Abstract
Dividing cells need to coordinate the separation of chromosomes with the formation of a cleavage plane. There is evidence that microtubule bundles in the interzone region of the anaphase spindle somehow control both the location and the assembly of the cleavage furrow [1–3]. A microtubule motor that concentrates in the interzone, MKLP1, has previously been implicated in the assembly of both the metaphase spindle and the cleavage furrow [4–6]. To gain insight into mechanisms that might underlie interdependence of the spindle and the cleavage furrow, we used RNA-mediated interference (RNAi) to study the effects of eliminating MKLP1 from Caenorhabditis elegans embryos. Surprisingly, in MKLP1(RNAi) embryos, spindle formation appears normal until late anaphase. Microtubule bundles form in the spindle interzone and the cleavage furrow assembles; anaphase and cleavage furrow ingression initially appear normal. The interzone bundles do not gather into a stable midbody, however, and furrow contraction always fails before complete closure. This sequence of relatively normal mitosis and a late failure of cytokinesis continues for many cell cycles. These and additional results suggest that the interzone microtubule bundles need MKLP1 to encourage the advance and stable closure of the cleavage furrow.
Results and discussion
The C. elegans ortholog of MKLP1 (CeMKLP1) was identified by using the predicted amino-acid sequence of human MKLP1 [7] to search the C. elegans genome database. A putative gene, M03D4.1, was identified that encodes 775 amino acids. Sequence analysis revealed two motor-domain insertions (amino acids 146–161 and 174–210) and a carboxy-terminal domain (amino acids 670–775) that are present in only MKLP1 proteins from other organisms.
We used RNAi to eliminate expression of CeMKLP1 by the maternal germline [8]. Two cDNA clones that encode most of CeMKLP1 were obtained [9] then double-stranded RNAs were prepared from each and microinjected into the gonads of C. elegans hermaphrodites. After 16–24 hours, embryos produced by the injected worms were observed by Nomarski microscopy (Figure 1). Pronuclear migration and pseudocleavage appeared normal. Assembly, orientation and function of the first mitotic spindle also appeared normal, except that the spindle appeared abnormally flexible; in wild-type embryos, it is rigid along the pole-to-pole axis and rocks back and forth as a unit during spindle elongation. In CeMKLP1(RNAi) embryos, rocking and elongation occurred, but the spindle often flexed at the equator. This effect is best seen in real time but can also be seen in Figure 1 (compare f with b). The increased flexibility is consistent with the idea that CeMKLP1 helps connect the two half-spindles by crosslinking their interdigitated microtubules [7,10].
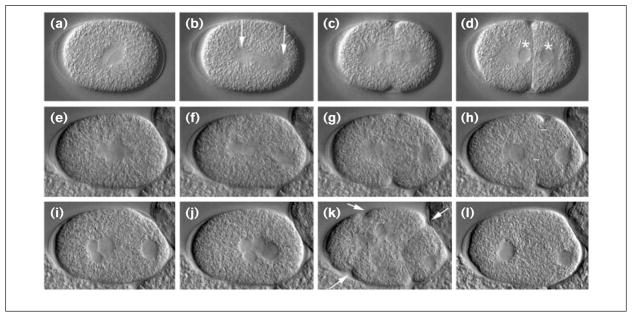
Figure 1.
The CeMKLP1(RNAi) phenotype in live embryos. The early development of a wild-type embryo (a–d) and a CeMKLP1(RNAi) embryo (e–l) were recorded by video Nomarski microscopy. Anterior is left. (a–h) First mitotic cycle. (a,e) The pronuclei have fused, and the spindle is beginning to elongate. (b,f) The spindle has started rocking as elongation continues; the spindle poles are the prominent, granule-free zones marked with arrows in (b). (c,g) Initiation of cleavage furrow invagination is evident. (d,h) Telophase; the granule-free zones are the two nuclei — marked with asterisks in (d)— each flanked by a centrosome (the anterior centrosome is spherical, posterior is disk-shaped). Cytokinesis is completed in the wild-type (d) but not the RNAi embryo (h); white bars show the maximum invagination of the cleavage furrow achieved in the RNAi embryo. (i,j) The furrow in the RNAi embryo regresses (i) and the nuclei migrate back to the center of the embryo (j). Reabsorbed polar body nuclei are often seen — two small anterior nuclei in (i). At anaphase–telophase of the second mitotic cycle, multiple cleavage furrows are evident (arrows in k). Invagination of the posterior-most furrow often appears complete, while invagination of the anterior furrows is less pronounced. (l) All furrows again retract, resulting in a multinucleate, single-cell embryo. Additional nuclei are out of the plane of focus. C. elegans embryos are ~50 μm long.
