Abstract
The transmitter phenotype of a neuron has long been thought to be stable for the lifespan. Much as eyes have one color and do not change it over time, neurons have been thought to have one neurotransmitter and retain it for their lifetime. Both principles, exclusivity and stability, are challenged by recent data. More and more neurons in different regions of the brain appear to coexpress two or more neurotransmitters. Moreover, the profile of neurotransmitter expression of a given neuron has been shown to change over time, both during development and in response to changes in activity. The present review summarizes recent studies of this neurotransmitter phenotype plasticity (NPP). Homeostatic mechanisms of plasticity are aimed at maintaining the system within a functional range. They appear to be critical for optimal network operations and have been thought to operate largely by regulating intrinsic excitability, synapse number and synaptic strength. NPP provides a new and unexpected level of regulation of network homeostasis. We propose that it provides the basis for NT coexpression and discuss emerging issues and new questions for further studies in the coming years.
Keywords: Neurotransmitter phenotype, transmitter specification, neuronal activity, homeostasis, transmitter coexpression, plasticity
INTRODUCTION
Hebbian plasticity mechanisms generate persistent changes in synaptic strength in response to repeated stimulation and are thought to be the biological substrate for long-term memory storage. However homeostatic plasticity mechanisms aimed at maintaining the system within a functional range are receiving increased attention, because they appear to be critical for optimal network operations. Like Hebbian mechanisms, they can regulate neurotransmission at different levels - principally through modulation of synaptic strength, synapse number and intrinsic excitability (Turrigiano and Nelson, 2000;Turrigiano and Nelson, 2004;Pozo and Goda, 2010;Grubb and Burrone, 2010;Burrone et al., 2002;Grashow et al., 2009;Marder, 2011).
Another level of regulation of homeostasis has been identified. Unexpectedly, it targets the neurotransmitter phenotype of a neuron, which was once believed to be static. This activity-dependent neurotransmitter phenotype plasticity (NPP) was first identified by Walicke and Patterson in rat superior cervical ganglion neurons (Walicke and Patterson, 1981) and later extensively characterized in the CNS of Xenopus laevis (Root et al., 2008;Dulcis and Spitzer, 2008;Borodinsky et al., 2004;Borodinsky and Spitzer, 2007;Marek et al., 2010;Demarque and Spitzer, 2010).
Interestingly, at the same time, information is becoming available about a complementary phenomenon, the coexpression of neurotransmitters in a given neuron (Seal and Edwards, 2006;Trudeau and Gutierrez, 2007). The neurotransmitter phenotype of neurons has been believed to be limited to only a single neurotransmitter, but more and more neuronal subtypes, in different regions of the brain, coexpress several neurotransmitters (Trudeau and Gutierrez, 2007). First described for neuropeptides coexpressed along with a classical transmitter (Hokfelt et al., 1977;Hokfelt et al., 1980), coexpression has now been demonstrated to exist among classical transmitters, in some cases triggered by contact with targets (Furshpan et al., 1976; Landis, 1990). GABA and glycine have been shown to be coexpressed together in interneurons in the spinal cord (Jonas et al., 1998) and in the medial nucleus of the trapezoid body (Gillespie et al., 2005). A subpopulation of serotonergic and dopaminergic neurons, as well as some motor neurons also express glutamate (Seal and Edwards, 2006).
Here we summarize the available data about NPP and propose it as a mechanism for homeostatic regulation of network activity. We suggest that NPP regulates cotransmission in the mammalian brain and discuss the perspectives for further research in coming years.
NEUROTRANSMITTER PHENOTYPE PLASTICITY IN THE XENOPUS BRAIN
Studies of the Xenopus central nervous system have provided information about the expression of NPP in specific brain areas, the molecular mechanisms that regulate it, the physiological triggers and its functional consequences.
Expression
The expression of four classical transmitters, excitatory glutamate and acetylcholine and inhibitory GABA and glycine, has been studied during the development of the Xenopus laevis spinal cord (Root et al., 2008;Borodinsky et al., 2004). Each neurotransmitter has a broad domain of expression at early stages of development, which is then refined during maturation (Root et al., 2008). This refinement is influenced by spontaneous calcium spike activity that occurs during a critical period of development. The activity-dependent changes in neurotransmitter phenotype expression follow a homeostatic principle. When spike activity is increased, the number of neurons expressing an excitatory transmitter is decreased and the number of neurons expressing an inhibitory transmitter is increased. The opposite is true when activity is decreased, with more neurons expressing excitatory transmitters and fewer neurons expressing inhibitory transmitters (Fig. 1A, H-J). When activity is increased using overexpression of mRNA encoding the rNav1.2 sodium channel, some motor neurons coexpress GABA and glycine along with acetylcholine. GABA and glycine can then be released at the neuromuscular junction and GABAergic and glycinergic mpscs can be recorded from muscle cells. Immunocytochemical and electrophysiological studies support the idea that receptors for different neurotransmitters, like transmitter phenotypes, are initially broadly expressed, and their expression is refined based on the neurotransmitter expressed presynaptically (Borodinsky and Spitzer, 2007).
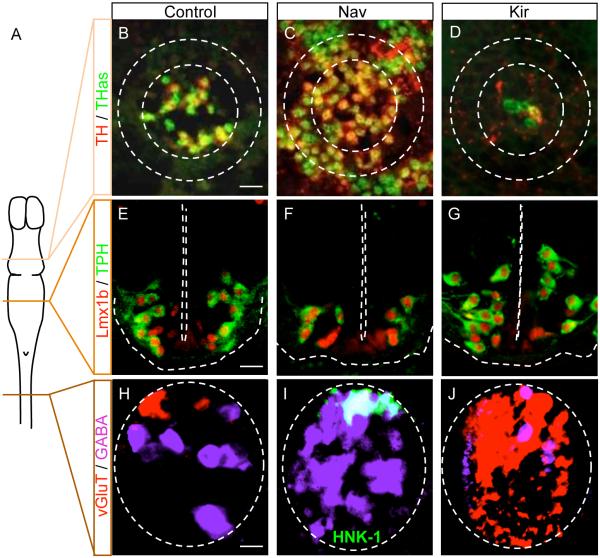
Figure 1. Xenopus neurons, expressing different neurotransmitters in different regions of the CNS, exhibit neurotransmitter phenotype plasticity.
