Several amino acids are considered to be essential nutrients for maintaining normal cardiovascular function; however, inadequate levels of these amino acids under different pathophysiological conditions are associated with heart dysfunction. Taurine, arginine and carnitine have shown some beneficial effects on the cardiovascular system during diabetes development, but the mechanisms of their actions are still under investigation along with the comparative effectiveness of these amino acid treatments. This study tested the hypothesis that diabetes-induced cardiac dysfunction is prevented by taurine, arginine and carnitine.
Keywords: Amino acids, Cardiac dysfunction, Diabetes, Dietary supplementation, Ultrastructure
Abstract
Although amino acid deficiencies are known to occur in diabetes patients and are considered to contribute to the occurrence of cardiomyopathy, the mechanisms of the impact of the restoration of amino acids on improved cardiac function are not completely understood. Accordingly, the present study was conducted to examine the beneficial effects of dietary supplementation of taurine, arginine and carnitine, individually or in combination, in an experimental model of chronic diabetes. For inducing diabetes, rats received a single injection of streptozotocin (65 mg/kg body weight). Experimental animals were treated (by oral gavage) daily for three weeks with amino acids before the induction of diabetes; this treatment was continued for an additional eight-week period. Diabetes was observed to induce cardiac dysfunction, myocardial cell damage, and changes in plasma glucose and lipid levels. Treatment of diabetic animals with taurine, unlike carnitine or arginine, attenuated alterations in cardiac function, as evidenced by echocardiography and in vivo catheterization techniques. Taurine, carnitine and arginine, individually or in combination, attenuated diabetes-induced cell damage as revealed by electron microscopy. While carnitine alone reduced plasma levels of triglycerides with an increase in high-density lipoprotein cholesterol, none of the amino acids, alone or in combination, had an effect on myocardial glycogen content, lipid accumulation or hyperglycemia. These results suggest that dietary supplementation of taurine attenuates diabetes-induced changes in cardiac contractile function and ultrastructure without any alterations in plasma lipid and glucose levels.
The occurrence of myocardial damage, reactive hypertrophy and cardiac dysfunction in diabetes patients increases the risk of mortality due to heart disease (1–7). High extracellular levels of glucose in diabetes patients disturb the cellular osmoregulation and formation of sorbitol intracellularly, which are suspected to be the key processes in the development of diabetic complications and associated organ dysfunctions. Several amino acids are considered to be essential nutrients for maintaining normal cardiovascular function because inadequate levels of some amino acids under different pathophysiological conditions are associated with heart dysfunction (8,9). Because intra cellular taurine is depleted during the development of diabetes (10), the role of changes in the level of this amino acid has been indicated in diabetes-induced cellular abnormalities. It has been pointed out that taurine is a sulphur-containing amino acid that is most abundant in the cell (11). The biosynthetic capacity of taurine is very low in humans and, thus, diet is its major source in the body (10). Taurine has been demonstrated to exhibit a diverse range of biological actions including protection against ischemia-reperfusion injury, modulation of intracellular calcium concentration, and antioxidant, antiatherogenic and blood pressure-lowering effects (9). Another amino acid, arginine, is a precursor for the synthesis of nitric oxide in virtually all cell types, and is also believed to exert cardiovascular benefits (12). Dietary intake of arginine remains the primary determinant of plasma arginine levels because the rate of arginine biosynthesis does not increase to compensate for its depletion or inadequate supply (13,14). The plasma levels of arginine are reduced in diabetic patients, and have been linked to disturbances in both fasting and postchallenge glucose levels (15). It should also be noted that carnitine deficiency occurs secondary to diabetes (16–18), and exogenous carnitine has been shown to improve cardiac metabolism and function (19,20). Carnitine is mainly synthesized endogenously, and cardiac muscle contains relatively high carnitine concentrations and plays an important regulatory role in energy metabolism (16).
Although some beneficial actions of taurine, arginine and carnitine on the cardiovascular system during the development of diabetes are known, the comparative effectiveness of these amino acid treatments as well as the mechanisms of their actions remain to be investigated. Furthermore, no studies have been performed to evaluate the potential effects of the combination of different amino acids on heart function and cellular integrity during diabetes. It was anticipated that a combination of amino acids would be more effective in attenuating cardiac dysfunction during diabetes than the individual amino acids. Accordingly, the present study was undertaken to test the hypothesis that diabetes-induced cardiac dysfunction is prevented by dietary taurine, arginine and carnitine, either alone or in combination. Furthermore, experiments were conducted to examine whether the beneficial effects of these amino acid treatments on cardiac performance in diabetes were associated with improved cardiac cellular structure as well as blood glucose and lipid profiles. The present study provides novel and direct evidence that amino acids can attenuate cell damage in the diabetic heart.
METHODS
Diabetes model
Diabetes was induced in male Sprague-Dawley rats (225 g to 250 g) with a single tail vein injection of streptozotocin (STZ) (65 mg/kg body weight, dissolved in 0.1 M citrate buffer, pH 4.5) as previously described (21–23). The STZ-rat model of type 1 diabetes is a well-established model that resembles human type 1 diabetes. Age-matched animals received citrate buffer and served as controls. All rats were fed ad libitum. Arginine 200 mg/kg body weight (24,25), taurine 400 mg/kg body weight (26,27) and carnitine 400 mg/kg body weight (19,20), either alone or in combination, were administered daily (by oral gavage) for three weeks before the induction of diabetes and then continued for an additional eight-week period following STZ injection. The selection of dosages for these amino acid treatments was based on the information available in the literature (19,20,24–27). Blood samples from diabetic animals treated with and without amino acids, individually or in combination, were taken at the time of sacrifice (eight weeks post-STZ) and analyzed for serum insulin, glucose, total cholesterol, triglycerides and high-density lipoprotein (HDL) cholesterol concentrations using standard methods by Laboratory Services at St Boniface Hospital, operated by Diagnostic Services of Manitoba (Winnipeg).
