Abstract
Our understanding of the pathophysiology of movement disorders, and associated changes in basal ganglia activities has significantly changed in the course of the last few decades. This process began with the development of detailed anatomical models of the basal ganglia, followed by studies of basal ganglia activity patterns in animal models of common movement disorders and electrophysiological recordings in movement disorder patients undergoing functional neurosurgical procedures. These investigations first resulted in an appreciation of global activity changes in the basal ganglia in parkinsonism and other disorders, and later in the detailed description of pathological basal ganglia activity patterns, specifically burst patterns and oscillatory synchronous discharge of basal ganglia neurons. In this review we critically summarize our current knowledge of the pathological discharge patterns of basal ganglia neurons in Parkinson’s disease, dystonia and dyskinesias.
Keywords: Striatum, globus pallidus, subthalamic nucleus, Parkinson’s disease, dystonia, dyskinesia
1. Introduction
The last decades have brought an enormous expansion of our knowledge of the pathophysiology of some of the most common movement disorders, specifically Parkinson’s disease (PD), dystonia and dyskinesias. We use the term ‘pathophysiology’ here to refer to the changes in neuronal electrical activity that are associated with these diseases. In part, the progress in this field of research was driven by the development of suitable animal models of these conditions. For instance, the development of dopamine depletion models such as the 6-hydroxydopamine (6-OHDA) treated rat, or the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treated monkey has been highly important for our current understanding of the changes underlying the dopamine-sensitive symptoms of PD. Due to the fact that recordings can be made in animals before and after the induction of movement disorders, such studies have enabled us to rigorously test hypotheses regarding the consequences of biochemical or structural changes in the brain, and the relevance of such changes for the development of behavioral signs of these conditions.
Another highly valuable source of information has been electrophysiologic recordings in patients undergoing neurosurgical treatments for movement disorders. Treatments such as deep brain stimulation (DBS) or lesioning of the basal ganglia are often guided by microelectrode recordings which can then be used to study basal ganglia activity patterns. Furthermore, implanted DBS macroelectrodes can be used to record local field potentials (LFP) from the basal ganglia (or thalamus) for a few days postoperatively until the DBS electrode leads are internalized. Such signals have been correlated with treatment responses or motor behavior.
In the following, we will provide a brief overview of the systems anatomy of the basal ganglia and then describe the current knowledge of disease-related changes in firing patterns and LFPs in the basal ganglia and associated structures. These discussions will focus on studies in non-human primates and human patients because of the immediate relevance of this research for our understanding of the human diseases. While non-motor symptoms may also arise from basal ganglia dysfunction, we will not further comment on these, reflecting our very limited knowledge on the pathophysiologic abnormalities that are underlying these problems.
2. Basal ganglia systems anatomy
It has been known since the mid-1980s that the basal ganglia are arranged in topographically and functionally specific circuits that also involve discrete regions of the thalamus and cortex (the ‘segregated circuit hypothesis’, see Alexander et al., 1986, Alexander et al., 1990, Middleton and Strick, 2000). These circuits are named after the function of the cortical areas from which they originate as ‘motor’, ‘associative’, ‘limbic’, and ‘oculomotor’ circuits. With some exceptions, the different circuits are similar in their general anatomical arrangement. In terms of the basal ganglia involvement in the pathophysiology of movement disorders, the motor circuit is of particular relevance, and will be described in some detail here.
This circuit arises from precentral motor fields (Figure 1, left). Massive projections from these cortical areas terminate in the motor portion of the striatum (the putamen), with sparser connections in the lateral and dorsal motor portion of the subthalamic nucleus (STN) (Hartmann-von Monakow et al., 1978, Inase et al., 1999, Takada et al., 2001, Nambu et al., 2002). Anatomical studies in rats have shown that corticostriatal projections arise in part as collaterals from corticospinal fibers (Lei et al., 2004). Anatomical single-cell tracing studies in primates have also found examples of corticofugal neurons with collaterals to the striatum (Parent and Parent, 2006), although half of the sample of cortical neurons of the latter study provided unbranched input to the striatum (and were, thus, separate from the corticospinal tract). The conclusion that many corticostriatal neurons do not project to the spinal cord is also supported by electrophysiologic studies (Bauswein et al., 1989, Turner and DeLong, 2000).
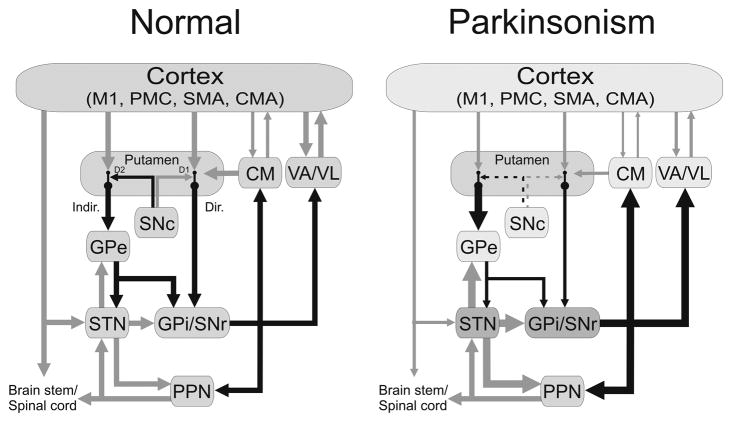
Figure 1.
Parkinsonism-related changes in overall activity (‘rate model’) in the basal ganglia-thalamocortical motor circuit. Black arrows indicate inhibitory connections; gray arrows indicate excitatory connections. The thickness of the arrows corresponds to their presumed activity. Abbreviations: CM, centromedian nucleus of thalamus; CMA, cingulate motor area; Dir., direct pathway; D1, D2, dopamine receptor subtypes; Indir., indirect pathway; M1, primary motor cortex; Pf, parafascicular nucleus of the thalamus; PMC, premotor cortex; PPN, pedunculopontine nucleus; SMA, supplementary motor area. See text for other abbreviations. Reprinted, with permission, from Galvan and Wichmann (2008).
The existing literature suggests that the corticosubthalamic pathway is separate from the corticostriatal pathway so that STN and striatum may receive different types of information (Parent and Parent, 2006) Data from experiments in cats and rats have suggested that the corticosubthalamic inputs may arise as axon collaterals of other corticofugal systems, such as the corticospinal tract (Iwahori, 1978, Kitai and Deniau, 1981, Giuffrida et al., 1985), but this fact has not been sufficiently clarified in primates. In fact, the aforementioned single-cell tracing study of corticofugal fibers (Parent and Parent, 2006) included only two motor cortical neurons that provided terminals in the STN. One of these was found to subsequently invade the cerebral peduncle while the other eventually terminated in the red nucleus. The important question of the nature of cortical inputs to the basal ganglia is further complicated by the fact that the motor and non-motor corticosubthalamic pathways are likely to differ substantially in terms of their status as being collaterals of other projections.
Striatal projections to the basal ganglia output nuclei, the internal segment of the globus pallidus (GPi, termed the entopeduncular nucleus in rodents), and the substantia nigra pars reticulata (SNr) can be grouped into the monosynaptic ‘direct’ pathway and the polysynaptic ‘indirect’ pathway which involves neurons in the external pallidal segment (GPe) and the STN (Figure 1). The degree of separation of these circuits remains debated. Most recent studies suggest that the direct and indirect pathways arise from different sets of neurons in the striatum (reviewed in Gerfen and Surmeier, 2010), but single-cell tracing studies have shown that at least some of the striatal projections terminate in both segments of the pallidum (Levesque and Parent, 2005), suggesting that the separation may not be complete.
Basal ganglia output from GPi/SNr is mostly directed towards the ventrolateral and ventral anterior nuclei of the thalamus (VL and VA, respectively). VL and VA send relatively minor projections to the basal ganglia (McFarland and Haber, 2000, 2001, Smith et al., 2004), but project massively to the frontal cortical areas from which the motor circuit arises, thereby at least partially closing the cortico-subcortico-cortical motor loop. Collaterals of the GPi/SNr projection to VL/VA reach the intralaminar thalamic nuclei, i.e., the ‘motor’ centromedian nucleus and the ‘non-motor’ parafascicular nucleus. These nuclei project back to motor and non-motor regions of the striatum, and may function as a feedback system by which striatal processing is influenced by basal ganglia output (Smith et al., 2004). One of the important additional functions of the intralaminar thalamic nuclei is to provide saliency information to the striatum during procedural learning (Kimura et al., 2004, Minamimoto et al., 2009).
In addition to their role as components of the cortico-cortical circuits, the basal ganglia have descending connections to brain stem targets such as the pedunculopontine nucleus (Mena-Segovia et al., 2004, Aravamuthan et al., 2007, Hamani et al., 2007) or the superior colliculus (see reviews by Hikosaka, 2007, Kaneda et al., 2008, Liu and Basso, 2008).
Dopamine has been assigned a pivotal role in almost all of the normal functions of the basal ganglia. Under physiologic conditions, dopamine release in the striatum appears to be strongly involved in reward processing (Schultz and Dickinson, 2000, Cragg, 2006, Calabresi et al., 2007, Hikosaka, 2007, Wickens et al., 2007, Surmeier et al., 2009, Morris et al., 2010). In addition, the ambient level of dopamine in the striatum may regulate the general flow of cortical information through direct/indirect pathway systems in the striatum. Dysfunction of this system has traditionally been linked to the development of motor abnormalities in the absence of dopamine (parkinsonism), or with abnormalities in dopaminergic transmission (as may occur in levodopa-induced dyskinesias and in some forms of dystonia, see below). Interestingly, the dopaminergic projections may also have functions other than the modulation of synaptic transmission. Thus, there is a considerable body of evidence that the absence of dopaminergic transmission may trigger changes in the density and morphology of dendritic spines on striatal projection neurons (Ingham et al., 1998, Gerfen, 2006, Day et al., 2008, Smith et al., 2009, Villalba et al., 2009) which, in turn, may influence corticostriatal transmission. Pathology studies have not identified major changes in the number of cortical projection neurons (with the exception of cortico-cortical projection neurons in the pre-supplementary motor area (MacDonald and Halliday, 2002, Halliday et al., 2005)), so that it seems likely that the dendritic spine changes are a primary striatal phenomenon, and not due to a reduction of the number of glutamatergic inputs to the striatum.
