Abstract
Localized irradiation is a common treatment modality for malignancies in the pelvic-abdominal cavity. We report here on the changes in bone mass and strength in mice 7–14 days after abdominal irradiation. Male C57BL/6 mice of 10–12 weeks of age were given a single-dose (0, 5, 10, 15 or 20 Gy) or fractionated (3 Gy × 2 per day × 7.5 days) X rays to the abdomen and monitored daily for up to 14 days. A decrease in the serum bone formation marker and ex vivo osteoblast differentiation was detected 7 days after a single dose of radiation, with little change in the serum bone resorption marker and ex vivo osteoclast formation. A single dose of radiation elicited a loss of bone mineral density (BMD) within 14 days of irradiation. The BMD loss was up to 4.1% in the whole skeleton, 7.3% in tibia, and 7.7% in the femur. Fractionated abdominal irradiation induced similar extents of BMD loss 10 days after the last fraction: 6.2% in the whole skeleton, 5.1% in tibia, and 13.8% in the femur. The loss of BMD was dependent on radiation dose and was more profound in the trabecula-rich regions of the long bones. Moreover, BMD loss in the total skeleton and the femurs progressed with time. Peak load and stiffness in the mid-shaft tibia from irradiated mice were 11.2–14.2% and 11.5–25.0% lower, respectively, than sham controls tested 7 days after a single-dose abdominal irradiation. Our data demonstrate that abdominal irradiation induces a rapid loss of BMD in the mouse skeleton. These effects are bone type- and region-specific but are independent of radiation fractionation. The radiation-induced abscopal damage to the skeleton is manifested by the deterioration of biomechanical properties of the affected bone.
INTRODUCTION
Within a few years after the 1895 discovery of X rays by German physicist Wilhelm Conrad Roentgen, ionizing radiation was used in treating malignant diseases and has since been proven to be an effective and indispensable treatment modality (1, 2). The efficacy of radiotherapy, however, comes at the expense of normal tissue injury, which has been the chief dose-limiting factor in radiotherapy (3, 4).
Various strategies have been developed to circumvent these dose-limiting side effects to increase the treatment efficacy of radiotherapy (2, 5). Despite these measures, the incidence of radiation normal tissue injury remains frequent, and the prevalence of these complications increases with time in cancer survivors, with the current overall postradiotherapy complication rate being approximately 18% (3).
Local irradiation is a common treatment modality for malignancies of the pelvic-abdominal cavities such as prostate, pancreatic, cervical, rectal and endometrial cancers (6–8). The pelvis-abdomen region is sensitive to radiation injury, partially because of the susceptibility of the fast-renewing epithelial cells in the gastrointestinal (GI) tract to ionizing radiation (9, 10). However, adverse effects of pelvic-abdominal irradiation on the skeleton are not rare. The frequently reported skeletal complications after pelvic-abdominal irradiation include regional and systemic osteoporosis, osteonecrosis and nonmalignancy fracture (11, 12). Diagnosis of these skeletal complications is often prompted by patient's complaint of pain from fractures at the affected bones. The median time for a fracture diagnosis is between 6 to 16.9 months after radiotherapy in female patients treated for pelvic malignancies, with a 5-year cumulative prevalence ranging from 13% to 45.2% (11, 13, 14). The respective numbers for male patients treated for prostate cancer are 20 months and 6.8% (12). Although a spatial proximity between the site of bone complications and the field of prior irradiation is present in some of the reported cases, the cause–effect link is noticeably missing in the others. For instance, in a randomized prospective study, femoral fracture was found in 6.7% of a total 1,716 breast cancer patients 1 year after treatment with local radiotherapy to the chest wall (15). This fracture rate is more than 20 times higher than the overall annualized hip fracture rate among breast cancer patients reported in another prospective study of 4,804 subjects being followed up for an average of 3.9 years after diagnosis (16). These latter studies clearly demonstrate that the high fracture rate in breast cancer patients receiving radiotherapy is not the consequence of significant direct radiation exposure of the bone, and thus the occurrence of nonmalignancy bone complications in cancer patients cannot be ascribed solely to the prior direct exposure of the affected bones to radiation.
The etiology of nonmalignancy bone complications in cancer patients treated with radiotherapy remains unclear and seems to be multifactorial. Cumulative evidence suggests the role of abscopal effects in the development of injuries in normal tissues including the skeleton after local radiotherapy. The term “abscopal” was first introduced into the field of radiation biology some 50 years ago by Mole to describe biological responses detected at sites distant from the site of radiation in the same organism (17). The occurrence of abscopal effects has been recorded, however scantly, in various systems from patients to experimental animals, in different types of tissues from cancerous to normal, and in a wide range of forms of life from humans to plants (18–24). We have previously reported a rapid abscopal suppression of the bone marrow stromal cell population in mice after abdominal irradiation (25). Bone marrow stroma hosts the progenitors of bone-forming and resorbing cells and regulates the survival and function of these progenitors and their progeny (26). Abscopal suppression of bone marrow stroma after abdominal irradiation would inevitably have consequences on these bone cells and the skeleton.
In the present study, we tested the hypothesis that abdominal irradiation can induce deterioration of the skeleton outside the radiation field in an abscopal fashion. Analyses were performed at two times after irradiation. At the early time (day 7), mice exposed to an intermediate dose (15 Gy) and a high dose (20 Gy) started to exhibit a clearly divergent path in their overall health conditions. A comparison of various analytical end points between these two radiation groups at this time could provide pivotal evidence on whether the suspected abscopal damage to the bone is the functions of radiation morbidity and mortality. This is particularly important when a clinically relevant dose is concerned. At a later time (day 10–14), the mice exposed to various doses of radiation were almost fully recovered from radiation illnesses. The skeleton was examined at this time to determine whether the radiation-induced skeletal response is associated with the overall health/physical conditions of the mice after the acute phase of radiation sickness has passed. We report here on the loss of bone mass and strength of the mouse skeleton 7–14 days after abdominal irradiation under a single-dose or fractionated radiation scheme at biologically equivalent doses (BEDs) that are clinically relevant.
MATERIALS AND METHODS
Mice
Male C57BL/6 mice were purchased from Harlan Laboratories (Indianapolis, IN) and fed ad libitum with clean water and standard laboratory rodent chow containing 23.5% protein, 6.5% fat, 3.8% fiber, 6.8% ash, 3.3 kcal/g, 3.3 IU vitamin D3/g, 1.0% calcium, and 0.65% phosphorus (Formulab Diet 5008, LabDiet, St. Louis, MO). The mice were group-housed in plastic cages, 4–5 mice per cage, under standard laboratory conditions with a 12:12-h light/dark cycle, humidity of 48%, and a constant temperature of 22°C. After an acclimatization period of 2 weeks, the mice were used for experiments at 10–12 weeks of age. At this age mice have ceased the rapid growth of the skeleton and the fast accumulation of bone mineral content but have not started to undergo the loss of bone mass that occurs at approximately 3.5–4 months of age. The size of mice at this age is almost uniform, with the coefficients of variation being 2.46% for body weight, 8.91% for fat content, 2.46% for total-body bone mineral density (BMD), and 4.55% for total-body bone area, as determined in a pilot experiment using a cohort of 22 age- and gender-matched intact mice (unpublished data). All procedures used in this study were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Arkansas for Medical Sciences.
