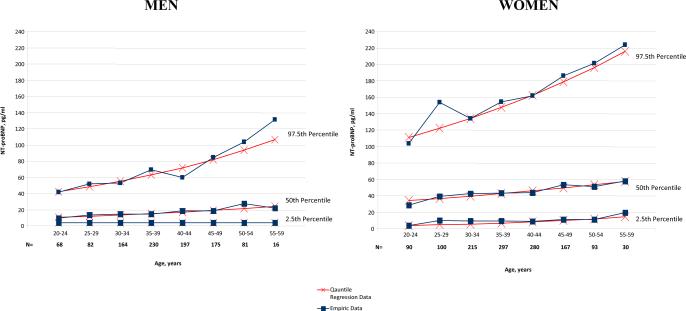
Characteristics of the individuals included in the reference sample are shown in Table 1. Mean age was 38 years, and 56% were women. Using both empiric and quantile regression approaches, we estimated NT-proBNP reference limits in men and women between the ages of 20 and 59 years. Upper (97.5th quantile), median (50th quantile) and lower (2.5th quantile) reference limits are presented by 5-year age intervals separately for men and women in Table 2 and Figure 1.
Table 1.
Characteristics of the Reference Sample
| Variable | Male (N=1,013) | Female (N=1,272) |
|---|---|---|
| Age (years)* | 38.6 (20–59) | 38.3 (20–59) |
| Body mass index (kg/m2) | 25.6 ± 2.6 | 23.4 ± 2.9 |
| Systolic Blood Pressure (mmHg) | 116 ± 9 | 109 ± 10 |
| Diastolic Blood Pressure (mmHg) | 75 ± 8 | 70 ± 8 |
| Creatinine (mg/dL) | 0.91 ± 0.12 | 0.70 ± 0.10 |
| N-terminal-pro-B-type Natriuretic Peptide (pg/mL)* | 16.5 (4.0–225.5) | 44.0 (4.0–510.3) |
All values represent mean ± standard deviation (SD) unless otherwise noted.
Median (minimum and maximum values) shown for these variables.
Table 2.
Reference Limits
| N-terminal-pro-B-type Natriuretic Peptide (pg/ml) | ||||||
|---|---|---|---|---|---|---|
| Males (M) | Females (F) | |||||
| Age Group, years (no. of individuals by sex) | 2.5th Quantile | 50th Quantile | 97.5th Quantile | 2.5th Quantile | 50th Quantile | 97.5th Quantile |
| Empirical Reference Limits | ||||||
| 20–24 (68 M, 90 F) | 4.0 | 9.6 | 41.8 | 4.0 | 29.0 | 103.5 |
| 25–29 (82 M, 100 F) | 4.0 | 13.5 | 51.6 | 10.3 | 39.4 | 153.7 |
| 30–34 (164 M, 215 F) | 4.0 | 14.7 | 53.1 | 9.5 | 42.6 | 134.1 |
| 35–39 (230 M, 297 F) | 4.0 | 14.6 | 69.2 | 9.7 | 43.2 | 154.2 |
| 40–44 (197 M, 280 F) | 4.0 | 18.5 | 59.8 | 8.9 | 44.1 | 161.5 |
| 45–49 (175 M, 167 F) | 4.0 | 18.7 | 84.7 | 11.0 | 53.5 | 185.7 |
| 50–54 (81 M, 93 F) | 4.0 | 27.7 | 103.5 | 11.5 | 51.0 | 201.0 |
| 55–59 (16 M, 30 F) | 4.0 | 22.1 | 131.2 | 19.8 | 57.8 | 223.8 |
| Quantile Regression Reference Limits | ||||||
| 20–24 | 4.0 | 10.5 | 42.5 | 4.1 | 34.2 | 111.0 |
| 25–29 | 4.0 | 11.8 | 48.5 | 4.9 | 36.8 | 122.1 |
| 30–34 | 4.0 | 13.3 | 55.3 | 5.8 | 39.7 | 134.3 |
| 35–39 | 4.0 | 15.0 | 63.0 | 7.0 | 42.8 | 147.6 |
| 40–44 | 4.0 | 16.8 | 71.8 | 8.4 | 46.1 | 162.4 |
| 45–49 | 4.0 | 18.9 | 81.9 | 10.0 | 49.7 | 178.5 |
| 50–54 | 4.0 | 21.3 | 93.3 | 12.0 | 53.6 | 196.3 |
| 55–59 | 4.0 | 24.0 | 106.4 | 14.4 | 57.7 | 215.9 |
Figure 1.