Recent research has shown that all of the other basal ganglia nuclei also receive dopaminergic inputs, although these projections and the dopamine concentrations in these nuclei are much smaller (Pifl et al., 1991, Rommelfanger and Wichmann, 2010). The importance of the dopaminergic input to the normal function of these extrastriatal areas (and to behavior) remains unclear, but it has been shown that dopamine receptor activation or blockade in these regions profoundly alters neuronal firing (reviewed in Rommelfanger and Wichmann, 2010). Furthermore, recent findings suggest that loss of dopamine can seriously interfere with the mechanisms underlying synaptic plasticity in the striatum and SNr (Picconi et al., 2003, Prescott et al., 2009), and it is possible that these changes contribute to the development of motor symptoms in PD or dyskinesias.
Other neuromodulators, such as serotonin, released in the striatum and other basal ganglia nuclei from projections of the brain stem raphe nuclei (Kalen et al., 1989, Di Matteo et al., 2008), or acetylcholine, released predominately in the striatum from terminals of interneurons, may also play a significant role in the regulation of basal ganglia activity and in the pathophysiology of movement disorders (Pisani et al., 2007, Di Matteo et al., 2008, Fox et al., 2009), but their function and the consequence of altered transmission at synapses involving these transmitters are less well characterized than those of dopamine.
The concept of the organization of the cortico-basal ganglia interactions into stable functionally segregated modules is gradually eroding, with increasing evidence that at least some information processing in the basal ganglia may undergo shifts between the different functional domains. This has been particularly shown for the involvement of the basal ganglia in procedural learning in which the striatal activation seems to gradually move from ‘non-motor’ to ‘motor’ areas (for instance, Miyachi et al., 2002). In reality, simultaneous involvement of motor and non-motor circuits in a given behavioral context is likely to be the norm rather than the exception, because few behaviors can be classified as being exclusively ‘motor’ or ‘non-motor’.
As a further qualifier of the segregated pathway hypothesis, there is increasing evidence for direct anatomical interactions between cerebellar and basal ganglia circuits (in addition to the known interaction between these circuits at the cortical level). Thus, virus tracing studies have suggested that the basal ganglia receive information from the deep cerebellar nuclei (Hoshi et al., 2005), and that they send projections to the cerebellar cortex (Bostan et al., 2010). Such interactions are not (yet) part of the pathophysiologic models mentioned below, but may become important in the future, particularly to explain aspects of tremor or dystonia.
3. Parkinson’s disease
PD is clinically defined by the presence of slowness of movement (bradykinesia), poverty of movement (akinesia), muscle stiffness (rigidity) and tremor at rest. This constellation of signs and symptoms is referred to as ‘parkinsonism’, is known to respond to dopamine replacement therapy, and is therefore considered to be a direct consequence of the pathological hallmark of the disease, the degeneration of dopaminergic neurons in the substantia nigra, pars compacta (SNc), and the loss of dopamine in the striatum. In order to develop a better understanding of the basal ganglia processes involved in the dopamine-responsive aspects of PD, a large number of studies have investigated the consequences of striatal dopamine loss on the electrical activities of basal ganglia neurons in animal models of the disease, or have characterized the electrical activities in the basal ganglia in patients.
Early studies of activity changes in the basal ganglia of MPTP-treated parkinsonian monkeys emphasized changes in the overall activity of basal ganglia nuclei (Miller and DeLong, 1987, Albin et al., 1989, DeLong, 1990). These studies were strongly influenced by previous 2-deoxyglucose imaging studies in the same animal model which had shown that the metabolic activity in GPe and VL/VA increases, while the activity in the STN and GPi was shown to be lower in MPTP-treated monkeys than in normal animals (Schwartzman et al., 1988, Mitchell et al., 1989). These metabolic changes were interpreted in terms of alterations in synaptic activity (Figure 1, right). Electrophysiological studies provided an explanation for these changes by demonstrating a reduction of activity in GPe, and increases in activity in the STN and GPi (Miller and DeLong, 1987, Albin et al., 1989, DeLong, 1990). Together with other studies, these findings resulted in the ‘rate model’ of movement disorders. Applied to PD, it explained the reduced firing rate in the GPe as a consequence of increased activity along the inhibitory indirect striato-pallidal pathway, which would then result in disinhibition of STN activities and facilitation of GPi firing. It was always understood that the increased activity in GPi could also be explained by reduced activity along the inhibitory direct pathway of the basal ganglia, but such changes have not been directly demonstrated. Downstream from GPi, increased basal ganglia output was thought to result in inhibition of thalamocortical projection neurons, reduced cortical activation, and slowing of movement.
Soon after its introduction, the rate model met with substantial criticism, because it did not explain some of the basic clinical findings in PD patients, including the fact that thalamotomy procedures did not result in worsening of parkinsonism (as one would expect based on the rate model), and that GPi lesions unexpectedly produced bradykinesia in normal monkeys while GPe lesions did not produce parkinsonism (Marsden and Obeso, 1994, Soares et al., 2004). Furthermore, although most studies to date have found that severe parkinsonism in MPTP-treated monkeys is indeed associated with reduced firing rates in GPe and increased firing rates in GPi (Miller and DeLong, 1987, Filion and Tremblay, 1991, Bergman et al., 1994, Boraud et al., 1996, Boraud et al., 1998, Heimer et al., 2002, Wichmann et al., 2002, Soares et al., 2004), other studies have reported unchanged GPe or GPi firing rates, or rate changes opposite to those predicted by the rate model (Wichmann et al., 1999, Raz et al., 2000, Wichmann and Soares, 2006, Leblois et al., 2007, Galvan et al., 2010). The reason for these differences is not clear, but may have to do with differences in the severity of parkinsonism induced by the dopaminergic lesion, the specific toxin treatment protocol, or the recording conditions used in these studies. Taken together, the available evidence suggests that changes in firing rates may play a role in the development of parkinsonism, but do not fully explain its appearance.
A significant factor interfering with the assessment of firing rate changes in parkinsonism is that firing rates throughout the basal ganglia are strongly dependent on the state of arousal of the subjects studied. Parkinsonism often results in profound state changes in patients (Menza et al., 2010) and monkeys (Daley et al., 2002, Fox and Brotchie, 2010), and such changes are often not well controlled in studies of firing rate changes in the basal ganglia in parkinsonism. Furthermore, many of the studies in rodents were done in anesthetized animals in which the depth of anesthesia was not (or could not be) adequately monitored. Thus, despite the fact that firing rates are technically easy to measure, the interpretation of firing rate changes in the parkinsonian state remains difficult.
Most authors have now turned away from exclusively firing rate-based models of the pathophysiology of parkinsonism, and towards models that take into account changes in basal ganglia firing patterns and synchrony. There are many different methods of characterizing firing patterns, but most involve some type of quantification of the degree of burstiness or regularity.
Increased burstiness (see example in Figure 2A) has emerged as one of the most reliable abnormalities of neuronal firing in the basal ganglia in parkinsonism, as shown in dopamine-depleted monkeys and in patients with Parkinson’s disease (Filion, 1979, Bergman et al., 1994, Hutchison et al., 1994, Wichmann et al., 1999, Magnin et al., 2000, Soares et al., 2004, Wichmann and Soares, 2006). Specifically, bursting activity in the STN develops relatively early in the course of dopamine depletion, along with changes in discharge rates and metabolic markers (Vila et al., 2000, Ni et al., 2001, Breit et al., 2007). Mechanisms that would lead to bursting in the STN may include unopposed constitutive activity at post-synaptic dopamine D5 receptors (Baufreton et al., 2005, Rommelfanger and Wichmann, 2010), and rebound burst phenomena, caused by synchronous GABAergic inputs from GPe (Baufreton et al., 2003, Shen and Johnson, 2005, Bevan et al., 2007).
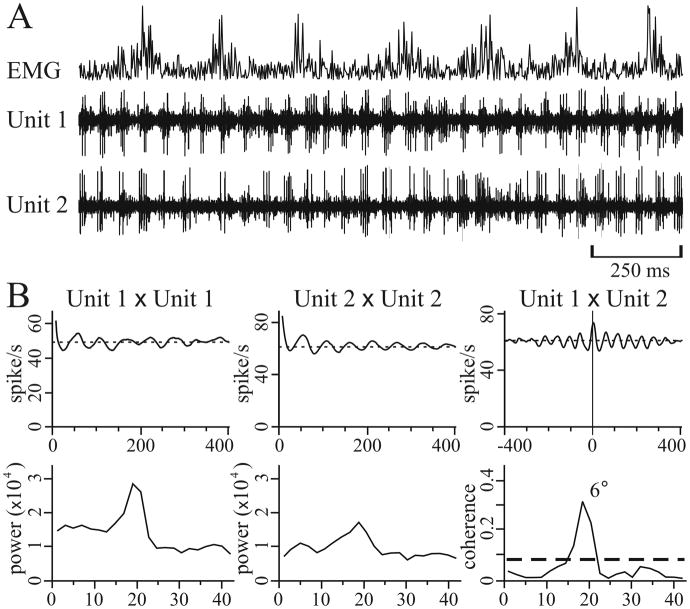
Figure 2.
Example of intraoperative microelectrode recordings in a patient with PD. A, The second and third trace (Unit 1 and Unit 2, respectively) show the discharge activity of two simultaneously recorded STN neurons during wrist tremor, as demonstrated with the recording of rectified wrist extensor electromyographic activity (EMG, top trace). The two neurons generated oscillatory bursts in synchrony with each other. B, Correlograms (top row) and spectra (bottom row) of the traces shown in A (the total sample time used to construct these plots was 29 sec). In all correlograms, the lines indicate mean firing rate. In the cross-correlogram (right panel of top row), Unit 1 is used as the trigger. The thick dashed line in the coherence function indicates the level of significant coherence, and the number by the peak is the phase difference. Reprinted, with permission, from Levy et al (2000).