Echocardiographic and hemodynamic assessment
An ultrasound imaging system (SONOS 5500 ultrasonograph; Agilent Technologies, USA) was used to measure cardiac output, ejection fraction, heart rate, and systolic and diastolic pressures as previously described (28). The left ventricle wall thickness and internal diameters during systole and diastole were also determined to gain information about cardiac remodelling in type 1 diabetic animals treated with and without amino acids at eight weeks following injection of STZ. At eight weeks post-STZ injection (just before animal sacrifice), left ventricular (LV) function and hemodynamics were measured using the in vivo catheterization technique described elsewhere (21). For this purpose, rats were anesthetized with 5% isoflurane, with an oxygen flow rate of 2 L/min (21). The right carotid artery was then exposed and a micromanometer-tipped catheter (2-0; model SPR-249, Miller Instruments, USA) was inserted, advanced into the left ventricle and secured with a silk ligature around the artery. After a 15 min stabilization of heart function, the heart rate, systolic pressure, diastolic pressure, mean arterial blood pressure, LV systolic pressure (LVSP), LV end diastolic pressure, rate of contraction (+dP/dt) and rate of relaxation (–dP/dt) were recorded as previously described (21). Hemodynamic data were computed and displayed using AcqKnowledge Software version 3.7.1 (MP System “Quick Start”, Biopac System Inc, USA).
Electron microscopy
Tissue samples of right and left ventricles were collected from all animals and fixed in 3% glutaraldehyde in 0.1 M phosphate buffer (pH 7.3) followed by postfixation in 1% osmium tetroxide in 0.1 M phosphate buffer. Samples were dehydrated and embedded in Epon 812 using standard techniques. Thin sections were stained with uranyl acetate and lead citrate, then viewed and photographed in a Philips CM 10 electron microscope (Philips, USA). To eliminate observer bias, tissues were examined using coded grids without foreknowledge of their source.
Statistical analysis
Microcal Origin version 7.5 (Origin Lab Corp, USA) was used for some of the statistical analyses of the data. All values are expressed as mean ± SE. The differences between all groups were evaluated by Student’s t test followed by Tukey’s post hoc multiple comparison tests. A probability of 95% or higher (P<0.05) was considered to be significant. For electron microscopy, specimens were assessed for alterations of cytoplasmic organelles and inclusions. The nonparametric comparison of scores, ranging from 0 to 3, was calculated using the Kruskal-Wallis test. P<0.05 was considered to be significant.
RESULTS
General characteristics of diabetic animals treated with or without amino acids
Table 1 shows that the body weight of the diabetic animals was significantly lower than the controls. Treatment of the diabetic rats with taurine, arginine and carnitine, either alone or in combination, did not change the body weight. Although a significant increase in the heart weight-to-body weight (HW/BW) ratio – an index of cardiac hypertrophy – was seen in the diabetic animals (Table 1), treatment of the diabetic animals with taurine alone partially attenuated the HW/BW ratio. While taurine or arginine had no effects on the elevated levels of blood glucose or lipid profile in diabetes, treatment with carnitine was observed to significantly lower plasma triglyceride levels and elevate plasma HDL cholesterol levels (Table 2). The combination of taurine, arginine and carnitine lowered triglyceride levels without any changes in blood glucose compared with nontreated diabetic animals (Table 2). Although diabetes resulted in a significant reduction in the plasma levels of insulin (control: 65.4±3.4 pM; versus diabetes: 13.7±5.1 pM), treatment of the diabetic rats with amino acids (taurine and carnitine) alone or in combination did not improve these levels (diabetes plus taurine: 17.2±6.8 pM; versus diabetes plus carnitine: 18.9±5.0 pM; versus diabetes plus combination [taurine and carnitine]: 12.0±1.7 pM). The plasma insulin level in the arginine-treated diabetic animals was not determined.
TABLE 1.
General characteristics of diabetic rats treated with taurine, arginine and carnitine alone or in combination
| BW, g | HW, mg | LVW, mg | RVW, mg | HW/BW | |
|---|---|---|---|---|---|
| Control | 595±17 | 1466±42 | 961±12 | 271±21 | 2.47±0.04 |
| Diabetes | 383±28* | 1314±87 | 820±63* | 223±14* | 3.45±0.10* |
| Diabetes + taurine | 399±12* | 1257±37* | 828±22* | 208±11* | 3.15±0.04*† |
| Diabetes + arginine | 386±30* | 1351±110 | 842±96* | 250±14 | 3.52±0.20* |
| Diabetes + carnitine | 371±22* | 1170±35* | 748±29* | 214±11* | 3.20±0.11* |
| Diabetes + combination | 399±21* | 1234±46* | 788±36* | 225±14* | 3.12±0.11* |
Data presented as mean ± SE of six to eight animals for each group. Treated diabetic rats were also pretreated with amino acids for three weeks before streptozotocin injection.
P<0.05 versus control value;
P<0.05 versus nontreated diabetic value. BW Body weight; HW Heart weight; LVW Left ventricular weight; RVW Right ventricular weight
TABLE 2.