A. Schematic view of the larval Xenopus CNS showing the different levels at which neurotransmitter phenotype plasticity is illustrated in B-J. B-J. Transverse sections immunostained with the antibodies or probes indicated at the left, in control larvae, larvae overexpressing Nav channels and larvae overexpressing Kir channels. Scale bars, 40 μm (B,E) and 20 μm (H). B-D. Expression of TH and TH transcripts (THas, TH antisense) in the midbrain increases with increasing activity and decreases with suppression of activity. From Dulcis and Spitzer, 2008, with permission. E-G. Expression of Lmx1b and TPH in the hindbrain decreases with increasing activity and increases with suppression of activity. From Demarque and Spitzer, 2010, with permission. H-J. Expression of glutamate and GABA in the spinal cord. The domain of expression of vGluT is reduced with increased activity and is dramatically increased with decreased activity while the number of neurons expressing GABA increases with increased activity and decreases with decreased activity. HNK1 is a cell identity marker for Rohon-Beard neurons. This marker is colocalized with vGluT in control or Kir overexpressing larvae (not shown) but colocalized with GABA in Nav-overexpressing embryos. From Borodinsky et al., 2004, with permission.
NPP has also been described for neuromodulators. The number of neurons expressing the monoamines, dopamine or serotonin, increases or decreases in response to different changes in the frequency of spontaneous calcium spikes (Dulcis and Spitzer, 2008; Demarque and Spitzer, 2010) (Fig. 1A-G). Suppression of activity leads to an increase in the number of serotonergic neurons, which generate excitatory inputs to motor neurons. Enhancement of activity leads to an increase in the number of dopaminergic neurons, which generate inhibitory inputs to their targets. Thus alterations of activity lead to responses that appear aimed at restoring a basal level of excitation. Interestingly the newly respecified neurons of a given transmitter phenotype are observed in the vicinity of those originally present, although sometimes in a different nucleus or structure. These observations suggest that plasticity of a given neurotransmitter phenotype involves molecular prerequisites that are not expressed in all neurons. These neurons potentially have a small repertoire of neurotransmitter phenotypes they can express, based on their activity. However, not all the neuronal populations tested change their transmitter in response to changes in activity. For instance, the population of dopaminergic neurons in the posterior tuberculum of Xenopus larvae remains stable despite alterations of calcium spiking (Velazquez-Ulloa et al., 2011).
Molecular mechanisms
These observations suggest that activity interacts with intrinsic factors to delimit domain of potential expression of each transmitter. The specification of a given neurotransmitter phenotype involves the coordinated synthesis of several classes molecules, from enzymes for transmitter anabolism and catabolism to specific vesicular transporters. The genetic components involved in this specification are only partially known but cascades of transcription factors controlling the synthesis of sets of molecules required for different transmitters have been described in rodents and chick (Cheng et al., 2003;Zhao et al., 2006;Scott and Deneris, 2005;Ding et al., 2003;Ang, 2006;Cheng et al., 2005). Most of them are also present in the Xenopus brain, although sometimes with slight differences in period of expression or importance for the phenotype (Alenina et al., 2006). Activity is hypothesized to act on some of them, designated “phenotypic checkpoints” - decision-making crossroads during maturation, but not on others (Ben-Ari and Spitzer, )(Fig. 2A). In the case of serotonin in the raphe, expression of the Lmx1b transcription factor is activity-dependent and is required for the expression of tryptophan hydroxylase. In contrast, expression of the Nkx2.2 transcription factor involved earlier in the cascade is independent of activity (Demarque and Spitzer, 2010)(Fig. 2B).
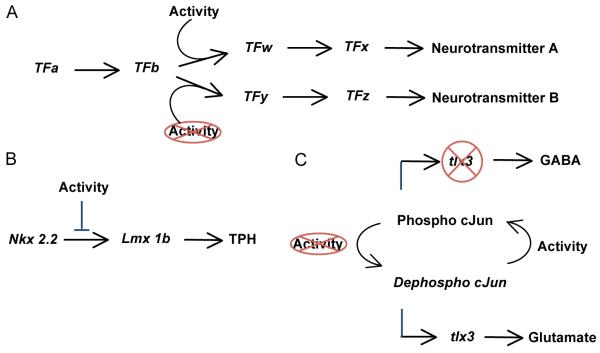
Figure 2. Activity-dependent modulation of transcription factor expression.
A. Cascades of transcription factors (TF) drive the expression of the enzymes and transporters required to specify neurotransmitter phenotype (A, B). Perturbations of activity influence the expression or activity of some (TFw,y) but not all (TFa,b) transcription factors. Activity-dependent transcription factors have been identified as phenotypic checkpoints (Ben-Ari and Spitzer, 2010). B. In the hindbrain, the influence of the frequency of intracellular calcium transients on the cascade of transcription factors leading to the serotonergic phenotype is downstream of Nkx2.2 and upstream of Lmx1b. Activity-dependent expression of Lxm1b regulates the serotonergic phenotype. C. In the spinal cord, the frequency of intracellular calcium transients determines the phosphorylation state of cJun, which in turn regulates the activation of tlx3. The subsequent changes in tlx3 expression promote the choice of fate toward the GABAergic or the glutamatergic phenotype.
