Abstract
Microbially Induced Carbonate Precipitation is proposed as an environmentally friendly method to protect decayed ornamental stone and introduced in the field of preservation of Cultural Heritage. Recent conservation studies performed under laboratory conditions on non-sterile calcarenite stones have successfully reported on the application of a suitable nutritional solution, inoculated and non-inoculated with Myxococcus xanthus, as a bioconsolidation treatment. Furthermore, this procedure has been applied in situ, very recently, to selected historical buildings in Granada, Spain. For the first time, we evaluate the efficiency and risks of the in situ application of the above mentioned treatments onto two historical buildings in Granada. The evaluation consists of a detailed investigation of the micro-biota actively growing during the seven days of the treatments – short-term monitoring and of that remaining on the stones after six and twelve months of the application – long-term monitoring. A molecular strategy, including DNA extraction, PCR amplification of 16S rRNA sequences, construction of clone libraries and fingerprinting by DGGE (Denaturing Gradient Gel Electrophoresis) analysis followed by sequencing was used to gain insight into the microbial diversity present on the differentially treated stones. The monitoring of M. xanthus was performed by PCR using species-specific primers. Similar dynamics were triggered on both buildings by the application of the nutritional solution (inoculated or non-inoculated). 16S rDNA sequencing revealed the dominant occurrence of members belonging to the Firmicutes and Proteobacteria during the seven days of the treatment, whereas after one year the order Bacillales of the phylum Firmicutes was the predominantly detected microorganisms. M. xanthus could be detected only during the seven days of the treatment. The treatments seem to activate no dangerous microorganisms and furthermore, to select the remainder of a homogeneous group of carbonatogenic bacteria on the stones after a long period of time.
Keywords: Molecular short- and long-term monitoring, Microbial community, Limestone, In situ bio-consolidation treatment, PCR-DGGE, Clone sequencing
Research Highlights
► Two historical buildings received two different in situ bioconsolidation treatments. ► The microbial communities were monitored with molecular techniques. ► Similar dynamics occurred on the buildings during a short- and long-time monitoring. ► The primordial heterogeneous micro-biota was changed by the treatments. ► A homogenous micro-biota of carbonatogenic bacteria was established (mainly Firmicutes).
1. Introduction
Microbially Induced Carbonate Precipitation (MICP) has been proposed as an environmentally friendly method of protecting decayed ornamental stone. This method can be applied as a process of bio-deposition, i.e. the deposition of a protective surface layer with consolidation properties as well as a process of bio-cementation, i.e. the generation of a biologically-induced binder (De Muynck et al., 2010).
There are two main approaches regarding bio-deposition processes, those employing the use of calcinogenic microorganisms on stone surfaces and those where no microorganisms are applied to the surface. In the first series of approaches, different microorganisms and metabolic pathways have been used for the precipitation of calcium carbonate (Adolphe et al., 1990; Castanier et al., 1999; Dick et al., 2006a; May, 2005; Rodriguez-Navarro et al., 2003). In the second series of approaches, there are studies in which inducing macromolecules are supplied to the stone together with a supersaturated solution of calcium carbonate (Tiano et al., 1999, 2006). In addition, other studies show carbonate precipitation by the micro-biota inhabiting the stone by the mere addition of an activator medium to the stone (González-Muñoz et al., 2008; González-Muñoz, 2008; Jimenez-Lopez et al., 2007, 2008).
Although MICP has been widely investigated under laboratory conditions, further tests are necessary to prove the viability and efficacy of this bacterial capability in situ under non-sterile conditions. This fact can consequently affect the microbial activity of the inoculated carbonatogenic bacteria as well as the activity of those microorganisms inhabiting the stone. To date, only a few published studies have implemented the in situ application of MICP as consolidation treatment and have addressed the question of how the consolidation treatment is affecting the micro-biota inhabiting the stone during and after the consolidation treatment (Castanier et al., 2000; Jimenez-Lopez et al., 2007, 2008; Jroundi et al., 2010; Le Métayer-Levrel et al., 1999; Orial, 2000; Piñar et al., 2010).
As mentioned in the review of De Muynck et al. (2010), the satisfactory application of MICP as a consolidation treatment by conservators requires further knowledge not only of the effectiveness of the method but also of the risk factors, especially the long term effects of the inoculated bacteria and the applied nutrient media. In our opinion, this additional evaluation parameter should be a pre-requisite to evaluating the effectiveness and risks of the treatments.
In the present study we have evaluated the effect on the microbial population inhabiting the stone during (short-term) and after (long-term) the in situ application of two different consolidation treatments. The applied treatments, namely the application of a sterile nutritional solution inoculated and non-inoculated with Myxococcus xanthus, have already been tested for their efficacy in the consolidation of ornamental stones under laboratory conditions (Jimenez-Lopez et al., 2007, 2008; Rodriguez-Navarro et al., 2003). Recently, this optimized consolidation treatment was applied, in a pilot-study, to two historical buildings in situ. The two locations selected were the Monastery of San Jerónimo (SJ; Diego de Siloé, XVI Century) (Jroundi et al., 2010) and the Royal Hospital (RH; Enrique Egas, XVI Century). Both buildings are built of buff-coloured bioclastic calcarenite stone extracted from the same quarry (Santa Pudia quarries, Escúzar, Granada), and located in the same city (Granada, Spain) and, therefore, subject to the same climatic conditions. In the first case, the authors performed enrichment cultures during the seven days of treatment and analyzed the dynamics of the microbial communities by culture-dependent techniques (Jroundi et al., 2010).
The results presented in this study are part of this first in situ pilot-study. With the samples obtained we conducted short-term monitoring, over the period corresponding to the seven days of the treatment, in addition to the long-term monitoring of the bacterial communities inhabiting the stones, using a molecular strategy, performed six and twelve months after the treatment, respectively.
The goals of the present study were to investigate and compare a) the microbial communities inhabiting ornamental limestone from two different buildings prior to any consolidation treatment, b) the dynamics of the community structure during the seven days of the application of two consolidation and one control treatments (short-term monitoring), c) the impact of the treatments on the inhabiting micro-biota after six months and one year (long-term monitoring) and d) the prevalence of the inoculated bacterium, M. xanthus, on both buildings over the period of one year.
The molecular strategy applied combined the fingerprinting by Denaturing Gradient Gel Electrophoresis (DGGE), the construction and screening of 16S rDNA clone libraries by DGGE and the sequencing of selected clones. Furthermore, species-specific primers were used for the PCR detection of the inoculated strain M. xanthus.
2. Materials and methods
2.1. Experimental procedure
In order to analyse the micro-biota inhabiting the non-treated stones of the two buildings under investigation, stone samples were collected from three selected sectors of each building prior to the application of any treatment. Thereafter, the three selected areas on the buildings each received a different treatment: (i) sterile water as a control; (ii) a M. xanthus-inoculated culture, and (iii) a sterile M-3P nutritional solution. Thereafter, to evaluate the impact of these conservation treatments on the microbial community inhabiting the stones, two strategies were applied. Firstly: short-term monitoring to identify the members of the microbial community able to be activated quickly by the different treatments and, therefore, potentially responsible for the production of calcium carbonate within the first week. To this end, enrichment cultures were performed on the 1st and 7th days of the application of the treatment. This monitoring was carried out by culture-dependent (Jroundi et al., 2010) and –independent techniques (this study).
Secondly: long-term molecular monitoring (6 and 12 months after the treatments) was performed to identify the fraction of the microbial communities remaining on the stones after this period of time. The bacteria identified during and after the treatments were compared with those inhabiting the non-treated stones. This monitoring was performed by molecular means.
2.2. Stone-consolidation treatment
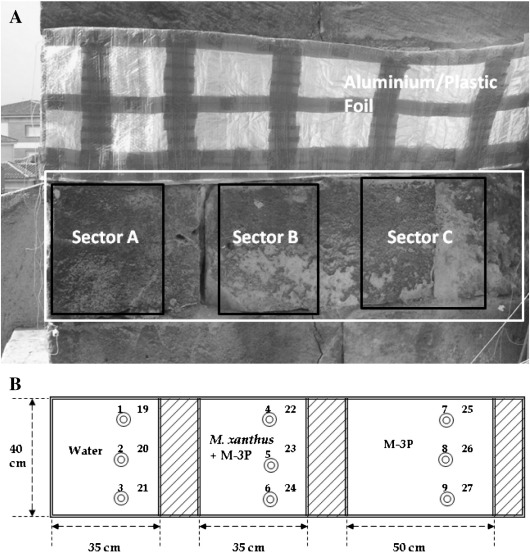
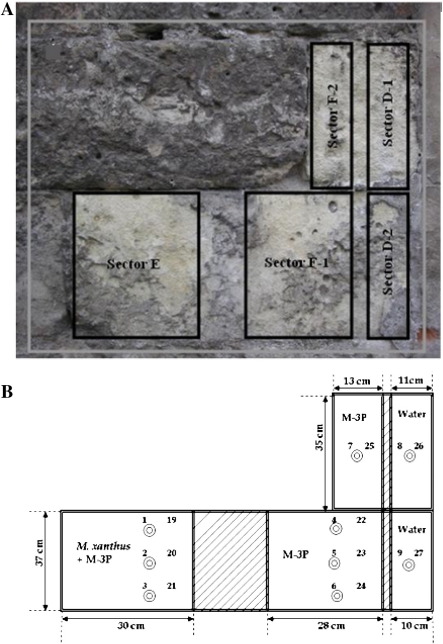
The decayed porous limestone (calcarenite) building blocks selected for the consolidation treatments in this study are part of an upright wall exposed for centuries to outdoor weathering in the church of San Jerónimo Monastery, and the Royal Hospital (Granada, Spain). In the case of SJ, the area selected for treatment has a southeast orientation and is located about 20 m above ground level. Treated sectors on the RH are located about 2 m above ground level on an outer wall with adjacent pavement (see Figs. 1 and 2).
Fig. 1.
Treated stone sectors of Monastery of San Jerónimo, Granada (Spain): A. Wall area showing the three sectors selected for the treatments with: A, water as a control; B, nutritional solution inoculated with M. xanthus the first day and with M-3P culture medium the rest of the days; C, liquid M-3P culture medium. B. Schematic representation of the sectors used for the treatments, indicating the sizes of the three areas. The circles and numbers 1 to 9 and 19 to 27 correspond to the sampling points for culture-dependent studies.
Fig. 2.
Treated stone sectors of the Royal Hospital, Granada (Spain): A. Wall area showing the three sectors selected for the treatments with: D (divided into the subsectors D-1 and D-2), water as a control; E, inoculated with M. xanthus the first day and with M-3P culture medium the rest of the days; F (also divided into the subsectors F-1 and F-2), liquid M-3P culture medium. B. Schematic representation of the sectors used for the treatments, indicating the sizes of the three areas, symbols as for Fig. 1.
Areas were delineated in three sectors and the treatment on both buildings was carried out as described in Jroundi et al. (2010). Briefly, sectors A (SJ) and D (RH) (the control areas) were treated with sterile distilled water; sectors B (SJ) and E (RH) received a culture of M. xanthus the first day of treatment and a sterile nutritional solution M-3P [1% Bacto Casitone, 1% Ca(CH3COO)2·4 H2O, 0.2% K2CO3·1/2 H2O in a 10 mM phosphate buffer, pH 8] (Rodriguez-Navarro et al., 2003) the remaining days of treatment; and sectors C (SJ) and F (RH) were only treated with the sterile nutritional solution M-3P. The treatment application was repeated twice every day over the seven days of treatment to keep the stone adequately damp.