Animal Treatments
The mice were individually tagged with an electronic identification chip (Bio Medic Data Systems, Inc., Seaford, DE), randomized and given a single-dose (5, 10, 15 or 20 Gy) or fractionated (3 Gy per fraction, 2 fractions per day for 7.5 consecutive days) X ray to the abdomen. The biologically equivalent doses (BEDs) were 7.5, 20, 37.5 and 60 Gy in the single-dose irradiation scheme and 58.5 Gy in the fractionated irradiation scheme, respectively. These BEDs are within the dose limit (<74 Gy) used in the clinic to treat malignancies residing in the pelvis-abdomen area (6–8, 27). Mice exposed to 0 Gy were used as sham controls. The mice were inspected twice a day and body weight was recorded daily for up to 14 days after the completion of irradiation. Radiation was delivered to the abdomen at a dose rate of 1.079 Gy/min (150 kVp and 6.6 mA) using a Faxitron X-ray Generating System (CP-160, Faxitron X-Ray Corp., Wheeling, IL) as described previously (25). Non-abdomen body parts including the skeleton were shielded with a custom-made cerrobend block. We have reported that the shielding efficacy is >97.5% at a targeted single dose of 20 Gy, and the backscattered radiation (approximately 50 cGy) under this shielding plan does not induce detectable changes in bone marrow cell viability and activity (25).
In a separate experiment, mice were placed under food restriction with free access to drinking water. The standard murine chow pellets were cut into small pieces weighing 0.2 g each (approximately 1% of the average body weight of the mice). The mice were fed once a day, one piece per mouse. Mice being fed the regular diet were used as controls. The mice were inspected twice a day and body weight was recorded daily for up to 7 days after the start of food restriction.
Longitudinal Measurement of Bone Mineral Density (BMD) and Body Mass Composition by Duel-Energy X-Ray Absorptiometry (DEXA)
Bone mineral content and body mass composition were determined in live mice 1 day prior to abdominal irradiation (baseline) and at day 7 to 14 after irradiation (final, specified in individual experiment). The early time (day 7) was when the mice exposed to a single dose (15 or 20 Gy) of radiation started to diverge in their overall health/physical conditions, while the later times (day 10 or 14) represented the time when the irradiated mice were close to a full recovery of their overall health from the radiation morbidity. BMD was determined using the standard DEXA technique as described previously (28, 29). Precision of the Piximus was determined by repeated measurements of five animals, five times each. Our mean intraindividual coefficients of variation were 1.7–2.0% for total-body BMD (28) and 1.7% for femoral BMD (29), consistent with previous reports (30). Briefly, mice under ketamine/xylazine anesthesia were scanned using the Lunar Piximus-II Bone Densitometer (GE Lunar Corp., Madison, WI) with the total-body mode and a resolution of 0.18 × 0.18 mm. Whole-body areal BMD (g/cm2) and body mass composition consisting of fat and lean mass were determined automatically by the software Lunar PIXImus 2.10. (GE Lunar Corp.) in a subcranial total-body region of interest (ROI) with the exclusion of the head and the electronic identification chip. BMD of specific sites of the skeleton or subregions of individual bones was acquired with manual placement of ROI as follows: ROI for the lumbar included lumbar vertebrae 1–5; the femur and tibia were measured between the articular joints at the proximal and distal ends of the bones; the proximal, mid-shaft and distal femur or tibia enclosed the corresponding one-third of the respective subregion of the bone. Baseline and final measurements of body mass composition, total-body BMD, BMD of individual bone, and BMD at a sub-region within an individual bone were used to determine the percentage change in BMD at the corresponding site of the skeleton for each mouse. The calculated percentage change in the BMD for the same type of long bone of both sides of each mouse or values of the same sub-region of each type of long bone of both sides of each mouse were averaged to determine the percentage change in BMD at that specific site of the skeleton for that mouse. These calculated values for each category for the mice of the same treatment group were then used to obtain the means and standard deviation/error for that group.
ELISA Measurement of Bone Turnover Markers in Serum
Blood was collected via retro-orbital bleeding prior to euthanasia at day 7 postirradiation after a single dose of radiation. Serum from each mouse was analyzed individually in duplicate for the bone formation marker osteocalcin (OCN) using the Mouse Osteocalcin EIA Kit (BTI Biomedical Technologies, Inc., Stoughton, MA) and bone resorption marker tartrate-resistant acid phosphatase 5b (TRAP5b) using the MouseTRAP Assay (Immunodiagnostic Systems Inc., Fountain Hills, AZ) following the instructions of the manufacturers. The average value of the duplicate measurements was obtained for each mouse.
Differentiation of Osteoblasts and Osteoclasts In Ex Vivo Bone Marrow Culture
After bleeding, the mice were euthanized by cervical dislocation. Bone marrow cells from the left tibia and left femur of the same mouse were pooled and processed individually in triplicate for ex vivo osteoblast and osteoclast formation as described previously (31, 32). The average value of colony/cell counts in the triplicate wells was determined for each mouse.
Biomechanical Test of the Femurs and Tibiae
At euthanasia, the right tibiae and femurs were excised and stored at 4°C in saline supplemented with antibiotics until testing. Bone strength at the mid-shaft femur and tibia was analyzed by the standard 3-point bending test following a published method (33, 34) with modifications made for murine bone testing (35). The method and nomenclature of biomechanical testing are in accordance with the published guidelines (36). In brief, the bones were brought to room temperature and remained in saline to prevent dehydration prior to testing. A 3-point bending test was performed using a servohydraulic 858 Mini Bionix II Test System load frame (MTS Systems, Eden Prairie, MN) with computer control, data logging and automatic calculations of load to failure using TestWorks 4.0 (MTS Systems). All bones were positioned in the same way with their anterior side (convex surface) down on two horizontal supports spaced 7 mm apart, with the central loading point contacted the posterior surface (concave surface) of the diaphysis at the midpoint of the bone length. This positioning method prevents the rotation of the bones. No additional stabilizing measure was applied to the bones. The tip of the loading point is rounded to avoid cutting into the bone surface during the test. After applying a pre-load of 0.1 N to the posterior center of the bone, the loading point was displaced downward (transverse to the long axis of the bone) at 0.1 mm/s until failure, generating bending in the anteroposterior plane. Load displacement data were recorded at 100 Hz (TestWorks 4.0, MTS), and from the load-displacement curve the following mechanical parameters were determined for the mid-shaft of the tested bones: peak load (Newtons) which is a measure of the resistance to permanent deformation, and stiffness (Newton/mm), which is a measure of rigidity or resistance to fracture. Stiffness was calculated from the slope of the linear portion of the load-displacement curve.
Statistical Analysis
The test of differences in the mean values among groups was performed by linear regression and by one-way ANOVA using the Student-Newman-Keuls post hoc comparison procedure, and the longitudinal comparison within the same group by t test. P < 0.05 was considered statistically significant.
