Abstract
NELL-1 is an osteoinductive molecule associated with premature calvarial suture closure. Here we identified APR3, a membrane protein known as a proliferation suppressor, as a binding protein of NELL-1 by biopanning. NELL-1 and APR3 colocalized on the nuclear envelope of human osteoblasts. NELL-1 significantly inhibited proliferation of osteoblasts co-transfected with APR3 through further down-regulation of Cyclin D1. The co-expression of NELL-1 and APR3 enhanced Ocn and Bsp expression and mineralization. RNAi of APR3 significantly reduced the differentiation effect of NELL-1. These findings suggest that the effects of NELL-1 on osteoblastic differentiation and proliferation are partly through binding to APR3.
Keywords: NELL-1, APR3, human osteoblast, Phage display, Cyclin D1
1. Introduction
NELL-1 is a novel protein that is overexpressed in the congenital craniofacial deformity craniosynostosis (CS) resulting in calvarial osteoblast differentiation and premature closure of cranial sutures [1]. Various animal models have confirmed NELL-1’s ability to promote osteoblastic differentiation and mineralization in vitro and bone regeneration in vivo [2,3]. In vitro studies have also shown that NELL-1 overexpression promotes osteoblastic differentiation and mineralization, while down regulation of NELL-1 inhibits osteoblastic differentiation [3]. Thus, NELL-1 is believed to be an important factor involved in osteogenesis-associated signaling pathways. It was reported that NELL-1 transiently activates the MAPK signaling cascade and induces Runx2 phosphorylation and also promotes the rapid intracellular accumulation of Tyrosine-phosphorylated proteins [4]. These findings suggest that NELL-1, a secreted protein [5], may bind to a specific membrane receptor(s) which then mediates the activation of intracellular signaling pathways. To identify the potential membrane associated receptor(s) or binding proteins of NELL-1, we screened a human osteoblast cDNA phage display system using NELL-1 as bait and found apoptosis related protein 3 (APR3) as the primary candidate.
APR3 was first cloned from HL60 cells treated with all-trans retinoic acid (ATRA) and was found to induce cell differentiation and apoptosis [6]. Although there have been very few studies on the function of APR3, it was reported that APR3 overexpression causes G1/S phase arrest, which may result from APR3’s dramatic reduction of Cyclin D1 expression [7]. APR3 overexpression was reported to significantly reduce Cyclin D1 promoter activity and decrease endogenous Cyclin D1 expression both at mRNA and protein levels. Structurally, APR3 is potentially a transmembrane protein. It is predicted to contain a signal sequence at the N-terminus, followed by an EGF-like domain, a transmembrane region and an intracellular region at the C-terminus [7–9]. APR3 was demonstrated on the cell surface of MCF-7 (human breast cancer cells) transfected in vitro with APR3 expression plasmid using immunofluorescence staining [7]. In our current study, we show a novel cellular distribution of APR3 on the nuclear envelope/membrane in human osteosarcoma cell lines Saos2 and U2OS, as well as in non-tumor cell lines COS-7 and 293T by confocal microscopy. The physical interaction of NELL-1 and APR3 demonstrated by co-immunoprecipitation and co-localization on the nuclear envelope in Saos2 and U2OS cells definitively confirm APR3 as a direct binding protein of NELL-1. Results of functional analysis on cell proliferation and osteoblast differentiation indicate APR3 binding as a potential mechanism by which NELL-1 promotes osteoblastic differentiation while suppressing cell proliferation.
2. Materials and Methods
2.1. Cell culture
Saos2, U2OS, Cos-7, 293T, HL-60, Raji and M-10B4 cells were cultured as described in Supplementary materials and methods.
2.2. T7 Saos2 cDNA library construction
Total RNA was isolated from Saos2 cells using Trizol reagent (Invitrogen) and mRNA was obtained using Oligotex Direct mRNA Mini Kit (Qiagen). cDNA was synthesized following manufacturer’s protocol from T7Select®10-3 OrientExpress™ cDNA Cloning System (Novagen) using a random primer. The cDNA inserts were ligated into T7Select 10-3b vector arms and packaged into T7 phages (Novagen). BLT5403 Escherichia coli strain was used as the phage library host. The resulting phage library contained 2.1 × 106 independent clones/ml and was amplified once to reach a titer of 1.6 × 1010 pfu/ml.
2.3. Phage library biopanning
An aliquot of the amplified phages was allowed to bind to NELL-1 purified protein and screened for several rounds of biopanning as described in Supplementary materials and methods.
2.4. Plaque PCR amplification and analysis
The phage DNA was then amplified by PCR and analyzed, as described in Supplementary materials and methods.
2.5. Plasmid construction
NELL-1 and APR3 expression plasmids were constructed and confirmed as described in Supplementary materials and methods.
2.6. Co-immunoprecipitation and western blotting
Overexpressed NELL-1 and/or APR3 were co-immunoprecipitated with biotinylated anti-NELL-1 monoclonal antibody and/or mouse anti-FLAG monoclonal antibody M2 (Sigma), and then analyzed by western blot, as described in Supplementary materials and methods.
2.7. Co-localization of NELL-1 and APR3
293T, Saos2 and U2OS cells were transfected with fluorescence tagged NELL-1 and/or APR3 plasmids and visualized by confocal microscope as described in Supplementary materials and methods.
2.8. MTS assay, alkaline phosphatase activity assay and Alizarin red staining
Saos2 and U2OS cells were transfected with Nell1-His and/or Apr3-FLAG or corresponding empty vectors. MTS assay for proliferation and ALP assay for differentiation as well as Alizarin red staining for mineralization were performed as described in Supplementary materials and methods.
2.9. Real-time PCR
Saos2 and U2OS cells were transfected as described and total RNA were isolated. Reverse transcription PCR and real-time PCR were performed, as described in Supplementary materials and methods.
