Abstract
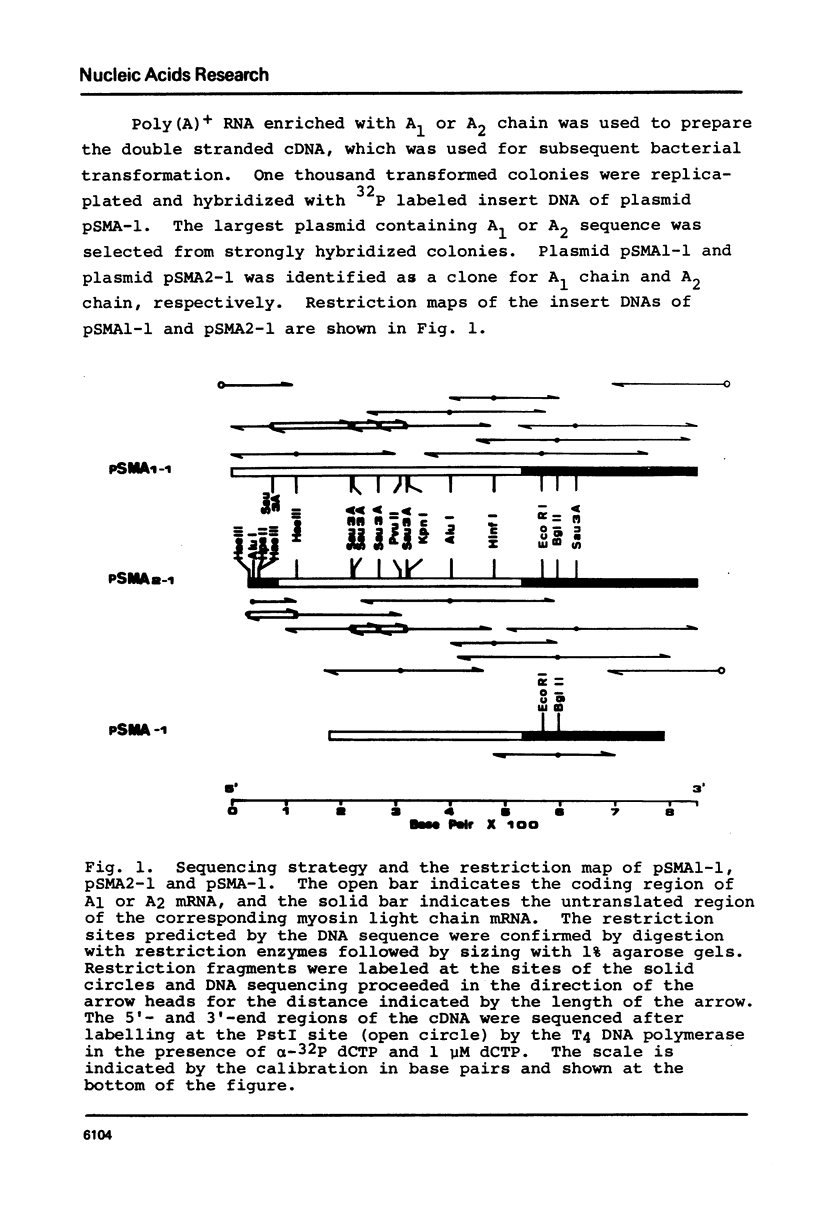
We report here the molecular cloning and sequence analysis of DNAs complementary to mRNAs for myosin alkali light chain of chicken embryo and adult leg skeletal muscle. pSMA2-1 contained an 818 base-pair insert that includes the entire coding region and 5' and 3' untranslated regions of A2 mRNA. pSMA1-1 contained a 848 base-pair insert that included the 3' untranslated region and almost all of the coding region except for the N-terminal 13 amino acid residues of the A1 light chain. The 741 nucleotide sequences of A1 and A2 mRNAs corresponding to C-terminal 141 amino acid residues and 3' untranslated regions were identical. The 5' terminal nucleotide sequences corresponding to N-terminal 35 amino acid residues of A1 chain were quite different from the sequences corresponding to N-terminal 8 amino acid residues and of the 5' untranslated region of A2 mRNA. These findings are discussed in relation to the structures of the genes for A1 and A2 mRNA.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold H. H., Siddiqui M. A. Cloning of synthetic deoxyribonucleic acid that codes for embryonic cardiac myosin light-chain polypeptide. Biochemistry. 1979 Dec 11;18(25):5641–5647. doi: 10.1021/bi00592a019. [DOI] [PubMed] [Google Scholar]
- Brack C., Hirama M., Lenhard-Schuller R., Tonegawa S. A complete immunoglobulin gene is created by somatic recombination. Cell. 1978 Sep;15(1):1–14. doi: 10.1016/0092-8674(78)90078-8. [DOI] [PubMed] [Google Scholar]
- Carmon Y., Neuman S., Yaffe D. Synthesis of tropomyosin in myogenic cultures and in RNA-directed cell-free systems: qualitative changes in the polypeptides. Cell. 1978 Jun;14(2):393–401. doi: 10.1016/0092-8674(78)90124-1. [DOI] [PubMed] [Google Scholar]
- Chi J. C., Fellini S. A., Holtzer H. Differences among myosins synthesized in non-myogenic cells, presumptive myoblasts, and myoblasts. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4999–5003. doi: 10.1073/pnas.72.12.4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi J. C., Rubinstein N., Strahs K., Holtzer H. Synthesis of myosin heavy and light chains in muscle cultures. J Cell Biol. 1975 Dec;67(3):523–537. doi: 10.1083/jcb.67.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. R., Coleman A. W. Muscle differentiation and macromolecular synthesis. J Cell Physiol. 1968 Oct;72(2 Suppl):19–34. doi: 10.1002/jcp.1040720404. [DOI] [PubMed] [Google Scholar]
- Duguid J. R., Steiner D. F., Chick W. L. Partial purification and characterization of the mRNA for rat preproinsulin. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3539–3543. doi: 10.1073/pnas.73.10.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Frank G., Weeds A. G. The amino-acid sequence of the alkali light chains of rabbit skeletal-muscle myosin. Eur J Biochem. 1974 May 15;44(2):317–334. doi: 10.1111/j.1432-1033.1974.tb03489.x. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Borst P., van den Burg J., Weissmann C., Cross G. A. The isolation of plasmids containing DNA complementary to messenger RNA for variant surface glycoproteins of Trypanosoma brucei. Gene. 1980 Mar;8(4):391–417. doi: 10.1016/0378-1119(80)90043-8. [DOI] [PubMed] [Google Scholar]