We are beginning to understand the way in which electrical activity influences transcription factor activity during NPP. For instance, tlx3, a selector gene that regulates the choice of glutamatergic versus GABAergic phenotype in mouse and chick (Cheng et al., 2005;Cheng et al., 2004), is expressed in Xenopus tropicalis. Ca spikes modulate its expression by changing the phosphorylation state of the transcription factor cJun. The phosphorylation status of cJun regulates its binding to a cAMP response element (TGATGTCA) in the tlx3 promoter that then regulates the expression of tlx3 (Marek et al., 2010) (Fig. 2C). These molecular mechanisms implement the process of activity-dependent homeostatic specification of neurotransmitters.
Triggers
Spontaneous tonic release of GABA and glutamate occurs before synapse formation in the Xenopus spinal cord (Root et al., 2008). Blockade of GABA or glutamate receptors leads to changes in spontaneous Ca spike incidence and frequency, suggesting early volumetric transmission (Fuxe et al., 2010), and hence the specification of neurotransmitters. Whether and how environmental stimuli influence this early release of neurotransmitter remains to be explored, but the paracrine action of these transmitters appears to be part of the mechanism that regulates expression or activity of transcription factors.
An environmental stimulus can trigger NPP at a later stage of development, however. Xenopus larvae escape predation through a chameleon-like camouflage behavior, which allows them to adapt the darkness of their skin to the ambient level of illumination and the light or dark level of the background on which they are positioned. Retinal ganglion cells project to dopaminergic neurons in the ventral suprachiasmatic nucleus (VSC) of the hypothalamus that then innervate melanotrope cells in the hypophysis to control the release of melanocyte stimulating hormone (MSH) into the blood stream. In bright light on a white background, higher firing of retinal ganglion cells increases dopaminergic inhibition of MSH release; melanocytes retract and the skin appears lighter. In the dark or on a dark background dopaminergic inhibition is reduced, more MSH is released, melanocytes expand and the skin appears darker. Ten minutes on a specific background is sufficient to trigger adaptation. Homeostatic plasticity of the dopaminergic phenotype enables the appearance of more dopaminergic neurons when larvae are placed on a light background for a sustained period whereas fewer dopaminergic neurons are present after sustained exposure to a dark background. Ambient light can therefore be considered as a physiological environmental stimulus able to trigger NPP in vivo (Dulcis and Spitzer, 2008).
Functional relevance of neurotransmitter phenotype plasticity
Camouflage behavior provides a good experimental model with which to test the function of newly dopaminergic neurons. Melanocyte retraction can no longer take place in the absence of the dopaminergic neurons in the VSC. Incubating larvae in a solution containing MPTP, a dopaminergic neurotoxin, kills the endogenous population dopaminergic neurons and blocks responses to changes in background illumination. Two hours’ illumination of MPTP-treated larvae induces the specification of dopamine in neighboring neurons and restores the behavior (Dulcis and Spitzer, 2008) (Fig. 3A-C). This result indicates that newly specified dopaminergic neurons are functionally integrated into existing neural circuitry. Indeed the neurons newly acquiring the dopaminergic phenotype share the same target as the endogenous dopaminergic population prior to transmitter respecification, constituting a reserve pool of neurons (Dulcis and Spitzer, 2008;Velazquez-Ulloa et al., 2011; Dulcis and Spitzer, in press).
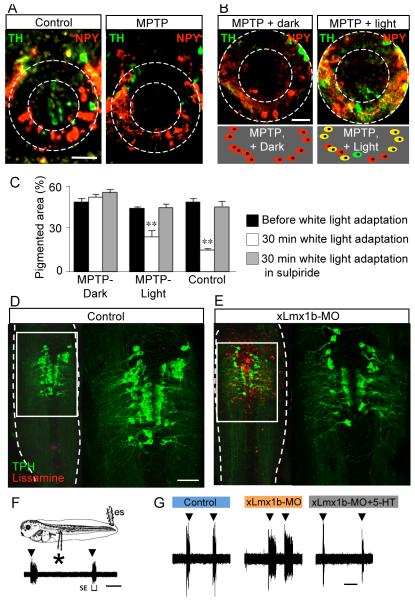
Figure 3. Neurotransmitter phenotype plasticity regulates two survival behaviors in Xenopus larvae.
A. MTPT treatment selectively kills the TH-positive neurons in the core of the ventral suprachiasmatic nucleus. B. Sustained exposure to white light following this treatment induces the expression of TH in NPY-positive neurons. C. Camouflage adaptation is abolished in MPTP-treated embryos but is largely restored after white light adaptation, indicating the role of newly TH-positive neurons. Scale bars, A,B, 50 μm. From Dulcis & Spitzer, 2008, with permission. D,E. Delivery of a lissamine-tagged Lmx1b-specific morpholino by focal electroporation leads to a decrease in the number of 5-HT neurons. F. Electrical stimulation (es, ▾) evokes swimming episodes (SE) recorded as action potentials from ventral roots (*). G. Reduction in number of 5-HT neurons causes an increase in the duration of evoked swimming episodes. Scale bars, D, 80 μm; F,G, 60 s. From Demarque & Spitzer, 2010, with permission.