Effect of taurine, arginine and carnitine alone or in combination on plasma glucose and lipid profile in diabetic rats
| Glucose, mM | Total cholesterol, mM | Triglycerides, mM | High-density lipoprotein cholesterol, mM | |
|---|---|---|---|---|
| Control | 25.3±1.5 | 2.13±0.12 | 1.60±0.21 | 1.28±0.06 |
| Diabetes | 31.9±1.3* | 2.87±0.24* | 9.18±2.56* | 1.20±0.03 |
| Diabetes + taurine | 34.8±1.2* | 3.34±0.40* | 12.66±2.90* | 1.06±0.07 |
| Diabetes + arginine | 33.3±1.4* | 3.10±0.8* | 12.95±3.10* | 1.29±0.60 |
| Diabetes + carnitine | 36.6±1.9* | 2.95±0.30* | 3.42±0.91*† | 1.72±0.12*† |
| Diabetes + combination | 40.7±1.5* | 2.46±0.39* | 4.11±1.13*† | 1.39±0.16 |
Data presented as mean ± SE of six to eight animals for each group. Treated diabetic rats were also pretreated with amino acids for three weeks before streptozotocin injection.
P<0.05 versus control value;
P<0.05 versus nontreated diabetes value
Echocardiographic and hemodynamic assessment of diabetic animals treated with or without amino acids
The data from echocardiographic assessment of diabetic rats treated with taurine, arginine and carnitine, alone or in combination, are shown in Table 3. The heart rate, fractional shortening and cardiac output were significantly reduced in the diabetic rats. Although treatment of the diabetic animals with arginine or carnitine did not improve these echocardiographic parameters, treatment with taurine normalized fractional shortening; however, a decrease in the LV internal diameter (LViD) during systole was evident (Table 3). The exact reason for the taurine-induced decrease in LViD during systole in diabetic animals is not clear at the present time. No other parameters for LV wall thickness or internal diameter during systole and diastole were altered in any of the treated or nontreated groups (Table 3). Diabetes was associated with a decrease in LVSP and a reduction in +dP/dt and –dp/dt, without any change in LV end diastolic pressure (Table 4). Treatment of the diabetic rats with taurine alone normalized +dP/dt and partially corrected –dP/dt, but LVSP remained depressed (Table 4). There was no hemodynamic improvement of the diabetic heart with arginine and carnitine, alone or in combination with taurine.
TABLE 3.
Echocardiographic assessment of diabetic rats treated with taurine, arginine and carnitine alone or in combination
| Systolic | Diastolic | ||||||
|---|---|---|---|---|---|---|---|
| Heart rate, beats/min | Fractional shortening, % | Cardiac output, L/min | LViD, cm | PWT, cm | LViD, cm | PWT, cm | |
| Control | 388±19 | 55.3±4.7 | 0.270±0.03 | 0.354±0.04 | 0.291±0.05 | 0.643±0.06 | 0.206±0.03 |
| Diabetes | 321±18* | 38.3±4.4* | 0.133±0.02* | 0.350±0.03 | 0.216 ±0.02 | 0.623±0.07 | 0.171±0.04 |
| Diabetes + taurine | 321±20* | 57.1±5.2† | 0.149±0.02* | 0.270±0.03*† | 0.242±0.04 | 0.610±0.04 | 0.140±0.04 |
| Diabetes + arginine | 319±23* | 40.9±4.2* | 0.171±0.03* | 0.398±0.04 | 0.261±0.04 | 0.674±0.05 | 0.167±0.02 |
| Diabetes + carnitine | 303±19* | 37.7±3.1* | 0.177±0.03* | 0.415±0.03 | 0.232±0.02 | 0.702±0.06 | 0.154±0.02 |
| Diabetes + combination | 289±32* | 37.3±3.7* | 0.152±0.02* | 0.421±0.04 | 0.223±0.02 | 0.667±0.05 | 0.144±0.03 |
Data presented as mean ± SE of six to eight animals for each group. Treated diabetic rats were also pretreated with amino acids for three weeks before streptozotocin injection.
P<0.05 versus control value;
P<0.05 versus nontreated diabetic value. LViD Left ventricular internal diameter; PWT Posterior wall thickness
TABLE 4.
Hemodynamic assessment of diabetic rats treated with taurine, arginine and carnitine alone or in combination
| +dP/dt, mmHg/s | –dP/dt, mmHg/s | LVSP, mmHg | LVEDP, mmHg | |
|---|---|---|---|---|
| Control | 6866±248 | 5848±125 | 142±11 | 5.5±0.8 |
| Diabetes | 5182±161* | 3599±286* | 108±8* | 4.8±0.6 |
| Diabetes + taurine | 6367±286† | 4263±143*† | 113±5* | 4.9±0.7 |
| Diabetes + arginine | 5364±230* | 3000±233* | 105±6* | 4.1±0.9 |
| Diabetes + carnitine | 4963±109* | 3192±182* | 103±11* | 5.3±0.8 |
| Diabetes + combination | 5211±187* | 3187±122* | 101±12* | 4.8±0.8 |
Data presented as mean ± SE of six to eight animals for each group. Treated diabetic rats were also pretreated with amino acids for three weeks before streptozotocin injection.