- Katcoff D., Nudel U., Zevin-Sonkin D., Carmon Y., Shani M., Lehrach H., Frischauf A. M., Yaffe D. Construction of recombinant plasmids containing rat muscle actin and myosin light chain DNA sequences. Proc Natl Acad Sci U S A. 1980 Feb;77(2):960–964. doi: 10.1073/pnas.77.2.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D. S., Heywood S. M. The role of muscle and reticulocyte initiation factor 3 on the translation of myosin and globin messenger RNA in a wheat germ cell-free system. FEBS Lett. 1976 Dec 31;72(2):314–318. doi: 10.1016/0014-5793(76)80994-5. [DOI] [PubMed] [Google Scholar]
- Low R. B., Vournakis J. N., Rich A. Identification of separate polysomes active in the synthesis of the light and heavy chains of myosin. Biochemistry. 1971 May 11;10(10):1813–1818. doi: 10.1021/bi00786a013. [DOI] [PubMed] [Google Scholar]
- Lowey S., Risby D. Light chains from fast and slow muscle myosins. Nature. 1971 Nov 12;234(5324):81–85. doi: 10.1038/234081a0. [DOI] [PubMed] [Google Scholar]
- Maki R., Roeder W., Traunecker A., Sidman C., Wabl M., Raschke W., Tonegawa S. The role of DNA rearrangement and alternative RNA processing in the expression of immunoglobulin delta genes. Cell. 1981 May;24(2):353–365. doi: 10.1016/0092-8674(81)90325-1. [DOI] [PubMed] [Google Scholar]
- Masaki T., Yoshizaki C. Differentiation of myosin in chick embryos. J Biochem. 1974 Jul;76(1):123–131. doi: 10.1093/oxfordjournals.jbchem.a130536. [DOI] [PubMed] [Google Scholar]
- Matsuda G., Maita T., Kato Y., Chen J. I., Umegane T. Amino acid sequences of the cardiac L-2A, L-2B and gizzard 17 000-Mr light chains of chicken muscle myosin. FEBS Lett. 1981 Dec 7;135(2):232–236. doi: 10.1016/0014-5793(81)80789-2. [DOI] [PubMed] [Google Scholar]
- Matsuda G., Maita T., Umegane T. The primary structure of L-1 light chain of chicken fast skeletal muscle myosin and its genetic implication. FEBS Lett. 1981 Apr 6;126(1):111–113. doi: 10.1016/0014-5793(81)81045-9. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford R. M., Wydro R. M., Nguyen H. T., Nadal-Ginard B. Cytoplasmic processing of myosin heavy chain messenger RNA: evidence provided by using a recombinant DNA plasmid. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5749–5753. doi: 10.1073/pnas.77.10.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima Y. I., Ogata K. Stimulation of the synthesis of ribosomal proteins in regenerating rat liver with special reference to the increase in the amounts of effective mRNAs for ribosomal proteins. Eur J Biochem. 1980 Jun;107(2):323–329. doi: 10.1111/j.1432-1033.1980.tb06032.x. [DOI] [PubMed] [Google Scholar]