Xenopus larvae also use swimming behavior to escape predators. Although 3-day old larvae do not yet swim spontaneously, brief dimming of the light can trigger a swimming episode. This reaction is believed to be related to an instinctive fear produced by the shadow of a bird flying over it. For experimental purposes, this behavior can be studied with recordings of fictive swimming. Larvae are anesthetized and the patterns of discharge of axons that would have induced muscle contraction in the unanaesthetized embryos are recorded. Fictive swimming episodes can be triggered by dimming illumination or by brief electrical shocks to the tail. The termination of swimming episodes in X. laevis is largely due to the progressive activation of Ca-sensitive potassium channels, KCa, which hyperpolarize neurons (Dale and Kuenzi, 1997). Because 5-HT increases burst duration and intensity (Sillar et al., 1998), it is expected to induce an increased buildup of intracellular Ca, leading to faster activation of KCa channels and more rapid hyperpolarization of interneurons that causes quicker arrest of swimming episodes. 5-HT reliably induces a decrease in episode duration mediated by activation of 5-HT1A receptors (Wedderburn and Sillar, 1994). Local downregulation of Lmx1b expression by electroporation of a specific translation-blocking morpholino decreases the number of 5HT neurons and animals with fewer 5HT neurons have longer swimming episodes (Fig. 3D-G). The expression of Lmx1b is activity-dependent and a decrease in Ca spike activity induces an increase in the incidence of Lmx1b expression, more 5HT neurons and shorter swimming episodes. Thus NPP regulates two key escape behaviors in Xenopus larvae, influencing their survival (Dulcis and Spitzer, 2008;Demarque and Spitzer, 2010).
NEUROTRANSMITTER PHENOTYPE PLASTICITY IN THE MAMMALIAN BRAIN
Several observations made in Xenopus have correlates in the rodent or chick brain (Gutierrez et al., 2003;Gillespie et al., 2005).
Early nonsynaptic activity
Mature synaptic network activity emerges through stereotyped steps of development, characterized by specific patterns of early spontaneous rhythmic activity in both the hippocampus and neocortex (Allene et al., 2008;Crepel et al., 2007). Activity is sporadic and uncoordinated initially, and the first synchronization is typically detected at the time of birth. Small neuronal ensembles fire together, synchronized by gap junctions. Progressively the first large-scale network activity appears, in the form of giant depolarizing potentials (GDPs) that are synaptic events involving GABAergic and glutamatergic synapses. Similar early spontaneous synchronized activity has been described in the retina and spinal cord (Hanson and Landmesser, 2003;Scain et al., 2010;McLaughlin et al., 2003). At earlier stages of development, migrating hippocampal neurons express GABA and glutamate receptors that are tonically activated by ambient transmitters. This early paracrine communication plays a role in the migration of pyramidal neurons (Demarque et al., 2002;Manent et al., 2005). Although the intracellular calcium transients in these systems have different durations and frequencies than in Xenopus (Blankenship and Feller, 2010), these forms of calcium entry precede synaptic input-regulated activity and may contribute to the normal refinement of transmitter phenotype as they do in Xenopus. Further studies are required to address this issue.
Initial broad expression of neurotransmitters
Developmentally transient expression of neurotransmitters has been reported at several locations in the mammalian brain. Young mesencephalic neurons have been shown to express a fleeting dopamine/glutamate phenotype (Berube-Carriere et al., 2009). At P15 but not P90, transcripts for tyrosine hydroxylase (TH) and vesicular glutamate transporter 2 (vGluT2) are detected in the ventral tegmental area (VTA) and the corresponding proteins are colocalized in axon terminals of the nucleus accumbens. This cotransmission has been shown to have important functional consequences: in a conditional vGluT2 knockout restricted to dopaminergic neurons, dopaminergic transmission is reduced in VTA neurons and the motor response to cocaine is abridged (Hnasko et al., 2010).
In the mature auditory system, neurons in the medial nucleus of the trapezoid body (MNTB) provide inhibition onto lateral superior olive neurons. A switch in the neurotransmitter mediating this inhibition from GABA to glycine takes place during the second postnatal week (Nabekura et al., 2004). In the same neurons, electrophysiological recordings showed that these MNTB neurons transiently express and release glutamate during the first two postnatal weeks. Immunohistochemistry experiments revealed the early expression of VGlut3 at synapses also expressing the vesicular transporter for GABA (Gillespie et al., 2005). A recent study demonstrated how instrumental this phenotype is for the refinement of an inhibitory map: the precision of the tonotopy in this inhibitory auditory pathway is reduced in a mouse model with a genetic deletion of vGluT3 (Noh et al., 2010). Neurotransmitter phenotype expression restricted to the immature brain has also been identified in the hippocampus. Granule cells of the dentate gyrus are traditionally described as glutamatergic. Their axons, the mossy fibers, synapse on the dendrites of CA3 pyramidal cells and constitute one of the inputs of the classical tri-synaptic hippocampal circuit. However, during early development these neurons express different phenotypes sequentially. They are initially GABergic and then express a dual GABAergic/glutamatergic phenotype before becoming purely glutamatergic. Physiological and morphological experiments demonstrate that, in normal conditions, specific markers for the GABAergic phenotype are transiently detected in these cells during the first postnatal weeks (Gutierrez et al., 2003;Safiulina et al., 2006;Safiulina et al., 2010). The basis for these transmitter switches during development remains to be identified, but are likely to entail activity-dependent modulation of genetic programs.
Interestingly, early neuronal networks frequently display spontaneous activity. Often these early forms of communication are not driven by the same transmitters as those mediating mature synaptic activity (Milner and Landmesser, 1999;Blankenship and Feller, 2010). Hence, these developmentally restricted phenotypes may be instrumental in the establishment of the normal patterns of synapse and network maturation.
Genetic machinery responsive to the environment
Tlx3 and Lmx1b are both expressed and have been implicated in neurotransmitter specification in mammalian systems (Cheng et al., 2003;Cheng et al., 2005). Furthermore, regulatory elements that enhance or repress gene expression, such as CREB and REST and the DNA motifs to which they bind allow adaptative responses to modifications of the environment (Abrajano et al., 2009). They may provide the basis for modulation of the expression of other transcription factors that influence the transmitter identity of neuronal populations.