When fluctuations in firing patterns occur in a regular repeating fashion then the pattern can also be characterized as oscillatory in nature and the frequency and magnitude of the oscillations can be quantified by methods such as spectral analysis (Fourier transforms) or autocorrelograms. Such oscillatory activity is frequently also observed as oscillatory LFPs and can be detected using large-tipped (macro-) electrodes such as for example the contacts of deep brain stimulation electrodes in patients. Analysis of oscillatory activity in neuronal spike trains and LFPs in animal models of PD and in PD patients, has revealed the frequent occurrence of significant oscillatory activity in the beta frequency range (approximately 10 – 35Hz) throughout the extrastriatal basal ganglia (Figures 2–4). Neurons within the individual basal ganglia show an increased level of synchrony (e.g., Bergman et al., 1994, Heimer et al., 2002, Goldberg et al., 2004, Rivlin-Etzion et al., 2006, Hammond et al., 2007). Furthermore, the oscillatory activity is in synchrony between STN, GPi and cortex and is suppressed by the administration of levodopa (Figures 2 and 3, and reviews by Brown, 2003, Gatev et al., 2006, Hammond et al., 2007). Several studies have revealed that neurons in the STN tend to fire in synchrony at beta frequencies and that this oscillatory activity is coherent with the beta-band LFP oscillatory activity and maximal in the motor region of the STN (Kuhn et al., 2005, Weinberger et al., 2006). This indicates that there is synchronous activity at the oscillation frequency in a large population of neurons. This could lead to a breakdown in the ability of individual neurons to process and relay specific information and thus to effectively control complex movements.
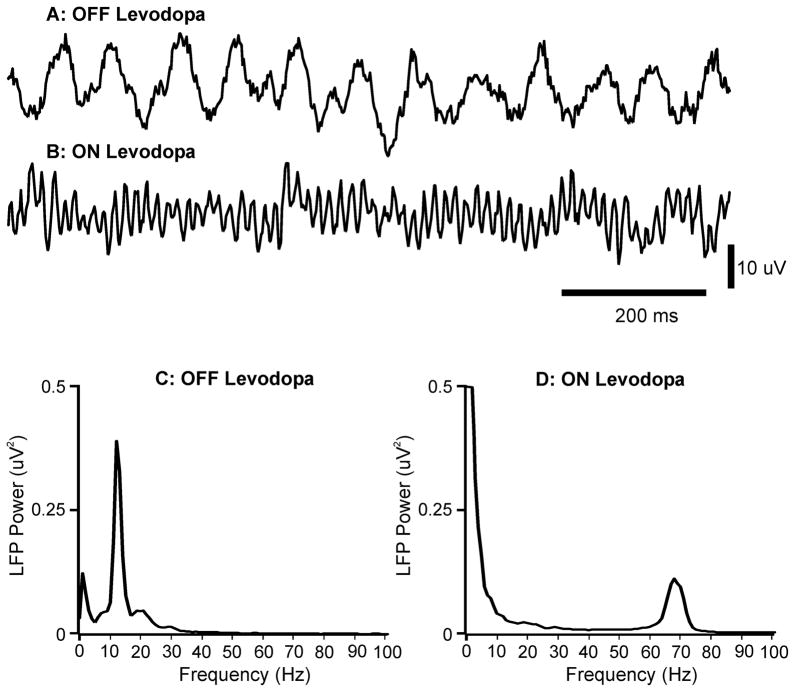
Figure 4.
Field potential signals recorded in a patient with PD with a macroelectrode positioned in the subthalamic area. A. Field potential signals recorded after overnight withdrawal of medication. B. Field potential signals recorded after subsequent levodopa challenge. C. Power spectrum of field potentials recorded after overnight withdrawal of medication (140 s record). D. Power spectrum of field potential signals recorded after subsequent levodopa challenge (140 s record). There was a spectral peak at around 13 Hz off medication, and at around 70 Hz after levodopa treatment. Reproduced, with permission from Brown and Williams (2005).
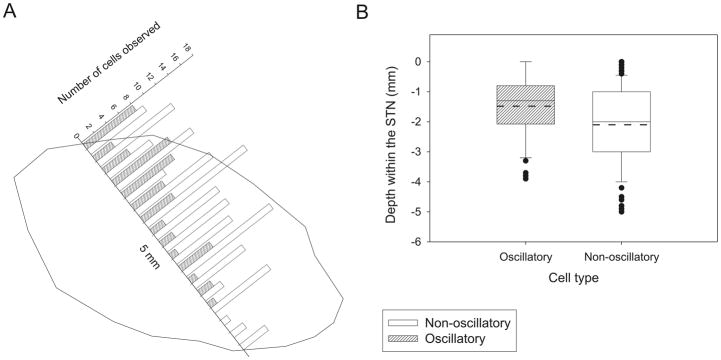
Figure 3.
Distribution of oscillatory and non-oscillatory cells located within the STN in a microelectrode recording study of 14 patients with PD. A. Histogram showing the distribution of oscillatory (n = 56) and nonoscillatory (n =144) cells located within the STN from top to bottom (0 to −5 mm, respectively), binned in 0.3-mm intervals. The x-axis also represents the typical trajectory of a microelectrode track through STN superimposed on the outline of the STN taken from the sagittal 12.0-mm lateral stereotactic STN map (Schaltenbrand and Wahren, 1977). Most of the oscillatory cells were found in the more dorsal portion of the STN, whereas the non-oscillatory cells were equally distributed along the nucleus. B: box plots of oscillatory and non-oscillatory cells’ distribution within the STN. Solid and dashed lines indicate the median and the mean depths, respectively (means ± SE: −1.5 ± 0.1 and −2.1 ± 0.1 mm for oscillatory and nonoscillatory cells, respectively; P < 0.001, t-test). Note the smaller number of observations in the last millimeter of STN attributed to the fact that in many cases the extent of the STN is < 5 mm. Reprinted, with permission, from Weinberger et al. (2006).
The generation of the oscillatory activity is still not clearly understood. It is unlikely that the oscillations are produced by a single oscillatory ‘driver’ in the basal ganglia, and more probable that they arise from network oscillatory resonance, in particular between GPe and STN (Plenz and Kitai, 1999, Holgado et al., 2010), or are driven from cortex via the hyperdirect pathway. Loss of dopamine in the basal ganglia produces changes in neuronal and synaptic properties that would tend to promote this type of oscillatory activity (see, Bevan et al., 2002, Terman et al., 2002, Weinberger and Dostrovsky, 2011).
In addition to the beta oscillatory activity, abnormalities in oscillations in two other bands have frequently been reported, i.e., low-frequency activity (<10Hz) and high-frequency gamma band activity (>60 Hz). Gamma band activity has been shown to be decreased in the dopamine depleted state, and to respond to dopaminergic treatment, and thus, its occurrence is believed to be prokinetic, whereas the increased beta and alpha activity is thought to be antikinetic (Figure 4, see also Brown, 2003, Brown and Williams, 2005).
It is very likely that dopamine loss in the basal ganglia causes or contributes to many of the motor signs of PD, and that it results in profound changes in basal ganglia neuronal activity (rate, pattern, synchronized oscillatory activity, synaptic plasticity). However, the link between specific changes in basal ganglia discharge and the behavioral manifestations of PD remains tenuous. This question has been addressed with studies in which animals were chronically (or multiple times) exposed to dopaminergic toxins (Bezard et al., 1999, Leblois et al., 2007). Early studies by Bezard et al. suggested that the neuronal activity in STN and GPi was increased prior to the onset of motor symptoms (Bezard et al., 1999). While these changes were interpreted as a potential sign for a compensatory role of the presymptomatic changes (Bezard et al., 2003), they could also be interpreted as evidence for a threshold of activity changes which has to be surpassed before parkinsonian signs can develop. More recent animal studies have found, however, that some of the neuronal activity changes (particularly oscillatory activities) appear only after the emergence of parkinsonism (Leblois et al., 2007), concluding that they cannot be interpreted as primary causes of the behavioral phenotype. Similar conclusions have also been drawn from studies in rodents in which nigrostriatal dopaminergic transmission was acutely disrupted by dopamine receptor blockade or by acute treatment with 6-hydroxydopamine. In distinction to chronically 6-OHDA-treated animals (e.g., Mallet et al., 2008a), these animals do not develop oscillatory activities (Mallet et al., 2008b). Likewise, despite the consistent finding of increased burst firing in the basal ganglia in parkinsonism, treatments with dopaminergic agents do not consistently reduce burst firing in the basal ganglia of parkinsonian animals or patients (compare Tseng et al., 2000, Lee et al., 2001, Levy et al., 2001). Local injections of dopamine D1-like receptor agonists into the primate GPi or SNr, or D5 receptor activation in the rodent STN were also found to increase rather than decrease burst firing in these nuclei (Baufreton et al., 2003, Kliem et al., 2007).
4. Dystonia
The pathophysiology of dystonia, a disorder in which normal movements are disrupted by co-contraction of agonist and antagonist muscles and by excessive activation of inappropriate muscle groups (overflow), is much less well understood than that of PD, primarily because the condition is caused by a large and heterogeneous group of diseases with presumably different pathophysiologic backgrounds, and because there are no phenomenologically reliable animal models for dystonia (Raike et al., 2005).
Despite a considerable amount of research, little is known about the brain abnormalities that underlie the dystonic phenotype. Most forms of dystonia are not caused by large-scale neurodegeneration and are therefore thought to be due to abnormal function of otherwise relatively intact brain circuits. Some forms of dystonia are probably caused by basal ganglia dysfunction, but others may result from disordered function at other brain locations, such as the cerebellum or cerebral cortex.
Early metabolic studies in primate models have suggested that dystonia may be associated with reduction of activity along the putamen-GPe connection, and increased inhibition of STN and GPi by GPe efferents (figure 1 and Hantraye et al., 1990, Mitchell et al., 1990). Pharmacologic studies of drug-induced dystonia suggested that the condition is associated with a shift of the balance towards the direct pathway (Casey, 1992, Gerlach and Hansen, 1997). Single-cell recording studies in patients undergoing functional neurosurgery have reported that discharge rates in GPe and GPi are low (Lenz et al., 1998, Vitek et al., 1999, Vitek, 2002, Zhuang et al., 2004, Starr et al., 2005, Tang et al., 2007), although in some patients firing rates have been found to be as high as in PD patients (Hutchison et al., 2003).
Dystonia can be associated with disturbances in dopaminergic transmission (Wichmann, 2008), and in these cases may arise from striatal pathology. For instance, dystonia may develop acutely or delayed in normal individuals treated with dopamine-receptor blocking agents. Dystonia also occurs in patients with PD, either as a manifestation of the disease, or as the result of exposure to dopaminergic drugs. Furthermore, in one form of hereditary dystonia (DYT-5), patients present with dystonia and parkinsonism which are eliminated by treatment with levodopa (so-called DOPA-responsive dystonia). Altered dopamine metabolism may also occur in the most common genetic form of dystonia, primary generalized dystonia (DYT1, Augood et al., 2002, Balcioglu et al., 2007, Zhao et al., 2008).