3. Results
3.1 Identification and confirmation of APR3 as a direct binding protein of NELL-1
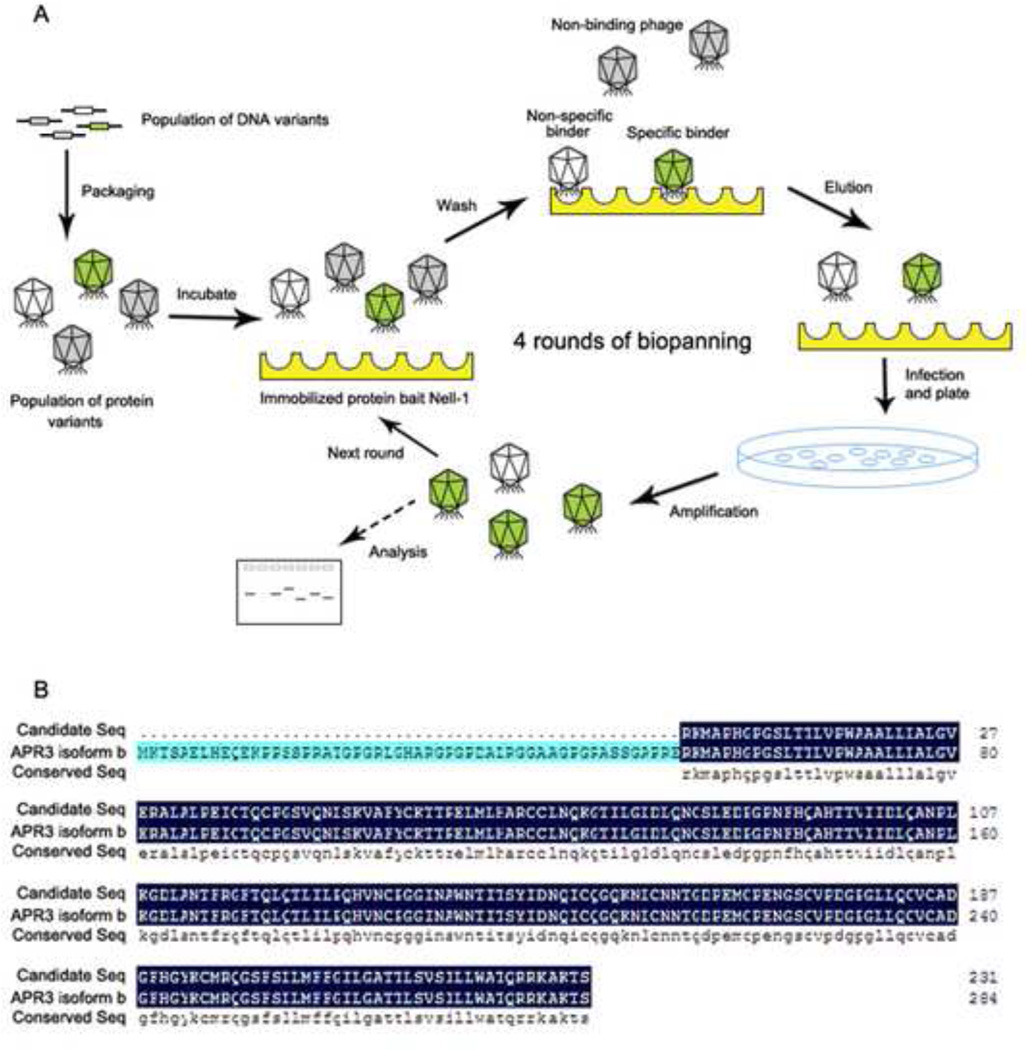
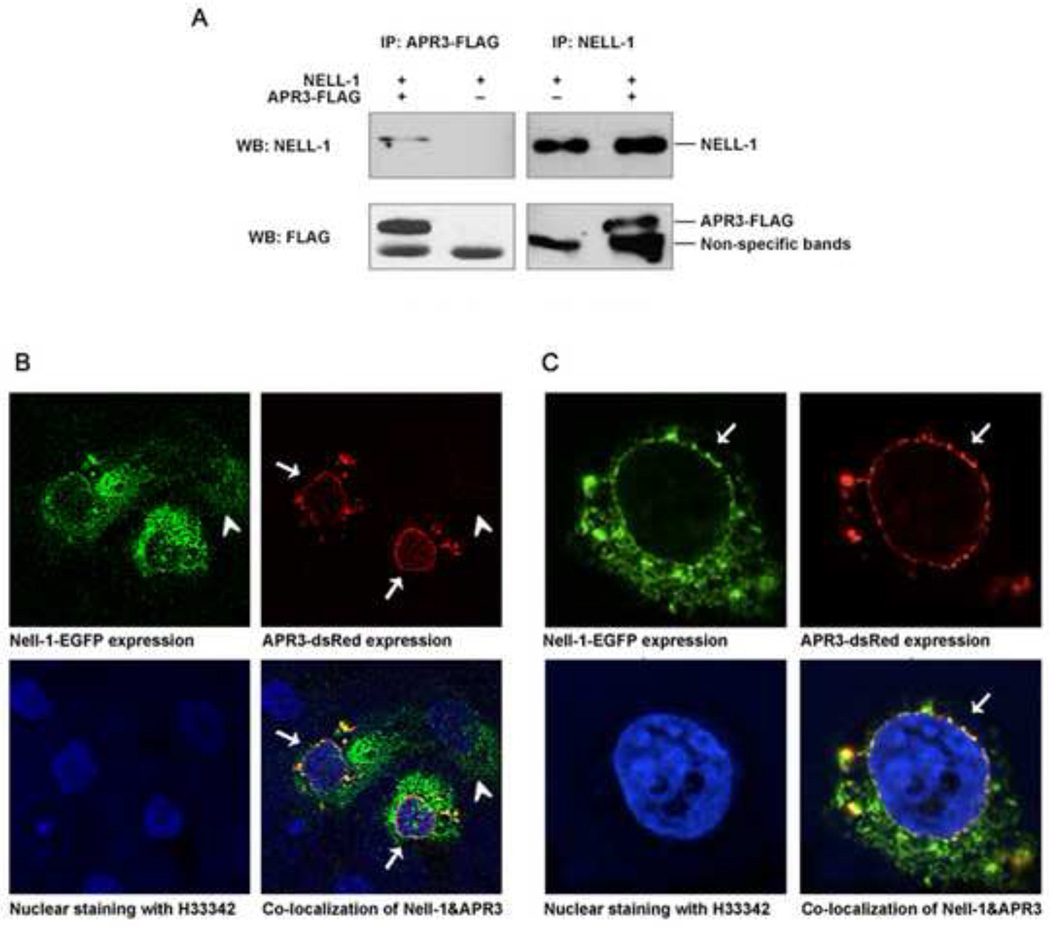
To determine how NELL-1 regulates osteoblastic differentiation and mineralization, we set a phage display system to identify the protein with which NELL-1 interacts (Fig. 1A). We constructed a T7 library that expressed different proteins and polypeptides from cDNA fragments of Saos2 cells, a human osteosarcoma cell line often used as osteoblastic cells. The quality of the library was examined by plaque assay. The titer was 2.1 × 106 pfu/ml originally and 1.6 × 1010 pfu/ml after one time amplification. With purified recombinant human NELL-1 protein (rhNELL-1) mobilized on an ELISA plate as bait. The library was screened, and candidates were chosen and analyzed. More than 100 candidates among all the single plaques after four rounds of biopanning were analyzed. Cellular distributions and functional properties of representative plaques are summarized in Supplemental data (Supplemental Table 1). Based on the hypothesis that NELL-1 initiates its signaling cascade through binding to a specific receptor or receptors on the cell surface, the identification of a transmembrane protein was our primary focus. Cross-referencing the candidates with information from structural molecule annotation, previous literature, and bioinformatics analysis enabled us to identify APR3, a potential transmembrane protein [7], as the candidate that warranted further characterization. The candidate clone of APR3 from our phage library covers amino acid 54 up to the stop codon (Fig. 1B), identical to APR3 isoform B. To further verify the physical interaction between NELL-1 and APR3, co-immunoprecipitation of NELL-1 by anti-APR3-FLAG or APR3 by anti-NELL-1 was performed and confirmed by co-overexpression of both proteins in Cos-7 cells (Fig. 2A). Similar results were obtained from 293T cells (data not shown). However, multiple attempts were unsuccessful in rhNELL-1 pull down experiments with different anti-APR3 antibodies currently available for use in human osteoblastic cells (data not shown). Unexpectedly, we detected the distinct cellular localization of the APR3-dsRed on the nuclear envelope/membrane of human osteoblastic cells with confocal microscope (Fig. 2B and C), which is distinctly different from its cell surface localization described in MCF-7 [7]. The nuclear membrane distribution of APR3 was also observed in Cos-7 and 293T cells (data not shown). Nell-1-EGFP was mainly distributed in the cytoplasm of transfected osteoblastic cells as previously reported [5]. However, the perinuclear condensation