Empirical and quantile regression reference limits for NT-proBNP in men and women.
For men, the lower (2.5th quantile) reference limit was equal to the assay lower limit of detection (4.0 pg/ml) across all age groups regardless of the statistical approach used. The overall percentage of men and women with values at the detection limit were 14% and 1% respectively. The median and upper reference limits for men increased with age for both sexes. NT-proBNP values were substantially higher in women compared with men at every age.
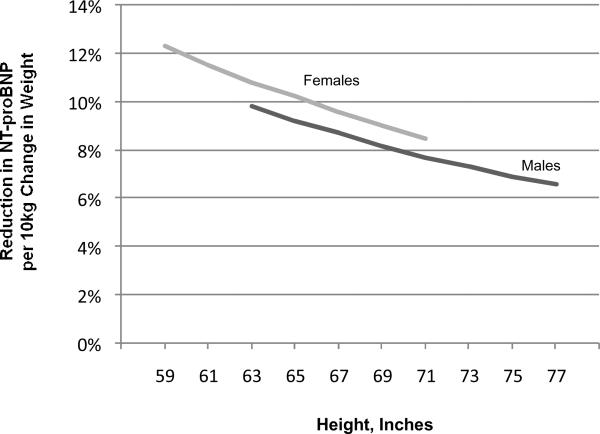
Clinical correlates of NT-proBNP in the reference sample are shown in Table 3. Age, sex, BMI, and blood pressure accounted for 33% of the variability in NT-proBNP concentrations. Most of this variation was attributable to sex, which accounted for 29% of the variability in NT-proBNP. Using modeled data, the percent reduction in NT-proBNP concentrations per 10 kg change in body weight at different heights for men and women is shown in Figure 2.
Table 3.
Clinical Correlates of Log N-terminal-pro-B-type Natriuretic Peptide in the Reference Sample
| Covariates Included in the Model* | Regression Coefficient | Standard Error | Percent Difference† | P value |
|---|---|---|---|---|
| Whole Sample | ||||
| Female Sex | 0.908 | 0.036 | +148% | <0.0001 |
| Age (per 8.4 years) | 0.180 | 0.016 | +20 | <0.0001 |
| Body Mass Index (per 3.0 kg/m2) | −0.077 | 0.018 | −7% | <0.0001 |
| Diastolic Blood Pressure (per 8 mm Hg) | −0.105 | 0.022 | −10% | <0.0001 |
| Systolic Blood Pressure (per 11 mm Hg) | 0.012 | 0.022 | +1% | 0.60 |
| Men | ||||
| Age (per 8.4 years) | 0.228 | 0.026 | +26% | <0.0001 |
| Body Mass Index (per 3.0 kg/m2) | −0.075 | 0.030 | −7% | 0.01 |
| Diastolic Blood Pressure (per 8 mm Hg) | −0.105 | 0.034 | −10% | 0.002 |
| Systolic Blood Pressure (per 11 mm Hg) | −0.007 | 0.036 | −1% | 0.83 |
| Women | ||||
| Age (per 8.4 years) | 0.138 | 0.021 | +15% | <0.0001 |
| Body Mass Index (per 3.0 kg/m2) | −0.083 | 0.022 | −8% | 0.0001 |
| Diastolic Blood Pressure (per 8 mm Hg) | −0.116 | 0.029 | −11% | <0.0001 |
| Systolic Blood Pressure (per 11 mm Hg) | 0.038 | 0.029 | +4% | 0.18 |
Regression coefficients represent the estimated change in log NT-proBNP per standard deviation (SD) change in continuous covariates, where SD values are displayed for each sample.
Values represent the estimated percent change in NT-proBNP per SD increment for the continuous variables or associated with the presence of the categorical variable. Percent change was obtained using the following equation: [(eβ)−1]×100%
Figure 2.
