The fruit of the Emblica officinalis plant has been reported to contain a high concentration of polyphenol with low and high molecular weight gallotannins. Polyphenols such as these are found in other plants that are known to have lipid-lowering, insulinomimetic and cardioprotective properties. Therefore, it was hypothesized that E officinalis polyphenols may have similar properties that would be effective in preventing or delaying the development of cardiomyopathy. This study investigated the effects of E officinalis fruit juice in male Wistar diabetic rats with regard to glycemia, lipidemia and cardiac dysfunction.
Keywords: Antidiabetic, Antihyperlipidemic, Emblica officinalis, Left ventricular hypertrophy, Myocardial dysfunction, Oxidative stress
Abstract
Normalization of hyperglycemia, hyperlipidemia and oxidative stress is an important objective in preventing diabetes-induced cardiac dysfunction. The present study investigated the effects of the fruit juice obtained from Emblica officinalis on myocardial dysfunction in diabetic rats. Diabetes was induced by streptozotocin (STZ), and the rats were treated with E officinalis fruit juice for eight weeks. Injection of STZ produced loss of body weight, polydypsia, polyphagia, hyperglycemia, hypoinsulinemia and dyslipidemia. It also produced hypertension, bradycardia, hypertrophy and myocardial functional alterations associated with an increase in serum lactate dehydrogenase and creatinine kinase-MB levels. Treatment with the fruit juice not only prevented STZ-induced loss of body weight, increases in water and food intake, increases in serum glucose levels and disturbed lipid profile, but also an increase in serum lactate dehydrogenase and creatinine kinase-MB levels, and increased myocardial hypertrophy and cardiomyopathy. There was an increase in the area under the curve (AUC) for glucose, and a decrease in AUCinsulin was observed in diabetic rats; treatment decreased AUCglucose but not AUCinsulin or hyperinsulinemia. There was a decrease in antioxidant enzyme levels (in superoxide dismutase, reduced glutathione and catalase) in diabetic hearts, which could be improved by treatment with fruit juice. The present data suggest that fruit juice may be beneficial for the treatment of myocardial damage associated with type 1 diabetes mellitus. The activity of E officinalis fruit juice can be attributed to the concentration of polyphenol present.
Diabetes mellitus increases the risk of cardiomyopathy and heart failure independent of underlying coronary artery disease (1). The pathogenesis of diabetes-induced cardiomyopathy involves metabolic derangements, such as hyperglycemia and hyperlipidemia, that produce glycation of interstitial proteins, such as collagen and, in turn, lead to myocardial stiffness and impaired contractility (2–4). There may be a decrease in insulin sensitivity associated with autonomic dysfunction, hypertension and left ventricular (LV) hypertrophy (5,6). An increase in oxidative stress contributes to the characteristic morphological and functional abnormalities that are also associated with diabetic cardiomyopathy (7). Various therapeutic strategies for the prevention or delay in the development of the above-mentioned complications include effective glycemic control, prevention of hyperlipidemia and oxidative stress.
The fruit of the Emblica officinalis (family: Euphorbiaceae) plant, commonly known as ‘Amla’ or the Indian gooseberry, has been reported to contain constituents with variable biological activity. Phytochemical investigations of the E officinalis fruit show that it has a high concentration of polyphenol with low and high molecular weight gallotannins such as L-malic acid 2-O-gallate; mucic acid 2-O-gallate; corilagin chebulagic acid; putrajivain A; elacocarpusin; mucic acid 1-O-galloyl-β-D-glucose; mucic acid 6-methyl ester 2-O-gallate; mucic acid 1,4-lactone 2-O-gallate; mucic acid 1-methyl ester 2-O-gallate; mucic acid 2-O-gallate; mucic acid 1,4-lactone 6-methyl ester 2-O-gallate; mucic acid 1,4-lactone 3-O-gallate; mucic acid 1,4-lactone 3,5-di-O-gallate; emblicanin A and B; punigluconin; pedunculagin; methyl gallate; corilagin; furosin and geraniin (8,9). Many such polyphenols are known to be present in a number of medicinal plants and are reported to have glucose-lowering (10), insulinomimetic (11), lipid-lowering (12–14), and antioxidant (15,16) and cardioprotective properties (17,18). It is possible that polyphenols present in E officinalis possess antidiabetic, antihyperlipidemic and antioxidant activities, which may be effective in preventing or delaying the development of cardiomyopathy. The present investigation was undertaken to examine whether chronic treatment with E officinalis fruit juice has beneficial effects on glycemia, lipidemia and cardiac dysfunction in the streptozotocin (STZ)-induced type 1 diabetic rat.