P<0.05 versus control value;
P<0.05 versus nontreated diabetes value. +dP/dt Rate of contraction; –dP/dt Rate of relaxation; LVEDP Left ventricular end diastolic pressure; LVSP Left ventricular systolic pressure
Ultrastructural examination of diabetic hearts
The morphological appearance of hearts from control and experimental animals are depicted in Figures 1 to 4, whereas the qualitative assessment of different changes is summarized in Table 5. Figure 1 shows normal myocardial architecture of control hearts; however, the most notable changes in hearts from diabetic animals were increases in glycogen and lipid droplets compared with controls (Figure 2). There were no differences in glycogen and lipid levels among diabetic and diabetic-treated animals (Table 5). Furthermore, loss of cellular integrity, notable disruption of myofibrils and swelling of sarcotubules was seen in the hearts of diabetic animals (Figure 3) compared with control hearts. In addition, loss of cellular integrity was greater in diabetic animals compared with diabetic-treated animals, but not within the different diabetic-treated groups. Figure 4 is a representative image showing the attenuation in the loss of cell integrity in the taurine-treated diabetic group. Treatment of diabetic animals with arginine and carnitine, either alone or in combination with taurine, was associated with images similar to the one shown in Figure 4. However, it should be noted that treatment with arginine alone showed a lower value for cell damage relative to the other treatment groups, but did not reach statistical significance (Table 5). Although intercalated discs are infrequently encountered in electron microscopy, there was evidence of disruption of the myofibril at the disc (data not shown). No changes in the nucleus or lysosomes in any of the groups were seen, but the mitochondria appeared larger in a cross-section orientation of the muscle compared with a longitudinal orientation.
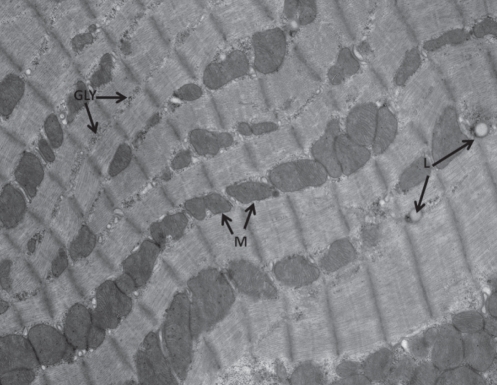
Figure 1).
Ventricular cell from control animal showing mitochondria (M), glycogen (GLY), lipid droplets (L) and cross-banding pattern typical of cardiac muscle. Original magnification ×10,500
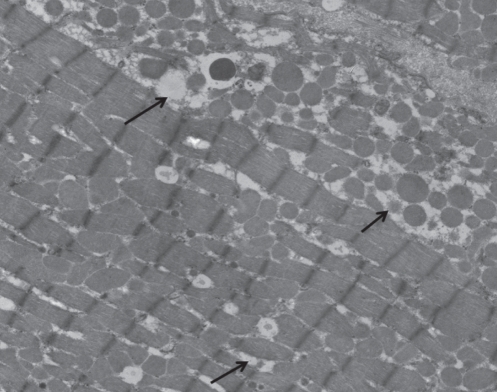
Figure 4).
Ventricular cell from a diabetic animal treated with taurine. Loss of cellular integrity (arrows) is lower than that seen in untreated diabetic animals. Original magnification ×5800. The morphological appearance of the diabetic group treated with arginine or carnitine was similar to that shown for a taurine-treated diabetic heart
TABLE 5.
Qualitative assessment of glycogen, lipid and myocardial cell damage in diabetic rats treated with taurine, arginine and carnitine alone or in combination
| Glycogen | Lipid | Cell damage | |
|---|---|---|---|
| Control | 0.1 | 0.5 | ND |
| Diabetes | 1.6* | 2.2* | 1.9* |
| Diabetes + taurine | 1.4* | 2.1* | 1.4*† |
| Diabetes + arginine | 1.5* | 1.8* | 0.9*† |
| Diabetes + carnitine | 1.2* | 1.9* | 1.2*† |
| Diabetes + combination | 1.6* | 1.9* | 1.1*† |
The qualitative averages are based on three blocks per chamber per animal; three animals per group. A scoring method, ranging from 0 to 3 to denote severity of the changes, was used. The score was derived by grading all cells in each section. Each section contains approximately 150 cells.
P<0.05 versus control value;
P<0.05 versus nontreated diabetes value. ND Not detected
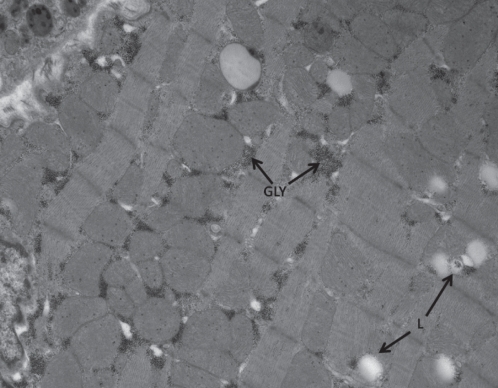
Figure 2).
Ventricular cell from a diabetic animal showing increased glycogen (GLY) and lipid droplets (L). Original magnification ×10,500 (A)
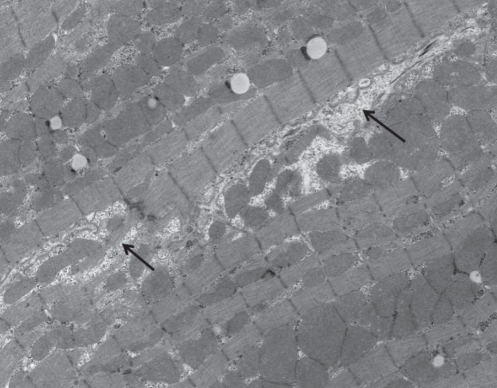
Figure 3).