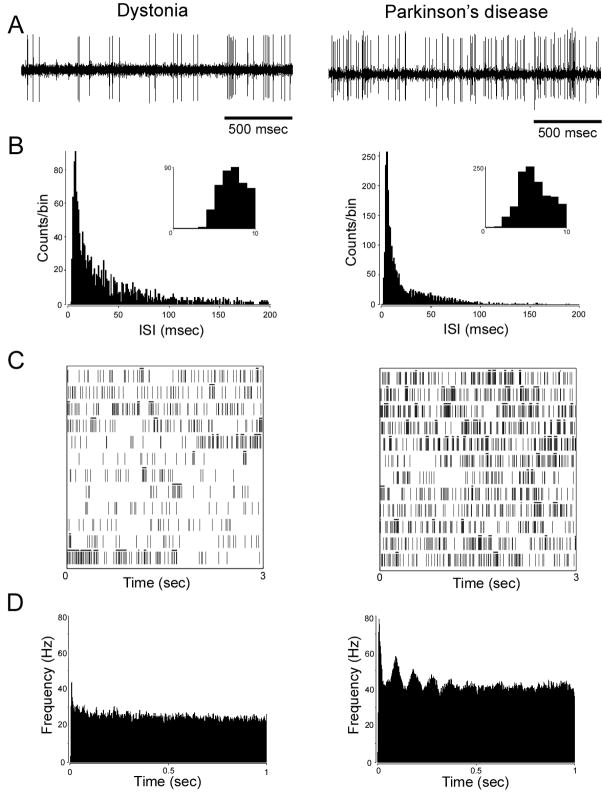
In contrast to PD, there have been relatively few studies examining oscillatory neuronal firing and LFPs in dystonia patients. Thus, much less is known about the possible role of such activity changes in mediating the symptoms. Oscillatory activity in the GPi in dystonic patients is typically different from that observed in PD. The predominant beta oscillations that are characteristic for PD are absent and instead there appears to be increased spectral power in the 4 – 10 Hz band, especially in GPe (Silberstein et al., 2003). However, Starr et al. reported that oscillatory activity of GPi neurons is fairly similar to that in PD and suggested that an important difference between dystonia and parkinsonism may be the coupling of abnormal synchrony and oscillatory activity with specific mean firing rate changes (lower rates in dystonia and higher rates in PD, see figure 5 and Starr et al., 2005, Schrock et al., 2009). As noted before, this is not necessarily the case in all patients (Hutchison et al., 2003).
Figure 5.
Examples of electrophysiological recordings from STN cells in a patient with cranial-cervical dystonia (left column) and a patient with akinetic-rigid parkinsonism (PD, right column). A: A 2-s interval of neuronal recordings. B: interspike interval (ISI) histograms, bin size of 1 ms. Inset: expanded timescale demonstrating the absence of ISIs of <3-ms duration, consistent with the neuronal refractory period. C: raster diagrams showing bursting discharge. Bursts as defined by the Poisson “surprise” method (surprise value = 5) are labeled with a black bar above spikes that constitute a burst. Note the higher proportion of bursts per total number of spikes shown in the dystonia neuron (0.40 vs. 0.26 in the PD neuron). Consecutive rows (3 s of data per row) from bottom to top represent continuous 36-s recordings. D: autocorrelograms. The right autocorrelogram shows oscillatory activity of about 11 Hz. The unit on the left was not found to have significant oscillations. Reprinted, with permission, from Schrock et al. (2009).
The final common pathway linking many of the different forms of dystonia may be abnormalities in cortical processing. Imaging studies in dystonic patients have demonstrated widespread changes in the activity of the primary motor cortex, SMA, anterior cingulate and dorsolateral prefrontal motor areas (for instance, Carbon et al., 2004, Asanuma et al., 2005). A large number of studies have demonstrated cortical sensory abnormalities (Berardelli et al., 1998, Hallett, 1998, Butefisch et al., 2005). Finally, intracortical excitability may also be increased (Deuschl et al., 1995, Kaji et al., 1995, Ikeda et al., 1996, Hamano et al., 1999, Sommer et al., 2002), and motor learning and plasticity may be disturbed (summarized in Breakefield et al., 2008). Given these extensive cortical activity changes, it is possible that the observed changes in the basal ganglia are in part not primary, but reflect some of the complex cortical alterations. For instance, altered sensorimotor maps similar to those in cortex, have been identified in the putamen (Delmaire et al., 2005) and thalamic levels (Lenz and Byl, 1999, Lenz et al., 1999). However, such changes were not seen in recent GPi recording studies (Chang et al., 2007, Tang et al., 2007).
5. Dyskinesias
Strictly speaking, the term ‘dyskinesia’ refers to an impairment in the ability of performing a voluntary movement. However, it is most commonly used to refer to involuntary spasmodic or repetitive movements such as those associated with chorea, i.e., irregular, brief and jerky involuntary movements. Unlike dystonia, there are no abnormal postures or sustained muscle contractions. Dyskinesias are heterogeneous in origin. They may arise without an identifiable cause, or secondary to other diseases. Examples for secondary types dyskinesia are ballismus, a characteristic form of large-amplitude proximal movements, which often occurs as a consequence of ischemic strokes in the STN (Ristic et al., 2002, Postuma and Lang, 2003, Lee et al., 2005), the involuntary movements associated with the use of dopaminergic drugs in PD (Fahn, 2000, Schrag and Quinn, 2000, Obeso et al., 2004), or movements seen with the long-term use of dopamine-receptor blocking drugs in patients with psychiatric or gastrointestinal diseases (tardive dyskinesias, see, e.g., review by Soares-Weiser and Fernandez, 2007). These varied conditions are grouped together here, because they share certain pathophysiologic features which will be described below.
Over the last decades, it has been shown that abnormalities in many of the basal ganglia nuclei can result in dyskinetic movements, including the striatum, GPe and STN. A potential causative role of striatal activity changes in some forms of dyskinesias or other hyperkinetic movement disorders (including myoclonus and tics) is supported by studies of the effects of GABA receptor antagonists in the striatum (Yoshida, 1991, Yoshida et al., 1991, Darbin and Wichmann, 2008, McCairn et al., 2009, Worbe et al., 2009). Furthermore, levodopa-induced dyskinesias are thought to arise from activity changes in the striatum (e.g. see review by Calabresi et al., 2010), subsequently resulting in altered firing rates and patterns of neurons throughout the rest of the basal ganglia-thalamocortical circuitry.
In terms of an involvement of GPe activity, GABA receptor blockade in this nucleus has been linked to dyskinetic movements (Grabli et al., 2004, Bronfeld et al., 2010). Similarly, STN inactivation is associated with ballismus, as shown through studies using electrolytic STN lesioning or injections of fiber-sparing excitotoxins or GABA-receptor agonists into the STN (Whittier and Mettler, 1949, Carpenter et al., 1950, Bergman et al., 1990, Hamada and DeLong, 1992a, b, Wichmann et al., 1994).
In contrast to the use of the rate model to explain the hypokinetic features of parkinsonism as a consequence of increased basal ganglia output to the thalamus (see above), the development of involuntary movements can be understood as the result of reductions in basal ganglia output to the thalamus. This is supported by studies in animals and humans with drug-induced dyskinesias which have provided evidence that the neuronal activity in the basal ganglia output nuclei is strongly reduced in animals or in humans with parkinsonism, who display dyskinesias after being treated with dopamine receptor agonists (Filion et al., 1991, Papa et al., 1999, Levy et al., 2001). Likewise, studies in the late 1980s and early 1990s showed that the ballistic movements seen with STN inactivation are accompanied by substantial reductions of firing in GPe and GPi, in this case easily understood as a consequence of reduced or eliminated glutamatergic STN inputs to these nuclei (Hamada and DeLong, 1992a, b). It is also conceivable that GABA-receptor antagonist injections in GPe results directly or indirectly in enhanced inhibition of GPi which may then contribute to dyskinetic movements.
In terms of the rate model it is paradoxical that GPi inactivation rarely (if ever) leads to dyskinesias. In fact, GPi lesioning is clinically used to abolish dyskinesias in patients with treatment-resistant hyperkinetic movements. This observation argues strongly against the possibility that cessation of GPi activity alone results in dyskinesias (e.g., Obeso et al., 2000), and suggest that a certain level of GPi activity, as well as abnormalities of basal ganglia output other than rate changes play an important role in the development of involuntary movements (Picconi et al., 2003, Silberstein et al., 2005, Alonso-Frech et al., 2006). The role of oscillatory activity in mediating dyskinesias has not been extensively studied. There are, however, a few reports in PD patients reporting significant alterations in oscillatory power in STN and GPi during periods of dyskinesias (Silberstein et al., 2005, Alonso-Frech et al., 2006) and these point to the possible involvement of such abnormal activity in the pathogenesis of dyskinesias.
6. Conclusion
The identification of abnormalities in firing rates or patterns in the basal ganglia and related areas in movement disorders remains a highly important field of research, because such findings may eventually help us to a better understanding of the involvement of the basal ganglia in parkinsonism, and the development of new treatments to help patients with these devastating diseases more specifically and with fewer side effects than currently possible. The facts described in the preceding sections demonstrate how much has been learned about changes in the basal ganglia activity in movement disorders. Most of these disorders are associated with changes in firing rates and firing patterns. The behavioral phenotypes of the individual disorders may depend strongly on the specific combination of rate and pattern abnormalities.
Of course, much still needs to be learned. For instance, we still do not know which of the rate or pattern changes cause specific disease phenotypes. We also need to better understand the importance of finding overlapping firing abnormalities in different movement disorders (such as PD and dystonia), to appreciate the regional specificity of firing rate and pattern changes, and to gauge the importance of subtle abnormalities (such as the duration or structure of bursts), as well as the development of synchronous network activities and deficits in synaptic plasticity. Furthermore, comparatively little is known about the downstream consequences of the firing rate and pattern abnormalities in the basal ganglia on information processing in thalamic, cortical and brainstem activities, yet these downstream effects will eventually determine the behavioral consequences of abnormal basal ganglia discharge.
Finally, researchers begin to realize that the assumption that a given disease is a ‘basal ganglia disease’ may need revision. We are gaining a much better appreciation for the widespread structural or biochemical changes that accompany diseases such as PD or dystonia which involve many brain regions other than the basal ganglia. It is therefore possible that some of the motor abnormalities in diseases such as dystonia or forms of dyskinesias do not originate in the basal ganglia, but in other areas, such as the thalamus, cortex, brain stem, or the cerebellum.