of Nell-1-EGFP became significantly prominent when APR3 was co-overexpressed in the same cells (Fig. 2B). The co-localization of NELL-1 and APR3 was clearly displayed predominantly around the nuclear envelope in positive co-transfected cells, which is indicative of formation of an intracellular binding complex by these two proteins (Fig. 2B and C). Furthermore, we studied the endogenous interaction of these two proteins in several relevant cell lines in which strong NELL-1 or APR3 RNA expression has been reported using a co-immunoprecipitation assay or IHC for co-localization. Unfortunately, no definitive data resulted from these experiments primarily because of lack of good cell lines expressing detectable levels of both NELL-1 and APR3 simultaneously, as well as a lack of good quality antibodies for APR3 available for this study. (Supplemental Fig. 2).
Figure 1. Identification of APR3 as a NELL-1 binding protein by phage display.
A. cDNA library was constructed from human osteosarcoma cell line Saos2 and packaged with T7 phage. The library was probed by purified human NELL-1 protein. After 4 rounds of biopanning, most of the non-specific binding phages were washed off; the remaining phages were considered binding candidates.
B. cDNA inserts from candidate plaques were amplified by PCR using specific primers from T7 phage vector. PCR products over 0.5 kb were sequenced and analyzed using bioinformatics method. Among the candidates, APR3 was selected as the primary membrane protein (NCBI blast tools and Human genomic plus transcript database). A candidate sequence was aligned precisely with full-length APR3 isoform b highlighted in dark blue.
Figure 2. Direct binding and intracellular co-localization of NELL-1 and APR3.
A. APR3 and NELL-1 expression plasmids or empty vectors were cotransfected into Cos-7 cells. APR3 protein with FLAG tag and NELL-1 protein were pulled down respectively by anti-FLAG affinity gel and Dynabeads Protein G binding with anti-NELL1 antibody. Western blot was performed after pull down assay. NELL-1 was detected in the APR3 pull down sample, but not in the vector sample. APR3 was detected in the NELL-1 pull down sample, but not in the vector sample.
B-C. APR3 expression plasmid with red fluorescence tag and NELL-1 expression plasmid with green fluorescence tag were cotransfected into Saos2 (B) and U2OS (C) cells. Samples were observed by confocal microscope after 48 hours of expression. NELL-1 was localized in the cytoplasm of single positive cells (arrowheads) while APR3 and NELL-1 were found to be co-localized in the perinuclear region and on the nuclear envelope of double positive cells as indicated by arrows.