Ventricular cell from a diabetic animal showing loss of cellular integrity (arrows). Original magnification ×5500
DISCUSSION
Diabetes was found to be associated with depressed cardiac function, increased HW/BW ratio, and elevated plasma glucose and lipid levels. These observations are in agreement with numerous reports in this regard (4–6,19–21). Although the LV weight was reduced in diabetic animals, the depression in cardiac function was not associated with LV remodelling because LV wall thickness or LViD was not altered. Nonetheless, the present study revealed three major findings: taurine supplementation prevented cardiac dysfunction during diabetes; taurine, carnitine and arginine attenuated the diabetes-induced cell damage; and carnitine reduced blood triglyceride levels and increased HDL cholesterol levels. Although taurine, arginine and carnitine, either individually or in combination, attenuated cell injury due to diabetes, only treatment with taurine alone improved cardiac function, as evidenced by echocardiography and the in vivo catheterization technique of the diabetic rat. The elevated taurine would be expected to modulate glycolytic capacity in the diabetic heart (29). However, in our study, taurine alone did not affect the plasma lipid profile and glucose levels, as well as the glycogen content and lipid accumulation in the diabetic heart. Taurine supplementation started after the development of cardiomyopathy was reported to prevent the increase in heart weight, and improve –dP/dt, but not +dP/dt (25). This is in contrast to the present study, because we found that taurine supplementation prevented the impairment of +dP/dt and partially prevented the reduction in –dP/dt. Such a preventive action of taurine in the present study is attributable to the fact that a pretreatment with this amino acid was instituted three weeks before the induction of diabetes with STZ injection. The observed beneficial effect of taurine on diabetic heart function may be due to depression in the HW/BW ratio and LViD during systole, although other parameters of cardiac remodelling, such as LV wall thickness and LViD during diastole, were not affected.
Treatment of the diabetic rats with amino acids, alone or in combination, did not affect blood glucose levels. With respect to taurine, You and Chang (30) demonstrated that taurine reduced triglyceride and low-density lipoprotein levels in the diabetic rat; however, the duration and dose of taurine treatment was an important determinant of the beneficial actions of taurine. Another study reported that supplementation of STZ-induced diabetic mice with taurine reduced serum low-density lipoprotein levels, but did not affect serum glucose levels (31). It should, however, be noted that taurine accumulation in the pancreas has been reported to suppress insulin secretion in STZ-induced diabetic mice (32), indicating that taurine could exert a negative effect on the regulation of the serum level of glucose. In this regard, we observed that taurine treatment did not affect plasma insulin levels in the diabetic rat. On the other hand, γ-amino butyric acid has been reported to restore β-cell mass and reverse diabetes; such effects on β-cell regeneration and regulation of glucose homeostasis may have clinical application for this particular amino acid (33). Although supplementation of taurine in diabetic patients has been reported to improve carbohydrate metabolism (34), another study has reported that the administration of taurine has no effect on glucose metabolism in insulin-dependent diabetes mellitus patients (35). Thus, in view of these conflicting results, the clinical usefulness of taurine with respect to glucose handling is still unclear.
It should be noted that carnitine, unlike taurine and arginine, was able to improve lipid profile and reduce triglyceride levels in the diabetic animals. Oral treatment of diabetic rats with carnitine (200 mg/kg/day) has been reported to exert no effect on plasma lipids or cardiac function (36); however, a high dose (3 g/kg/day) of carnitine (intraperitoneal) was associated with reduced plasma glucose and lipid levels, and improved cardiac performance (18). Although we observed an improved lipid profile with carnitine, cardiac function remained depressed; thus, it is possible that the lack of effect of carnitine on cardiac function is related to dosage and route of administration. These findings suggest that depressed cardiac function in diabetic subjects may be independent of elevated blood lipid and glucose levels in the short term; however, the impact on cardiac function of long-term diabetes remains to be determined. Indeed, our findings suggest that the improved function of the diabetic heart due to taurine is not related to any changes in glucose or lipid profile because carnitine markedly reduced the increases in triglyceride levels and increased HDL cholesterol levels, but did not improve cardiac function. Interestingly, oral carnitine administration (1 g orally three times a day before meals for a period of four weeks) has been reported to exert no effect on insulin sensitivity and lipid profile albeit in subjects with type 2 diabetes (37). Thus, it would appear that there are some inconsistencies with respect to the influence of carnitine on blood glucose and lipid levels; additional studies are required.
While cell damage was expectedly observed in the diabetic heart, all amino acids, either individually or in combination, attenuated cell damage. However, improved function of the diabetic heart was seen with taurine alone, and appears to be independent of the attenuation of the loss of cell integrity. It should be noted that taurine, arginine and carnitine are known to exhibit antioxidant properties, and provide protection against oxidative damage and cell death (38–41). Indeed, treatment of diabetic rats with arginine has been previously reported to reduce diabetes-induced myocardial structural remodelling (42). On the other hand, taurine supplementation has been shown to suppress the reduction in expression of the antiapoptotic protein Bcl-2 in STZ-diabetic rats, which indicates that the antiapoptotic action of taurine may be involved in the protective effect of taurine against diabetes-induced cardiac dysfunction (25).
Because there is an overlap of the beneficial effects of amino acids on cell damage during diabetes, other mechanisms can be proposed to explain the beneficial actions of taurine on diabetic heart function. For example, it is well known that the reduced contractile performance in diabetes is associated with abnormal cardiomyocyte Ca2+ handling (21,22,43–48). Accordingly, it could be suggested that the beneficial effects of taurine on heart function in diabetes are linked to improved cardiomyocyte Ca2+ homeostasis. In this regard, the effect of taurine on intracellular Ca2+ concentration in cardiomyocytes has been well documented (9,49–51). Other studies have shown that Ca2+ is an important factor in mediating the effects of taurine in the heart (52,53). With respect to diabetes, taurine has been reported to increase intracellular Ca2+ concentration and the amplitude of the Ca2+ transient in diabetic cardiomyocytes (49). The Ca2+ paradox is an important phenomenon to study cell injury induced by Ca2+ overload in myocardium, and has been shown to be attenuated by taurine (38). In fact, taurine exerts a cardioprotective effect on Ca2+ paradox in STZ-induced diabetic rat hearts (54). Because the increase of intracellular Na+ is a critical step in cardiac damage due to the Ca2+ paradox (38), taurine supplementation may reduce the intracellular Na+ concentration, and subsequently reduce Ca2+ overload by inhibition of the Na+-Ca2+ exchanger. This effect offers another possible mechanism that explains how taurine may protect the heart from diabetes-induced damage. Although carnitine is known to correct the diabetes-induced depression of sarcoplasmic reticular Ca2+ uptake (55), no improvement of cardiac function was observed; thus, sarcoplasmic reticular Ca2+ uptake alone cannot account for the functional alterations of the diabetic heart. This suggests the possibility of multiple sites of action of taurine with respect to Ca2+ handling and cardiac function, which could explain the higher efficacy of taurine compared with arginine and carnitine. While the exact mechanism of action of taurine in relation to improved cardiac function still remains to be completely defined, the improved function of the diabetic heart in response to taurine may be due to a combination of effects of cell damage and Ca2+ handling.