Highlights.
Movement disorders are associated with varying combinations of changes in discharge rate and oscillatory synchronized bursting activities
The specific combination of these changes may determine the eventual behavioral disease manifestations
The link(s) between basal ganglia firing abnormalities and movement disorder symptoms are not (yet) established
Acknowledgments
The preparation of this review article was supported through grants from the NIH/NINDS (R01-NS054976, R01-NS071074 and P50-NS071669 (TW), and R01-NS40872 (JOD)), by NIH/NCRR grant RR-000165 (Yerkes National Primate Center), and by grants from the Canadian Institutes of Health Research (MOP-42505 and MOP-98006 (JOD)).
Abbreviations
- 6-OHDA
6-hydroxy dopamine
- CM
centromedian nucleus of thalamus
- CMA
cingulate motor area
- DBS
deep brain stimulation
- EMG
electromyogram
- GPe
external pallidal segment
- GPi
internal segment of the globus pallidus
- LFP
local field potentials
- M1
primary motor cortex
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- PD
Parkinson’s disease
- Pf
parafascicular nucleus of the thalamus
- PMC
premotor cortex
- PPN
pedunculopontine nucleus
- SMA
supplementary motor area
- SNc
substantia nigra, pars compacta
- SNr
substantia nigra pars reticulata
- STN
subthalamic nucleus
- VL
ventrolateral nucleus of the thalamus
- VA
ventral anterior nuclei of the thalamus
Footnotes
7. Conflicts of interests
The authors declare no actual or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, ‘prefrontal’ and ‘limbic’ functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alonso-Frech F, Zamarbide I, Alegre M, Rodriguez-Oroz MC, Guridi J, Manrique M, Valencia M, Artieda J, Obeso JA. Slow oscillatory activity and levodopa-induced dyskinesias in Parkinson’s disease. Brain. 2006;129:1748–1757. doi: 10.1093/brain/awl103. [DOI] [PubMed] [Google Scholar]
- Aravamuthan BR, Muthusamy KA, Stein JF, Aziz TZ, Johansen-Berg H. Topography of cortical and subcortical connections of the human pedunculopontine and subthalamic nuclei. Neuroimage. 2007;37:694–705. doi: 10.1016/j.neuroimage.2007.05.050. [DOI] [PubMed] [Google Scholar]
- Asanuma K, Carbon-Correll M, Eidelberg D. Neuroimaging in human dystonia. J Med Invest. 2005;52(Suppl):272–279. doi: 10.2152/jmi.52.272. [DOI] [PubMed] [Google Scholar]
- Augood SJ, Hollingsworth Z, Albers DS, Yang L, Leung JC, Muller B, Klein C, Breakefield XO, Standaert DG. Dopamine transmission in DYT1 dystonia: a biochemical and autoradiographical study. Neurology. 2002;59:445–448. doi: 10.1212/wnl.59.3.445. [DOI] [PubMed] [Google Scholar]
- Balcioglu A, Kim MO, Sharma N, Cha JH, Breakefield XO, Standaert DG. Dopamine release is impaired in a mouse model of DYT1 dystonia. J Neurochem. 2007;102:783–788. doi: 10.1111/j.1471-4159.2007.04590.x. [DOI] [PubMed] [Google Scholar]
- Baufreton J, Garret M, Rivera A, de la Calle A, Gonon F, Dufy B, Bioulac B, Taupignon A. D5 (not D1) dopamine receptors potentiate burst-firing in neurons of the subthalamic nucleus by modulating an L-type calcium conductance. J Neurosci. 2003:816–825. doi: 10.1523/JNEUROSCI.23-03-00816.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baufreton J, Zhu ZT, Garret M, Bioulac B, Johnson SW, Taupignon AI. Dopamine receptors set the pattern of activity generated in subthalamic neurons. Faseb J. 2005;19:1771–1777. doi: 10.1096/fj.04-3401hyp. [DOI] [PubMed] [Google Scholar]
- Bauswein E, Fromm C, Preuss A. Corticostriatal cells in comparison with pyramidal tract neurons: contrasting properties in the behaving monkey. Brain Res. 1989;493:198–203. doi: 10.1016/0006-8993(89)91018-4. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M, Marsden CD. The pathophysiology of primary dystonia. Brain. 1998;121:1195–1212. doi: 10.1093/brain/121.7.1195. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Hallworth NE, Baufreton J. GABAergic control of the subthalamic nucleus. Prog Brain Res. 2007;160:173–188. doi: 10.1016/S0079-6123(06)60010-1. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Magill PJ, Terman D, Bolam JP, Wilson CJ. Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network. Trends in Neurosciences. 2002;25:525–531. doi: 10.1016/s0166-2236(02)02235-x. [DOI] [PubMed] [Google Scholar]
- Bezard E, Boraud T, Bioulac B, Gross CE. Involvement of the subthalamic nucleus in glutamatergic compensatory mechanisms. Eur J Neurosci. 1999;11:2167–2170. doi: 10.1046/j.1460-9568.1999.00627.x. [DOI] [PubMed] [Google Scholar]
- Bezard E, Gross CE, Brotchie JM. Presymptomatic compensation in Parkinson’s disease is not dopamine-mediated. Trends in Neurosciences. 2003;26:215–221. doi: 10.1016/S0166-2236(03)00038-9. [DOI] [PubMed] [Google Scholar]
- Boraud T, Bezard E, Bioulac B, Gross C. High frequency stimulation of the internal Globus Pallidus (GPi) simultaneously improves parkinsonian symptoms and reduces the firing frequency of GPi neurons in the MPTP-treated monkey. Neuroscience Letters. 1996;215:17–20. doi: 10.1016/s0304-3940(96)12943-8. [DOI] [PubMed] [Google Scholar]
- Boraud T, Bezard E, Guehl D, Bioulac B, Gross C. Effects of L-DOPA on neuronal activity of the globus pallidus externalis (GPe) and globus pallidus internalis (GPi) in the MPTP-treated monkey. Brain Res. 1998;787:157–160. doi: 10.1016/s0006-8993(97)01563-1. [DOI] [PubMed] [Google Scholar]
- Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci U S A. 2010;107:8452–8456. doi: 10.1073/pnas.1000496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9:222–234. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- Breit S, Bouali-Benazzouz R, Popa RC, Gasser T, Benabid AL, Benazzouz A. Effects of 6-hydroxydopamine-induced severe or partial lesion of the nigrostriatal pathway on the neuronal activity of pallido-subthalamic network in the rat. Exp Neurol. 2007;205:36–47. doi: 10.1016/j.expneurol.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Bronfeld M, Belelovsky K, Erez Y, Bugaysen J, Korngreen A, Bar-Gad I. Bicuculline induced chorea manifests in focal rather than globalized abnormalities in the activation of the external and internal globus pallidus. J Neurophysiol. 2010 doi: 10.1152/jn.00093.2010. [DOI] [PubMed] [Google Scholar]
- Brown P. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson’s disease. Mov Disord. 2003;18:357–363. doi: 10.1002/mds.10358. [DOI] [PubMed] [Google Scholar]
- Brown P, Williams D. Basal ganglia local field potential activity: character and functional significance in the human. Clin Neurophysiol. 2005;116:2510–2519. doi: 10.1016/j.clinph.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Boroojerdi B, Chen R, Battaglia F, Hallett M. Task-dependent intracortical inhibition is impaired in focal hand dystonia. Mov Disord. 2005;20:545–551. doi: 10.1002/mds.20367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Di Filippo M, Ghiglieri V, Tambasco N, Picconi B. Levodopa-induced dyskinesias in patients with Parkinson’s disease: filling the bench-to-bedside gap. Lancet Neurol. 2010;9:1106–1117. doi: 10.1016/S1474-4422(10)70218-0. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Carbon M, Trost M, Ghilardi MF, Eidelberg D. Abnormal brain networks in primary torsion dystonia. Adv Neurol. 2004;94:155–161. [PubMed] [Google Scholar]
- Carpenter MB, Whittier JR, Mettler FA. Analysis of choreoid hyperkinesia in the rhesus monkey: surgical and pharmacological analysis of hyperkinesia resulting from lesions in the subthalamic nucleus of Luys. J Comp Neurol. 1950;92:293–332. doi: 10.1002/cne.900920303. [DOI] [PubMed] [Google Scholar]
- Casey DE. Dopamine D1 (SCH 23390) and D2 (haloperidol) antagonists in drug-naive monkeys. Psychopharmacol (Berlin) 1992;107:18–22. doi: 10.1007/BF02244960. [DOI] [PubMed] [Google Scholar]
- Chang EF, Turner RS, Ostrem JL, Davis VR, Starr PA. Neuronal responses to passive movement in the globus pallidus internus in primary dystonia. J Neurophysiol. 2007;98:3696–3707. doi: 10.1152/jn.00594.2007. [DOI] [PubMed] [Google Scholar]
- Cragg SJ. Meaningful silences: how dopamine listens to the ACh pause. Trends Neurosci. 2006;29:125–131. doi: 10.1016/j.tins.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Daley J, Turner R, Bliwise D, Rye D. Excessive daytime sleepiness following MPTP-induced dopamine depletion in Rhesus monkeys. J Sleep Res. 2002;11:44–45. [Google Scholar]
- Darbin O, Wichmann T. Effects of Striatal GABAA-Receptor Blockade on Striatal and Cortical Activity in Monkeys. J Neurophysiol. 2008;99:1294–1305. doi: 10.1152/jn.01191.2007. [DOI] [PubMed] [Google Scholar]
- Day M, Wokosin D, Plotkin JL, Tian X, Surmeier DJ. Differential excitability and modulation of striatal medium spiny neuron dendrites. J Neurosci. 2008;28:11603–11614. doi: 10.1523/JNEUROSCI.1840-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmaire C, Krainik A, Tezenas du Montcel S, Gerardin E, Meunier S, Mangin JF, Sangla S, Garnero L, Vidailhet M, Lehericy S. Disorganized somatotopy in the putamen of patients with focal hand dystonia. Neurology. 2005;64:1391–1396. doi: 10.1212/01.WNL.0000158424.01299.76. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends in Neurosciences. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Toro C, Matsumoto J, Hallett M. Movement-related cortical potentials in writer’s cramp. Ann Neurol. 1995;38:862–868. doi: 10.1002/ana.410380606. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Pierucci M, Esposito E, Crescimanno G, Benigno A, Di Giovanni G. Serotonin modulation of the basal ganglia circuitry: therapeutic implication for Parkinson’s disease and other motor disorders. Prog Brain Res. 2008;172:423–463. doi: 10.1016/S0079-6123(08)00921-7. [DOI] [PubMed] [Google Scholar]