3.2 APR3 inhibits osteoblast proliferation enhanced by binding to NELL-1
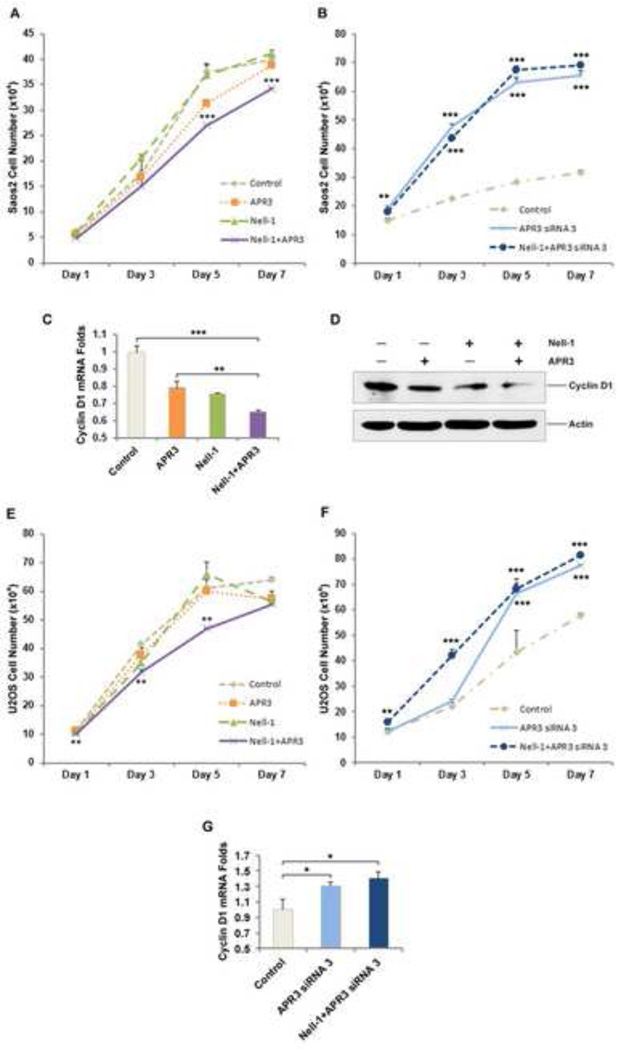
APR3 was reported to have inhibitory effects on proliferation of Hela cells [7]. It is of interest to us to determine whether NELL-1 binding to APR3 affects human osteoblastic cell proliferation. While no significant inhibitory effect was observed with NELL-1 single transfection, we found that transfection of APR3 alone can significantly suppress Saos2 cell proliferation (Fig. 3A) and APR3 siRNA, inversely, can promote Saos2 proliferation (Fig. 3B). Parallel experiments were done using U2OS cells and similar results were obtained (Fig. 3E-F). In addition, the proliferation of Saos2 and U2OS cells were inhibited by co-transfection of NELL-1 and APR3 far more significantly than by APR3 transfection alone (Fig. 3A and E). The alteration of Cyclin D1 mRNA and protein expression was also investigated in Saos2 cells transfected with APR3, NELL-1 or APR3 + NELL-1 using real time PCR and western blot. It was previously demonstrated that the cell cycle arrest mediated by APR3 involves suppression of Cyclin D1 which plays a major role in the control of cell cycle progression [7,10–12]. We detected that the expression levels of Cyclin D1 mRNA and protein were significantly lower in Saos2 cells transfected with either NELL-1 or APR3 in comparison to cells transfected with corresponding empty vectors. Furthermore, this down-regulation of Cyclin D1 was significantly enhanced in Saos2 cells co-transfected with NELL-1 and APR3 and much greater than in either single transfection (Fig. 3C-D). When endogenous APR3 was silenced in U2OS, the mRNA level of Cyclin D1 was found to be increased, and that it increased even more with NELL-1 overexpression and APR3 siRNA co-treatment (Fig. 3G). These data suggest the possibility that NELL-1 and APR3 exert a synergistic inhibitory effect on human osteosarcoma cell proliferation through significant down-regulation of the critical cell cycle molecule Cyclin D1. Collectively, APR3 and NELL-1, through binding to each other, significantly suppress human osteosarcoma Saos2 and U2OS cell proliferation, and instead drive these cells toward osteoblastic differentiation and terminal mineralization.
Figure 3. APR3 inhibition of osteoblastic proliferation was enhanced by co-overexpression of NELL-1.
A. APR3 and NELL-1 expression plasmids or empty vectors were cotransfected into Saos2 cells. MTS assay was performed at indicated time points.
B. APR3 siRNA increased Saos2 cell proliferation.
C. Effects on mRNA level of Cyclin D1 in Saos2 cells were examined by real-time PCR. APR3 and NELL-1 interaction downregulated Cyclin D1 significantly.
D. Effects on Cyclin D1 protein level were tested by western blot 48 hours after APR3 and NELL-1 transfection in Saos2 cells. Cyclin D1 was dramatically downregulated after APR3 and NELL-1 co-transfection.