METHODS
The fresh fruit of E officinalis were purchased from Gaziabad (India), and authenticated by the Department of Pharmacognosy, LM College of Pharmacy, Gujarat University (India). The juice from the fruit was prepared in the laboratory; standardization of the juice was performed through estimation of the polyphenolic content, as per the method described by Gao et al (19). STZ was purchased from Sigma-Aldrich (USA), and the glucose GOD-POD kit, as well as the triglyceride and total cholesterol kits were purchased from Span Diagnostics Ltd (India). The radioimmunoassay kit for rat insulin was obtained from Bhabha Atomic Research Centre (India). The cholesterol-high density lipoprotein (HDL) kit was purchased from Lab-care Diagnostics Pvt Ltd (India), and the lactate dehydrogenase (LDH) and the CK-MB kits were purchased from Bayer Diagnostics Ltd, India. Other chemicals used were of analytical reagent grade.
Male Wistar rats weighing 200 g to 250 g were obtained from the animal facility of the Zydus Research Centre (India). They were maintained under standard environmental conditions (12 h light/dark cycle at 20°C to 25°C, and controlled humidity) and provided with feed and purified water ad libitum. All experiments and protocols described in the present study were approved by the Institution of Animal Ethics Committee, and are in accordance with guidelines as per the Guide for the Care and Use of Laboratory Animals. Permission was obtained from the Committee for the Purpose of Control and Supervision of Experiments on Animals.
Induction of diabetes and treatment protocol
Diabetes was induced by a single-tail vein injection of STZ (50 mg/kg intravenously) dissolved in normal saline. The control animals were injected with equal volumes of the vehicle. Forty-eight hours after STZ injection, animals showing glycosuria (>2%) were considered to be diabetic. Animals were divided into four groups: normal control, diabetic control, normal animals treated with E officinalis fruit juice (1 mL/kg/orally/day) and diabetic animals treated with fruit juice (1 mL/kg/orally/day). Treatment was started three days after STZ injection, and was given daily for eight weeks. Food intake, water intake and body weight gain were measured. Mean and systolic blood pressures and heart rate were measured noninvasively by tail-cuff method using the Harward blood pressure monitor in all the above-mentioned groups at the end of the eight-week treatment period.
At the end of the eight-week treatment period, the animals were kept on an overnight fast, and the blood samples were collected. The blood was allowed to clot for 30 min at room temperature and then centrifuged at 5000 rpm for 20 min. The serum was separated and stored at −20° C until analysis was complete. Serum samples were analyzed spectrometrically (Shimadzu UV-1601, Japan) for serum glucose, triglyceride, total cholesterol, HDL-cholesterol, LDH and CK-MB using their respective kits. Very low-density lipoprotein (VLDL) and low-density lipoprotein (LDL) were calculated from the total cholesterol, triglyceride and HDL values. Serum insulin was estimated by radioimmunoassay technique using a gamma counter (Riastar, Packard, USA).
Oral glucose tolerance test
The same animals were subjected to an oral glucose tolerance test (OGTT). To perform the OGTT, the animals were orally administered 1.5 g/kg of glucose, and blood samples were collected from the tail vein under light ether anesthesia before (ie, 0 min) and 30 min, 60 min and 120 min after oral glucose administration. Samples were analyzed for glucose and insulin. Plotting glucose concentration versus time results in a curve showing the rise and fall in glucose and insulin levels with time, and is expressed as an integrated area under the curve for glucose and insulin (AUCglucose, AUCinsulin).
Isolated heart experiment
Three to four days following OGTT, the animals were euthanized and the hearts were isolated and mounted as per the Langendorff heart technique. The hearts were perfused with Chenoweth-Koelle buffer (119.8 mmol/L NaCl, 5.6 mmol/L KCl, 2.88 mmol/L CaCl2, 4.5 mmol/L MgCl2, 3.8 mmol/L NaHCO3 and 5 mmol/L glucose), and were continuously infused with 95% O2 and 5% CO2 carbogen. The responses were recorded using a transducer attached to the students’ physiographs. At the end of the study, the hearts were blotted with filter paper to remove excess water and the remaining extraneous tissues were removed. The weight of the hearts were noted to calculate the index of hypertrophy as wet left ventricular weight to body weight (LV/BW) and heart weight to body weight (HW/BW), and were subjected to assessment of antioxidant parameters and estimation of LV collagen and protein content.