RESULTS
Loss of Body Mass after Abdominal Irradiation
Consistent with our previous report (25), loss of body weight was evident after a single dose of radiation to the abdomen starting 1 day after irradiation regardless of dose (Fig. 1A and B). Weight loss in mice exposed to radiation at 20 Gy progressed with time until the end of the experiment, i.e., day 7 after irradiation, when the mice in this radiation group would soon have started to die, as established previously (25). Mice exposed to radiation at 15 Gy were able to regain the lost weight starting at day 5 postirradiation and would have all survived (25). Weight loss in mice exposed to 5 or 10 Gy radiation was much milder, and body weight recovery started 3 days after irradiation. The maximum weight loss was dependent on radiation dose.
FIG. 1.
Changes in body weight and body mass composition after abdominal irradiation (AI). Panels A–C: Postirradiation changes in body weight. Mice were exposed to single-dose (panels A and B) or fractionated (panel C) X rays to the abdomen. Body weight was recorded daily. Weight loss was calculated individually for each mouse as percentage change from baseline values. Data points are means + SD. Panel A: n = 14 (sham), 20 (15 Gy) and 12 (20 Gy), pooled from two experiments with similar results. Panel B: n = 11 per group; experiment was performed once. Panel C: n = 8 per group; experiment was performed once. Panel D: Postirradiation changes in body mass composition. Body mass composition was determined in the same mice shown in panel A at baseline and postirradiation day 7. Data points are means ± SE. n = 14 (sham), 20 (15 Gy) and 12 (20 Gy), pooled from two experiments with similar results. #: P < 0.05 compared to sham; ##: P < 0.05 20 Gy compared to 15 Gy.
Similar weight loss also occurred in mice after fractionated abdominal irradiation (Fig. 1C). Although the biologically equivalent dose (BED) of the fractionated abdominal irradiation scheme was virtually identical to that from 20 Gy given in a single fraction (58.5 Gy compared to 60 Gy) and the maximum weight loss was similar for both groups, mice given fractionated abdominal irradiation started to regain their body weight 4 days after the last fraction and reached 91.5% of the baseline body weight by day 10 days after the completion of irradiation. All animals survived this irradiation scheme.
Regression analysis of the body mass composition (Fig. 1D) measured in mice 7 days after 15 or 20 Gy abdominal irradiation showed that the loss of total body weight was proportional to the loss of lean mass (R2 = 0.9508, P < 0.001), while the extent of the loss of fat mass was similar between the two radiation groups (P > 0.05) at day 7 after irradiation.
Suppressed Bone Formation Activity after Abdominal Irradiation
ELISA analysis of bone turnover markers in serum revealed a dose-dependent suppression of the serum bone formation marker osteocalcin (OCN) at day 7 postirradiation, while the serum bone resorption marker tartrate-resistant acid phosphatase 5b (TRAP5b) unaffected (Fig. 2A). These results were consistent with the ex vivo differentiation capacity of the progenitors of the bone-forming osteoblastic cells and bone-resorbing osteclasts (Fig. 2B). These findings illustrate a dramatic suppression of bone-formation activity but virtually unchanged bone resorption activity after abdominal irradiation.
FIG. 2.
Changes in bone formation and resorption activities 7 days after a single dose of radiation to the abdomen. Panel A: Serum levels of the bone formation marker osteocalcin (OCN) and bone resorption marker tartrate-resistant acid phosphatase 5b (TRAP5b) were measured by ELISA at day 7 postirradiation. Data points are means ± SE, n = 5. #: P < 0.05 compared to sham; ##: P < 0.05 20 Gy compared to 15 Gy. Panel B: Ex vivo formation of alkaline phosphatase (ALP)-positive osteoblastic colonies and tartrate-resistant acid phosphatase (TRAP)-positive multinucleated osteoclasts from bone marrows harvested at day 7 postirradiation. Data points are the mean ± SE, n = 5. #: P < 0.05 compared to sham; ##: P < 0.05 20 Gy compared to 15 Gy.
Loss of Total-Body Bone Mineral Density (BMD) after Abdominal Irradiation
Seven days after a single dose of 15 or 20 Gy (37.5 and 60 Gy, respectively, in BED), a respective 2.2% and 3.1% net loss in total-body BMD was detected as opposed to a 0.5% net gain in the sham controls (Fig. 3). Regression analysis showed a strong correlation between the loss of fat mass and the loss of total-body BMD (R2 = 0.475, P < 0.001), but no correlation was found between the loss of total-body BMD and the loss of either total mass (R2 = 0.1002, P > 0.05) or lean mass (R2 = 0.0267, P > 0.05), indicating that the loss of total-body BMD after abdominal irradiation was not simply a result of reduced mechanical loading on the skeleton due to decreased weight bearing and/or muscle strength, two common causes of disuse osteoporosis as a result of skeletal adaptation (37).
FIG. 3.
Loss of total-body bone mineral density (BMD) 7 days after a single dose of radiation. Mice were subjected to a single dose of X rays to the abdomen. BMD was determined at baseline and at day 7 postirradiation. Data points are means ± SE. n = 14 (sham), 20 (15 Gy) and 12 (20 Gy), pooled from two experiments with similar results. #: P < 0.05 compared to sham.
To verify the lack of a relationship between the rapid decrease in body weight and the loss of total-body BMD after abdominal irradiation, changes in BMD were measured in mice placed under food restriction. Weight loss in these mice started from day 1 of food restriction, progressed with time, and reached a weight loss of 30.4% (–29.1% to –33.5%, n = 7) from baseline by day 7. The pace and extent of weight loss in food-restricted mice were comparable with those in mice given abdominal irradiation at a single dose of 20 Gy. Changes in lean mass and fat mass were –28.1% (SD = 3.5%) and –39.0% (SD = 6.0%), respectively, by day 7 of food restriction, also comparable to those after 20 Gy abdominal irradiation. Total-body BMD increased 0.21% (SD = 1.71%) by day 7 of food restriction, similar to the BMD changes in sham controls (P > 0.05 compared to sham controls) but significantly different from the irradiated groups (P < 0.05 compared to 15 Gy or 20 Gy). Together, these data demonstrate the minimal contribution of weight loss to the rapid loss of BMD in mice 7 days after abdominal irradiation.
To determine whether the loss of total-body BMD occurred at lower doses of radiation, mice were exposed to a single fraction of radiation to the abdomen at 5, 10 and 15 Gy (7.5, 20 and 37.5 Gy, respectively, in BED) and assessed for BMD change 14 days later. A decrease in total-body BMD was induced by radiation at 10 and 15 Gy but not at 5 Gy, and the change was dependent on dose (Fig. 5A). Compared with the extend of BMD change at day 7 postirradiation, the loss of BMD was greater at day 14 postirradiation after a single dose of 15 Gy (P < 0.05 compared to day 7).
FIG. 5.
Changes in BMD 14 days after a single dose of radiation. Mice were subjected to a single dose of X rays to the abdomen (AI). BMD was determined at baseline and at day 14 postirradiation. Panel A: Changes in total-body BMD. Panel B: Changes in BMD in the femur, tibia and lumbar vertebrae. Data points are means ± SE, n = 12, experiment was performed once. #: P < 0.05 compared to sham, ##: P < 0.05 compared to 5 Gy, ###: P < 0.05 compared to 10 Gy.