Studies in other systems have suggested that MKLP1 is a motor for spindle elongation during anaphase B [4,5,10]. During the first mitotic division, no significant differences were detected in either the duration (n = 5 for controls; n = 6 for RNAi) or the extent (n = 6 for controls, n = 9 for RNAi) of anaphase elongation (data not shown). This result, and the normal appearance of most other aspects of spindle function, contrasts with the dramatic disruption of mitosis caused by antibody injection into mammalian cells and sea urchin embryos [4,5]. Our results are, however, consistent with the observation that elimination of MKLP1 in Drosophila pav mutants does not affect mitosis [6]. It is possible that the antibody injections caused non-specific effects; it is also possible that MKLP1 is employed somewhat differently by different organisms.
The most striking defect caused by CeMKLP1 (RNAi) was a late failure of cytokinesis. In all RNAi one-cell embryos observed (n = 60), the first cleavage furrow appeared at the proper time and at the proper location (Figure 1, compare g with c). The furrow contracted substantially, achieving 40–100% closure before arrest (Figure 1h); it then retracted, and the telophase nuclei moved to the center of the embryo (Figure 1 i,j). During telophase, one, two or three additional small nuclei usually appeared in the anterior end of the embryo and migrated to join the anterior daughter nucleus (Figure 1i). The position and size of the additional nuclei plus the concurrent disappearance of polar bodies suggested that those ectopic nuclei were the haploid products of female meiosis and indicated a failure of meiotic as well as mitotic cleavage-furrow closure.
The second round of mitotic spindle assembly was synchronous. Second division spindles often shared chromosomes or were multipolar. In cases in which the two spindles appeared separate, their assembly, structure, orthogonal positions, and function appeared fairly normal. During anaphase of the second cell cycle, one cleavage furrow formed close to the site of the first division furrow and commonly advanced to what appeared to be complete closure (Figure 1k) before reversing. Other, less vigorous cleavage furrows also formed, contracted and regressed, usually at positions consistent with spindle interzone equators (Figure 1k–l). This cycle of mitosis and failed cytokinesis continued for many rounds, producing single-cell embryos with many nuclei.
The late failure of cleavage-furrow function caused by MKLP1 depletion in C. elegans contrasts with the early inhibition of cleavage-furrow assembly [6] and spindle assembly [4,5] reported in other systems. It is possible that our RNAi approach did not cause complete elimination of CeMKLP1; the late failure might simply represent a mild case of the disruption of furrow assembly that has been seen in Drosophila pav (MKLP1) mutants. To test the effectiveness of CeMKLP1 elimination by RNAi and to determine the distribution of CeMKLP1 in untreated embryos, two anti-CeMKLP1 antisera were generated. Both antisera and antibodies affinity-purified from them showed identical staining patterns in fixed wild-type embryos. Staining was reduced below detectability by RNAi (Figure 2h), indicating that RNAi effectively eliminates CeMKLP1 from embryos, consistent with the observation that RNAi mimics null mutations for many C. elegans genes that have been tested [8]. It is therefore likely that the late failure of cleavage-furrow advance that we saw reflects a complete or near-complete loss of CeMKLP1 function. The contrast between previously reported spindle assembly and cleavage furrow phenotypes and ours suggests that some aspects of MKLP1 function have diverged in different organisms. Perhaps MKLP1 has an evolutionarily conserved role in furrow advance that is phenotypically detectable in C. elegans but masked by the earlier phenotypes seen in the other organisms studied.