2.3. Sampling
Sampling was carried out by two different methods: 1) taking stone grains directly from the surface of the stone before treatment and 1 year after (in the case of San Jerónimo, samples were also taken 6 months after treatment) and 2) applying filter paper pieces to the stone on the first and last days of treatments, as described below, to obtain bacterial pellets.
2.3.1. Stone sampling
Stone grains (about 300 mg) were taken from 3 different spots of each sector prior to the application of any treatment (hereafter referred to as ‘non-treated stone’) and after the treatments (at the times indicated above). These grains were added to 1 ml of sterile distilled water, mixed, and aliquots were taken to perform serial dilutions and inoculations of different selective media. The remaining water together with the stone grains was dried at 37 °C in sterile tubes sealed with a 45 μm pore size sterile filter. Dried stone grains were frozen at −80 °C for molecular analysis.
The three stone samples corresponding to each individual sector were pooled prior to DNA extraction. In the case of SJ samples they were called SJ-NT (non-treated stone), SA6, SB6, and SC6 for samples after 6 months of treatment, and SB12 and SC12 for samples after one year (see Fig. 3). Regarding the Royal Hospital sample RH-NT (non-treated stone) was taken before the treatment; samples SD12-1 and SD12-2 (sector D), sample SE12 (sector E) and samples SF12-1 and SF12-2 (sector F) were collected one year after the consolidation treatment (Fig. 4).
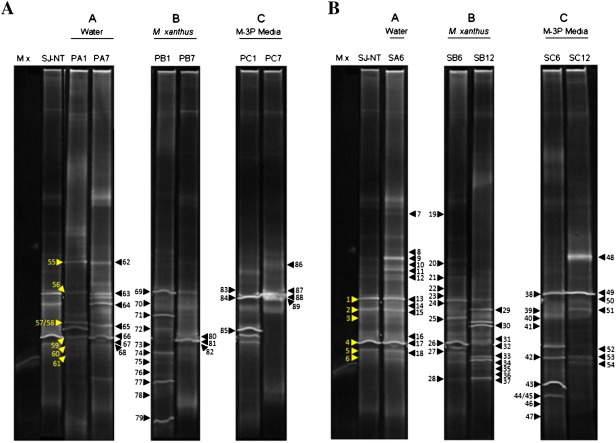
Fig. 3.
DGGE profiles of pellet and stone samples derived from the Monastery of San Jerónimo showing the succession in the bacterial community structure, as well as the monitoring during the time course experiment. The samples were loaded according to their corresponding sector. Capital letters A, B and C correlate to the different treated areas: sectors A (water treatment), sector B (M. xanthus-inoculated medium) and sector C (M-3P medium). Lane M.x.: M. xanthus strain 422 DNA for comparision. Lane SJ-NT: non-treated stone sample. A. DGGE fingerprints derived from pellet samples. Lane PA1: pellet sample from sector A from the 1st day of treatment. Lane PA7: pellet sample from sector A from the 7th day of treatment. Lane PB1: pellet sample from sector B from the 1st day of treatment. Lane PB7: pellet sample from sector B from the 7th day of treatment. Lane PC1: pellet sample from sector C from the 1st day of treatment. Lane PC7: pellet sample from sector C from the 7th day of treatment. B. DGGE fingerprints derived from stone samples. Lane SA6: stone sample from sector A after six months. Lane SB6: stone sample from sector B after six months. Lane SB12: stone sample from sector B after one year. Lane SC6: stone sample from sector C after six months. Lane SC12: stone sample from sector C after one year. Dominant, faint- and in the DGGE profile of the original sample not visible bands, identified from the 16S rDNA clone libraries were numbered and marked with arrowheads. The bands are explained in the Supplemantary Tables 3 and 4.
DGGE profiles of pellet and stone samples derived from the Monastery of San Jerónimo showing the succession in the bacterial community structure, as well as the monitoring during the time course experiment. The samples were loaded according to their corresponding sector. Capital letters A, B and C correlate to the different treated areas: sectors A (water treatment), sector B (M. xanthus-inoculated medium) and sector C (M-3P medium). Lane M.x.: M. xanthus strain 422 DNA for comparision. Lane SJ-NT: non-treated stone sample. A. DGGE fingerprints derived from pellet samples. Lane PA1: pellet sample from sector A from the 1st day of treatment. Lane PA7: pellet sample from sector A from the 7th day of treatment. Lane PB1: pellet sample from sector B from the 1st day of treatment. Lane PB7: pellet sample from sector B from the 7th day of treatment. Lane PC1: pellet sample from sector C from the 1st day of treatment. Lane PC7: pellet sample from sector C from the 7th day of treatment. B. DGGE fingerprints derived from stone samples. Lane SA6: stone sample from sector A after six months. Lane SB6: stone sample from sector B after six months. Lane SB12: stone sample from sector B after one year. Lane SC6: stone sample from sector C after six months. Lane SC12: stone sample from sector C after one year. Dominant, faint- and in the DGGE profile of the original sample not visible bands, identified from the 16S rDNA clone libraries were numbered and marked with arrowheads. The bands are explained in the Supplemantary Tables 3 and 4.
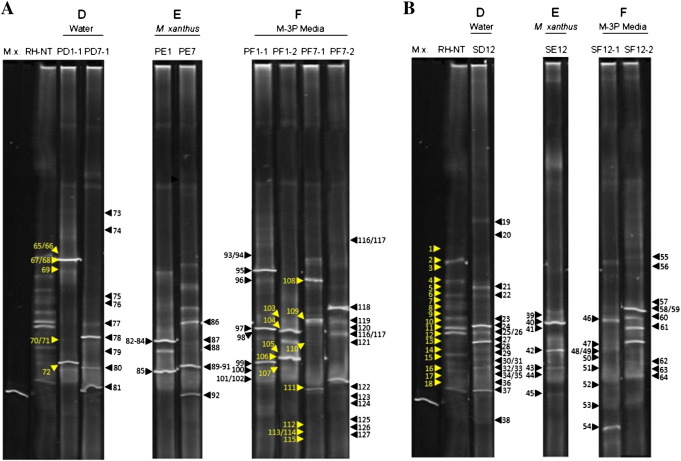
Fig. 4.
DGGE profiles of pellet and stone samples derived from the Royal Hospital showing the succession in the bacterial community structure, as well as the monitoring during the time course experiment. The samples were loaded according to their corresponding sector. Capital letters D, E and F correlate to the different treated areas: sectors D (water treatment), sector E (M. xanthus-inoculated medium) and sector F (M-3P medium). Lane M.x.: M. xanthus strain 422 DNA for comparision. Lane RH-NT: non-treated stone sample. A. DGGE fingerprints derived from pellet samples. Lane PD1-1: pellet sample from sector D-1 from the 1st day of treatment. Lane PD7-1: pellet sample from sector D-1 from the 7th day of treatment. Lane PE1: pellet sample from sector E from the 1st day of treatment. Lane PE7: pellet sample from sector E from the 7th day of treatment. Lane PF1-1: pellet sample from sector F-1 from the 1st day of treatment. Lane PF1-2: pellet sample from sector F-2 from the 1st day of treatment. Lane PF7-1: pellet sample from sector F-1 from the 7th day of treatment. Lane PF7-2: pellet sample from sector F-2 from the 7th day of treatment. B. DGGE fingerprints derived from stone samples. Lane SD12: stone sample from sector D after one year. Lane SE12: stone sample from sector E after one year. Lane SF12-1: stone sample from sector F-1 after one year. Lane SF12-2: stone sample from sector F-2 after one year. As for Fig. 3, the marked arrowheads are explained in the Supplemantary Tables 3 and 4.
DGGE profiles of pellet and stone samples derived from the Royal Hospital showing the succession in the bacterial community structure, as well as the monitoring during the time course experiment. The samples were loaded according to their corresponding sector. Capital letters D, E and F correlate to the different treated areas: sectors D (water treatment), sector E (M. xanthus-inoculated medium) and sector F (M-3P medium). Lane M.x.: M. xanthus strain 422 DNA for comparision. Lane RH-NT: non-treated stone sample. A. DGGE fingerprints derived from pellet samples. Lane PD1-1: pellet sample from sector D-1 from the 1st day of treatment. Lane PD7-1: pellet sample from sector D-1 from the 7th day of treatment. Lane PE1: pellet sample from sector E from the 1st day of treatment. Lane PE7: pellet sample from sector E from the 7th day of treatment. Lane PF1-1: pellet sample from sector F-1 from the 1st day of treatment. Lane PF1-2: pellet sample from sector F-2 from the 1st day of treatment. Lane PF7-1: pellet sample from sector F-1 from the 7th day of treatment. Lane PF7-2: pellet sample from sector F-2 from the 7th day of treatment. B. DGGE fingerprints derived from stone samples. Lane SD12: stone sample from sector D after one year. Lane SE12: stone sample from sector E after one year. Lane SF12-1: stone sample from sector F-1 after one year. Lane SF12-2: stone sample from sector F-2 after one year. As for Fig. 3, the marked arrowheads are explained in the Supplemantary Tables 3 and 4.
2.3.2. Sampling to obtain bacterial pellets
Sterile and absorbent filter paper (AFP, ANOIA, Spain) pieces (ca. 1 × 2 cm in size), that can absorb a volume of 15 μl/cm2 of solution, were used to collect the bacteria from the stone (Jroundi et al., 2010). As soon as the treated areas were humidified on the first day and just after the last application (day 7) of treatment, the paper pieces were placed for 5 minutes on several spots (from 1 to 9 and 19 to 27, see Figs. 1B and 2B) on the surface of the stone blocks. Each aseptically collected AFP piece was immediately added to 1 ml of sterile M-3P nutritional solution and transported to the laboratory for cultivation analysis. The tubes with the absorbent paper strips were subsequently made up to 5 ml with M-3P culture medium and incubated for 24 h. After this incubation time, the enrichment cultures were centrifuged (at 10,000 × g for 10 min) and the pellets were frozen (− 80 °C) for further molecular analysis.
2.4. DNA extraction and PCR analyses
DNA from stone slabs as well as from the collected bacterial pellets was extracted according to the protocol described by Schabereiter-Gurtner et al. (2001).
For all PCR reactions 2× PCR Master Mix from Promega [50 units/ml of TaqDNA Polymerase in a supplied reaction buffer (pH 8.5), 400 μM dATP, 400 μM dGTP, 400 μM dCTP, 400 μM dTTP, 3 mM MgCl2] was diluted to 1x and 50 pmol/μl of each primer was applied to the reaction volumes. Two PCR reactions were performed to amplify eubacterial 16S rDNA fragments for genetic fingerprinting by DGGE. First, primers 341f (Muyzer et al., 1993) and 907r (Teske et al., 1996) were used for the DNA amplification. Second, a semi-nested PCR using primers 341GC and 518r (Neefs et al., 1990) was performed for DGGE analyses. PCR products were pooled, precipitated in ethanol and 20 μl was loaded onto the DGGE gel. All PCR reactions were performed in an MJ Research PTC-200 Peltier Thermal Cycler with the thermocycling programs described by Schabereiter-Gurtner et al., 2001. Seven μl of each PCR product was run on a 2% (w/v) agarose gel for about 35 min at 110 V, stained in an ethidium bromide solution [1 μg/ml; stock: 10 mg/ml] for 15–25 min and visualized by a UVP documentation system (BioRad Transilluminator, Universal Hood; Mitsubishi P93D-printer). A negative control was carried out in all PCR reactions, where no template was added, to exclude the possibility of cross-contamination.