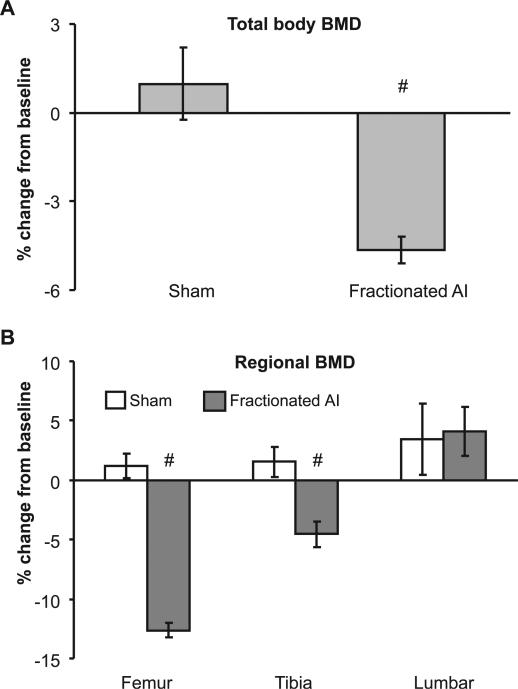
To further assess the skeletal response to local irradiation that mimics the clinical setting of radiotherapy, mice were given fractionated abdominal irradiation with a BED of 58.7 Gy. A 6.2% loss of total-body BMD was detected in the irradiated mice 10 days after the last fraction (Fig. 6A) despite the fact that the mice had regained 91.5% of the preirradiation body weight (Fig. 1C), and all survived to this point with the overall health conditions greatly improved.
FIG. 6.
Changes in BMD 10 days after the completion of fractionated irradiation. Mice were subjected to fractionated X irradiation of the abdomen at 3 Gy per fraction, 2 fractions per day for 7.5 consecutive days. BMD was determined at baseline and at 10 days after the last fraction. Panel A: Changes in total-body BMD. Panel B: Changes in BMD in the femur, tibia and lumbar vertebrae. Data points are means ± SE, n = 8; experiment was performed once. #: P < 0.05 compared to sham, P < 0.05 compared to 10 Gy.
Postirradiation Bone Loss Was Bone Type- and Region-Specific
Examination of three bones (lumbar vertebrae, femurs and tibiae) that are most relevant to osteoporosis-related fracture revealed that the changes in BMD after abdominal irradiation differed among skeletal sites. Tibiae and femurs exhibited a consistent loss of BMD detected 7 and 14 days after a single dose of radiation to the abdomen, and the dose dependence of the loss of BMD was clearly present at day 14 postirradiation (Fig. 4 and Fig. 5B). Compared with the BMD change at day 7 after irradiation, the loss of BMD in the femur deteriorated at day 14 postirradiation after a single dose of 15 Gy (P < 0.05 compared to day 7), while the extent of loss of BMD in tibia remained the same. Similar to the bone loss induced by single doses of radiation, fractionated doses of radiation also resulted in a loss of femoral and tibial BMD 10 days after the last fraction, with the femur exhibiting a greater loss (Fig. 6B). Unlike the tibia and femur, changes in lumbar vertebral BMD after abdominal irradiation under both radiation schemes were mainly a reduction in BMD accumulation, and the difference between the irradiated groups and sham-irradiated controls was not significant (Figs. 4, 5B and 6B).
FIG. 4.
Loss of BMD in the femur, tibia and lumbar vertebra 7 days after a single dose of radiation. Mice were subjected to a single dose of X rays to the abdomen. BMD was determined at baseline and at day 7 postirradiation. Data points are means ± SE. n = 14 (sham), 20 (15 Gy) and 12 (20 Gy), pooled from two experiments with similar results. #: P < 0.05 compared to sham.
When a femur or tibia was further divided along the axis into three regions with equal length, a greater loss of BMD was detected in regions rich in cancellous bone (i.e., the proximal and distal thirds of the femur as well as the proximal two-third of the tibia) 7 days after a single dose of radiation at 15 or 20 Gy (Fig. 7). The BMD loss in these regions showed a trend of dose dependence. The regions dominant with cortical bone (i.e., the mid-shaft femur and distal third of the tibia) showed only a modest decrease in BMD 7 days after a single dose of radiation at 15 or 20 Gy (P > 0.05 compared to sham) (Fig. 7).
FIG. 7.
Changes in regional BMD 7 days after a single dose of radiation. Mice were subjected to a single-dose X irradiation of the abdomen. BMD was determined at baseline and at day 7 postirradiation. Data points are means ± SE. n = 14 (sham), 20 (15 Gy) and 12 (20 Gy), pooled from two experiments. #: P < 0.05 compared to sham.
Deterioration of Bone Strength after Abdominal Irradiation
To test whether abdominal irradiation also affected the biomechanical properties of the bones, peak deformation load and stiffness were measured at the mid-shaft tibia (rich in cancellous bone) and mid-shaft femur (rich in cortical bone) using a standard 3-point bending. Seven days after exposure to a single dose of radiation at 15 Gy and 20 Gy, the respective values of peak load were 11.2% and 14.2% lower and the respective stiffness values were 11.5% and 25.0% lower in the two irradiated groups than in sham-irradiated controls for the mid-shaft tibia (Fig. 8). Regression analysis showed that the values for peak load and stiffness were inversely correlated with the percentage loss of BMD at the mid-shaft tibia (peak load as a function of percentage decrease in BMD: R2 = 0.3066, P < 0.001; stiffness as a function of percentage decrease in BMD: R2 = 0.1979, P = 0.003). There was no detectable loss of either peak load or stiffness at the mid-shaft femur (data not shown).
FIG. 8.
Effects of abdominal irradiation on bone strength at the mid-shaft tibia. Mice were subjected to a single-dose X irradiation of the abdomen. Tibiae were dissected at day 7 postirradiation and subjected to 3-point bending to determine the peak load and stiffness. The value for each bone was transformed to the percentage difference from the mean value of the sham control. Data points are means ± SE of the transformed values. n = 14 (sham), 20 (15 Gy) and 12 (20 Gy), pooled from 2 experiments. #: P < 0.05 compared to sham, ##: P < 0.05 20 Gy compared to 15 Gy.
DISCUSSION
Radiotherapy-related bone complications have been recognized since the application of ionizing radiation on patients nearly a century ago, and they continue to be one of the treatment-related nonmalignant conditions that could considerably compromise the quality of life of patients (38). Retrospective study is the most common method to examine the relationship between radiotherapy and the development of bone complications. However, the majority of these studies examined the adverse impact of radiotherapy only on the skeletal sites directly exposed to radiotherapy (11, 12, 39). Whether local radiotherapy could result in systemic adverse effects on the unirradiated skeleton, however, remains unclear (15, 16). Using a mouse model, we have obtained unequivocal evidence that abdominal irradiation induced a rapid deterioration of not only the quantity (BMD) but more importantly the quality (strength) of the unexposed skeleton. This finding demonstrates that radiation damage to the bone does not require a direct radiation exposure of the bone. This form of radiation damage to the skeleton presents a previously unrecognized side effect of radiotherapy.