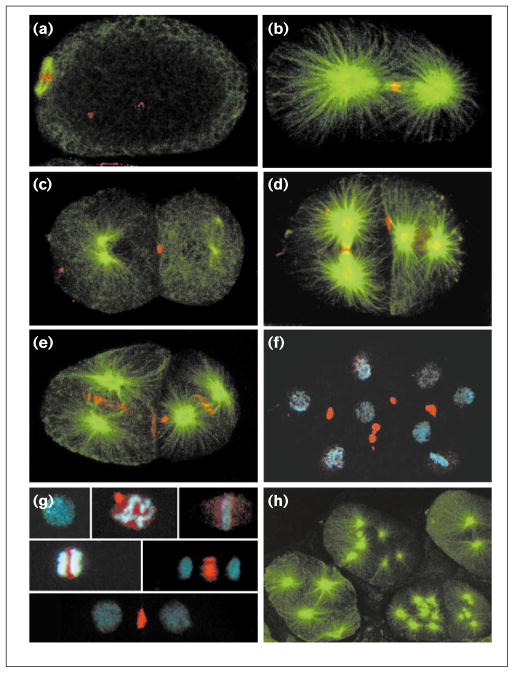
Figure 2.
CeMKLP1 distribution in embryos. Wild-type embryos were fixed and double-stained with rabbit anti-CeMKLP1 antiserum at 1:4000 (red) and either mouse 4A1 anti-tubulin at 1:40 (green; gift of M. Fuller) or mouse PA3 anti-nucleosome at 1:100 (blue; gift of M. Monestier). Projections of multiple confocal optical sections are shown. CeMKLP1 is seen (a) at the equator of the meiotic spindle (left), (b) at the equator of the first mitotic spindle interzone at anaphase, and (c) as a component of the midbody. (d,e) Similar patterns are seen in subsequent cell cycles: (d) the left cell in this two-cell embryo is in late anaphase/telophase and the right cell is in prometaphase; (e) both cells are in early anaphase. (f) CeMKLP1 staining of midbodies persists through many cell cycles. (g) CeMKLP1 localization relative to DNA staining at various stages of the cell cycle. From top left to lower right: interphase, prophase (the bright CeMKLP1 spot is a polar body), metaphase, early anaphase, anaphase, telophase. (h) CeMKLP1 staining was not detectable in CeMKLP1(RNAi) embryos. In all panels except (g,h) anterior is to the left.
Consistent with the findings in other systems, in wild-type embryos CeMKLP1 was seen amongst the chromosomes during prophase and in the interzones and midbodies of meiotic and mitotic spindles (Figure 2). In contrast to MKLP1 localization in other systems [6,7,10–12], however, CeMKLP1 was not concentrated in the nucleus during interphase nor at the spindle poles during mitosis. This result supports the idea that the evolutionarily conserved functions of MKLP1 rely on its localization at the interzone and midbody.
Previous work suggests that signals from the interzone to the cortex, or physical contact of interzone microtubule bundles with the cortex, is required for cleavage-furrow assembly [2,6]. Cleavage furrows clearly assembled in CeMKLP1(RNAi) embryos; to determine whether they were normal, RNAi embryos were fixed and stained for actin and cytoplasmic myosin. RNAi did not prevent the accumulation of these proteins in ring-like structures of normal appearance (Figure 3). Thus, CeMKLP1 seems not to be required for assembly of the actomyosin ring nor for initial inward advance of the cleavage furrow. It clearly is required for the final stages of furrow advance, however, and perhaps also for stabilization of the closed state.
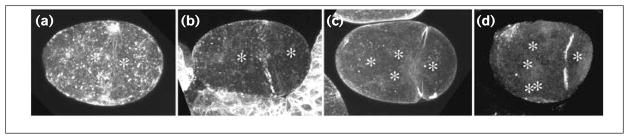
Figure 3.