Assessment of oxidative stress-related markers in the heart
Heart tissues were finely sliced and homogenized in chilled tris buffer. The homogenates were centrifuged and the clear supernant was used for estimation of various antioxidant parameters such as superoxide dismutase (SOD), catalase, lipid peroxidation or malondialdehyde (MDA), and reduced glutathione (GSH). SOD was determined by the Misra and Fridovich (20) method, catalase was determined by the Aebi (21) method, and GSH was determined according to the method described by Moron et al (22). MDA formation was determined using the Slater and Sawyer (23) method. Antioxidant activity in the liver was expressed in terms of protein content, which was measured as per the method reported by Lowry et al (24).
Statistical analysis
Values were expressed as mean ± SEM. The results were analyzed using one-way factorial ANOVA followed by Tukey’s multiple comparison test. P<0.05 was considered to be statistically significant.
RESULTS
Effect of E officinalis fruit juice on general features and biochemical parameters
STZ administration produced the cardinal signs of diabetes such as loss of body weight and increase in food and water intake compared with control animals. Chronic treatment with E officinalis fruit juice prevented the loss of body weight and elevated food and water intake significantly (P<0.05) in diabetic rats (Table 1). Diabetic rats were found to exhibit significant hyperglycemia and hypoinsulinemia, with hypertriglyceridemia and hypercholesteremia. Treatment with E officinalis fruit juice also produced an increase in serum LDL and VLDL cholesterol levels and a decrease in HDL cholesterol levels in diabetic rats. Additionally, it produced a significant decrease in the elevated serum glucose, triglyceride, total cholesterol, LDL cholesterol and VLDL cholesterol levels of diabetic rats. There was a slight increase in insulin and HDL cholesterol levels at the same dose, but it was not statistically significant (Table 2).
TABLE 1.
Effect of Emblica officinalis fruit juice on the general features of nondiabetic control and diabetic rats
| Parameters | Nondiabetic control | Diabetic control | Nondiabetic treated with fruit juice | Diabetic treated with fruit juice |
|---|---|---|---|---|
| Body weight after treatment, g | 214.8±0.38 | 168.6±2.45 | 200.40±0.63 | 199.80±0.77* |
| Food intake, g/animal/day | 23.16±0.39 | 43.49±0.83 | 22.51±0.46 | 35.66±0.87* |
| Water intake, mL/animal/day | 24.88±0.43 | 59.73±1.35 | 29.30±0.69 | 47.88±1.66* |
Data presented as mean ± SEM.
Significantly different from diabetic control rats (P<0.05)
TABLE 2.
Effect of Emblica officinalis fruit juice on biochemical parameters of nondiabetic control and diabetic rats
| Parameters | Nondiabetic control | Diabetic control | Nondiabetic treated with fruit juice | Diabetic treated with fruit juice |
|---|---|---|---|---|
| Serum glucose, mmol/L | 6.62±0.33 | 25.02±1.23 | 5.50±0.31 | 14.47±0.94* |
| Serum insulin, pmol/L | 318.8±30.0 | 161.8±25.4 | 292.9±28.0 | 202.6±26.0 |
| AUCglucose, mmol/L·min ×103 | 6.75±0.27 | 29.64±1.09 | 6.57±0.24 | 15.26±1.37* |
| AUCinsulin, pmol/L ×103 | 39.57±1.67 | 22.78±1.88 | 40.28±2.92 | 25.90±1.46 |
| Serum triglyceride, mmol/L | 0.91±0.12 | 2.23±0.22 | 0.97±0.10 | 1.33±0.10* |
| Serum cholesterol, mmol/L | 2.24±0.13 | 3.22±0.13 | 2.11±0.13 | 2.62±0.1* |
| HDL cholesterol, mmol/L | 0.98±0.09 | 0.82±0.05 | 0.98±0.08 | 0.98±0.11 |
| LDL cholesterol, mmol/L | 0.98±0.04 | 0.82±0.14 | 0.98±0.11 | 0.98±0.16* |
| VLDL cholesterol, mmol/L | 0.42±0.06 | 1.02±0.10 | 0.45±0.05 | 0.61±0.04* |
Data presented as mean ± SEM.
Significantly different from diabetic control rats (P<0.05). AUC Area under the curve; HDL High-density lipoprotein; LDL Low-density lipoprotein; VLDL Very low-density lipoprotein
OGTT
Administration of glucose produced a significant increase in AUCglucose of the diabetic control group compared with that of the normal control group. Treatment with E officinalis fruit juice produced a significant (P<0.05) decrease in AUCglucose of diabetic rats compared with that of the diabetic controls (Table 2).