E. Parallel experiment in U2OS cells. Cell proliferation was decreased when APR3 and NELL-1 were overexpressed.
F. Endogenous APR3 knockdown increased U2OS cell proliferation.
G. The mRNA level of Cyclin D1 in U2OS cells increased under APR3 silencing condition. Samples were prepared in triplicate. (* p < 0.05, ** p < 0.01, *** p < 0.005)
3.3 NELL-1 promotes osteoblastic differentiation and mineralization partially through binding to APR3
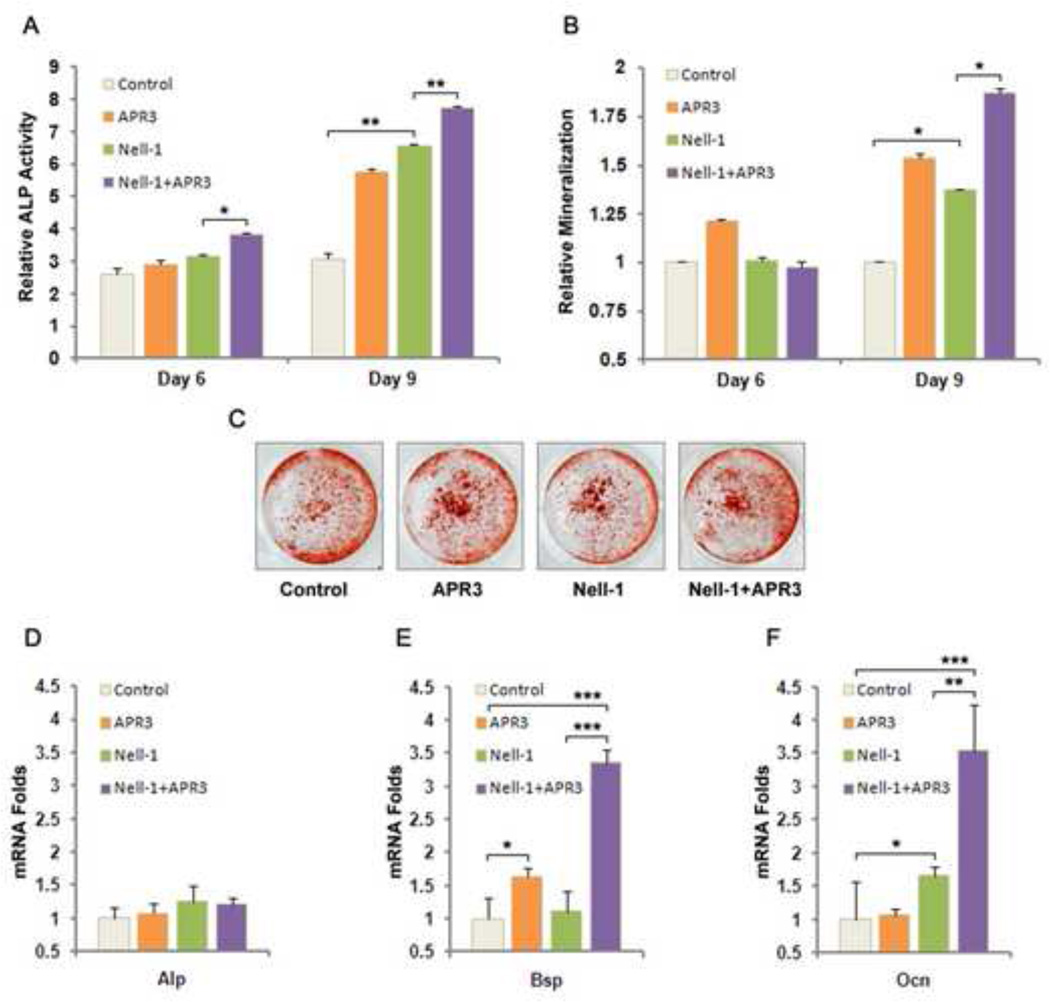
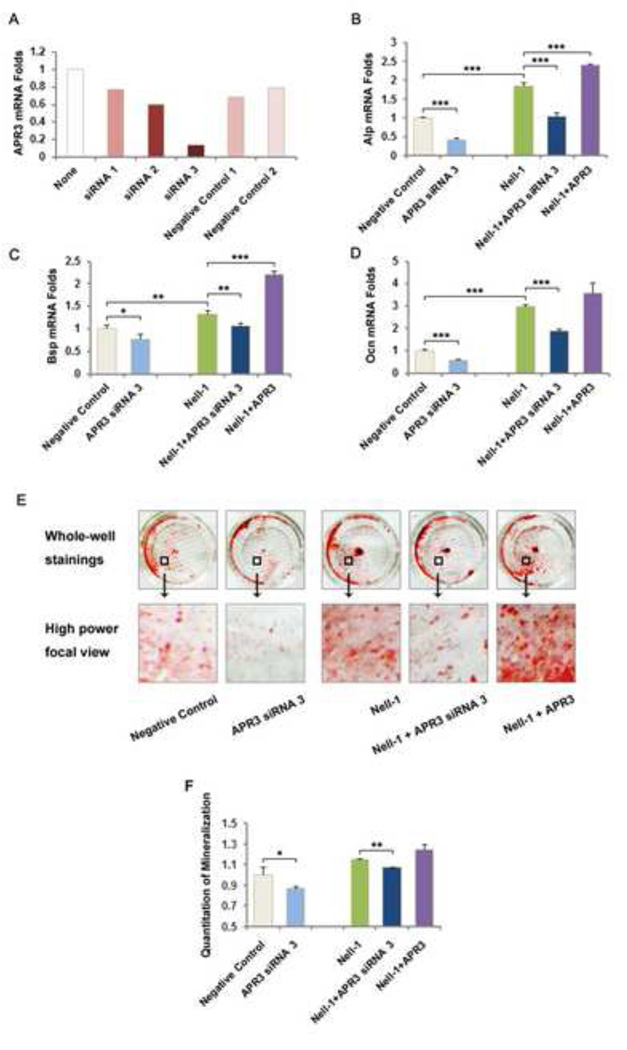
In our previous study, we reported that NELL-1 is capable of inducing osteoblastic differentiation and mineralization of preosteoblasts in vitro [3]. In this study, we were interested in determining whether APR3 is required or significantly critical for NELL-1 to mediate its osteogenic effects on human Saos2 and U2OS cells. Alkaline phosphatase (ALP) assay revealed that NELL-1 and APR3 co-transfection increased ALP activity to the highest level in Saos2 cells at both day 6 and day 9 compared to either NELL-1 or APR3 single transfection (Fig. 4A). At day 9, the most intense mineralization was induced in Saos2 cells co-transfected with NELL-1 and APR3 although the moderate mineralization was also observed in Saos2 cells transfected with either NELL-1 or APR3 alone (Fig. 4B-C). On a molecular level, we detected mRNA of bone marker genes Alp, osteocalcin (Ocn) and bone sialoprotein (Bsp) in Saos2 cells at day 3 post-transfection. Specifically, the expression of Ocn and Bsp was increased significantly in Saos2 cells co-transfected with NELL-1 and APR3, and also much higher than that with NELL-1 single transfection (Fig. 4E-F). To further examine APR3’s role in NELL-1 mediated osteoblastic differentiation, we knocked down the endogenous expression of APR3 by siRNA oligos and investigated bone marker gene expression and mineralization in Saos2 cells transfected with NELL-1. As shown in Fig. 5A, the best knockdown efficacy of over 80% was achieved with APR3 siRNA-3 oligo. In contrast to overexpression, the knockdown of APR3 in Saos2 cells resulted in lower level expression of Alp, Ocn and Bsp with or without NELL-1 transfection (Fig. 5B-D). Furthermore, NELL-1 induced mineralization was partially blocked by knocking down endogenous APR3 (Fig. 5E-F). These data indicate that APR3 is an important co-factor of NELL-1 in promoting osteoblastic differentiation and terminal mineralization of human osteosarcoma Saos2 cells.