CONCLUSION
It can be suggested that taurine could serve as an adjunct to standard therapy in patients with diabetes and at risk for heart disease. Furthermore, supplementation of the diet with carnitine, unlike taurine and arginine, may provide a useful nonpharmacological strategy for reduced risk of atherosclerosis and coronary heart disease in diabetic patients. Nonetheless, the data on combination therapy of diabetic animals with taurine, carnitine and arginine did not provide any evidence for the synergetic actions or added beneficial effects of these amino acids on diabetes-induced changes in cardiac structure, metabolism and function.
Acknowledgments
This study was supported by a research grant from CheemoCree Pharma Inc (Winnipeg, Manitoba). Infrastructural support was provided by the St Boniface Hospital Research Foundation.
REFERENCES
- 1.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035–8. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 2.Bell DS. Diabetic cardiomyopathy. Diabetes Care. 2003;26:2949–51. doi: 10.2337/diacare.26.10.2949. [DOI] [PubMed] [Google Scholar]
- 3.Bergner DW, Goldberger JJ. Diabetes mellitus and sudden cardiac death: What are the data? Cardiol J. 2010;17:117–29. [PubMed] [Google Scholar]
- 4.Pierce GN, Beamish RE, Dhalla NS. Heart Dysfunction in Diabetes. CRC Press; Boca Raton: 1988. pp. 1–245. [Google Scholar]
- 5.Regan TJ. Congestive heart failure in the diabetic. Annu Rev Med. 1983;34:161–8. doi: 10.1146/annurev.me.34.020183.001113. [DOI] [PubMed] [Google Scholar]
- 6.Dhalla NS, Pierce GN, Innes IR, Beamish RE. Pathogenesis of cardiac dysfunction in diabetes mellitus. Can J Cardiol. 1985;1:263–81. [PubMed] [Google Scholar]
- 7.Gargiulo P, Jacobellis G, Vaccari V, Andreani D. Diabetic cardiomyopathy: Pathophysiological and clinical aspects. Diab Nutr Metab. 1998;11:336–46. [Google Scholar]
- 8.Kendler BS. Supplemental conditionally essential nutrients in cardiovascular disease therapy. J Cardiovasc Nurs. 2006;21:9–16. doi: 10.1097/00005082-200601000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Xu Y-J, Arneja AS, Tappia PS, Dhalla NS. The potential health benefits of taurine in cardiovascular disease. Exp Clin Cardiol. 2008;13:57–65. [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen SH. The role of taurine in diabetes and the development of diabetic complications. Diabetes Metab Res Rev. 2001;17:330–46. doi: 10.1002/dmrr.229. [DOI] [PubMed] [Google Scholar]
- 11.Bouckenooghe T, Remacle C, Reusens B. Is taurine a functional nutrirent? Curr Opin Clin Nutr Metab Care. 2006;9:728–33. doi: 10.1097/01.mco.0000247469.26414.55. [DOI] [PubMed] [Google Scholar]
- 12.Appleton J. Arginine: Clinical potential of a semi-essential amino acid. Altern Med. 2002;7:512–22. [PubMed] [Google Scholar]
- 13.Castillo L, Chapman TE, Sanchez M, et al. Plasma arginine and citruline kinetics in adults given adequate and arginine-free diets. Proc Natl Acad Sci USA. 1993;90:7749–53. doi: 10.1073/pnas.90.16.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castilo L, Ajami A, Branch S, et al. Plasma arginine kinetics in adult man: Response to an arginine-free diet. Metabolism. 1994;43:114–22. doi: 10.1016/0026-0495(94)90166-x. [DOI] [PubMed] [Google Scholar]
- 15.Menge BA, Schrader H, Ritter PR, et al. Selective amino acid deficiency in patients with impaired glucose tolerance and type 2 diabetes. Regul Pept. 2010;160:75–80. doi: 10.1016/j.regpep.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Kendler BS. Carnitine: An overview of its role in preventive medicine. Prev Med. 1986;15:373–90. doi: 10.1016/0091-7435(86)90005-8. [DOI] [PubMed] [Google Scholar]
- 17.Malone JI, Schocken DD, Morrison AD, Gilbert-Barness E. Diabetic cardiomyopathy and carnitine deficiency. J Diabetes Complications. 1999;13:86–90. doi: 10.1016/s1056-8727(99)00039-2. [DOI] [PubMed] [Google Scholar]
- 18.Mamoulakis D, Galanakis E, Dionyssopoulou E, Evangeliou A, Sbyrakis S. Carnitine deficiency in children and adolescents with type 1 diabetes. J Diabetes Complications. 2004;18:271–4. doi: 10.1016/S1056-8727(03)00091-6. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigues B, Xiang H, McNeill JH. Effect of L-carnitine treatment on lipid metabolism and cardiac performance in chronically diabetic rats. Diabetes. 1988;37:1358–64. doi: 10.2337/diab.37.10.1358. [DOI] [PubMed] [Google Scholar]