Effect on hemodynamic parameters
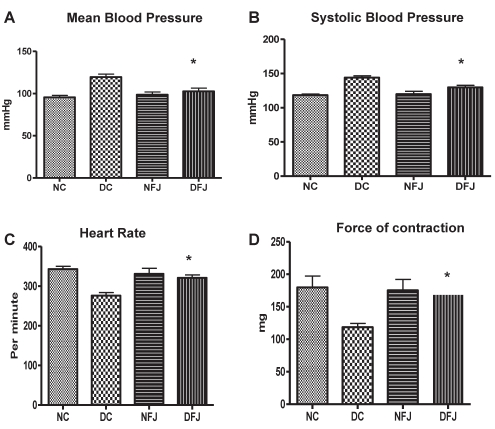
Mean blood pressure was found to be increased, and heart rate and force of contraction were found to be decreased in diabetic rats compared with the control animals. Chronic treatment with E officinalis fruit juice showed a significant (P<0.05) increase in heart rate and force of contraction and a decrease in blood pressure compared with diabetic control animals (Figure 1).
Figure 1).
Effect of Emblica officinalis fruit juice on hemodynamic parameters of nondiabetic control and diabetic rats. Each bar represents the mean ± SEM of six animals. DC Diabetic control; DFJ Diabetic animals treated with E officinalis fruit juice 1 mL/kg orally; NC Nondiabetic control; NFJ Normal animals treated with E officinalis fruit juice 1 mL/kg orally. *Significantly different from diabetic control rats (P<0.05)
Cardiac hypertrophy index
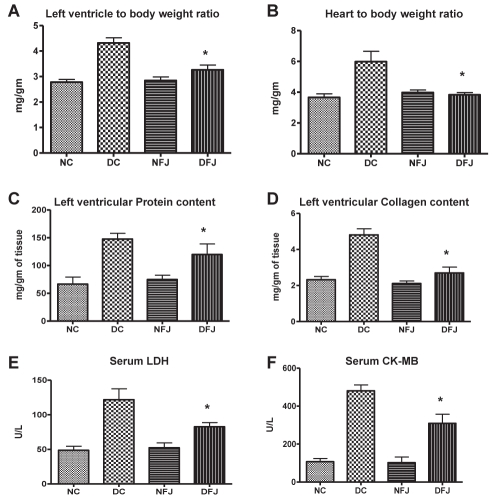
The ratio of wet LV/BW and HW/BW served as an index of cardiac hypertrophy. The LV/BW, HW/BW, LV collagen and protein content were found to be significantly increased in diabetic rats. Treatment with E officinalis fruit juice significantly (P<0.05) decreased the LV/BW ratio, left ventricular collagen and protein content (Figure 2).
Figure 2).
Effect of Emblica officinalis fruit juice on serum lactate dehydrogenase (LDH), creatine kinase-MB, and morphological parameters of nondiabetic control and diabetic rats. Each bar represents the mean ± SEM of six animals. DC Diabetic control; DFJ Diabetic animals treated with E officinalis fruit juice 1 mL/kg orally; NC Nondiabetic control; NFJ Normal animals treated with E officinalis fruit juice 1 mL/kg orally. *Significantly different from diabetic control rats (P<0.05)
Effect on serum LDH and CK-MB
Diabetic rats showed a significant increase in serum CK-MB activity compared with control rats. Administration of E officinalis fruit juice significantly decreased the diabetes-induced increase in serum CK-MB activity in diabetic animals. Serum LDH levels of diabetic animals were also significantly increased compared with control animals. Administration of E officinalis fruit juice to diabetic rats significantly (P<0.05) attenuated LDH serum activity level compared with diabetic control animals (Figure 2).
Effect on antioxidant parameters in the heart
STZ diabetic rats were found to have decreased SOD, GSH and catalase enzyme levels in the heart compared with control rats. Treatment with E officinalis fruit juice produced a significant increase in these enzyme levels. STZ diabetic rats were also found to exhibit a significant increase in MDA levels in the heart compared with control rats. Treatment with E officinalis fruit juice produced significant (P<0.05) decreases in MDA levels (Table 3).
TABLE 3.