Modification of neurotransmitter phenotype in response to activity
The cholinergic phenotype of developing hypothalamic neurons is regulated by activity. Chronic blockade of NMDA receptors, both in vitro and in vivo, upregulates the expression of choline acetyltransferase in hypothalamic neurons (Belousov et al., 2002; Liu et al., 2008). Inactivation of NMDA receptors also induces cholinergic synaptic activity in hypothalamic cultures. Physiological patterns of electrical stimulation lead to an increase in the percent of neurons that express tyrosine hydroxylase mRNA and protein in fetal dissociated primary sensory neurons (Brosenitsch and Katz, 2001). Activity-dependent expression of the dopaminergic phenotype in embryonic and postnatal sensory neurons appears to depend on expression of Phox2a/2b transcription factors both in vitro and in vivo (Brosenitsch & Katz, 2002).
Although mature hippocampal granule cells are glutamatergic, they respond to chemically induced epileptic activity or LTP-like stimulation by expressing GABAergic markers (along with axonal sprouting), thus resuming a phenotype they expressed early in development (Gutierrez, 2002;Gutierrez and Heinemann, 2006). These changes are likely to occur through synaptic calcium-dependent regulation of gene expression (Gomez-Lira et al., 2005). In seizure-prone gerbils, 5-HT1A receptor immunoreactivity is decreased in CA1 interneurons. Because changes in transmitter specification lead to changes in postsynaptic receptor expression (Borodinsky and Spitzer, 2007; Dulcis and Spitzer, 2008), this change in receptor expression raises the possibility of downregulation of the serotonergic phenotype in the innervating neurons (Kim et al., 2007). These changes in transmitter specification are consistent with homeostatic logic, with an increase in inhibition or decrease of excitation occurring to balance the hyperactivity of the system. Further studies are required to confirm this hypothesis, examining the effect of graded levels of activity on specification of excitatory and inhibitory transmitters.
PERSPECTIVES
Both the similarities between developmental processes observed in Xenopus and in mammals and the early direct evidence in mammals point to the presence of NPP in the mammalian brain. We propose that this plasticity plays an important role in network development and that it provides flexibility for transmitter coexpression. Analysis of coexpression has often focused on single points in time, either during development or in the adult nervous system, under conditions of standard activity. Perturbations of activity are now revealing that the complement of neurotransmitter expression can change.
Neurotransmitter coexpression in the mammalian brain
Coexpression of transmitters and cotransmission in mammals have been the focus of substantial recent attention. Characterization of the vesicular transporters specific for glutamate has been useful in studies of the glutamatergic phenotype and has revealed coexpression with other transmitters that is more widespread than anticipated (Boulland et al., 2004). Probably the most striking example is the mammalian spinal motor neuron, long been believed to release only acetylcholine. Immunocytochemical detection of vGluT2 and electrophysiological recordings (Nishimaru et al., 2005) have demonstrated that glutamate is a functional cotransmitter in these cells. Interestingly, the release of the two neurotransmitters seems to be compartmentalized in these cells. Acetylcholine is the exclusive neurotransmitter at the neuromuscular junction, with glutamate being used for central synaptic contacts with interneurons such as Renshaw cells or with other motor neurons. Whether this is the result of target-derived influences or due to different patterns of activity remains to be determined.
VGluT2 has also recently been detected in a subset of GABAergic supramamillary-hippocampal connections, traditionally described as GABAergic (Soussi et al., 2010). VGluT3, another member of the glutamate vesicular transporter family, has been found in most central serotonergic neurons (Gras et al., 2002). The glutamate component of the synaptic activity of serotonergic neurons is involved in fast excitatory transmission and in presynaptic modulation of 5-HT release (Amilhon et al., 2010). Taken together these observations suggest that cotransmission may be a very general process. Further research is required to increase our understanding of neurotransmitter coexpression as well as potential pathophysiological consequences of NPP.
Technological considerations
Screening for NPP with constitutive global increases or decreases in activity is a useful experimental approach, but precise temporal regulation is a key parameter for all developmental processes, whether it involves the timing of a period of activity in relation to other developmental landmarks or the duration and timing of individual events within each period of activity. The importance of global timing is illustrated by the differential plasticity of synapse formation dependent on the timing of suppression of activity in an individual neuron. Early suppression of activity, when the targeted neuron is fighting to become integrated into a network, leads to less synapse formation on that neuron. In contrast, later manipulation, when a neuron is already an active part of a network, leads to a compensatory increase in synapse formation (Burrone et al., 2002). The importance of internal timing is illustrated by regulatory processes that are successfully triggered by bursting activity when equivalent activation by single stimuli is not effective (McLaughlin et al., 2003;Grubb and Burrone, 2010). The current development of optogenetic tools provides ways to control both aspects of timing. The growing number of members of the channelrhodopsin and halorhodopsin families enables temporal control over neuronal firing, with channels that specifically increase or decrease neuronal activity (Mancuso et al., 2011;Li et al., 2005). A bacterial potassium-selective ionotropic glutamate receptor that reversibly inhibits neuronal activity in response to light will be useful for such studies (Janovjak et al., 2010).
In parallel, our increasing knowledge of molecular markers of cell identity facilitates targeted introduction of molecules of interest in subpopulations of neurons. Tools to target neuronal populations in spinal networks and in serotonergic neurons are now available (Goulding, 2009;Scott et al., 2005). Electroporation provides a useful technical alternative for local introduction of molecules (Demarque and Spitzer, 2010;LoTurco et al., 2009). This approach has recently been used to restore correct patterning of the dorsoventral projection of motor neurons in the chick spinal cord after blockade of GABAA receptors (LoTurco et al., 2009;Kastanenka and Landmesser, 2010). The development of genetically targeted light-activated channels (Gradinaru et al., 2007;Arenkiel et al., 2007;Airan et al., 2007) is creating many opportunities for the investigation of activity-dependent transmitter specification.