Distribution of (a–c) actin and (d) myosin in CeMKLP1(RNAi) embryos. CeMKLP1(RNAi) embryos were stained either with C4 mouse anti-actin at 1:500 (gift of J. Lessard) or rabbit anti-NMY-2 myosin at 1:200 (gift of K. Kemphues) and imaged by confocal fluorescence microscopy. DNA was visualized using DAPI and standard epifluorescence. The positions of nuclei or chromosome masses are shown with asterisks. Actin rings are shown at (a) first division anaphase, (b) telophase, and (c) at second division telophase; (d) a cytoplasmic myosin ring at second division telophase.
The concentration of CeMKLP1 in the interzone, the inhibition of furrow advance by CeMKLP1 depletion, and the previous finding that furrow advance in some organisms depends on the presence of interzone microtubule bundles [2] raised the possibility that CeMKLP1 depletion prevents the formation of interzone microtubule bundles. To test this possibility, fixed CeMKLP1(RNAi) embryos were co-stained for microtubules and AIR-2, a kinase that concentrates at the interzone equator and midbody during anaphase/telophase, thereby serving as a marker for the organization of interzone microtubule bundles (J. Schumacher and A. Golden, personal communication). Microtubule and AIR-2 localization appeared normal through the first mitotic cycle until late anaphase; interzone microtubules were present and formed small bundles with AIR-2 associated (Figure 4d). The bundles did not compact to form the dense telophase columns seen in wild-type embryos, however (Figure 4e). It is possible that this poor organization of the interzone causes or contributes to the failure of furrow advance. Strong compaction of the telophase interzone was often seen during the second and subsequent mitotic divisions (for example, see Figure 4f), yet furrow advance always failed. The failure in furrow ingression and closure is not, therefore, due to the absence or complete disorganization of interzone microtubule bundles; it may instead be due to a mild disorganization of interzone bundles or the absence of specific bundle components.
Figure 4.
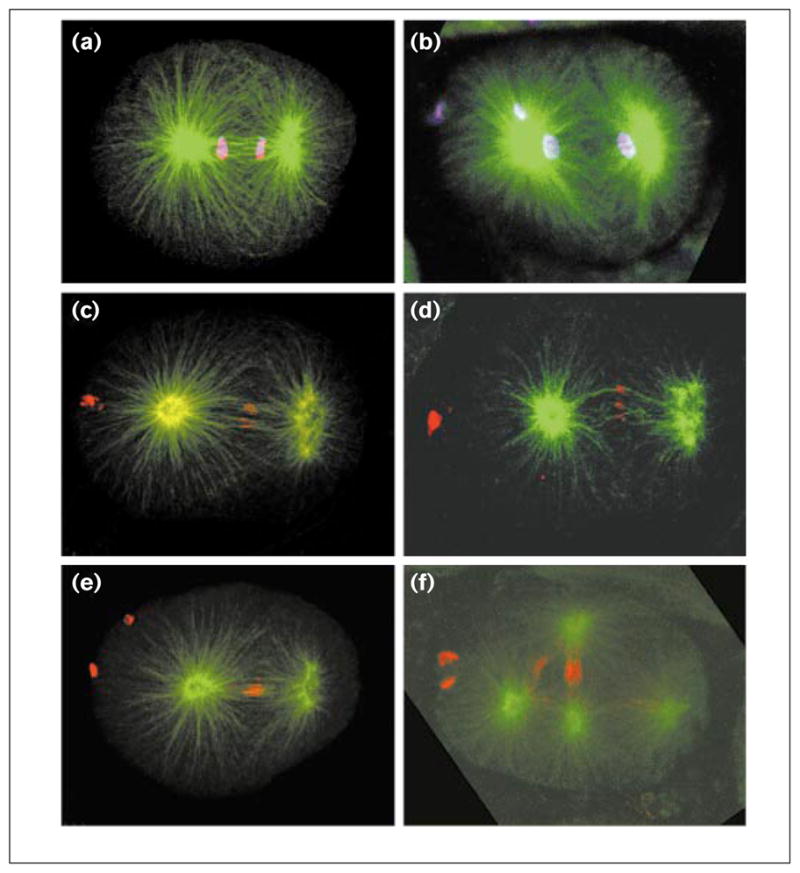
Interzone microtubule bundles in CeMKLP1(RNAi) embryos. Wild-type and RNAi embryos were fixed and double-stained with mouse 4A1 anti-tubulin (green) and either rabbit anti-AIR-2 at 1:200 (red; gift of J. Schumacher) or rabbit anti-acetylated-histone H4 at 1:500 (purple; gift of D. Allis) and imaged by confocal microscopy. Comparison of (a) wild-type anaphase and (b) RNAi telophase embryos shows that CeMKLP1 depletion inhibits the compaction of interzone microtubules during the first division. AIR-2 staining of (c,e) wild-type and (d,f) RNAi embryos reveals, however, that CeMKLP1 depletion does not prevent the formation of bundles of presumably interdigitated microtubules. Further, during the second division (f) and subsequent cell cycles, RNAi embryos often do show substantial compaction of some interzone microtubules. Bright spots of DNA and AIR-2 staining on the left sides of embryos are polar bodies.