Effect of Emblica officinalis fruit juice on antioxidant parameters of nondiabetic control and diabetic rats
| Parameters | Nondiabetic control | Diabetic control | Nondiabetic treated with fruit juice | Diabetic treated with fruit juice |
|---|---|---|---|---|
| SOD, units/min/mg protein | 12.1±1.28 | 4.81±0.34 | 11.61±1.40 | 9.44±0.82* |
| Catalase, units/min/mg protein | 12.29±0.71 | 5.43±0.46 | 11.73±0.99 | 9.24±1.01* |
| MDA, nmol/mg protein | 0.58±0.08 | 1.86±0.26 | 0.63±0.15 | 0.73±0.08* |
| GSH, μg/mg protein | 11.58±0.94 | 4.39±0.40 | 11.44±0.96 | 9.54±0.98* |
Data presented as mean ± SEM.
Significantly different from diabetic control rats (P<0.05). GSH Reduced glutathione; MDA Malondialdehyde; SOD Superoxide dismutase
DISCUSSION
STZ diabetic rats were found to exhibit significant hyperglycemia and hypoinsulinemia with hypertriglyceridemia and hypercholesteremia. Treatment with fruit juice produced significant decreases in elevated serum glucose, triglyceride, total cholesterol, LDL cholesterol and VLDL cholesterol levels in diabetic rats. The results of the present study showed that there was a decrease in the glucose level without a significant increase in insulin output, indicating that treatment with E officinalis fruit juice produced increased sensitivity of peripheral tissue to insulin or direct insulin-like effects. The results of the OGTT further support these findings.
Several studies have suggested that carbohydrate and lipid metabolic abnormalities, such as hyperglycemia and hyperlipidemia, may contribute to the development of cardiac dysfunction in diabetes mellitus (25). A significant reduction in myocardial glucose supply and utilization has been observed in isolated diabetic cardiomyocytes, which could be the primary injury in the pathogenesis of this specific heart muscle disease (26,27). Therefore, it is necessary to increase glucose utilization or the rate of glucose transport in the diabetic heart. Thus, the study suggests that diabetes results in progressive, marked changes in the myocardium that can be prevented by treatment with fruit juice either by increasing glucose utilization or glucose transport in the diabetic heart.
Earlier studies have shown that in STZ diabetic rats, hyperglycemia is associated with hypercholesterolemia and hypertriglyceridemia (26). Abnormalities in lipid metabolism have been demonstrated in cardiomyopathy in which the rate of free fatty acid uptake by myocardium is inversely proportional to the severity of the myocardial dysfunction (28); correction of lipid abnormalities can improve heart function in STZ diabetic rats (29). Thus, the strategy used to produce improvement in cardiac function is to improve on these metabolic derangements. In the present study, it was found that the oral administration of E officinalis fruit juice decreases glucose levels and elevates lipid profiles, which may be a major contributing factor in the improvement of cardiac dysfunction. Polyphenolic compounds are present in a number of medicinal plants that are reported to possess antidiabetic and antihyperlipidemic activity, thus it is possible that polyphenols abundant in E officinalis fruit juice (541.3 mg gallic acid equivalent/1 g extract) might be responsible for glucose-lowering and the lipid-lowering effects of fruit juice, which may be responsible for cardioprotective effects.
Diabetes also produces various hemodynamic changes such as hypertension, decrease in the force of contraction and bradycardia (24,30). Chronic treatment with E officinalis fruit juice showed a significant increase in heart rate and decrease in blood pressure compared with diabetic control animals, which is consistent with previous reports that show that medicinal plants containing polyphenol produce hypertensive effects by decreasing blood pressure and increasing heart rate (31,32).
In the present study, diabetic rats were found to exhibit increased LV weight and HW. Both the ratio of wet LV/BW and HW/BW serve as an index of cardiac hypertrophy. LV dysfunction has also been associated with increased LV/BW and heart weight to body weight (33). In our study, LV/BW and HW/BW were found to be increased in diabetic hearts. This is in agreement with previous reports in which it has been shown that there is an increase in fibrous tissue formation and accumulation of collagen in diabetic rats, causing an increase in the LV weight (34). A correlation with a biopsy study in diabetic patients has also supported these findings in animals (35). Treatment with fruit juice produced an improvement in the wet LV/BW and HW/BW ratio. It also produced decreases in LV collagen and protein content in diabetic rats. Various studies have shown that experimentally induced diabetes causes an increase in collagen formation and accumulation of protein within the interstitium, which in turn, results in anatomical and physiological changes in the myocardium (36). Furthermore, various studies have demonstrated that an increase in LV collagen and protein content may produce cardiac stiffness and fibrosis, resulting in cardiac dysfunction (37,38). Thus, E officinalis fruit juice may provide protection against cardiac stiffness and fibrosis in cardiac dysfunction.