Relation of NT-proBNP to height and weight. Based on regression models in Framingham reference sample. Range of heights restricted to central 95% of heights for men and women in the study sample.
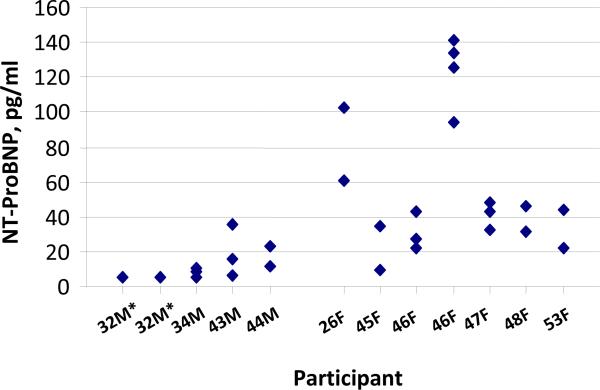
Intra-individual variability was examined in individuals in the MGH sample. Mean age was 41, and 58% were women. Serial NT-proBNP measures are displayed in Figure 3, by participant. Mean NT-proBNP concentrations were 13.4 pg/ml (range 5–36 pg/ml) in men, and 61.3 pg/ml (range 27–141 pg/ml) in women. The mean CV for intra-individual measurements was 36%. The ICC was 0.85. Thus, 85% of the variability in NT-proBNP measurements was attributed to among-person differences and 15% to within-person differences. The RCV was 100%.
Figure 3.
Intra-individual NT-proBNP measurements in the MGH sample.
F=Female; M=Male
*These data represent 4 separate NT-proBNP measurements all of which were the same value.
DISCUSSION
In summary, the present study establishes reference limits for NT-proBNP in healthy, young to middle-aged adults, and examines factors that contribute to “normal” variation in circulating concentrations. These data provide a useful context for interpreting NT-proBNP measurements obtained in clinical practice and for research studies.
Several studies have proposed reference limits for NT-proBNP, but these have been based on older populations8–10 or selected samples.11–13 For the age groups that overlap, our median reference values are generally similar to those of prior studies. For instance, we noted median NT-proBNP values of 21.3 pg/ml and 53.6 pg/ml for men and women respectively, in the 50–54 year old group. Costello-Boerrigter and colleagues studied healthy individuals aged 45 and older from Olmsted County, Minnesota, reporting values of 13 pg/ml and 54 pg/ml.9 Similarly, Galasko and colleagues reported values of 20 pg/ml and 49 pg/ml for healthy men and women (aged 45 to 59) from general medical practices in the United Kingdom.10 While our upper reference values are also similar to those reported in the above studies, they are lower than those reported by Hess and colleagues in a study of 2,000 middle-aged German blood donors.11 Our use of detailed medical assessments, including physician-administered examinations, laboratory testing, and stringent criteria to define the healthy reference sample, may have contributed to these differences. Other strengths of the current study include the large sample size and broad distribution of ages, increasing precision and generalizability.
Our study underscores the fact that “normal” concentrations of NT-proBNP (e.g. the range observed in healthy individuals) are largely a function of gender and age. This observation is consistent with results of studies evaluating other populations or other natriuretic peptides.8, 9, 11 The upper reference limit for NT-proBNP implied by our data is more than 2-fold higher in women than men. Although gender differences in circulating natriuretic peptides have been previously reported, the underlying mechanisms remain unclear.9, 14 Our findings underscore the importance of further studies to examine biological factors, such as gonadal steroids, that modulate the synthesis, secretion, or clearance of natriuretic peptides.15–17
Upper reference limits also increased 2-fold between the ages of 20 and 60 years. While subtle changes in cardiac or arterial function could contribute to the increase, the burden of subclinical abnormalities in our study sample should be low given the exclusion of individuals with obesity, hypertension, diabetes, or other comorbidities. It has been suggested that receptor-mediated clearance of BNP is reduced with aging but this is unlikely to affect the circulating N-terminal pro-peptide, which does not bind to the clearance receptor.18, 19 Older individuals also have lower androgen levels, which could play a role given the apparent inhibitory effects of androgens on natriuretic peptide secretion.15, 17 However, age-related increases in NT-proBNP are proportionally similar in women, despite much smaller changes in androgens with aging.