Functional interest of NPP
Acquisition of a neurotransmitter phenotype requires the assembly of a host of components. The transmitter must be synthesized, transported into vesicles, released into the synaptic cleft and inactivated by membrane transporters or degradative enzymes. Although it seems metabolically expensive to target this level of regulation, the gain may be substantial. NPP may provide changes in network output that are more widespread than those provided by synapse-specific mechanisms, since it affects whole sets of synapses simultaneously. It may shift overall network activity up or down to keep it in an operating range, while preserving the specific differences between synapses previously built up through Hebbian plasticity mechanisms. We conclude that neurotransmitter specification can now be added to the list of neuronal properties that are be modulated by activity to adjust the efficacy of neurotransmission. NPP appears to provide flexibility to fine tune the activity in neuronal networks. Knowing whether different kinds of network events can trigger different kinds of homeostatic mechanisms should help to understand the contribution of each of them to the network processing of information. Future work will provide a more detailed understanding of the extent of NPP and its implications for brain development, plasticity and disease.
Acknowledgements
We thank the members of our lab for useful discussions. Supported by NIH NS15918 and MH74702 to N.C.S.
Reference List
- Abrajano JJ, Qureshi IA, Gokhan S, Zheng D, Bergman A, Mehler MF. REST and CoREST modulate neuronal subtype specification, maturation and maintenance. PLoS One. 2009;4:e7936. doi: 10.1371/journal.pone.0007936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airan RD, Hu ES, Vijaykumar R, Roy M, Meltzer LA, Deisseroth K. Integration of light-controlled neuronal firing and fast circuit imaging. Curr Opin Neurobiol. 2007;17:587–592. doi: 10.1016/j.conb.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alenina N, Bashammakh S, Bader M. Specification and differentiation of serotonergic neurons. Stem Cell Rev. 2006;2:5–10. doi: 10.1007/s12015-006-0002-2. [DOI] [PubMed] [Google Scholar]
- Allene C, Cattani A, Ackman JB, Bonifazi P, Aniksztejn L, Ben-Ari Y, Cossart R. Sequential generation of two distinct synapse-driven network patterns in developing neocortex. J Neurosci. 2008;28:12851–12863. doi: 10.1523/JNEUROSCI.3733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amilhon B, Lepicard E, Renoir T, Mongeau R, Popa D, Poirel O, Miot S, Gras C, Gardier AM, Gallego J, Hamon M, Lanfumey L, Gasnier B, Giros B, El MS. VGLUT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety. J Neurosci. 2010;30:2198–2210. doi: 10.1523/JNEUROSCI.5196-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang SL. Transcriptional control of midbrain dopaminergic neuron development. Development. 2006;133:3499–3506. doi: 10.1242/dev.02501. [DOI] [PubMed] [Google Scholar]
- Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ, Ehlers MD, Feng G. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron. 2007;54:205–218. doi: 10.1016/j.neuron.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belousov AB, Hunt ND, Raju RP, Denisova JV. Calcium-dependent regulation of cholinergic cell phenotype in the hypothalamus in vitro. J Neurophysiol. 2002;88:1352–1362. doi: 10.1152/jn.2002.88.3.1352. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Spitzer NC. Phenotypic checkpoints regulate neuronal development. Trends in Neurosciences. 33:485–492. doi: 10.1016/j.tins.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berube-Carriere N, Riad M, Dal BG, Levesque D, Trudeau LE, Descarries L. The dual dopamine-glutamate phenotype of growing mesencephalic neurons regresses in mature rat brain. J Comp Neurol. 2009;517:873–891. doi: 10.1002/cne.22194. [DOI] [PubMed] [Google Scholar]
- Blankenship AG, Feller MB. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci. 2010;11:18–29. doi: 10.1038/nrn2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodinsky LN, Root CM, Cronin JA, Sann SB, Gu X, Spitzer NC. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature. 2004;429:523–530. doi: 10.1038/nature02518. [DOI] [PubMed] [Google Scholar]
- Borodinsky LN, Spitzer NC. Activity-dependent neurotransmitter-receptor matching at the neuromuscular junction. Proc Natl Acad Sci U S A. 2007;104:335–340. doi: 10.1073/pnas.0607450104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulland JL, Qureshi T, Seal RP, Rafiki A, Gundersen V, Bergersen LH, Fremeau RT, Jr., Edwards RH, Storm-Mathisen J, Chaudhry FA. Expression of the vesicular glutamate transporters during development indicates the widespread corelease of multiple neurotransmitters. J Comp Neurol. 2004;480:264–280. doi: 10.1002/cne.20354. [DOI] [PubMed] [Google Scholar]
- Brosenitsch TA, Katz DM. Physiological patterns of electrical stimulation can induce neuronal gene expression by activating N-type calcium channels. J Neurosci. 2001;21:2571–2579. doi: 10.1523/JNEUROSCI.21-08-02571.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosenitsch TA, Katz DM. Expression of Phox2 transcription factors and induction of the dopaminergic phenotype in primary sensory neurons. Mol Cell Neurosci. 2002;20:447–457. doi: 10.1006/mcne.2002.1135. [DOI] [PubMed] [Google Scholar]
- Burrone J, O’Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–418. doi: 10.1038/nature01242. [DOI] [PubMed] [Google Scholar]
- Cheng L, Arata A, Mizuguchi R, Qian Y, Karunaratne A, Gray PA, Arata S, Shirasawa S, Bouchard M, Luo P, Chen CL, Busslinger M, Goulding M, Onimaru H, Ma Q. Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates. Nat Neurosci. 2004;7:510–517. doi: 10.1038/nn1221. [DOI] [PubMed] [Google Scholar]