- Fahn S. The spectrum of levodopa-induced dyskinesias. Ann Neurol. 2000;47:S2–9. discussion S9–11. [PubMed] [Google Scholar]
- Filion M. Effects of interruption of the nigrostriatal pathway and of dopaminergic agents on the spontaneous activity of globus pallidus neurons in the awake monkey. Brain Res. 1979;178:425–441. doi: 10.1016/0006-8993(79)90704-2. [DOI] [PubMed] [Google Scholar]
- Filion M, Tremblay L. Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res. 1991;547:142–151. [PubMed] [Google Scholar]
- Filion M, Tremblay L, Bedard PJ. Effects of dopamine agonists on the spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res. 1991;547:152–161. [PubMed] [Google Scholar]
- Fox SH, Brotchie JM. The MPTP-lesioned non-human primate models of Parkinson’s disease. Past, present, and future. Prog Brain Res. 2010;184:133–157. doi: 10.1016/S0079-6123(10)84007-5. [DOI] [PubMed] [Google Scholar]
- Fox SH, Chuang R, Brotchie JM. Serotonin and Parkinson’s disease: On movement, mood, and madness. Mov Disord. 2009;24:1255–1266. doi: 10.1002/mds.22473. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hu X, Smith Y, Wichmann T. Localization and function of GABA transporters in the globus pallidus of parkinsonian monkeys. Exp Neurol. 2010;223:505–515. doi: 10.1016/j.expneurol.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clin Neurophysiol. 2008;119:1459–474. doi: 10.1016/j.clinph.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatev P, Darbin O, Wichmann T. Oscillations in the basal ganglia under normal conditions and in movement disorders. Mov Disord. 2006;21:1566–1577. doi: 10.1002/mds.21033. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. Indirect-pathway neurons lose their spines in Parkinson disease. Nat Neurosci. 2006;9:157–158. doi: 10.1038/nn0206-157. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of Striatal Projection Systems by Dopamine. Annu Rev Neurosci. 2010 doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach J, Hansen L. Clozapine and D1/D2 antagonism in extrapyramidal functions. BrJPychiatr. 1997;17 (Suppl):34–37. [PubMed] [Google Scholar]
- Giuffrida R, Li Volsi G, Maugeri G, Perciavalle V. Influences of pyramidal tract on the subthalamic nucleus in the cat. Neurosci Lett. 1985;54:231–235. doi: 10.1016/s0304-3940(85)80084-7. [DOI] [PubMed] [Google Scholar]
- Goldberg JA, Rokni U, Boraud T, Vaadia E, Bergman H. Spike synchronization in the cortex/basal-ganglia networks of Parkinsonian primates reflects global dynamics of the local field potentials. J Neurosci. 2004;24:6003–6010. doi: 10.1523/JNEUROSCI.4848-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabli D, McCairn K, Hirsch EC, Agid Y, Feger J, Francois C, Tremblay L. Behavioural disorders induced by external globus pallidus dysfunction in primates: I. Behavioural study. Brain. 2004 Sep;127:2039–2054. doi: 10.1093/brain/awh220. [DOI] [PubMed] [Google Scholar]
- Hallett M. The neurophysiology of dystonia. Arch Neurol. 1998;55:601–603. doi: 10.1001/archneur.55.5.601. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Macdonald V, Henderson JM. A comparison of degeneration in motor thalamus and cortex between progressive supranuclear palsy and Parkinson’s disease. Brain. 2005;128:2272–2280. doi: 10.1093/brain/awh596. [DOI] [PubMed] [Google Scholar]
- Hamada I, DeLong MR. Excitotoxic acid lesions of the primate subthalamic nucleus result in reduced pallidal neuronal activity during active holding. J Neurophysiol. 1992a;68:1859–1866. doi: 10.1152/jn.1992.68.5.1859. [DOI] [PubMed] [Google Scholar]
- Hamada I, DeLong MR. Excitotoxic acid lesions of the primate subthalamic nucleus result in transient dyskinesias of the contralateral limbs. J Neurophysiol. 1992b;68:1850–1858. doi: 10.1152/jn.1992.68.5.1850. [DOI] [PubMed] [Google Scholar]
- Hamani C, Stone S, Laxton A, Lozano AM. The pedunculopontine nucleus and movement disorders: anatomy and the role for deep brain stimulation. Parkinsonism Relat Disord. 2007;13(Suppl 3):S276–280. doi: 10.1016/S1353-8020(08)70016-6. [DOI] [PubMed] [Google Scholar]
- Hamano T, Kaji R, Katayama M, Kubori T, Ikeda A, Shibasaki H, Kimura J. Abnormal contingent negative variation in writer’s cramp. Clin Neurophysiol. 1999;110:508–515. doi: 10.1016/s1388-2457(98)00045-5. [DOI] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci. 2007;30:357–364. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Hantraye P, Riche D, Maziere M, Isacson O. A primate model of Huntington’s disease: behavioral and anatomical studies of unilateral excitotoxic lesions of the caudate-putamen in the baboon. Exp Neurol. 1990;108:91–104. doi: 10.1016/0014-4886(90)90014-j. [DOI] [PubMed] [Google Scholar]
- Hartmann-von Monakow K, Akert K, Kunzle H. Projections of the precentral motor cortex and other cortical areas of the frontal lobe to the subthalamic nucleus in the monkey. Exp Brain Res. 1978;33:395–403. doi: 10.1007/BF00235561. [DOI] [PubMed] [Google Scholar]
- Heimer G, Bar-Gad I, Goldberg JA, Bergman H. Dopamine replacement therapy reverses abnormal synchronization of pallidal neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of parkinsonism. J Neurosci. 2002;22:7850–7855. doi: 10.1523/JNEUROSCI.22-18-07850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O. Basal ganglia mechanisms of reward-oriented eye movement. Ann N Y Acad Sci. 2007;1104:229–249. doi: 10.1196/annals.1390.012. [DOI] [PubMed] [Google Scholar]
- Holgado AJ, Terry JR, Bogacz R. Conditions for the generation of beta oscillations in the subthalamic nucleus-globus pallidus network. J Neurosci. 2010;30:12340–12352. doi: 10.1523/JNEUROSCI.0817-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nature Neuroscience. 2005;8:1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- Hutchison WD, Lang AE, Dostrovsky JO, Lozano AM. Pallidal neuronal activity: implications for models of dystonia. Ann Neurol. 2003;53:480–488. doi: 10.1002/ana.10474. [DOI] [PubMed] [Google Scholar]
- Hutchison WD, Lozano AM, Davis K, Saint-Cyr JA, Lang AE, Dostrovsky JO. Differential neuronal activity in segments of globus pallidus in Parkinson’s disease patients. Neuroreport. 1994;5:1533–1537. doi: 10.1097/00001756-199407000-00031. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Shibasaki H, Kaji R, Terada K, Nagamine T, Honda M, Hamano T, Kimura J. Abnormal sensorimotor integration in writer’s cramp: study of contingent negative variation. Mov Disord. 1996;11:683–690. doi: 10.1002/mds.870110614. [DOI] [PubMed] [Google Scholar]
- Inase M, Tokuno H, Nambu A, Akazawa T, Takada M. Corticostriatal and corticosubthalamic input zones from the presupplementary motor area in the macaque monkey: comparison with the input zones from the supplementary motor area. Brain Res. 1999;833:191–201. doi: 10.1016/s0006-8993(99)01531-0. [DOI] [PubMed] [Google Scholar]
- Ingham CA, Hood SH, Taggart P, Arbuthnott GW. Plasticity of synapses in the rat neostriatum after unilateral lesion of the nigrostriatal dopaminergic pathway. J Neurosci. 1998;18:4732–4743. doi: 10.1523/JNEUROSCI.18-12-04732.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahori N. A Golgi study on the subthalamic nucleus of the cat. J Comp Neurol. 1978;182:383–397. doi: 10.1002/cne.901820303. [DOI] [PubMed] [Google Scholar]
- Kaji R, Ikeda A, Ikeda T, Kubori T, Mezaki T, Kohara N, Kanda M, Nagamine T, Honda M, Rothwell JC, et al. Physiological study of cervical dystonia. Task-specific abnormality in contingent negative variation. Brain. 1995;118:511–522. doi: 10.1093/brain/118.2.511. [DOI] [PubMed] [Google Scholar]
- Kalen P, Strecker RE, Rosengren E, Bjorklund A. Regulation of striatal serotonin release by the lateral habenula-dorsal raphe pathway in the rat as demonstrated by in vivo microdialysis: role of excitatory amino acids and GABA. Brain Res. 1989;492:187–202. doi: 10.1016/0006-8993(89)90901-3. [DOI] [PubMed] [Google Scholar]
- Kaneda K, Isa K, Yanagawa Y, Isa T. Nigral inhibition of GABAergic neurons in mouse superior colliculus. J Neurosci. 2008;28:11071–11078. doi: 10.1523/JNEUROSCI.3263-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Minamimoto T, Matsumoto N, Hori Y. Monitoring and switching of cortico-basal ganglia loop functions by the thalamo-striatal system. Neurosci Res. 2004;48:355–360. doi: 10.1016/j.neures.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Kitai ST, Deniau JM. Cortical inputs to the subthalamus: intracellular analysis. Brain Res. 1981;214:411–415. doi: 10.1016/0006-8993(81)91204-x. [DOI] [PubMed] [Google Scholar]
- Kliem MA, Maidment NT, Ackerson LC, Chen S, Smith Y, Wichmann T. Activation of nigral and pallidal dopamine D1-like receptors modulates basal ganglia outflow in monkeys. J Neurophysiol. 2007;98:1489–1500. doi: 10.1152/jn.00171.2007. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Trottenberg T, Kivi A, Kupsch A, Schneider GH, Brown P. The relationship between local field potential and neuronal discharge in the subthalamic nucleus of patients with Parkinson’s disease. Exp Neurol. 2005;194:212–220. doi: 10.1016/j.expneurol.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Leblois A, Meissner W, Bioulac B, Gross CE, Hansel D, Boraud T. Late emergence of synchronized oscillatory activity in the pallidum during progressive Parkinsonism. Eur J Neurosci. 2007;26:1701–1713. doi: 10.1111/j.1460-9568.2007.05777.x. [DOI] [PubMed] [Google Scholar]