- Nudel U., Katcoff D., Carmon Y., Zevin-Sonkin D., Levi Z., Shaul Y., Shani M., Yaffe D. Identification of recombinant phages containing sequences from different rat myosin heavy chain genes. Nucleic Acids Res. 1980 May 24;8(10):2133–2146. doi: 10.1093/nar/8.10.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B., Strohman R. C. Myosin synthesis in cultures of differentiating chicken embryo skeletal muscle. Dev Biol. 1972 Oct;29(2):113–138. doi: 10.1016/0012-1606(72)90050-4. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- REPORTER M. C., KONIGSBERG I. R., STREHLER B. L. Kinetics of accumulation of creatine phosphokinase activity in developing embryonic skeletal muscle in vivo and in monolayer culture. Exp Cell Res. 1963 Apr;30:410–417. doi: 10.1016/0014-4827(63)90313-6. [DOI] [PubMed] [Google Scholar]
- Robbins J., Freyer G. A., Chisholm D., Gilliam T. C. Isolation of multiple genomic sequences coding for chicken myosin heavy chain protein. J Biol Chem. 1982 Jan 10;257(1):549–556. [PubMed] [Google Scholar]
- Robert B., Weydert A., Caravatti M., Minty A., Cohen A., Daubas P., Gros F., Buckingham M. cDNA recombinant plasmid complementary to mRNAs for light chains 1 and 3 of mouse skeletal muscle myosin. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2437–2441. doi: 10.1073/pnas.79.8.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Jan;3(1):101–116. doi: 10.1093/nar/3.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Sreter F. A., Gergely J. Light chains of myosins from white, red, and cardiac muscles. Proc Natl Acad Sci U S A. 1971 May;68(5):946–950. doi: 10.1073/pnas.68.5.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shainberg A., Yagil G., Yaffe D. Alterations of enzymatic activities during muscle differentiation in vitro. Dev Biol. 1971 May;25(1):1–29. doi: 10.1016/0012-1606(71)90017-0. [DOI] [PubMed] [Google Scholar]
- Staynov D. Z., Pinder J. C., Gratzer W. B. Molecular weight determination of nucleic acids by gel electrophoresis in non-aqueous solution. Nat New Biol. 1972 Jan 26;235(56):108–110. doi: 10.1038/newbio235108a0. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Pope B. Studies on the chymotryptic digestion of myosin. Effects of divalent cations on proteolytic susceptibility. J Mol Biol. 1977 Apr;111(2):129–157. doi: 10.1016/s0022-2836(77)80119-8. [DOI] [PubMed] [Google Scholar]
- Whalen R. G., Sell S. M., Butler-Browne G. S., Schwartz K., Bouveret P., Pinset-Härstöm I. Three myosin heavy-chain isozymes appear sequentially in rat muscle development. Nature. 1981 Aug 27;292(5826):805–809. doi: 10.1038/292805a0. [DOI] [PubMed] [Google Scholar]
- Young R. A., Hagenbüchle O., Schibler U. A single mouse alpha-amylase gene specifies two different tissue-specific mRNAs. Cell. 1981 Feb;23(2):451–458. doi: 10.1016/0092-8674(81)90140-9. [DOI] [PubMed] [Google Scholar]