- Cheng L, Chen CL, Luo P, Tan M, Qiu M, Johnson R, Ma Q. Lmx1b, Pet-1, and Nkx2.2 coordinately specify serotonergic neurotransmitter phenotype. J Neurosci. 2003;23:9961–9967. doi: 10.1523/JNEUROSCI.23-31-09961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Samad OA, Xu Y, Mizuguchi R, Luo P, Shirasawa S, Goulding M, Ma Q. Lbx1 and Tlx3 are opposing switches in determining GABAergic versus glutamatergic transmitter phenotypes. Nat Neurosci. 2005;8:1510–1515. doi: 10.1038/nn1569. [DOI] [PubMed] [Google Scholar]
- Crepel V, Aronov D, Jorquera I, Represa A, Ben-Ari Y, Cossart R. A parturition-associated nonsynaptic coherent activity pattern in the developing hippocampus. Neuron. 2007;54:105–120. doi: 10.1016/j.neuron.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Demarque M, Represa A, Becq H, Khalilov I, Ben-Ari Y, Aniksztejn L. Paracrine intercellular communication by a Ca2+- and SNARE-independent release of GABA and glutamate prior to synapse formation. Neuron. 2002;36:1051–1061. doi: 10.1016/s0896-6273(02)01053-x. [DOI] [PubMed] [Google Scholar]
- Demarque M, Spitzer NC. Activity-Dependent Expression of Lmx1b Regulates Specification of Serotonergic Neurons Modulating Swimming Behavior. Neuron. 2010;67:321–334. doi: 10.1016/j.neuron.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YQ, Marklund U, Yuan W, Yin J, Wegman L, Ericson J, Deneris E, Johnson RL, Chen ZF. Lmx1b is essential for the development of serotonergic neurons. Nat Neurosci. 2003;6:933–938. doi: 10.1038/nn1104. [DOI] [PubMed] [Google Scholar]
- Dulcis D, Spitzer NC. Illumination controls differentiation of dopamine neurons regulating behaviour. Nature. 2008;456:195–201. doi: 10.1038/nature07569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulcis E, Spitzer NC. Reserve pool neuron transmitter respecification: novel neuroplasticity. Dev Neurobiol. 2011 doi: 10.1002/dneu.20920. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie DC, Kim G, Kandler K. Inhibitory synapses in the developing auditory system are glutamatergic. Nat Neurosci. 2005;8:332–338. doi: 10.1038/nn1397. [DOI] [PubMed] [Google Scholar]
- Gomez-Lira G, Lamas M, Romo-Parra H, Gutierrez R. Programmed and induced phenotype of the hippocampal granule cells. J Neurosci. 2005;25:6939–6946. doi: 10.1523/JNEUROSCI.1674-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci. 2009;10:507–518. doi: 10.1038/nrn2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Thompson KR, Zhang F, Mogri M, Kay K, Schneider MB, Deisseroth K. Targeting and readout strategies for fast optical neural control in vitro and in vivo. J Neurosci. 2007;27:14231–14238. doi: 10.1523/JNEUROSCI.3578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El MS. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22:5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashow R, Brookings T, Marder E. Reliable neuromodulation from circuits with variable underlying structure. Proc Natl Acad Sci U S A. 2009;106:11742–11746. doi: 10.1073/pnas.0905614106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MS, Burrone J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature. 2010;465:1070–1074. doi: 10.1038/nature09160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez R. Activity-dependent expression of simultaneous glutamatergic and GABAergic neurotransmission from the mossy fibers in vitro. J Neurophysiol. 2002;87:2562–2570. doi: 10.1152/jn.2002.87.5.2562. [DOI] [PubMed] [Google Scholar]
- Gutierrez R, Heinemann U. Co-existence of GABA and Glu in the hippocampal granule cells: implications for epilepsy. Curr Top Med Chem. 2006;6:975–978. doi: 10.2174/156802606777323692. [DOI] [PubMed] [Google Scholar]
- Gutierrez R, Romo-Parra H, Maqueda J, Vivar C, Ramirez M, Morales MA, Lamas M. Plasticity of the GABAergic phenotype of the “glutamatergic” granule cells of the rat dentate gyrus. J Neurosci. 2003;23:5594–5598. doi: 10.1523/JNEUROSCI.23-13-05594.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MG, Landmesser LT. Characterization of the circuits that generate spontaneous episodes of activity in the early embryonic mouse spinal cord. J Neurosci. 2003;23:587–600. doi: 10.1523/JNEUROSCI.23-02-00587.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, Rayport S, Edwards RH. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65:643–656. doi: 10.1016/j.neuron.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokfelt T, Elfvin LG, Elde R, Schultzberg M, Goldstein M, Luft R. Occurrence of somatostatin-like immunoreactivity in some peripheral sympathetic noradrenergic neurons. Proc Natl Acad Sci U S A. 1977;74:3587–3591. doi: 10.1073/pnas.74.8.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokfelt T, Johansson O, Ljungdahl A, Lundberg JM, Schultzberg M. Peptidergic neurones. Nature. 1980;284:515–521. doi: 10.1038/284515a0. [DOI] [PubMed] [Google Scholar]
- Janovjak H, Szobota S, Wyart C, Trauner D, Isacoff EY. A light-gated, potassium-selective glutamate receptor for the optical inhibition of neuronal firing. Nat Neurosci. 2010;13:1027–1032. doi: 10.1038/nn.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, hler J. Corelease of Two Fast Neurotransmitters at a Central Synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- Kastanenka KV, Landmesser LT. In vivo activation of channelrhodopsin-2 reveals that normal patterns of spontaneous activity are required for motoneuron guidance and maintenance of guidance molecules. J Neurosci. 2010;30:10575–10585. doi: 10.1523/JNEUROSCI.2773-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Kim JE, Kwak SE, Kim DW, Choi SY, Kwon OS, Kang TC. Seizure activity selectively reduces 5-HT1A receptor immunoreactivity in CA1 interneurons in the hippocampus of seizure-prone gerbils. Brain Res. 2007;1154:181–193. doi: 10.1016/j.brainres.2007.03.084. [DOI] [PubMed] [Google Scholar]
- Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, Chiel H, Hegemann P, Landmesser LT, Herlitze S. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc Natl Acad Sci U S A. 2005;102:17816–17821. doi: 10.1073/pnas.0509030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Popescu IR, Denisova JV, Neve RL, Corriveau RA, Belousov AB. Regulation of cholinergic phenotype in developing neurons. J Neurophysiol. 2008;99:2443–2455. doi: 10.1152/jn.00762.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoTurco J, Manent JB, Sidiqi F. New and improved tools for in utero electroporation studies of developing cerebral cortex. Cereb Cortex. 2009;19(Suppl 1):i120–i125. doi: 10.1093/cercor/bhp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso JJ, Kim J, Lee S, Tsuda S, Chow NB, Augustine GJ. Optogenetic probing of functional brain circuitry. Exp Physiol. 2011;96:26–33. doi: 10.1113/expphysiol.2010.055731. [DOI] [PubMed] [Google Scholar]
- Manent JB, Demarque M, Jorquera I, Pellegrino C, Ben-Ari Y, Aniksztejn L, Represa A. A noncanonical release of GABA and glutamate modulates neuronal migration. J Neurosci. 2005;25:4755–4765. doi: 10.1523/JNEUROSCI.0553-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E. Quantification of Behavior Sackler Colloquium: Variability, compensation, and modulation in neurons and circuits. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1010674108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek KW, Kurtz LM, Spitzer NC. cJun integrates calcium activity and tlx3 expression to regulate neurotransmitter specification. Nat Neurosci. 2010;13:944–950. doi: 10.1038/nn.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin T, Torborg CL, Feller MB, O’Leary DD. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron. 2003;40:1147–1160. doi: 10.1016/s0896-6273(03)00790-6. [DOI] [PubMed] [Google Scholar]
- Milner LD, Landmesser LT. Cholinergic and GABAergic inputs drive patterned spontaneous motoneuron activity before target contact. J Neurosci. 1999;19:3007–3022. doi: 10.1523/JNEUROSCI.19-08-03007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabekura J, Katsurabayashi S, Kakazu Y, Shibata S, Matsubara A, Jinno S, Mizoguchi Y, Sasaki A, Ishibashi H. Developmental switch from GABA to glycine release in single central synaptic terminals. Nat Neurosci. 2004;7:17–23. doi: 10.1038/nn1170. [DOI] [PubMed] [Google Scholar]
- Nishimaru H, Restrepo CE, Ryge J, Yanagawa Y, Kiehn O. Mammalian motor neurons corelease glutamate and acetylcholine at central synapses. Proc Natl Acad Sci U S A. 2005;102:5245–5249. doi: 10.1073/pnas.0501331102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh J, Seal RP, Garver JA, Edwards RH, Kandler K. Glutamate co-release at GABA/glycinergic synapses is crucial for the refinement of an inhibitory map. Nat Neurosci. 2010;13:232–238. doi: 10.1038/nn.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66:337–351. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root CM, Velazquez-Ulloa NA, Monsalve GC, Minakova E, Spitzer NC. Embryonically expressed GABA and glutamate drive electrical activity regulating neurotransmitter specification. J Neurosci. 2008;28:4777–4784. doi: 10.1523/JNEUROSCI.4873-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safiulina VF, Caiati MD, Sivakumaran S, Bisson G, Migliore M, Cherubini E. Control of GABA Release at Mossy Fiber-CA3 Connections in the Developing Hippocampus. Front Synaptic Neurosci. 2010;2:1. doi: 10.3389/neuro.19.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safiulina VF, Fattorini G, Conti F, Cherubini E. GABAergic signaling at mossy fiber synapses in neonatal rat hippocampus. J Neurosci. 2006;26:597–608. doi: 10.1523/JNEUROSCI.4493-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scain AL, Le CH, Allain AE, Muller E, Rigo JM, Meyrand P, Branchereau P, Legendre P. Glycine release from radial cells modulates the spontaneous activity and its propagation during early spinal cord development. J Neurosci. 2010;30:390–403. doi: 10.1523/JNEUROSCI.2115-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MM, Deneris ES. Making and breaking serotonin neurons and autism. Int J Dev Neurosci. 2005;23:277–285. doi: 10.1016/j.ijdevneu.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S, Jiang W, Conlon RA, Strowbridge BW, Deneris ES. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci U S A. 2005;102:16472–16477. doi: 10.1073/pnas.0504510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Edwards RH. Functional implications of neurotransmitter co-release: glutamate and GABA share the load. Curr Opin Pharmacol. 2006;6:114–119. doi: 10.1016/j.coph.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Soussi R, Zhang N, Tahtakran S, Houser CR, Esclapez M. Heterogeneity of the supramammillary-hippocampal pathways: evidence for a unique GABAergic neurotransmitter phenotype and regional differences. Eur J Neurosci. 2010 doi: 10.1111/j.1460-9568.2010.07329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau LE, Gutierrez R. On cotransmission & neurotransmitter phenotype plasticity. Mol Interv. 2007;7:138–146. doi: 10.1124/mi.7.3.5. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol. 2000;10:358–364. doi: 10.1016/s0959-4388(00)00091-x. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Velazquez-Ulloa NA, Spitzer NC, Dulcis D. Contexts for dopamine specification by calcium spike activity in the CNS. J Neurosci. 2011;31:78–88. doi: 10.1523/JNEUROSCI.3542-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walicke PA, Patterson PH. On the role of Ca2+ in the transmitter choice made by cultured sympathetic neurons. J Neurosci. 1981;1:343–350. doi: 10.1523/JNEUROSCI.01-04-00343.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ, Scott M, Chiechio S, Wang JS, Renner KJ, Gereau RW, Johnson RL, Deneris ES, Chen ZF. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci. 2006;26:12781–12788. doi: 10.1523/JNEUROSCI.4143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]