- Lee HS, Kim SW, Yoo IS, Chung SP. Common causes of hemiballism. Am J Emerg Med. 2005;23:576–578. doi: 10.1016/j.ajem.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Lee JI, Shin HJ, Nam DH, Kim JS, Hong SC, Park K, Eoh W, Kim JH, Lee WY. Increased burst firing in substantia nigra pars reticulata neurons and enhanced response to selective D2 agonist in hemiparkinsonian rats after repeated administration of apomorphine. J Korean Med Sci. 2001;16:636–642. doi: 10.3346/jkms.2001.16.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W, Jiao Y, Del Mar N, Reiner A. Evidence for differential cortical input to direct pathway versus indirect pathway striatal projection neurons in rats. J Neurosci. 2004;24:8289–8299. doi: 10.1523/JNEUROSCI.1990-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz FA, Byl NN. Reorganization in the cutaneous core of the human thalamic principal somatic sensory nucleus (Ventral caudal) in patients with dystonia. J Neurophysiol. 1999;82:3204–3212. doi: 10.1152/jn.1999.82.6.3204. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Jaeger CJ, Seike MS, Lin YC, Reich SG, DeLong MR, Vitek JL. Thalamic single neuron activity in patients with dystonia: dystonia-related activity and somatic sensory reorganization. J Neurophysiol. 1999;82:2372–2392. doi: 10.1152/jn.1999.82.5.2372. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Suarez JI, Metman LV, Reich SG, Karp BI, Hallett M, Rowland LH, Dougherty PM. Pallidal activity during dystonia: somatosensory reorganisation and changes with severity. J Neurol Neurosurg Psychiatry. 1998;65:767–770. doi: 10.1136/jnnp.65.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque M, Parent A. The striatofugal fiber system in primates: a reevaluation of its organization based on single-axon tracing studies. Proc Natl Acad Sci (USA) 2005;102:11888–11893. doi: 10.1073/pnas.0502710102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Dostrovsky JO, Lang AE, Sime E, Hutchison WD, Lozano AM. Effects of apomorphine on subthalamic nucleus and globus pallidus internus neurons in patients with Parkinson’s disease. J Neurophysiol. 2001;86:249–260. doi: 10.1152/jn.2001.86.1.249. [DOI] [PubMed] [Google Scholar]
- Liu P, Basso MA. Substantia nigra stimulation influences monkey superior colliculus neuronal activity bilaterally. J Neurophysiol. 2008;100:1098–1112. doi: 10.1152/jn.01043.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald V, Halliday GM. Selective loss of pyramidal neurons in the pre-supplementary motor cortex in Parkinson’s disease. Mov Disord. 2002;17:1166–1173. doi: 10.1002/mds.10258. [DOI] [PubMed] [Google Scholar]
- Magnin M, Morel A, Jeanmonod D. Single-unit analysis of the pallidum, thalamus and subthalamic nucleus in parkinsonian patients. Neurosci. 2000;96:549–564. doi: 10.1016/s0306-4522(99)00583-7. [DOI] [PubMed] [Google Scholar]
- Mallet N, Pogosyan A, Marton LF, Bolam JP, Brown P, Magill PJ. Parkinsonian beta oscillations in the external globus pallidus and their relationship with subthalamic nucleus activity. J Neurosci. 2008a;28:14245–14258. doi: 10.1523/JNEUROSCI.4199-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Pogosyan A, Sharott A, Csicsvari J, Bolam JP, Brown P, Magill PJ. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J Neurosci. 2008b;28:4795–4806. doi: 10.1523/JNEUROSCI.0123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden CD, Obeso JA. The functions of the basal ganglia and the paradox of stereotaxic surgery in Parkinson’s disease. Brain. 1994;117:877–897. doi: 10.1093/brain/117.4.877. [DOI] [PubMed] [Google Scholar]
- McCairn KW, Bronfeld M, Belelovsky K, Bar-Gad I. The neurophysiological correlates of motor tics following focal striatal disinhibition. Brain. 2009;132:2125–2138. doi: 10.1093/brain/awp142. [DOI] [PubMed] [Google Scholar]
- McFarland NR, Haber SN. Convergent inputs from thalamic motor nuclei and frontal cortical areas to the dorsal striatum in the primate. J Neurosci. 2000;20:3798–3813. doi: 10.1523/JNEUROSCI.20-10-03798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland NR, Haber SN. Organization of thalamostriatal terminals from the ventral motor nuclei in the macaque. J Comp Neurol. 2001;429:321–336. doi: 10.1002/1096-9861(20000108)429:2<321::aid-cne11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Mena-Segovia J, Bolam JP, Magill PJ. Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci. 2004;27:585–588. doi: 10.1016/j.tins.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Menza M, Dobkin RD, Marin H, Bienfait K. Sleep disturbances in Parkinson’s disease. Mov Disord. 2010;25(Suppl 1):S117–122. doi: 10.1002/mds.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Miller WC, DeLong MR. Altered tonic activity of neurons in the globus pallidus and subthalamic nucleus in the primate MPTP model of parkinsonism. In: Carpenter MB, Jayaraman A, editors. The Basal Ganglia II. New York: Plenum Press; 1987. pp. 415–427. [Google Scholar]
- Minamimoto T, Hori Y, Kimura M. Roles of the thalamic CM-PF complex-Basal ganglia circuit in externally driven rebias of action. Brain Res Bull. 2009;78:75–79. doi: 10.1016/j.brainresbull.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Mitchell IJ, Clarke CE, Boyce S, Robertson RG, Peggs D, Sambrook MA, Crossman AR. Neural mechanisms underlying parkinsonian symptoms based upon regional uptake of 2-deoxyglucose in monkeys exposed to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurosci. 1989;32:213–226. doi: 10.1016/0306-4522(89)90120-6. [DOI] [PubMed] [Google Scholar]
- Mitchell IJ, Luquin R, Boyce S, Clarke CE, Robertson RG, Sambrook MA, Crossman AR. Neural mechanisms of dystonia: evidence from a 2-deoxyglucose uptake study in a primate model of dopamine agonist-induced dystonia. Mov Disord. 1990;5:49–54. doi: 10.1002/mds.870050113. [DOI] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, Lu X. Differential activation of monkey striatal neurons in the early and late stages of procedural learning. Exp Brain Res. 2002;146:122–126. doi: 10.1007/s00221-002-1213-7. [DOI] [PubMed] [Google Scholar]
- Morris G, Schmidt R, Bergman H. Striatal action-learning based on dopamine concentration. Exp Brain Res. 2010;200:307–317. doi: 10.1007/s00221-009-2060-6. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci Res. 2002;43:111–117. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- Ni ZG, Bouali-Benazzouz R, Gao DM, Benabid AL, Benazzouz A. Time-course of changes in firing rates and firing patterns of subthalamic nucleus neuronal activity after 6-OHDA-induced dopamine depletion in rats. Brain Res. 2001;899:142–147. doi: 10.1016/s0006-8993(01)02219-3. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz M, Marin C, Alonso F, Zamarbide I, Lanciego JL, Rodriguez-Diaz M. The origin of motor fluctuations in Parkinson’s disease: importance of dopaminergic innervation and basal ganglia circuits. Neurology. 2004;62:S17–30. doi: 10.1212/wnl.62.1_suppl_1.s17. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Rodriguez M, Macias R, Alvarez L, Guridi J, Vitek J, DeLong MR. Pathophysiologic basis of surgery for Parkinson’s disease. Neurology. 2000;55:S7–12. [PubMed] [Google Scholar]
- Papa SM, Desimone R, Fiorani M, Oldfield EH. Internal globus pallidus discharge is nearly suppressed during levodopa-induced dyskinesias. Ann Neurol. 1999;46:732–738. doi: 10.1002/1531-8249(199911)46:5<732::aid-ana8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Parent M, Parent A. Single-axon tracing study of corticostriatal projections arising from primary motor cortex in primates. J Comp Neurol. 2006;496:202–213. doi: 10.1002/cne.20925. [DOI] [PubMed] [Google Scholar]
- Picconi B, Centonze D, Hakansson K, Bernardi G, Greengard P, Fisone G, Cenci MA, Calabresi P. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat Neurosci. 2003;6:501–506. doi: 10.1038/nn1040. [DOI] [PubMed] [Google Scholar]
- Pifl C, Schingnitz G, Hornykiewicz O. Effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine on the regional distribution of brain monoamines in the rhesus monkey. Neurosci. 1991;44:591–605. doi: 10.1016/0306-4522(91)90080-8. [DOI] [PubMed] [Google Scholar]
- Pisani A, Bernardi G, Ding J, Surmeier DJ. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci. 2007;30:545–553. doi: 10.1016/j.tins.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Plenz D, Kitai S. A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature. 1999;400:677–682. doi: 10.1038/23281. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Lang AE. Hemiballism: revisiting a classic disorder. Lancet Neurol. 2003;2:661–668. doi: 10.1016/s1474-4422(03)00554-4. [DOI] [PubMed] [Google Scholar]
- Prescott IA, Dostrovsky JO, Moro E, Hodaie M, Lozano AM, Hutchison WD. Levodopa enhances synaptic plasticity in the substantia nigra pars reticulata of Parkinson’s disease patients. Brain. 2009;132:309–318. doi: 10.1093/brain/awn322. [DOI] [PubMed] [Google Scholar]
- Raike RS, Jinnah HA, Hess EJ. Animal models of generalized dystonia. NeuroRx. 2005;2:504–512. doi: 10.1602/neurorx.2.3.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A, Vaadia E, Bergman H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. J Neurosci. 2000;20:8559–8571. doi: 10.1523/JNEUROSCI.20-22-08559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristic A, Marinkovic J, Dragasevic N, Stanisavljevic D, Kostic V. Long-term prognosis of vascular hemiballismus. Stroke. 2002;33:2109–2111. doi: 10.1161/01.str.0000022810.76115.c0. [DOI] [PubMed] [Google Scholar]