- 20.Rodrigues B, Ross JR, Farahbakshian S, McNeill JH. Effects of in vivo and in vitro treatment with L-carnitine on isolated hearts from chronically diabetic rats. Can J Physiol Pharmacol. 1990;68:1085–92. doi: 10.1139/y90-163. [DOI] [PubMed] [Google Scholar]
- 21.Tappia PS, Asemu G, Aroutiounova N, Dhalla NS. Defective sarcolemmal phospholipase C signaling in diabetic cardiomyopathy. Mol Cell Biochem. 2004;261:193–9. doi: 10.1023/b:mcbi.0000028756.31782.46. [DOI] [PubMed] [Google Scholar]
- 22.Clark TA, Maddaford TG, Tappia PS, Heyliger CE, Ganguly PK, Pierce GN. Restoration of cardiomyocyte function in streptozotocin-induced diabetic rats after treatment with vanadate in a tea decoction. Curr Pharm Biotechnol. 2010;11:906–10. doi: 10.2174/138920110793261999. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Suzuki H, Sethi R, Tappia PS, Takeda N, Dhalla NS. Blockade of the renin-angiotensin system attenuates sarcolemma and sarcoplasmic reticulum remodeling in chronic diabetes. Ann N Y Acad Sci. 2006;1084:141–54. doi: 10.1196/annals.1372.003. [DOI] [PubMed] [Google Scholar]
- 24.El-Missiry MA, Othman AI, Amer MA. L-arginine ameliorates oxidative stress in alloxan induced experimental diabetes mellitus. J Appl Toxicol. 2004;24:93–7. doi: 10.1002/jat.952. [DOI] [PubMed] [Google Scholar]
- 25.Khaider A, Marx M, Lubec B, Lubec G. L-arginine reduces heart collagen accumulation in the diabetic db/db mouse. Circulation. 1994;90:479–83. doi: 10.1161/01.cir.90.1.479. [DOI] [PubMed] [Google Scholar]
- 26.Li C, Cao L, Zeng Q, et al. Taurine may prevent diabetic rats from developing cardiomyopathy also by downregulating angiotensin II type 2 receptor expression. Cardiovasc Drugs Therap. 2005;18:105–12. doi: 10.1007/s10557-005-0443-x. [DOI] [PubMed] [Google Scholar]
- 27.Song XD, Chen CZ, Dong B, et al. Study on the intervening mechanism of taurine on streptozotocin-induced diabetic cataracts. Zhonghua Yan Ke Za Zhi. 2003;39:605–9. [PubMed] [Google Scholar]
- 28.Cheema SK, Dent MR, Saini HK, Aroutiounova N, Tappia PS. Prenatal exposure to maternal undernutrition induces adult cardiac dysfunction. Br J Nutr. 2005;93:471–7. doi: 10.1079/bjn20041392. [DOI] [PubMed] [Google Scholar]
- 29.Militante JD, Lombardini JB, Schaffer SW. The role of taurine in the pathogenesis of the cardiomyopathy of insulin-dependent diabetes mellitus. Cardiovasc Res. 2000;46:393–402. doi: 10.1016/s0008-6363(00)00025-0. [DOI] [PubMed] [Google Scholar]
- 30.You J, Chang KJ. Effects of taurine supplementation on lipid peroxidation, blood glucose and blood lipid metabolism in streptozotocin-induced diabetic rats. Adv Exp Med Biol. 1998;442:163–8. doi: 10.1007/978-1-4899-0117-0_21. [DOI] [PubMed] [Google Scholar]
- 31.Kamata K, Sugiura M, Kojima S, Kasuya Y. Restoration of endothelium-dependent relaxation in both hypercholesterolemia and diabetes by chronic taurine. Eur J Pharmacol. 1996;303:47–53. doi: 10.1016/0014-2999(96)00094-5. [DOI] [PubMed] [Google Scholar]
- 32.Tokunaga H, Yoneda Y, Kuriyama K. Streptozotocin-induced elevation of pancreatic taurine content and suppressive effect of taurine on insulin secretion. Eur J Pharmacol. 1983;87:237–43. doi: 10.1016/0014-2999(83)90333-3. [DOI] [PubMed] [Google Scholar]
- 33.Soltani N, Qiu H, Aleksic M, et al. GABA exerts protective and regenerative effects on islet β cells and reverses diabetes. Proc Natl Acad Sci USA. 2011;108:11692–7. doi: 10.1073/pnas.1102715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elizarova EP, Nedosugova LV. First experiments in taurine administration for diabetes mellitus. The effect of erythrocyte membranes. Adv Exp Med Biol. 1996;403:583–8. doi: 10.1007/978-1-4899-0182-8_63. [DOI] [PubMed] [Google Scholar]
- 35.Franconi F, Bennardini F, Mattana A, et al. Plasma and platelet taurine are reduced in subjects with insulin-dependent diabetes mellitus: Effects of taurine supplementation. Am J Clin Nutr. 1995;5:1115–9. doi: 10.1093/ajcn/61.4.1115. [DOI] [PubMed] [Google Scholar]
- 36.Rodrigues B, Seccombe D, McNeill JH. Lack of effect of oral L-carnitine treatment on lipid metabolism and cardiac function in chronically diabetic rats. Can J Physiol Pharmacol. 1990;66:1601–8. doi: 10.1139/y90-244. [DOI] [PubMed] [Google Scholar]