In the present study we compared the changes in the mouse skeleton under two radiation schemes. The single-dose scheme delivers a desirable BED in a simplified manner, and the fractionated scheme bears more resemblance to the clinical use of local radiation (6–8). Abdominal irradiation at clinically relevant BEDs (<74 Gy) (6–8, 27) under either scheme triggered a substantial loss of BMD, and a dose-dependent decrease of BMD was clearly present at all sites of the skeleton tested under the single-dose radiation scheme. This observation indicates that the abscopal damage to the skeleton elicited by radiation is an intrinsic component of the side effects of local irradiation. Our findings from the fractionated radiation experiment are more intriguing. It is generally believed that the severity of tissue injury after irradiation decreases with increasing fraction number and/or intervals between fractions (40, 41). The abscopal damage to the skeleton by radiation, however, did not seem to be eased by fractionation, because similar extents of bone loss were elicited by a BED of approximately 60 Gy given in either a single dose of 20 Gy or 15 fractions of 3 Gy each. Together, these observations suggest that the radiation-induced abscopal damage to the skeleton is little affected by the extent of overall radiation morbidity and mortality, and the intervals between fractions intended to reduce normal tissue radiation injury in the clinic may not be effective at minimizing the abscopal damage to the skeleton.
The two postirradiation times selected for skeletal assessment represent two phases of normal tissue radiation injury. The early time (day 7) approximately resembles the final stage of acute radiation morbidity, when the mice exposed to the intermediate dose (15 Gy) and high dose (20 Gy) of radiation started to exhibit clearly divergent directions in their overall health/physical conditions. At the later times (day 10–14), mice exposed to radiation were almost fully recovered from radiation illnesses. Our findings at these two postirradiation times indicate that the abscopal degradation of the skeleton after abdominal irradiation is not only a detrimental event that occurs early but also an injury that progresses with time (at least in the total skeleton and the femur after a single dose of radiation at 15 Gy, or a BED of 37.5 Gy). More importantly, this progression of postirradiation bone loss is present at the time that the overall health conditions of the irradiated individual have greatly improved. This observation is clinically relevant, because this later time with an overall improved health/physical conditions in mice resembles the post-therapy stage in the clinic when the initial phase of tumor control and radiation morbidity management is successful and the quality of life of patients becomes a primary focus in patient care. We are investigating the long-term impact of abdominal irradiation on the mouse skeleton and are trying to address questions such as how long the progression of the loss of BMD persists after irradiation and whether the lost BMD will be regained over time as in the case of maternal BMD changes during lactation and after weaning (42, 43).
It is important to point out the rapidity and severity of the loss of BMD observed in our model compared with that induced by two common causes of osteoporosis. The annual loss of BMD ranges from 7.8% for the whole skeleton and 0.7% to 9.9% at the femoral neck in patients with glucocorticoid excess-induced osteoporosis (44, 45). In the case of sex steroid deficiency-induced osteoporosis, the annual rate of BMD loss in postmenopausal women ranges from 0.7% in the total skeleton to 4% in the spine (46), and approximately 4 weeks is required in gonadectomized rodents to show a 3–4% loss of total-body BMD and 5.3–10% loss of BMD in the lower limbs (47, 48). In contrast, it took only 7–14 days for the mice to lose BMD of this magnitude in the total skeleton and the long bones after abdominal irradiation. Our findings suggest that the bone loss after irradiation is more severe and occurs at a much faster rate compared with bone loss associated with menopause or glucocorticoid excess.
We detected a greater loss of BMD in the trabecula-rich regions than in the cortex-rich regions in long bones. Regional variations in bone turnover activities between cancellous and cortical bone are commonly observed (49, 50). It is unclear whether this regional difference in abscopal bone loss after abdominal irradiation is due to a more active bone resorption on the trabecular surface and whether endosteal/periosteal resorption of the cortical bone also contributes to the detected bone loss in the femur and tibia. We also noticed that the BMD of the lumbar vertebrae, a site rich in cancellous bone, was only modestly affected by radiation. This is in strong contrast to the decrease in BMD detected in the trabecula-rich regions of tibiae and femurs. Differences between lumbar vertebrae and long bones in their responsiveness to pathophysiological challenges have been observed elsewhere (46, 51–53). It is unclear at present whether the site-specific difference in the extent of BMD loss in our model is due to a delayed response of lumbar vertebrae as seen in the anabolic effects of PTH on the skeleton (54) or represents a true resistance of lumbar vertebrae to the abscopal actions after abdominal irradiation. Further investigation is needed to address these important questions.
Severe loss of bone mass is often manifested as insufficiency fractures when the biomechanical strength of a bone decreases below the threshold required for the bone to perform normal activities (55, 56). Although local and systemic osteopenia and osteoporosis are the common forms of postradiotherapy nonmalignancy bone complications, they often go undiagnosed until the development of clinical symptom, i.e., nonmalignancy fractures at the affected bones (11, 12, 38, 39). In the present study, the rate of deterioration of bone strength after abdominal irradiation was at least two to three times faster than in mouse models of primary and secondary osteoporosis (31, 47). Our demonstration of a rapid (day 7) loss of bone strength in the unirradiated tibia after abdominal irradiation provides the first direct evidence that postradiotherapy fractures could result from the abscopal impact of local irradiation on the unirradiated skeleton.
In the present study, bone strength was tested only at the mid-shaft of the tibia and femur. The exclusion of the distal and proximal regions of these bones from biomechanical testing, however, is not an indication of the insignificance of the bone strength in these regions but rather a reflection of our technical limitations. The proximal region of the femur, for instance, is in fact one of the most commonly diagnosed fracture sites after local radiotherapy (38). Given that the extent of BMD loss in these regions was similar to or even greater than that in the mid-shaft tibia, we suspect that a loss of bone strength also occurred in these regions after abdominal irradiation. Furthermore, since the loss of BMD in the femur and tibia after fractionated irradiation was at least as severe as the loss after a single dose (15 Gy), we suspect that a similar or greater degree of decrease in bone strength occurred in these bones after fractionated irradiation.
The loss of bone strength after abdominal irradiation was twice as great as the loss of BMD at the mid-shaft tibia 7 days after a single dose, and only a weak, although significant, correlation was found between the loss of bone strength and the loss of BMD at the tested mid-shaft tibia. These observations suggest that other factors in addition to the loss of BMD might also contribute to the impaired bone strength. An alteration in bone geometry (e.g. the size of a bone) and/or microarchitecture (e.g. trabecula connectivity) of the bone, for instance, can result in a change in bone strength with or without BMD change (57). We do not know at present how much of the detected loss of bone strength is attributable to the loss of BMD and whether the radiation exposure also significantly altered the geometric and structural features of the tested bones.