2.5. Fingerprint analysis by DGGE — Denaturing Gradient Gel Electrophoresis
For DGGE fingerprinting of the bacterial communities, the pooled PCR products supplemented with Loading Dye Solution (Fermentas), were run on gels in 0.5x TAE buffer [20 mM Tris, 10 mM acetate, 0.5 mM Na2EDTA; pH 8.0] for 3.5 hours at 200 V and 60 °C in a BIORAD-DCODE™ — Universal Mutation Detection System (Muyzer et al., 1993).
A chemical gradient ranging from 30 to 55% of urea and formamide in an 8% (w/v) polyacrylamide gel (BioRad, Munich, Germany) was used to separate the DNA bands from the different bacterial members. Staining of the denaturant polyacrylamide gels was done in a 1 μg/ml ethidium bromide solution [stock: 10 mg/ml] for 15–25 min and afterwards visualized by a UVP documentation system (BioRad Transilluminator, Universal Hood; Mitsubishi P93D-printer).
2.6. Construction of 16S rDNA clone libraries
For the construction of clone libraries 2 × 3 μl DNA templates of each sample were amplified in 2 × 50 μl reaction volumes using the universal primers 341f and 907r. Aliquots of PCR products were analysed on a 2% agarose gel and further purified using the QIAquick PCR Purification Kit (Qiagen), following the protocol of the manufacturer. The purified DNA was again visualized on a 2% agarose gel.
A volume of 5.5 μl of the purified PCR product was used as a ligation template for the pGEM — T easy Vector system (Promega) following the manufacturer's instructions. One shot TOP10 cells (Invitrogen) were used for the transformation reactions. These cells allow the identification of recombinants (white colonies) on an indicator LB medium containing ampicilline (100 μg/ml), streptomycine (25 μg/ml) and X-Gal (5-bromo-4-chloro-3-indolyl-ß-1-galactopyranoside; 0.1 mM) (Sambrook et al., 1989).
2.7. Screening of the bacterial clones
About 50 to 150 white clones from each clone library were harvested, and screened on DGGE as described by Schabereiter-Gurtner et al. (2001). The band positions of the bacterial clones were compared with each other and with the DGGE profile of the original samples. Inserts of clones matching the most intense bands and with faint bands of the DGGE fingerprint of the original were selected for sequencing.
Selected bacterial clones were stored in glycerol at −80 °C as bacterial suspensions.
2.8. 16S rDNA sequencing and phylogenetic analyses
Sequencing of selected clone inserts was done as described by Ettenauer et al. (2010). Comparative sequence analyses was done by comparing pair-wise insert sequences with those available in the online databases provided by the NCBI (National Centre for Biotechnology Information), respectively EMBL (European Molecular Biology Laboratory) using the BLAST and FASTA search programs (Altschul et al., 1997; Pearson, 1994). The ribosomal sequences of the bacterial clones have been deposited at the EMBL nucleotide database. To implement a continuous monitoring and a comparison of the micro-biota inhabiting the stone of the two buildings during the time of the course of treatment, clone sequences were deposited in the EMBL database and search results showing the highest similarity ranking were hunted for matches with clone sequences derived from the same or other sectors at different sampling times of both buildings (shown in the Supplementary Tables 3 and 4).
2.9. PCR-amplification of M. xanthus-DNA with specific primers
Stone and pellet samples derived from the sectors treated with M. xanthus of both buildings (Sectors B and E) were screened for the presence of M. xanthus-DNA. The strain-specific primers Frz799 and Frz1147 (Piñar et al., 2010) were used to amplify a 349 bp fragment of the frzABCD gene. PCR was performed as described by Piñar et al., 2010. Aliqouts of PCR product were analysed on 2% agarose gels and visualized by a UVP documentation system.
3. Results
3.1. Molecular monitoring of the application of treatments on the Monastery of San Jerónimo
DGGE fingerprints derived from pellet samples as well as from non-treated and treated stones of SJ are shown in Fig. 3A and B, respectively. Note that, due to restoration works at the Monastery, it was not possible to take stone samples from sector A after one year. The DNA of M. xanthus was loaded in the gel as a control.
To obtain detailed phylogenetic information of the bacteria colonising the stones and pellets on all three sectors, clone libraries were constructed from all samples shown in Fig. 3. A total of 89 clones, grouped into 19 different genera were retrieved and sequence results obtained from inserted clones showed similarities consistent between 92 and 100% of sequences from the EMBL (see Supplementary Table 3). The relative abundance of the detected genera and phyla (number of clones) and their distribution on the different samples over the time course of the experiment are shown in Tables 1 and 2. For easy tracking of the changes in the microbial communities, the band numbers of the identified clones are indicated in the text and can be compared with their band positions in the DGGE profiles in Fig. 3.
Table 1.
Overview of the different genera detected during the short-time monitoring performed with pellets samples from the Monastery of San Jerónimo and the Royal Hospital of Granada. Numbers of clones and calculated percentages indicate the amount of sequenced clones related to the corresponding genus and phylum. SJ: Monastery of San Jerónimo; RH: Royal Hospital of Granada.
| Pellet samples | Non-treated | Treatments |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water | M. xanthus | M-3P media | |||||||||||||||
| Phylum | Building | SJ | RH | SJ | SJ | RH | RH | SJ | SJ | RH | RH | SJ | SJ | RH | RH | RH | Rh |
| Time of sampling | 0 | 0 | 1st day | 7th day | 1st day | 7th day | 1st day | 7th day | 1st day | 7th day | 1st day | 7th day | 1st day | 1st day | 7th day | 7th day | |
| Sample Genera | SJ-NT | RH-NT | PA1 | PA7 | PD1-1 | PD7-1 | PB1 | PB7 | PE1 | PE7 | PC1 | PC7 | PF1-1 | PF1-2 | PF7-1 | PF7-2 | |
| Proteobacteria | 83.3% | 39% | 71.5% | 85.7% | 100% | 55.5% | 0% | 100% | 100% | 71.4% | 33% | 25% | 90% | 100% | 12.5% | 25% | |
| Alpha-Proteobacteria | Rhizobiaceae/Agrobacterium | 1 (16.7%) | 1 (5.6%) | 3 (42.9%) | 1 (14.3%) | 1 (11.1%) | 1 (33.3%) | 1 (10%) | |||||||||
| Brevundimonas | 2 (28.6%) | 1 (12.5%) | 1 (14.3%) | 2 (20%) | |||||||||||||
| Beta-Proteobacteria | Comamonas | 2 (33.3%) | 1 (5.6%) | 2 (28.6%) | 2 (22.2%) | 3 (100%) | 3 (42.9%) | 1 (12.5%) | 3 (25%) | ||||||||
| Delftia | 1 (16.7%) | 3 (42.9%) | 1 (11.1%) | 3 (75%) | 2 (40%) | ||||||||||||
| Neisseria | 1 (5.6%) | ||||||||||||||||
| Uncultured beta Proteobacteria | 1 (25%) | ||||||||||||||||
| Gamma-Proteoabcteria | Acinetobacter | 1 (11.1%) | |||||||||||||||
| Pseudomonas | 1 (5.6%) | 5 (62.5%) | 5 (50%) | ||||||||||||||
| Stenotrophomonas | 1 (25%) | 1 (14.3%) | 1 (10%) | 3 (60%) | |||||||||||||
| Enterobacter | 1 (5.6%) | ||||||||||||||||
| Pseudoalteromonas | 2 (11.1%) | ||||||||||||||||
| Xanthomonas | 1 (16.7%) | ||||||||||||||||
| Uncultured gamma Proteobacteria | 2 (25%) | ||||||||||||||||
| Actinobacteria | 0% | 16.5% | 0% | 0% | 0% | 0% | 18.2% | 0% | 0% | 14.3% | 0% | 0% | 0% | 0% | 12.5% | 0% | |
| Arthrobacter | 1 (9.1%) | 1 (14.3%) | 1 (12.5%) | ||||||||||||||
| Micrococcus | 1 (5.6%) | ||||||||||||||||
| Kocuria | 1 (5.6%) | 1 (9.1%) | |||||||||||||||
| Corynebacterium | 1 (5.6%) | ||||||||||||||||
| Firmicutes | 16.7% | 44.5% | 0% | 14.3% | 0% | 44.5% | 81.8% | 0% | 0% | 14.3% | 66% | 75% | 10% | 0% | 75% | 75% | |
| Bacillus | 1 (16.7%) | 4 (22.2%) | 1 (14.3%) | 4 (36.4%) | 2 (66.7%) | 2 (50%) | 1 (10%) | ||||||||||
| Solibacillus | 1 (9.1%) | 1 (25%) | |||||||||||||||
| Exiguobacterium | 4 (44.5%) | 1 (14.3%) | 4 (50%) | 3 (25%) | |||||||||||||
| Planococcaceae | 3 (27.3%) | 2 (25%) | 5 (41.7%) | ||||||||||||||
| Brevibacillus | 1 (5.6%) | ||||||||||||||||
| Streptococcus | 1 (5.6%) | ||||||||||||||||
| Uncultured bacterium | 2 (11.1%) | 1 (9.1%) | 1 (8.3%) | ||||||||||||||
| Bacteroidetes | 0% | 0% | 28.6% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| Flavobacterium | 1 (14.3%) | ||||||||||||||||
| Sphingobacterium | 1 (14.3%) | ||||||||||||||||
| Total number of clones | 6 (100%) | 18 (100%) | 7 (100%) | 7 (100%) | 8 (100%) | 9 (100%) | 11 (100%) | 3 (100%) | 4 (100%) | 7 (100%) | 3 (100%) | 4 (100%) | 10 (100%) | 5 (100%) | 8 (100%) | 12 (100%) | |
Table 2.
Overview of the different genera detected on non-treated and treated stones during the long-time monitoring performed at the Monastery of San Jerónimo and the Royal Hospital of Granada. Legend as for Table 1.