- 37.González-Ortiz M, Hernández-González SO, Hernández-Salazar E, Martínez-Abundis E. Effect of oral L-carnitine administration on insulin sensitivity and lipid profile in type 2 diabetes mellitus patients. Ann Nutr Metab. 2008;52:335–8. doi: 10.1159/000151488. [DOI] [PubMed] [Google Scholar]
- 38.Xu YJ, Saini HK, Zhang M, Elimban V, Dhalla NS. MAPK activation and apoptosis alterations in hearts subjected to calcium paradox are attenuated by taurine. Cardiovasc Res. 2006;72:163–74. doi: 10.1016/j.cardiores.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 39.Ito T, Schaffer SW, Azuma J. The potential usefulness of taurine on diabetes mellitus and its complications. Amino Acids. 2011 Mar 25; doi: 10.1007/s00726-011-0883-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wink DA, Cook JA, Pacelli R, et al. Nitric oxide (NO) protects against cellular damage by reactive oxygen species. Toxicol Lett. 1995:82–83. 221–6. doi: 10.1016/0378-4274(95)03557-5. [DOI] [PubMed] [Google Scholar]
- 41.Rizzo AM, Berselli P, Zava S, et al. Endogenous antioxidants and radical scavengers. Adv Exp Med Biol. 2011;698:52–67. doi: 10.1007/978-1-4419-7347-4_5. [DOI] [PubMed] [Google Scholar]
- 42.Okruhlicova L, Sotnikova R, Stefek M, et al. L-arginine reduces structural remodeling in the diabetic rat myocardium. Methods Find Exp Clin Pharmacol. 2002;24:201–7. doi: 10.1358/mf.2002.24.4.678451. [DOI] [PubMed] [Google Scholar]
- 43.Pierce GN, Kutryk MJB, Dhalla NS. Alterations in Ca2+ binding by and composition of the cardiac sarcolemmal membrane in chronic diabetes. Proc Natl Acad Sci USA. 1983;80:5412–6. doi: 10.1073/pnas.80.17.5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ganguly PK, Pierce GN, Dhalla KS, Dhalla NS. Defective sarcoplasmic reticular calcium transport in diabetic cardiomyopathy. Am J Physiol. 1983;244:E528–35. doi: 10.1152/ajpendo.1983.244.6.E528. [DOI] [PubMed] [Google Scholar]
- 45.Heyliger CE, Prakash A, McNeill JH. Alterations in cardiac sarcolemmal Ca2+ pump activity during diabetes mellitus. Am J Physiol. 1987;252:H540–4. doi: 10.1152/ajpheart.1987.252.3.H540. [DOI] [PubMed] [Google Scholar]
- 46.Penpargkul S, Schaibe T, Yipinstol T, Scheuer J. The effect of diabetes on performance and metabolism of rat hearts. Circ Res. 1980;47:911–21. doi: 10.1161/01.res.47.6.911. [DOI] [PubMed] [Google Scholar]
- 47.Fein FS, Kornstein LB, Strobeck JE, Capasso JM, Sonnenblick EH. Altered myocardial mechanics in diabetic rat. Circ Res. 1980;47:922–33. doi: 10.1161/01.res.47.6.922. [DOI] [PubMed] [Google Scholar]
- 48.Dhalla NS, Liu X, Panagia V, Takeda N. Subcellular remodeling and heart dysfunction in chronic diabetes. Cardiovasc Res. 1998;40:239–47. doi: 10.1016/s0008-6363(98)00186-2. [DOI] [PubMed] [Google Scholar]
- 49.Holloway C, Kotsanas G, Wendt I. Acute effects of taurine on intracellular calcium in normal and diabetic cardiac myocytes. Pflugers Arch. 1999;438:384–91. doi: 10.1007/s004240050925. [DOI] [PubMed] [Google Scholar]
- 50.Yamauchi-Takihara K, Azuma J, Kishimoto S, Onishi S, Sperelakis N. Taurine prevention of calcium paradox-related damage in cardiac muscle. Its regulatory action on intracellular cation contents. Biochem Pharmacol. 1988;37:2651–8. doi: 10.1016/0006-2952(88)90259-6. [DOI] [PubMed] [Google Scholar]
- 51.Franconi F, Stendardi I, Matucci R, et al. Inotropic effect of taurine in guinea-pig ventricular strips. Eur J Pharmacol. 1984;102:511–4. doi: 10.1016/0014-2999(84)90572-7. [DOI] [PubMed] [Google Scholar]
- 52.Satoh H, Nakatani T, Tanaka T, Haga S. Cardiac functions and taurine’s actions at different extracellular calcium concentrations in forced swimming stress-loaded rats. Biol Trace Elem Res. 2002;87:171–82. doi: 10.1385/BTER:87:1-3:171. [DOI] [PubMed] [Google Scholar]
- 53.Franconi F, Martini F, Stendardi I, et al. Effect of taurine on calcium levels and contractility in guinea pig ventricular strips. Biochem Pharmacol. 1982;31:3181–5. doi: 10.1016/0006-2952(82)90547-0. [DOI] [PubMed] [Google Scholar]
- 54.Tatsumi T, Matoba S, Kawahara A, et al. Cardioprotective effect of taurine on calcium paradox in streptozotocin-induced diabetic rat hearts. Adv Exp Med Biol. 1996;403:539–49. doi: 10.1007/978-1-4899-0182-8_58. [DOI] [PubMed] [Google Scholar]
- 55.Tahiliani AG, McNeill JH. Effects of triiodothyronine and carnitine on myocardial dysfunction in diabetic rats. Can J Physiol Pharmacol. 1986;64:669–72. doi: 10.1139/y86-110. [DOI] [PubMed] [Google Scholar]