The mechanisms for the radiation-induced abscopal response in the skeleton are unclear. In recent years, a number of candidate molecules have been proposed as intermediates between localized radiation and abscopal tissue responses (58, 59). We showed earlier that the abscopal suppression of bone marrow after abdominal irradiation is mediated by elevated oxidative stress (25). Reactive oxygen species (ROS) modulate bone cell survival and activity as well bone metabolism (60, 61) and could play a role in the abscopal response of the skeleton after abdominal irradiation. In the present study, the strong association between the loss of total-body BMD and the loss of fat mass after abdominal irradiation suggests the involvement of adipose-derived signaling in postirradiation bone loss. However, food restriction also induced a similar loss of fat mass but did not cause bone loss in our control experiment, suggesting that the adipose-derived signaling alone is not sufficient to lead to a rapid bone loss or the adipose-derived signaling after irradiation differs from that induced by fasting in bone metabolism regulation. Serotonin is another candidate molecule. The gut-derived serotonin exerts deleterious effects on bone (62, 63), and its secretion is stimulated by radiation (64). Whatever the case, further investigation is required to elucidate the mechanism(s) responsible for radiation-induced local systemic compromise of the skeleton.
In the present study, total-abdomen irradiation was used as a tool to elicit biological responses. Caution must be used when extrapolating the results from this study into clinical implication, because total-pelvis or total-abdomen irradiation is rarely used in radiotherapy (6–8) and radiation dose–volume effects (65–67) could have well been amplified in our model. Young male mice were used in the present study. Whether the observed abscopal effects of abdominal irradiation on the skeleton are age- and/or gender-dependent are unknown.
Localized irradiation is a frequently used treatment modality for malignancies in the pelvic-abdominal cavity. Nonmalignancy bone complications, frequently in the form of bone loss and in many cases diagnosed only after the occurrence of fractures, are common postirradiation complications with elusive causes and few treatment options. We present evidence that abdominal irradiation at clinically relevant BEDs induces a rapid loss of BMD in mice after either single-dose or fractionated irradiation. This loss of BMD is accompanied by an even larger decrease in bone strength. The observed effects of abdominal irradiation on the mouse skeleton illustrate a robust but previously overlooked response of the bone/bone marrow to local non-bone irradiation. Our findings suggest that abscopal degradation of the skeleton is an important risk factor for nonmalignancy fracture in unirradiated bones after local radiotherapy to the pelvic-abdominal area. Skeletal radiation damage of this form might present a novel therapeutic target for the prevention and treatment of skeletal complications associated with localized radiotherapy.
ACKNOWLEDGMENTS
We thank Ms. Nisreen S. Akel for performing DEXA scanning and Mr. William R. Hogue for biomechanical tests. This work was funded by a seed grant from the Department of Radiation Oncology, University of Arkansas for Medical Sciences, the Kaufman's Foundation through Central Arkansas Radiation Therapy Institute (CARTI), the Carl. L Nelson endowed Chair in Orthopaedic Creativity, the RadCCORE Pilot Project (parent U19 AI067798), and R21 AI085438.
REFERENCES
- 1.Orton CG. Uses of therapeutic x rays in medicine. Health Phys. 1995;69:662–76. doi: 10.1097/00004032-199511000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Bernier J, Hall EJ, Giaccia A. Radiation oncology: a century of achievements. Nat Rev Cancer. 2004;4:737–47. doi: 10.1038/nrc1451. [DOI] [PubMed] [Google Scholar]
- 3.Cohen L, Schultheiss TE, Hendrickson FR, Mansell J, Saroja KR, Lennox A. Normal tissue reactions and complications following high-energy neutron beam therapy: I. Crude response rates. Int J Radiat Oncol Biol Phys. 1989;16:73–8. doi: 10.1016/0360-3016(89)90012-6. [DOI] [PubMed] [Google Scholar]
- 4.Stone HB, Coleman CN, Anscher MS, McBride WH. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4:529–36. doi: 10.1016/s1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 5.Smith RP, Heron DE, Huq MS, Yue NJ. Modern radiation treatment planning and delivery—from Röntgen to real time. Hematol Oncol Clin N Am. 2006;20:45–62. doi: 10.1016/j.hoc.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Golis G, Zanaboni F, Vanoli P, Russo A, Franchi M, Scarfone G, et al. The impact of whole-abdomen radiotherapy on survival in advanced ovarian cancer patients with minimal residual disease after chemotherapy. Gynecol Oncol. 1990;39:150–4. doi: 10.1016/0090-8258(90)90423-i. [DOI] [PubMed] [Google Scholar]
- 7.Liu L, Glicksman AS, Coachman N, Kuten A. Low acute gastrointestinal and genitourinary toxicities in whole pelvic irradiation of prostate cancer. Int J Radiat Oncol Biol Phys. 1997;38:65–71. doi: 10.1016/s0360-3016(96)00580-9. [DOI] [PubMed] [Google Scholar]
- 8.Dorr W, Kost S, Keinert K, Glaser FH, Endert G, Herrmann T. Early intestinal changes following abdominal radiotherapy comparison of endpoints. Strahlenther Onkol. 2006;182:1–8. doi: 10.1007/s00066-006-1471-6. [DOI] [PubMed] [Google Scholar]
- 9.Somosy Z, Horvath G, Telbisz A, Rez G, Palfia Z. Morphological aspects of ionizing radiation response of small intestine. Micron. 2002;33:167–78. doi: 10.1016/s0968-4328(01)00013-0. [DOI] [PubMed] [Google Scholar]
- 10.Keefe DM, Gibson RJ, Hauer-Jensen M. Gastrointestinal mucositis. Semin Oncol Nurs. 2004;20:38–47. doi: 10.1053/j.soncn.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Kwon JW, Huh SJ, Yoon YC, Choi SH, Jung JY, Oh D, et al. Pelvic bone complications after radiation therapy of uterine cervical cancer: evaluation with MRI. AJR Am J Roentgenol. 2008;191:987–94. doi: 10.2214/AJR.07.3634. [DOI] [PubMed] [Google Scholar]
- 12.Igdem S, Alco G, Ercan T, Barlan M, Ganiyusufoglu K, Unalan B, et al. Insufficiency fractures after pelvic radiotherapy in patients with prostate cancer. Int J Radiat Oncol Biol Phys. 2010;77:818–23. doi: 10.1016/j.ijrobp.2009.05.059. [DOI] [PubMed] [Google Scholar]