We determined NT-proBNP reference limits using both empirical and quantile regression approaches. Empirical limits are conceptually straightforward and commonly utilized, but can be affected by a few outlying values. Accordingly, we employed quantile regression models, which are not sensitive to outliers.4 There was excellent agreement in approaches for the 2.5th and 50th quantile values. We observed more variability for the 97.5th quantile cutpoints however, which we would expect to be most sensitive to outliers in the empirical approach. Thus, we recommend the cutpoints obtained from quantile regression for laboratories interested in obtaining reference limits for the Roche NT-proBNP assay or for studies in ambulatory cohorts. Additionally, laboratories should consider reporting age and sex specific reference ranges.
Prior studies in both healthy patients and in those with chronic stable heart failure have reported significant intra-individual variability in NT-proBNP measurements.5–7, 20 In our study, the intra-individual CV of 36% and the RCV of 100% were similar to those reported in the previous studies. As discussed by Wu and colleagues, these values suggest that relatively large proportional differences in NT-proBNP concentrations are necessary to distinguish biological change from normal variability.6 Further, caution should be exercised when attempting to interpret changes between serial measurements of less than 24.2 pg/ml (approximately twice the SD of change in our sample). That said, the calculated ICC of 85% suggests that most of the variability in NT-proBNP in groups of individuals are due to among-person variability, rather than within-person variability.
Several limitations of the study deserve mention. There were few elderly individuals in the cohort, so we restricted the analyses to those individuals between the ages of 20 and 59 years. Our results cannot be generalized to individuals outside this age range, nor do they necessarily apply to other racial/ethnic groups, or results obtained from other NT-proBNP assays.
Acknowledgments
From the Framingham Heart Study of the National Heart Lung and Blood Institute of the National Institutes of Health and Boston University School of Medicine. This work was supported by the National Heart, Lung and Blood Institute's Framingham Heart Study (NO1-HC-25195) and grant R01-HL086875.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures Dr. Wang has been a co-investigator on studies that have received assay support from Siemens Healthcare Diagnostics and Brahms.
REFERENCES
- 1.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB, Sr, Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 2.Karl J, Borgya A, Gallusser A, Huber E, Krueger K, Rollinger W, Schenk J. Development of a novel, N-terminal-proBNP (NT-proBNP) assay with a low detection limit. Scand J Clin Lab Invest Suppl. 1999;230:177–181. [PubMed] [Google Scholar]
- 3.SAS Institute . SAS/STAT user's guide. version 8 SAS Institute; Cary, NC: 1999. [Google Scholar]
- 4.Wei Y, Pere A, Koenker R, He X. Quantile regression methods for reference growth charts. Stat Med. 2006;25:1369–1382. doi: 10.1002/sim.2271. [DOI] [PubMed] [Google Scholar]
- 5.Melzi d'Eril G, Tagnochetti T, Nauti A, Klersy C, Papalia A, Vadacca G, Moratti R, Merlini G. Biological variation of N-terminal pro-brain natriuretic peptide in healthy individuals. Clin Chem. 2003;49:1554–1555. doi: 10.1373/49.9.1554. [DOI] [PubMed] [Google Scholar]
- 6.Wu AH. Serial testing of B-type natriuretic peptide and NTpro-BNP for monitoring therapy of heart failure: the role of biologic variation in the interpretation of results. Am Heart J. 2006;152:828–834. doi: 10.1016/j.ahj.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Wu AH, Smith A, Wieczorek S, Mather JF, Duncan B, White CM, McGill C, Katten D, Heller G. Biological variation for N-terminal pro- and B-type natriuretic peptides and implications for therapeutic monitoring of patients with congestive heart failure. Am J Cardiol. 2003;92:628–631. doi: 10.1016/s0002-9149(03)00741-0. [DOI] [PubMed] [Google Scholar]