| Stone samples | Non-treated | Treatments |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water | M.xanthus | M-3P media | ||||||||||
| Phylum | Building | SJ | RH | SJ | RH | SJ | SJ | RH | SJ | SJ | RH | RH |
| Time of sampling | 0 | 0 | 6 months | 12 months | 6 months | 12 months | 12 months | 6 months | 12 months | 12 months | 12 months | |
| Sample Genera | SJ-NT | RH-NT | SA6 | SD12 | SB6 | SB12 | SE12 | SC6 | SC12 | SF12-1 | SF12-2 | |
| Proteobacteria | 83.3% | 39% | 58.4% | 60% | 30% | 0% | 0% | 0% | 14.3% | 0% | 0% | |
| Alpha-Proteobacteria | Rhizobiaceae/Agrobacterium | 1 (16.7%) | 1 (5.6%) | 1 (8.3%) | 1 (5%) | 1 (10%) | ||||||
| Porphyrobacter | 1 (5%) | |||||||||||
| Bosea | 1 (5%) | |||||||||||
| Beta-Proteobacteria | Comamonas | 2 (33.3%) | 1 (5.6%) | 2 (16.7%) | 1 (10%) | 1 (14.3%) | ||||||
| Delftia | 1 (16.7%) | 1 (8.3%) | 2 (10%) | |||||||||
| Thiobacillus | 1 (5%) | |||||||||||
| Methylobacterium | 1 (5%) | |||||||||||
| Hydrogenophaga | 1 (5%) | |||||||||||
| Neisseria | 1 (5.6%) | |||||||||||
| Bacterium J10 | 1 (5%) | |||||||||||
| Gamma-Proteobacteria | Pseudomonas | 1 (5.6%) | 3 (25%) | 2 (10%) | ||||||||
| Stenotrophomonas | 1 (5%) | |||||||||||
| Enterobacter | 1 (5.6%) | |||||||||||
| Pseudoalteromonas | 2 (11.1%) | 1 (10%) | ||||||||||
| Xanthomonas | 1 (16.7%) | |||||||||||
| Actinobacteria | 0% | 16.5% | 0% | 15% | 10% | 0% | 0% | 0% | 0% | 0% | 20% | |
| Micrococcus | 1 (5.6%) | |||||||||||
| Kocuria | 1 (5.6%) | 1 (10%) | ||||||||||
| Corynebacterium | 1 (5.6%) | 1 (10%) | ||||||||||
| Brachybacterium | 1 (5%) | |||||||||||
| Uncultured Actinobacteria | 2 (10%) | 1 (10%) | ||||||||||
| Firmicutes | 16.7% | 44.5% | 25% | 25% | 50% | 100% | 100% | 100% | 85.7% | 100% | 80% | |
| Bacillus | 1 (16.7%) | 4 (22.2%) | 2 (16.7%) | 1 (5%) | 5 (50%) | 9 (100%) | 7 (100%) | 1 (10%) | 2 (28.6%) | 1 (11.1%) | ||
| Exiguobacterium | 1 (5%) | |||||||||||
| Virgibacillus | 2 (20%) | |||||||||||
| Planococcaceae | 1 (8.3%) | 3 (15%) | 7 (70%) | 4 (57.1%) | 7 (77.8%) | 7 (70%) | ||||||
| Brevibacillus | 1 (5.6%) | |||||||||||
| Desemzia | 1 (10%) | |||||||||||
| Streptococcus | 1 (5.6%) | |||||||||||
| Uncultured bacterium | 2 (11.1%) | 1 (11.1%) | ||||||||||
| Bacteroidetes | 0% | 0% | 16.6% | 0% | 10% | 0% | 0% | 0% | 0% | 0% | 0% | |
| Flavobacterium | 1 (8.3%) | 1 (10%) | ||||||||||
| Uncultured Bacteroidetes bacterium | 1 (8.3%) | |||||||||||
| Total number of clones | 6 (100%) | 18 (100%) | 12 (100%) | 20 (100%) | 10 (100%) | 9 (100%) | 7 (100%) | 10 (100%) | 7 (100%) | 9 (100%) | 10 (100%) | |
3.1.1. Identification of the inhabiting micro-biota — phylogenetic analysis of sequenced clones derived from non-treated stone — sample SJ-NT
From the non-treated stone of SJ, six different clones were selected for sequencing. Clones showed similarities between 97 and 100% with sequences from the EMBL and arranged into 5 different genera. They belonged to the Proteobacteria (α-, β- and γ-subdivisions; 83.3%). These were comprised of one clone sequence (16.7%) each of Delftia sp. (band number, bn 2), Agrobacterium sp. (bn 4) and an uncultured Xanthomonas sp. (bn 3). The rest of the 16S rDNA sequences (33.3%) could be associated with Comamonas sp. (bn 5 and 6). The phylum Firmicutes (16.7%) was represented by one clone sequence affiliating with Bacillus sp. (bn 1).
3.1.2. Short-term monitoring — phylogenetic analysis of sequenced clones derived from enrichment cultures conducted during the seven days of the consolidation treatments
During the seven days of the application of the treatments, the 16S rDNA sequences of 35 obtained clones showed similarities ranging from 92 to 100% to known sequences of the database and 13 different genera were identified in the pellet samples of SJ (Table 1).
3.1.2.1. Control sector A — water treatment — samples PA1 and PA7
A total of 14 clones phylogenetically affiliated with the phyla Proteobacteria, Firmicutes and Bacteroidetes (Table 1).
On the first day 7 clone sequences could be obtained. Five of them belonged to the phylum Proteobacteria (71.5%). Thereof 42.9% were related to uncultured Rhizobiaceae bacteria (bn 59–61) and 28.6% to cultured and uncultured Brevundimonas sp. (bn 57 and 58). The two additional clones affiliated with the phylum Bacteroidetes (28.6%), namely with Sphingobacterium sp. (bn 55) and Flavobacterium sp. (bn 56) (each 14.3%). This phylum was not detected before the treatment, proving to be activated by the mere application of water. In parallel, members of the Firmicutes, observed in the non-treated stone, were not detectable (Fig. 3A-A, lane PA1).
On the seventh day 6 out of 7 clones were highly related to members of the Proteobacteria (85.8%), namely to uncultured Delftia sp. (bn 64–66) (42.9%), uncultured Comamonas sp. (bn 62 and 68) (28.6%) and to an uncultured Rhizobiaceae bacterium (bn 67) (14.3%). The Bacteroidetes group, detected on the first day, vanished and instead a Firmicutes representative, an uncultured Bacillus sp. (bn 63) (14.3%) re-emerged (Fig. 3A-A, lane PA7).
3.1.2.2. Sector B — M. xanthus-inoculated nutritional solution treatment — samples PB1 and PB7
The DGGE fingerprints derived from this sector clearly showed different profiles on the first and seventh days of the treatment (Fig. 3A, sector B). During the first day of the treatment, all members of the Proteobacteria inhabiting the non-treated stone vanished. Instead, 2 out of a total of 7 16S rDNA sequences showed affiliations with the Actinobacteria (18.2%), namely with an uncultured Kocuria sp. (bn 79) and with an Arthrobacter sp. (bn 75) (each 9.1%), not detectable on the non-treated stone. The remaining 9 clones showed sequence similarities to the Firmicutes (81.8%), namely to cultured and uncultured Bacillus sp. (bn 69, 71–73) (36.4%), cultured and uncultured members of the Planococcaceae (bn 74, 76 and 77) (27.3%), Solibacillus sp. (bn 78) and to an uncultured bacterium (bn 70) (each 9.1%) (Fig. 3A-B, lane PB1).
After 7 days, sequence results revealed a new shift in the microbial community, showing the total disappearance of the Firmicutes and the Actinobacteria found on the first day. All 3 obtained clones were characterized as members of the β-Proteobacteria. The most dominant band and the two surrounding faint bands were identical to uncultured Comamonas sp. (bn 80, 81 and 82) (100%) (Fig. 3A-B, lane PB7).
It is worth noting that no clone sequence corresponding to M. xanthus could be retrieved from this sector during the seven days of the treatment.
3.1.2.3. Sector C — M-3P sterile nutritional solution treatment — samples PC1 and PC7
One out of the 3 clones, obtained during the first day of the application of this treatment, could be associated with the phylum Proteobacteria (33.3%), to an uncultured Rhizobiaceae bacterium (bn 85), and the other 2 clones were identified as Bacillus sp. (bn 83 and 84) of the Firmicutes (66.7%) (Fig. 3A-C, lane PC1).
On the seventh day, 75% of the identified clones showed high similarities to members of the Firmicutes, to uncultured Bacillus sp. (bn 87 and 89) (50%), and to Solibacillus sp. (bn 88) (25%), whereas the additional clone affiliated to an uncultured β-Proteobacterium (bn 86) (25%) (Fig. 3A-C, lane PC7).
3.1.3. Long-term monitoring — phylogenetic analysis of sequenced clones derived from the treated stones
The 16S rDNA fragments of 48 selected clones obtained from treated stone samples of SJ showed similarities between 93 and 100% with known sequences in the database (see Supplementary Table 3) and grouped the clones into 11 different genera (Table 2).
3.1.3.1. Control sector A — water treatment — sample SA6
Six months after the application of water, 12 clones were obtained. Of these, seven clones belonged to the phylum Proteobacteria (58.3%), affiliating with members of the Rhizobiaceae (bn 16) and with species of the genera Delftia (bn 15) (each 8.3%), Comamonas (bn 17 and 18) (16.7%) and Pseudomonas (bn 8, 11 and 12) (25%); this last genus not being identified on the non-treated stone. Three clones (25%) affiliated with the Firmicutes, namely with Bacillus sp. (bn 10 and 14) (16.7%) and Planococcaceae (bn 13) (8.3%). This last family was not found in the primordial micro-biota. Two further clones were newly detected on the water-treated but not identified on the non-treated stone. They affiliated with a Flavobacteriaceae (bn 7) and an uncultured Bacteroidetes bacterium (bn 9) (each 8.3%) of the phylum Bacteroidetes (16.6%) (Fig. 3B-A, lane SA6).
3.1.3.2. Sector B — M. xanthus-inoculated nutritional solution treatment
3.1.3.2.1. After six months — sample SB6
From this sector 10 clones belonging to 4 different phyla could be identified. Three clones were identified as members of the Proteobacteria (30%), namely Rhizobiaceae bacterium (bn 26), Comamonas sp. (bn 27) and Pseudoalteromonas sp. (bn 21) (each 10%). The last sequence could not be detected in the non-treated stone. Five clones showed relatedness to the Firmicutes (50%) — with cultured and uncultured Bacillus sp. (bn 20, 22–25). One clone affiliated with the Flavobacteriaceae (bn 19), a member of the Bacteroidetes (10%) and one clone affiliated with the genus Corynebacterium sp. (bn 28), a member of the Actinobacteria (10%). These two last phyla were not identified on the non-treated stone of this sector (Fig. 3B-B, lane SB6).
3.1.3.2.2. After one year — sample SB12
After one year all 9 sequenced clones showed affiliations with the order Bacillales within the phylum Firmicutes (100%). All representatives of the other phyla previously detected on the non-treated stone vanished. Most of the identified clone sequences (55.6%) were related to cultured Bacillus sp. (bn 29, 31, 33, 35 and 37) whereas 33.3% were associated with uncultured Bacillus sp. (bn 32, 34 and 36). One clone (11.1%) affiliated with the Bacillaceae bacterium MOLA 662 (bn 30) (Fig. 3B-B, lane SB12).
No clone derived from the samples taken from this sector after six or twelve months showed similarities to the inoculated strain M. xanthus.
3.1.3.3. Sector C — M-3P sterile nutritional solution treatment
3.1.3.3.1. After six months — sample SC6
Six months after the application of this treatment, all representatives of the Proteobacteria detected on the non-treated stone completely disappeared and all 10 obtained clones clustered into the phylum Firmicutes (100%). The majority of the sequences (70%) were identified as uncultured Planococcaceae bacteria (bn 41–47) — not identified in the primordial micro-biota. Two clones (20%) affiliated with Virgibacillus sp. (bn 39 and 40) and one additional clone (10%) was related to Bacillus sp. (bn 38) (Fig. 3B-C, lane SC6).
3.1.3.3.2. After one year — sample SC12
One out of the 7 clones identified on this sector was related to the phylum Proteobacteria (14.3%), namely to an uncultured Comamonas sp.(bn 52), which was already found on the non-treated stone. The remaining 6 clones affiliated with the Firmicutes (85.7%), of which 57.1% were classified as uncultured Planomicrobium sp. (bn 48–51) and 28.6% as uncultured Bacillus sp. (bn 53 and 54) (Fig. 3B-C, lane SC12).