- 13.Baxter NN, Habermann EB, Tepper JE, Durham SB, Virnig BA. Risk of pelvic fractures in older women following pelvic irradiation. JAMA. 2005;294:2587–93. doi: 10.1001/jama.294.20.2587. [DOI] [PubMed] [Google Scholar]
- 14.Ikushima H, Osaki K, Furutani S, Yamashita K, Kishida Y, Kudoh T, et al. Pelvic bone complications following radiation therapy of gynecologic malignancies: clinical evaluation of radiation-induced pelvic insufficiency fractures. Gynecol Oncol. 2006;103:1100–4. doi: 10.1016/j.ygyno.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 15.Kirstensen B, Ejlertsen B, Mouridsen HT, Andersen KW, Lauritzen JB. Femoral fractures in postmenopausal breast cancer patients treated with adjuvant tamoxifen. Breast Cancer Res Treat. 1996;39:321–6. doi: 10.1007/BF01806160. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, Maraicic M, Aragaki AK, Mouton C, Arendell L, Lopez AM, et al. Fracture risk increases after diagnosis of breast or other cancers in postmenopausal women: results from the Women's Health Initiative. Osteoporos Int. 2009;20:527–36. doi: 10.1007/s00198-008-0721-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol. 1953;26:234–41. doi: 10.1259/0007-1285-26-305-234. [DOI] [PubMed] [Google Scholar]
- 18.Phillips RD, Kimeldorf DJ. Local and systemic effects of ionizing radiation on bone growth. Am J Physiol. 1966;210:1096–100. doi: 10.1152/ajplegacy.1966.210.5.1096. [DOI] [PubMed] [Google Scholar]
- 19.Gompertz RH, Man WK, Li SK, Baron JH, Spencer J, Michalowski AS. Gastric histamine in a chronic duodenal ulcer model induced by partial thoracic irradiation. Agents Actions. 1989;27:177–9. doi: 10.1007/BF02222232. [DOI] [PubMed] [Google Scholar]
- 20.Van der Meeren A, Monti P, Vandamme M, Squiban C, Wysocki J, Griffiths N. Abdominal radiation exposure elicits inflammatory responses and abscopal effects in the lungs of mice. Radiat Res. 2005;163:144–52. doi: 10.1667/rr3293. [DOI] [PubMed] [Google Scholar]
- 21.Robin HI, AuBuchon J, Varanasi VR, Weinstein AB. The abscopal effect: demonstration in lymphomatous involvement of kidneys. Med Pediatr Oncol. 1981;9:473–6. doi: 10.1002/mpo.2950090510. [DOI] [PubMed] [Google Scholar]
- 22.Petrovic N, Perovic J, Karanovic D, Todorovic L, Petrovic V. Abscopal effects of local fractionated X-irradiation of face and jaw region. Strahlentherapie. 1982;158:40–2. [PubMed] [Google Scholar]
- 23.Koturbash I, Rugo RE, Hendricks CA, Loree J, Thibault B, Kutanzi K, et al. Irradiation induces DNA damage and modulates epigenetic effectors in distant bystander tissue in vivo. Oncogene. 2006;25:4267–75. doi: 10.1038/sj.onc.1209467. [DOI] [PubMed] [Google Scholar]
- 24.Yang G, Wu L, Chen L, Pei B, Wang Y, Zhan F, et al. Targeted irradiation of shoot apical meristem of Arabidopsis embryos induces long-distance bystander/abscopal effects. Radiat Res. 2007;167:298–305. doi: 10.1667/RR0710.1. [DOI] [PubMed] [Google Scholar]
- 25.Jia D, Koonce NA, Griffin RJ, Jackson C, Corry PM. Prevention and mitigation of acute death of mice after abdominal irradiation by the antioxidant N-acetyl-cysteine (NAC). Radiat Res. 2010;173:579–89. doi: 10.1667/RR2030.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999;181:67–73. doi: 10.1002/(SICI)1097-4652(199910)181:1<67::AID-JCP7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 27.De Meerleer GO, Vakaet LA, De Gersem WR, De Wagter C, De Naeyer B, De Neve W. Radiotherapy of prostate cancer with or without intensity modulated beams: a planning comparison. Int J Radiat Oncol Biol Phys. 2000;47:639–48. doi: 10.1016/s0360-3016(00)00419-3. [DOI] [PubMed] [Google Scholar]
- 28.Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B. Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology. 2004;145:401–6. doi: 10.1210/en.2003-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fluckey JC, Dupont-Versteegden EE, Montague DC, Knox M, Tesch P, Peterson CA, et al. A rat resistance exercise regimen attenuates losses of musculoskeletal mass during hindlimb suspension. Acta Physiol Scand. 2002;176:293–300. doi: 10.1046/j.1365-201X.2002.01040.x. [DOI] [PubMed] [Google Scholar]
- 30.Nagy TR, Clair AL. Precision and accuracy of dual-energy X-ray absorptiometry for determining in vivo body composition of mice. Obes Res. 2000;8:392–8. doi: 10.1038/oby.2000.47. [DOI] [PubMed] [Google Scholar]
- 31.Weinstein RS, Jia D, Powers CC, Stewart SA, Jilka RL, Parfitt AM, et al. The skeletal effects of glucocorticoid excess override those of orchidectomy in mice. Endocrinology. 2004;145:1980–7. doi: 10.1210/en.2003-1133. [DOI] [PubMed] [Google Scholar]
- 32.Jia D, O'Brien CA, Stewart SA, Manolagas SC, Weinstein RS. Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology. 2006;147:5592–9. doi: 10.1210/en.2006-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aronson J, Hogue WR, Flahiff CM, Gao GG, Shen XC, Skinner RA, et al. Development of tensile strength during distraction osteogenesis in a rat model. J Orthop Res. 2001;19:64–9. doi: 10.1016/S0736-0266(00)00002-4. [DOI] [PubMed] [Google Scholar]
- 34.Brown EC, Berrien DS, Fletcher TW, Irby DJ, Aronson J, Gao GG, et al. Skeletal toxicity associated with chronic ethanol exposure in a rat model using total enteral nutrition. J Pharmacol Exp Ther. 2002;301:1132–8. doi: 10.1124/jpet.301.3.1132. [DOI] [PubMed] [Google Scholar]
- 35.Suva LJ, Hartman E, Dilley JD, Russell S, Akel NS, Skinner RA, et al. Platelet dysfunction and a high bone mass phenotype in a murine model of platelet-type von Willebrand disease. Am J Pathol. 2008;172:430–9. doi: 10.2353/ajpath.2008.070417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner CJ, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
- 37.Giangregorio L, Blimkie CJ. Skeletal adaptations to alterations in weight-bearing activity: a comparison of models of disuse osteoporosis. Sports Med. 2002;32:459–76. doi: 10.2165/00007256-200232070-00005. [DOI] [PubMed] [Google Scholar]
- 38.Asknes LH, Bruland OS. Some musculo-skeletal sequelae in cancer survivors. Acta Oncol. 2007;46:490–6. doi: 10.1080/02841860701218642. [DOI] [PubMed] [Google Scholar]
- 39.Huh SJ, Kim B, Kang MK, Lee JE, Lim DH, Wark W, et al. Pelvic insufficiency fracture after pelvic irradiation in uterine cervix cancer. Gynecol Oncol. 2002;86:264–8. doi: 10.1006/gyno.2002.6756. [DOI] [PubMed] [Google Scholar]
- 40.Wiernik G. Fractionation in radiotherapy. Anticancer Res. 1983;3:283–97. [PubMed] [Google Scholar]