Figure 4. NELL-1 induced osteoblast differentiation and mineralization were significantly enhanced by APR3 co-overexpression.
A. APR3 and NELL-1 expression plasmids or empty vectors were co-transfected into Saos2 cells. ALP activities were examined at day 6 and day 9.
B-C. Mineralization was examined by Alizarin red staining at day 6 and day 9 after co-transfection indicating that NELL-1 and APR3 have greater effects when co-overexpressed in Saos2 cells in promoting osteoblast terminal differentiation. Representative Alizarin red staining images indicate mineralization level at day 9.
D-F. Bone marker genes Bsp and Ocn were significantly upregulated in NELL-1 and APR3 co-transfected Saos2 cells while mRNA expression of Alp remained unchanged at day 3 post-transfection.
Samples were prepared in triplicate. (* p < 0.05, ** p < 0.01, *** p < 0.005)
Figure 5. Endogenous APR3 knock-down decreases NELL-1 upregulation of osteoblastic mineralization.
A. APR3 knock down efficiency test in Saos2 cells showed that siRNA3 among three APR3 siRNA oligos provided by Invitrogen was most effective with over 80% endogenous APR3 mRNA knock down 48 hours post-transfection using current experimental conditions.
B-D. Bone marker gene levels were examined 9 days after endogenous APR3 knock down in Saos2 cells. Consistent with mineralization results, mRNA levels of Bsp and Ocn were significantly decreased after APR3 knock down.
E-F. Mineralization of Saos2 was significantly reduced when endogenous APR3 was knocked down by siRNA3 with and without NELL-1 overexpression at day 9. Nonspecific negative control siRNA and pcDNA 3.1-his-myc empty vector were transfected as control.
Samples were prepared in triplicate. (* p < 0.05, ** p < 0.01, *** p < 0.005)
4. Discussion
NELL-1 is a novel osteogenic factor identified from its upregulation in cranial bones of patients with craniosynostosis [1]. Previous studies have demonstrated that NELL-1 is a secreted protein [5] which likely interact with certain cell surface membrane receptor(s) thereby mediating its downstream signaling pathways. The original purpose of our work was to identify the membrane receptor(s) that physically interacts with NELL-1 and mediates NELL-1’s function on human osteosarcoma cell lines Saos2 and U2OS proliferation and osteoblastic differentiation. We constructed a phage library encoding various protein fragments from the Saos2 cell line and screened binding proteins using recombinant human NELL-1 protein as bait by multiple rounds of biopanning. Among the over 100 identified candidates, APR3, previously reported as a transmembrane protein and located on the cell surface of MCF-7 cells [7], was a promising candidate as a “receptor-like” NELL-1 binding protein. Unexpectedly, our results indicated that APR3 was located dispersedly in the cytoplasm, but predominantly concentrated around/on the nuclear envelope in Saos2 and U2OS cells as well as in other non-osteoblastic cell lines including 293T and Cos-7. To make certain that the relatively large size of fluorescent tag in our APR3 construct would not affect its subcellular localization, we also used APR3 with small FLAG tag for transfection and observed a similar localization pattern in those same cell lines (Supplemental Fig. 1A). Interestingly, the cytoplasmic localization of APR3 was also the major distribution pattern in MFC-7 cells transfected with our APR3 constructs although the cell membrane localization was seen in some transfected cells (Supplemental Fig. 1B). This finding suggests that APR3 cellular localization may vary in different cell types, allowing it to regulate different signaling pathways for cell-type specific functions. Nevertheless, the finding of a nuclear envelope/membrane localization of APR3 directed our current study towards exploring an intracellular protein, rather than a cell surface protein, that interacts with NELL-1 by using plasmid co-transfection strategy. The subsequent effects of this interaction on proliferation and osteoblastic differentiation of Saos2 and U2OS cells were also analyzed. It is noteworthy that human NELL-1 has been identified not only as an extracellular signaling molecule [4,13,14], but also as an intracellular protein capable of directly interacting with a specific subtype of protein kinase C (PKC) [5]. It would not be surprising if the existence of a cytoplasmic form of human NELL-1 protein, similar to human NELL-2 [15], is verified experimentally since an alternative promoter utility has been identified on transcription of human NELL-1 (DBTSS: http://dbtss.hgc.jp/). Therefore, the intracellular interaction of NELL-1 with APR3 is unexpected, but not entirely unlikely, and its functional significance worthy of further exploration. The physical interaction between NELL-1 and APR3 was further confirmed by co-immunoprecipitation with Cos-7 cells co-transfected with NELL-1 and APR3-FLAG expression plasmids. However, the critical experiment to demonstrate the interaction between endogenous NELL-1 and APR3 in human Saos2 or U2OS cells was severely hampered due to the lack of specificity of all three anti-APR3 antibodies currently available in the field. Furthermore, the multiple cell lines with detectable RNA levels of either NELL-1 (Raji cells [5]) or APR3 (HL-60 and 293T [7]) were used to maximize the chance of endogenous binding of these two proteins. Unfortunately, these experiments did not result in any definitive data either (Supplemental Fig. 2). Using confocal microscope imaging on Saos2 cells co-transfected with red and green fluorescent-tagged APR3 and NELL-1, the distinct alteration in the subcellular distribution of NELL-1 in NELL-1/APR3 double positive cells in comparison to NELL-1 single positive cells strongly indicated the formation of an intracellular NELL-1/APR3 complex on the nuclear envelope. We speculate that this direct intracellular interaction between NELL-1 and APR3 may play a significant role in regulating human osteoblastic cell differentiation as both molecules have been identified previously as critical components in the regulation of cell proliferation and differentiation [2–4,7,13,16]. Notably, a subset of preliminary data presented in supplemental Fig. 3, the TSPN and EGF-like domains of NELL-1 did not appear to play crucial roles in binding of NELL-1 to APR3 as the binding capacity was not changed significantly when the fragment-deleted NELL-1 constructs were transfected. However, the binding of NELL-1 without TSPN domain showed a relative weak affinity, suggesting that TSPN domain may be more important than the other examined domains for further investigation. More defined deletion of NELL-1 domains of TSPN and EGF will be carefully constructed and investigated in future studies for identifying interactive components of NELL-1 and APR3.