3.2. Molecular monitoring of the application of treatments on the Royal Hospital
DGGE fingerprints derived from pellet samples as well as from non-treated and treated stones collected from the RH are shown in Fig. 4A and B. As in Fig. 3, the DNA of M. xanthus was loaded in the gel as a control. DGGE analysis revealed that the micro-biota inhabiting the non-treated stone of the RH (Fig. 4, lane RH-NT) showed per se a higher diversity of 16S rDNA sequences than that of SJ (Fig. 3, lane SJ-NT). Thereafter, the application of the consolidation treatments led to a visible change in the microbial community structure on the different sectors. DGGE profiles from the water-treated control sector D, divided into sub-sectors D-1 and D-2, proved to be identical during the seven days of the treatment and after one year (data not shown), and therefore only the profiles of one sector are shown, named as PD-1 and SD12 in Fig. 4. In contrast, DGGE fingerprints derived from the M-3P nutritional solution-treated sector F, divided into subsectors F-1 and F-2, displayed different community profiles and both of them are shown in Fig. 4.
Clone libraries from all samples shown in Fig. 4 were constructed to phylogenetically identify the microorganisms present in the pellet and stone samples. A total of 127 sequences were obtained showing similarities between 89 and 100% with sequences from EMBL (see Supplementary Table 4). In summary, 31 different genera were identified from all samples of the RH. The relative abundance of the detected genera and phyla (number of clones) and their distribution on the different samples over the time course of the experiment are shown in Tables 1 and 2. As in Section 3.1 the band numbers of the clones from the Royal Hospital are indicated in the text and the community changes can be followed in the DGGE profiles shown in Fig. 4.
3.2.1. Identification of the inhabiting micro-biota — phylogenetic analysis of sequenced clones derived from non-treated stone — sample RH-NT
Eighteen 16S rDNA sequences obtained from the non-treated stone showed similarities between 98 and 99% consistent with sequences from the EMBL (see Supplementary Table 4) and could be arranged into 13 different genera belonging to 3 different phyla: the Proteobacteria (39%), the Firmicutes (44.5%) and the Actinobacteria (16.5%).
Single sequences belonging to the Proteobacteria were related to Agrobacterium sp. (bn 13), Comamonas sp. (bn 14), Neisseria sp. (bn 5), Pseudomonas sp. (bn 3), Enterobacter sp. (bn 15) (each 5.6%) and 2 additional sequences to Pseudoalteromonas sp. (bn 6 and 7) (11.1%). Belonging to the Firmicutes, 16S rDNA sequences associated with Bacillus sp. (bn 8, 9, 11 and 12) (22.2%), Brevibacillus sp. (bn 10), Streptococcus sp. (bn 4) (each 5.6%) and to 2 uncultured bacteria (bn 1 and 2) (11.1%). Three single sequences affiliated with the Actinobacteria: Micrococcus sp. (bn 16), Kocuria sp. (bn 17) and Corynebacterium sp. (bn 18) (each 5.6%) (Fig. 4, lane RH-NT).
3.2.2. Short-term monitoring — phylogenetic analysis of sequenced clones derived from enrichment cultures conducted during the seven days of the consolidation treatment
The 16S rDNA sequences of 63 selected clones displayed 89–100% relatedness to known sequences of the EMBL (see Supplementary Table 4) and a total of 13 different genera were detected (Table 1).
3.2.2.1. Control Sector D — water treatment — samples PD1-1 and PD7-1
On the first day of the application of sterile water, a broad range of the microorganisms detected on the non-treated stone disappeared and members of the Proteobacteria rose (100%). Out of the 8 sequenced clones, five 16S rDNA sequences were related to Pseudomonas sp. (bn 65, 66, 69–71) (62.5%). Even though this genus was already present on the non-treated stone, its dominance on the first day is noticeable. In addition, 2 clones showed high similarity rankings to uncultured bacteria (bn 67 and 68) (25%) and one sequence affiliated with an uncultured Brevundimonas sp. (bn 72) (12.5%) (Fig. 4A-D, lane PD1-1).
Nine clone sequences were obtained on the seventh day. The general dominance of the Proteobacteria remained (55.5%), but the community structure shifted: all previously detected members of this phylum disappeared, whereas 5 clones proved to be related to uncultured Comamonas sp. (bn 76 and 78) (22.2%), an Acinetobacter sp. (bn 73), an uncultured Rhizobiaceae bacterium (bn 74) and an uncultured Delftia sp. (bn 75) (each 11.1%). The remaining 4 sequences were members of the Firmicutes and affiliated with uncultured Exiguobacterium sp. (bn 77, 79–81). The relative abundance of this phylum (44.5%) was almost identical to that found on the non-treated stones. Furthermore, the phylum Actinobacteria, detected on the non-treated stone completely vanished (Fig. 4A-D, lane PD7-1).
3.2.2.2. Sector E — M. xanthus-inoculated nutritional solution treatment - Samples PE1 and PE7
During the seven days of this treatment, 11 different clones obtained from this sector were related to the phyla Proteobacteria, Firmicutes and Actinobacteria (Table 1).
On the first day all clone inserts were identical in their sequence to partial rRNA genes of different Proteobacteria (100%). 75% of the sequences were identified as uncultured Delftia sp. (bn 82–84) and the remaining 25% affiliated to an uncultured Stenotrophomonas sp. (bn 85). All members of the other phyla detected on the non-treated stone vanished (Fig. 4A-E, lane PE1).
On the seventh day the dominance of the Proteobacteria (71.4%) remained, even though the detected genera changed, with the disappearance of the genus Delftia. Sequences were related to uncultured Comamonas sp. (bn 89–91) (42.9%), to a cultured Brevundimonas sp. (bn 87) and an uncultured Stenotrophomonas sp. (bn 88) (each 14.3%). Two additional clone sequences could be identified as members of the Firmicutes, namely an uncultured Exiguobacterium sp. (bn 92) and of the Actinobacteria, namely an uncultured Arthrobacter sp. (bn 86) (each 14.3%) (Fig. 4A-E, lane PE7).
As observed in SJ, no clone corresponding to M. xanthus could be retrieved from this sector during this period of time.
3.2.2.3. Sector F — M-3P sterile nutritional solution treatment — samples PF1-1, PF1-2, PF7-1 and PF7-2
On the first day of the application of the M-3P solution, most of the clones obtained from sector F-1 were members of the Proteobacteria (90%), related to cultured and uncultured Pseudomonas sp. (bn 93–96, 99) (50%), uncultured Brevundimonas sp. (bn 98, 101) (20%), Stenotrophomonas sp. (bn 100) and Rhizobiaceae (bn 102) (each representing 10%). Only one clone was a relative of the Firmicutes (10%), namely a Bacillus sp. (bn 97). From subsector F-2 only uncultured members of the Proteobacteria (100%) were detected. Out of the 5 sequenced clones, 3 affiliated with uncultured Stenotrophomonas sp. (bn 105–107) (60%). The other two 16S rDNA sequences (40%) associated with uncultured Delftia sp. (bn 103 and 104) (Fig. 4A-F, lanes PF1-1, PF1-2).
On the seventh day the micro-biota shifted further, leading to an attenuation of the Proteobacteria with a simultaneous increase of the Firmicutes in both subsectors F-1 and F-2. Eight clones were identified from the subsector F-1. Of these one clone was related to a Comamonas sp. (bn 111), a member of the Proteobacteria (12.5%). Six clones affiliated with the Firmicutes (75%): with cultured and uncultured Exiguobacterium sp. (bn 108, 112–114) (50%) and with members of the Planococcaceae (bn 109 and 110) (25%). One further clone was identified as an uncultured Arthrobacter sp. (bn 115), a member of the Actinobacteria (12.5%) (Fig. 4A-F, lane PF7-1).
Twelve clones were obtained from sample PF7-2. Three of them were shown to be members of the Proteobacteria (25%) and were related to uncultured Comamonas sp. (bn 122–124). The remaining 9 clones belonged to the Firmicutes (75%) and had the highest similarity ranking with cultured and uncultured members of the Planococcaceae (bn 116–120) (41.7%), uncultured Exiguobacterium sp. (bn 125–127) (25%) and with an uncultured bacterium (bn 121) (8.3%) (Fig. 4A-F, lane PF7-2).
Despite the different DGGE profiles derived from the two sub-sectors, the obtained clones correlated with each other at the family level and partly at the genus level. The matching results of the independent sub-sectors indicate a similar community development during the 7 days of the treatment with the M-3P nutritional solution.
3.2.3. Long-term monitoring — phylogenetic analysis of sequenced clones derived from the treated stones
A total of 46 sequences were obtained from the treated stone samples of the RH, showing similarities between 93 and 100% with known sequences of the database (see Supplementary Table 4). The clones were grouped into 18 different genera and their distribution and relative abundance are shown in Table 2.
3.2.3.1. Control sector D — water treatment — sample SD12
After one year of the application of water, the micro-biota re-established on the stone and 16S rDNA sequences derived from 20 clones affiliated with the same 3 phyla already identified on the non-treated stone (Table 2). Sixty percent of the identified sequences were members of the Proteobacteria showing similarities to representatives of the Rhizobiaceae (bn 33) (5%) and to Pseudomonas sp. (bn 20 and 22) (10%), already found on the non-treated stones. Additional sequences were related to species of the genera Porphyrobacter (bn 25), Bosea (bn 27), Thiobacillus (bn 32), Methylobacterium (bn 34), Hydrogenophaga (bn 30), Stenotrophomonas (bn 31) (each 5%), Delftia (bn 24 and 26) (10%) as well as to one non-classified Bacterium J10 (bn 19) (5%), all not detected in the non-treated stone. The Firmicutes phylum was represented by 25% of the total sequences and they affiliated with Bacillus sp. (bn 29), an uncultured Exiguobacterium sp. (bn 36) (each 5%) and with uncultured Planococcaceae bacteria (bn 23, 28 and 35) (15%). The remaining 15% of the 16S rDNA sequences matched in the BLAST search with members of the Actinobacteria, namely uncultured Actinobacteria (bn 21 and 38) (10%) and Brachybacterium sp. (bn 37)(5%) (Fig. 4B-D, lane SD12).
3.2.3.2. Sector E — M. xanthus-inoculated nutritional solution treatment — sample SE12
One year after the application of a M. xanthus-inoculated medium all 7 obtained clone sequences affiliated with the phylum Firmicutes (100%). The other previously detected phyla completely vanished. Interestingly, all characterized clone sequences associated with the genus Bacillus (bn 39–45), which was the most dominant representative of this phylum on the non-treated stone (Fig. 4B-E, lane SE12).
No clone obtained from this sector after one year of the application of the treatment showed phylogenetic affiliations with M. xanthus.
3.2.3.3. Sector F — M-3P sterile nutritional solution treatment — sample SF12-1 and SF12-2
From the two sampled sub-sectors, altogether 19 clones were identified. From sample F-1 all 9 clones belonged to the Firmicutes (100%), of which seven 16S rDNA sequences affiliated with uncultured Planococcus sp. (bn 47–49, 51–54) (77.8%). One clone sequence was related to an uncultured Bacillus sp. (bn 46), respectively to an uncultured Firmicutes bacterium (bn 50) (each 11.1%). From sample F-2, 80% of the 16S rDNA sequences showed similarities to the phyla Firmicutes, and were related to cultured and uncultured members of the Planococcaceae (bn 56–62) (70%), and Desemzia sp. (bn 55) (10%). The remaining 20% were members of the Actinobacteria and the detected clones affiliated with an uncultured Actinobacterium (bn 63) and an uncultured Kocuria sp. (bn 64) (each 10%) (Fig. 4B-F, lanes SF12-1 and SF12-2).