- 41.Saunders MI, Dische S. Fractionation in radiotherapy: a view from the clinic. Br J Radiol. 1997;70:S17–24. doi: 10.1259/bjr.1997.0004. Spec No. [DOI] [PubMed] [Google Scholar]
- 42.Nishiwaki M, Yasumizu T, Hoshi K, Ushijima Effect of pregnancy, lactation and weaning on bone mineral density in rats as determined by dual-energy X-ray absorptiometry. Endocr J. 1999;46:711–6. doi: 10.1507/endocrj.46.711. [DOI] [PubMed] [Google Scholar]
- 43.Yasumizu T, Nakamura Y, Hoshi K, Iijima S, Asaka A. Bone metabolism after human parturition and the effect of lactation: longitudinal analysis of serum bone-related proteins and bone mineral content of the lumbar spine. Endocr J. 1998;45:679–86. doi: 10.1507/endocrj.45.679. [DOI] [PubMed] [Google Scholar]
- 44.Alesci S, De Martino MU, Ilas I, Gold PW, Chrousos GP. Glucocorticoid-induced osteoporosis: from basic mechanisms to clinical aspects. Neuroimmunomodulation. 2005;12:1–19. doi: 10.1159/000082360. [DOI] [PubMed] [Google Scholar]
- 45.Shaker JL, Lukert BP. Osteoporosis associated with excess glucocorticoids. Endocrinol Metab Clin North Am. 2005;34:341–56. doi: 10.1016/j.ecl.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 46.Moschonis G, Manios Y. Skeletal site-dependent response of bone mineral density and quantitative ultrasound parameters following a 12-month dietary intervention using dairy products fortified with calcium and vitamin D: the Postmenopausal Health Study. Br J Nutr. 2006;96:1140–8. doi: 10.1017/bjn20061977. [DOI] [PubMed] [Google Scholar]
- 47.Kousteni S, Chen JR, Bellido T, Han L, Ali AA, O'Brien CA, et al. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science. 2002;298:843–6. doi: 10.1126/science.1074935. [DOI] [PubMed] [Google Scholar]
- 48.Nagashima H. Longitudinal study of in vivo bone mineral changes in rats using dual X-ray absorptiometry—effect of ovariectomy and prednisolone. Nippon Seikeigeka Gakkai Zasshi. 1993;67:1151–61. [PubMed] [Google Scholar]
- 49.Hamrick MW, Skedros JG, Pennington C, McNeil PL. Increased osteogenic response to exercise in metaphyseal versus diaphyseal cortical bone. J Musculoskelet Neuronal Interact. 2006;6:258–63. [PubMed] [Google Scholar]
- 50.Lloyd SA, Yuan YY, Simske SJ, Riffle SE, Ferguson VL, Bateman TA. Administration of high-dose macrophage colony-stimulating factor increases bone turnover and trabecular volume fraction. J Bone Miner Metab. 2009;27:546–54. doi: 10.1007/s00774-009-0071-9. [DOI] [PubMed] [Google Scholar]
- 51.McCarthy I, Goodship A, Herzog R, Oganov V, Stussi E, Vahlensieck M. Investigation of bone changes in microgravity during long and short duration space flight: comparison of techniques. Eur J Clin Invest. 2000;30:1044–54. doi: 10.1046/j.1365-2362.2000.00719.x. [DOI] [PubMed] [Google Scholar]
- 52.Seeman E, Wahner HW, Offord KP, Kumar R, Johnson WJ, Riggs BL. Differential effects of endocrine dysfunction on the axial and the appendicular skeleton. J Clin Invest. 1982;69:1302–9. doi: 10.1172/JCI110570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Willey JS, Livingston EW, Robbins ME, Bourland JD, Tirado-Lee L, Smith-Sielicki H, et al. Risedronate prevents early radiation-induced osteoporosis in mice at multiple skeletal locations. Bone. 2010;46:101–11. doi: 10.1016/j.bone.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iida-Klein A, Zhou H, Lu SS, Levine R, Ducayen-Knowles M, Dempster DW, et al. Anabolic action of parathyroid hormone is skeletal site specific at the tissue and cellular levels in mice. J Bone Miner Res. 2002;17:808–16. doi: 10.1359/jbmr.2002.17.5.808. [DOI] [PubMed] [Google Scholar]
- 55.Soubrier M, Dubost JJ, Boisgard S, Sauvezie B, Gaillard P, Michel JL, et al. Insufficiency fracture. A survey of 60 cases and review of the literature. Joint Bone Spine. 2003;70:209–18. doi: 10.1016/s1297-319x(03)00024-1. [DOI] [PubMed] [Google Scholar]
- 56.Guggenbuhl P, Meadeb J, Chales G. Osteoporotic fractures of the proximal humerus, pelvis, and ankle: epidemiology and diagnosis. Joint Bone Spine. 2005;72:372–5. doi: 10.1016/j.jbspin.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Chavassieux P, Seeman E, Delmas PD. Insights into material and structural basis of bone fragility from diseases associated with fractures: how determinants of the biomechanical properties of bone are compromised by disease. Endocr Rev. 2007;28:151–64. doi: 10.1210/er.2006-0029. [DOI] [PubMed] [Google Scholar]
- 58.Camphausen K, Moses MA, Menard C, Sproull M, Beecken WD, Folkman J, et al. Radiation abscopal antitumor effect is mediated through p53. Cancer Res. 2003;63:1990–3. [PubMed] [Google Scholar]
- 59.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–70. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 60.Isomura H, Fujie K, Shibata K, Inoue N, Iizuka T, Takebe G, et al. Bone metabolism and oxidative stress in postmenopausal rats with iron overload. Toxicology. 2004;197:93–100. doi: 10.1016/j.tox.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 61.Banfí G, Iorio EL, Corsi MM. Oxidative stress, free radicals and bone remodeling. Clin Chem Lab Med. 2008;46:1550–5. doi: 10.1515/CCLM.2008.302. [DOI] [PubMed] [Google Scholar]
- 62.Bliziotes M. Update in serotonin and bone. J Clin Endocrinol Metab. 2010;95:4124–32. doi: 10.1210/jc.2010-0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ducy P, Karsenty G. The two faces of serotonin in bone biology. J Cell Biol. 2010;191:7–13. doi: 10.1083/jcb.201006123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scarantino CW, Ornitz RD, Hoffman LG, Anderson RF., Jr On the mechanism of radiation-induced emesis: the role of serotonin. Int J Radiat Oncol Biol Phys. 1994;30:825–30. doi: 10.1016/0360-3016(94)90356-5. [DOI] [PubMed] [Google Scholar]
- 65.Kavanagh BD, Pan CC, Dawson LA, Das SK, Li XA, Ten Haken RK, et al. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys. 2010;76:S101–7. doi: 10.1016/j.ijrobp.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 66.Fiorino C, Alongi F, Perna L, Broggi S, Cattaneo GM, Cozzarini C, et al. Dose-volume relationships for acute bowel toxicity in patients treated with pelvic nodal irradiation for prostate cancer. Int J Radiat Oncol Biol Phys. 2009;75:27–35. doi: 10.1016/j.ijrobp.2008.10.086. [DOI] [PubMed] [Google Scholar]
- 67.Mameghan H, Fisher R, Mameghan J, Watt WH, Tynan A. Bowel complications after radiotherapy for carcinoma of the prostate: the volume effect. Int J Radiat Oncol Biol Phys. 1990;18:315–20. doi: 10.1016/0360-3016(90)90095-2. [DOI] [PubMed] [Google Scholar]