In ATRA treated cells, APR3 was significantly upregulated resulting in G1/S phase cell arrest leading to cell differentiation [7]. It also has been reported that NELL-1 can promote osteoblastic differentiation without promoting cellular proliferation in adenoviral NELL-1 transduced MC3T3 and BMSCs [3]. However, the underlying mechanisms and signal transduction pathways remain unclear. In this study, we focused on the changes on Cyclin D1 at the mRNA and protein levels after NELL-1 and APR3 were introduced into Saos2 and U2OS human osteosarcoma cells since Cyclin D1 has been identified as a critical factor in APR3 mediated suppression of cell cycle in G1/S phase. Interestingly, the significant inhibition of cell proliferation occurred only when APR3 and NELL-1 were co-transfected into target cells. Both APR3 and NELL-1 single transfection could also reduce the expression of Cyclin D1 to a certain extent however, this modest reduction, in contrast to the greater reduction achieved by co-transfection with both APR3 and NELL-1, was incapable of affecting cell proliferation significantly although slight suppression of cell growth was observed in both single transfection at certain time points. MTS assay was done after knock down of endogenous APR3 by siRNA, which further verified APR3’s inhibitory function on osteoblastic proliferation (Fig. 3B and G). These results clearly demonstrate that binding to APR3 is required for NELL-1’s inhibitory role on the proliferation of Saos2 and U2OS human osteosarcoma cells partially through further down-regulation of Cyclin D1 expression.
In addition to their critical roles on cell proliferation, the significance of APR3 binding on NELL-1’s osteogenic effects was further evaluated by in vitro osteoblastic differentiation of Saos2 cells and confirmed with APR3 siRNA knock down strategy. NELL-1 was previously identified as one of the downstream mediators of Runt-related transcription factor 2 (Runx2), which is essential for osteogenesis [16,17]. We found that the mRNA levels of important bone marker genes such as Bsp and Ocn were significantly upregulated by APR3 and NELL-1 respectively, while ALP activity was increased by single transfection of APR3 or NELL-1, and co-transfection of both. Notably, a synergistic increase in both Bsp and Ocn mRNA was observed only when NELL-1 and APR3 were co-overexpressed in Saos2 cells. Moreover, the mineralization of Saos2 cells was induced by either NELL-1 or APR3 alone at moderate levels, but co-transfection induced the highest level of mineralization among all treatment groups. On the other hand, the application of APR3 siRNA significantly inhibited the upregulation of bone marker mRNA expression and mineralization induced by either APR3 alone or co-transfection of APR3 and NELL-1 in Soas2 cells. Collectively, these data indicate for the first time that APR3, a molecule directly regulated by two critical transcriptional factors NFAT and NFκB [18], is capable of inducing critical bone marker genes and terminal osteoblastic differentiation of Saos2 cells in osteoblastic differentiation medium. More importantly, the synergistic effects on promoting osteoblastic differentiation of Saos2 cells by co-transfection of APR3 with NELL-1 further demonstrated the functional significance of NELL-1 binding to APR3 with respect to NELL-1’s osteogenic property.
One of the proposed mechanisms underlying NELL-1’s osteogenic effects involves increasing the phosphorylation of Runx2, which in turn actively regulates osteogenesis [4]. It has been reported that the level of Runx2 can be downregulated by Cyclin D1 through promotion of its degradation [19]. It remains to be seen whether the significant decrease in Cyclin D1 level caused by APR3 binding to NELL-1 results in further stabilization of Runx2 and enhancement of its bioactivity, ultimately resulting in its synergistic effects in promoting Saos2 cell differentiation.
Prospectively, it is also of interest to further investigate whether the nuclear membrane localization of the NELL-1 and APR3 complex could function as the signaling bridge to the nucleus for cytoplasmic retinoic acid (RA), which is the core molecule that acts through binding nuclear receptors, in ATRA signaling pathway [20]. In addition to its pro-osteoblastic property, the critical roles of the direct interaction between APR3 and NELL-1 in inducing tumor cell differentiation and/or apoptosis through ATRA and/or other pathways would not be surprising as both NELL-1 and APR3 are suggested to have such capacities in previous reports [7,13,21].
Highlights.
-
➢
We constructed a cDNA library of Saos2 cells to identify NELL-1 interacting proteins.
-
➢
APR3 was identified as a direct binding protein of NELL-1 through phage display.
-
➢
The inhibitory effect of APR3 on osteoblast proliferation is enhanced by NELL-1.
-
➢
NELL-1 and APR3 binding can promote osteoblast differentiation and mineralization.