3.3. Comparison of the micro-biota detected on both buildings
3.3.1. Comparison of the micro-biota detected on non-treated stones
Regarding the diversity of the genera detected on non-treated stones of both buildings, with the exception of Delftia and Xanthomonas, all identified genera from SJ could be retrieved from the RH. In addition, a further 10 genera were found on this last building, namely Pseudomonas, Streptococcus, Neisseria, Pseudoalteromonas, Brevibacillus, Enterobacter, Micrococcus, Kocuria, Corynebacterium and uncultured Firmicutes clones (see Tables 1 and 2).
3.3.2. Short-term molecular monitoring — comparison of the micro-biota activated during the seven days of the treatment
Sequencing results derived from the pellet samples of SJ allowed the detection and identification of 35 different clones from 6 clone libraries, grouped into 13 different genera. From pellet samples of the RH, 63 clone sequences obtained from 8 clone libraries clustered into 13 different genera (see Table 1). Twenty-one out of the 35 clone sequences detected at the Monastery showed the highest relatedness to clone sequences previously detected in the non-treated stones or in pellet samples of other sectors of both buildings. In the case of the Royal Hospital 44 such sequences were identified.
During the first week of the application of treatments, out of the 5 genera detected on the non-treated stone of SJ, only the genus Xanthomonas could not be detected on this building. In addition to the primordial bacteria, the genera Brevundimonas, Arthrobacter, Kocuria, Solibacillus, Flavobacterium, Sphingobacterium as well as members of the family Planococcaceae emerged in the pellet samples of SJ.
In the case of the RH, the genera Neisseria, Enterobacter, Pseudoalteromonas, Micrococcus, Kocuria, Corynebacterium, Brevibacillus and Streptococcus, detected on the non-treated stone of this building, vanished in pellet samples. Newly emerged genera detected in pellet samples of this building affiliated with Brevundimonas, Delftia, Acinetobacter, Stenotrophomonas, Arthrobacter, Exiguobacterium and members of the family Planococcaceae.
Throughout the whole short-term monitoring 7 genera were found in pellet samples of both buildings, namely Brevundimonas, Comamonas, Delftia, Arthrobacter, Bacillus and members of the families Rhizobiaceae and Planococcaceae (Table 1).
3.3.3. Long-term molecular monitoring — comparison of the micro-biota remaining on the stones after one year of the application of treatments
One year after the application of treatments, a total of 48 clones were obtained from SJ. 16S rDNA analysis clustered the sequences into 11 genera, belonging to the phyla Firmicutes, Proteobacteria (α-, β- and γ-subdivisions), Actinobacteria and Bacteroidetes. Forty-six clones were obtained from the RH, which affiliated with 18 genera of the phyla Proteobacteria (α-, β- and γ-subdivisions), Firmicutes and Actinobacteria (Table 2). Out of the 5 genera detected on the non-treated stone of SJ, only the genus Xanthomonas could not be detected on this building. In addition to the primordial bacteria, the genera Planomicrobium, Planococcus, Pseudomonas, Flavobacterium, Pseudoalteromonas, Corynebacterium and Virgibacillus were detected after 6 and 12 months, respectively.
In the case of the RH, the genera Streptococcus, Neisseria, Pseudoalteromonas, Brevibacillus, Enterobacter, Micrococcus, Comamonas and Corynebacterium, inhabiting the non-treated stone, were never again detected on this building. Newly emerged and detected sequences affiliated with Desemzia, Brachybacterium, Methylobacterium, Thiobacillus, Hydrogenophaga, Bosea, Porphyrobacter, Exiguobacterium, Planococcus, Planomicrobium, Stenotrophomonas, Delftia, uncultured Actinobacteria and a non-classified Bacterium J10.
Throughout the whole long-term monitoring 6 genera were found on both buildings, namely Planomicrobium, Planococcus, Bacillus, Pseudomonas, Delftia and Rhizobiaceae (Table 2).
3.4. Detection of the inoculated M. xanthus during the time course experiment
As mentioned before, no clone corresponding to M. xanthus could be retrieved from sectors inoculated with this strain in any building. Furthermore, no DGGE-band corresponding to the DNA of M. xanthus, loaded on the DGGE-gels as positive control (lane M.x. in Figs. 3 and 4), could be identified on the DGGE-gels.
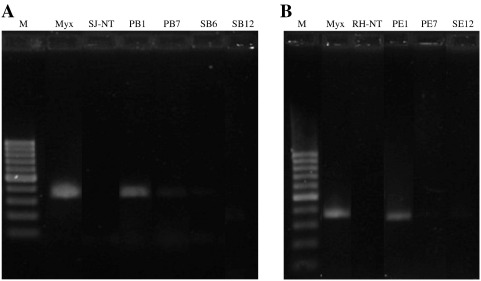
To overcome this problem, a second molecular strategy was applied for a more sensitive detection of M. xanthus, using the species-specific primer pair Frz799/Frz1147. This molecular method allowed the detection of M. xanthus in pellet samples PB1 and PB7 from SJ as well as in pellet sample PE1 from the RH (Fig. 5). All these samples were taken during the first seven days, the further detection of M. xanthus not being possible after a longer period of time.
Fig. 5.
Screening of Myxococcus xanthus-DNA in crude DNA extracts derived from pellet and stone samples from the Monastery of San Jerónimo A and the Royal Hospital of Granada B Lane M: molecular marker (100 bp DNA Ladder, Fermentas). Lane M.x.: M. xanthus strain 422 DNA as positive control. For the description of sample names see Figs. 3 and 4.
4. Discussion
4.1. Evaluation of the effect of the consolidation treatments on the micro-biota of both buildings
This study evaluates and compares the effect on the microbial population inhabiting the stone of two historical buildings before, during (short-term) and after (long-term) the in situ application of two different consolidation treatments.
Concerning the micro-biota detected on the non-treated stones of both buildings, our results derived from DGGE fingerprints revealed a far more complex community structure on the RH, than on SJ. This fact can be explained by the location of the treated area at the RH. The ground floor situated sectors are more exposed to environmental influences, such as traffic, animals, humans, etc, and, therefore, the availability of additional nutrients supporting the growth of different microorganisms is increased. Nevertheless, we cannot rule out that the reason for the higher diversity found on the non-treated stone of this building could be its greater degree of deterioration, with a rougher surface that can offer protective sites for the inhabiting bacteria. Because of the different degrees of deterioration and the surface irregularity of the treated stone on the RH, two sectors were selected for the treatment with water and M-3P nutritional solution.
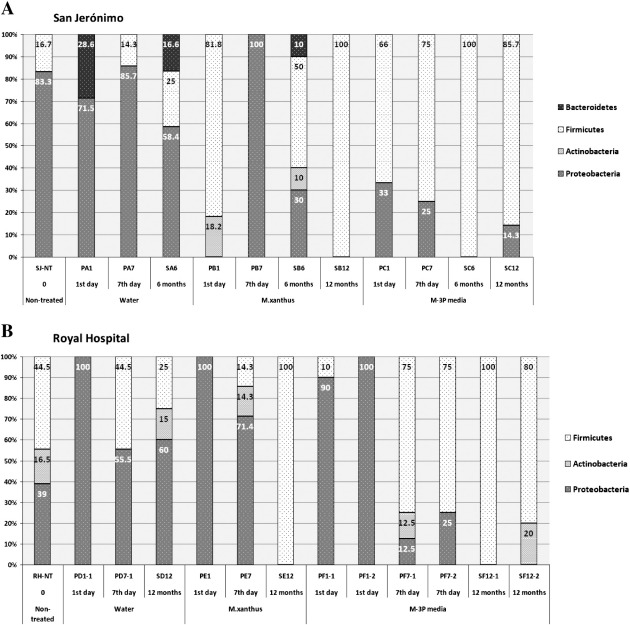
The short-term monitoring revealed that the application of the two different consolidation treatments triggered similar microbial dynamics on both buildings (Fig. 6). However, interestingly, the mere application of water as a control resulted in more differentiated microbial dynamics when comparing the two buildings. In the case of SJ, the relative abundance of the Proteobacteria in the water treated sector was very similar to that observed in the non-treated stone (85.8% in pellet sample vs. 83.3% in NT-stone). In the case of the RH, the relative abundance of Proteobacteria increased during the seven days. On the first day we observed a great stimulation of this phylum (100%) with the simultaneous disappearance of a broad range of microorganisms inhabiting the non-treated stone. Throughout the week the relative abundance of the Proteobacteria increased in comparison with that observed in the non-treated stone (from 39% in NT-stone to 55.5% in pellet sample). Nevertheless, the microbial dynamics of the Firmicutes phylum was identical in both buildings (Fig. 6). This group temporary vanished on the first day of the water application, but re-established through the rest of the week showing a relative abundance very similar to that found on the non-treated stones of SJ (14.3% in pellet sample vs. 16.7% in NT-stone) and even identical in the case of the RH (44.5% in pellet and NT-stone samples). The Actinobacteria, detected on the non-treated stone of the RH, completely vanished during the seven days, probably due to a slower growth of these bacteria.
Fig. 6.
General overview of the detected phyla and its dynamics over the timecourse of the investigation on both buildings. The community structure of the samples from each building was chronologically ordered according to the treated sectors and is displayed on the phylum level (in percentages). A. Community dynamics of San Jerónimo. B. Community dynamics of the Royal Hospital. For the explanation of the abbreviations see Figs. 3 and 4.
The water supplied can dissolve those soluble contaminants present on the stone which can act as nutrients and boost the growth of the fast growing inhabiting bacteria. This scenario can be compared to that established after intensive periods of rain. However, the activation of these bacteria did not result in the consolidation of the stone, as shown by Jroundi et al. (2010) in the peeling tape test performed on sectors treated with water. This could be due to the lack of the appropriate nutritional medium and its special constituents that induce the production of calcium carbonate.
The application of a M. xanthus-inoculated culture triggered similar microbial dynamics on both buildings at the end of the short-term monitoring, resulting in the boosting of the Proteobacteria (Fig. 6). This phylum accounted for 100% and 71.4% of the total sequences detected in SJ and in RH, respectively. In the latter case the evolution of members of the phyla Firmicutes and Actinobacteria, each accounting for 14.3% of the total sequences, was observed at the end of the week. However, results from the first day showed differences on both buildings. At SJ, the Proteobacteria inhabiting the non-treated stone vanished. Instead, representatives of the Actinobacteria, not detectable on the non-treated stone, as well as dormant Bacillales members were activated and dominated on the first day. In contrast, at the RH there was a strong activation of the Proteobacteria (100%) on the first day, whereas members of the other phyla detected on the non-treated stone vanished.
The application of the nutritional solution M-3P also led to similar microbial dynamics on both buildings (Fig. 6). A boost of the Firmicutes (75% of the total sequences in both buildings) at the end of the week was visible. Compared to the non-treated stone, the Proteobacteria strongly decreased in the community (from 83.3% to 25% in the case of SJ and from 39% to 12.5% in sub-sector F-1 and to 25% in sub-sector F-2 of RH). Additionally in the latter building, the Actinobacteria were observed in sector F-2 with a relative abundance similar to that found on the non-treated stone (12.5% in pellet sample vs. 16.5% in NT-stone). Nevertheless, results from the first day showed differences in the microbial dynamics in both buildings, as already observed in the sectors treated with a M. xanthus-inoculated culture. In the case of SJ, the application of the M-3P solution resulted in the gradual decrease of the Proteobacteria from the first day on, whereas at the RH, the Proteobacteria (90% and 100%, respectively for subsectors F-1 and F-2) were activated on the first day. This phenomenon was also observed on the other two sectors of this building.