A genetic analysis published by Raich et al. [13] (while this paper was under revision) has identified M03D4.1 as zen-4, named for its zygotic enclosure defect during morphogenesis. Their genetic mosaic and RNAi analyses also indicate a late failure of cytokinesis. They suggest that the failure occurs because CeMKLP1 deprivation eliminates the presence of interzone microtubules. Our analysis of tubulin and AIR-2 localization shows that interzone microtubules and microtubule bundles are present after RNAi, however, suggesting that CeMKLP1 function is more directly required for furrow advance. We suggest the following model to explain our results. CeMKLP1 is required for the plus-end-directed, microtubule-based transport of cell components that are essential for persistent furrow advance. As those components move toward the interzone equator in early anaphase, they accumulate in the zone of microtubule plus-end interdigitation, perhaps because of the propensity of MKLP1 to crosslink microtubules of opposite polarity [10]. This accumulation of CeMKLP1 cargoes (and perhaps CeMKLP1 itself) then promotes positive signaling or structural interactions between interzone microtubule bundles and the advancing cleavage furrow; and these interactions are necessary for furrow advance, stable furrow closure, and the formation of a midbody.
Materials and methods
The CeMKLP1 cDNA clones yk35d10 and yk36e3 were obtained from Yuji Kohara. Phagemid DNA was prepared as described in the Stratagene ExAssist protocol. Sense and antisense strands of RNA were synthesized using the MEGAscript in vitro transcription kit (Ambion) and annealed before injection at 0.5 mg/ml [8]. The extent of cleavage furrow closure in one-cell RNAi embryos was assessed from videotapes. The percentage closure was calculated as the sum of furrow advance from each side divided by the embryo’s width; the extent of anaphase B was assessed in one-cell embryos by measuring the distance between the outer edges of the chromosome masses at the point of maximum separation and dividing by embryo length. The duration of anaphase B rate was estimated by measuring the duration of spindle rocking, which is concurrent with spindle elongation in first-division embryos. Antisera to C. elegans MKLP1 were generated against a peptide made by fusing codons 577–775 of M03D4.1 to six histidine codons in the pET28b expression vector (Novagen). A fusion protein of the appropriate size (~30 kDa) was expressed and purified from Escherichia coli using a nickel–agarose column (Novagen). SDS–polyacrylamide gel bands containing the fusion protein were injected into two rabbits by Cocalico Biologicals, Inc.. Both sera were specific to a single band with a relative molecular weight of ~80 kDa on standard western blots of C. elegans embryo homogenates. To visualize protein distribution in embryos, adult hermaphrodites were cut, fixed and stained as described by Strome and Wood [14], except that to visualize actin and cytoplasmic myosin, embryos were fixed and stained using method III of Strome [15], and for actin staining, 0.16μM Texas Red phalloidin (Molecular Probes, Inc.) was included in the fix.