Serum LDH and CK-MB activities were found to be increased in STZ diabetic rats, possibly due to myocardial dysfunction because it has been previously reported that serum LDH and CK-MB activities were found to be increased in cardiomyopathy (39). Serum CK-MB and LDH levels were also reported to increase in diabetic patients, and may serve as a marker for cardiovascular risk and cardiac muscular damage (40). In the present study, there was a significant decrease in serum LDH and CK-MB levels observed with treatment of fruit juice, indicating good cardioprotection.
Increased reactive oxygen species (ROS) production in the diabetic heart is a contributing factor in the development and progression of diabetic cardiomyopathy (41). Increased ROS amplifies hyperglycemia-induced activation of protein kinase C isoforms, increased formation of glucose-derived advanced glycation end products, and glucose flux through the aldose reductase pathways (42,43). All of these factors may contribute to the development of cardiac complications in diabetes mellitus. Increased ROS generation may activate maladaptive signalling pathways, which may lead to cell death and could promote abnormal cardiac remodelling, which ultimately may contribute to the characteristic morphological and functional abnormalities that are associated with diabetic cardiomyopathy. Thus, the levels of antioxidant enzymes, such as GSH, SOD or catalase, are decreased in the diabetic heart. The strategies either to reduce ROS or augment myocardial antioxidant defense mechanisms might have therapeutic efficacy in improving myocardial function in diabetes mellitus. Treatment with fruit juice increased the levels of endogenous antioxidants and decreased lipid peroxidation in diabetic rats. The phenolic content of E officinalis fruit juice is reported to have strong antioxidant properties, which can be beneficial in the prevention of diabetes-induced cardiomyopathy (44,45). In our study, an increase in antioxidant enzyme levels in the heart were observed after treatment with fruit juice in diabetic animals, which can reverse diabetic cardiomyopathy. Thus, diabetics patients who are at an increased risk of cardiac dysfunction may benefit both from an improvement in glucose and lipid homeostasis, as well as from the antioxidant cardioprotective effect of E officinalis fruit juice.
CONCLUSION
E officinalis fruit juice treatment ameliorates hyperglycemia, hyperlipidemia, oxidative stress and the development of cardiac dysfunction associated with STZ diabetes.
Acknowledgments
The authors thank the animal facility at Zydus Research Centre for providing the animals for the present research. This study was supported by the National Facilities in Engineering and Technology with Industrial collaboration (NAFETIC) scheme, All India Collegium for Technical Education, New Delhi.
REFERENCES
- 1.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–23. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 2.Garvey WT, Hardin D, Juhaszova M, et al. Effects of diabetes on myocardial glucose transport system in rats: Implications for diabetic cardiomyopathy. Am J Physiol. 1993;264:H837–44. doi: 10.1152/ajpheart.1993.264.3.H837. [DOI] [PubMed] [Google Scholar]
- 3.Capasso JM, Robinson TF, Anversa P. Alterations in collagen cross-linking impair myocardial contractility in the mouse heart. Circ Res. 1989;65:1657–64. doi: 10.1161/01.res.65.6.1657. [DOI] [PubMed] [Google Scholar]
- 4.Berg TJ, Snorgaard O, Faber J, et al. Serum levels of advanced glycation end products are associated with left ventricular diastolic function in patients with type 1 diabetes. Diabetes Care. 1999;22:186–90. doi: 10.2337/diacare.22.7.1186. [DOI] [PubMed] [Google Scholar]
- 5.Eiro M, Katoh T, Sakuma Y, et al. Insulin resistance highly associates with hypertension in IgA nephropathy. Clin Nephrol. 1999;59:71–8. doi: 10.5414/cnp59071. [DOI] [PubMed] [Google Scholar]
- 6.Hirayama H, Sugano M, Abe N, et al. Troglitazone, an antidiabetic drug, improves left ventricular mass and diastolic function in normotensive diabetic patients. Int J Cardiol. 2001;77:75–9. doi: 10.1016/s0167-5273(00)00411-3. [DOI] [PubMed] [Google Scholar]