Results derived from the long-term monitoring showed that, in general, the application of water as a control resulted in the detection of the same phyla already observed on the non-treated stone, namely Proteobacteria, Firmicutes as well as the Actinobacteria in the case of the RH. Nevertheless, the relative abundances of the detected phyla changed substantially compared to that found on the non-treated stones (Fig. 6). At SJ, 6 months after the application, the Proteobacteria decreased (from 83.3% in NT-stone to 58.4% in treated stone) and members of the Bacteroidetes (16.6%), already found in the pellet samples, re-emerged. The relative abundance of the Firmicutes rose (from 16.7% in NT-stone to 25% in treated stone), with the appearance of the Planococcaceae. After one year the micro-biota partly re-established at the RH and all 3 phyla originally found were detected again. Compared to the non-treated stone, the relative amount and the diversity of the Proteobacteria rose (from 39% to 60%). The relative abundance of the Firmicutes decreased (from 44.5% to 25%). Instead, the relative frequency of occurrence of the Actinobacteria (15%) remained very similar to the non-treated stone (16.5%). We believe that these changes can be more dependent on the rain periods over the year than on the water applied during the seven days.
On both buildings the application of the nutritional solution, irrespective of its inoculation with the M. xanthus strain, resulted in the establishment of very similar micro-biota after one year (Fig. 6).
Compared to the non-treated stone, six months after the application of the M. xanthus-inoculated culture on SJ, members of Firmicutes (Bacillus) dominated (50% of the total sequences), whereas the identified Proteobacteria decreased (from 83.3% in NT-stone to 30% in treated stone). Additionally, representatives of the Actinobacteria, already detected in pellet samples from this sector, as well as the Bacteroidetes, not previously found on this sector, were detected (each accounting for 10% of the total sequences). Interestingly, one year after the application of the M. xanthus-inoculated culture, on both buildings the Firmicutes (100% of the total detected sequences) dominated. The Firmicutes dominance contradicted the dominance of the Proteobacteria observed in the pellet samples.
At SJ, 6 months after the application of the M-3P nutritional solution resulted in the absolute dominance of the Firmicutes (100% of the detected sequences). The dominance of this phylum remained stable after one year (85.7%) and, additionally, the Proteobacteria, found on the non-treated stone, emerged again accounting for 14.3% of the sequences. At the RH, one year after the application, the Proteobacteria completely vanished from the stone and instead the Firmicutes was the dominant phylum (100% on subsector F-1, respectively 80% on subsector F-2; mostly associated with the family Planococcaceae). Also, representatives of the Actinobacteria (20% of sequences) were detectable on subsector F-2, making its relative abundance similar to that found on the non-treated stone (16.5%).
Our results agree with those previously obtained from Jimenez-Lopez et al. (2007, 2008) which demonstrated that despite the presence/absence of M. xanthus, there is an activation of a fraction of the inhabiting bacteria which has the potential to produce new calcium carbonate cement on the stone that was compatible with the substrate and to consolidate the porous limestone without pore plugging. Moreover, the long-term dominance of the Firmicutes after the application of treatments observed in our study agrees with the observations of Jimenez-Lopez et al. (2008) and Piñar et al. (2010). These authors immersed non-sterile calcarenite stone slabs, for a period of a month, in Erlenmeyer flasks containing the M-3P nutritional solution non-inoculated (Jimenez-Lopez et al., 2008) and inoculated (Piñar et al., 2010) with M. xanthus. Both studies revealed the dominance of the Proteobacteria group in the non-treated stone slabs, as well as within the first stages of the application of the treatments (mainly Pseudomonas sp.), which were found to be replaced during the treatment by representatives of the Firmicutes (mainly Bacillales), both of which are well known for their capability of CaCO3 precipitation (Boquet et al., 1973).
Taking together the results derived from this study (in situ application), with those derived from the studies of Jimenez-Lopez et al. (2008) and Piñar et al. (2010), performed under laboratory conditions, it seems clear that the application of the M-3P nutritional solution (inoculated or non-inoculated with M. xanthus) triggers an antagonistic behaviour among members of the phyla Proteobacteria and Firmicutes. Though there is an initial activation of the Proteobacteria, the proliferation of the Firmicutes produces a gradual disappearance of the first group. Piñar et al. (2010) suggested different strategies to explain this phenomenon. Firstly, the capability of some sporulating bacteria to develop predation behaviours in mixed cultures under nutritional stress by using an antibacterial factor (Kumar Nandy et al., 2007). Secondly, the capability of spore-forming bacteria to resist dry conditions on the stones (Shida et al., 1996; Sneath, 1986), thus being the microbial fraction of the total bacterial community able to dominate on the treated stone and be detectable by PCR-DGGE after long periods of time.
However, as mentioned before, it is difficult to compare our data with those derived from other investigations on the in situ application of stone consolidation treatments due to the lack of published data on long-term monitoring. One of the few published studies, performed by Le Métayer-Levrel et al. (1999), shows a long-term investigation concerning bacterial carbonatogenesis and its in situ application on the tower of the Saint Mėdar Church in Thouars. An area of 50 m2 on the Tuffeau limestone was treated with carbonatogenic bacteria and nutritional medium and further monitored after 6 months and one year. The authors analysed the quality of the biocalcin layer and mentioned that the development of carbonatogenic bacterial populations prevented the development of autochthonous acidifying bacteria. However no details concerning the identified bacteria before, during and after the treatments are provided.
A similar procedure was applied on Tuffeau and Saint-Maximim limestone statuaries placed under different climatic conditions, i.e. rural, urban, and littoral environments (Le Métayer-Levrel et al., 1999). The long-term monitoring of this procedure showed that in the urban site, the statues performed better. After four years of exposure to urban environment the formed biocalcin retained its protective effect (Orial, 2000) and only fungal colonization could be observed (Castanier et al., 2000). However, once more, no details of the identity of the microbial communities colonising these statuaries during and after the treatment are available.
Most of the methods proposed so far to consolidate ornamental stone based on the application of a bacterially-inoculated culture medium use culture media containing a considerable amount of carbohydrates (Adolphe et al., 1990; Castanier et al., 2000; Le Métayer-Levrel et al., 1999). In contrast, the culture medium used in our study (M-3P) had a particular composition lacking carbohydrates, while a pancreatic digest of casein was introduced as a source of carbon and nitrogen. The removal of carbohydrates as a source of carbon excludes the growth of organic-acid-producing microorganisms, while the growth of bacteria that use amino acids as a source of carbon and nitrogen is enhanced. The latter is of particular importance, because the oxidative deamination of the amino acids results in a release of ammonia, which alkalinizes the culture medium. This alkalinization favours the formation of calcium carbonate and in addition, limits the growth of fungi, which preferentially grow in more acidic conditions (Jimenez-Lopez et al., 2007, 2008). The inclusion of carbohydrates in the culture media used by Le Métayer-Levrel et al. (1999) explains the fungal colonization observed by Castanier et al. (2000) mentioned above. Fungi have to be avoided because they can produce undesirable acids due to their metabolic activity (Strzelczyk, 1981) and can colonize the treated stone and seriously interfere with the conservation treatment.
In our study, the successful long-term consolidation effects on the treated stones are noteworthy and in both buildings, to date, no detrimental or negative effects (colour change or fungi growth) have been observed. On the other hand, because of the good consolidation results obtained by using the sole application of a nutritional solution (González-Muñoz et al., 2008; Jimenez-Lopez et al., 2007, 2008; Jroundi et al., 2010) we advise the use of the product and method of González-Muñoz et al. (2008).
4.2. Comparison of the short term monitoring performed at the Monastery of San Jerónimo by culture-dependent and independent techniques
As mentioned in the Introduction section, Jroundi et al. (2010) performed a parallel short-term monitoring of the micro-biota of SJ by using culture-dependent techniques. Comparing the results obtained in the present study with those of Jroundi et al. (2010), that is: molecular versus conventional cultivation techniques, at the family level 60% of the cultured microorganisms could also be detected with molecular techniques during the first week of the application of the treatments. Species of 7 cultivated genera could not be found by molecular means, namely Sphingomonas, Diaphorobacter, Variovorax, Acidovorax, Stenotrophomonas and Pseudomonas. Nevertheless, in this study 2 new genera, namely Sphingobacterium and Flavobacterium were detected. Jroundi et al. (2010) isolated a total of 116 bacterial strains, which were tested on solid M-3P medium for their MICP ability. With the exception of 3 strains, 113 isolates apparently showed a high capacity to produce CaCO3 after 2 days of incubation. These results proved that the application of the conservation treatments boosted the growth of CaCO3 producing bacteria. Due to the sequence similarities between the isolated strains and our obtained clones, it is reasonable to postulate that the microorganisms identified by molecular means are also able to produce calcium carbonate.
4.3. Detection of M. xanthus on the sectors treated with a culture medium inoculated with this strain
Piñar et al. (2010) had already shown the limitations of using PCR-DGGE analysis to detect M. xanthus in a mixed population. In the present study, on both buildings, no band corresponding to the band of M. xanthus could be detected on the DGGE-profiles derived from sectors treated with the M. xanthus-inoculated culture (see DGGE profiles of sectors B and E). The construction of clone libraries and the isolation of different clones did not yield positive results for the detection of this microorganism. This can be explained by the difficulties of extracting the large genome of M. xanthus (9454 kbp) (Chen et al., 1991) from an environmental sample. The differences in the relative abundance of DNA fragments from variable microorganisms compete in a PCR reaction, making it much more difficult to amplify this specific DNA (Muyzer et al., 1993, and unpublished data from Ettenauer et al., 2010). In addition to the differences in genome size and copy number of 16S rRNA genes and hence the resulting variable relative abundance of the targeted sequence in the total microbial community, the differential or preferential amplification of rRNA genes by PCR using universal primers (Reysenbach et al., 1992) makes it even more difficult to monitor M. xanthus.
To overcome these limitations, the species-specific primers designed by Piñar et al. (2010) were used to detect the inoculated strain. By using these species-specific primers, we succeeded in detecting the targeted microorganism in the enrichment cultures of samples from the first and the seventh days of treatment in the case of SJ and the enrichment cultures of the first day of treatment in the RH. However, it is worth noting that cultivation assays performed by Jroundi et al. (2010) with the enrichment cultures from the Monastery of San Jerónimo yielded no positive results in the isolation of M. xanthus during the seven days of the treatment. Those results were to be expected considering the longer generation time of M. xanthus compared to that of other activated bacteria (Jimenez-Lopez et al., 2008) and hence the resulting higher cell numbers of the competitive bacteria. These results supported the results obtained by Piñar et al. (2010) showing the unfeasibility of isolating M. xanthus from a mixed culture, even if the detection of this microorganism is possible by using molecular techniques.
5. Conclusions
The molecular strategy used in this study offers a reliable tool to monitor the community dynamics occurring during and after the application of two independent in situ consolidation treatments. In addition, this strategy allows follow up of the dynamics of the inoculated M. xanthus strain.
Results derived from the short-term monitoring reveal the activation of calcium carbonate-producing bacteria. The application of the M. xanthus-inoculated medium resulted in rapid boosting of members of the Proteobacteria, whereas the sole M-3P nutritional solution triggered the growth of the Firmicutes, irrespective of the investigated building.
Furthermore, results derived from the long-term monitoring revealed that the consolidation treatments used in this study significantly changed the micro-biota remaining on the treated stones. On both buildings and irrespective of the presence of M. xanthus, similar dynamics could be observed leading to the disappearance of a heterogeneous microbial community which was replaced by a more homogeneous micro-biota, mainly composed of bacteria belonging to the Firmicutes. On the sectors treated with a M. xanthus-inoculated medium only Bacillus sp. could be detected, while members of the family Planococcaceae were dominant on the sectors treated with the M-3P solution. These bacteria are known for their capability to precipitate calcium carbonate and are used by other authors for the MICP on stones.