- 8.Alehagen U, Goetze JP, Dahlstrom U. Reference intervals and decision limits for B-type natriuretic peptide (BNP) and its precursor (Nt-proBNP) in the elderly. Clin Chim Acta. 2007;382:8–14. doi: 10.1016/j.cca.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Costello-Boerrigter LC, Boerrigter G, Redfield MM, Rodeheffer RJ, Urban LH, Mahoney DW, Jacobsen SJ, Heublein DM, Burnett JC., Jr. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: determinants and detection of left ventricular dysfunction. J Am Coll Cardiol. 2006;47:345–353. doi: 10.1016/j.jacc.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galasko GI, Lahiri A, Barnes SC, Collinson P, Senior R. What is the normal range for N-terminal pro-brain natriuretic peptide? How well does this normal range screen for cardiovascular disease? Eur Heart J. 2005;26:2269–2276. doi: 10.1093/eurheartj/ehi410. [DOI] [PubMed] [Google Scholar]
- 11.Hess G, Runkel S, Zdunek D, Hitzler WE. Reference interval determination for N-terminal-B-type natriuretic peptide (NT-proBNP): a study in blood donors. Clin Chim Acta. 2005;360:187–193. doi: 10.1016/j.cccn.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 12.Mir TS, Flato M, Falkenberg J, Haddad M, Budden R, Weil J, Albers S, Laer S. Plasma concentrations of N-terminal brain natriuretic peptide in healthy children, adolescents, and young adults: effect of age and gender. Pediatr Cardiol. 2006;27:73–77. doi: 10.1007/s00246-005-1022-4. [DOI] [PubMed] [Google Scholar]
- 13.de Lemos JA, McGuire DK, Khera A, Das SR, Murphy SA, Omland T, Drazner MH. Screening the population for left ventricular hypertrophy and left ventricular systolic dysfunction using natriuretic peptides: results from the Dallas Heart Study. Am Heart J. 2009;157:746–753. e742. doi: 10.1016/j.ahj.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Wang TJ, Larson MG, Levy D, Leip EP, Benjamin EJ, Wilson PW, Sutherland P, Omland T, Vasan RS. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am J Cardiol. 2002;90:254–258. doi: 10.1016/s0002-9149(02)02464-5. [DOI] [PubMed] [Google Scholar]
- 15.Chang AY, Abdullah SM, Jain T, Stanek HG, Das SR, McGuire DK, Auchus RJ, de Lemos JA. Associations among androgens, estrogens, and natriuretic peptides in young women: observations from the Dallas Heart Study. J Am Coll Cardiol. 2007;49:109–116. doi: 10.1016/j.jacc.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 16.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC., Jr. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol. 2002;40:976–982. doi: 10.1016/s0735-1097(02)02059-4. [DOI] [PubMed] [Google Scholar]
- 17.Saenger AK, Dalenberg DA, Bryant SC, Grebe SK, Jaffe AS. Pediatric brain natriuretic peptide concentrations vary with age and sex and appear to be modulated by testosterone. Clin Chem. 2009;55:1869–1875. doi: 10.1373/clinchem.2009.123778. [DOI] [PubMed] [Google Scholar]
- 18.Giannessi D, Andreassi MG, Del Ry S, Clerico A, Colombo MG, Dini N. Possibility of age regulation of the natriuretic peptide C-receptor in human platelets. J Endocrinol Invest. 2001;24:8–16. doi: 10.1007/BF03343802. [DOI] [PubMed] [Google Scholar]
- 19.Kawai K, Hata K, Tanaka K, Kubota Y, Inoue R, Masuda E, Miyazaki T, Yokoyama M. Attenuation of biologic compensatory action of cardiac natriuretic peptide system with aging. Am J Cardiol. 2004;93:719–723. doi: 10.1016/j.amjcard.2003.11.054. [DOI] [PubMed] [Google Scholar]
- 20.Bruins S, Fokkema MR, Romer JW, Dejongste MJ, van der Dijs FP, van den Ouweland JM, Muskiet FA. High intraindividual variation of B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with stable chronic heart failure. Clin Chem. 2004;50:2052–2058. doi: 10.1373/clinchem.2004.038752. [DOI] [PubMed] [Google Scholar]