- 7.Cai L, Wang Y, Zhou G, et al. Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J Am Coll Cardiol. 2006;48:1688–97. doi: 10.1016/j.jacc.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Zhang YJ, Tanaka T, Yang CR, et al. New phenolic constituents from the fruit juice of Phyllanthus emblica. Chem Pharm Bull. 2001;49:537–40. doi: 10.1248/cpb.49.537. [DOI] [PubMed] [Google Scholar]
- 9.Kumaran A, Karunakaran RJ. Nitric oxide radical scavenging active components from Phyllanthus emblica L. Plant Foods Hum Nutr. 2006;61:1–5. doi: 10.1007/s11130-006-0001-0. [DOI] [PubMed] [Google Scholar]
- 10.Chandrika UG, Wedage WS, Wickramasinghe SMDN, et al. Hypoglycaemic action of the flavonoid fraction of artocarpus heterophyllus leaf. Afr J Tradit Complement Altern Med. 2006;3:42–50. [Google Scholar]
- 11.Yugarani T, Tan BKH, Das NP. Effects of polyphenolic natural products on the lipid profiles of rats fed high fat diets. Lipids. 1992;27:181–6. doi: 10.1007/BF02536175. [DOI] [PubMed] [Google Scholar]
- 12.Yunsheng L, Jaekyung K, Jing L, et al. Natural anti-diabetic compound 1,2,3,4,6-penta-O-galloyl-D-glucopyranose binds to insulin receptor and activates insulin-mediated glucose transport signaling pathway. Biochem Biophys Res Commun. 2005;336:430–7. doi: 10.1016/j.bbrc.2005.08.103. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Jae-kyung K, Yunsheng L, et al. Tannic acid stimulates glucose transport and inhibits adipocyte differentiation in 3T3-L1 cells biochemical and molecular actions of nutrients. J Nutr. 2005;135:165–71. doi: 10.1093/jn/135.2.165. [DOI] [PubMed] [Google Scholar]
- 14.Anila L, Vijayalakshmi NR. Flavonoids from Emblica officinalis and Mangifera indica – effectiveness for dyslipidemia. J Ethnopharmacol. 2002;79:81–7. doi: 10.1016/s0378-8741(01)00361-0. [DOI] [PubMed] [Google Scholar]
- 15.Croft KD. The chemistry and biological effects of flavonoids and phenolic acids. Ann N Y Acad Sci. 1998;854:435–42. doi: 10.1111/j.1749-6632.1998.tb09922.x. [DOI] [PubMed] [Google Scholar]
- 16.Cuvrelier ME, Richard H, Berset C. Comparison of the antioxidant activity of some acid phenols, structure-activity relationship. Biosci Biotechnol Biochem. 1992;56:324–5. [Google Scholar]
- 17.Zenebe W, Pechanova O. Effects of red wine polyphenolic compounds on the cardiovascular system. Bratisl Lek Listy. 2002;103:159–65. [PubMed] [Google Scholar]
- 18.Aneja R, Hake PW, Burroughs TJ, et al. Epigallocatechin, a green tea polyphenol, attenuates myocardial ischemia reperfusion injury in rats. Mol Med. 2004;10:55–62. doi: 10.2119/2004-00032.aneja. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao X, Ohlander M, Jeppsson N, et al. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J Agri Food Chem. 2000;48:1485–90. doi: 10.1021/jf991072g. [DOI] [PubMed] [Google Scholar]
- 20.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 21.Aebi H. Oxidoreductases acting on groups other than CHOH: Catalase. In: Colowick SP, Kaplan NO, Packer L, editors. Methods in Enzymology. London: Academic Press; 1984. pp. 121–5. [Google Scholar]
- 22.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochem Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 23.Slater TF, Sawyer BC. The stimulatory effects of carbon tetrachloride and other halogenoalkanes or peroxidative reactions in rat liver fractions in vitro. J Biochem. 1971;123:805–14. doi: 10.1042/bj1230805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowry OH, Rosenbrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;93:265–75. [PubMed] [Google Scholar]
- 25.Dhalla NS, Pierce GN, Inncs IR, et al. Pathogenesis of cardiac dysfunction in diabetes mellitus. Can J Cardiol. 1985;1:263–81. [PubMed] [Google Scholar]
- 26.Rodrigues B, Goyal RK, McNeill JH. Effects of hydralazine on STZ-induced diabetes rats – prevention of hyperlipidemia and improvement in cardiac function. J Pharmcol Exp Ther. 1986;237:299–307. [PubMed] [Google Scholar]
- 27.Chen V, Ianuzzo CD, Fong BC, et al. The effects of acute and chronic diabetes on myocardial metabolism in rats. Diabetes. 1984;33:1078–84. doi: 10.2337/diab.33.11.1078. [DOI] [PubMed] [Google Scholar]