The finding of a micro-biota mainly consisting of carbonatogenic bacteria can further boost the consolidation treatments used in this study and enhance the protection and strengthening of the stone materials leading to long lasting effects. On the contrary, the micro-biota inhabiting the control sectors of both buildings, treated with water, remained heterogeneous when samples were monitored long-term, proving to be unaltered by this treatment.
In summary, this study calls for the necessity to include monitoring of the dynamics of the microbial communities inhabiting stone-works when they are subjected to bioconsolidation treatments as a parameter to evaluate the risks and benefits of such treatments. This evaluation should not only take into account the characteristics and appearance of the treated stone but also the ecology of the microbial communities remaining on the stone after the treatment.
The following are the supplementary materials related to this article.
Explanation of marked and numbered bands shown in Fig. 3A and B. The bands are explained in this table, describing the clone designation, the sequence length, the closest relative — as determined by comparative sequence analysis (partly including reference), the phylum of the clone, the similarity ranking, the accession numbers of each identified clone and additional information concerning the relativeness of this clone to sequences of clones found on other sectors of this building or on the Royal Hospital of Granada. A. Description of clones derived from non-treated and treated stone samples. B. Description of clones derived from pellet samples.
Explanation of marked and numbered bands from Fig. 4A and B. The bands are explained in this table, describing the clone designation, the sequence length, the closest relative — as determined by comparative sequence analysis (partly including reference), the phylum of the clone, the similarity ranking, the accession numbers of each identified clone and additional information concerning the relativeness of this clone to sequences of clones found on other sectors of this building or on the Monastery of San Jerónimo. A. Description of clones derived from non-treated and treated stone samples. B. Description of clones derived from pellet samples.
Acknowledgements
This work was financed by the “Proyecto de excelencia RNM-3943” of the Junta de Andalucía (Spain) and by the “Hertha-Firnberg-Nachwuchsstelle (T137)” of the FWF (Austrian Science Fund).
Contributor Information
Jörg Ettenauer, Email: Joerg.Ettenauer@boku.ac.at.
Guadalupe Piñar, Email: guadalupe.pinar@boku.ac.at.
Katja Sterflinger, Email: Katja.Sterflinger@boku.ac.at.
Maria Teresa Gonzalez-Muñoz, Email: mgonzale@ugr.es.
Fadwa Jroundi, Email: fadwa@ugr.es.
References
- Adolphe JP, Loubière JF, Paradas J, Soleilhavoup F. Procédé de traitement biologique d'une surface artificielle. European patent 90400G97.0 1990 (after French patent 8903517); 1989.
- Altschul S.F., Madden T.L., Schäffer A.A., Zhang J., Zhang Z., Miller W. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boquet E., Boronat A., Ramos-Cormenzana A. Production of calcite (calcium carbonate) crystals by soil bacteria is a common phenomenon. Nature. 1973;246:527–529. [Google Scholar]
- Castanier S., Le Metayer-Levrel G., Perthuisot J.P. Ca-carbonates precipitation and limestone genesis—the microbiogeologist point of view. Sediment Geol. 1999;126:9–23. [Google Scholar]
- Castanier S., Le Métayer-Levrel G., Orial G., Loubière J.F., Perthuisot J.P. Bacterial carbonatogenesis and applications to preservation and restoration of historic property. Of Microbes and Art. In: Ciferri O., Tiano P., Mastromei G., editors. The Role of Microbial Communities in the Degradation and Protection of Cultural Heritage. Kluwer Academic/Plenum Publisher; New York: 2000. pp. 201–218. [Google Scholar]
- Chen H., Kuspa A., Keseler I.M., Shimkets L.J. Physical map of the Myxococcus xanthus chromosome. J Bacteriol. 1991;173:2109–2115. doi: 10.1128/jb.173.6.2109-2115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Muynck W., De Belie N., Verstreate W. Microbial carbonate precipitation in construction materials: a review. Ecol Eng. 2010;36:118–136. [Google Scholar]
- Dick J., De Windt W., De Graef B., Saveyn H., Van der Meeren P., De Belie N. Bio-deposition of a calcium carbonate layer on degraded limestone by Bacillus species. Biodegradation. 2006;17:357–367. doi: 10.1007/s10532-005-9006-x. [DOI] [PubMed] [Google Scholar]
- Ettenauer J.D., Sterflinger K., Piñar G. Cultivation and molecular monitoring of halophilic microorganisms inhabiting an extreme environment presented by a salt-attacked monument. Int J Astrobiol. 2010;9:59–72. [Google Scholar]
- González-Muñoz M.T. Bacterial biomineralization applied to the protection-consolidation of ornamental stone: current development and perspectives. Coalition. 2008;15:p. 12–p. 18. [Google Scholar]
- González-Muñoz MT, Rodriguez-Navarro C, Jimenez-Lopez C, Rodriguez-Gallego M. Method and product for protecting and reinforcing construction and ornamental materials, publication number (Spanish patent, nº P200602030, WO2008009771); 2008.
- Jimenez-Lopez C., Rodriguez-Navarro C., Piñar G., Carrillo-Rosua F.J., Rodriguez-Gallego M., Gonzalez-Muñoz M.T. Consolidation of degraded ornamental porous limestone stone by calcium carbonate precipitation induced by the micro-biota inhabiting the stone. Chemosphere. 2007;68:1929–1936. doi: 10.1016/j.chemosphere.2007.02.044. [DOI] [PubMed] [Google Scholar]
- Jimenez-Lopez C., Jroundi F., Pascolini C., Rodriguez-Navarro C., Piñar G., Rodriguez-Gallego M. Consolidation of quarry calcarenite by calcium carbonate precipitation induced by bacteria activated among the micro-biota that inhabits the stone. Int Biodeterior Biodegrad. 2008;62:352–363. [Google Scholar]
- Jroundi F., Fernández-Vivas A., Rodriguez-Navarro C., Bedmar E.J., Gonzalez-Muñoz M.T. Bioconservation of deteriorated monumental calcarenite stone and identification of bacteria with carbonatogenic activity. Microb Ecol. 2010;60:39–54. doi: 10.1007/s00248-010-9665-y. [DOI] [PubMed] [Google Scholar]
- Kumar Nandy S., Bapat P.M., Venkatesh K.V. Sporulating bacteria prefers predation to cannibalism in mixed cultures. FEBS Lett. 2007;581:151–156. doi: 10.1016/j.febslet.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Le Métayer-Levrel G., Castanier S., Orial G., Loubière J.F., Perthuisot J.P. Application of bacterial carbonatogenesis to the protection and regeneration of limestone in buildings and historic patrimony. Sediment Geol. 1999;126:25–34. [Google Scholar]
- May E. 2005. Biobrush research monograph: novel approaches to conserve our European heritage. EVK4-CT-2001-00055. [Google Scholar]
- Muyzer G., De Waal E.C., Uitterlinden A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neefs J.M., Van de Peer Y., Hendriks L., De Wachter R. Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Res. 1990;18:2237–2317. doi: 10.1093/nar/18.suppl.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orial G. Restaurar la memoria. Valladolid; Spain: 2000. La biomineralisation appliquée à la conservation du patrimoine: bilan de dix ans d'experimentation. [Google Scholar]
- Pearson W.R. Rapid and sensitive sequence comparison with FAST and FASTA. Methods Enzymol. 1994;183:63–98. doi: 10.1016/0076-6879(90)83007-v. [DOI] [PubMed] [Google Scholar]
- Piñar G., Jimenez-Lopez C., Sterflinger K., Ettenauer J., Jroundi F., Fernández-Vivas A. Bacterial community dynamics during the application of a Myxococcus xanthus-inoculated culture medium used for consolidation of ornamental limestone. Microb Ecol. 2010;60:15–28. doi: 10.1007/s00248-010-9661-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reysenbach A.L., Giver L.J., Wickham G.S., Pace N.R. Differential amplification of rRNA genes by polimerase chain reaction. Appl Environ Microbiol. 1992;58:3417–3418. doi: 10.1128/aem.58.10.3417-3418.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Navarro C., Rodriguez-Gallego M., Ben Chekroun K., Gonzalez-Muñoz M.T. Conservation of ornamental stone by Myxococcus xanthus-induced carbonate biomineralization. Appl Environ Microbiol. 2003;69:2182–2193. doi: 10.1128/AEM.69.4.2182-2193.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. 2nd ed. Cold Spring Harbor Laboratory; Cold Spring Harbor, New York: 1989. Molecular Cloning: A Laboratory Manual. [Google Scholar]
- Schabereiter-Gurtner C., Piñar G., Lubitz W., Rölleke S. An advanced molecular strategy to identify bacterial communities on art objects. J Microbiol Methods. 2001;45:77–87. doi: 10.1016/s0167-7012(01)00227-5. [DOI] [PubMed] [Google Scholar]
- Shida O., Takagi H., Kadowaki K., Komagata K. Proposal for two new genera, Brevibacillus gen. nov. and Aneurinibacillus gen. nov. Int J Syst Bacteriol. 1996;46:939–946. doi: 10.1099/00207713-46-4-939. [DOI] [PubMed] [Google Scholar]
- Sneath P.H.A. 1st ed. vol. 2:1110. 1986. Endospore-forming Gram-Positive Rods and Cocci. (Bergey´s Manual of Systematic Bacteriology). Section 13. [Google Scholar]
- Strzelczyk A.B. Stone. In: Rose A.H., editor. Microbial Deterioration. Academic Press; New York: 1981. pp. 61–80. [Google Scholar]
- Teske A., Wawer C., Muyzer G., Ramsing N.B. Distribution of sulphate-reducing bacteria in a stratified fjord (Mariager Fjord, Denmark) as evaluated by most-probable-number counts and DGGE of PCR-amplified ribosomal DNA fragments. Appl Environ Microbiol. 1996;62:1405–1415. doi: 10.1128/aem.62.4.1405-1415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiano P., Biagiotti L., Mastromei G. Bacterial bio-mediated calcite precipitation for monumental stones conservation: methods of evaluation. J Microbiol Methods. 1999;36:139–145. doi: 10.1016/s0167-7012(99)00019-6. [DOI] [PubMed] [Google Scholar]
- Tiano P., Cantisani E., Sutherland I., Paget J.M. Biomediated reinforcement of weathered calcareous stones. J Cult Herit. 2006;7:49–55. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Explanation of marked and numbered bands shown in Fig. 3A and B. The bands are explained in this table, describing the clone designation, the sequence length, the closest relative — as determined by comparative sequence analysis (partly including reference), the phylum of the clone, the similarity ranking, the accession numbers of each identified clone and additional information concerning the relativeness of this clone to sequences of clones found on other sectors of this building or on the Royal Hospital of Granada. A. Description of clones derived from non-treated and treated stone samples. B. Description of clones derived from pellet samples.
Explanation of marked and numbered bands from Fig. 4A and B. The bands are explained in this table, describing the clone designation, the sequence length, the closest relative — as determined by comparative sequence analysis (partly including reference), the phylum of the clone, the similarity ranking, the accession numbers of each identified clone and additional information concerning the relativeness of this clone to sequences of clones found on other sectors of this building or on the Monastery of San Jerónimo. A. Description of clones derived from non-treated and treated stone samples. B. Description of clones derived from pellet samples.