- Rivlin-Etzion M, Marmor O, Heimer G, Raz A, Nini A, Bergman H. Basal ganglia oscillations and pathophysiology of movement disorders. Curr Opin Neurobiol. 2006;16:629–637. doi: 10.1016/j.conb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Rommelfanger KS, Wichmann T. Extrastriatal dopaminergic circuits of the Basal Ganglia. Front Neuroanat. 2010;4:139. doi: 10.3389/fnana.2010.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaltenbrand G, Wahren W. Atlas for Stereotaxy of the Human Brain. Stuttgart, Germany: Thieme-Verlag; 1977. [Google Scholar]
- Schrag A, Quinn N. Dyskinesias and motor fluctuations in Parkinson’s disease. A community-based study. Brain. 2000;123 (Pt 11):2297–2305. doi: 10.1093/brain/123.11.2297. [DOI] [PubMed] [Google Scholar]
- Schrock LE, Ostrem JL, Turner RS, Shimamoto SA, Starr PA. The subthalamic nucleus in primary dystonia: single-unit discharge characteristics. J Neurophysiol. 2009;102:3740–3752. doi: 10.1152/jn.00544.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Schwartzman RJ, Alexander GM, Ferraro TN, Grothusen JR, Stahl SM. Cerebral metabolism of parkinsonian primates 21 days after MPTP. Exp Neurol. 1988;102:307–313. doi: 10.1016/0014-4886(88)90224-5. [DOI] [PubMed] [Google Scholar]
- Shen KZ, Johnson SW. Dopamine depletion alters responses to glutamate and GABA in the rat subthalamic nucleus. Neuroreport. 2005;16:171–174. doi: 10.1097/00001756-200502080-00021. [DOI] [PubMed] [Google Scholar]
- Silberstein P, Kuhn AA, Kupsch A, Trottenberg T, Krauss JK, Wohrle JC, Mazzone P, Insola A, Di Lazzaro V, Oliviero A, Aziz T, Brown P. Patterning of globus pallidus local field potentials differs between Parkinson’s disease and dystonia. Brain. 2003;126:2597–2608. doi: 10.1093/brain/awg267. [DOI] [PubMed] [Google Scholar]
- Silberstein P, Oliviero A, Di Lazzaro V, Insola A, Mazzone P, Brown P. Oscillatory pallidal local field potential activity inversely correlates with limb dyskinesias in Parkinson’s disease. Exp Neurol. 2005;194:523–529. doi: 10.1016/j.expneurol.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Smith Y, Raju D, Nanda B, Pare JF, Galvan A, Wichmann T. The thalamostriatal systems: anatomical and functional organization in normal and parkinsonian states. Brain Res Bull. 2009;78:60–68. doi: 10.1016/j.brainresbull.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27:520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Soares-Weiser K, Fernandez HH. Tardive dyskinesia. Semin Neurol. 2007;27:159–169. doi: 10.1055/s-2007-971169. [DOI] [PubMed] [Google Scholar]
- Soares J, Kliem MA, Betarbet R, Greenamyre JT, Yamamoto B, Wichmann T. Role of external pallidal segment in primate parkinsonism: comparison of the effects of MPTP-induced parkinsonism and lesions of the external pallidal segment. J Neurosci. 2004;24:6417–6426. doi: 10.1523/JNEUROSCI.0836-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer M, Ruge D, Tergau F, Beuche W, Altenmuller E, Paulus W. Intracortical excitability in the hand motor representation in hand dystonia and blepharospasm. Mov Disord. 2002;17:1017–1025. doi: 10.1002/mds.10205. [DOI] [PubMed] [Google Scholar]
- Starr PA, Rau GM, Davis V, Marks WJ, Jr, Ostrem JL, Simmons D, Lindsey N, Turner RS. Spontaneous pallidal neuronal activity in human dystonia: comparison with Parkinson’s disease and normal macaque. J Neurophysiol. 2005;93:3165–3176. doi: 10.1152/jn.00971.2004. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Plotkin J, Shen W. Dopamine and synaptic plasticity in dorsal striatal circuits controlling action selection. Curr Opin Neurobiol. 2009;19:621–628. doi: 10.1016/j.conb.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada M, Tokuno H, Hamada I, Inase M, Ito Y, Imanishi M, Hasegawa N, Akazawa T, Hatanaka N, Nambu A. Organization of inputs from cingulate motor areas to basal ganglia in macaque monkey. Eur J Neurosci. 2001;14:1633–1650. doi: 10.1046/j.0953-816x.2001.01789.x. [DOI] [PubMed] [Google Scholar]
- Tang JK, Moro E, Mahant N, Hutchison WD, Lang AE, Lozano AM, Dostrovsky JO. Neuronal firing rates and patterns in the globus pallidus internus of patients with cervical dystonia differ from those with Parkinson’s disease. J Neurophysiol. 2007;98:720–729. doi: 10.1152/jn.01107.2006. [DOI] [PubMed] [Google Scholar]
- Terman D, Rubin JE, Yew AC, Wilson CJ. Activity patterns in a model for the subthalamopallidal network of the basal ganglia. J Neurosci. 2002;22:2963–2976. doi: 10.1523/JNEUROSCI.22-07-02963.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Riquelme LA, Belforte JE, Pazo JH, Murer MG. Substantia nigra pars reticulata units in 6-hydroxydopamine-lesioned rats: responses to striatal D2 dopamine receptor stimulation and subthalamic lesions. Eur J Neurosci. 2000;12:247–256. doi: 10.1046/j.1460-9568.2000.00910.x. [DOI] [PubMed] [Google Scholar]
- Turner RS, DeLong MR. Corticostriatal activity in primary motor cortex of the macaque. J Neurosci. 2000;20:7096–7108. doi: 10.1523/JNEUROSCI.20-18-07096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila M, Perier C, Feger J, Yelnik J, Faucheux B, Ruberg M, Raisman-Vozari R, Agid Y, Hirsch EC. Evolution of changes in neuronal activity in the subthalamic nucleus of rats with unilateral lesion of the substantia nigra assessed by metabolic and electrophysiological measurements. Eur J Neurosci. 2000;12:337–344. doi: 10.1046/j.1460-9568.2000.00901.x. [DOI] [PubMed] [Google Scholar]
- Villalba RM, Lee H, Smith Y. Dopaminergic denervation and spine loss in the striatum of MPTP-treated monkeys. Exp Neurol. 2009;215:220–227. doi: 10.1016/j.expneurol.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitek JL. Pathophysiology of dystonia: a neuronal model. Mov Disord. 2002;17(Suppl 3):S49–62. doi: 10.1002/mds.10142. [DOI] [PubMed] [Google Scholar]
- Vitek JL, Chockkan V, Zhang JY, Kaneoke Y, Evatt M, DeLong MR, Triche S, Mewes K, Hashimoto T, Bakay RA. Neuronal activity in the basal ganglia in patients with generalized dystonia and hemiballismus. Ann Neurol. 1999;46:22–35. doi: 10.1002/1531-8249(199907)46:1<22::aid-ana6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Weinberger M, Dostrovsky JO. A basis for the pathological oscillations in basal ganglia: the crucial role of dopamine. Neuroreport. 2011;22:151–156. doi: 10.1097/WNR.0b013e328342ba50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger M, Mahant N, Hutchison WD, Lozano AM, Moro E, Hodaie M, Lang AE, Dostrovsky JO. Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in Parkinson’s disease. J Neurophysiol. 2006;96:3248–3256. doi: 10.1152/jn.00697.2006. [DOI] [PubMed] [Google Scholar]
- Whittier JR, Mettler FA. Studies of the subthalamus of the rhesus monkey. II. Hyperkinesia and other physiologic effects of subthalamic lesions with special references to the subthalamic nucleus of Luys. J Comp Neurol. 1949;90:319–372. doi: 10.1002/cne.900900304. [DOI] [PubMed] [Google Scholar]
- Wichmann T. Commentary: Dopaminergic dysfunction in DYT1 dystonia. Exp Neurol. 2008;212:242–246. doi: 10.1016/j.expneurol.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, DeLong MR. The primate subthalamic nucleus. III. Changes in motor behavior and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:521–530. doi: 10.1152/jn.1994.72.2.521. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, Starr PA, Subramanian T, Watts RL, DeLong MR. Comparison of MPTP-induced changes in spontaneous neuronal discharge in the internal pallidal segment and in the substantia nigra pars reticulata in primates. Exp Brain Res. 1999;125:397–409. doi: 10.1007/s002210050696. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Kliem MA, Soares J. Slow oscillatory discharge in the primate basal ganglia. J Neurophysiol. 2002;87:1145–1148. doi: 10.1152/jn.00427.2001. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Soares J. Neuronal firing before and after burst discharges in the monkey basal ganglia is predictably patterned in the normal state and altered in parkinsonism. J Neurophysiol. 2006;95:2120–2133. doi: 10.1152/jn.01013.2005. [DOI] [PubMed] [Google Scholar]
- Wickens JR, Budd CS, Hyland BI, Arbuthnott GW. Striatal contributions to reward and decision making: making sense of regional variations in a reiterated processing matrix. Ann N Y Acad Sci. 2007;1104:192–212. doi: 10.1196/annals.1390.016. [DOI] [PubMed] [Google Scholar]
- Worbe Y, Baup N, Grabli D, Chaigneau M, Mounayar S, McCairn K, Feger J, Tremblay L. Behavioral and movement disorders induced by local inhibitory dysfunction in primate striatum. Cereb Cortex. 2009;19:1844–1856. doi: 10.1093/cercor/bhn214. [DOI] [PubMed] [Google Scholar]
- Yoshida M. The neuronal mechanism underlying parkinsonism and dyskinesia: differential roles of the putamen and caudate nucleus. NeurosciRes. 1991;12:31–40. doi: 10.1016/0168-0102(91)90097-i. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Nagatsuka Y, Muramatsu S, Niijima K. Differential roles of the caudate nucleus and putamen in motor behavior of the cat as investigated by local injection of GABA antagonists. Neurosci Res. 1991;10:34–51. doi: 10.1016/0168-0102(91)90018-t. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Decuypere M, Ledoux MS. Abnormal motor function and dopamine neurotransmission in DYT1 DeltaGAG transgenic mice. Exp Neurol. 2008;210:719–730. doi: 10.1016/j.expneurol.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Bartol M, Shen K, Johnson SW. Excitatory effects of dopamine on subthalamic nucleus neurons: in vitro study of rats pretreated with 6-hydroxydopamine and levodopa. Brain Res. 2002;945:31–40. doi: 10.1016/s0006-8993(02)02543-x. [DOI] [PubMed] [Google Scholar]
- Zhuang P, Li Y, Hallett M. Neuronal activity in the basal ganglia and thalamus in patients with dystonia. Clin Neurophysiol. 2004;115:2542–2557. doi: 10.1016/j.clinph.2004.06.006. [DOI] [PubMed] [Google Scholar]