- 28.Yazaki Y, Isobe M, Takahashi W, et al. Assessment of myocardial fatty acid abnormalities in patients with idiopathic dilated cardiomyopathy using I123 BMIPP SPECT: Correlation with clinicopathological findings and clinical course. Heart. 1999;81:153–9. doi: 10.1136/hrt.81.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuen VG, Orvig C, Thompson KH, et al. Improvement in cardiac dysfunction in streptozotocin-induced diabetic rats following chronic oral administration of bis(maltolato)oxovanadium(IV) Can J Physiol Pharmacol. 1993;71:270–6. doi: 10.1139/y93-042. [DOI] [PubMed] [Google Scholar]
- 30.Zarich SW, Nesto RW. Diabetic cardiomyopathy. Am Heart J. 1989;118:1000–12. doi: 10.1016/0002-8703(89)90236-6. [DOI] [PubMed] [Google Scholar]
- 31.Mohammad GI, Zia-ul-Arifeen S, Dilawer AB, et al. Hypotensive potential of aqueous extract of Emblica officinalis on anaesthetized dogs. Exp Med. 2005;12:213–5. [Google Scholar]
- 32.Hantamalala RR, Myriam D, Ramaroson A. Wine polyphenols induce hypotension, and decrease cardiac reactivity and infarct size in rats: Involvement of nitric oxide. Br J Pharmacol. 2004;142:671–8. doi: 10.1038/sj.bjp.0705833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balakumar P, Manjeet S. The possible role of caspase-3 in pathological and physiological cardiac hypertrophy in rats. Basic Clin Pharmacol Toxicol. 2006;99:418–24. doi: 10.1111/j.1742-7843.2006.pto_569.x. [DOI] [PubMed] [Google Scholar]
- 34.Joffe II, Travers KE, Perreault-Micale CL, et al. Abnormal cardiac function in the streptozotocin-induced non-insulin-dependent diabetic rat: Noninvasive assessment with Doppler echocardiography and contribution of the nitric oxide pathway. J Am Coll Cardiol. 1999;34:2111–9. doi: 10.1016/s0735-1097(99)00436-2. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu M, Umeda K, Sugihara N, et al. Collagen remodelling in myocardia of patients with diabetes. J Clin Pathol. 1993;46:32–6. doi: 10.1136/jcp.46.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poornima IG, Parikh P, Shannon RP. Diabetic cardiomyopathy: The search for a unifying hypothesis. Circ Res. 2006;98:596–605. doi: 10.1161/01.RES.0000207406.94146.c2. [DOI] [PubMed] [Google Scholar]
- 37.Weber KT, Sun Y, Tyagi SC, et al. Collagen network of the myocardium: Function, structure remodeling and regulatory mechanisms. J Mol Cell Cardiol. 1994;26:279–92. doi: 10.1006/jmcc.1994.1036. [DOI] [PubMed] [Google Scholar]
- 38.Nagai R, Low RB, Stirewalt WS, et al. Efficiency and capacity of protein synthesis are increased in pressure overload cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 1988;255:325–8. doi: 10.1152/ajpheart.1988.255.2.H325. [DOI] [PubMed] [Google Scholar]
- 39.Hall RL. Clinical pathology of laboratory animals. In: Gad SC, Chengelis CP, editors. Animal Models in Toxicology. New York: Marcel Dekker Inc; 1991. pp. 765–811. [Google Scholar]
- 40.Huang E, Kuo W, Chen Y, et al. Homocysteine and other biochemical parameters in type 2 diabetes mellitus with different diabetic duration or diabetic retinopathy. Clinica Himica Acta. 2006;366:293–8. doi: 10.1016/j.cca.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 41.Cai L. Suppression of nitrative damage by metallothionein in diabetic heart contributes to the prevention of cardiomyopathy. Free Radic Biol Med. 2006;41:851–61. doi: 10.1016/j.freeradbiomed.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 42.Brownlee M. Advanced protein glycosylation in diabetes and aging. Ann Rev Med. 1995;46:223–34. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- 43.Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47:859–66. doi: 10.2337/diabetes.47.6.859. [DOI] [PubMed] [Google Scholar]
- 44.Ghosal S, Tripathi VK, Chauhan S. Active constituents of Emblica officinalis: Part I. The chemistry and antioxidant activity of two new hydrolysable tannins, emblicanin A and B. Indian J Chem. 1996;35B:941–8. [Google Scholar]
- 45.Bhattacharya A, Ghosal S, Bhattacharya SK. Antioxidant activity of tannoid principles of Emblica officinalis (amla) in chronic stress induced changes in rat brain. Indian J Exp Biol. 2000;38:877–80. [PubMed] [Google Scholar]