Supplementary Material
Acknowledgements
We would like to thank Mariam Razi for editorial help on this manuscript. This work was supported by the NIH/NIDCR (grants R21 ED0177711 and RO1 DE01607), UC Discovery Grant 07-10677 and the Thomas R. Bales Endowed Chair. C.S., K.T and X.Z. are inventors of NELL-1 related patents filed from UCLA.
Abbreviations
- NELL-1
Nel-like protein 1
- APR3
apoptosis related protein 3
- CS
craniosynostosis
- Alp
alkaline phosphatase
- Bsp
bone sialo protein
- Ocn
osteocalcin
- ATRA
all-trans-retinoic acid
- Runx2
runt-related transcription factor 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ting K, et al. Human NELL-1 expressed in unilateral coronal synostosis. J Bone Miner Res. 1999;14:80–89. doi: 10.1359/jbmr.1999.14.1.80. [DOI] [PubMed] [Google Scholar]
- 2.Aghaloo T, et al. A study of the role of nell-1 gene modified goat bone marrow stromal cells in promoting new bone formation. Mol Ther. 2007;15:1872–1880. doi: 10.1038/sj.mt.6300270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang XL, et al. Craniosynostosis in transgenic mice overexpressing Nell-1. Journal of Clinical Investigation. 2002;110:861–870. doi: 10.1172/JCI15375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bokui N, et al. Involvement of MAPK signaling molecules and Runx2 in the NELL1-induced osteoblastic differentiation. FEBS Lett. 2008;582:365–371. doi: 10.1016/j.febslet.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuroda S, et al. Biochemical characterization and expression analysis of neural thrombospondin-1-like proteins NELL1 and NELL2. Biochem Biophys Res Commun. 1999;265:79–86. doi: 10.1006/bbrc.1999.1638. [DOI] [PubMed] [Google Scholar]
- 6.Zhu F, et al. Improved PCR-based subtractive hybridization strategy for cloning differentially expressed genes. Biotechniques. 2000;29:310–313. doi: 10.2144/00292st06. [DOI] [PubMed] [Google Scholar]
- 7.Yu F, et al. Apoptosis related protein 3, an ATRA-upregulated membrane protein arrests the cell cycle at G1/S phase by decreasing the expression of cyclin D1. Biochem Biophys Res Commun. 2007;358:1041–1046. doi: 10.1016/j.bbrc.2007.05.049. [DOI] [PubMed] [Google Scholar]
- 8.Mulder NJ, et al. New developments in the InterPro database. Nucleic Acids Res. 2007;35:D224–D228. doi: 10.1093/nar/gkl841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hulo N, Bairoch A, Bulliard V, Cerutti L, De Castro E, Langendijk-Genevaux PS, Pagni M, Sigrist CJ. The PROSITE database. Nucleic Acids Res. 2006;34:D227–D230. doi: 10.1093/nar/gkj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beumer TL, Roepers-Gajadien HL, Gademan IS, Kal HB, de Rooij DG. Involvement of the D-type cyclins in germ cell proliferation and differentiation in the mouse. Biol Reprod. 2000;63:1893–1898. doi: 10.1095/biolreprod63.6.1893. [DOI] [PubMed] [Google Scholar]
- 11.Pajalunga D, Mazzola A, Salzano AM, Biferi MG, De Luca G, Crescenzi M. Critical requirement for cell cycle inhibitors in sustaining nonproliferative states. J Cell Biol. 2007;176:807–818. doi: 10.1083/jcb.200608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arsenijevic T, Degraef C, Dumont JE, Roger PP, Pirson I. G1/S Cyclins interact with regulatory subunit of PKA via A-kinase anchoring protein, AKAP95. Cell Cycle. 2006;5:1217–1222. doi: 10.4161/cc.5.11.2802. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, et al. Overexpression of Nell-1, a craniosynostosis-associated gene, induces apoptosis in osteoblasts during craniofacial development. J Bone Miner Res. 2003;18:2126–2134. doi: 10.1359/jbmr.2003.18.12.2126. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Zara J, Siu RK, Ting K, Soo C. The role of NELL-1, a growth factor associated with craniosynostosis, in promoting bone regeneration. J Dent Res. 2010;89:865–878. doi: 10.1177/0022034510376401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang EM, et al. Alternative splicing generates a novel non-secretable cytosolic isoform of NELL2. Biochem Biophys Res Commun. 2007;353:805–811. doi: 10.1016/j.bbrc.2006.12.115. [DOI] [PubMed] [Google Scholar]
- 16.Truong T, Zhang X, Pathmanathan D, Soo C, Ting K. Craniosynostosis-associated gene nell-1 is regulated by runx2. J Bone Miner Res. 2007;22:7–18. doi: 10.1359/jbmr.061012. [DOI] [PubMed] [Google Scholar]
- 17.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 18.Yang G, et al. Identification of the distinct promoters for the two transcripts of apoptosis related protein 3 and their transcriptional regulation by NFAT and NFkappaB. Mol Cell Biochem. 2007;302:187–194. doi: 10.1007/s11010-007-9440-7. [DOI] [PubMed] [Google Scholar]
- 19.Zhang M, et al. PTHrP prevents chondrocyte premature hypertrophy by inducing cyclin-D1-dependent Runx2 and Runx3 phosphorylation, ubiquitylation and proteasomal degradation. J Cell Sci. 2009;122:1382–1389. doi: 10.1242/jcs.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levadoux-Martin M, Li Y, Blackburn A, Chabanon H, Hesketh JE. Perinuclear localisation of cellular retinoic acid binding protein I mRNA. Biochem Biophys Res Commun. 2006;340:326–331. doi: 10.1016/j.bbrc.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Jin Z, et al. Hypermethylation of the nel-like 1 gene is a common and early event and is associated with poor prognosis in early-stage esophageal adenocarcinoma. Oncogene. 2007;26:6332–6340. doi: 10.1038/sj.onc.1210461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.