Acknowledgments
This work was supported by grants from NIH (GM46295 to W.S.; GM34059 to S.S.) and ACS (FRA-399 to S.S.). W.S. is an Established Investigator of the American Heart Association (AHA) with funds provided in part by the AHA Indiana Affiliate, Inc.
References
- 1.Cao LG, Wang YL. Signals from the spindle midzone are required for the stimulation of cytokinesis in cultured epithelial cells. Mol Biol Cell. 1996;7:225–232. doi: 10.1091/mbc.7.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wheatley SP, Wang Y. Midzone microtubule bundles are continuously required for cytokinesis in cultured epithelial cells. J Cell Biol. 1996;135:981–989. doi: 10.1083/jcb.135.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams BC, Riedy MF, Williams EV, Gatti M, Goldberg ML. The Drosophila kinesin-like protein KLP3A is a midbody component required for central spindle assembly and initiation of cytokinesis. J Cell Biol. 1995;129:709–723. doi: 10.1083/jcb.129.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright BD, Terasaki M, Scholey JM. Roles of kinesin and kinesin-like proteins in sea urchin embryonic cell division: evaluation using antibody microinjection. J Cell Biol. 1993;123:681–689. doi: 10.1083/jcb.123.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nislow C, Sellitto C, Kuriyama R, McIntosh JR. A monoclonal antibody to a mitotic microtubule-associated protein blocks mitotic progression. J Cell Biol. 1990;111:511–522. doi: 10.1083/jcb.111.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams RR, Tavares AA, Salzberg A, Bellen HJ, Glover DM. pavarotti encodes a kinesin–like protein required to organize the central spindle and contractile ring for cytokinesis. Genes Dev. 1998;12:1483–1494. doi: 10.1101/gad.12.10.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuriyama R, Dragas-Granoic S, Maekawa T, Vassilev A, Khodjakov A, Kobayashi H. Heterogeneity and microtubule interaction of the CHO1 antigen, a mitosis-specific kinesin-like protein. Analysis of subdomains expressed in insect sf9 cells. J Cell Sci. 1994;107:3485–3499. doi: 10.1242/jcs.107.12.3485. [DOI] [PubMed] [Google Scholar]
- 8.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 9.Waterston RH, Sulston JE, Coulson AR. The genome. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C elegans II. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1997. pp. 23–45. [PubMed] [Google Scholar]
- 10.Nislow C, Lombillo VA, Kuriyama R, McIntosh JR. A plus-end-directed motor enzyme that moves antiparallel microtubules in vitro localizes to the interzone of mitotic spindles. Nature. 1992;359:543–547. doi: 10.1038/359543a0. [DOI] [PubMed] [Google Scholar]
- 11.Sellitto C, Kuriyama R. Distribution of a matrix component of the midbody during the cell cycle in Chinese hamster ovary cells. J Cell Biol. 1988;106:431–439. doi: 10.1083/jcb.106.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu W, Sharp DJ, Kuriyama R, Mallik P, Baas PW. Inhibition of a mitotic motor compromises the formation of dendrite-like processes from neuroblastoma cells. J Cell Biol. 1997;136:659–668. doi: 10.1083/jcb.136.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raich WB, Moran AN, Rothman JH, Hardin J. Cytokinesis and midzone microtubule organization in Caenorhabditis elegans require the kinesin-like protein ZEN-4. Mol Biol Cell. 1998;9:2037–2049. doi: 10.1091/mbc.9.8.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strome S, Wood WB. Generation of asymmetry and segregation of germ-line granules in early C. elegans embryos. Cell. 1983;35:15–25. doi: 10.1016/0092-8674(83)90203-9. [DOI] [PubMed] [Google Scholar]
- 15.Strome S. Fluorescence visualization of the distribution of microfilaments in gonads and early embryos of the nematode Caenorhabditis elegans. J Cell Biol. 1986;103:2241–2252. doi: 10.1083/jcb.103.6.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]